Abstract
Background
Approximately 450,000 children worldwide die of pneumococcal infections each year. The development of bacterial resistance to antimicrobials adds to the difficulty of treatment of diseases and emphasizes the need for a preventive approach. Newborn vaccination schedules could substantially reduce the impact of pneumococcal disease in immunized children, but do not have an effect on the morbidity and mortality of infants less than three months of age. Pneumococcal vaccination during pregnancy may be a way of preventing pneumococcal disease during the first months of life before the pneumococcal vaccine administered to the infant starts to produce protection.
Objectives
To assess the effect of pneumococcal vaccination during pregnancy for preventing infant infection.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 July 2014) and reference lists of retrieved studies.
Selection criteria
Randomized controlled trials in pregnant women comparing pneumococcal vaccine with placebo or doing nothing, or with another vaccine to prevent infant infections.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. We contacted study authors for additional information.
Main results
Seven trials were included, but only six trials (919 participants) contributed data. There was no evidence that pneumococcal vaccination during pregnancy reduces the risk of neonatal infection (risk ratio (RR) 0.66; 95% confidence interval (CI) 0.30 to 1.46; two trials, 241 pregnancies, low quality evidence). Although the data suggest an effect in reducing pneumococcal colonization in infants by 16 months of age (average RR 0.33; 95% CI 0.11 to 0.98; one trial, 56 pregnancies), there was no evidence of this effect in infants at two to three months of age (average RR 1.13; 95% CI 0.46 to 2.78; two trials, 146 pregnancies, low quality evidence) or by six to seven months of age (average RR 0.67, 95% CI 0.22 to 2.08; two trials, 148 pregnancies, low quality evidence). None of the trials included in this review reported neonatal death as a result of pneumococcal infection.
Neonatal antibody levels were reported as geometric mean and 95% CI. There were inconsistent results between studies. Two studies showed significantly higher immunoglobulin G (IgG) levels in cord blood in the pneumococcal vaccine group when compared with the control group for all serotypes. In contrast, another trial showed no difference in neonatal antibody levels between the pneumococcal vaccine group and the control group.
Maternal antibody levels were also reported as geometric mean and 95% CI. One study showed significantly higher IgG levels in maternal serum in women immunized with pneumococcal vaccine when compared with control vaccine regardless of any serotypes. Another study showed significantly higher maternal antibody levels only for serotype 14, but no evidence of an effect for other serotypes.
The percentage of women with seroprotection was measured in one trial at delivery and at 12 months post‐delivery. At delivery, results favored the intervention group for serotype 6 (RR 1.49, 95% CI 1.31 to 1.69), serotype 14 (RR 1.40, 95% CI 1.25 to 1.56) and serotype 19 (RR 2.29, 95% CI 1.89 to 2.76). There were no group differences seen at 12 months post‐delivery for serotypes 6 or 14 (RR 1.06, 95% CI 1.00 to 1.12 and RR 1.06, 95% CI 0.98 to 1.15, respectively), but results favored the intervention group for serotype 19 (RR 1.59, 95% CI 1.37 to 1.85).
No significant difference for tenderness at the injection site between women who received pneumococcal vaccine and those who received control vaccine (average RR 3.20; 95% CI 0.32 to 31.54; two trials, 130 women).
The overall quality of evidence is low for primary outcomes. Most outcomes had wide confidence intervals crossing the line of no effect, and most of the included trials had small numbers of participants and few events which led to downgrading evidence for imprecision of findings.
Authors' conclusions
There is insufficient evidence to assess whether pneumococcal vaccination during pregnancy could reduce infant infections.
Plain language summary
Pneumococcal vaccination during pregnancy for preventing infant infection
There is not enough evidence to assess whether using pneumococcal vaccination during pregnancy can prevent infant infections.
Although the incidence of invasive pneumococcal disease is variable across the world, the rate of serious illness or death is high in children who get this infection. The Streptococcus pneumoniae (pneumococcus) organism colonizes the upper respiratory tract and can cause bacteremia, meningitis, pneumonia and other lower respiratory tract, and upper respiratory tract infections, including otitis media and sinusitis. Newborn vaccination schedules of three primary doses with a booster dose could reduce the impact of pneumococcal disease in immunized children, but these vaccinations have no protective effect in infants less than three months of age. Maternal pneumococcal immunization during pregnancy may be a way of preventing pneumococcal disease during the infant's first months of life. We included seven randomized controlled trials. A total of 919 pregnant women participated in the six randomized controlled trials that contributed data to this review. The trials compared 23‐valent pneumococcal polysaccharide vaccine with control vaccine. All women received a single injection of pneumococcal or control vaccine (where used). The women’s mean gestational age at the time of immunization was between 27 and 38 weeks, where stated. Only two trials with 241 pregnancies reported on neonatal infections. This was not enough information to say whether pneumococcal vaccination during pregnancy led to fewer infant infections. Two trials with 146 pregnancies reported on infant nasal carriage of pneumococci (pneumococcal colonization), which was not enough evidence to show an effect in reducing colonization at two to three months of age or six to seven months of age. The included trials were of reasonable quality. There was no difference between pneumococcal vaccine and control vaccine for tenderness at the injection site. No serious adverse events were reported in the trials.
Summary of findings
Summary of findings for the main comparison. Pneumococcal vaccine versus control vaccine for preventing infant infection.
| Pneumococcal vaccine versus control vaccine for preventing infant infection | ||||||
| Patient or population: Pregnant women undergoing vaccination to prevent infant infection Settings: Studies were located in Bangladesh, Brazil, The Gambia and the USA. Intervention: Pneumococcal vaccine versus control vaccine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Pneumococcal vaccine versus control vaccine | |||||
| Neonatal death due to pneumococcal infection | 0 | 0 | Not estimable | None of the included studies in this review measured the primary outcome of neonatal death due to pneumococcal infection. | ||
|
Neonatal infection ‐ Pneumonia Follow‐up: 1 year |
Study population | RR 0.58 (0.18 to 1.9) | 149 (1 study) | ⊕⊕⊝⊝ low1 | ||
| 93 per 1000 | 54 per 1000 (17 to 177) | |||||
|
Neonatal infection ‐ Meningitis Follow‐up: 1 year |
Study population | RR 3.04 (0.13 to 73.44) | 149 (1 study) | ⊕⊕⊝⊝ low1 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
|
Neonatal infection ‐ Otitis media Follow‐up: 1 year |
Study population | RR 0.14 (0.01 to 2.75) | 149 (1 study) | ⊕⊕⊝⊝ low1 | ||
| 40 per 1000 | 6 per 1000 (0 to 110) | |||||
|
Neonatal infection ‐ All infections Follow‐up: 3‐12 months |
Study population | RR 0.66 (0.3 to 1.46) | 241 (2 studies) | ⊕⊕⊝⊝ low1 | ||
| 115 per 1000 | 76 per 1000 (34 to 168) | |||||
|
Pneumococcal colonization ‐ At 2‐3 months of age Follow‐up: 2‐3 months |
Study population | RR 1.13 (0.46 to 2.78) | 146 (2 studies) | ⊕⊕⊝⊝ low1 | ||
| 133 per 1000 | 150 per 1000 (61 to 368) | |||||
|
Pneumococcal colonization ‐ By 6‐7 months of age Follow‐up: 6‐7 months |
Study population | RR 0.67 (0.22 to 2.08) | 148 (2 studies) | ⊕⊕⊝⊝ low1 | ||
| 271 per 1000 | 181 per 1000 (60 to 563) | |||||
| *The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Wide confidence interval crossing the line of no effect, few events and small sample size (‐2).
Background
Description of the condition
Infections caused byStreptococcus pneumoniae (pneumococcus) are a major cause of morbidity and mortality throughout the world (WHO 2012). Pneumococcus is a leading cause of illness in young children and causes illness and death among the elderly and persons who have certain underlying medical conditions. The organism colonizes the upper respiratory tract and can cause the following types of illnesses: (a) invasive pneumococcal infections, including bacteremia and meningitis; (b) pneumonia and other lower respiratory tract infections; and (c) upper respiratory tract infections, including otitis media and sinusitis. Invasive pneumococcal diseases are less common than non‐invasive manifestations, but cause high mortality. Each year, approximately 330,000 to 529,000 children worldwide, mostly in low‐ to middle‐income countries, die of pneumococcal infections (WHO 2012). The development of resistance to antimicrobials by the bacteria adds to the difficulty of treatment of diseases and emphasizes the need for a preventive approach.
Regional differences in incidence of invasive pneumococcal disease have been noted. A 10‐year surveillance in the Oxfordshire region of England, found that the annual incidence of invasive pneumococcal disease among children under five years of age prior to implementation of the pneumococcal vaccine was 24.3 per 100,000 persons (95% confidence interval 21.0 to 27.7) (Foster 2008). A population‐based study carried out in a rural area of Bangladesh estimated the incidence of invasive pneumococcal disease among children to be 86 cases per 100,000 person‐years (Arifeen 2009). In the United States, the routine use of pneumococcal vaccine beginning in 2000 has had a substantial impact on the epidemiology of pneumococcal disease in children. In infants younger than five years, rates of invasive pneumococcal disease have decreased from 98.7 cases per 100,000 population during 1998 and 1999 to 23.6 cases per 100,000 population in 2007 (Pilishvili 2010). Similar findings were reported in European countries. After widespread pneumococcal vaccination, there was a mean decline in the incidence of invasive pneumococcal disease in children aged less than two years from 32.5 to 23.4/100,000 (Isaacman 2010).
Description of the intervention
There are two types of pneumococcal vaccine available, polysaccharide vaccines and polysaccharide/protein conjugate vaccines. The 23‐valent polysaccharide vaccine (PPV23) contains polysaccharide antigen from 23 types of pneumococcal bacteria (serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B,17F, 18C, 19A, 19F, 20, 22F, 23F and 33F), which cause 85% to 90% of bacteremic pneumococcal disease in adults. More than 80% of healthy adults who receive PPV23 develop antibodies against the serotypes contained in the vaccine within two to three weeks after vaccination; however, the vaccine is relatively poor at producing immunity in children less than two years old (Klouwenberg 2008). The pneumococcal conjugated vaccine (PCV) is much better at producing immunity in infants than pure polysaccharide vaccines (Black 2000; Klouwenberg 2008). In 2000, the 7‐valent pneumococcal conjugated vaccine (PCV7) was recommended in the United States for immunization of infants (CDC 2000). PCV‐7 includes the seven most frequent polysaccharides (serotypes 4, 6B, 9V, 14, 18C, 19F, 23F) in pneumococcal infections of the young child. Newborn vaccination schedules consist of three primary doses routinely plus a booster dose. After four doses, more than 90% of healthy infants develop antibodies to all seven serotypes contained in the vaccine. In a large trial, PCV7 was shown to reduce invasive disease caused by vaccine serotypes by 97%; however, it was less effective against acute otitis media (Black 2000). Local reactions following PCV7 occur in 10% to 20% of recipients and are more common with the fourth dose than the first three doses. No severe adverse events attributable to PCV7 have been reported. The vaccine could substantially reduce the impact of pneumococcal disease in immunized children (Isaacman 2010; Pilishvili 2010); however, it does not have an effect on the morbidity and mortality of the younger infants, especially those less than three months of age. This may be due to the fact that serum IgG (immunoglobulin G) antibodies against polysaccharides increase only after the second and third vaccine doses are administered (Rennels 1998).
PCV7 given to the children has had a substantial effect on pneumococcal disease in the United States since its introduction in 2000. A large, population‐based surveillance system monitoring invasive pneumococcal disease in a population of nearly 20 million persons in the United States, found that rates of invasive pneumococcal disease in children younger than two years of age were 68.6% lower in 2001 compared with rates of disease before the vaccine was introduced (Whitney 2003). Furthermore, widespread vaccination of children with PCV7 has shown a 'herd effect' in decreasing the carriage rate of S. pneumoniae in children, who are an important vector of S. pneumoniae to other children and adults (Dagan 2000; Pilishvili 2010). Data from the same surveillance indicate that unvaccinated adults are benefiting from the vaccination of children. Infection rates in adults fell 8% to 32% when compared with the average rates in 1998 and 1999 (Whitney 2003). However, invasive pneumococcal disease caused by non‐PCV7 serotypes has increased and partially offset the reductions (CDC 2010; Pilishvili 2010). Overall, rates of invasive pneumococcal disease have remained stable at 22 to 25 cases per 100,000 since 2002 (CDC 2010; Pilishvili 2010). An increase in disease due to non‐PCV‐7 serotypes and the need for broader serotype coverage to address the global disease burden provides a rationale for a second‐generation conjugate vaccine, 10‐valent pneumococcal conjugated vaccine (PCV10, including serotypes 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F and 23F) and 13‐valent pneumococcal conjugated vaccine (PCV13, including serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F) (Grijalva 2011; WHO 2012). The Advisory Committee on Immunization Practices in the United States has recommended the use of PCV13 for immunization of infants since 2010 (CDC 2010) and the World Health Organization has recommended to replace PCV7 by PCV10 or PCV13 for immunization of infants in 2012 (WHO 2012).
How the intervention might work
Maternal immunization could help to prevent the two to three million neonatal and early infant deaths that occur in low‐ to middle‐income countries each year (Greenwood 2003). Maternal pneumococcal immunization may be a way of preventing pneumococcal disease during the first months of life before infant‐administered pneumococcal conjugate vaccine starts to produce protection. This strategy has the potential to impact on public health, as has been seen by the prevention of tetanus neonatorum through maternal immunization (Khan 2013). An effective delivery system for maternal immunization already exists and, because of the success of maternal tetanus immunization, this approach to the prevention of serious illness or death in young infants is widely accepted by the general population.
Why it is important to do this review
There are clinical studies on maternal pneumococcal immunization to prevent infant infection, but information regarding effectiveness of the intervention is not known.
Objectives
To assess the effectiveness of pneumococcal vaccine administered to pregnant women in preventing pneumococcal infection in the infant.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials. Quasi‐randomized controlled trials were not included.
Types of participants
Healthy women with uncomplicated pregnancies.
Types of interventions
Pneumococcal vaccine (polysaccharide or conjugate) compared with placebo or doing nothing, or with another vaccine.
Types of outcome measures
Primary outcomes
(1) Neonatal pneumococcal infection:
pneumonia (diagnosed by clinical findings and radiological or laboratory findings);
meningitis (diagnosed by clinical findings and laboratory findings);
bacteremia/sepsis (diagnosed by clinical findings and laboratory findings);
neonatal death (due to pneumococcal infection);
otitis media (diagnosed by clinical findings and laboratory findings).
(2) Neonatal pneumococcal colonization:
at two to three months of age;
by six to seven months of age.
Secondary outcomes
(1) Neonatal antibody levels. (2) Adverse neonatal effects. (3) Maternal antibody levels. (4) Incidence of maternal pneumococcal colonization during labor. (5) Adverse maternal effects. (6) Neonatal pneumococcal colonization by 16 months of age.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 July 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
(Please see Appendix 1 for details of additional searches carried out in the initial version of the review (Chaithongwongwatthana 2006).)
Searching other resources
We searched cited references from retrieved articles for additional studies. We reviewed abstracts and letters to the editor to identify randomized controlled trials that have not been published. If we identified a randomized controlled trial, we contacted the primary investigator directly to obtain further data. We reviewed editorials, indicating expert opinion, to identify and ensure that no key studies were missed for inclusion in this review.
We did not apply any language restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeChaithongwongwatthana 2012.
For this update, the following methods were used for assessing the two reports that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we contacted authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomization);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
For this update, the quality of the evidence was assessed using the GRADE approach (Schunemann 2009) in order to assess the quality of the body of evidence relating to the following outcomes for the main comparisons.
Neonatal infection ‐ pneumonia
Neonatal infection ‐ meningitis
Neonatal infection ‐ otitis media
Neonatal infection ‐ all
Neonatal infection ‐ neonatal death
Pneumococcal colonization ‐ two to three months
Pneumococcal colonization ‐ six to seven months
GRADE profiler (GRADE 2008) was used to import data from Review Manager 5.3 (RevMan 2014) in order to create a ’Summary of findings’ table. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
The primary outcomes were included in the Table 1. For neonatal infection, none of the included trials in this review reported the outcome of neonatal death. Neonatal pneumococcal colonization by 16 months of age was reported in one trial, but it was not included as a primary outcome or in the 'Summary of findings' table because the colonization at this time point may be confounded by neonatal vaccination.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We did not identify an continuous outcomes. We planned to use the mean difference if outcomes were measured in the same way between trials. We would have used the standardized mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomized trials
For this update we did not include any cluster‐randomized trials in the review. If in future updates eligible cluster‐randomized trials are identified, we will include these cluster‐randomized trials in the analyses along with individually‐randomized trials. We will adjust their sample sizes or standard errors using the methods described in the Handbook using an estimate of the intra‐cluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomized trials and individually‐randomized trials, we plan to synthesize the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomization unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomization unit and perform a sensitivity analysis to investigate the effects of the randomization unit.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if more eligible studies are included, the impact of including studies with high levels of missing data in the overall assessment of treatment effect will be explored by using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis i.e. we attempted to include all participants randomized to each group in the analyses. The denominator for each outcome in each trial was the number randomized minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. Had we identified substantial heterogeneity (above 30%), we planned to explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
Where there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. Where we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
Had we identified substantial heterogeneity, we planned to investigate it using subgroup analyses and sensitivity analyses. We would have considered whether an overall summary was meaningful, and if it was, we planned to use random‐effects analysis to produce it.
We planned to carry out the following subgroup analyses:
types of pneumococcal vaccine (polysaccharide versus conjugate);
countries of participants (high‐income versus low‐ to middle‐income).
The following outcomes were planned for use in subgroup analyses:
neonatal pneumococcal infection;
neonatal pneumococcal colonization.
There were too few trials included in this review to conduct meaningful subgroup analysis.
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this makes any difference to the overall result.
There were too few trials included in this review to conduct meaningful sensitivity analysis.
Results
Description of studies
Results of the search
We identified 10 studies as potentially eligible for inclusion in this review. We included seven trials and excluded two trials, one trial is ongoing (Dunbar 2007). Seven new reports were identified in the updated search. All of them reported new information from the two included trials (Lopes 2009; Zaman 2008).
Included studies
One trial (Obaro 2004) did not contribute data toward the analyses because it did not report the outcomes of interest. A total of 919 pregnant women participated in the other six included studies comparing 23‐valent pneumococcal polysaccharide vaccine with control vaccine (O' Dempsey 1996; Munoz 2001; Shahid 1995; Zaman 2008) or no vaccine (Lopes 2009; Quiambao 2003). The O' Dempsey 1996 trial included 75 women in each group. The Munoz 2001 trial used a 2:1 randomization scheme with 20 women in the vaccine group and 40 women in control group. The Shahid 1995 trial included 36 women in vaccine group and 34 women in control group. The Zaman 2008 trial included 168 women in vaccine group and 172 women in control group. Lopes 2009 was a trial with three arms. We have included data from two of the arms: 47 women in a control group (no vaccine) and 45 women who received vaccine at 30 to 34 weeks of gestation. The third arm involved 47 women who received vaccine after delivery; these data were not included from our analyses. Quiambao 2003 employed a 2:1 randomization scheme and included 106 women in vaccine group and 54 women in control group.
Participants
This review includes data from six trials with a total of 919 pregnant women. A total of 497 women were randomized to be immunized with pneumococcal polysaccharide vaccine and 422 women were randomized to control vaccines. The included trials were conducted in various settings; one in Brazil (Lopes 2009), one in the United States (Munoz 2001), two in the Gambia (O' Dempsey 1996; Obaro 2004), one in the Philippines (Quiambao 2003), and two in Bangladesh (Shahid 1995; Zaman 2008). All participants were healthy women with an uncomplicated pregnancy. Lopes 2009 and Obaro 2004 had no available data on participants' mean age but for the other included trials (Munoz 2001; O' Dempsey 1996; Quiambao 2003; Shahid 1995; Zaman 2008), the mean age of the women in each study was 30.2 (Munoz 2001), 22.0 (O' Dempsey 1996), 26.8 (Quiambao 2003), 25.6 (Shahid 1995), and 24.9 (Zaman 2008) years.
Interventions
All trials used a 23‐valent pneumococcal polysaccharide vaccine compared with control vaccine or no vaccine. One trial (Munoz 2001) used Hemophilus influenzae conjugate vaccine as the control; two trials (O' Dempsey 1996; Shahid 1995) used meningococcal vaccine as the control; one trial (Zaman 2008) used inactivated influenza vaccine as the control while the other two trials (Lopes 2009; Quiambao 2003) had no control vaccine. All women received a single injection of pneumococcal or control vaccine (where used). The mean gestational age at the time of immunization in each study was no available data (Lopes 2009), 33.3 (Munoz 2001), 38.0 (O' Dempsey 1996), 27.3 weeks (Quiambao 2003), 32.3 (Shahid 1995), and no available data (Zaman 2008). The mean interval between immunization and delivery in each study was 43.6 (Munoz 2001), 44.1 (O' Dempsey 1996), 51.4 (Shahid 1995), 54.9 (Zaman 2008), not available data (Lopes 2009) and 76.3 days (Quiambao 2003) respectively.
Outcomes
Two studies reported the incidence of neonatal infection (Lopes 2009; O' Dempsey 1996). Two trials reported the incidence of neonatal pneumococcal colonization (Lopes 2009; Munoz 2001). Three trials (Munoz 2001; O' Dempsey 1996; Quiambao 2003) reported neonatal antibody levels as geometric mean with 95% confidence interval. Three trials (Munoz 2001; O' Dempsey 1996; Shahid 1995) reported maternal antibody levels. One trial reported (Zaman 2008) the percentage of mothers with seroprotection. No serious adverse reactions attributable to the vaccines were observed in all studies (seeCharacteristics of included studies).
Excluded studies
We excluded two trials (Daly 2003; Glezen 2000). SeeCharacteristics of excluded studies table.
Risk of bias in included studies
One trial was at low risk of bias (Zaman 2008). All the remaining trials were at unclear risk of selection bias. High risk for attrition bias was noted in the two trials (Quiambao 2003; Shahid 1995). The risk of bias in the included trials is summarized in the 'Risk of bias' graph (Figure 1).
1.
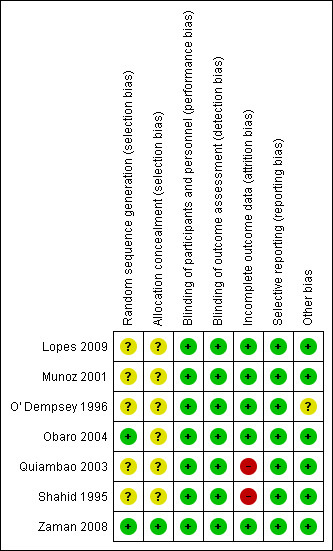
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Only one trial (Zaman 2008) described the method of random allocation and allocation concealment. The other six trials had an unclear risk of selection bias.
Blinding
Four trials (Munoz 2001; O' Dempsey 1996; Shahid 1995; Zaman 2008) were double‐blind studies. Although the other two trials were not double‐blind studies, the risk of detection bias was low because the reported outcomes were objective outcomes.
Incomplete outcome data
Only one trial (Lopes 2009) had complete follow‐up. There were two trials (Quiambao 2003; Shahid 1995) that had high risk for attrition bias due to high proportion of incomplete outcome data.
Selective reporting
All trials had low risk for reporting bias.
Other potential sources of bias
None.
Effects of interventions
See: Table 1
Comparison
Primary outcomes
Neonatal infections
Only two trials (Lopes 2009; O' Dempsey 1996) with 241 pregnancies reported these outcomes. There was insufficient evidence to show an effect of maternal pneumococcal vaccine during pregnancy in reduction of neonatal infections (risk ratio (RR) 0.66; 95% confidence interval (CI) 0.30 to 1.46) including pneumonia (RR 0.58; 95% CI 0.18 to 1.90), meningitis (RR 3.04; 95% CI 0.13 to 73.44), and otitis media (RR 0.14; 95% CI 0.01 to 2.75). SeeAnalysis 1.1.
1.1. Analysis.
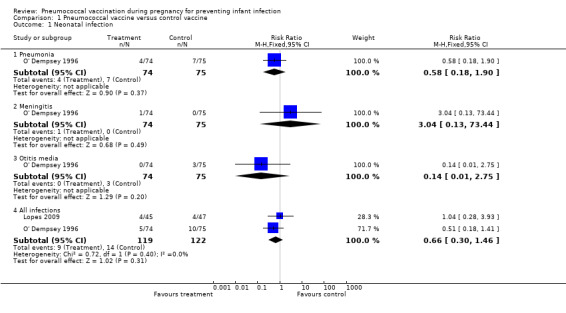
Comparison 1 Pneumococcal vaccine versus control vaccine, Outcome 1 Neonatal infection.
None of the included trials in this review reported the outcome of neonatal death due to pneumococcal infection.
Neonatal pneumococcal colonization
Two studies (Lopes 2009; Munoz 2001) with 148 pregnancies reported this outcome at several time points and according to three serotypes (6, 14 and 19). There was not enough evidence to show an effect of maternal pneumococcal vaccination in reduction of neonatal nasal carriage of pneumococci at two to three months of age (average RR 1.13; 95% CI 0.46 to 2.78) or by six to seven months of age (average RR 0.67; 95% CI 0.22 to 2.08, I² = 54%, Tau² = 0.38). Nevertheless, the results showed a statistically significant decrease in the incidence of pneumococcal colonization in infants by 16 months of age (RR 0.33; 95% CI 0.11 to 0.98; one study, 56 infants). SeeAnalysis 1.2. One study with 92 women (Lopes 2009) found no group differences in pneumococcal colonization according to serotype 6, 14 or 19 (RR 0.12, 95% CI 0.01 to 2.09; not estimable; and RR 2.09, 95% CI 0.40 to 10.85, respectively).
1.2. Analysis.
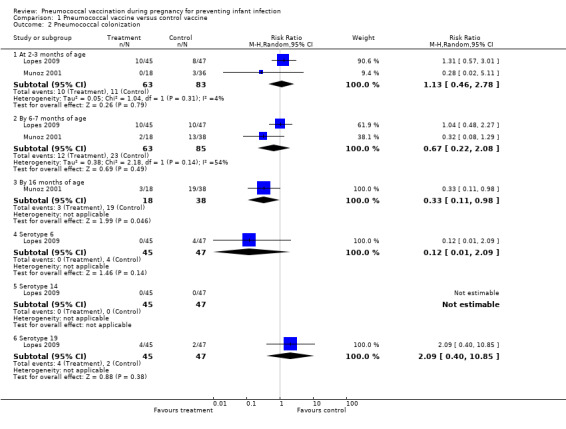
Comparison 1 Pneumococcal vaccine versus control vaccine, Outcome 2 Pneumococcal colonization.
Heterogeneity was noted for neonatal colonization at the time point six to seven months of age. This substantial heterogeneity may be from participants from different settings. Lopes 2009 conducted the trial in Brazil while Munoz 2001 conducted the trial in the United States. However, no statistical significant difference between the vaccine group and control group was found in either trial (RR 1.04; 95% CI 0.48 to 2.27 in Lopes 2009 and RR 0.32; 95% CI 0.08 to 1.29 in Munoz 2001).
Secondary outcomes
Neonatal antibody levels
Antibody levels were reported as geometric means and 95% CIs. There were inconsistent results between studies. Two studies (Munoz 2001; Quiambao 2003) showed significantly higher immunoglobulin G (IgG) levels in cord blood in the pneumococcal vaccine group when compared with the control group for all serotypes. In contrast, O' Dempsey 1996 showed no difference in neonatal antibody levels between the pneumococcal vaccine group and the control group. SeeAnalysis 1.3.
1.3. Analysis.
Comparison 1 Pneumococcal vaccine versus control vaccine, Outcome 3 Neonatal antibody levels at birth.
| Neonatal antibody levels at birth | ||||||
|---|---|---|---|---|---|---|
| Study | Treatment N | Treatment IgG GM | Treatment 95% CI | Control N | Control IgG GM | Control 95% CI |
| Serotype 6 | ||||||
| Munoz 2001 | 20 | 3.7 | 2.6 to 5.3 | 40 | 1.1 | 0.8 to 1.5 |
| O' Dempsey 1996 | 43 | 2.7 | 1.7 to 4.4 | 26 | 5.7 | 2.9 to 11.3 |
| Quiambao 2003 | 82 | 5.3 | 4.0 to 7.1 | 42 | 0.87 | 0.58 to 1.3 |
| Serotype 14 | ||||||
| Munoz 2001 | 19 | 13.4 | 7.3 to 25.1 | 39 | 3.0 | 2.2 to 3.9 |
| O' Dempsey 1996 | 41 | 13.1 | 8.6 to 20.0 | 23 | 7.1 | 3.7 to 13.3 |
| Quiambao 2003 | 82 | 8.1 | 6.1 to 10.7 | 42 | 2.4 | 1.6 to 3.7 |
| Serotype 19 | ||||||
| Munoz 2001 | 19 | 3.6 | 2.3 to 5.6 | 39 | 1.5 | 1.1 to 2.1 |
| O' Dempsey 1996 | 43 | 4.1 | 2.7 to 5.9 | 24 | 3.6 | 1.8 to 7.3 |
| Quiambao 2003 | 82 | 21.9 | 15.2 to 31.6 | 42 | 3.9 | 2.5 to 6.1 |
Maternal antibody levels
Antibody levels were reported as geometric means and 95% CIs. One study (Munoz 2001) showed significantly higher IgG levels in maternal serum in women immunized with pneumococcal vaccine when compared with control vaccine regardless of any serotypes. The other study (O' Dempsey 1996) showed significantly higher maternal antibody levels only for serotype 14, but no evidence of an effect of the pneumococcal vaccine resulting in an increase in maternal antibody levels in the other serotypes. SeeAnalysis 1.4.
1.4. Analysis.
Comparison 1 Pneumococcal vaccine versus control vaccine, Outcome 4 Maternal antibody levels post vaccination.
| Maternal antibody levels post vaccination | ||||||
|---|---|---|---|---|---|---|
| Study | Treatment N | Treatment IgG GM | Treatment 95% CI | Control N | Control IgG GM | Control 95% CI |
| Serotype 6 | ||||||
| Munoz 2001 | 20 | 4.4 | 2.7 ‐ 7.1 | 40 | 0.9 | 0.6 ‐ 1.3 |
| O' Dempsey 1996 | 49 | 7.3 | 4.4 ‐ 12.0 | 26 | 14.4 | 6.4 ‐ 32.7 |
| Shahid 1995 | 29 | 13.8 | NA | 24 | 5.3 | NA |
| Serotype 14 | ||||||
| Munoz 2001 | 20 | 16.8 | 8.0 ‐ 35.5 | 40 | 3.0 | 2.2 ‐ 4.0 |
| O' Dempsey 1996 | 49 | 43.1 | 39.5 ‐ 70.6 | 26 | 18.8 | 9.2 ‐ 38.8 |
| Serotype 19 | ||||||
| Munoz 2001 | 20 | 3.7 | 2.3 ‐ 6.0 | 40 | 1.4 | 1.0 ‐ 1.9 |
| O' Dempsey 1996 | 49 | 11.8 | 7.7 ‐ 18.2 | 26 | 10.3 | 4.3 ‐ 25.2 |
| Shahid 1995 | 29 | 17.4 | NA | 24 | 4.7 | NA |
Percentage of mothers with seroprotection was also reported in one trial with 340 women (Zaman 2008). This outcome was measured according to serotype (6, 14 or 19) and at two time points (delivery and 12 months post‐delivery). Results favored the intervention group for serotype 6 at delivery (RR 1.49, 95% CI 1.31 to 1.69); serotype 14 at delivery (RR 1.40, 95% CI 1.25 to 1.56) and serotype 19 at delivery (RR 2.29, 95% CI 1.89 to 2.76). There were no group differences seen at 12 months post‐delivery for serotypes 6 or 14 (RR 1.06, 95% CI 1.00 to 1.12 and RR 1.06, 95% CI 0.98 to 1.15, respectively). Results favored the intervention group for serotype 19 measured at 12 months post delivery (RR 1.59, 95% CI 1.37 to 1.85). See Analysis 1.5.
1.5. Analysis.
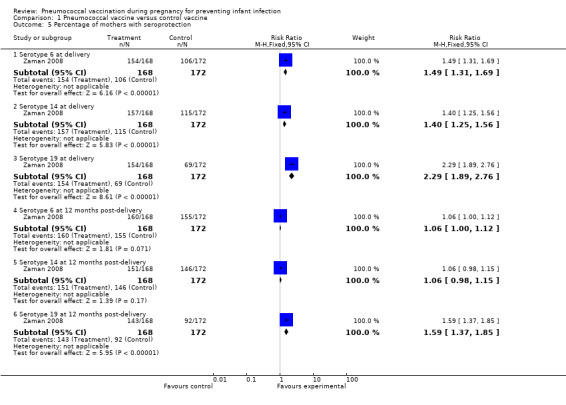
Comparison 1 Pneumococcal vaccine versus control vaccine, Outcome 5 Percentage of mothers with seroprotection.
Adverse maternal effects
Two studies (Munoz 2001; Shahid 1995) reported tenderness at the injection site but this was not significantly different between the intervention and control groups (average RR 3.20; 95% CI 0.32 to 31.54, I² = 79%, Tau² = 2.24 (Analysis 1.6)). The substantial heterogeneity may be from the different control vaccine used in the trials. Munoz 2001 used Hemophilus influenzae type b conjugate vaccine as control and found significant higher rate of tenderness in the pneumococcal vaccine group (RR 12.00; 95% CI 1.55 to 93.01). Shahid 1995 used meningococcal vaccine as control and found no significant difference of tenderness between the groups (RR 1.28; 95% CI 0.77 to 2.13).
1.6. Analysis.
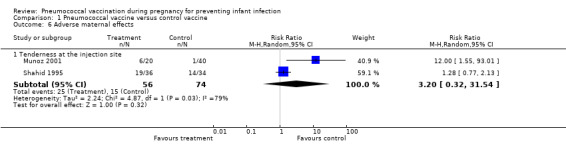
Comparison 1 Pneumococcal vaccine versus control vaccine, Outcome 6 Adverse maternal effects.
Discussion
Summary of main results
There was no evidence of an effect of pneumococcal vaccination during pregnancy in preventing neonatal infections; however, there were only two trials (Lopes 2009; O' Dempsey 1996) reporting this outcome. The power to detect this effect might be too low because of a small sample size. Results of one study (Munoz 2001) suggested that maternal pneumococcal vaccination can reduce the risk of pneumococcal colonization by 16 months of age, but no effect was shown at earlier ages.
An inconsistent result between two trials (Munoz 2001; Shahid 1995) was shown regarding tenderness at the injection site. This may due to the different control vaccines used in the studies. Although there tends to be increased tenderness among women injected with pneumococcal vaccine, this symptom lasted only a few days after injection and no serious adverse events were reported in all of the trials.
Overall completeness and applicability of evidence
There are some limitations that should be considered when interpreting the results of this review. First, neonatal infection, the most significant outcome, was only reported in two trials with small numbers of participants. Neonatal death due to pneumococcal infections was not reported in any included trial. Second, the effect on neonatal pneumococcal colonization at 16 months of age with no effect demonstrated at two to seven months may not be due to the vaccine administered during pregnancy. There was no detail regarding the confounding factors or co‐intervention reported in the trial. The included studies did not report on breastfeeding or antibody level in breast milk that may impact on antibody transfer to the neonates.
Quality of the evidence
Only one trial (Zaman 2008) described the precise method of random allocation and demonstrated method of allocation concealment. Four trials (Munoz 2001; O' Dempsey 1996; Shahid 1995; Zaman 2008) were double‐blind studies. Only one trial (Lopes 2009) had complete follow‐up.
Overall, the quality of evidence is low for all outcomes (Table 1). Outcomes had wide confidence intervals crossing the line of no effect, and included trials had small numbers of participants and few events, which led to downgrading evidence for imprecision of findings. There were no included studies that reported the primary outcome of neonatal death due to pneumococcal infection, but this important outcome is included in the GRADE table to highlight this gap in the evidence.
Potential biases in the review process
None.
Agreements and disagreements with other studies or reviews
A non‐randomized study in Papua New Guinea (Lehmann 2002) compared neonatal antibody level between women who were immunized with 23‐valent pneumococcal polysaccharide vaccine at 28 to 38 weeks' gestation and unimmunized women. The results were similar to the two included studies (Munoz 2001; Quiambao 2003). Geometric mean antibody titers were significantly higher in children of the immunized mothers than in those of the unimmunized group.
Authors' conclusions
Implications for practice.
There is insufficient evidence from randomized controlled trials to support the use of pneumococcal vaccination during pregnancy for preventing infant infections. Pneumococcal vaccine is recommended for administration to pregnant women when they have underlying medical conditions for preventing maternal infections (ACOG 2003). None of the studies reported risk from the vaccine to fetus. Risk to a developing fetus from vaccination of the mother during pregnancy is primarily theoretical. No evidence exists of risk from vaccinating pregnant women with inactivated virus or bacterial vaccines or toxoids (CDC 2011). The benefits of vaccinating pregnant women usually outweigh potential risks when the likelihood of disease exposure is high, when infection would pose a risk to the mother or fetus, and when the vaccine is unlikely to cause harm.
One of the strategies to prevent infection in the neonatal and early infant period is neonatal vaccination. Two recent trials (Pomat 2013; Scott 2011) showed impressive results of earlier PCV7 administration to the newborn (at birth or shortly after birth). Although no clinical outcomes have yet been reported, both trials demonstrated that neonatal PCV7 vaccination was safe, immunogenic and not associated with immune tolerance. Vaccination beginning at birth may be a considerable option to minimize invasive pneumococcal disease in these young infants.
Implications for research.
The review included a small number of randomized controlled trials on pneumococcal vaccination during pregnancy, therefore, they may not have enough power to detect the effectiveness on preventing infant infections. Future trials need to choose the vaccine type as well as the timing of vaccination that could maximize maternal immunogenicity and antibody transfer to the fetus. A new approach using recombinant pneumococcal surface protein A (PspA) as a protein‐based vaccine has been investigated in mice (Kono 2011).
None of the included studies in this review reported the primary outcome of neonatal death due to pneumococcal infection.
As previously discussed, the future vaccine formulations containing additional serotypes and earlier administration to the newborn needs to be evaluated for its effectiveness in reducing invasive pneumococcal disease in children. If it is the case, trials of pneumococcal vaccination during pregnancy for preventing infant infection may not be needed.
What's new
| Date | Event | Description |
|---|---|---|
| 30 September 2014 | New citation required but conclusions have not changed | Review updated. |
| 30 September 2014 | New search has been performed | Search updated. Seven new reports were identified. All of them reported new information for two included studies (Lopes 2009; Zaman 2008). Data from the new reports were added. A 'Summary of findings' table has been incorporated. |
History
Protocol first published: Issue 3, 2004 Review first published: Issue 1, 2006
| Date | Event | Description |
|---|---|---|
| 31 December 2011 | New search has been performed | Search updated. One new trial identified (Zaman 2008) and included. Trial reports previously awaiting classification, have now been incorporated into the review as two new included studies (Lopes 2009; Obaro 2004) and one ongoing study (Dunbar 2007). One study, previously classified as excluded (Quiambao 2003) has now been included. This updated review is now comprised of seven included studies, two excluded studies and one ongoing study. |
| 31 December 2011 | New citation required but conclusions have not changed | For this update we have added two new included studies. The overall results and conclusions have not changed. |
| 31 December 2011 | New search has been performed | Updated search, adding two included studies. |
| 6 July 2011 | Amended | Search updated. Eight reports added to Studies awaiting classification (Deubzer 2004; Dunbar 2007a; Henkle 2010; Holmlund 2011; Lopes 2009a; Obaro 2004a; Quiambao 2007; Steinhoff 2010). |
| 8 May 2008 | Amended | Converted to new review format. |
Acknowledgements
We would like to thank Nancy Medley for her help with the production of the 'Summary of findings' table. Nancy Medley's work was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization. The named authors alone are responsible for the views expressed in this publication.
Appendices
Appendix 1. Search strategy
In the initial version of the review, we searched the Cochrane Central Register of Controlled Trials (The Cochrane Library 2004, Issue 2), MEDLINE (January 1966 to June 2004) and EMBASE (January 1985 to June 2004).
CENTRAL (The Cochrane Library 2004, Issue 2)
#1 PREGNANCY*:ME #2 PREGNAN* #3 MATERN* #4 ANTEPART* #5 PRENATAL #6 ANTENATAL #7 PERINATAL #8 ((((((#1 or #2) or #3) or #4) or #5) or #6) or #7) #9 PNEUMOCOCC* #10 PNEUMOCOCCAL*:ME #11 (#9 or #10) #12 VACCIN* #13 VACCINE*:ME #14 IMMUNUNIZATION*:ME #15 ((#12 or #13) or #14) #16 (#11 and #15) #17 (#8 and #16)
We adapted the above search strategy to search MEDLINE (January 1966 to June 2004) and EMBASE (January 1985 to June 2004) by selecting appropriate MeSH and/or keywords from their respective thesauri.
Data and analyses
Comparison 1. Pneumococcal vaccine versus control vaccine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Neonatal infection | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Pneumonia | 1 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.18, 1.90] |
| 1.2 Meningitis | 1 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.04 [0.13, 73.44] |
| 1.3 Otitis media | 1 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.75] |
| 1.4 All infections | 2 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.30, 1.46] |
| 2 Pneumococcal colonization | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 At 2‐3 months of age | 2 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.46, 2.78] |
| 2.2 By 6‐7 months of age | 2 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.22, 2.08] |
| 2.3 By 16 months of age | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.11, 0.98] |
| 2.4 Serotype 6 | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.01, 2.09] |
| 2.5 Serotype 14 | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Serotype 19 | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 2.09 [0.40, 10.85] |
| 3 Neonatal antibody levels at birth | Other data | No numeric data | ||
| 3.1 Serotype 6 | Other data | No numeric data | ||
| 3.2 Serotype 14 | Other data | No numeric data | ||
| 3.3 Serotype 19 | Other data | No numeric data | ||
| 4 Maternal antibody levels post vaccination | Other data | No numeric data | ||
| 4.1 Serotype 6 | Other data | No numeric data | ||
| 4.2 Serotype 14 | Other data | No numeric data | ||
| 4.3 Serotype 19 | Other data | No numeric data | ||
| 5 Percentage of mothers with seroprotection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Serotype 6 at delivery | 1 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [1.31, 1.69] |
| 5.2 Serotype 14 at delivery | 1 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.25, 1.56] |
| 5.3 Serotype 19 at delivery | 1 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.29 [1.89, 2.76] |
| 5.4 Serotype 6 at 12 months post‐delivery | 1 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [1.00, 1.12] |
| 5.5 Serotype 14 at 12 months post‐delivery | 1 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.98, 1.15] |
| 5.6 Serotype 19 at 12 months post‐delivery | 1 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.37, 1.85] |
| 6 Adverse maternal effects | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Tenderness at the injection site | 2 | 130 | Risk Ratio (M‐H, Random, 95% CI) | 3.20 [0.32, 31.54] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Lopes 2009.
| Methods | Pregnant women were randomly assigned to one of three groups for the study. | |
| Participants | Country: Brazil.
Number: 47 women in the control group (no vaccine); 45 women received vaccine at 30‐34 weeks of gestation; and 47 women received vaccine after delivery. Mean age: data not available. Mean gestational age: data not available. |
|
| Interventions | 23‐valent pneumococcal polysaccharide vaccine. | |
| Outcomes | Incidence of neonatal infection. Incidence of infant pneumococcal colonization. Maternal antibody levels (GM). |
|
| Notes | Data from women received vaccine after delivery were excluded from analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details were not reported. |
| Allocation concealment (selection bias) | Unclear risk | Details were not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lack of blinding would be unlikely to affect results. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Lack of blinding would be unlikely to affect results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All infants completed follow‐up at 3 months old. |
| Selective reporting (reporting bias) | Low risk | All outcomes were predefined and reported. |
| Other bias | Low risk | The trial appears to be free of industry sponsorship. |
Munoz 2001.
| Methods | The study was a prospective, double‐blind, randomized, controlled trial. | |
| Participants | Country: United States.
Number: 20 women in the intervention group and 40 women in the control group. Mean age: 30.2 years. Mean gestational age: 33.3 weeks. |
|
| Interventions | 23‐valent pneumococcal polysaccharide vaccine vs Hemophilus influenzae type b conjugate vaccine. | |
| Outcomes | Incidence of neonatal and infant pneumococcal colonization. Neonatal antibody levels (GM). Maternal antibody levels (GM). Incidence of tenderness at the injection site. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details were not reported. |
| Allocation concealment (selection bias) | Unclear risk | Details were not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “double blind”. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “double blind”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/60 were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes were predefined and reported. |
| Other bias | Low risk | The trial appears to be free of industry sponsorship. |
O' Dempsey 1996.
| Methods | Women were randomized to receive either pneumococcal vaccine or control vaccine. | |
| Participants | Country: The Gambia.
Number: 75 women in the intervention group and 75 women in the control group. Mean age: 22.0 years. Mean gestational age: 38.0 weeks. |
|
| Interventions | 23‐valent pneumococcal polysaccharide vaccine vs meningococcal vaccine. | |
| Outcomes | Incidence of neonatal pneumonia. Incidence of neonatal meningitis. Incidence of neonatal otitis media. Neonatal antibody levels (GM). Maternal antibody levels (GM). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details were not reported. |
| Allocation concealment (selection bias) | Unclear risk | Details were not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lack of blinding would be unlikely to affect results. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Lack of blinding would be unlikely to affect results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/150 were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes were predefined and reported. |
| Other bias | Unclear risk | Vaccines were provided by industry sponsorship, but none of the authors affiliated with the company. |
Obaro 2004.
| Methods | The study was a randomized, double‐blind, controlled trial. | |
| Participants | Country: The Gambia.
Number: 56 women in the intervention group and 57 women in the control group. Mean age: data not available. Mean gestational age: data not available. |
|
| Interventions | 23‐valent pneumococcal polysaccharide vaccine vs meningococcal vaccine. | |
| Outcomes | Pneumococcal polysaccharide‐specific s‐IgA antibody concentrations in breast milk. | |
| Notes | Did not contribute data to analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were vaccinated with 1 dose of either a pneumococcal vaccine or control by computer‐generated random assignment. |
| Allocation concealment (selection bias) | Unclear risk | Details were not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “double blind”. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “double blind”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two mothers withdrew their consent before the completion of the study, and 6 mothers traveled out of the study area and did not return before the completion of the study. |
| Selective reporting (reporting bias) | Low risk | All outcomes were predefined and reported. |
| Other bias | Low risk | The trial appears to be free of industry sponsorship. |
Quiambao 2003.
| Methods | The study was a randomized, controlled trial. | |
| Participants | Country: Philippines.
Number: 106 women in the intervention group and 54 women in the control group. Mean age: 26.8 years. Mean gestational age: 27.3 weeks. |
|
| Interventions | 23‐valent pneumococcal polysaccharide vaccine. | |
| Outcomes | Neonatal antibody levels (GM). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details were not reported. |
| Allocation concealment (selection bias) | Unclear risk | Details were not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lack of blinding would be unlikely to affect results. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Lack of blinding would be unlikely to affect results. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Cord blood was obtained from 42/54 in the control group and 82/106 in the intervention group. |
| Selective reporting (reporting bias) | Low risk | All outcomes were predefined and reported. |
| Other bias | Low risk | The trial appears to be free of industry sponsorship. |
Shahid 1995.
| Methods | The study was a prospective double‐blind controlled trial. | |
| Participants | Country: Bangladesh.
Number: 36 women in the intervention group and 34 women in the control group. Mean age: 25.6 years. Mean gestational age: 32.3 weeks. |
|
| Interventions | 23‐valent pneumococcal polysaccharide vaccine vs meningococcal vaccine. | |
| Outcomes | Maternal antibody levels (GM). Incidence of tenderness at the injection site. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details were not reported. |
| Allocation concealment (selection bias) | Unclear risk | Details were not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “double blind”. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “double blind”. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7/36 missing from intervention group; 10/34 missing from control group. |
| Selective reporting (reporting bias) | Low risk | All outcomes were predefined and reported. |
| Other bias | Low risk | The trial appears to be free of industry sponsorship. |
Zaman 2008.
| Methods | The study was a prospective, controlled, blinded, randomized trial. | |
| Participants | Country: Bangladesh.
Number: 168 women in the intervention group and 172 women in the control group. Mean age: 24.9 years. Mean gestational age: data not available. |
|
| Interventions | 23‐valent pneumococcal polysaccharide vaccine vs influenza vaccine. | |
| Outcomes | Incidence of neonatal influenza. Incidence of maternal influenza. Percentage of mothers with seroprotection. |
|
| Notes | The main purpose of the trial was to assess the effectiveness of influenza vaccine. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomization sequence was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | The randomization sequentially numbered opaque envelopes with data regarding assignments to study groups were provided to each clinic. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “double blind”. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “double blind”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the mother–infant pairs, 316 were observed for the full 24‐week period. |
| Selective reporting (reporting bias) | Low risk | All outcomes were predefined and reported. |
| Other bias | Low risk | The trial appears to be free of industry sponsorship and the conflict of interest was declared. |
GM: geometric mean vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Daly 2003 | The objective of this pilot study was to estimate enrolment rate for the phase III trial, not for the determination of the effectiveness of maternal immunization. |
| Glezen 2000 | It was an abstract only and there was not enough information in the abstract. |
Characteristics of ongoing studies [ordered by study ID]
Dunbar 2007.
| Trial name or title | PneuMum. |
| Methods | Randomized controlled trial. |
| Participants | Country: Australia. Number: 210 women aged 18–39 years who have an uncomplicated pregnancy: 70 women will receive pneumococcal vaccine in the third trimester; 70 at delivery; and 70 at 7 months after childbirth (the control group). |
| Interventions | 23‐valent pneumococcal polysaccharide vaccine. |
| Outcomes | Incidence of neonatal otitis media.
Incidence of neonatal pneumococcal colonization. Neonatal antibody levels. |
| Starting date | 2007. |
| Contact information | melissa.dunbar@menzies.edu.au. |
| Notes |
Differences between protocol and review
For the initial version of the review, we searched the Cochrane Central Register of Controlled Trials (The Cochrane Library 2004 Issue 2), MEDLINE (January 1966 to June 2004) and EMBASE (January 1985 to June 2004). This additional searching has not been carried out for this update.
The outcomes have been separated into 'Primary' and 'Secondary' outcomes and the methods have been updated to reflect the latest Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Contributions of authors
Surasith Chaithongwongwatthana (SC) designed the review and wrote the protocol. Waralak Yamasmit (WY), Sompop Limpongsanurak (SL), Pisake Lumbiganon (PL), and Jorge Tolosa (JT) provided general advice and approved the published version. SC and WY conducted and drafted the review. SL, PL, and JT gave intellectual comments on the review and approved the final version.
For the 2014 update, SC and WY contributed to data extraction and assessment of risk of bias in studies. SC conducted data analysis and updated the main text. The final version of the updated review was approved by all review authors.
Sources of support
Internal sources
Chulalongkorn University, Thailand.
Faculty of Medicine Vajira Hospital, Navamindradhiraj University, Thailand.
Khon Kaen University, Thailand.
Oregon Health Science University, USA.
Global Network for Perinatal and Reproductive Health (GNPRH), USA.
External sources
Thailand Research Fund (Senior Research Scholar), Thailand.
UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Lopes 2009 {published data only}
- Lopes CC, Berezin EN, Scheffer D, Huziwara R, Sliva MI, Brandao A, et al. Pneumococcal nasopharyngeal carriage in infants of mothers immunized with 23V non‐conjugate pneumococcal polysaccharide vaccine. Journal of Tropical Pediatrics 2012;58(5):348‐52. [PUBMED: 22238137] [DOI] [PubMed] [Google Scholar]
- Lopes CR, Berezin EN, Ching TH, Canuto Jde S, Costa VO, Klering EM. Ineffectiveness for infants of immunization of mothers with pneumococcal capsular polysaccharide vaccine during pregnancy. Brazilian Journal of Infectious Diseases 2009;13(2):104‐6. [PUBMED: 20140352] [PubMed] [Google Scholar]
Munoz 2001 {published data only}
- Munoz FM, Englund JA, Cheesman CC, Maccato ML, Pinell PM, Nahm MH, et al. Maternal immunization with pneumococcal polysaccharide vaccine in the third trimester of gestation. Vaccine 2001;20(5‐6):826‐37. [PUBMED: 11738746] [DOI] [PubMed] [Google Scholar]
O' Dempsey 1996 {published data only}
- O'Dempsey TJ, McArdle T, Ceesay SJ, Banya WA, Demba E, Secka O, et al. Immunization with a pneumococcal capsular polysaccharide vaccine during pregnancy. Vaccine 1996;14(10):963‐70. [PUBMED: 8873389] [DOI] [PubMed] [Google Scholar]
Obaro 2004 {published data only}
- Deubzer HE, Obaro SK, Newman VO, Adegbola RA, Greenwood BM, Henderson DC. Colostrum obtained from women vaccinated with pneumococcal vaccine during pregnancy inhibits epithelial adhesion of Streptococcus pneumoniae. Journal of Infectious Diseases 2004;190(10):1758‐61. [PUBMED: 15499530] [DOI] [PubMed] [Google Scholar]
- Obaro SK, Deubzer HE, Newman VO, Adegbola RA, Greenwood BM, Henderson DC. Serotype‐specific pneumococcal antibodies in breast milk of Gambian women immunized with a pneumococcal polysaccharide vaccine during pregnancy. Pediatric Infectious Disease Journal 2004;23(11):1023‐9. [PUBMED: 15545857] [DOI] [PubMed] [Google Scholar]
Quiambao 2003 {published data only}
- Holmlund E, Nohynek H, Quiambao B, Ollgren J, Kayhty H. Mother‐infant vaccination with pneumococcal polysaccharide vaccine: Persistence of maternal antibodies and responses of infants to vaccination. Vaccine 2011;29(28):4565‐75. [PUBMED: 21550374] [DOI] [PubMed] [Google Scholar]
- Quiambao BP, Nohynek H, Kayhty H, Ollgren J, Gozum L, Gepanayao CP, et al. Maternal immunization with pneumococcal polysaccharide vaccine in the Philippines. Vaccine 2003;21(24):3451‐4. [PUBMED: 12850358] [DOI] [PubMed] [Google Scholar]
- Quiambao BP, Nohynek HM, Kayhty H, Ollgren JP, Gozum LS, Gepanayao CP, et al. Immunogenicity and reactogenicity of 23‐valent pneumococcal polysaccharide vaccine among pregnant Filipino women and placental transfer of antibodies. Vaccine 2007;25(22):4470‐7. [PUBMED: 17442467] [DOI] [PubMed] [Google Scholar]
Shahid 1995 {published data only}
- Shahid NS, Steinhoff MC, Hoque SS, Begum T, Thompson C, Siber GR. Serum, breast milk, and infant antibody after maternal immunisation with pneumococcal vaccine. Lancet 1995;346(8985):1252‐7. [PUBMED: 7475716] [DOI] [PubMed] [Google Scholar]
Zaman 2008 {published data only}
- Henkle E, Steinhoff MC, Omer SB, Roy OE, Arifeen SE, Raqib R, et al. Incidence of influenza infection in early infancy in Southern Asia. Pediatric Academic Societies' 2010 Annual Meeting; 2010 May 1‐4; Vancouver, Canada. 2010.
- Omer SB, Zaman K, Roy E, Arifeen SE, Raqib R, Noory L, et al. Combined effects of antenatal receipt of influenza vaccine by mothers and pneumococcal conjugate vaccine receipt by infants: Results from a randomized, blinded, controlled trial. Journal of Infectious Diseases 2013;207(7):1144‐7. [PUBMED: 23300160] [DOI] [PubMed] [Google Scholar]
- Schlaudecker EP, Steinhoff MC, Omer SB, McNeal MM, Roy E, Arifeen SE, et al. IgA and neutralizing antibodies to influenza a virus in human milk: a randomized trial of antenatal influenza immunization. PLoS ONE 2013;8(8):e70867. [PUBMED: 23967126] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaudecker EP, Steinhoff MC, Omer SB, Roy E, Arifeen SE, Dodd CN, et al. Antibody persistence in mothers one year after pneumococcal immunization in pregnancy. Vaccine 2012;30(34):5063‐6. [PUBMED: 22709949] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff MC, Omer SB, Roy E, Arifeen SE, Raqib R, Altaye M, et al. Influenza immunization in pregnancy and birth weights: observations from a randomized controlled trial. Pediatric Academic Societies' 2010 Annual Meeting; 2010 May 1‐4; Vancouver, Canada. 2010.
- Steinhoff MC, Omer SB, Roy E, Arifeen SE, Raqib R, Dodd C, et al. Neonatal outcomes after influenza immunization during pregnancy: A randomized controlled trial. Canadian Medical Association Journal 2012;184(6):645‐53. [PUBMED: 22353593] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff MC, Schlaudecker EP, Omer SB, Roy E, Altaye M, Zaman K. Antibody persistence in young adults one year after pneumococcal immunization in pregnancy. Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2011 April 30‐May 3; Denver, Colorado, USA. 2011:4150.2.
- Steinhoff MC, Schlaudecker EP, Omer SB, Roy E, Zaman K. Influenza IgA antibody in human milk: a randomized trial of maternal influenza immunization. Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2011 April 30‐May 3; Denver, Colorado, USA. 2011:3420A.4.
- Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, et al. Effectiveness of maternal influenza immunization in mothers and infants. New England Journal of Medicine 2008;359(15):1555‐64. [PUBMED: 18799552] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Daly 2003 {published data only}
- Daly KA, Toth JA, Giebink GS. Pneumococcal conjugate vaccines as maternal and infant immunogens: challenges of maternal recruitment. Vaccine 2003;21(24):3473‐8. [DOI] [PubMed] [Google Scholar]
Glezen 2000 {published data only}
- Glezen W, Munoz F, Piedra P, Maccato M, Pinell P. Administration of pneumococcal polysaccharide vaccine (PCNP) and the purified fusion protein (PFP‐2) for respiratory syncytial virus to pregnant women. Infectious Diseases in Obstetrics & Gynecology 2000;8(3/4):200. [Google Scholar]
References to ongoing studies
Dunbar 2007 {published data only}
- Dunbar M, Moberley S, Nelson S, Leach AJ, Andrews R. Clear not simple: an approach to community consultation for a maternal pneumococcal vaccine trial among indigenous women in the Northern Territory of Australia. Vaccine 2007;25(13):2385‐8. [DOI] [PubMed] [Google Scholar]
Additional references
ACOG 2003
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion: immunization during pregnancy. Obstetrics & Gynecology 2003;101(1):207‐12. [DOI] [PubMed] [Google Scholar]
Arifeen 2009
- Arifeen SE, Saha SK, Rahman S, Rahman KM, Rahman SM, Bari S, et al. Invasive pneumococcal disease among children in rural Bangladesh: results from a population‐based surveillance. Clinical Infectious Diseases 2009;48(Suppl 2):S103‐S113. [DOI: 10.1086/596543] [DOI] [PubMed] [Google Scholar]
Black 2000
- Black SB, Shinefield HR, Ling S, Hansen J, Fireman B, Spring D, et al. Efficacy safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatric Infectious Disease Journal 2000;19:187‐95. [DOI] [PubMed] [Google Scholar]
CDC 2000
- Centers for Disease Control and Prevention. Preventing pneumococcal disease among infants and young children: recommendations of the Advisory Committee on Immunization Practice (ACIP). Morbidity and Mortality Weekly Report 2000;49(RR‐9):1‐29. [PubMed] [Google Scholar]
CDC 2010
- Centers for Disease Control and Prevention. Prevention of pneumococcal disease among infants and children ‐ use of 13‐valent pneumococcal conjugate vaccine and 23‐valent pneumococcal polysaccharide vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report 2010;59(RR‐11):1‐18. [PUBMED: 21150868] [PubMed] [Google Scholar]
CDC 2011
- Centers for Disease Control and Prevention. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices. Morbidity and Mortality Weekly Report 2011;60(RR‐2):26. [PUBMED: 21293327] [PubMed] [Google Scholar]
Dagan 2000
- Dagan R, Fraser D. Conjugate pneumococcal vaccine and antibiotic‐resistant Streptococcus pneumoniae: herd immunity and reduction of otitis morbidity. Pediatric Infectious Diseases Journal 2000;19(5 Suppl):S79‐88. [DOI] [PubMed] [Google Scholar]
Foster 2008
- Foster D, Knox K, Walker AS, Griffiths DT, Moore H, Haworth E, et al. Invasive pneumococcal disease: epidemiology in children and adults prior to implementation of the conjugate vaccine in the Oxfordshire region, England. Journal of Medical Microbiology 2008;57(Pt 4):480‐7. [DOI] [PubMed] [Google Scholar]
GRADE 2008
- GRADEpro [Computer program]. Jan Brozek, Andrew Oxman, Holger Schünemann. Version 3.6 for Windows 2008.
Greenwood 2003
- Greenwood B. Maternal immunisation in developing countries. Vaccine 2003;21:3436‐41. [DOI] [PubMed] [Google Scholar]
Grijalva 2011
- Grijalva CG, Pelton SI. A second‐generation pneumococcal conjugate vaccine for prevention of pneumococcal diseases in children. Current Opinion in Pediatrics 2011;23(1):98‐104. [PUBMED: 21191300] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Isaacman 2010
- Isaacman DJ, McIntosh ED, Reinert RR. Burden of invasive pneumococcal disease and serotype distribution among Streptococcus pneumoniae isolates in young children in Europe: impact of the 7‐valent pneumococcal conjugate vaccine and considerations for future conjugate vaccines. International Journal of Infectious Diseases 2010;14(3):e197‐e209. [DOI: 10.1016/j.ijid.2009.05.010] [DOI] [PubMed] [Google Scholar]
Khan 2013
- Khan AA, Zahidie A, Rabbani F. Interventions to reduce neonatal mortality from neonatal tetanus in low and middle income countries‐a systematic review. BMC Public Health 2013;13:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klouwenberg 2008
- Klouwenberg PK, Bont L. Neonatal and infantile immune responses to encapsulated bacteria and conjugate vaccines. Clinical and Developmental Immunology 2008;2008:628963. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kono 2011
- Kono M, Hotomi M, Hollingshead SK, Briles DE, Yamanaka N. Maternal immunization with pneumococcal surface protein A protects against pneumococcal infections among derived offspring. PLoS One 2011;6(10):e27102. [PUBMED: 22073127] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lehmann 2002
- Lehmann D, Pomat WS, Combs B, Dyke T, Alpers MP. Maternal immunization with pneumococcal polysaccharide vaccine in the highlands of Papua New Guinea. Vaccine 2002;20(13‐14):1837–45. [PUBMED: 11906773] [DOI] [PubMed] [Google Scholar]
Pilishvili 2010
- Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. Journal of Infectious Diseases 2010;201(1):32‐41. [DOI: 10.1086/648593] [DOI] [PubMed] [Google Scholar]
Pomat 2013
- Pomat WS, Biggelaar AH, Phuanukoonnon S, Francis J, Jacoby P, Siba PM, et al. Safety and immunogenicity of neonatal pneumococcal conjugate vaccination in Papua New Guinean children: a randomised controlled trial. PLoS One 2013;8(2):e56698. [PUBMED: 23451070] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rennels 1998
- Rennels MB, Edwards KM, Keyserling HL, Reisinger KS, Hogerman DA, Madore DV, et al. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM197 in United States infants. Pediatrics 1998;101:604‐11. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schunemann 2009
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [PUBMED: 19839216] [DOI] [PubMed] [Google Scholar]
Scott 2011
- Scott JA, Ojal J, Ashton L, Muhoro A, Burbidge P, Goldblatt D. Pneumococcal conjugate vaccine given shortly after birth stimulates effective antibody concentrations and primes immunological memory for sustained infant protection. Clinical Infectious Diseases 2011;53(7):663‐70. [DOI: 10.1093/cid/cir444] [DOI] [PMC free article] [PubMed] [Google Scholar]
Whitney 2003
- Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, et al. Decline in invasive pneumococcal disease after the introduction of protein‐polysaccharide conjugate vaccine. New England Journal of Medicine 2003;348:1737‐46. [DOI] [PubMed] [Google Scholar]
WHO 2012
- World Health Organization. Pneumococcal vaccines WHO position paper ‐ 2012. Weekly Epidemiological Record 2012;87(14):129‐144. [PUBMED: 24340399] 24340399 [Google Scholar]
References to other published versions of this review
Chaithongwongwatthana 2004
- Chaithongwongwatthana S, Yamasmit W, Limpongsanurak S, Lumbiganon P, DeSimone JA, Baxter JK, et al. Pneumococcal vaccination during pregnancy for preventing infant infection. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD004903] [DOI] [PubMed] [Google Scholar]
Chaithongwongwatthana 2006
- Chaithongwongwatthana S, Yamasmit W, Limpongsanurak S, Lumbiganon P, DeSimone JA, Baxter JK, et al. Pneumococcal vaccination during pregnancy for preventing infant infection. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD004903.pub2] [DOI] [PubMed] [Google Scholar]
Chaithongwongwatthana 2012
- Chaithongwongwatthana S, Yamasmit W, Limpongsanurak S, Lumbiganon P, DeSimone JA, Baxter JK, et al. Pneumococcal vaccination during pregnancy for preventing infant infection. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD004903.pub3] [DOI] [PubMed] [Google Scholar]


