Abstract
Lycopene is a group of phytochemicals found in nature, primarily in fruits and vegetables. Lycopene is thought to protect against a variety of diseases attributed to its antioxidant capabilities. Lycopene has anti-inflammatory, anti-cancer, and immunity-boosting qualities, among other biological and pharmacological benefits. COVID-19 (coronavirus disease 19) is an infectious disease caused by the SARS-CoV-2 virus, which has recently emerged as one of the world's leading causes of death. Patients may be asymptomatic or show signs of respiratory, cytokine release syndrome, gastrointestinal, or even multiple organ failure, all of which can lead to death. In COVID-19, inflammation, and cytokine storm are the key pathogenic mechanisms, according to SARS-CoV-2 infection symptoms. ARDS develops in some vulnerable hosts, which is accompanied by an inflammatory "cytokine syndrome" that causes lung damage. Immunological and inflammatory markers were linked to disease severity in mild and severe COVID-19 cases, implying that inflammatory markers, including IL-6, CRP, ESR, and PCT were significantly linked with COVID-19 severity. Patients with severe illness have reduced levels of several immune subsets, including CD4 + T, NK, and CD8 + cells. As a result, lycopene can be commended for bolstering physiological defenses against COVID-19 infections
Keywords: COVID-19, Lycopene, Pro-inflammatory cytokine, Immune response, Oxidative stress
Introduction
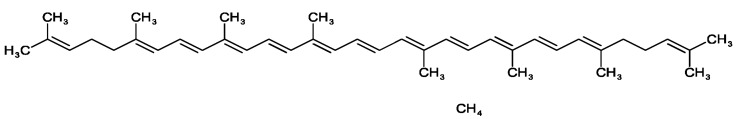
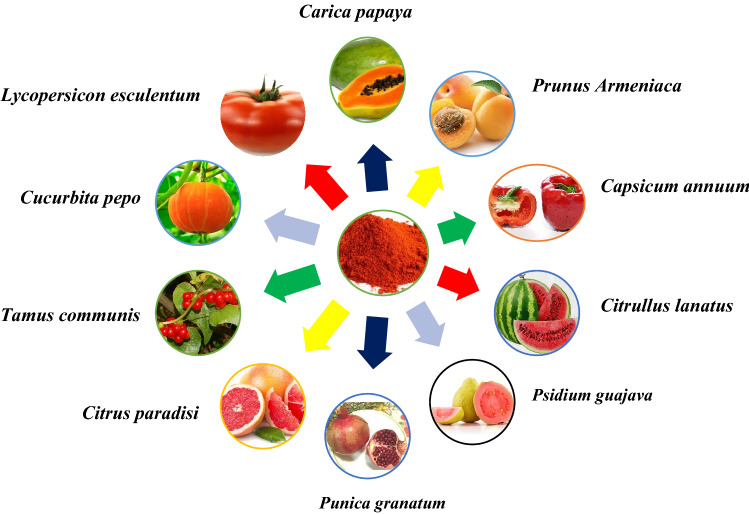
Wackenroder discovered an orange pigment in carrots (Daucus Carota) in 1831 and named it "Carotene" after the Latin term Carota (Pfander 1992). Carotenoids are antioxidants that may have an impact on the immune system (Mihailovic et al. 2021), exhibiting a wide range of biological effects in addition to acting as an immunomodulator, influencing T and B cell activity, inducing differentiation, and inhibiting cell proliferation in some cells, and influencing humoral and cellular immunity (Zhao et al. 2017). Lycopene is a dietary component that can be derived from a variety of plant sources. They are potential antioxidants and have been found to possess free radical scavenging activities and antioxidative (George et al. 2004; Shami and Moreira 2004). Color is the first thing a customer notices about a product, and it sets expectations for quality and flavor (Di Mascio et al. 1989; Magne et al. 2022). Lycopene and other hydrocarbon carotenoids are made completely of hydrogen and carbon. Lycopene has two non-conjugated double bonds and eleven conjugated double bonds. It's lipophilic, meaning it dissolves better in fat. Cis–trans isomerization happens due to their double bonds (see Fig. 1) (Rao and Rao 2007). Lycopene is also high in vitamins K, A, C, fiber, and carbohydrates, as well as a little amount of iron, potassium, phosphorus, and sulfur. Furthermore, it has a minimal sodium, fat, and calorie content. Tomatoes are a good source of lycopene, while other popular sources include watermelon, grapefruit, and guava (Fig. 2).
Fig. 1.
Chemical structure of lycopene (C40H56, Mw: 536.873 g/mL)
Fig. 2.
The main sources of lycopene
COVID-19 was discovered in Wuhan, China in December 2019 from a cluster of pneumonia patients linked to a seafood wholesale market (Nandan et al. 2021; Adelodun et al. 2022). The COVID-19 epidemic has infected practically every country and territory in the world (Tavakoli et al. 2020; Zhu 2019). Acute respiratory distress syndrome (ARDS) in COVID-19 patients is caused by the activation of a cytokine storm and inflammatory signaling pathways. The innate immune system is dysregulated when chemokines and pro-inflammatory cytokines are secreted in excess. This is observed in both COVID-19 and SARS, and viral load is unrelated to symptoms worsening in SARS (Mehta et al. 2020; Stebbing et al. 2020). A loss of beneficial lung surfactants, as well as increased oxidative stress and inflammation, characterizes ARDS (Wu et al. 2020).
A total of 6,324,112 deaths and 539,893,858 confirmed cases of COVID-19 have been reported to WHO as of June 23, 2022. SARS-CoV-2 is a single-stranded RNA virus with a genomic length of around 30,000 nucleotides belonging to the Betacoronavirus genus, one of four Coronaviridae genera that cause respiratory and enteric infections affecting both humans and animals (Zhu 2019). It has a modest substitution rate per site, ranging from 1.5 to 10–4. A prevalence of cytosine (C) to uracil (U) transitions characterizes sequence alterations, which can be attributed to pyrimidine deamination. There is a nearly twofold excess of U over C, resulting in 32% uracil and 18% cytosine (Hou 2020). When compared to SARS-CoV, the nucleocapsid proteins and envelope of 2019-n CoV/SARS-CoV-2 are two evolutionarily conserved regions, with 89.6% and 96% of sequence identity, respectively (Hou 2020). SARS-CoV-2 primarily infects bronchial epithelial cells and alveolar epithelial angiotensin-converting enzyme-2 (ACE2) pneumocytes, while immunodetection and RNA sequencing have shown that vascular muscle cells (VSMC) contain ACE2 (Barbry et al. 2020; Hamming et al. 2004; Nicin et al. 2020; Thum 2020).
In the initial stages of COVID-19 infection, delayed cytokine production was observed in respiratory epithelial cells, macrophages, and DCs. Pericytes and microvascular smooth muscle cells, but not endothelial cells, express ACE2, which could reveal significant details about COVID-19 pathology (He et al. 2020). The receptor-binding domain (RBD) of SARS-spike CoV-2's protein was quite similar to that of bat SARS-like coronaviruses. The discovery of several SARS-like COVs in bats has led to the hypothesis that these species serve as natural reservoirs for these viruses. Endocytosis of the SARS-CoV-2 virion is triggered by RBD binding to ACE2, exposing it to endosomal proteases (Wu et al. 2020b). A cytokine storm occurs in one out of every two fatal COVID-19 cases, with 82% of those over 60 years old (Paranjpe et al. 2020). Upregulation of several other pro-inflammatory mediators, including ACE2, is seen in the endothelial response to pericyte loss. Such pro-inflammatory endothelium changes, on the other hand, appear to occur even when there is no significant vascular inflammation. It's possible that if pericytes become infected with SARS-CoV-2, they'll become immunological targets. The increase in inflammation in severe COVID-19 cases could be due to the spread of microvascular inflammation (Varga et al. 2020).
Currently there are no treatment medicines that have been demonstrated to be effective against the virus (Ofuyatan et al. 2022). As a result of the pandemic, many supportive medications, such as ivermectin, and remdesivir which reduce viral RNA activity, were deployed. In combination with favipiravir, nafamostat mesylate therapy may allow for virus entry and replication suppression as well as prevention of the pathogenic host response, i.e. hyper-coagulopathy (Ikeda et al. 2020). The most promising antiviral agent is remdesivir, which has been licensed by the FDA for emergency use, although clinical trials are still underway (Mahboobipour and Baniasadi 2021; Wang et al. 2021a).
Immunotherapy can be employed in the most serious and life-threatening situations. Immunotherapy should not be given to patients who are currently unwell, such as those who have tuberculosis (Prattes et al. 2021).
The MERS and SARS outbreaks generated interest in such viruses, and researchers have found several antiviral targets, including polymerases, entry proteins, and viral proteases. However, more work is still needed to create drugs that target these pathways and can inhibit viral replication. Because SARS-CoV2 is an RNA virus, it can be detected using any of the existing RNA detection formats. When nasopharyngeal swabs are initially negative, repeat testing can be done to boost the chances of discovering SARS-CoV-2 in the nasopharynx (Loeffelholz and Tang 2020). Globally, COVID-19 treatment strategies are still limited. Vitamin (VA) has pharmacological efficacy in the treatment of pneumonia, it could be used in a SARS-CoV-2 antiviral treatment. VA's methods of action against SARS-CoV-2, according to bioinformatics research, include immunoreaction enrichment, inflammatory reaction suppression, and biological processes involving reactive oxygen species (Li et al. 2020b).
In humans, high amounts of lycopene can be found in the prostate, testes human plasma, and adrenal gland. It has been determined to be a safe substance for daily dietary use, with no adverse health effects even at the highest dose of 3 g/kg/day (Gupta et al. 2015). Lycopene was shown to improve the status of enzymatic activity in the cell and act as an important antioxidant—both enzymatic (catalase, glutathione reductase, and superoxide dismutase) and nonenzymatic (vitamins B, E, and C, carotenoids, and carnitines) antioxidants under normal operating conditions (Durairajanayagam et al. 2014). Lycopene is a natural antioxidant, anti-inflammatory, and antiviral agent that can help prevent and treat inflammatory diseases like COVID-19. Lycopene is a non-provitamin A and is well known for its antioxidant properties. Lycopene could be an effective pharmacological therapy for treating liver damage induced by long-term CS treatment (Rocha et al. 2021). In finishing pigs, dietary lycopene supplementation improved intestinal shape, and tight junction function inhibited inflammatory response and increased antioxidant capacity (Liu et al. 2021).
Currently, there is no effective anti-SARS-CoV-2 drug available to treat COVID-19 patients worldwide. In many diverse cultures, natural herbal treatments have been utilized for a long time to treat ailments. The effectiveness of traditional remedies produced from medicinal plants has contributed to the success of modern medicine. Antiviral drugs given early after the onset of symptoms can reduce viral shedding in patients' respiratory secretions, which lowers the risk of infection for contacts of those who are receiving treatment. The most prevalent clinical problems associated with the antiviral drug have been reported to be decreased appetite, constipation, and diarrhea (Ağagündüz et al. 2021). Remdesivir has been shown to have viral activity against coronaviruses in both Vitro and Vivo studies. Remdesivir inhibits SARS-CoV-2 (SARS-CoV-2 RdRp) replication, suppresses viral load, and protects animals infected with SARS-CoV-2 (Frediansyah et al. 2021). Therefore, the most promising Remdesivir antiviral drugs were taken into consideration to assess both their effectiveness and potential side effects on individuals with varying levels of COVID-19. Additionally, type I interferons are immunomodulatory medications that have been effective in treating COVID-19 early on because they have antiviral and pro-inflammatory properties (Hossain et al. 2021). The most common side effects of some patients who are taking remdesivir are increased aminotransferases and gastrointestinal symptoms (vomiting and/or nausea) (Fan et al. 2020). Understanding food-drug interactions are essential for improving the health of patients. Some antiviral drugs, including hydroxychloroquine, umifenovir, remdesvir, favipiravir, ribavirin, lopinavir, nitazoxanide, and oseltamivir, have the capability to interfere with food-drug and nutrition-drug interactions. However, it has been noted that the use of these drugs may have some negative adverse effects and comorbidities. Furthermore, the pharmacokinetic and pharmacodynamic alterations generated by these supportive care drugs can impact nutrient metabolism both acutely and chronically (Ağagündüz et al. 2021).
The molecular docking analysis revealed that 20 naturally occurring molecules from popular Iranian medicinal plants (such as astragalin, adlumidine, chelidimerine, catechin gallate, rutin, fumariline, somniferine, etc.) have antiviral properties that could protect individuals from contracting SARS-CoV-2 due to their potent binding patterns to the pocket of SARS-CoV-2-RdRp. As a result, these molecules act as inhibitors of the SARS-CoV-2 Mpro and RBD receptors (Mousavi et al. 2021). Lycorine, gemcitabine, and oxysophoridine are examples of bioactive alkaloids extracted from Chinese herbal remedies that have antiviral activity against SARS-CoV-2. The three compounds have also been reported to inhibit SARS-CoV-2 replication in Vero E6 cells at noncytotoxic concentrations. (Zhang et al. 2020b). Phytochemicals extracted from Avicennia officinalis have excellent antioxidant and cytotoxic properties. Using molecular docking assays, the three compounds (hydrocinnamic acid, dihydroartemisinin, and phenethyl alcohol) reported the highest binding affinities against the main protease of SARS-CoV-2. As a result, A. officinalis fruit and leaf extract compounds could be employed therapeutically in response to COVID-19 (Mahmud et al. 2021). Andrographis paniculate extracts and their andrographolide component significantly inhibited the SARS-CoV-2 in Calu-3 cells through their anti-SARS-CoV-2 activities (Sa-Ngiamsuntorn et al. 2021). The phytochemicals extracted from Oxalix pes-caprae have antiviral potential, and molecular docking analysis revealed that four natural compounds (Caeruleanone A, vadimezan, matairesinol, and 2′,4′-Dihydroxy-2′′-(1-hydroxy-1-methylethyl) dihydrofuro [2,3-h] flavanone) are promising SARS CoV-2 Mpro (6LU7 Mpro) inhibitors due to their strong binding affinity (Gul et al. 2022). Scutellaria baicalensis Georgi, a traditional medicine with several pharmacological properties, contains the major active component baicalein. In hACE2 transgenic mice, baicalein (200 mg/kg/day) significantly decreased SARS-CoV-2 virus replication and increase body weight. As a result, baicalein may be a potential therapeutic solution for COVID-19 treatment (Song et al. 2021). Curcumin has antiviral and anti-inflammatory properties, and it could be used to treat COVID-19 patients by reducing pro-inflammatory cytokine levels in the blood (Amalraj et al. 2017; Pawar et al. 2021). Flavonoids and the medicinal plants from which they are derived have a wide range of biological applications, including antioxidants, antivirals, antifungals, antibacterial, anti-inflammatory agents, and so on. Flavonoids and antiviral drugs (mundulinol and luteolin) have the maximum binding affinity and can act as potential inhibitors for the Mpro and SP of SARS-CoV-2 as compared to the antiviral drugs that are being used in COVID-19 treatment (Abd El-Mageed et al. 2021). The polyphenols extracted from the leaves and bud of Camellia sinensis were found to have potent antiviral activities and powerful Mpro inhibitors. It has also been reported that a large number of C. sinensis polyphenols (such as theaflavin (TF1), theaflavin-3O-O-gallate (TF2a), theaflavin-3O-gallate (TF2b), theaflavin-3,3O-gallate (TF3), epicatechin-3-gallate (EGCG), myricetin, hesperidin, and quercetin) serve as SARS-CoV-2 RdrP inhibitors (Tallei et al. 2021; Wang et al. 2021b; Zhang et al. 2021). Isorhamnetin, a flavonoid derived from sea buckthorn berries, inhibited the entry of SARS-CoV-2 spike pseudo-typed virus into HEK293/ACE2 cells in vitro and could be a new treatment for COVID-19 control (Zhan et al. 2021). SARS-CoV-2 S protein enters 293/hACE2 cells predominantly by endocytosis, and virus entry requires TPC2, PIKfyve, and cathepsin L. Tetrandrine was found to inhibit the entry of SARS-CoV-2-S pseudoviruses into HEK293/ACE2 cells, most likely as a result of its ability to suppress TPC2, which may be involved in the SARS-CoV-2 endocytic pathway (Ou et al. 2020). Naringenin exhibited moderate anti-SARS-CoV-2 efficacy and its treatment potential for COVID-19 infection. It was observed that it reduced the expression of ACE2, SARS-CoV-2 3CLpro inhibition, and targeted TPCs in Vero E6 cells (Abdallah et al. 2021).
COVID-19 symptomatology, epidemiology, and pathogenesis
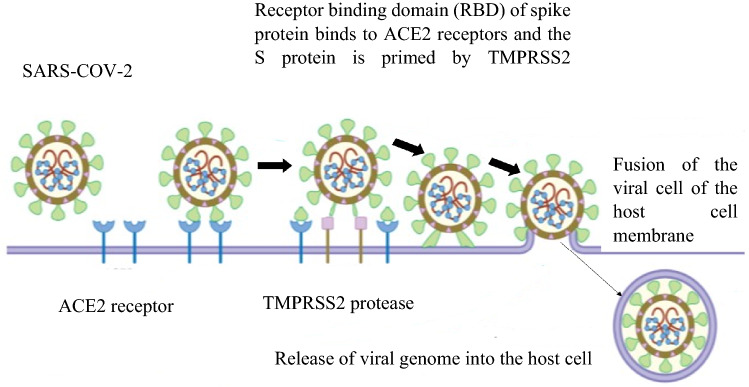
COVID-19 patients have been linked to a wide spectrum of symptoms, from moderate to severe. The most common are headache, cough, fever or chill, vomiting, difficulty breathing, lack of smell or taste, body aches, and fatigue. However, many patients also have congestion, dyspnea, cardiac damage, multi-organ failure, and shock. Symptoms emerge 2–14 days after exposure to the virus (Zhong et al. 2020). A nasopharyngeal or oropharyngeal swab is used to diagnose a patient, which permits the virus to be isolated and verifies the infection (Holshue et al. 2020; To 2020). Severe COVID-19 patients might suffer a second set of symptoms and problems later in the disease's progression, including neurological manifestations, pulmonary embolism, stroke, myocardial infection, acute renal injury, and venous and arterial thrombosis. In COVID-19, extrapulmonary symptoms have a substantially higher death rate than pulmonary signs (Diao et al. 2021; Helms et al. 2020; Mao et al. 2020; Mehta et al. 2020; Tang et al. 2020). When the virus enters the host, the viral RNA genome is released into the host cell cytoplasm and then replicates. The ACE2 receptor is being used by the SARS-CoV-2 virus to enter cells (Fig. 3). SARS-CoV-2 can be transmitted through direct (human-to-human) and indirect contact (airborne contagion and contaminated objects). This virus can persist in the human body for at least four days (Bandyopadhyay 2021; Chu et al. 2020; Li et al. 2020b). The spread of COVID-19 to healthcare personnel poses the greatest danger. Healthcare workers made up 21% of those infected by the SARS pandemic in 2002 (Chang et al. 2020). The spike protein's furin-cleaved S1 component binds directly to NRP1 on the cell surface, and utilizing a small-molecule inhibitor or monoclonal antibodies to block this interaction prevented virus replication in cell culture (Cantuti-Castelvetri et al. 2020; Daly et al. 2020; Haslberger et al. 2020).
Fig. 3.
SARS-CoV-2 binds to ACE 2 as the host target cell receptor, resulting in viral cell fusion with the host cell membrane via transmembrane serine protease 2
The pathogenic characteristics of COVID-19 are strikingly similar to those of SARS and MERS coronavirus infections but have a lower overall fatality rate. In severe cases of COVID-19 infection, the cytokine storm could trigger a strong immune system attack on the host, resulting in ARDS, multiple organ failure, and death (Li et al. 2020b). Currently, it is considered that these coronaviruses can only survive for a few days outside of living cells in the environment, but this may be enough time for them to spread to other organisms, mutate and change their properties, and so on. For the foreseeable future, a variety of situations should be addressed (Núñez-Delgado 2020). In DT-induced mice, lycopene therapy improved mitochondrial performance by reducing oxidative stress, heart hypertrophy, cardiac fatty acid accumulation and viral infection (Wang et al. 2022). Lycopene has a protective effect against an increase in the serum level of creatine kinase MB in individuals undergoing percutaneous coronary intervention (PCI). This could point to lycopene's possible function in preventing PCI-induced myocardial damage (Arakeri et al. 2020).
Risk factors of COVID-19
Age, male gender, and underlying health issues like asthma, diabetes, cardiovascular disease, obesity, neurological, diabetes, hypertension, chronic lung diseases, and kidney diseases, all of which are thought to be the risk factors for COVID 19 (Table 1). The majority of the patients exhibited lymphopenia, thrombocytopenia, abnormal liver functions, and immune responses (Goyal et al. 2020; Lazzerini et al. 2020; Quartuccio et al. 2020). SARS-CoV-2 is mostly a respiratory infection, but it has the potential to impact other organ systems as well. COVID-19 has been demonstrated to disrupt practically every organ system, including the heart, brain, vascular, hematopoietic, liver, skin, and others, causing concerns such as abrupt cardiac failure, thrombosis, immunological dysfunction, and metabolic abnormalities (Li et al. 2021a). Diabetes, dyslipidemia, and oxidative stress are examples of proatherogenic stimuli that can cause endothelial dysfunction and, as a result, vascular dysfunction, leading to the development of atherosclerotic arterial disease, which can lead to coronary artery disease, acute coronary syndrome, stroke, and thrombosis. The disorders have also been linked to severe COVID-19 infections (Chistiakov et al. 2015; Fang et al. 2020; Stefan et al. 2020; Yuan et al. 2007). COVID-19 patients with severe CKD have a far higher risk of mortality than other known high-risk categories, such as those with obesity, hypertension, chronic heart disease, or lung illness (Gansevoort and Hilbrands 2020). Pregnant women are also at a high risk of contracting COVID-19, because the presence of ACE2 receptors in the placenta may further increase the risk of virus transmission from mother to baby. Normal pregnancy and COVID-19 both have decreased lymphocytes and NKG2A inhibitory receptors, as well as elevated ACE2 (Fig. 4), IL-8, IL-10, and IP-10 (Phoswa and Khaliq 2020). Obesity was linked to a threefold increase in the risk of COVID-19. Each unit of BMI increased the chance of severe COVID-19 by 12% (Alberca et al. 2021; Gao et al. 2020). Diabetes patients are more likely to develop an inflammatory storm, which leads to the rapid deterioration of COVID-19 (Guo et al. 2020). Increased levels of ACE 2 could be one of the mechanisms by which COVID-19 patients are affected by cardio-cerebrovascular illness. Patients with the cardio-cerebrovascular disease were more prone to developing a myocardial injury, increasing their risk of becoming critically ill and dying (Yu et al. 2021a). Severe patients had lower levels of hemoglobin, monocytes, lymphocytes, eosinophils, and platelet counts than non-severe patients while having higher neutrophil counts among the CBC indices. Severe and non-severe COVID-19 cases had significantly different renal function tests and liver, glucose levels inflammatory/infection indicators, and serum electrolytes (Feng et al. 2020). Innate and adaptive immunity in women is stronger, and they are more resistant to viral infections than men (Flanagan et al. 2017). In children with COVID-19, blood levels of TNF-α and IL-6 were increased at diagnosis than in children with other infectious illnesses. However, these cytokine levels did not correspond with disease severity, which is likely due to the fact that the small cohort included mostly moderate cases (Curatola et al. 2021). Children who contract the SARS-CoV-2 have better results than adults (Qian et al. 2021). Due to a less robust immune response, older age was linked to a higher risk of developing ARDS and death. Although the high temperature was linked to the development of ARDS, it was also linked to improved outcomes in ARDS patients. Furthermore, methylprednisolone medication may be advantageous for people who develop ARDS (Wu et al. 2020a).
Table 1.
A risk factor for COVID-19
| Risk factor | Virus | Mortality rate | Remark | Cited |
|---|---|---|---|---|
| Older age | SARS-CoV-2, SARS-CoV-1, MERS | 10.0, vs. 4.9% among those with an age greater than 65 years | Elderly people have a higher risk of developing severe COVID-19 | (Mehra et al. 2020; Petrilli et al. 2020; Williamson et al. 2020; Zhu 2019) |
| Cardiovascular diseases | SARS-CoV-2 | 10.2, vs. 5.2% among those without disease | COVID-19 mortality is significantly high in patients with cardiovascular disease | (Galbadage et al. 2020; Petrilli et al. 2020; Williamson et al. 2020; Zhu et al. 2020) |
| Coronary artery diseases (CAD) | SARS-CoV-2 | 15.3, vs. 5.6% among those without diseases | Patients with CAD had more severe conditions than non-CAD patients, according to studies | (Mehra et al. 2020) |
| Current vs former smoking | SARS-CoV-2 | 9.4, vs. 5.6% among former smokers or non-smokers | COVID-19 infection is more common in smokers | (Mehra et al. 2020; Williamson et al. 2020) |
| Cardiac arrhythmia | SARS-CoV-2 | 11.5, vs. 5.6% among those without arrhythmia | In patients with COVID-19, there is an increased risk of cardiac arrhythmia | (Mehra et al. 2020; Williamson et al. 2020) |
| Type 2 diabetes (T2D) | SARS-CoV-2 | 7.8 vs. 2.7% | Diabetes patients are more likely to have a severe COVID-19 clinical course and a higher mortality rate | (Williamson et al. 2020; Zhu et al. 2020) |
| Obesity | SARS-CoV-2 | The case-fatality rate (CFR) was 2.3% among the 44,672, with obesity accounting for 7.3% (1,023) of the deaths with COVID-19 | Through immunological, biochemical, and anatomical mechanisms, obesity is a highly risk factor for severe COVID-19 | (Petrilli et al. 2020; Williamson et al. 2020; Yu et al. 2021b) |
| Sex | SARS-CoV-2, MERS | Men 60% versus 40% women | When compared to women, men have a higher risk factor for COVID-19 | (Docherty et al. 2020; Galbadage et al. 2020; Williamson et al. 2020) |
| Kidney diseases | SARS-CoV-2, MERS | 16% (2830 deaths out of 17,506 cases confirmed) | Chronic kidney disease was associated with higher mortality in hospitals | (Bhatraju et al. 2020; Cheng et al. 2020; Docherty et al. 2020; Williamson et al. 2020) |
| Hypertension | SARS-CoV-2, MERS | The CFR was 6.0 percent (1023 deaths out of 44,672 cases confirmed) | Hypotension might face a greater risk of developing into a critical or mortal condition | (Williamson et al. 2020; Wu and McGoogan 2020) |
| Cancer | SARS-CoV-2, MERS | The CFR was 5.6% (1023 deaths out of 44,672 confirmed cases) | Covid-19 causes severe problems in cancer patients | (Wu and McGoogan 2020) (Moni and Liò 2014; Williamson et al. 2020) |
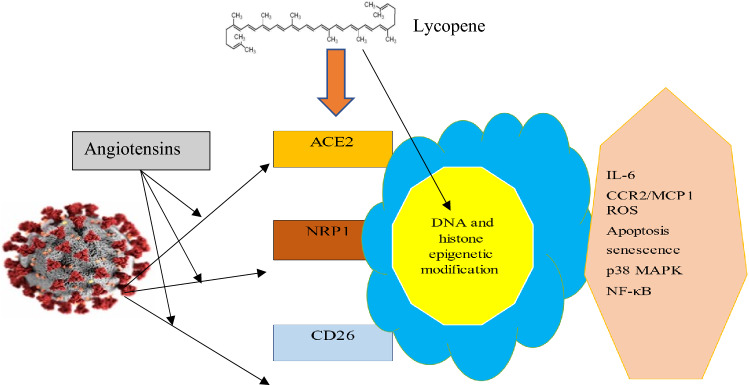
Fig. 4.
This diagram demonstrates the entry of SARS-CoV-2 into the cell, as well as potential lycopene-based prevention measures (Cantuti-Castelvetri et al. 2020; Haslberger et al. 2020; Raha et al. 2020)
Lycopene suppresses inflammatory reactions during COVID-19 infection
Severe COVID-19 infection is characterized by a large pro-inflammatory response or cytokine storm, which ultimately in ARDS and multi-organ failure. During COVID-19 infection, proinflammatory cytokines are released, causing infection of endothelial inflammation. When pro-inflammatory cytokines activate endothelial cells, they become more permeability to macromolecules that might cause extensive lung damage (Varga et al. 2020). The cytokine release syndrome (CRS) can occur directly as a result of viral damage or indirectly by overactivation of the immune system that triggers the infiltration of immune cells such as neutrophils and macrophages into the tissue. COVID-19 Patients may experience mild to moderate symptoms due to massive cytokine production, which typically leads to elevated inflammation markers, and disease severity (Darif et al. 2021; Griffith et al. 2014).
A cytokine release syndrome (CRS) was linked to COVID-19. The inflammatory pathway that promotes lung parenchymal injury and thromboembolic events appears to be triggered by CRS. Interleukin-6 is a key cytokine in the development of CRS, which causes hyper-inflammation, ALI, and, in some cases, irreversible lung tissue damage (Alharthy et al. 2020). SARS-CoV-2 infection increases the number of ACE2-positive endothelial cells in COVID-19-induced respiratory failure (ARDS). SARS-CoV-2 infection of endothelial cells produces endothelial cell swelling and disintegration, abnormal microvascular architecture, and endothelial dysfunction (Ackermann et al. 2020a). ACE2 is a protein found in various human organs that serves as a negative regulator of the renin-angiotensin system. Patients with aberrant pulmonary inflammation and immune responses can have either very high or very low ACE2 levels (Sodhi et al. 2019).
COVID-19 severity is linked to secondary haemophagocytic lymphohistiocytosis (sHLH), and several studies have found a significant increase in levels of various cytokines and chemokines such as TNF-α, IL-2, IL-7, macrophage inflammatory protein 1-α, interferon-γ inducible protein 10, granulocyte colony-stimulating factor, and monocyte chemoattractant protein 1 (Huang et al. 2020b). IL-6 is a key proinflammatory cytokine that also plays a role in inflammation, immune system modulation, and hematopoiesis. As a result, IL-6 could be used to initiate therapies and monitor pro-and anti-inflammatory responses (Ritchie and Singanayagam 2020). Indeed, inhibiting the NF-κB pathway could regulate the number of NOS and NO content, as well as diminish the production of cytokines and chemokines (Li et al. 2017).
When the SARS-CoV-2 S protein binds to the ACE2 receptor, the pro-inflammatory pathway is activated, with the Ang II hormone directly activating pro-inflammatory responses and the release of cytokines. The SARS-CoV-2 virus has recently been shown to use the TNF-α pathway to release pro-inflammatory cytokines such as TNF-α and IL-6. TNF-α overproduction is caused by ADAM-17 angiotensin II overactivation, which is mediated by virus-induced ACE2 deficiency (Mahmud-Al-Rafat et al. 2020; Scialo et al. 2020).
NF-κB is a key transcription factor for a wide range of genes involved in cellular immunity and inflammation. Activated NF-κB is crucial for a full-fledged cytokine storm because it regulates the transcription and synthesis of chemokines and inflammatory cytokines that are involved in the acute inflammatory response (LIAO et al. 2005). As a result of cytokine storms and inflammatory responses, many COVID-19 patients may die. As a result, anti-inflammatory drugs may be able to reduce the severity of the condition and the rate of fatalities (Dhama et al. 2020; Huang et al. 2020a; Mehta et al. 2020). The release of IL-1β triggers the production of IL-6, a cytokine that raises C reactive protein (CRP) and has been associated with the COVID-19 cytokine storm as a significant proinflammatory factor (Qin et al. 2020; Zhang et al. 2020a). Patients with COVID-19 infection have been shown to have high amounts of pro-and anti-inflammatory cytokines in their blood than those with uncomplicated SARS. Airway epithelial cells infected with SARS-CoV also produce a high amount of CXCL-10, IL-6, CXCL8, IFN-α, and CCL5. Severe SARS patients, on the other hand, had relatively low levels of the anti-inflammatory cytokine IL-10 (Cameron et al. 2008; Huang et al. 2005; Zhang et al. 2004).
The inflammatory response was triggered by ATR (atrazine), which increased NO (nitric oxide) generation and caused heart injury. By regulating NO (nitric oxide) and NOS (nitric oxide synthase) generating systems and inhibiting the TRAF6-NF-κB pathway, supplement lycopene dramatically reduces heart damage (Li et al. 2017). Lycopene suppresses the production of proinflammatory cytokines and chemokines in macrophages (Lee et al. 2012; Marcotorchino et al. 2012). Lycopene improved blood cell and hepatic lipid function, boosted high-density lipoprotein cholesterol, reduced TNF-α and malondialdehyde, and increased hepatic antioxidant activity (Róvero Costa et al. 2019). Lycopene's anti-inflammatory actions are mediated via a reduction in TNF-α, NO, and IL-6 release, which leads to a reduction in uveal inflammation. Lycopene's anti-inflammatory and antioxidant properties may make it useful in the treatment of eye inflammation (Göncü et al. 2016). Lycopene's anti-inflammatory properties were linked to an M2-dominant phenotype in adipose and hepatic macrophages. Lycopene reduced HFD-stimulated insulin resistance and inflammation in epididymal white adipose tissues and the liver by easing M2-dominant polarization in adipose tissue macrophages (Chen et al. 2019). Interleukin-1, Interleukin-6, and NF-κB-p65 expression were downregulated in the PA control, but Interleukine-10 expression was elevated owing to lycopene therapy. Lycopene normalized severe brain vacuolation was seen in the histopathology of PA control rats (Ugbaja et al. 2021). Lycopene possesses anti-obesity and anti-diabetic properties in a variety of organs and tissues, including adipose tissue, liver, kidney, pancreas, brain, ovaries, stomach, and eyes. Several studies have revealed that lycopene consumption has antioxidant properties as well as immunological and inflammatory functions (Agarwal and Rao 2000; Zhao et al. 2020; Zou et al. 2013).
A dysfunctional immune response to COVID-19 could be enhanced by lycopene
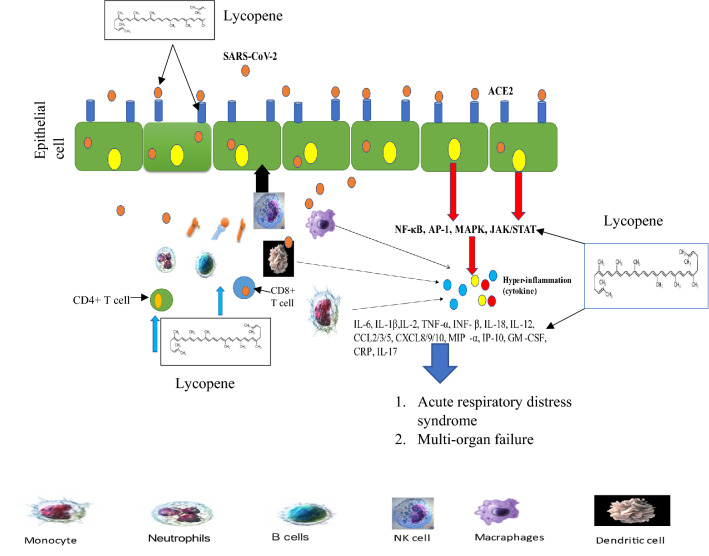
COVID-19 disease, which is occurred by the SARS-CoV-2 virus, is linked to several physiopathological mechanisms that mobilize a wide range of biomolecules, most of which are immunological (Fig. 5). In its most serious conditions, hyperproduction of primarily proinflammatory cytokines, which preferentially target lung tissue, can significantly worsen the prognosis (Costela-Ruiz et al. 2020). During SARS-CoV2 infection, CD4 + and CD3 + T cells and the total lymphocyte count were low, and IL-6, ESR, and CRP were elevated, especially in severe COVID-19 patients (Iwamura et al. 2021). SARS-CoV-2 induces lymphopenia, dysfunction, lymphocyte activation, higher cytokine levels, as well as monocyte and granulocyte abnormalities, increase in immunoglobulin G (IgG) and total antibodies. As a result, decreasing inflammation and improving lymphopenia may be beneficial therapeutic methods for COVID-19 patients (Yang et al. 2020).
Fig. 5.
A diagram showing COVID-19 pathogenesis can lead to ARDS and multi-organ failure. Lycopene can disrupt excessive inflammatory cytokine production
Both T and B cell responses to SARS-CoV-2 are identified in the blood approximately one week after the start of COVID-19 symptoms. CD4 + and CD8 + T cells are important in acute infection. COVID 19 has a hyper-inflammatory innate immune system and a defective adaptive immune system due to severe lymphopenia, decreased functioning, and exhausted T lymphocytes. CD8 + T cells are important for directly attacking and killing virus-infected cells, whereas CD4 + T cells are crucial for priming both B and CD8 + T cells and also for cytokine production to drive immune cell recruitment (Zheng et al. 2020). Severe COVID-19 patients have higher levels of ferritin, D-dimer, CRP, IL-6, and TNF-α in their blood, as well as a lower total number of NK cells, CD8 + cells, and CD4 + cells, as well as lower levels of basophils, monocytes, and eosinophils (Catanzaro et al. 2020; Janssen et al. 2021; Tay et al. 2020).
Furthermore, SARS-COV-2 was seen in the peripheral blood to impair components of host immunity, including an increase in fatigued CD8 + T cells and a decrease in CD4 + T cell activity, all of which point to a Th17 (Tay et al. 2020). When SARS-CoV-2 attaches to Toll-like Receptors (TLR), pro-IL1β is released, which leads to the production of mature IL-1β, which induces pulmonary inflammation, fever, and fibrosis. Most members of the IL-1 family of cytokines produce inhibitory cytokines such as IL-38 and IL-37. Immunological cells include B cells and macrophages. IL-38 is a cytokine that could be utilized to treat inflammation caused by viral infections, particularly coronavirus-19 infections. IL-37 performs its anti-inflammatory function via interacting with the IL-R5 or IL-18Ra receptors and may be explored as a therapeutic drug (Conti et al. 2020). The N-COV virus enters the cell by latching onto the protein receptor ACE2 and slinking within, after which it replicates and weakens our immune system. The lungs and colon have the most receptors. When the virus stays in the respiratory tract, it causes cough and cold, and when it inflames the hypothalamus, it causes diarrhea. When the virus inflames the hypothalamus, the fever rises (Koff and Williams 2020).
Innate immune cells have a vital role in activating a specific and effective immune response to SARS-CoV-2 infection, which is used as a biomarker for tissue damage in COVID-19 cases with severe tissue damage. The inflammatory response to SARS-CoV-2 has numerous clinical trials exploring the effectiveness of anti-inflammatory therapeutics against COVID-19. Lycopene also influences immunoglobulin production, increases IgA, IgG, and IgM levels in the blood, and boosts immunity (Kaneko et al. 2008), promoting cell-to-cell communication and increasing immunological response (Olson et al. 2008). Lycopene has immunomodulatory effects, as evidenced by the circulating percentage of lymphocytes, blood IgG concentrations, and ewe colostrum. Corn treated with lycopene increased the level of immunoglobulin G in sheep's blood, which facilitated passive immunity transfer to newborn lambs (Fallah et al. 2021). Dietary lycopene supplementation may increase antioxidant activity and ameliorate AFB1-induced intestinal immune function and barrier function impairment in the intestinal mucosa (Sarker et al. 2021). In diabetic rats, lycopene has anti-anemic properties and improves immunity. Low platelet counts, a low neutrophil–lymphocyte ratio, steady albumin, a drop in neutrophil counts, and low globulin content were among the findings. Lycopene may aid in the regulation of basic hemato-physiological variables (Eze et al. 2019). Lycopene is an anti-inflammatory antioxidant with antioxidant effects. Lycopene reduced intestinal damage, intestinal immunoglobulin A depletion, and bacterial translocation when given before the ischemia–reperfusion event (İkiz et al. 2021). In severe COVID-19 cases, there was increased pro-inflammatory cytokine and chemokine production. Lycopene inhibits the synthesis of proinflammatory cytokines and chemokines in macrophages, reducing inflammation in a variety of organs (Lee et al. 2012; Marcotorchino et al. 2012). Aside from regulating chronic immunological and inflammatory processes and delaying dendritic cell maturation (Kim et al. 2004).
Oxidative stress of COVID-19
Severe COVID-19 frequently experiences respiratory distress that is treated by oxygen medication and may result in oxidative stress and ARDS. It has been reported that increased nitro-oxidative stress contributes to the development of ARDS (Mach et al. 2011; Park et al. 2009). Even in cases where people are dying from chronic ARDS, there is nitrosative stress. SARS-CoV-2 appears to be localized to macrophages and type I pneumocytes (Massaro et al. 1975). Nearly all patients with viral infections have chronic oxidative stress, which has an impact on disease pathogenesis including impaired immune function and inflammatory response (Nin 2012). According to some researchers, the development of severe lung damage in SARS-CoV patients is related to oxidative stress produced by cell activation, which may have a substantial effect on the pathogenesis of COVID-19. In general, cytokine production, inflammation, and cell death are linked to respiratory virus infections, which could be associated with oxidative (Delgado-Roche and Mesta 2020).
The Fenton reaction,
The first step: Fe3+ + •O2 − → Fe2+ + O2.
The second step: Fe2+ + H2O2 → Fe3+ + OH − + •OH (Khomich et al. 2018; Sies 2020).
ROS is well-known for both its beneficial and harmful characteristics. Free radicals produced by RNA virus infections may have a function in a range of features of viral illness pathogenesis. During allergic and nonallergic inflammation, the inflammatory cells produce ROS (Chernyak et al. 2020).
However, ROS released by viral illness should not be regarded only as destructive agents, since they are crucial for the eradication of viruses phagocytosed by immune cells and also contribute to signaling between immune cells. Antioxidants may also help to reduce the viral load by minimizing oxidative stress. Most importantly, antioxidants tend to be appropriate during the stage of COVID-19 when inflammatory reactions must be inhibited. Such therapy is believed to protect organs and tissues from cytokine storms or oxidative stress (Assimakopoulos and Marangos 2020; Yang et al. 2013). ACE2 and TMPRSS2 gene expression has been found mostly in alveolar epithelial type II cells, which play a role in SARS-CoV pathogenesis (Sungnak et al. 2020; Ziegler et al. 2020). During COVID-19, increased levels of angiotensin-converting enzyme 2 (ACE2) served as an entrance receptor for SARS-CoV-2. Ang II is involved in the initiation and progression of inflammatory response. Low ANG II levels and high tACE2 levels give SARS-CoV-2 greater pathogenicity; a high ratio suggests the virus is less harmful and causes oxidative stress (free RONS) (Ackermann et al. 2020b; Fodor et al. 2021). When a patient is infected with SARS-CoV-2, phagocytes get activated, causing reactive oxygen species (ROS) to generate. Angiogenesis appears to be caused by endothelial dysfunction and hypoxia within lung injury foci during COVID19 (Varga et al. 2020).
Endothelial NOX2 is a prominent ROS producer, and AT1R is a key ATII receptor that stimulates multi-layered signaling in endothelial cells (Forrester et al. 2018). The possible causes of the pore opening of the mitochondrial permeability transition pore (mPTP), which is regulated by such a protein in the mitochondria, may increase the level of mtROS generated by NOX2. As a result, ATII increased mtROS generation, which in turn increased NOX2 activity (Bernardi et al. 2015; Wacquier et al. 2020).
Endothelial cells may undergo ROS-dependent apoptosis as a result of cytokine storms. The activation of endogenous antioxidant mechanisms is another strategy for combating oxidative stress during COVID 19. The transcription factor Nrf2, for example, regulates the production of antioxidants and other cell defense mechanisms (Zinovkin and Grebenchikov 2020). The generation of ROS is a typical element of cellular aerobic metabolism, which involves the respiratory and oxidizing of nutrients to generate energy. antioxidant mimetics or Nrf2 inducers could be used to treat viral-induced infections including respiratory problems and other infections that are correlated to a decrease in cellular antioxidative (Komaravelli and Casola 2014).
Hepcidin is important for maintaining iron homeostasis. Although iron is essential, its free form is likely to be involved in oxidation–reduction reactions, resulting in free radical production and oxidative stress (Daher et al. 2017; Kell and Pretorius 2014). The hyperferritinemia seen in COVID-19 patients could be a result of inflammation. In an era of multidrug-resistant viruses, iron chelation appears to be a promising and logical adjuvant therapy for viral infections (Schmidt 2020).
Lycopene is an extremely strong antioxidant, and this is a key mechanism of lycopene action. Its exceptional ability to quench ROS is due to its eleven conjugated double bonds (Heber and Lu 2002). Lycopene's nutraceutical effects have been reported in cancer, infertility, metabolic syndrome, and liver damage patients (Grabowska et al. 2019). In the livers of obese rats, dietary lycopene (50 mg/kg) reduced oxidative stress, inflammation, and metabolic problems caused by long-term consumption of a high-fat diet (HFD) (Albrahim and Alonazi 2021). For the rise in the COVID-19-related risk of death, Nrf2 has powerful antiapoptotic effects and anti-inflammatory in endothelial cells. Lycopene reduced the oxidative damage caused by zearalenone (ZEA) in Sertoli cells (SCs) by increasing Nrf2 pathway expression and lowering autophagy and apoptosis in piglet SCs (Cao et al. 2021). In HFD-fed mice, lycopene (10 mg/kg) treatment had better anti-lipidemic and anti-antioxidant benefits than Moringa (400 mg/kg), most likely due to its ability to reduce oxidative stress (Greish et al. 2021). Lycopene alleviated palmitic acid (PA)-induced neuroinflammation in female Wistar rats through lowering oxidative stress and downregulation of NF-ĸB-p65 expression (Ugbaja et al. 2021).
During transient ischemia, lycopene has been proven to protect the brain from ischemia-induced damage. Lycopene protected SHSY5Y cells from oxygen–glucose deprivation (OGD)-induced autophagic death by decreasing oxidative stress-dependent activation of the AMPK/mTOR pathway (Li et al. 2021b). In vitro, neuronal damage was alleviated, reactive oxygen species generation was reduced, and cell viability was successfully increased by lycopene pre-treatment. As a result, lycopene can be useful in the treatment of oxidative stress-related AD (Huang et al. 2019).
Lycopene (20 mg/kg) therapy for 10 days reduced testicular function and produced oxidative stress in Swiss albino mice to reduce the adverse effects of ZEA exposure. In the kidneys and testes, ZEA decreased GST activity while increasing SOD activity in the liver (Boeira et al. 2014). The anti-inflammatory and antioxidant effects of lycopene have been demonstrated to reduce oxidative stress brought on by an excess of APAP and the ensuing liver damage in C57BL/6 mice (Bandeira et al. 2017). Lycopene can lower the risk of varicocele brought on by oxidative stress by boosting TAC levels and TAC enzymatic activities (Babaei et al. 2021) (Table 2).
Table 2.
Various health benefits of lycopene
| Model | Lycopene (route/dose) | Analysis | Main outcomes | Duration | Conclusion | Cited |
|---|---|---|---|---|---|---|
| Male C57BL/6 mice | 25 and 50 mg/kg/day | ELISA | TNFα↓, IL-6↓, IL-10↓, ROS production↓, SOD↓, NF-κB↓ | 12 h | Lycopene, via reducing oxidative stress and inflammation, was effective in preventing liver damage induced by long-term CS exposure | (Rocha et al. 2021) |
| Male Sprague–Dawley rats | 5 mg/kg BW | ELISA | ↑CAT, ↑SOD, ↑GPx, TNF-α↓, CRP↓ | 10 weeks | In T2DM rats, lycopene could reduce oxidative stress by reducing glucose and lipid peroxidation, as well as reduce inflammatory events | (Zheng et al. 2019) |
| STZ-induced diabetic Wistar rats | 10 or 20 mg/kg/BW | ELISA | SOD↑, GSH-Px↑, MDA↓ | 10 weeks | In diabetic rats, lycopene enhances glycolipid metabolism by reducing oxidative stress | (Yin et al. 2019) |
| Wistar male rats | 4 mg/kg | ELISA, spectrophotometer | MDA↓, TOS↓, NF-κB↓, TNFα↓ | 3 months | Lycopene can alleviate inflammatory responses by reducing oxidative stress | (Icel et al. 2019) |
| Adult male Wistar albino rats | 50 mg/kg | ELISA, spectrophotometer, qPCR | TNFα↓, iNOS↓, MPO↓, NO↓, GSH↑, | 10 days | Lycopene prior treatment proved anti-inflammatory and antioxidant effects against acute pancreatitis (AP) rat | (El-Ashmawy et al. 2018) |
| Female Wistar–Albino rats | 4 mg/kg/BW | Spectrophotometer | MDA↓, CAT↑, SOD↑, GPx↑, and GST↑ | 28 days | Lycopene possesses antioxidant properties that protect ovarian tissue against the toxicity of diabetic furans and non-diabetic | (Uçar and Pandir 2017) |
| Weanling ICR Male mice | 60 and 1 mg/kg | ELISA, Immunoblotting | IL-1β↓, TNF-α↓, IL-6↓, HO-1↓ | 6–24 h | Lycopene can reduce LPS-induced inflammation and depression-like behavior by inhibiting the production of pro-inflammatory cytokines | (Zhang et al. 2016) |
| Male C57BL/6 mice | 50 or 25 mg/kg/day | ELISA | IL-10↓, IFN-γ↓, TNF-α↓, SOD↑, CAT↑, GSH↑ | 60 days | lycopene has anti-inflammatory and antioxidant properties; it may be a promising novel treatment for acute lung inflammation and emphysema in mice exposed to CS | (Campos et al. 2019) |
| Sprague–Dawley rats | 25 or 50 mg/kg | ELISA, Western blotting | TNF-α↑, IL-1β↑, IL-6↑, and IL-8↑, HO-1↑, COX-2↓ | 14 days | The anti-inflammatory properties of lycopene may be used as a therapy to protect against spinal cord ischemia/reperfusion injury (SCII) | (Hua et al. 2019) |
| Male mice | 20 mg/kg | ELISA, Western blotting | ALT↓, AST↓, TNF-α↓, IL-6↓, MDA ↓, SOD↑, Nrf2↑, COX-2↓, NF-κB↓ | 2 weeks | Lycopene has been reported to lessen the liver damage brought on by LPS by reducing oxidative stress and inflammatory damage | (Dong et al. 2019) |
| Adult male C57BL/6j mice | 2 μM | Western blotting, luminex kit | IL-6↓, MCP-1↓, IL-1β↓, TNFα↓, NF-κB↑ | 24 h | Lycopene could prevent or reduce the prevalence of obesity-related diseases by reducing the expression of pro-inflammatory cytokines and chemokines in adipose tissues | (Gouranton et al. 2011) |
| Macrophage and adipocyte cells | 0.5–2 µM | ELISA, Western blot | ↓IL-1β, ↓TNF-α, ↓IL-6, JNK ↑, NF-κB↑ | 24 h | Lycopene has the potential to disrupt the vicious loop that occurs in adipose tissue between adipocytes and macrophages during obesity | (Marcotorchino et al. 2012) |
| Eight-week-old male C57BL/6 J mice | Powder | ELISA | TNFα↓, IL-6↓, CCL2↓, CCL5↓, MCP-1↓, NF-κB↓, IκB↓, p65↓ | 12 weeks | Lycopene has beneficial effects on inflammatory responses, obesity, and metabolic disorders | (Fenni et al. 2017) |
| Airway epithelial cells | 2.5 μg/ml | ELISA | IL-6↓, IP-10↓, NF-κB↓ | 48 h | Lycopene may also be effective in reducing rhinovirus-induced airway inflammation | (Saedisomeolia et al. 2009) |
| Sprague–Dawley rats | 2 or 4 mg/kg BW | ELISA | ALT ↓, MDA ↓, TNF-α ↓, GSH ↑, HSC activation ↓ | Orally for 6 weeks | Lycopene supplementation may help to prevent the progression of steatohepatitis by reducing high-fat diet-induced oxidative stress in the cells | (Bahcecioglu et al. 2010) |
| C57BL/6 mice | 10 or 100 mg/kg | RT-PCR, spectrophotometer | CAT ↑, GSH ↑, TBARS ↓, IL1β ↓, Redox imbalance ↓ | 2 weeks | Lycopene can reduce the oxidative stress caused due to APAP overdose and subsequent liver damage in C57BL/6 mice | (Bandeira et al. 2017) |
| Sprague–Dawley rats | 20 mg/kg diet | SOD ↑, CAT ↑, LDL ↓, GSHPX ↑ | 4 weeks | Lycopene's potential health advantages against non-alcoholic fatty liver disease (NAFLD) | (Piña-Zentella et al. 2016) | |
| Sprague–Dawley rats | 2.5, 5 mg/kg BW | Spectrophotometer | CAT ↑, MDA ↓, ALT ↓, AST ↓, LDH ↓ | Lycopene's antioxidant properties protect against ischemia/reperfusion injury | (Bayramoglu et al. 2015) | |
| Wistar rats and C57BL/6 mice | Rats: 1.1 mg/kg Mice: 1.1 mg/kg | Western blot, real time-PCR | IL-6 ↓, IL-1β ↓ IFNγ ↓,, Hepatic inflammatory foci ↓ | 4 and 3 weeks | Dietary lycopene powder can prevent alcohol-induced liver impairment and oxidative damage by inhibiting cytochrome p450 | (Stice et al. 2015) |
| MI mice | 10 mg/kg/day | Western blot, qPCR | TNFα ↓, IL-1β↓, NF-κB p65 ↓ | 4 weeks | Lycopene has been shown to have a cardioprotective effect by inhibiting local myocardial inflammation and apoptosis | (He et al. 2015) |
Interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) interleukin-1β (IL-1β), interleukin-10 (IL-10), heme oxygenase (HO), monocyte chemotactic protein 1 (MCP-1), reactive protein (CRP), JNK (c-Jun N-terminal kinase), interferon-gamma induced protein-10 (IP-10), Quantitative polymerase chain reaction (qPCR), Enzyme-linked immunosorbent Assay (ELISA), Superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), inducible nitric oxide synthase (iNOS), glutathione (GSH), Glutathione-S-transferase (GST), catalase (CAT), glutathione peroxidase (GSH-Px), malondialdehyde (MDA), non-alcoholic fatty liver disease (NAFLD), Overall case-fatality rate (CFR), SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), MERS (Middle East respiratory syndrome), RNA-dependent RNA polymerase (RdRp), main protease (Mpro) and receptors binding domain (RBD), two-pore calcium channel 2 (TPC2).
Clinical significance of lycopene
Lycopene has been suggested to have clinical efficacy in the treatment of diabetes. The average daily intake of dietary lycopene among diabetics was 0.04 mg/kg BW/d, and lycopene could lower oxidative stress and enhance the pathophysiology of T2DM (Leh et al. 2021). Lycopene's anti-cancer effects on oral cancer (OC) cells reveal that it might activate the PI3K/AKT/m-TOR signaling pathway to suppress EMT and induce apoptosis in OC cells. These data support the clinical use of lycopene in the treatment of OC (R. Wang et al. 2020). Lycopene (0.03 percent w/w of normal chow for five weeks) can reduce LPS-induced (0.25 mg/kg for nine days) neuroinflammation, oxidative stress, amyloidogenesis, and cognitive deficits in male C57BL/6 J mice, perhaps by modulating MAPKs, NFB, and Nrf2 signaling pathways, suggesting that LYC could be a nutritional preventive strategy in neuroinflammation-related disorders like Alzheimer's (AD) (Jia Wang et al. 2018a). In male mice, lycopene suppressed dependent MAPKs (ERK1/2, p38, and JNK1/ 2) and Akt/ GSK-3β signaling pathways to reduce oxidative stress and ameliorate cardiac hypertrophy. Therefore, it was considered that the antioxidant capabilities of lycopene would prevent cardiac hypertrophy (Zeng et al. 2019). Lycopene (5–10 mg/kg body weight/day for 10 days) in male SD rats could alleviate LPS-stimulated oxidative stress via enhanced total antioxidant and HDL-associated PON-1 activity, as well as down-regulating hepatocyte PCSK-9 expression via HNF-1α downregulation and plasma levels of inflammatory mediators (Alvi et al. 2017). In Wistar rats, lycopene (10 mg/kg/day) treatment prevented the angiotensin-II-induced remodeling of the cardiovascular system. Lycopene enhanced angiotensin-II-induced vascular superoxide anion, cardiac fibrosis, hypertrophy, lipid peroxidation, and antioxidant enzyme activity. Interestingly, lycopene showed antihypertensive potential without causing hypotension in normotensive rats (Ferreira-Santos et al. 2018). Mice were treated with lycopene (5 mg/kg) which significantly protected the heart against ATR-induced environmental cardiology and alleviated cardiac injury via modulating the NO and NO-generating systems and blocking the TRAF6-NF-κB pathway (Zeng et al. 2019). Lycopene treatment (23 mg/kg BW per day) could alleviate oxidative stress-induced neuroinflammation and cognitive impairments via mediating Nrf2/NF-κB transcriptional pathway in CD-1 mice by improving neuronal damage, restoring antioxidant activities, and down-regulating inflammatory responses (Zhao et al. 2017). Lycopene (5–125 mg/kg for four weeks) treatment in hyperlipidemic rats could alleviate the damage to the blood–brain barrier and the loss of neurons in the brain induced by hyperlipidemia. These results suggest an inverse U-shape relation between dose and serum concentration of lycopene (Wang et al. 2018b). Mice treated with lycopene (10 mg/kg) exhibited inhibition of p38 activation and MMP-9 expression, which indicates that lycopene inhibits myocardial fibrosis to improve cardiac function and ventricular remodeling (Wang et al. 2014).
Lycopene (10 mg/kg/day) was given to the mice to inhibit the NFB signaling pathway, which attenuated the mice's inflammatory response and cardiomyocyte apoptosis post-MI. As a result, lycopene may have a cardioprotective impact by inhibiting local myocardial inflammation and apoptosis (He et al. 2015). Treatment with lycopene (5 mg/kg BW) in T2DM rats was able to reduce inflammation and oxidative stress, which protected against the progression of diabetes and associated consequences (Zheng et al. 2019). Lycopene (16 mg) treatment for individuals with oral submucous fibrosis considerably lowered the expression levels of the IGF1 pathway, suggesting that it could be a promising drug for the pre-treatment of oral squamous cell carcinoma (OSCC) and significantly induced cell apoptosis, which inhibits tumor growth and cell migration (Kumar et al. 2007). Lycopene exhibits suppressive inhibitory effects on WSU-HN6 and CAL-27 cells in vivo and in vitro, similar to prior findings. This study might treat oral squamous cell carcinoma with lycopene by acting as an anti-cancer agent (Tao et al. 2021).
Side effects/toxicity
A clinical disorder called lycopenemia is characterized by yellowish-orange skin pigmentation. It results from the overconsumption of lycopene resources obtained through diet or caused by the deposition of lycopene in the stratum corneum (Shaw and Koti 2009). The anti-oxidant lycopene does not prevent pre-eclampsia in healthy primigravidas, but it helps lessen pre-eclampsia and intrauterine development retardation. As a result, there have been more premature and underweight babies born. Therefore, it is advisable to refrain from using lycopene supplements as well as lycopene found in food during pregnancy and lactation (Banerjee et al. 2009; Sharma et al. 2006). Docetaxel with lycopene treatment was well tolerated, with no dose-limiting toxicities seen in the first six patients participating in the research. Two patients (15%) required dose reduction because of peripheral neuropathy. The most frequent side reactions (all low grade) that affected at least 15% of patients were diarrhea, nausea, vomiting, or both, peripheral neuropathy, weight loss, appetite changes, weariness, onycholysis, alopecia, and anemia (Zhuang et al. 2021). Lycopene is not benign. A significant number of individuals experienced gastrointestinal adverse symptoms such as nausea, vomiting, anorexia, flatulence, and abdominal distension. Although the majority of these were minor, they nevertheless highlight the fact that even natural, nutrition-based medicines might have unintended repercussions (Jatoi et al. 2007). Lycopene supplements were well tolerated. One patient discontinued treatment due to diarrhea (grade 2 toxicity) caused by lycopene supplementation. Other toxicities were observed, but these were unlikely to be caused by lycopene supplementation (Clark et al. 2006).
Lycopene has not been linked to any negative side effects. One patient reported an intense burning sensation and oral ulcers one week after starting treatment (Kumar et al. 2007). According to the Common Toxicity Criteria of the National Cancer Institute, none of the investigations found any serious toxicity or intolerance associated with lycopene supplementation (Kaba et al. 2004). Lycopene appears to be a perfectly safe dietary component from a safety point of view. It has no harmful effect on populations, ranging from an optimal dose of 12 mg/day to a very high dose of 150 mg/100 g (Przybylska and Tokarczyk 2022).
Conclusion
This review has presented the various mechanisms of SARS-CoV-2 infection along with the inflammatory, immunity, and oxidative properties of lycopene as COVID-19 preventative measures in light of the COVID-19 pandemic. COVID-19 cytokinemia differs from other kinds of pneumonia in that it causes organ failure and ICU hospitalization. SARS-CoV-2-induced hyper-inflammatory and immune responses are critical for not only understanding the pathogenesis of severe COVID-19 disease but also for vaccine evaluation and design. Many immunomodulatory, anti-inflammatory, and antiviral drugs have been tested in patients with COVID-19 as well. We conclude that lycopene could be used as a drug for treating the inflammation of COVID-19 infection because of its antioxidant, anti-inflammatory and antivirals properties.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
The study did not utilize a dataset.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Pankaj Kumar Chauhan, Email: chauhanbiochem084@gmail.com.
Joshua O. Ighalo, Email: oshea.ighalo@yahoo.com, Email: jo.ighalo@unizik.edu.ng
References
- Abd El-Mageed HR, Abdelrheem DA, Rafi M, Sarker M, Al-Khafaji K, Hossain M, Capasso R, Emran TB. In silico evaluation of different flavonoids from medicinal plants for their potency against SARS-CoV-2. Biologics. 2021;1(3):416–434. doi: 10.3390/biologics1030024. [DOI] [Google Scholar]
- Abdallah HM, El-Halawany AM, Sirwi A, El-Araby AM, Mohamed GA, Ibrahim SRM, Koshak AE, Asfour HZ, Awan ZA, Elfaky MA. Repurposing of some natural product isolates as SARS-COV-2 main protease inhibitors via in vitro cell free and cell-based antiviral assessments and molecular modeling approaches. Pharmaceuticals. 2021;14(3):213. doi: 10.3390/ph14030213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann M, Mentzer SJ, Kolb M, Jonigk D. Inflammation and intussusceptive angiogenesis in COVID-19: everything in and out of flow. Eur Resp J. 2020;56(5):2003147. doi: 10.1183/13993003.03147-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelodun B, Ajibade FO, Ibrahim RG, Ighalo JO, Bakare HO, Kumar P, Eid EM, Kumar V, Odey G, Choi KS. Insights into hazardous solid waste generation during COVID-19 pandemic and sustainable management approaches for developing countries. J Mater Cycle Waste Manage. 2022;23(6):2077–2086. doi: 10.1007/s10163-021-01281-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ağagündüz D, Çelik MN, Çıtar Dazıroğlu ME, Capasso R. Emergent drug and nutrition interactions in COVID-19: a comprehensive narrative review. Nutrients. 2021;13(5):1550. doi: 10.3390/nu13051550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Rao AV. Carotenoids and chronic diseases. Drug Metab Drug Interact. 2000;17(1–4):189–210. doi: 10.1515/dmdi.2000.17.1-4.189. [DOI] [PubMed] [Google Scholar]
- Alberca RW, de Oliveira LM, Branco ACCC, Pereira NZ, Sato MN. Obesity as a risk factor for COVID-19: an overview. Crit Rev Food Sci Nutr. 2021;61(13):2262–2276. doi: 10.1080/10408398.2020.1775546. [DOI] [PubMed] [Google Scholar]
- Albrahim T, Alonazi MA. Lycopene corrects metabolic syndrome and liver injury induced by high fat diet in obese rats through antioxidant, anti-inflammatory, antifibrotic pathways. Biomed Pharmacother. 2021;141:111831. doi: 10.1016/j.biopha.2021.111831. [DOI] [PubMed] [Google Scholar]
- Alharthy A, Faqihi F, Memish ZA, Karakitsos D. Lung injury in COVID-19—an emerging hypothesis. ACS Chem Neurosci. 2020;11(15):2156–2158. doi: 10.1021/acschemneuro.0c00422. [DOI] [PubMed] [Google Scholar]
- Alvi SS, Ansari IA, Ahmad MK, Iqbal J, Khan MS. Lycopene amends LPS induced oxidative stress and hypertriglyceridemia via modulating PCSK-9 expression and Apo-CIII mediated lipoprotein lipase activity. Biomed Pharmacother. 2017;96:1082–1093. doi: 10.1016/j.biopha.2017.11.116. [DOI] [PubMed] [Google Scholar]
- Amalraj A, Pius A, Gopi S, Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives–a review. J Tradit Complement Med. 2017;7(2):205–233. doi: 10.1016/j.jtcme.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakeri G, Patil S, Maddur N, Rao USV, Subash A, Patil S, Gao S, Brennan PA. Long-term effectiveness of lycopene in the management of oral submucous fibrosis (OSMF): a 3-years follow-up study. J Oral Pathol Med. 2020;49(8):803–808. doi: 10.1111/jop.13085. [DOI] [PubMed] [Google Scholar]
- Assimakopoulos SF, Marangos M. N-acetyl-cysteine may prevent COVID-19-associated cytokine storm and acute respiratory distress syndrome. Med Hypotheses. 2020;140:109778. doi: 10.1016/j.mehy.2020.109778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaei A, Asadpour R, Mansouri K, Sabrivand A, Kazemi-Darabadi S. Lycopene protects sperm from oxidative stress in the experimental varicocele model. Food Sci Nutr. 2021;9(12):6806–6817. doi: 10.1002/fsn3.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahcecioglu IH, Kuzu N, Metin K, Ozercan IH, Ustündag B, Sahin K, Kucuk O. Lycopene prevents development of steatohepatitis in experimental nonalcoholic steatohepatitis model induced by high-fat diet. Vet Med Int. 2010;2010:1–8. doi: 10.4061/2010/262179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeira ACB, da Silva RC, Júnior JVR, Figueiredo VP, Talvani A, Cangussú SD, Bezerra FS, Costa DC. Lycopene pretreatment improves hepatotoxicity induced by acetaminophen in C57BL/6 mice. Bioorg Med Chem. 2017;25(3):1057–1065. doi: 10.1016/j.bmc.2016.12.018. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay P. Role of functional foods in COVID-19 situation. Int Res J Mod Eng Technol Sci. 2021;3:2582–5208. [Google Scholar]
- Banerjee S, Jeyaseelan S, Guleria R. Trial of lycopene to prevent pre-eclampsia in healthy primigravidas: results show some adverse effects. J Obst Gynaecol Res. 2009;35(3):477–482. doi: 10.1111/j.1447-0756.2008.00983.x. [DOI] [PubMed] [Google Scholar]
- Barbry P, Muus C, Luecken M, Eraslan G, Waghray A, Heimberg G, Sikkema L, Kobayashi Y, Vaishnav ED, Subramanian A. Integrated analyses of single-cell atlases reveal age, gender, and smoking status associations with cell type-specific expression of mediators of SARS-CoV-2 viral entry and highlights inflammatory programs in putative target cells. bioRxiv. 2020 doi: 10.1101/2020.04.19.049254v2. [DOI] [Google Scholar]
- Bayramoglu G, Bayramoglu A, Altuner Y, Uyanoglu M, Colak S. The effects of lycopene on hepatic ischemia/reperfusion injury in rats. Cytotechnology. 2015;67(3):487–491. doi: 10.1007/s10616-014-9706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi P, Rasola A, Forte M, Lippe G. The mitochondrial permeability transition pore: channel formation by F-ATP synthase, integration in signal transduction, and role in pathophysiology. Physiol Rev. 2015;95(4):1111–1155. doi: 10.1152/physrev.00001.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, Greninger AL, Pipavath S, Wurfel MM, Evans L. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeira SP, Borges Filho C, Del’Fabbro L, Roman SS, Royes LFF, Fighera MR, Jessé CR, Oliveira MS, Furian AF. Lycopene treatment prevents hematological, reproductive and histopathological damage induced by acute zearalenone administration in male Swiss mice. Exp Toxicol Pathol. 2014;66(4):179–185. doi: 10.1016/j.etp.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Cameron MJ, Bermejo-Martin JF, Danesh A, Muller MP, Kelvin DJ. Human immunopathogenesis of severe acute respiratory syndrome (SARS) Virus Res. 2008;133(1):13–19. doi: 10.1016/j.virusres.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos KKD, de Oliveira Ramos C, Martins TL, de Paula Costa G, Talvani A, Garcia CCM, Oliveira LAM, Cangussú SD, Costa DC, Bezerra FS. Lycopene mitigates pulmonary emphysema induced by cigarette smoke in a murine model. J Nutr Biochem. 2019;65:93–100. doi: 10.1016/j.jnutbio.2018.12.008. [DOI] [PubMed] [Google Scholar]
- Cantuti-Castelvetri L, Ojha R, Pedro LD, Djannatian M, Franz J, Kuivanen S, van der Meer F, Kallio K, Kaya T, Anastasina M. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370(6518):856–860. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Zhao J, Ma L, Chen J, Xu J, Rahman SU, Feng S, Li Y, Wu J, Wang X. Lycopene attenuates zearalenone-induced oxidative damage of piglet sertoli cells through the nuclear factor erythroid-2 related factor 2 signaling pathway. Ecotoxicol Environ Saf. 2021;225:112737. doi: 10.1016/j.ecoenv.2021.112737. [DOI] [PubMed] [Google Scholar]
- Catanzaro M, Fagiani F, Racchi M, Corsini E, Govoni S, Lanni C. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct Target Ther. 2020;5(1):1–10. doi: 10.1038/s41392-020-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D, Xu H, Rebaza A, Sharma L, Cruz Dela CS. Protecting health-care workers from subclinical coronavirus infection. Lancet Respir Med. 2020;8(3):e13. doi: 10.1016/S2213-2600(20)30066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Ni Y, Nagata N, Zhuge F, Xu L, Nagashimada M, Yamamoto S, Ushida Y, Fuke N, Suganuma H. Lycopene alleviates obesity-induced inflammation and insulin resistance by regulating M1/M2 status of macrophages. Mol Nutr Food Res. 2019;63(21):1900602. doi: 10.1002/mnfr.201900602. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyak BV, Popova EN, Prikhodko AS, Grebenchikov OA, Zinovkina LA, Zinovkin RA. COVID-19 and oxidative stress. Biochem Mosc. 2020;85(12):1543–1553. doi: 10.1134/S0006297920120068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistiakov DA, Orekhov AN, Bobryshev YV. Endothelial barrier and its abnormalities in cardiovascular disease. Front Physiol. 2015;6:365. doi: 10.3389/fphys.2015.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DK, Akl EA, Duda S, Solo K, Yaacoub S, Schünemann HJ, El-harakeh A, Bognanni A, Lotfi T, Loeb M. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. The Lancet. 2020;395(10242):1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PE, Hall MC, Borden LS, Jr, Miller AA, Hu JJ, Lee WR, Stindt D, D’Agostino R, Jr, Lovato J, Harmon M. Phase I-II prospective dose-escalating trial of lycopene in patients with biochemical relapse of prostate cancer after definitive local therapy. Urology. 2006;67(6):1257–1261. doi: 10.1016/j.urology.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Conti P, Ronconi G, Caraffa AL, Gallenga CE, Ross R, Frydas I, Kritas SK. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2):327–331. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultate TP. Food: the chemistry of its components. Royal Society of Chemistry; 2009. [Google Scholar]
- Curatola A, Chiaretti A, Ferretti S, Bersani G, Lucchetti D, Capossela L, Sgambato A, Gatto A. Cytokine response to SARS-CoV-2 infection in children. Viruses. 2021;13(9):1868. doi: 10.3390/v13091868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher R, Manceau H, Karim Z. Iron metabolism and the role of the iron-regulating hormone hepcidin in health and disease. La Presse Médicale. 2017;46(12):e272–e278. doi: 10.1016/j.lpm.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Daly JL, Simonetti B, Klein K, Chen KE, Williamson MK, Antón-Plágaro C, Shoemark DK, Simón-Gracia L, Bauer M, Hollandi R, Greber UF, Horvath P, Sessions RB, Helenius A, Hiscox JA, Teesalu T, Matthews DA, Davidson AD, Collins BM, Yamauchi Y. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;370(6518):861–865. doi: 10.1126/science.abd3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darif D, Hammi I, Kihel A, Saik IEI, Guessous F, Akarid K. The pro-inflammatory cytokines in COVID-19 pathogenesis: what goes wrong? Microb Pathog. 2021;153:104799. doi: 10.1016/j.micpath.2021.104799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Roche L, Mesta F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch Med Res. 2020;51(5):384–387. doi: 10.1016/j.arcmed.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhama K, Patel SK, Pathak M, Yatoo MI, Tiwari R, Malik YS, Singh R, Sah R, Rabaan AA, Bonilla-Aldana DK. An update on SARS-CoV-2/COVID-19 with particular reference to its clinical pathology, pathogenesis, immunopathology and mitigation strategies. Travel Med Infect Dis. 2020;37:101755. doi: 10.1016/j.tmaid.2020.101755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mascio P, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys. 1989;274(2):532–538. doi: 10.1016/0003-9861(89)90467-0. [DOI] [PubMed] [Google Scholar]
- Diao B, Wang C, Wang R, Feng Z, Zhang J, Yang H, Tan Y, Wang H, Wang C, Liu L. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nat Commun. 2021;12(1):1–9. doi: 10.1038/s41467-021-22781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, Holden KA, Read JM, Dondelinger F, Carson G. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020 doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Li W, Cheng L-M, Wang G-G. Lycopene attenuates LPS-induced liver injury by inactivation of NF-κB/COX-2 signaling. Int J Clin Exp Pathol. 2019;12(3):817. [PMC free article] [PubMed] [Google Scholar]
- Durairajanayagam D, Agarwal A, Ong C, Prashast P. Lycopene and male infertility. Asian J Androl. 2014;16(3):420. doi: 10.4103/1008-682X.126384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ashmawy NE, Khedr NF, El-Bahrawy HA, Hamada OB. Suppression of inducible nitric oxide synthase and tumor necrosis factor-alpha level by lycopene is comparable to methylprednisolone in acute pancreatitis. Dig Liver Dis. 2018;50(6):601–607. doi: 10.1016/j.dld.2018.01.131. [DOI] [PubMed] [Google Scholar]
- Eze ED, Afodun AM, Kasolo J, Kasozi KI. Lycopene improves on basic hematological and immunological parameters in diabetes mellitus. BMC Res Notes. 2019;12(1):1–6. doi: 10.1186/s13104-019-4841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallah R, Kiani A, Khaldari M. Supplementing lycopene combined with corn improves circulating IgG concentration in pregnant ewes and their lambs. Trop Anim Health Prod. 2021;53(3):1–9. doi: 10.1007/s11250-021-02802-3. [DOI] [PubMed] [Google Scholar]
- Fan Q, Zhang B, Ma J, Zhang S. Safety profile of the antiviral drug remdesivir: an update. Biomed Pharmacother. 2020;130:110532. doi: 10.1016/j.biopha.2020.110532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Li S, Sun Q, Zhu J, Chen B, Xiong M, Cao G. Immune-inflammatory parameters in COVID-19 cases: a systematic review and meta-analysis. Front Med. 2020;7:301. doi: 10.3389/fmed.2020.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenni S, Hammou H, Astier J, Bonnet L, Karkeni E, Couturier C, Tourniaire F, Landrier J. Lycopene and tomato powder supplementation similarly inhibit high-fat diet induced obesity, inflammatory response, and associated metabolic disorders. Mol Nutr Food Res. 2017;61(9):1601083. doi: 10.1002/mnfr.201601083. [DOI] [PubMed] [Google Scholar]
- Ferreira-Santos P, Aparicio R, Carrón R, Sevilla MÁ, Monroy-Ruiz J, Montero MJ. Lycopene-supplemented diet ameliorates cardiovascular remodeling and oxidative stress in rats with hypertension induced by Angiotensin II. J Funct Foods. 2018;47:279–287. doi: 10.1016/j.jff.2018.06.002. [DOI] [Google Scholar]
- Flanagan KL, Fink AL, Plebanski M, Klein SL. Sex and gender differences in the outcomes of vaccination over the life course. Annu Rev Cell Dev Biol. 2017;33:577–599. doi: 10.1146/annurev-cellbio-100616-060718. [DOI] [PubMed] [Google Scholar]
- Fodor A, Tiperciuc B, Login C, Orasan OH, Lazar AL, Buchman C, Hanghicel P, Sitar-Taut A, Suharoschi R, Vulturar R. Endothelial dysfunction, inflammation, and oxidative stress in COVID-19—mechanisms and therapeutic targets. Oxid Med Cell Longev. 2021;2021:1–15. doi: 10.1155/2021/8671713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester SJ, Booz GW, Sigmund CD, Coffman TM, Kawai T, Rizzo V, Scalia R, Eguchi S. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev. 2018;98(3):1627–1738. doi: 10.1152/physrev.00038.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frediansyah A, Nainu F, Dhama K, Mudatsir M, Harapan H. Remdesivir and its antiviral activity against COVID-19: a systematic review. Clin Epidemiol Global Health. 2021;9:123–127. doi: 10.1016/j.cegh.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbadage T, Peterson BM, Awada J, Buck AS, Ramirez DA, Wilson J, Gunasekera RS. Systematic review and meta-analysis of sex-specific COVID-19 clinical outcomes. Front Med. 2020;7:348. doi: 10.3389/fmed.2020.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gansevoort RT, Hilbrands LB. CKD is a key risk factor for COVID-19 mortality. Nat Rev Nephrol. 2020;16(12):705–706. doi: 10.1038/s41581-020-00349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Zheng KI, Wang X-B, Sun Q-F, Pan K-H, Wang T-Y, Chen Y-P, Targher G, Byrne CD, George J. Obesity is a risk factor for greater COVID-19 severity. Diabetes Care. 2020;43(7):e72–e74. doi: 10.2337/dc20-0682. [DOI] [PubMed] [Google Scholar]
- George B, Kaur C, Khurdiya DS, Kapoor HC. Antioxidants in tomato (Lycopersium esculentum) as a function of genotype. Food Chem. 2004;84(1):45–51. doi: 10.1016/S0308-8146(03)00165-1. [DOI] [Google Scholar]
- Göncü T, Oğuz E, Sezen H, Koçarslan S, Oğuz H, Akal A, Adıbelli FM, Çakmak S, Aksoy N. Anti-inflammatory effect of lycopene on endotoxin-induced uveitis in rats. Arq Bras Oftalmol. 2016;79(6):357–362. doi: 10.5935/0004-2749.20160102. [DOI] [PubMed] [Google Scholar]
- Gouranton E, Thabuis C, Riollet C, Malezet-Desmoulins C, El Yazidi C, Amiot MJ, Borel P, Landrier JF. Lycopene inhibits proinflammatory cytokine and chemokine expression in adipose tissue. J Nutr Biochem. 2011;22(7):642–648. doi: 10.1016/j.jnutbio.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, Satlin MJ, Campion TR, Jr, Nahid M, Ringel JB. Clinical characteristics of Covid-19 in New York city. N Engl J Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowska M, Wawrzyniak D, Rolle K, Chomczyński P, Oziewicz S, Jurga S, Barciszewski J. Let food be your medicine: nutraceutical properties of lycopene. Food Funct. 2019;10(6):3090–3102. doi: 10.1039/C9FO00580C. [DOI] [PubMed] [Google Scholar]
- Greish SM, Kader GSA, Abdelaziz EZ, Eltamany DA, Sallam HS, Abogresha NM. Lycopene is superior to moringa in improving fertility markers in diet-induced obesity male rats. Saudi J Biol Sci. 2021;28(5):2956–2963. doi: 10.1016/j.sjbs.2021.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32(1):659–702. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- Gul F, Khan I, Iqbal J, Abbasi BA, Shahbaz A, Capasso R, Amaro-Estrada I, Jardan YAB, Cossio-Bayugar R, Mahmood T. Phytochemistry, biological activities and in silico molecular docking studies of Oxalis pes-caprae L. compounds against SARS-CoV-2. J King Saud Univ-Sci. 2022;34(6):102136. doi: 10.1016/j.jksus.2022.102136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, Qin R, Wang H, Shen Y, Du K. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020;36(7):e3319. doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Jawanda MK, Arora V, Mehta N, Yadav V. Role of lycopene in preventing oral diseases as a nonsurgical aid of treatment. Int J Prev Med. 2015;6:1–70. doi: 10.4103/2008-7802.162311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I, Timens W, Bulthuis MLC, Lely AT, van Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslberger A, Jacob U, Hippe B, Karlic H. Mechanisms of selected functional foods against viral infections with a view on COVID-19: mini review. Funct Foods Health Dis. 2020;10(5):195–209. doi: 10.31989/ffhd.v10i5.707. [DOI] [Google Scholar]
- He L, Mäe MA, Muhl L, Sun Y, Pietilä R, Nahar K, Liébanas EV, Fagerlund MJ, Oldner A, Liu J. Pericyte-specific vascular expression of SARS-CoV-2 receptor ACE2–implications for microvascular inflammation and hypercoagulopathy in COVID-19. bioRxiv. 2020 doi: 10.1101/2020.05.11.088500v2. [DOI] [Google Scholar]
- He QIN, Zhou WEI, Xiong C, Tan G, Chen M. Lycopene attenuates inflammation and apoptosis in post-myocardial infarction remodeling by inhibiting the nuclear factor-κB signaling pathway. Mol Med Rep. 2015;11(1):374–378. doi: 10.3892/mmr.2014.2676. [DOI] [PubMed] [Google Scholar]
- Heber D, Lu Q-Y. Overview of mechanisms of action of lycopene. Exp Biol Med. 2002;227(10):920–923. doi: 10.1177/153537020222701013. [DOI] [PubMed] [Google Scholar]
- Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MJ, Jannat T, Brishty SR, Roy U, Mitra S, Rafi MO, Islam MR, Nesa ML, Islam MA, Emran TB. Clinical efficacy and safety of antiviral drugs in the extended use against COVID-19: what we know so Far. Biologics. 2021;1(2):252–284. doi: 10.3390/biologics1020016. [DOI] [Google Scholar]
- Hou W. Characterization of codon usage pattern in SARS-CoV-2. Virol J. 2020;17(1):1–10. doi: 10.1186/s12985-020-01395-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Y, Xu N, Ma T, Liu Y, Xu H, Lu Y. Anti-inflammatory effect of lycopene on experimental spinal cord ischemia injury via cyclooxygenase-2 suppression. NeuroImmunoModulation. 2019;26(2):84–92. doi: 10.1159/000495466. [DOI] [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wen C, Yang M, Gan D, Fan C, Li A, Li Q, Zhao J, Zhu L, Lu D. Lycopene protects against t-BHP-induced neuronal oxidative damage and apoptosis via activation of the PI3K/Akt pathway. Mol Biol Rep. 2019;46(3):3387–3397. doi: 10.1007/s11033-019-04801-y. [DOI] [PubMed] [Google Scholar]
- Huang K, Su I, Theron M, Wu Y, Lai S, Liu C, Lei H. An interferon-γ-related cytokine storm in SARS patients. J Med Virol. 2005;75(2):185–194. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Wu X, Zheng X, Luo S, Xu S, Weng J. Targeting inflammation and cytokine storm in COVID-19. Pharmacol Res. 2020;159:105051. doi: 10.1016/j.phrs.2020.105051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icel E, Icel A, Uçak T, Karakurt Y, Elpeze B, Keskin Çimen F, Süleyman H. The effects of lycopene on alloxan induced diabetic optic neuropathy. Cutan Ocul Toxicol. 2019;38(1):88–92. doi: 10.1080/15569527.2018.1530258. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Hayase N, Moriya K, Morimura N. Nafamostat mesylate treatment in combination with favipiravir for patients critically ill with Covid-19: a case series. Crit Care. 2020;24(1):1–4. doi: 10.1186/s13054-020-03078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- İkiz Ö, Kahramansoy N, Erkol H, Koçoğlu E, Fırat T. Effects of lycopene in intestinal ischemia reperfusion injury via intestinal immunoglobulin A. J Surg Res. 2021;267:63–70. doi: 10.1016/j.jss.2021.04.039. [DOI] [PubMed] [Google Scholar]
- Iwamura APD, Tavares da Silva MR, Hümmelgen AL, Soeiro Pereira PV, Falcai A, Grumach AS, Goudouris E, Neto AC, Prando C. Immunity and inflammatory biomarkers in COVID-19: a systematic review. Rev Med Virol. 2021;31(4):e2199. doi: 10.1002/rmv.2199. [DOI] [PubMed] [Google Scholar]
- Janssen NAF, Grondman I, de Nooijer AH, Boahen CK, Koeken VACM, Matzaraki V, Kumar V, He X, Kox M, Koenen HJPM. Dysregulated innate and adaptive immune responses discriminate disease severity in COVID-19. J Infect Dis. 2021;223(8):1322–1333. doi: 10.1093/infdis/jiab065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatoi A, Burch P, Hillman D, Vanyo JM, Dakhil S, Nikcevich D, Rowland K, Morton R, Flynn PJ, Young C. A tomato-based, lycopene-containing intervention for androgen-independent prostate cancer: results of a Phase II study from the North Central Cancer Treatment Group. Urology. 2007;69(2):289–294. doi: 10.1016/j.urology.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Kaba H, Fukuda H, Yamamoto S, Ohashi Y. Reliability at the National Cancer Institute-Common Toxicity Criteria version 2.0. Gan to Kagaku Ryoho. Cancer & Chemotherapy. 2004;31(8):1187–1192. [PubMed] [Google Scholar]
- Kaneko JJ, Harvey JW, Bruss ML. Clinical biochemistry of domestic animals. Academic Press; 2008. [Google Scholar]
- Kell DB, Pretorius E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics. 2014;6(4):748–773. doi: 10.1039/C3MT00347G. [DOI] [PubMed] [Google Scholar]
- Khomich OA, Kochetkov SN, Bartosch B, Ivanov AV. Redox biology of respiratory viral infections. Viruses. 2018;10(8):392. doi: 10.3390/v10080392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G, Kim J, Ahn S, Lee H, Moon D, Lee C, Park Y. Lycopene suppresses the lipopolysaccharide-induced phenotypic and functional maturation of murine dendritic cells through inhibition of mitogen-activated protein kinases and nuclear factor-κB. Immunology. 2004;113(2):203–211. doi: 10.1111/j.1365-2567.2004.01945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff WC, Williams MA. Covid-19 and immunity in aging populations—a new research agenda. N Engl J Med. 2020;383(9):804–805. doi: 10.1056/NEJMp2006761. [DOI] [PubMed] [Google Scholar]
- Komaravelli N, Casola A. Respiratory viral infections and subversion of cellular antioxidant defenses. J Pharmacogenom Pharmacoproteom. 2014;5(4):1000141. doi: 10.4172/2153-0645.1000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Bagewadi A, Keluskar V, Singh M. Efficacy of lycopene in the management of oral submucous fibrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(2):207–213. doi: 10.1016/j.tripleo.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Lazzerini PE, Boutjdir M, Capecchi PL. COVID-19, arrhythmic risk, and inflammation: mind the gap! Circulation. 2020;142(1):7–9. doi: 10.1161/CIRCULATIONAHA.120.047293. [DOI] [PubMed] [Google Scholar]
- Lee W, Ku S-K, Bae JW, Bae J-S. Inhibitory effects of lycopene on HMGB1-mediated pro-inflammatory responses in both cellular and animal models. Food Chem Toxicol. 2012;50(6):1826–1833. doi: 10.1016/j.fct.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Leh HE, Mohd Sopian M, Abu Bakar MH, Lee LK. The role of lycopene for the amelioration of glycaemic status and peripheral antioxidant capacity among the Type II diabetes mellitus patients: a case–control study. Ann Med. 2021;53(1):1060–1066. doi: 10.1080/07853890.2021.1943515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Wu K, Li Y, Liang X, Tse WKF, Yang L, Lai KP. Revealing the targets and mechanisms of vitamin A in the treatment of COVID-19. Aging (albany NY) 2020;12(15):15784. doi: 10.18632/aging.103888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Ma F, Yokota T, Garcia G, Jr, Palermo A, Wang Y, Farrell C, Wang Y-C, Wu R, Zhou Z. Metabolic reprogramming and epigenetic changes of vital organs in SARS-CoV-2–induced systemic toxicity. JCI Insight. 2021 doi: 10.1172/jci.insight.145027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Zhang Y, Qi Y, Liu H. Lycopene prevents oxygen-glucose deprivation-induced autophagic death in SH-SY5Y cells via inhibition of the oxidative stress-activated AMPK/mTOR pathway. Mol Med Rep. 2021;24(2):1–10. doi: 10.3892/mmr.2021.12233. [DOI] [PubMed] [Google Scholar]
- Li X-N, Lin J, Xia J, Qin L, Zhu S-Y, Li J-L. Lycopene mitigates atrazine-induced cardiac inflammation via blocking the NF-κB pathway and NO production. J Funct Foods. 2017;29:208–216. doi: 10.1016/j.jff.2016.12.029. [DOI] [Google Scholar]
- Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharmaceutical Analysis. 2020;10(2):102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Q, Ye L, Timani KA, Zeng Y, She Y, Ye L, Wu Z. Activation of NF-κB by the full-length nucleocapsid protein of the SARS coronavirus. Acta Biochim Biophys Sin. 2005;37(9):607–612. doi: 10.1111/j.1745-7270.2005.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Chen X, Huang Z, Chen D, Yu B, Chen H, He J, Yan H, Zheng P, Yu J. Effects of dietary lycopene supplementation on intestinal morphology, antioxidant capability and inflammatory response in finishing pigs. Anim Biotechnol. 2021;33(3):563–570. doi: 10.1080/10495398.2021.2009490. [DOI] [PubMed] [Google Scholar]
- Loeffelholz MJ, Tang Y-W. Laboratory diagnosis of emerging human coronavirus infections–the state of the art. Emerg Microb Infect. 2020;9(1):747–756. doi: 10.1080/22221751.2020.1745095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach WJ, Thimmesch AR, Pierce JT, Pierce JD. Consequences of hyperoxia and the toxicity of oxygen in the lung. Nurs Res Pract. 2011;2011:1–7. doi: 10.1155/2011/260482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magne TM, da Silva de Barros AO, de Almeida Fechine PB, Alencar LMR, Ricci-Junior E, Santos-Oliveira R. Lycopene as a multifunctional platform for the treatment of cancer and inflammation. Rev Bras. 2022 doi: 10.1007/s43450-022-00250-0. [DOI] [Google Scholar]
- Mahboobipour AA, Baniasadi S. Clinically important drug–drug interactions in patients admitted to hospital with COVID-19: drug pairs, risk factors, and management. Drug Metab Personal Ther. 2021;36(1):9–16. doi: 10.1515/dmpt-2020-0145. [DOI] [PubMed] [Google Scholar]
- Mahmud-Al-Rafat A, Asim MMH, Taylor-Robinson AW, Majumder A, Muktadir A, Muktadir H, Karim M, Khan I, Ahasan MM, Billah MM. A combinational approach to restore cytokine balance and to inhibit virus growth may promote patient recovery in severe COVID-19 cases. Cytokine. 2020;136:155228. doi: 10.1016/j.cyto.2020.155228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmud S, Paul GK, Afroze M, Islam S, Gupt SBR, Razu MH, Biswas S, Zaman S, Uddin MS, Khan M. Efficacy of phytochemicals derived from Avicennia officinalis for the management of COVID-19: a combined in silico and biochemical study. Molecules. 2021;26(8):2210. doi: 10.3390/molecules26082210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotorchino J, Romier B, Gouranton E, Riollet C, Gleize B, Malezet-Desmoulins C, Landrier J. Lycopene attenuates LPS-induced TNF-α secretion in macrophages and inflammatory markers in adipocytes exposed to macrophage-conditioned media. Mol Nutr Food Res. 2012;56(5):725–732. doi: 10.1002/mnfr.201100623. [DOI] [PubMed] [Google Scholar]
- Massaro GD, Gail DB, Massaro D. Lung oxygen consumption and mitochondria of alveolar epithelial and endothelial cells. J Appl Physiol. 1975;38(4):588–592. doi: 10.1152/jappl.1975.38.4.588. [DOI] [PubMed] [Google Scholar]
- Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med. 2020;382(25):e102. doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihailovic V, Katanic Stankovic JS, Selakovic D, Rosic G. An overview of the beneficial role of antioxidants in the treatment of nanoparticle-induced toxicities. Oxid Med Cell Longev. 2021;2021:1–21. doi: 10.1155/2021/7244677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moni MA, Liò P. Network-based analysis of comorbidities risk during an infection: SARS and HIV case studies. BMC Bioinformatics. 2014;15(1):1–23. doi: 10.1186/1471-2105-15-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi SS, Karami A, Haghighi TM, Tumilaar SG, Idroes R, Mahmud S, Celik I, Ağagündüz D, Tallei TE, Emran TB. In silico evaluation of Iranian medicinal plant phytoconstituents as inhibitors against main protease and the receptor-binding domain of SARS-CoV-2. Molecules. 2021;26(18):5724. doi: 10.3390/molecules26185724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandan A, Siddiqui NA, Singh C, Aeri AWG, Ighalo JO, Nagliate PC, Meili L, Singh P, Chaukura N, Rangabhashiyam S. COVID-19 pandemic in Uttarakhand, India: environmental recovery or degradation? J Environ Chem Eng. 2021;9(6):106595. doi: 10.1016/j.jece.2021.106595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicin L, Abplanalp WT, Mellentin H, Kattih B, Tombor L, John D, Schmitto JD, Heineke J, Emrich F, Arsalan M. Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur Heart J. 2020;41(19):1804–1806. doi: 10.1093/eurheartj/ehaa311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nin N, et al. Lung histopathological findings in fatal pandemic influenza A (H1N1)Resultados histopatológicos pulmonares en la gripe a (H1N1) pandémica letal. Med Intensiva. 2012;36(1):24–31. doi: 10.1016/j.medin.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Núñez-Delgado A. What do we know about the SARS-CoV-2 coronavirus in the environment? Sci Total Environ. 2020;727:138647. doi: 10.1016/j.scitotenv.2020.138647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofuyatan MO, Ighalo JO, Olukanni D, Adeniyi AG, Oluwafemi J. Implications of COVID-19 pandemic on energy and environment research in Nigeria. In: Folarin SF, Akinlabi E, Atayero A, editors. The United Nations and sustainable development goals. Palgrave Macmillan; 2022. pp. 91–101. [Google Scholar]
- Olson JB, Ward NE, Koutsos EA. Lycopene incorporation into egg yolk and effects on laying hen immune function. Poult Sci. 2008;87(12):2573–2580. doi: 10.3382/ps.2008-00072. [DOI] [PubMed] [Google Scholar]
- Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, Guo L, Guo R, Chen T, Hu J. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11(1):1–12. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranjpe I, Russak AJ, De Freitas JK, Lala A, Miotto R, Vaid A, Johnson KW, Danieletto M, Golden E, Meyer D. Clinical characteristics of hospitalized Covid-19 patients in New York City. MedRxiv. 2020 doi: 10.1101/2020.04.19.20062117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Kim SR, Lee YC. Impact of oxidative stress on lung diseases. Respirology. 2009;14(1):27–38. doi: 10.1111/j.1440-1843.2008.01447.x. [DOI] [PubMed] [Google Scholar]
- Pawar KS, Mastud RN, Pawar SK, Pawar SS, Bhoite RR, Bhoite RR, Kulkarni MV, Deshpande AR. Oral curcumin with piperine as adjuvant therapy for the treatment of COVID-19: a randomized clinical trial. Front Pharmacol. 2021 doi: 10.3389/fphar.2021.66936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, Tobin KA, Cerfolio RJ, Francois F, Horwitz LI. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. MedRxiv. 2020;69(12):343. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfander H. [1] Carotenoids: an overview. Methods Enzymol. 1992;213:3–13. doi: 10.1016/0076-6879(92)13105-7. [DOI] [Google Scholar]
- Phoswa WN, Khaliq OP. Is pregnancy a risk factor of COVID-19? Eur J Obst Gynecol Reprod Biol. 2020;252:605–609. doi: 10.1016/j.ejogrb.2020.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piña-Zentella RM, Rosado JL, Gallegos-Corona MA, Madrigal-Pérez LA, García OP, Ramos-Gomez M. Lycopene improves diet-mediated recuperation in rat model of nonalcoholic fatty liver disease. J Med Food. 2016;19(6):607–614. doi: 10.1089/jmf.2015.0123. [DOI] [PubMed] [Google Scholar]
- Prattes J, Valentin T, Hoenigl M, Talakic E, Reisinger AC, Eller P. Invasive pulmonary aspergillosis complicating COVID-19 in the ICU-a case report. Med Mycol Case Rep. 2021;31:2–5. doi: 10.1016/j.mmcr.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybylska S, Tokarczyk G. Lycopene in the prevention of cardiovascular diseases. Int J Mol Sci. 2022;23(4):1957. doi: 10.3390/ijms23041957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian G, Zhang Y, Xu Y, Hu W, Hall IP, Yue J, Lu H, Ruan L, Ye M, Mei J. Reduced inflammatory responses to SARS-CoV-2 infection in children presenting to hospital with COVID-19 in China. EClinicalMedicine. 2021;34:100831. doi: 10.1016/j.eclinm.2021.100831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartuccio L, Semerano L, Benucci M, Boissier M-C, De Vita S. Urgent avenues in the treatment of COVID-19: targeting downstream inflammation to prevent catastrophic syndrome. Joint Bone Spine. 2020;87(3):191. doi: 10.1016/j.jbspin.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raha AA, Chakraborty S, Henderson J, Mukaetova-Ladinska E, Zaman S, Trowsdale J, Raha-Chowdhury R. 2020. Investigation of CD26, a potential SARS-CoV-2 receptor, as a biomarker of age and pathology. Biosci Rep. [DOI] [PMC free article] [PubMed]
- Rao AV, Rao L. Carotenoids and human health. Pharmacol Res. 2007;55:207–216. doi: 10.1016/j.phrs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Ritchie AI, Singanayagam A. Immunosuppression for hyperinflammation in COVID-19: a double-edged sword. Lancet. 2020;395(10230):1111. doi: 10.1016/S0140-6736(20)30691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha DFA, Machado-Junior PA, Souza ABF, de Castro TF, de Costa PG, Talvani A, Bezerra FS, Cangussú SD. Lycopene ameliorates liver inflammation and redox status in mice exposed to long-term cigarette smoke. BioMed Res Int. 2021;2021:1–11. doi: 10.1155/2021/7101313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Róvero Costa M, Leite Garcia J, de Almeida Silva CCV, Ferron AJT, Francisqueti-Ferron FV, Hasimoto FK, Gregolin CS, de Campos DHS, de Andrade CR, Ferreira ALDS, Corrêa CR, Moreto F. Lycopene modulates pathophysiological processes of non-alcoholic fatty liver disease in obese rats. Antioxidants (basel, Switzerland) 2019 doi: 10.3390/antiox8080276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sa-Ngiamsuntorn K, Suksatu A, Pewkliang Y, Thongsri P, Kanjanasirirat P, Manopwisedjaroen S, Charoensutthivarakul S, Wongtrakoongate P, Pitiporn S, Chaopreecha J. Anti-SARS-CoV-2 activity of Andrographis paniculata extract and its major component Andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives. J Nat Prod. 2021;84(4):1261–1270. doi: 10.1021/acs.jnatprod.0c01324. [DOI] [PubMed] [Google Scholar]
- Saedisomeolia A, Wood LG, Garg ML, Gibson PG, Wark PAB. Lycopene enrichment of cultured airway epithelial cells decreases the inflammation induced by rhinovirus infection and lipopolysaccharide. J Nutr Biochem. 2009;20(8):577–585. doi: 10.1016/j.jnutbio.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Sarker MT, Wan X, Yang H, Wang Z. Dietary lycopene supplementation could alleviate aflatoxinB1 induced intestinal damage through improving immune function and anti-oxidant capacity in broilers. Animals. 2021;11(11):3165. doi: 10.3390/ani11113165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt SM. The role of iron in viral infections. Front Biosci. 2020;25(4):893–911. doi: 10.2741/4839. [DOI] [PubMed] [Google Scholar]
- Scialo F, Daniele A, Amato F, Pastore L, Matera MG, Cazzola M, Castaldo G, Bianco A. ACE2: the major cell entry receptor for SARS-CoV-2. Lung. 2020;198(6):867–877. doi: 10.1007/s00408-020-00408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shami NJIE, Moreira EAM. Licopeno como agente antioxidante. Rev Nutr. 2004;17:227–236. doi: 10.1590/S1415-52732004000200009. [DOI] [Google Scholar]
- Sharma JB, Sharma A, Bahadur A, Vimala N, Satyam A, Mittal S. Oxidative stress markers and antioxidant levels in normal pregnancy and pre-eclampsia. Int J Gynecol Obstet. 2006;94(1):23–27. doi: 10.1016/j.ijgo.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Shaw JA, Koti M. Clinical images. Canad Med Assoc J. 2009;180(8):895. doi: 10.1503/cmaj.071335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H. Findings in redox biology: from H2O2 to oxidative stress. J Biol Chem. 2020;295(39):13458–13473. doi: 10.1074/jbc.X120.015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi CP, Nguyen J, Yamaguchi Y, Werts AD, Lu P, Ladd MR, Fulton WB, Kovler ML, Wang S, Prindle T. A dynamic variation of pulmonary ACE2 is required to modulate neutrophilic inflammation in response to Pseudomonas aeruginosa lung infection in mice. J Immunol. 2019;203(11):3000–3012. doi: 10.4049/jimmunol.1900579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Zhang L, Xu Y, Yang D, Yang S, Zhang W, Wang J, Tian S, Yang S, Yuan T. The comprehensive study on the therapeutic effects of baicalein for the treatment of COVID-19 in vivo and in vitro. Biochem Pharmacol. 2021;183:114302. doi: 10.1016/j.bcp.2020.114302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, Smith D, Richardson P. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20(4):400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan N, Birkenfeld AL, Schulze MB, Ludwig DS. Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol. 2020;16(7):341–342. doi: 10.1038/s41574-020-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice CP, Liu C, Aizawa K, Greenberg AS, Ausman LM, Wang X-D. Dietary tomato powder inhibits alcohol-induced hepatic injury by suppressing cytochrome p450 2E1 induction in rodent models. Arch Biochem Biophys. 2015;572:81–88. doi: 10.1016/j.abb.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, Talavera-López C, Maatz H, Reichart D, Sampaziotis F. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallei TE, Niode NJ, Idroes R, Zidan BM, Mitra S, Celik I, Nainu F, Ağagündüz D, Emran TB, Capasso R. A comprehensive review of the potential use of green tea polyphenols in the management of COVID-19. Evidence-Based Complement Altern Med. 2021;2021:1–13. doi: 10.1155/2021/7170736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao A, Wang X, Li C. Effect of Lycopene on oral squamous cell carcinoma cell growth by inhibiting IGF1 pathway. Cancer Manage Res. 2021;13:723. doi: 10.2147/CMAR.S283927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakoli A, Vahdat K, Keshavarz M. Novel coronavirus disease 2019 (COVID-19): an emerging infectious disease in the 21st century. ISMJ. 2020;22(6):432–450. [Google Scholar]
- Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thum T. SARS-CoV-2 receptor ACE2 expression in the human heart: cause of a post-pandemic wave of heart failure? Eur Heart J. 2020;41(19):1807–1809. doi: 10.1093/eurheartj/ehaa410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To KK-W, Tsang OT-Y, Leung W-S, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uçar S, Pandir D. Furan induced ovarian damage in non-diabetic and diabetic rats and cellular protective role of lycopene. Arch Gynecol Obstet. 2017;296(5):1027–1037. doi: 10.1007/s00404-017-4521-7. [DOI] [PubMed] [Google Scholar]
- Ugbaja RN, James AS, Ugwor EI, Akamo AJ, Thomas FC, Kosoko AM. Lycopene suppresses palmitic acid-induced brain oxidative stress, hyperactivity of some neuro-signalling enzymes, and inflammation in female Wistar rat. Sci Rep. 2021;11(1):1–13. doi: 10.1038/s41598-021-94518-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z, Flammer AAJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. The Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacquier B, Combettes L, Dupont G. Dual dynamics of mitochondrial permeability transition pore opening. Sci Rep. 2020;10(1):1–10. doi: 10.1038/s41598-020-60177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Li L, Wang Z, Cui Y, Tan X, Yuan T, Liu Q, Liu Z, Liu X. Supplementation of lycopene attenuates lipopolysaccharide-induced amyloidogenesis and cognitive impairments via mediating neuroinflammation and oxidative stress. J Nutr Biochem. 2018;56:16–25. doi: 10.1016/j.jnutbio.2018.01.009. [DOI] [PubMed] [Google Scholar]
- Wang J, Tang X, Lu Y, Zheng Y, Zeng F, Shi W, Zhou P. Lycopene regulates dietary dityrosine-induced mitochondrial-lipid homeostasis by increasing mitochondrial complex activity. Mol Nutr Food Res. 2022;66(1):2100724. doi: 10.1002/mnfr.202100724. [DOI] [PubMed] [Google Scholar]
- Wang R, Lu X, Yu R. Lycopene inhibits epithelial-mesenchymal transition and promotes apoptosis in oral cancer via PI3K/AKT/m-TOR signal pathway. Drug Des Dev Ther. 2020;14:2461–2471. doi: 10.2147/DDDT.S251614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Ye J, Kang Z, Peng H, Mackey V, Sun L. The COVID-19 pandemic and the potential treatment of the novel coronavirus SARS-CoV-2. Am J Transl Res. 2021;13(3):871. [PMC free article] [PubMed] [Google Scholar]
- Wang W, Yang W, Shen Z, Wen S, Hu M. The dose-response effect of lycopene on cerebral vessel and neuron impairment induced by hyperlipidemia. J Agric Food Chem. 2018;66(50):13173–13182. doi: 10.1021/acs.jafc.8b05232. [DOI] [PubMed] [Google Scholar]
- Wang X, Lv H, Gu Y, Wang X, Cao H, Tang Y, Chen H, Huang C. Protective effect of lycopene on cardiac function and myocardial fibrosis after acute myocardial infarction in rats via the modulation of p38 and MMP-9. J Mol Histol. 2014;45(1):113–120. doi: 10.1007/s10735-013-9535-2. [DOI] [PubMed] [Google Scholar]
- Wang Y-Q, Li Q-S, Zheng X-Q, Lu J-L, Liang Y-R. Antiviral effects of green tea EGCG and its potential application against COVID-19. Molecules. 2021;26(13):3962. doi: 10.3390/molecules26133962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson E, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P. OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. MedRxiv. 2020 doi: 10.1101/2020.05.06.20092999v1. [DOI] [Google Scholar]
- Wu C, Chen X, Cai Y, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Zhao S, Yu B, Chen Y-M, Wang W, Song Z-G, Hu Y, Tao Z-W, Tian J-H, Pei Y-Y. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Yang L, Liu S, Liu J, Zhang Z, Wan X, Huang B, Chen Y, Zhang Y. COVID-19: immunopathogenesis and Immunotherapeutics. Signal Transduct Target Ther. 2020;5(1):1–8. doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Bazhin AV, Werner J, Karakhanova S. Reactive oxygen species in the immune system. Int Rev Immunol. 2013;32(3):249–270. doi: 10.3109/08830185.2012.755176. [DOI] [PubMed] [Google Scholar]
- Yin Y, Zheng Z, Jiang Z. Effects of lycopene on metabolism of glycolipid in type 2 diabetic rats. Biomed Pharmacother. 2019;109:2070–2077. doi: 10.1016/j.biopha.2018.07.100. [DOI] [PubMed] [Google Scholar]
- Yu J-N, Wu B-B, Yang J, Lei X-L, Shen W-Q. Cardio-cerebrovascular disease is associated with severity and mortality of COVID-19: a systematic review and meta-analysis. Biol Res Nurs. 2021;23(2):258–269. doi: 10.1177/1099800420951984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Rohli KE, Yang S, Jia P. Impact of obesity on COVID-19 patients. J Diab Complications. 2021;35(3):107817. doi: 10.1016/j.jdiacomp.2020.107817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan SY, Breslin JW, Perrin R, Gaudreault N, Guo M, Kargozaran H, Wu MH. Microvascular permeability in diabetes and insulin resistance. Microcirculation. 2007;14(4–5):363–373. doi: 10.1080/10739680701283091. [DOI] [PubMed] [Google Scholar]
- Zeng J, Zhao J, Dong B, Cai X, Jiang J, Xue R, Yao F, Dong Y, Liu C. Lycopene protects against pressure overload-induced cardiac hypertrophy by attenuating oxidative stress. J Nutr Biochem. 2019;66:70–78. doi: 10.1016/j.jnutbio.2019.01.002. [DOI] [PubMed] [Google Scholar]
- Zhan Y, Ta W, Tang W, Hua R, Wang J, Wang C, Lu W. Potential antiviral activity of isorhamnetin against SARS-CoV-2 spike pseudotyped virus in vitro. Drug Dev Res. 2021;82(8):1124–1130. doi: 10.1002/ddr.21815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Wu Z, Li J-W, Zhao H, Wang G-Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55(5):105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Fu Y, Zhou X, Pan W, Shi Y, Wang M, Zhang X, Qi D, Li L, Ma K. Depression-like behaviors and heme oxygenase-1 are regulated by Lycopene in lipopolysaccharide-induced neuroinflammation. J Neuroimmunol. 2016;298:1–8. doi: 10.1016/j.jneuroim.2016.06.001. [DOI] [PubMed] [Google Scholar]
- Zhang Y-N, Zhang Q-Y, Li X-D, Xiong J, Xiao S-Q, Wang Z, Zhang Z-R, Deng C-L, Yang X-L, Wei H-P. Gemcitabine, lycorine and oxysophoridine inhibit novel coronavirus (SARS-CoV-2) in cell culture. Emerg Microb Infect. 2020;9(1):1170–1173. doi: 10.1080/22221751.2020.1772676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li J, Zhan Y, Wu L, Yu X, Zhang W, Ye L, Xu S, Sun R, Wang Y. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect Immun. 2004;72(8):4410–4415. doi: 10.1128/IAI.72.8.4410-4415.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhang X, Bi K, He Y, Yan W, Yang CS, Zhang J. Potential protective mechanisms of green tea polyphenol EGCG against COVID-19. Trends Food Sci Technol. 2021;114:11–24. doi: 10.1016/j.tifs.2021.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Ren B, Guo R, Zhang W, Ma S, Yao Y, Yuan T, Liu Z, Liu X. Supplementation of lycopene attenuates oxidative stress induced neuroinflammation and cognitive impairment via Nrf2/NF-κB transcriptional pathway. Food Chem Toxicol. 2017;109:505–516. doi: 10.1016/j.fct.2017.09.050. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Yang F, Meng L, Chen D, Wang M, Lu X, Chen D, Jiang Y, Xing N. Lycopene attenuates chronic prostatitis/chronic pelvic pain syndrome by inhibiting oxidative stress and inflammation via the interaction of NF-κB, MAPKs, and Nrf2 signaling pathways in rats. Andrology. 2020;8(3):747–755. doi: 10.1111/andr.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H-Y, Zhang M, Yang C-X, Zhang N, Wang X-C, Yang X-P, Dong X-Q, Zheng Y-T. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol. 2020;17(5):541–543. doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Yin Y, Lu R, Jiang Z. Lycopene ameliorated oxidative stress and inflammation in type 2 diabetic rats. J Food Sci. 2019;84(5):1194–1200. doi: 10.1111/1750-3841.14505. [DOI] [PubMed] [Google Scholar]
- Zhong J, Tang J, Ye C, Dong L. The immunology of COVID-19: is immune modulation an option for treatment? The Lancet Rheumatol. 2020;2(7):e428–e436. doi: 10.1016/S2665-9913(20)30120-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, She Z-G, Cheng X, Qin J-J, Zhang X-J, Cai J, Lei F, Wang H, Xie J, Wang W. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31(6):1068–1077. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N. A Novel coronavirus from patients with pneumonia in China. N Engl J Med. 2019;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang E, Uchio E, Lilly M, Zi X, Fruehauf JP. A phase II study of docetaxel plus lycopene in metastatic castrate resistant prostate cancer. Biomed Pharmacother. 2021;143:112226. doi: 10.1016/j.biopha.2021.112226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, Cao Y, Yousif AS, Bals J, Hauser BM. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181(5):1016–1035. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinovkin RA, Grebenchikov OA. Transcription factor Nrf2 as a potential therapeutic target for prevention of cytokine storm in COVID-19 patients. Biochem Mosc. 2020;85(7):833–837. doi: 10.1134/S0006297920070111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Feng D, Ling W-H, Duan R-D. Lycopene suppresses proinflammatory response in lipopolysaccharide-stimulated macrophages by inhibiting ROS-induced trafficking of TLR4 to lipid raft-like domains. J Nutr Biochem. 2013;24(6):1117–1122. doi: 10.1016/j.jnutbio.2012.08.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Raha AA, Chakraborty S, Henderson J, Mukaetova-Ladinska E, Zaman S, Trowsdale J, Raha-Chowdhury R. 2020. Investigation of CD26, a potential SARS-CoV-2 receptor, as a biomarker of age and pathology. Biosci Rep. [DOI] [PMC free article] [PubMed]
Data Availability Statement
The study did not utilize a dataset.