Abstract
Interactions between the nervous and immune systems were recognized long ago, but recent studies show that this crosstalk occurs more frequently than was previously appreciated. Moreover, technological advances have enabled the identification of the molecular mediators and receptors that enable the interaction between these two complex systems and provide new insights on the role of neuroimmune crosstalk in organismal physiology. Most neuroimmune interaction occurs at discrete anatomical locations in which neurons and immune cells colocalize. Here, we describe the interactions of the different branches of the peripheral nervous system with immune cells in various organs, including the skin, intestine, lung, and adipose tissue. We highlight how neuroimmune crosstalk orchestrates physiological processes such as host defense, tissue repair, metabolism, and thermogenesis. Unraveling these intricate relationships is invaluable to explore the therapeutic potential of neuroimmune interaction.
Keywords: neuroimmune interactions, neuroimmunology, mucosal immunology
BRIEF HISTORICAL OVERVIEW
The field of neuroimmunology is rapidly growing, garnering the attention of several communities, including neuroscientists, immunologists, and microbiologists. Knowledge about connections between the nervous and immune systems dates back to Aulus Cornelius Celsus (25 BC–50 AD), who described the cardinal signs of inflammation: swelling (tumor), redness (rubor), heat (calor), and pain (dolor). All four hallmarks can be caused by neurons via neurogenic inflammation (Chiu et al. 2012), and pain, the unpleasant sensation, is often connected with immune responses in the inflamed tissue. As work over the last decades has uncovered, it is increasingly clear that branches of the peripheral nervous system (PNS), including sensory, sympathetic, vagal, and enteric neurons, signal to immune cells to impact their function. While central nervous system (CNS) and neuroendocrine (e.g., hypothalamic-pituitary-adrenal axis) control of immunity have been evaluated elsewhere (Bellavance & Rivest 2014, Schiller et al. 2021, Webster et al. 2002), the goal of this review is to highlight the bidirectional communication between the PNS and the immune system in establishing homeostasis, inflammation, and disease.
The nervous and immune systems are evolutionarily ancient and likely coevolved to use common molecular mediators and receptors for intersystem communication (Kraus et al. 2021). Molecular crosstalk between neurons and immune cells impacts neurological function, barrier homeostasis, and host defense (Chesné et al. 2019, Chu et al. 2020, Huh & Veiga-Fernandes 2020) while also playing roles in diseases such as cancer and neurodegeneration (Pavlov & Tracey 2017, Shurin et al. 2020). The PNS and immune system are each enormously complex, both being represented by cell types whose gene expression patterns are dependent upon cell state, tissue residence, and interactions with neighboring cells. The white blood cells of the immune system are derived from hematopoietic precursors circulating throughout the body via the vasculature and lymphatics to survey tissues (Supplemental Appendix). At barrier sites, notably in the skin, gut, and lungs, immune cells are often in close proximity to PNS nerve fibers and express receptors for neuropeptides and neurotransmitters, thus rendering the immune cells amenable to neuromodulation (Godinho-Silva et al. 2019, Tamari et al. 2021) (Figure 1). As is true of immune cells, neuronal cells also harbor a high degree of diversity, with molecular changes that occur after tissue injury and inflammation. Additionally, neurons express immune-related receptors such as pattern recognition receptors and cytokine receptors. Thus, these systems reciprocally respond to and influence each other’s behaviors through shared receptors and mediators, prompting the emergent collaboration between immunologists and neuroscientists.
Figure 1.
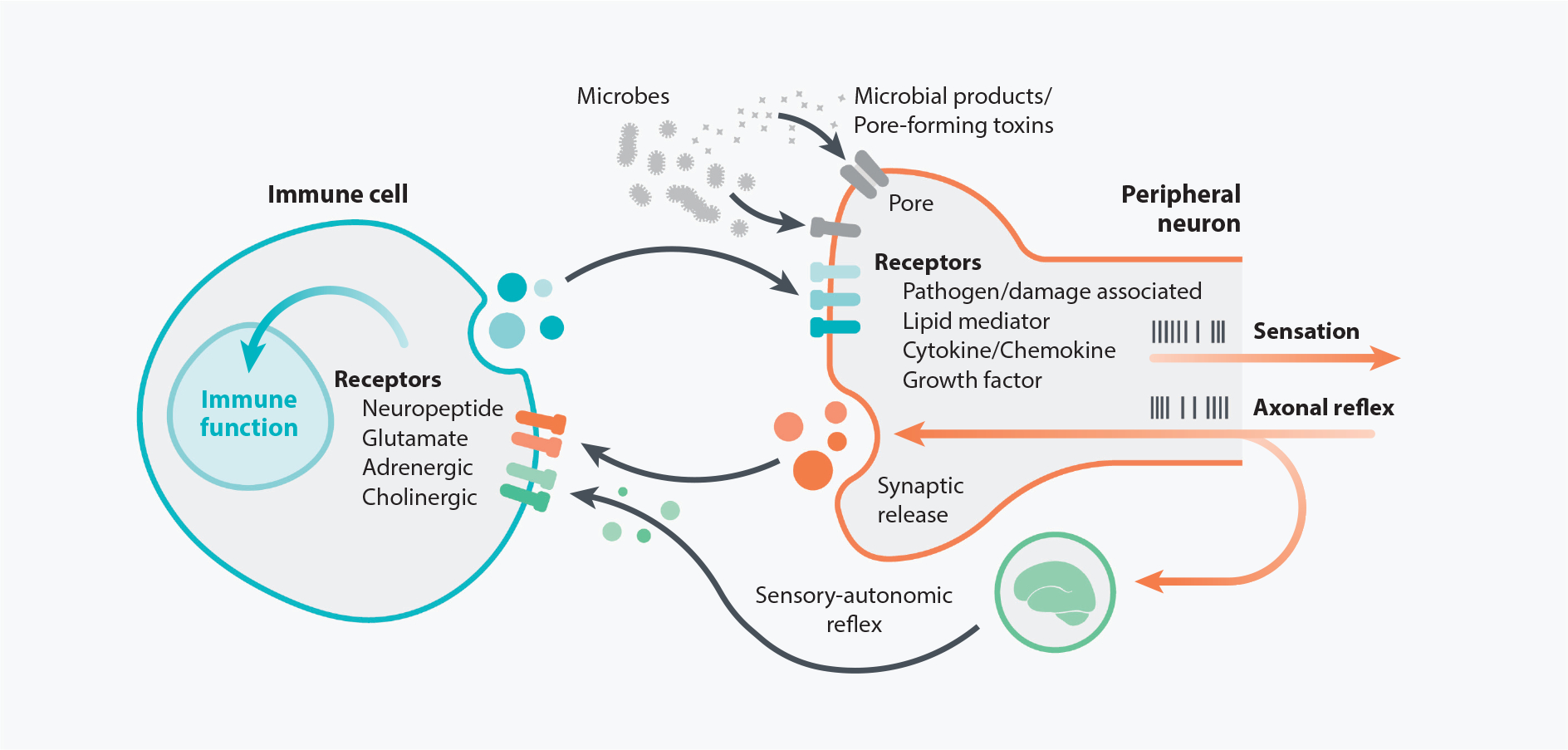
Direct communication between peripheral neurons and immune cells. Immune cell function can be modulated by factors produced from the PNS, as many immune cell types express receptors for neuropeptides and neurotransmitters. Reciprocally, the PNS can be regulated by the immune system since peripheral nerve terminals express canonical immune-related receptors for molecules such as cytokines, chemokines, and lipids. Nociceptive neurons also express receptors that detect microbes and/or their products, thereby allowing the nervous system to sense potential infections. In response to environmental cues such as inflammatory cytokines acting on receptors expressed by peripheral nerves, action potentials are generated to produce a variety of neuronal outputs, including activating reflex circuits and sensations such as pain and itch. One potential outcome is an antidromic axonal reflex that results in synaptic vesicle fusion and release of neuronal factors, which can in turn affect immune cell behavior and function. Signals from peripheral sensory nerves can also integrate with the central nervous system to produce a sensory-autonomic reflex and synaptic vesicle release. Abbreviation: PNS, peripheral nervous system.
Historically, much of neuroimmunology was relegated to the study of autoimmune attack in multiple sclerosis or correlative work showing that psychological or neurological changes impact immunity without clear mechanistic insights (Nutma et al. 2019). Presently, neuroimmunology is undergoing a renaissance with the advent of tools such as optogenetics, chemogenetics, viral tracing, and molecular profiling that have aided in deciphering the ways in which nerves and immune cells communicate. Here, we discuss the interactions between the nervous and immune systems that occur at distinct anatomical locations in peripheral organs where nerve terminals and immune cells colocalize in neuroimmune cell units.
SENSORY NEURON CROSSTALK WITH IMMUNE CELLS
Somatosensory neurons in the dorsal root ganglia (DRG) and trigeminal ganglia (TG) innervate barrier sites, including the skin and gut, and mediate perceptions, including touch, pain, and itch. Vagal sensory neurons in the nodose/jugular ganglia innervate visceral organs. Sensory neurons respond to inflammation by detecting immune mediators and then releasing neuropeptides, including calcitonin gene–related peptide (CGRP), substance P (SP), and vasoactive intestinal peptide (VIP). These factors regulate immune cell function and impact host defense in the skin, lungs, and gut.
Sensory Neuron Detection of Immune Factors
The sensory nervous system is finely tuned by inflammation. DRG and TG neurons respond to immune factors to drive the sensations of pain and itch. While pain is acutely protective by driving avoidance behaviors and facilitating wound healing, pain becomes pathological when it reaches a chronic state. During injury and infection, immune cells release a cocktail of factors, including cytokines, histamine, lipids (e.g., prostaglandins, leukotrienes), and growth factors (e.g., NGF). Nociceptive sensory neurons, the neuronal subset that mediates pain, can detect nearly all these classes of immune molecules (Cook et al. 2018). Many of these factors can cause neuronal sensitization and action potential generation to produce pain, and a subsequent local axonal reflex causes the release of neuropeptides from peripheral terminals that can induce neurogenic inflammation (Cook et al. 2018). Some examples of pain-mediating cytokines include IL-1 and IL-17, which are produced in chronic inflammatory diseases. IL-1β is sensed by nociceptive neurons through interleukin-1 receptor (IL-1R) signaling (Binshtok et al. 2008). IL-1β increases neuronal excitability by changing the properties of voltage-gated sodium channels (Navs) that are phosphorylated by p38 MAPK, lowering the threshold for action potential firing (Binshtok et al. 2008). IL-17, a cytokine necessary for the elimination of extracellular pathogens and implicated in multiple sclerosis, mediates pain in response to injury (Kim & Moalem-Taylor 2011). In a model of chronic pain caused by sciatic nerve ligation, IL-17-deficient mice exhibited reduced mechanical allodynia, decreased T cell and macrophage recruitment to the sciatic nerve and DRG, and diminished microglial activation (Kim & Moalem-Taylor 2011). Beyond these examples, sensory neurons express receptors for a range of other immune molecules that are extensively discussed elsewhere (Cook et al. 2018, Pinho-Ribeiro et al. 2018).
Vagal sensory neurons relay sensory input from the gut, lungs, and other visceral organs to the brainstem to drive sensory-autonomic reflexes. Like DRG neurons, vagal neurons possess receptors for immune molecules. One key question is whether distinct immune-derived molecules induce differing vagal sensory inputs to activate differential neuroimmune reflexes. For example, vagal neurons express receptors for the inflammatory cytokines IL-1β and tumor necrosis factor alpha (TNFα) produced during infection and injury. Yet, although both cytokines can induce vagal activation, whole-nerve recordings in mice exposed to IL-1β or TNFα in vivo show cytokine-specific compound action potentials (Zanos et al. 2018). Vagal sensory neurons in IL-1R knockout mice fail to respond to IL-1β but maintain responses to TNFα, suggesting that each cytokine directs distinct neural outputs. Therefore, the action of cytokines on sensory neurons can positively or negatively feedback via neuroimmune circuits to affect immunity.
DRG Sensory Neurons in Skin Immunity
The skin is in constant contact with the environment, serving as a barrier against pathogens and as a sensory interface that is innervated by DRG sensory neurons. These neurons signal to immune cells to regulate the outcome of infection, allergic responses, and wound repair.
Skin host defense.
During infection, skin-innervating DRG sensory neurons crosstalk with microbes and immune cells to direct local immune defenses (Figure 2). Microbes can directly act on nociceptive neurons through pathogen receptors to induce pain. For example, nociceptive neurons express receptors to microbial molecules, including formylated-peptide receptors and Toll-like receptors (TLRs). Lipopolysaccharide (LPS), a component of Gram-negative bacterial cell walls, binds to its canonical receptors TLR4 and CD14 expressed by transient receptor potential cation channel subfamily V member 1 (TRPV1)+ sensory neurons (Diogenes et al. 2011). TLR4 signaling may couple to TRPV1 ion channel sensitization to induce pain. LPS also acts directly on transient receptor potential cation channel subfamily A member 1 (TRPA1) channels on nociceptors to cause calcium flux, CGRP release, and local skin edema (Meseguer et al. 2014). Myelinated sensory Aβ fibers from the DRG also express TLR5, the receptor for bacterial flagellin (Xu et al. 2015). In subcutaneous Staphylococcus aureus infection, bacterial products, including α-hemolysin and N-formylated peptides, induced calcium flux in DRG Nav1.8+ nociceptors to produce pain (Chiu et al. 2013). In addition, the S. aureus toxins phenol-soluble modulin α3 and leukocidin γ-hemolysin AB induce DRG firing and pain, which is blocked by lidocaine derivative QX-314 (Blake et al. 2018). In response to bacterial triggers, Nav1.8+ nociceptors release CGRP, which acts on macrophages to suppress TNFα production, decreasing neutrophil and monocyte recruitment to the infection site (Chiu et al. 2013). Draining lymph nodes of S. aureus–infected, Nav1.8+-deficient mice become larger, with increased numbers of B cells, T cells, and monocytes (Chiu et al. 2013), suggesting a role for neurons in negatively regulating adaptive immunity.
Figure 2.
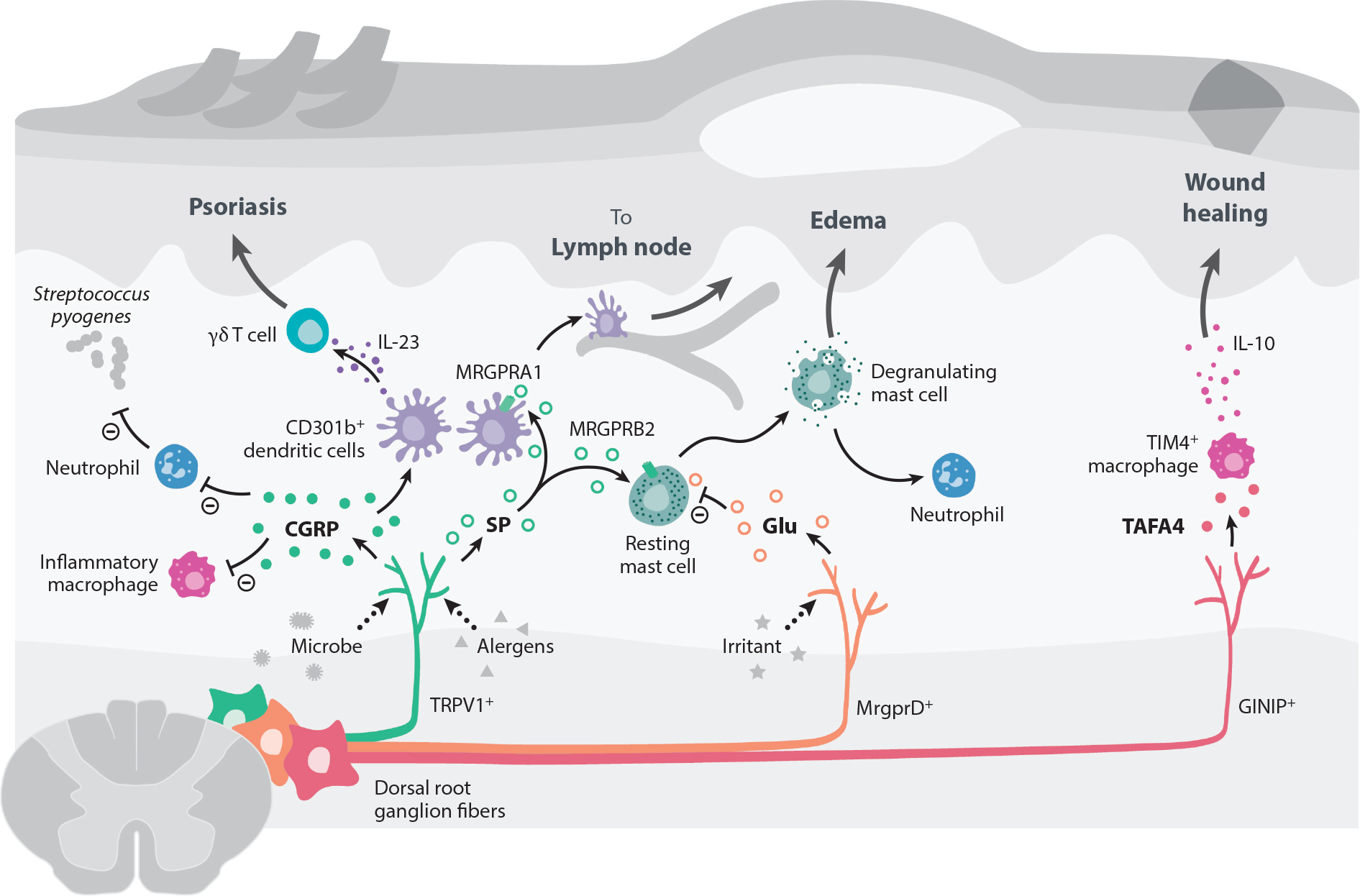
Peripheral neuroimmune interactions in the skin. Most of the skin is innervated by sensory fibers originating from dorsal root ganglia, which have been shown to communicate with skin-resident and infiltrating immune cells. In bacterial infections caused by Staphylococcus aureus or Streptococcus pyogenes, pore-forming toxins activate Nav1.8+ and TRPV1+ nociceptor neurons to release CGRP. CGRP can subsequently suppress macrophages from producing inflammatory cytokines like TNFα and decrease neutrophil recruitment and function. This inhibitory activity of CGRP leads to the loss of control of bacterial infection. TRPV1+ fibers can also be activated by Candida albicans to release CGRP during infection. CGRP acts on dermal CD301b+ DCs to produce IL-23, which promotes dermal γδ T cell proliferation and secretion of IL-17 to control fungal infection. However, this DC/γδ T cell/IL-17 circuit can also promote inflammation and pathology in a mouse model of psoriasis. Active proteases in allergens trigger TRPV1+ neurons to release SP, which acts on MRGPRA1 on DCs to promote their migration to draining lymph nodes and prime a Th2 immune response. SP release from TRPV1+ sensory neurons also trigger mast cell degranulation via binding MRGPRB2. The SP-MRGPRB2 mast cell axis drives tissue edema and neutrophil recruitment. By contrast, nonpeptidergic MRGPRD+ sensory neurons release glutamate to suppress mast cell degranulation. GINIP+ sensory fibers promote tissue healing by releasing the neuropeptide TAFA4 to promote release of the anti-inflammatory cytokine IL-10 by TIM4+ macrophages. Abbreviations: CGRP, calcitonin gene–related peptide; DC, dendritic cell; GINIP, Gαi-interacting protein; MRGPR, Mas-related G protein–coupled receptor; Nav, voltage-gated sodium channel; SP, substance P; TAFA4, TAFA chemokine like family member 4; Th2, T-helper type 2; TIM4, T cell immunoglobulin and mucin domain containing 4; TNFα, tumor necrosis factor alpha; TRPV1, transient receptor potential cation channel V member 1.
Sensory neurons also mediate antifungal immunity. TRPV1+ fibers are activated by cell wall components of the fungal pathogen Candida albicans and signal pain via the Dectin-1 β-glucan receptor (Maruyama et al. 2018). CGRP is released by sensory neurons and acts on dermal CD301b+ dendritic cells (DCs) to drive IL-23 production; IL-23 in turn promotes the proliferation of dermal γδ T cells, IL-17 secretion, and control of fungal burden of C. albicans (Kashem et al. 2015).
Pain and sensory neuron activation can prime an anticipatory immune response through an axonal reflex arc (Cohen et al. 2019). Optogenetic stimulation of channel rhodopsin–expressing TRPV1+ neurons in the dorsal ear induced cutaneous inflammation through a type 17 inflammatory response characterized by γδ and CD4+ T cell activation, infiltration of neutrophils, and an increase of IL-6, IL-23, and TNFα. In turn, this inflammatory environment results in a greater control of C. albicans and S. aureus at the stimulation site and adjacent tissue. However, TRPV1+ neurons can also be coopted by microbes to mediate immune suppression and promote pathogen survival. The bacterium Streptococcus pyogenes, a causative agent of necrotizing fasciitis (flesh-eating disease), produces the toxin streptolysin S (SLS), which activates TRPV1+ DRG neurons to induce pain and suppress neutrophil recruitment (Pinho-Ribeiro et al. 2018). In response to SLS, CGRP released by TRPV1+ fibers suppressed the bactericidal activity of neutrophils by decreasing myeloperoxidase (MPO) activity. Ablation of TRPV1+ neurons, treatment with a CGRP antagonist, or administering the botulinum neurotoxin A to inhibit neuronal vesicle release significantly improved disease severity of S. pyogenes infection (Pinho-Ribeiro et al. 2018).
Skin allergic responses and psoriasis.
Sensory neuron–mast cell interactions play an important role in allergic inflammation (Figure 2). Mast cells form intimate clusters with sensory neurons in the skin and contain dense vesicles full of molecules, including histamine and heparin, that promote inflammation, swelling, and edema (Serhan et al. 2019). TRPV1+ sensory neuron activation rapidly leads to mast cell degranulation via the neuropeptide SP, which binds to mast cell receptor Mas-related G protein–coupled receptor member X2 (MrgprX2) in humans and MrgprB2 in mice (McNeil et al. 2015). In a mouse model of wound healing, neuron–mast cell signaling through SP-MrgprB2 drove local tissue edema, cytokine production, and monocyte and neutrophil recruitment (Green et al. 2019). In a model relevant to atopic dermatitis, house dust mites (HDMs) directly activate peptidergic TRPV1+ nociceptive neurons to induce MrgprB2+ mast cell–dependent allergic skin inflammation (Serhan et al. 2019). Mast cells are in close proximity to TRPV1+ sensory fibers, and SP release promotes MrgprB2+ mast cell degranulation, release of inflammatory cytokines, and skin pathology (Serhan et al. 2019). Many common allergens harbor enzymatically active proteases, including bee venom and HDMs (Lamhamedi-Cherradi et al. 2008, Palm et al. 2013, Porter et al. 2009, Sokol et al. 2008, Van Dyken & Locksley 2018). Upon injection with the model skin allergen papain, TRPV1+ neurons trigger DC migration to draining lymph nodes to prime a T-helper type 2 (Th2) immune response in a SP-MrgprA1-dependent manner (Perner et al. 2020). Conversely, MrgprD+ skin sensory neurons, which are distinct from TRPV1+ neurons, promote tissue homeostasis by releasing glutamate to suppress mast cell degranulation, inflammatory gene programs, and expression of MrgprB2 (Zhang et al. 2021). Yet, although at baseline the skin is more inflamed, MrgprD neuron–deficient mice display smaller lesions and control dermonecrotic S. aureus infection more efficiently (Zhang et al. 2021).
In a mouse model of psoriasis caused by the chemical imiquimod, nociceptors promote skin inflammation, neutrophil and inflammatory monocyte recruitment, and draining lymph node enlargement (Riol-Blanco et al. 2014). Notably, Nav1.8+ nociceptors induce closely associated DCs to produce IL-23, which in turn promotes IL-17 and IL-22 production by γδ T cells to drive this inflammation (Riol-Blanco et al. 2014).
Wound repair.
DRG sensory neurons also promote wound repair (Figure 2). Tissue-resident macrophages can promote inflammation or tissue healing depending on environmental cytokines and cues. C-fiber sensory neurons expressing Gαi-interacting protein (GINIP) are protective in a mouse model of ultraviolet burn, as deletion of GINIP+ fibers caused worse fibrosis, increased numbers of inflammatory macrophages, and decreased numbers of dermal anti-inflammatory IL-10-producing TIM4+ macrophages (Hoeffel et al. 2021). The neuropeptide TAFA4 produced by a subset of GINIP+ neurons regulated IL-10 production by dermal macrophages (Hoeffel et al. 2021). Thus, depending upon stimuli, sensory neurons in concert with immune cells can promote protective or pathological dermal responses.
Vagal Sensory Neurons in Lung Immunity
In the lungs, neuroimmune signaling maintains protective barrier functions while limiting pathological inflammation (Figure 3). The vagus nerve supplies much of the sensory innervation of the airways, mediating breathing, bronchoconstriction, and protective reflexes such as cough (Canning et al. 2006, Carr & Undem 2003, Chang et al. 2015, Coleridge & Coleridge 2011, Tränkner et al. 2014, Widdicombe 2001). Both vagal sensory neurons and immune-derived mediators contribute to the airway constriction observed in asthmatic disease. In ovalbumin and HDM models of induced airway inflammation, ablation of Nav1.8+ sensory neurons or silencing vagal neurons using QX314 leads to decreased airway inflammation (Talbot et al. 2015). At steady state, the lungs contain populations of group 2 innate lymphoid cells (ILC2s). IL-5 released during the allergic airway challenge acts on sensory neurons to release VIP, resulting in recruitment and activation of CD4+ T cells and ILC2s (Talbot et al. 2015). Another neuropeptide, neuromedin U (NMU), acts on ILC2s expressing the receptor Nmur1 (Cardoso et al. 2017, Klose et al. 2017, Wallrapp et al. 2017). When administered together with IL-25, NMU potentiates ILC2 numbers and functions, leading to allergic inflammation (Wallrapp et al. 2017). In a mouse model of S. aureus lethal pneumonia, TRPV1+ nociceptive neurons limit antibacterial immunity in the lung via CGRP (Baral et al. 2018). Deletion of vagal TRPV1+ nociceptive neurons enhanced host survival and bacterial clearance but also coincided with increased levels of inflammatory molecules such as TNFα and CXCL-1 in the lungs. These inflammatory mediators also correspond to the increased recruitment of neutrophils and γδ T cells (Baral et al. 2018). In DCs, CGRP suppresses TLR-signaled production of TNFα and CCL4 by cAMP/protein kinase A (PKA) downstream of calcitonin receptor–like receptor (CALCRL) and receptor activity–modifying protein 1 (RAMP1). CGRP signaling activates the inducible cAMP early repressor (ICER) to transcriptionally regulate inflammatory cytokine promoters such as the tumor necrosis factor (Tnf) locus (Harzenetter et al. 2007). Thus, sensory fibers in the lung can potentiate airway inflammation by activating ILC2s but suppress inflammation and immune cell recruitment in bacterial infection. It remains to be determined whether other types of lung inflammation (e.g., viral infection, fibrosis) are regulated via distinct neuroimmune axes.
Figure 3.
Peripheral neuroimmune interactions in the lungs. The vagus nerve supplies most of the sensory and cholinergic innervation of the airways. Allergic inflammation in the lungs stimulates the production of the type 2 cytokine IL-5 that directly stimulates sensory neurons to release VIP. VIP recruits and activates ILC2s and CD4+ T cells, which further potentiate airway inflammation. TRPV1+ pulmonary fibers restrict antibacterial immunity through secretion of CGRP, which restricts recruitment of neutrophils and γδ T cells. CGRP, released by sensory fibers and neuroendocrine cells that are found interspersed in the lung epithelium, also regulates IL-5 production by ILC2s. Cholinergic fibers release the neuropeptide NMU, which is a potent activator of ILC2s via NMUR1 that responds by producing the type 2 effector cytokines IL-5, IL-13, and Areg that are important for helminth immunity. In turn, acetylcholine inhibits ILC2 function via NACHR7. Sympathetic fibers release NE that acts on β2AR expressed by ILC2s, downmodulating the production of IL-5 and IL-13. Abbreviations: β2AR, β2 adrenergic receptor; Areg, amphiregulin; CGRP, calcitonin gene–related peptide; ILC2, group 2 innate lymphoid cell; NACHR7, nicotinic acetylcholine receptor 7; NE, norepinephrine; NMU, neuromedin U; NMUR1, neuromedin U receptor 1; TRPV1, transient receptor potential cation channel V member 1; VIP, vasoactive intestinal peptide.
Sensory Neurons in Gastrointestinal Immunity
Primary sensory afferents emanating from vagal ganglia and lumbar-sacral DRG innervate the gastrointestinal tract to detect harmful substances and mediate protective neural reflexes such as nausea and visceral pain (Blackshaw & Gebhart 2002, Di Giovangiulio et al. 2015, Grundy et al. 2019) (Figure 4). Gut-innervating DRG neurons mediate protection to Salmonella enterica serovar Typhimurium (STm) in a CGRP-dependent manner by regulating the intestinal barrier and the microbiota (Lai et al. 2020). Microfold (M) cells, specialized epithelial cells in Peyer’s patches, facilitate translocation of microbes and antigens across the gut barrier from the lumen to lamina propria. STm coopts M cells as entry points to establish infection in mice. DRG TRPV1+ and Nav1.8+ fibers release CGRP to negatively regulate M cell numbers to decrease STm uptake and burden. M cells also regulate the density of segmented filamentous bacteria, a beneficial microbe that protects against STm infection (Lai et al. 2020). Potentially, SP may also modulate immune responses to STm, as mice lacking its receptor, neurokinin-1 receptor (NK1R), exhibit enhanced protection and immunoglobulin A (IgA) responses to STm infection (Walters et al. 2005). In an STm oral challenge model, NK1R-deficient mice produce greater levels of serum IgA, increased IgA-producing cells in the lamina propria, and CD4+ T cells producing IL-5 and IL-6 (Walters et al. 2005). However, the mechanisms as to how SP/NK1R mediates these effects remain unknown.
Figure 4.
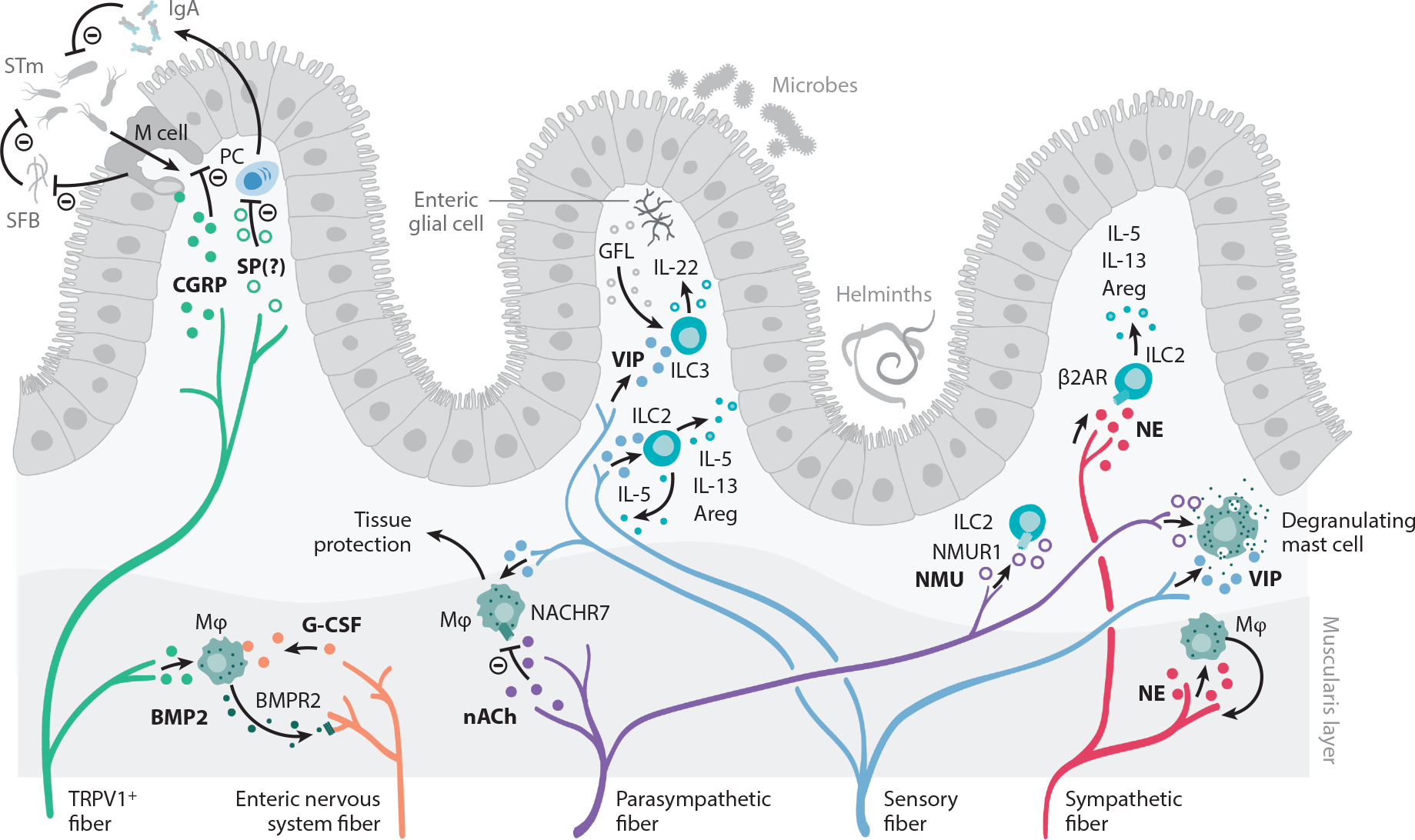
Peripheral neuroimmune interactions in the gut. The intestine is innervated by sensory fibers and the autonomic nervous system, composed of enteric neurons and sympathetic and parasympathetic fibers. Sensory afferents from vagal ganglia and lumbar-sacral DRG innervate the gastrointestinal tract and mediate protection to STm. M cells in Peyer’s patches and mucosa-associated lymphoid tissue facilitate STm transfer to establish an infection. Sensing of STm by DRG TRPV1+ and Nav1.8+ fibers cause CGRP release. CGRP acts on M cells to decrease their numbers and to limit STm uptake. M cells also negatively regulate SFB density, which further antagonizes STm infection. SP may also negatively regulate immune responses to STm, as NK1R-deficient mice exhibit enhanced levels of serum IgA and increased numbers of IgA-producing cells in the lamina propria. Sensory fibers also release VIP, which acts on ILC2s and ILC3s that release type 2 cytokines and tissue-protective IL-22, respectively. ILC3s are also stimulated by the RET ligand GFL, released by enteric glial cells. Enteric nervous system fibers release G-CSF, which promotes muscularis macrophage maintenance. In turn, macrophages produce BMP2, which activates neurons via the BMPR2. Furthermore, acetylcholine acts as a negative regulator of tissue-protective muscularis macrophages via the NACHR7. NE released by sympathetic fibers promotes macrophage-induced protection of neurons during infection. The function of intestinal ILC2 is also regulated by NMU, which acts as a strong stimulator of ILC2 function, and NE, which signals through the β2AR. Finally, intestinal mast cells can integrate a wide variety of neuronal cues and respond by producing a range of inflammatory mediators, which is covered in other excellent reviews and is beyond the scope of this piece. Abbreviations: β2AR, β2 adrenergic receptor; Areg, amphiregulin; BMP2, bone morphogenetic protein 2; BMPR2, bone morphogenetic protein receptor type 2; CGRP, calcitonin gene–related peptide; DRG, dorsal root ganglia; G-CSF, granulocyte colony stimulating factor; GFL, GDNF family of ligands; IgA, immunoglobulin A; ILC2, group 2 innate lymphoid cell; ILC3, group 3 innate lymphoid cell; M, microfold; Mϕ, macrophage; NACHR7, nicotinic acetylcholine receptor 7; Nav, voltage-gated sodium channel; NE, norepinephrine; NK1R, neurokinin-1 receptor; NMU, neuromedin U; NMUR1, neuromedin U receptor; PC, Plasma cell; RET, rearranged during transfection; SFB, segmented filamentous bacteria; SP, substance P; STm, Salmonella enterica serovar Typhimurium; TRPV1, transient receptor potential cation channel subfamily V member 1; VIP, vasoactive intestinal peptide.
INTERACTIONS OF THE AUTONOMIC NERVOUS SYSTEM WITH IMMUNE CELLS
The three subdivisions of the autonomic nervous system—sympathetic, parasympathetic, and enteric nervous system (ENS)—engage in bidirectional communication with the immune system (Huh & Veiga-Fernandes 2020, Chesné et al. 2019). Sympathetic signals originate from preganglionic neurons in the intermediolateral cell column of the spinal cord. Segmental efferents projecting from the brainstem and hypothalamus provide cholinergic excitatory input to the sympathetic ganglia and adrenal medulla. Norepinephrine is the major neurotransmitter of the sympathetic nervous system and acts on several classes of α and β adrenergic receptors (ARs) that are also expressed by immune cells (Bellinger & Lorton 2014). In addition, some sympathetic fibers also influence immune cell function via the secretion of neuropeptides such as neuropeptide Y (NPY) (Felten & Felten 1987).
Parasympathetic signals are delivered to peripheral organs from local and intramural ganglia, which are controlled by cholinergic input from the brainstem and sacral spinal cord. In the thorax and abdominal cavity, the preganglionic input is transmitted through the vagus nerve, while pelvic ganglia are controlled by the sacral parasympathetic nucleus. The main neurotransmitter of the parasympathetic system and ENS is acetylcholine (ACh), which can be integrated by muscarinic and nicotinic receptors expressed by immune cells (Bellinger & Lorton 2014).
Proper intestinal function relies on the ENS—a complex neuronal network that contains as many neurons as the spinal cord (Furness et al. 2014). This so-called second brain engages in bidirectional communication with the diverse pool of immune cells, microbiota, epithelium, and muscle cells (Veiga-Fernandes & Pachnis 2017).
In the following sections, we consider how functional interactions of the autonomic nervous system with immune cells regulate homeostasis in various peripheral organs. In addition, the effects of individual neuronal cues on immune cells have also been reviewed (Bellinger & Lorton 2014), but these are not considered here.
Spleen: The Inflammatory Reflex and the Cholinergic
Anti-Inflammatory Pathway
The innervation to the spleen has been extensively studied: Postganglionic sympathetic fibers from the celiac and superior mesenteric ganglia have nerve endings in close proximity to T and B lymphocytes, DCs, macrophages, and natural killer cells (Bellinger et al. 1989, Bellinger & Lorton 2014). These fibers mainly release norepinephrine, which acts on the adrenoreceptor expressed in this organ and exerts anti-inflammatory responses (Bratton et al. 2012, Oke & Tracey 2009), which was termed the inflammatory reflex (Oke & Tracey 2009). In addition, the sympathetic tone in the spleen is indirectly controlled by the vagus nerve via the cholinergic anti-inflammatory pathway (Pavlov et al. 2003, Tracey 2002). However, it has been shown that there is no direct parasympathetic innervation in the spleen, and therefore this model has been challenged. Furthermore, in other experimental settings, vagotomy did not exacerbate inflammation (Bratton et al. 2012, Martelli et al. 2014).
Recently, a brain-spleen circuit was described that directly impacts on immune defenses by regulating the formation of immunoglobulin-producing plasma cells (Zhang et al. 2020). The stress-responsive central amygdala and paraventricular nucleus of the hypothalamus stimulate splenic T cells via noradrenaline released by the splenic nerve. These T cells in turn secrete ACh, which binds to splenic B cells, driving its differentiation into immunoglobulin-producing plasma cells.
Bone Marrow: Neuroimmune Communication in Hematopoiesis
Hematopoiesis, the formation of new blood cells that continues throughout life and takes place in the bone marrow, is tightly controlled by the autonomous nervous system (Warr et al. 2011). The bone marrow mainly receives sympathetic input, and the relevance of this innervation was established more than 20 years ago (Afan et al. 1997, Warr et al. 2011). Neuronal cues were shown to interact with stromal cells in the bone marrow, controlling circadian mobilization of hematopoietic stem cells into the bloodstream (Méndez-Ferrer et al. 2008, 2010). Stromal cells release catecholamines that control the diurnal expression of CXCL12, a chemokine that retains stem cells in bone marrow (Méndez-Ferrer et al. 2008, 2010). Sympathetic fibers also secrete NPY, which in mice induces stromal cytokine release and promotes hematopoietic stem cell mobilization via activation of matrix metallopeptidase 9 (MMP-9) (Park et al. 2015).
In humans, aging leads to decreased numbers of sympathetic fibers in the bone marrow and diminished β3-adrenergic signaling, which is associated with decreased HSC function, reflected as decreased regenerative capacity and diminished multilineage differentiation potential (Maryanovich et al. 2018). Myeloid leukemia in humans can also lead to sympathetic neuropathy that disrupts mesenchymal homeostasis, leading to expansion of malignant progenitors (Arranz et al. 2014, Hanoun et al. 2014).
Parasympathetic fibers in the bone marrow are largely outnumbered by sympathetic fibers and nociceptor neurons. Consequently, the effects of parasympathetic cues in hematopoiesis are not well understood. A recent study demonstrated that signaling via the muscarinic receptor type 1 (Chrm1) in the brain promotes HSC mobilization (Pierce et al. 2017). This cholinergic signaling in the hypothalamus primes the hypothalamic-pituitary-adrenal axis, inducing glucocorticoid release, which in turn promotes egression of HSCs from the bone marrow via the glucocorticoid receptor NR3C1. Though this study demonstrates a role for central cholinergic signaling, the role of local parasympathetic fibers remains unknown.
Recently, sensory fibers were also shown to regulate HSC mobilization. CGRP secreted by bone marrow nociceptive fibers acts on HSCs expressing RAMP1/CALCRL to promote HSC egress via activation of the G protein Gαs (Gao et al. 2021). Feeding mice with capsaicin also induced increased BM CGRP levels and increased HSC mobilization into the blood (Gao et al. 2021). Interestingly, DRG sensory nerve fibers were also found to innervate the lymph node capsule (Huang et al. 2021). Transcriptionally these fibers express innate immune receptors, including TLRs, and optogenetic activation leads to changes in lymph node stromal cells and neutrophils (Huang et al. 2021). The observation that sensory fibers exist in lymphoid tissues opens opportunities by which the sensory nervous system could modulate host immunity.
The glial cells that ensheath bone marrow nerves have also been shown to support the hematopoietic stem cell niche. Transforming growth factor beta (TGF-β) is an important factor for maintenance of HSC that is produced by various bone marrow cells (Yamazaki et al. 2011). Nonmyelinating Schwann cells were shown to critically mediate the activation of TGF-β production in these cells (Yamazaki et al. 2011). Glial cells are also the most likely source of rearranged during transfection (RET) ligands. These neurotrophic factors are required for survival, expansion, and robustness of HSC in a B cell lymphoma 2 (Bcl2)- and BCL2-like 1 (Blc2l1)-dependent manner (Fonseca-Pereira et al. 2014).
Intestine
The intestine is a vast mucosal surface that relies on the largest immune compartment of the body and an extensive neuronal network to exert its functions. The diverse microbiota is separated from the inner milieu by only a single layer of epithelium. Intestinal neurons are classified as intrinsic with cell bodies in the gut, i.e., the ENS, and as extrinsic with cell bodies located outside the intestine, i.e., the autonomous nervous system. We first describe intrinsic and extrinsic innervation before discussing functional neuroimmune interactions in the intestine.
Intrinsic innervation: the enteric nervous system.
The mammalian ENS is composed of two layers of interconnected neurons and glia: the myenteric plexus, located between the intestinal muscle layers, and the submucosal plexus, located above the circular muscle layer. The myenteric plexus mainly coordinates smooth muscle contractions, while the submucosal plexus is involved in gut secretions and absorption (Furness 2000, Matteoli et al. 2014). The neuronal cell bodies of both plexuses are located in ganglia surrounded by glia that outnumber neurons by three to five times (Kulkarni et al. 2018). There are neuronal projections that interconnect the ganglia and project to nonneuronal targets, including immune cells and epithelium (Furness 2000) (Figure 4).
Enteric glia are a heterogeneous population with diverse morphology, distribution, and genetic profiles that include markers of Schwann cells or astrocytes (Veiga-Fernandes & Pachnis 2017). Glial cells are present within the intrinsic ganglia but also form a dense network that spreads from the crypts to the tips of intestinal villi. Intestinal glial cells interact with neuroendocrine cells, immune cells, blood vessels, and nerve fibers.
The development of the ENS and enteric immune system is closely related and interdependent. The neuroregulatory RET is a key player in the development of the ENS and intestinal secondary lymphoid structures called Peyer’s patches. RET signals in cis via GDNF family receptor alpha 1 (GFRα1) and glial cell-derived neurotrophic factor (GDNF), which are essential for ENS formation (Patel et al. 2012, Veiga-Fernandes et al. 2007), while RET signaling in trans through GFRα3 is required for lymphoid tissue initiator cell–induced formation of Peyer’s patches (Veiga-Fernandes et al. 2007).
Extrinsic innervation: sympathetic and parasympathetic regulation.
The extrinsic autonomic innervation of the intestine, part of the gut-brain axis, includes both parasympathetic and sympathetic branches (Veiga-Fernandes & Pachnis 2017). Sympathetic nerve endings containing norepinephrine densely innervate various parts of the intestine, including the serosa, mucosa, muscularis, myenteric plexus, and Peyer’s patches (Capurso et al. 1968, Fu et al. 2013, Phillips et al. 2006).
Inflammation
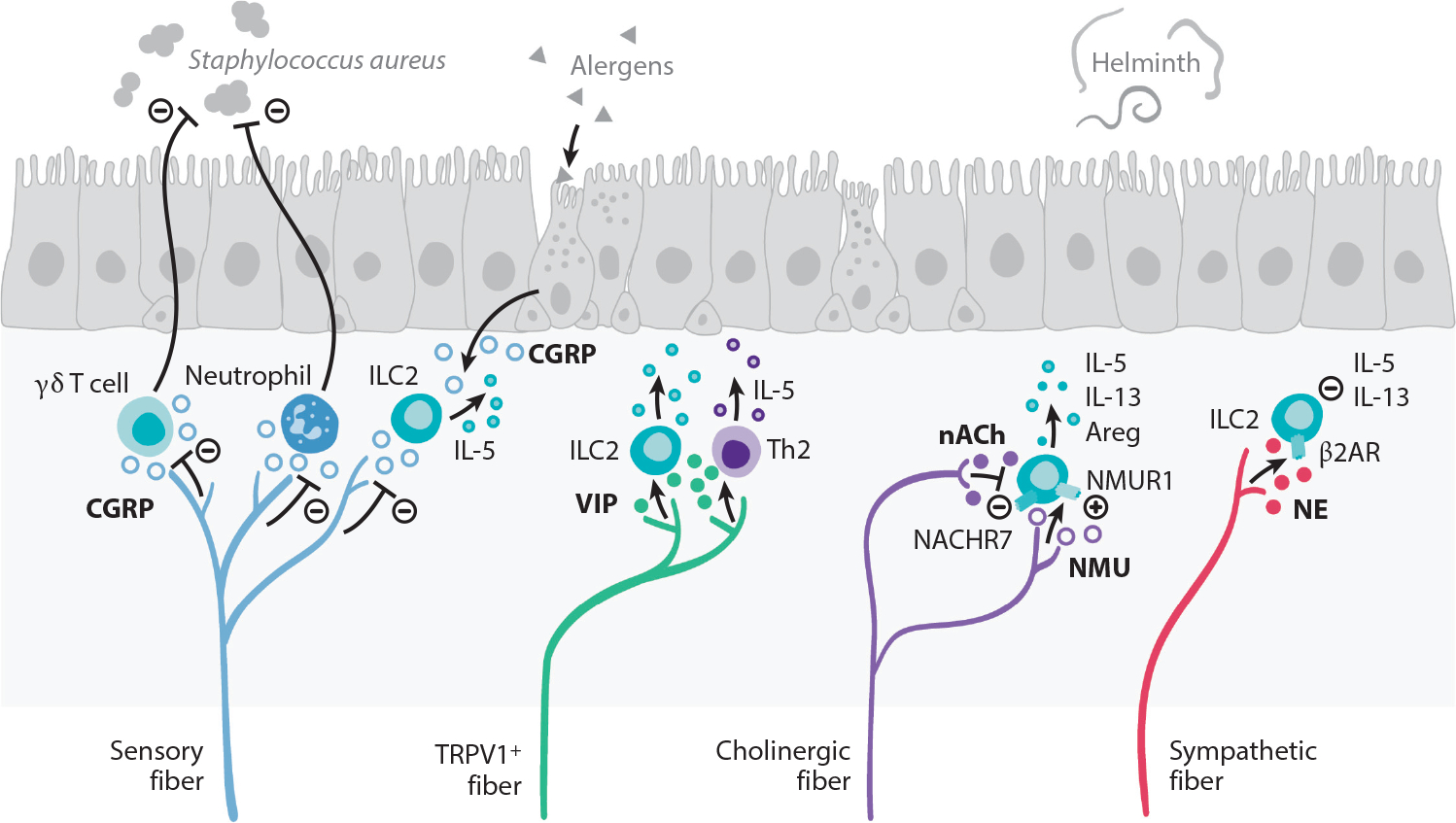
So-called type 2 immune responses have evolved to provide immunity against helminthic infections. T helper 2 cells and ILC2s are key regulators of this type of inflammatory response, which can also lead to chronic intestinal inflammatory disorders such as inflammatory bowel disease (IBD) (Veiga-Fernandes & Artis 2018). ILC2s have also emerged as critical integrators of neuronderived signals (Figure 4). ILC2s express various receptors for neuropeptides and the β2AR. In the intestine, ILC2s are found to colocalize with adrenergic neurons, and deletion of the β2AR enhanced type 2 responses by ILC2s (Moriyama et al. 2018). Conversely, treatment with β2 receptor agonists enhanced the infection burden in a helminth infection model in mice (Moriyama et al. 2018).
Mast cells are myeloid cells that are present in connective tissue, including the intestine. They are often implicated in allergic and inflammatory responses, as they are activated by allergy-associated mediators such as IgE. Upon activation, mast cells release inflammatory mediators such as histamine, proteases, and a wide variety of immune-regulating cytokines that also impact on sensory neurons (Krystel-Whittemore et al. 2015, van Diest et al. 2012). Mast cells are often found close to autonomic nerves (Williams et al. 1997), and adrenergic stimulation via β2 receptors on mast cells inhibits the release of histamine and other inflammatory mediators (Butchers et al. 1991). Mouse and human mast cells express nicotinic acetylcholine receptors (nAChRs) (Kageyama-Yahara et al. 2008, Mishra et al. 2010) and ACh-promoted histamine release (Masini et al. 1985).
Neuroimmune interaction has also been linked to human disease. In Crohn’s disease, altered sympathetic innervation is observed (Belai et al. 1997). Likewise, in animal models of IBD, enteric inflammation impacts on sympathetic and sensory innervation, leading to IBD-associated hypersensitivity (Bai et al. 2009, Xia et al. 2011).
Host Defense and Tissue Protection
ILC3s are another member of the innate lymphoid cell family and are recognized to engage in neuronal crosstalk (Veiga-Fernandes & Artis 2018) (Figure 4). Intestinal ILC3s and glial cells colocalize in specialized locations where their function is controlled by neurotrophic factors (Ibiza et al. 2016). Glial cells respond to microbial and host alarmin cues by production of neurotrophic factors that act on RET+ ILC3s, inducing the production of tissue-protective IL-22. In the context of various inflammatory and infectious insults, these neuroimmune cell units are required to maintain tissue homeostasis by promoting tissue repair (Ibiza et al. 2016). Further studies show that enteric neuronal circuits tune ILC responses via neuroregulators. Enteric glia and neurons were demonstrated to respond to environmental alterations such as microbial cues, worm products, and host alarmins by the production of neuromediators (Cardoso et al. 2017, Ibiza et al. 2016).
In addition to neurotrophic factors, ILC3s were also found to integrate vagal-derived ACh (Dalli et al. 2017). In a model of Escherichia coli infection of the peritoneal cavity, unilateral disruption of the vagus reduced ILC3 numbers and decreased the concentration of ILC3-derived tissue-protective protectin conjugates in tissue regeneration 1 (PCTR1), leading to an increased infection burden (Dalli et al. 2017). Together, these studies demonstrate how neuron-ILC units induce fast responses to external aggressions, required for maintaining tissue homeostasis.
Close interactions between neurons and macrophages have also been recognized to play an important role in protection of the integrity of the intestinal barrier (Gabanyi et al. 2016, Matteoli et al. 2014). In a mouse model of postoperative injury, stimulation of the vagus nerve reduced inflammation and promoted bowel transit (Matteoli et al. 2014). The most likely target of the protective cholinergic mediators were muscularis-resident macrophages expressing α7nAChR. Intestinal macrophages show compartmentalization and specialization: Proinflammatory macrophages are located in the lamina propria, with tissue-protective anti-inflammatory subsets in the muscularis layer (Gabanyi et al. 2016). The function of the latter, regulatory-like subtype of macrophages is stimulated via β2AR to integrate sympathetic norepinephrine signaling (Gabanyi et al. 2016). In steady-state conditions, enteric neurons also mediate the maintenance of muscularis macrophages via the secretion of a growth factor required for macrophage development, macrophage colony-stimulating factor (M-CSF) (Muller et al. 2014). In turn, muscularis macrophages regulate neuronal function via secretion of bone morphogenetic protein 2 (BMP2), thereby activating bone morphogenetic protein receptor (BMPR)-expressing enteric neurons (Muller et al. 2014). This bidirectional interaction is further regulated by commensal microbe-derived signals that modulate CSF1 and BMP2 expression levels. Thus, this microbe-macrophage-neuron triangle contributed to tissue homeostasis in steady state by regulating colonic motility (Muller et al. 2014). Importantly, muscularis macrophages also have a role in maintaining intrinsic enteric-associated neurons during infection (Matheis et al. 2020). Sympathetic signaling via the β2 receptor in muscularis macrophages was shown to induce a neuronal protection program through an arginase-1-polyamine axis using murine intestinal infection models. Thus, functional crosstalk between neurons and macrophages in the intestine could play an important role in the long-term postinfectious symptoms in irritable bowel syndrome or IBD.
Lung
The lung receives sympathetic inputs from the superior cervical and stellate ganglia. While sympathetic fibers can produce nonclassical neuromediators such as NPY, nitric oxide synthase (NOS), and ATP, noradrenaline is the main sympathetic neurotransmitter and is responsible for bronchodilation. The nucleus ambiguous sends parasympathetic fibers to the lung that travel through the vagus nerve (Kummer et al. 1992, McGovern & Mazzone 2010). ACh acts on the pulmonary nicotinergic and cholinergic receptors, inducing smooth muscle contraction and bronchoconstriction (Yang et al. 2014). Some parasympathetic fibers also secrete nitric oxide (NO) and VIP, inducing relaxation of smooth muscles (Fischer et al. 1998).
Functional neuroimmune cell units in the lung have been best described in the context of type 2 immune responses (Figure 3). IL-4, IL-5, and IL-13 are the prototypic effector cytokine responses of type 2 immune responses. Typically, these cytokines induce smooth muscle contraction, for example, of airways, increased mucus production, and recruitment and activation of other immune cells. Depending on the context, these responses induce allergic reactions such as airway hyperreactivity or are protective, for instance, in helminth infections in the lung or intestine (Hammad & Lambrecht 2015).
ILC2s are prototypical cells mediating type 2 responses and have been found to engage in bidirectional communication with neurons. Cholinergic neurons control ILC2-mediated allergic and helminth responses via secretion of the neuropeptide NMU, which acts on the neuromedin U receptor 1 (NMUR1) on ILC2s (Cardoso et al. 2017, Klose et al. 2017, Wallrapp et al. 2017). NMU/NMUR1 signaling induces rapid production of the effector cytokines IL-5 and IL-13 and tissue-protective amphiregulin. This neuroimmune cell unit functions to provide immediate tissue protection against helminth infection. Conversely, cholinergic neurons are also capable of dampening ILC2 function via the α7nAChR (Galle-Treger et al. 2016).
ILC2s have also been reported to be capable of producing ACh itself (Chu et al. 2021, Roberts et al. 2021). Release of ACh by ILC2s increases during helminth infection, allergic airway inflammation, and stimulation with alarmins IL-25 and IL-33. The ILC2-ACh pathway is required for optimal helminth immunity, as the parasite burden increases upon disruption of the pathway. ILC2s thus also constitute a nonneuronal source of ACh in the lung and intestine.
The sympathetic nervous system also regulates ILC2 biology. Pulmonary and intestinal ILC2s also express the β2AR (Moriyama et al. 2018). In the intestine, ILC2s colocalize with adrenergic fibers. Deletion of β2AR resulted in enhanced type 2 inflammation in the lung and intestine, while β2 agonists dampened ILC2 responses.
Thus, ILC2s can integrate a wide variety of neuronal cues, including parasympathetic and sympathetic signals. It is now clear that they interact in complex regulatory networks comprising local neurons that are important for tuning protective and pathological mucosal responses.
Next to ILC2s, mast cells are important immune cells in type 2 responses. Mast cells are found throughout the airways, mostly along the airway lumen and less frequently in the lung parenchyma (Andersson & Grundström 1987, Barnes 1986, Carr & Undem 2003). Mast cells are also localized in close proximity to airway nerves (Kowalski et al. 1997, Undem et al. 1995). Antigen-induced contraction of mouse trachea requires both parasympathetic nerve–derived ACh and mast cell–derived serotonin, although it is unclear whether mast cell–produced serotonin acts directly on smooth muscle cells (Cyphert et al. 2009, Weigand et al. 2009).
Adipose Tissue
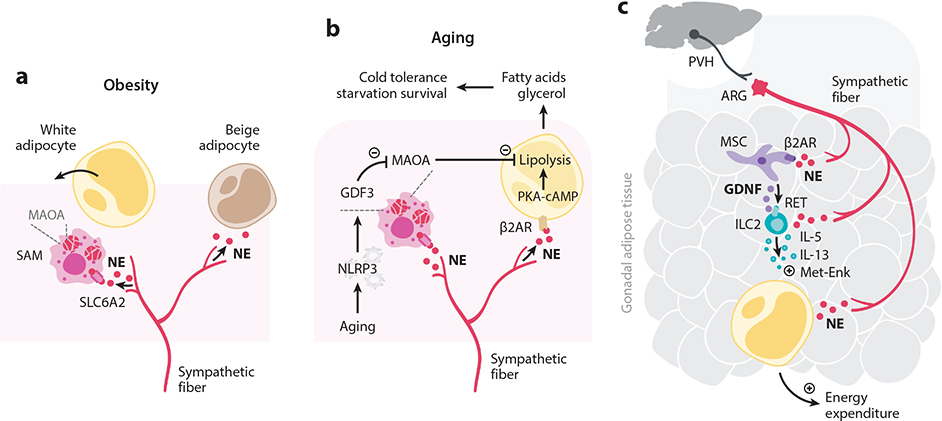
Adipocyte function is known to be tightly controlled by neuronal cues (Bartness et al. 2010) (Figure 5). In addition, several studies have shown that fat metabolism is also controlled by neuroimmune cell units involving tissue-resident macrophages. A recently described population of white adipose tissue macrophages colocalizes with sympathetic fibers (Pirzgalska et al. 2017) (Figure 5a). These sympathetic neuroassociated macrophages (SAMs) clear norepinephrine through SLC6A2, a transporter of norepinephrine, and the degrading enzyme monoamine oxidase A (MAOA). Optogenetic activation of the sympathetic nervous system stimulated SAM-mediated uptake of norepinephrine. Deletion of Slc6a2 in SAMs induced browning of white fat and increased thermogenesis, leading to weight loss in obese mice (Pirzgalska et al. 2017). Similarly, a recent study described an age-related reduction of lipolysis by adipose tissue adipocytes due to lower noradrenaline bioavailability (Camell et al. 2017) (Figure 5b). Aging correlated with higher gene expression levels of catecholamine-degrading enzymes, possibly explaining reduced lipolysis at advanced age. Likewise, tissue-resident macrophages were also found in brown adipose tissue that is also innervated by sympathetic fibers (Wolf et al. 2017). Mutations in the transcription factor Mecp2 in macrophages correlated with metabolic imbalance due to decreased sympathetic fat innervation. Brown adipose tissue macrophages thus steer sympathetic innervation, thereby indirectly controlling the bioavailability of catecholamines. Together, these studies demonstrate that tissue-resident macrophages control tissue homeostasis by relaying neuronal cues.
Figure 5.
Peripheral neuroimmune interactions in the adipose tissue. (a) SAMs localized in adipose tissue around sympathetic fibers express the NE transporter SLC6A2 and the degradation enzyme MAOA. Thus, these macrophages act to remove NE from the interstitium, thereby lowering the amount of NE available to adipocytes. Normally, NE stimulates adipocyte lipolysis and fatty acid oxidation, giving rise to beige adipocytes in a process called browning. (b) Aging stimulates the NLRP3 inflammasome, inducing GDF3 that promotes MAOA expression in NE-degrading macrophages. Aging dampens NE release and lipolysis in adipose tissue through increased removal of NE, contributing to increased availability of fatty acids, which are required to survive starvation and to tolerate exercise. (c) A sympathetic aorticorenal-adipose circuit connects to the brain and regulates group 2 innate lymphoid cells (ILC2s) via mesenchymal stem cells. Gonadal adipose tissue mesenchyme units translate NE cues to expression of the RET ligand GDNF. In turn, this neurotrophic fact controls adipose tissue ILC2 function via the neuroregulatory receptor RET. ILC2s control adipocyte metabolism via the effector cytokines IL-5, IL-13, and Met-Enk. Abbreviations: β2AR, β2 adrenergic receptor; ARG, aorticorenal ganglion; Enk, enkephalin; GDF3, growth differentiation factor 3; GDNF, glial-derived neurotrophic factor; ILC2, group 2 innate lymphoid cell; MAOA, monoamine oxidase A; MSC, mesenchymal stem cell; NE, norepinephrine; NLRP3, NLR family pyrin domain containing 3; PVH, paraventricular nucleus of hypothalamus; RET, rearranged during transfection; SAM, sympathetic neuron–associated macrophage.
Recently, interactions between the nervous system, mesenchymal stem cells, and ILC2s were shown to cooperate to regulate metabolism and obesity (Cardoso et al. 2021) (Figure 5c). In mice, sympathetic nerve terminals in visceral adipose tissue were shown to act on local mesenchymal stem cells via β2ARs to control the release of glial-derived neurotrophic factor (GDNF). This RET ligand in turn regulates adipose tissue ILC2 activity, as manipulation of GDNF machinery altered ILC2 function, energy expenditure, insulin resistance, and obesity. Retrograde tracing studies coupled with functional manipulation revealed that these adipose tissue circuits are controlled by sympathetic fibers from the aorticorenal ganglion and connect to higher-order brain areas, including the paraventricular nucleus of the hypothalamus (Cardoso et al. 2021). This study thus demonstrates control of metabolism and obesity by a neuro-mesenchymal unit via tissue-resident ILC2s by integrating cues from the CNS.
CONCLUDING REMARKS
The study of peripheral neuroimmune interactions has revealed a complex network that regulates health and disease, both at the local level and at long distance. Recent technological advances have spurred the study of these intricate interactions between two systems that engage in crosstalk much more frequently than previously appreciated. Many studies have revealed neuroimmune interactions at the barrier surfaces where organisms interact with the outside world such as the gut, skin, and lungs. Neuroimmune units have emerged as hubs that integrate a wide variety of environmental cues to maintain or to reestablish tissue homeostasis. As such, neuroimmune units are an interesting target for the development of new therapeutic strategies (Klose & Veiga-Fernandes 2021). Indeed, the vagus nerve has already been targeted using electrical stimulation (de Jonge et al. 2005, Ji et al. 2014).
To further explore the therapeutic potentials of neuroimmune interactions, a better understanding of the molecular makeup of the neuroimmune interface is required. In addition, we require a better understanding of the transcriptional and epigenetic changes and the resulting effects of neuroimmune interactions: How do neuropeptides and neurotransmitters affect immune cell function, and vice versa, what are the effects of immune mediators on neurons? How do the neuronal receptors expressed by immune cells behave, and do immune receptors expressed on axonal terminals signal similarly in neurons as they do in immune cells?
Over the last several years, many efforts have been made to map the neuronal interactome in the brain, but little is known about the PNS. A detailed atlas of peripheral neurons, including information on the various neuronal subsets and the neurotransmitters involved, will be instrumental for the further development of this field. Similarly, how discrete brain networks regulate and/or are regulated by peripheral neuroimmune axes remains poorly understood. These are open questions that are among the most promising and exciting endeavors in the realm of brain-body axes.
These and others are some of the exciting frontiers to explore in uncovering the language that these two ancient systems use to communicate.
Supplementary Material
ACKNOWLEDGMENTS
We thank Gil Castro for help with the illustrations. R.G.J.K.W. was supported by a Marie Skłodowska-Curie Individual fellowship, a Cancer Research Institute/Irvington Postdoctoral Fellowship, and a Postdoctoral Junior Leader fellowship from La Caixa Foundation. H.V.-F. is funded by the European Research Council, Paul G. Allen Frontiers Group, Chan Zuckerberg Initiative (CZI), Fundación la Caixa, and Fundação para a Ciência e Tecnologia. I.M.C. is funded by the National Institutes of Health(R01AI130019 and R01DK127257), CZI, Burroughs Wellcome Fund, Kenneth Rainin Foundation, Allergan Pharmaceuticals, Ning Drako Family Foundation, and Food Allergy Sciences Initiative.
Footnotes
DISCLOSURE STATEMENT
I.M.C. is on scientific advisory boards for GSK Pharmaceuticals and LiMM Therapeutics. His lab receives funding from Allergan Pharmaceuticals for sponsored research. H.V.-F. is on the board of LiMM Therapeutics.
LITERATURE CITED
- Afan AM, Broome CS, Nicholls SE, Whetton AD, Miyan JA. 1997. Bone marrow innervation regulates cellular retention in the murine haemopoietic system. Br. J. Haematol. 98(3):569–77 [DOI] [PubMed] [Google Scholar]
- Andersson RG, Grundström N. 1987. Innervation of airway smooth muscle. Efferent mechanisms. Pharmacol. Ther. 32(2):107–30 [DOI] [PubMed] [Google Scholar]
- Arranz L, Sánchez-Aguilera A, Martín-Pérez D, Isern J, Langa X, et al. 2014. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature 512(7512):78–81 [DOI] [PubMed] [Google Scholar]
- Bai A, Lu N, Guo Y, Chen J, Liu Z. 2009. Modulation of inflammatory response via α2-adrenoceptor blockade in acute murine colitis. Clin. Exp. Immunol. 156(2):353–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baral P, Umans BD, Li L, Wallrapp A, Bist M, et al. 2018. Nociceptor sensory neurons suppress neutrophil and γδ T cell responses in bacterial lung infections and lethal pneumonia. Nat. Med. 24(4):417–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. 1986. Non-adrenergic non-cholinergic neural control of human airways. Arch. Int. Pharmacodyn. Ther 280(2 Suppl.):208–28 [PubMed] [Google Scholar]
- Bartness TJ, Vaughan CH, Song CK. 2010. Sympathetic and sensory innervation of brown adipose tissue. Int. J. Obes. 34(Suppl. 1):S36–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belai A, Boulos PB, Robson T, Burnstock G. 1997. Neurochemical coding in the small intestine of patients with Crohn’s disease. Gut 40(6):767–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellavance M-A, Rivest S 2014. The HPA–immune axis and the immunomodulatory actions of glucocorticoids in the brain. Front. Immunol. 5:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DL, Felten SY, Lorton D, Felten DL. 1989. Origin of noradrenergic innervation of the spleen in rats. Brain Behav. Immun. 3(4):291–311 [DOI] [PubMed] [Google Scholar]
- Bellinger DL, Lorton D. 2014. Autonomic regulation of cellular immune function. Auton. Neurosci. 182:15–41 [DOI] [PubMed] [Google Scholar]
- Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, et al. 2008. Nociceptors are interleukin-1β sensors. J. Neurosci. 28(52):14062–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw LA, Gebhart GF. 2002. The pharmacology of gastrointestinal nociceptive pathways. Curr. Opin. Pharmacol. 2(6):642–49 [DOI] [PubMed] [Google Scholar]
- Blake KJ, Baral P, Voisin T, Lubkin A, Pinho-Ribeiro FA, et al. 2018. Staphylococcus aureus produces pain through pore-forming toxins and neuronal TRPV1 that is silenced by QX-314. Nat. Commun. 9(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratton BO, Martelli D, McKinley MJ, Trevaks D, Anderson CR, McAllen RM. 2012. Neural regulation of inflammation: no neural connection from the vagus to splenic sympathetic neurons. Exp. Physiol. 97(11):1180–85 [DOI] [PubMed] [Google Scholar]
- Butchers PR, Vardey CJ, Johnson M. 1991. Salmeterol: a potent and long-acting inhibitor of inflammatory mediator release from human lung. Br. J. Pharmacol. 104(3):672–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camell CD, Sander J, Spadaro O, Lee A, Nguyen KY, et al. 2017. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature 550(7674):119–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ, Mori N, Mazzone SB. 2006. Vagal afferent nerves regulating the cough reflex. Respir. Physiol. Neurobiol. 152(3):223–42 [DOI] [PubMed] [Google Scholar]
- Capurso L, Friedmann CA, Parks AG. 1968. Adrenergic fibres in the human intestine. Gut 9(6):678–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso F, Klein Wolterink RGJ, Godinho-Silva C, Domingues RG, Ribeiro H, et al. 2021. Neuro-mesenchymal units control ILC2 and obesity via a brain-adipose circuit. Nature 597:410–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso V, Chesné J, Ribeiro H, García-Cassani B, Carvalho T, et al. 2017. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 549(7671):277–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr MJ, Undem BJ. 2003. Bronchopulmonary afferent nerves. Respirology 8(3):291–301 [DOI] [PubMed] [Google Scholar]
- Chang RB, Strochlic DE, Williams EK, Umans BD, Liberles SD. 2015. Vagal sensory neuron subtypes that differentially control breathing. Cell 161(3):622–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesné J, Cardoso V, Veiga-Fernandes H. 2019. Neuro-immune regulation of mucosal physiology. Mucosal Immunol. 12:10–20 [DOI] [PubMed] [Google Scholar]
- Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, et al. 2013. Bacteria activate sensory neurons that modulate pain and inflammation. Nature 501(7465):52–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu IM, von Hehn CA, Woolf CJ. 2012. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 15(8):1063–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C, Artis D, Chiu IM. 2020. Neuro-immune interactions in the tissues. Immunity 52(3):464–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C, Parkhurst CN, Zhang W, Zhou L, Yano H, et al. 2021. The ChAT-acetylcholine pathway promotes group 2 innate lymphoid cell responses and anti-helminth immunity. Sci. Immunol. 6(57):eabe3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JA, Edwards TN, Liu AW, Hirai T, Jones MR, et al. 2019. Cutaneous TRPV1+ neurons trigger protective innate type 17 anticipatory immunity. Cell 178(4):919–32.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JCG. 2011. Reflexes evoked from tracheobronchial tree and lungs. In Comprehensive Physiology, ed. Terjung R, pp. 395–429. Rockville, MD: Am. Physiol. Soc. [Google Scholar]
- Cook AD, Christensen AD, Tewari D, McMahon SB, Hamilton JA. 2018. Immune cytokines and their receptors in inflammatory pain. Trends Immunol. 39(3):240–55 [DOI] [PubMed] [Google Scholar]
- Cyphert JM, Kovarova M, Allen IC, Hartney JM, Murphy DL, et al. 2009. Cooperation between mast cells and neurons is essential for antigen-mediated bronchoconstriction. J. Immunol. 182(12):7430–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalli J, Colas RA, Arnardottir H, Serhan CN. 2017. Vagal regulation of group 3 innate lymphoid cells and the immunoresolvent PCTR1 controls infection resolution. Immunity 46(1):92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, et al. 2005. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat. Immunol. 6(8):844–51 [DOI] [PubMed] [Google Scholar]
- Di Giovangiulio M, Verheijden S, Bosmans G, Stakenborg N, Boeckxstaens GE, Matteoli G. 2015. The neuromodulation of the intestinal immune system and its relevance in inflammatory bowel disease. Front. Immunol. 6:590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diogenes A, Ferraz CCR, Akopian AN, Henry MA, Hargreaves KM. 2011. LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J. Dent. Res. 90(6):759–64 [DOI] [PubMed] [Google Scholar]
- Felten DL, Felten SY. 1987. Immune interactions with specific neural structures. Brain Behav. Immun. 1(4):279–83 [DOI] [PubMed] [Google Scholar]
- Fischer A, Canning BJ, Undem BJ, Kummer W. 1998. Evidence for an esophageal origin of VIP-IR and NO synthase-IR nerves innervating the guinea pig trachealis: a retrograde neuronal tracing and immunohistochemical analysis. J. Comp. Neurol. 394(3):326–34 [PubMed] [Google Scholar]
- Fonseca-Pereira D, Arroz-Madeira S, Rodrigues-Campos M, Barbosa IAM, Domingues RG, et al. 2014. The neurotrophic factor receptor RET drives haematopoietic stem cell survival and function. Nature 514(7520):98–101 [DOI] [PubMed] [Google Scholar]
- Fu Y-Y, Peng S-J, Lin H-Y, Pasricha PJ, Tang S-C. 2013. 3-D imaging and illustration of mouse intestinal neurovascular complex. Am. J. Physiol. Gastrointest. Liver Physiol 304(1):G1–11 [DOI] [PubMed] [Google Scholar]
- Furness JB. 2000. Types of neurons in the enteric nervous system. J. Auton. Nerv. Syst. 81(1–3):87–96 [DOI] [PubMed] [Google Scholar]
- Furness JB, Callaghan BP, Rivera LR, Cho H-J. 2014. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv. Exp. Med. Biol. 817:39–71 [DOI] [PubMed] [Google Scholar]
- Gabanyi I, Muller PA, Feighery L, Oliveira TY, Costa-Pinto FA, Mucida D. 2016. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell 164(3):378–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galle-Treger L, Suzuki Y, Patel N, Sankaranarayanan I, Aron JL, et al. 2016. Nicotinic acetylcholine receptor agonist attenuates ILC2-dependent airway hyperreactivity. Nat. Commun. 7:13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Zhang D, Xu C, Li H, Caron KM, Frenette PS. 2021. Nociceptive nerves regulate haematopoietic stem cell mobilization. Nature 589(7843):591–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinho-Silva C, Cardoso F, Veiga-Fernandes H. 2019. Neuro-immune cell units: a new paradigm in physiology. Annu. Rev. Immunol. 37:19–46 [DOI] [PubMed] [Google Scholar]
- Green DP, Limjunyawong N, Gour N, Pundir P, Dong X. 2019. A mast-cell-specific receptor mediates neurogenic inflammation and pain. Neuron 101(3):412–20.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy L, Erickson A, Brierley SM. 2019. Visceral pain. Annu. Rev. Physiol. 81:261–84 [DOI] [PubMed] [Google Scholar]
- Hammad H, Lambrecht BN. 2015. Barrier epithelial cells and the control of type 2 immunity. Immunity 43(1):29–40 [DOI] [PubMed] [Google Scholar]
- Hanoun M, Zhang D, Mizoguchi T, Pinho S, Pierce H, et al. 2014. Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell Stem Cell 15(3):365–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harzenetter MD, Novotny AR, Gais P, Molina CA, Altmayr F, Holzmann B. 2007. Negative regulation of TLR responses by the neuropeptide CGRP is mediated by the transcriptional repressor ICER. J. Immunol. 179(1):607–15 [DOI] [PubMed] [Google Scholar]
- Hoeffel G, Debroas G, Roger A, Rossignol R, Gouilly J, et al. 2021. Sensory neuron-derived TAFA4 promotes macrophage tissue repair functions. Nature 594(7861):94–99 [DOI] [PubMed] [Google Scholar]
- Huang S, Ziegler CGK, Austin J, Mannoun N, Vukovic M, et al. 2021. Lymph nodes are innervated by a unique population of sensory neurons with immunomodulatory potential. Cell 184(2):441–59.e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JR, Veiga-Fernandes H. 2020. Neuroimmune circuits in inter-organ communication. Nat. Rev. Immunol. 20(4):217–28 [DOI] [PubMed] [Google Scholar]
- Ibiza S, García-Cassani B, Ribeiro H, Carvalho T, Almeida L, et al. 2016. Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature 535(7612):440–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Rabbi MF, Labis B, Pavlov VA, Tracey KJ, Ghia JE. 2014. Central cholinergic activation of a vagus nerve-to-spleen circuit alleviates experimental colitis. Mucosal Immunol. 7(2):335–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama-Yahara N, Suehiro Y, Yamamoto T, Kadowaki M. 2008. IgE-induced degranulation of mucosal mast cells is negatively regulated via nicotinic acetylcholine receptors. Biochem. Biophys. Res. Commun. 377(1):321–25 [DOI] [PubMed] [Google Scholar]
- Kashem SW, Riedl MS, Yao C, Honda CN, Vulchanova L, Kaplan DH. 2015. Nociceptive sensory fibers drive interleukin-23 production from CD301b+ dermal dendritic cells and drive protective cutaneous immunity. Immunity 43(3):515–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CF, Moalem-Taylor G. 2011. Interleukin-17 contributes to neuroinflammation and neuropathic pain following peripheral nerve injury in mice. J. Pain 12(3):370–83 [DOI] [PubMed] [Google Scholar]
- Klose CSN, Mahlakõiv T, Moeller JB, Rankin LC, Flamar A-L, et al. 2017. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 549:282–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose CSN, Veiga-Fernandes H. 2021. Neuroimmune interactions in peripheral tissues. Eur. J. Immunol. 51(7):1602–14 [DOI] [PubMed] [Google Scholar]
- Kowalski ML, Didier A, Lundgren JD, Igarashi Y, Kaliner MA. 1997. Role of sensory innervation and mast cells in neurogenic plasma protein exudation into the airway lumen. Respirology 2(4):267–74 [DOI] [PubMed] [Google Scholar]
- Kraus A, Buckley KM, Salinas I. 2021. Sensing the world and its dangers: an evolutionary perspective in neuroimmunology. eLife 10:e66706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystel-Whittemore M, Dileepan KN, Wood JG. 2015. Mast cell: a multi-functional master cell. Front. Immunol. 6:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni S, Ganz J, Bayrer J, Becker L, Bogunovic M, Rao M. 2018. Advances in enteric neurobiology: the “brain” in the gut in health and disease. J. Neurosci. 38(44):9346–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer W, Fischer A, Kurkowski R, Heym C. 1992. The sensory and sympathetic innervation of guinea-pig lung and trachea as studied by retrograde neuronal tracing and double-labelling immunohistochemistry. Neuroscience 49(3):715–37 [DOI] [PubMed] [Google Scholar]
- Lai NY, Musser MA, Pinho-Ribeiro FA, Baral P, Jacobson A, et al. 2020. Gut-innervating nociceptor neurons regulate Peyer’s patch microfold cells and SFB levels to mediate salmonella host defense. Cell 180(1):33–49.e22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamhamedi-Cherradi S-E, Martin RE, Ito T, Kheradmand F, Corry DB, et al. 2008. Fungal proteases induce Th2 polarization through limited dendritic cell maturation and reduced production of IL-12. J. Immunol. 180(9):6000–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli D, Yao ST, McKinley MJ, McAllen RM. 2014. Reflex control of inflammation by sympathetic nerves, not the vagus. J. Physiol. 592(7):1677–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K, Takayama Y, Sugisawa E, Yamanoi Y, Yokawa T, et al. 2018. The ATP transporter VNUT mediates induction of Dectin-1-triggered Candida nociception. iScience 6:306–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryanovich M, Zahalka AH, Pierce H, Pinho S, Nakahara F, et al. 2018. Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat. Med. 24(6):782–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masini E, Fantozzi R, Conti A, Blandina P, Brunelleschi S, Mannaioni PF. 1985. Mast cell heterogeneity in response to cholinergic stimulation. Int. Arch. Allergy Appl. Immunol. 77(1–2):184–85 [DOI] [PubMed] [Google Scholar]
- Matheis F, Muller PA, Graves CL, Gabanyi I, Kerner ZJ, et al. 2020. Adrenergic signaling in muscularis macrophages limits infection-induced neuronal loss. Cell 180(1):64–78.e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoli G, Gomez-Pinilla PJ, Nemethova A, Di Giovangiulio M, Cailotto C, et al. 2014. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut 63(6):938–48 [DOI] [PubMed] [Google Scholar]
- McGovern AE, Mazzone SB. 2010. Characterization of the vagal motor neurons projecting to the Guinea pig airways and esophagus. Front. Neurol. 1:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, et al. 2015. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 519(7542):237–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez-Ferrer S, Lucas D, Battista M, Frenette PS. 2008. Haematopoietic stem cell release is regulated by circadian oscillations. Nature 452(7186):442–47 [DOI] [PubMed] [Google Scholar]
- Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, et al. 2010. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466(7308):829–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meseguer V, Alpizar YA, Luis E, Tajada S, Denlinger B, et al. 2014. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat. Commun. 5:3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra NC, Rir-sima-ah J, Boyd RT, Singh SP, Gundavarapu S, et al. 2010. Nicotine inhibits FcεRI-induced cysteinyl leukotrienes and cytokine production without affecting mast cell degranulation through α7/α9/α10-nicotinic receptors. J. Immunol. 185(1):588–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama S, Brestoff JR, Flamar A-L, Moeller JB, Klose CSN, et al. 2018. β2-adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science 359(6379):1056–61 [DOI] [PubMed] [Google Scholar]
- Muller PA, Koscsó B, Rajani GM, Stevanovic K, Berres M-L, et al. 2014. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 158(2):300–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutma E, Willison H, Martino G, Amor S. 2019. Neuroimmunology—the past, present and future. Clin. Exp. Immunol. 197(3):278–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oke SL, Tracey KJ. 2009. The inflammatory reflex and the role of complementary and alternative medical therapies. Ann. N. Y. Acad. Sci. 1172:172–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm NW, Rosenstein RK, Yu S, Schenten DD, Florsheim E, Medzhitov R. 2013. Bee venom phospholipase A2 induces a primary type 2 response that is dependent on the receptor ST2 and confers protective immunity. Immunity 39(5):976–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MH, Jin HK, Min W-K, Lee WW, Lee JE, et al. 2015. Neuropeptide Y regulates the hematopoietic stem cell microenvironment and prevents nerve injury in the bone marrow. EMBO J. 34(12):1648–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Harker N, Moreira-Santos L, Ferreira M, Alden K, et al. 2012. Differential RET signaling pathways drive development of the enteric lymphoid and nervous systems. Sci. Signal. 5(235):ra55. [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ. 2017. Neural regulation of immunity: molecular mechanisms and clinical translation. Nat. Neurosci. 20(2):156–66 [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. 2003. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol. Med. 9(5–8):125–34 [PMC free article] [PubMed] [Google Scholar]
- Perner C, Flayer CH, Zhu X, Aderhold PA, Dewan ZNA, et al. 2020. Substance P release by sensory neurons triggers dendritic cell migration and initiates the type-2 immune response to allergens. Immunity 53(5):1063–77.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RJ, Rhodes BS, Powley TL. 2006. Effects of age on sympathetic innervation of the myenteric plexus and gastrointestinal smooth muscle of Fischer 344 rats. Anat. Embryol. 211(6):673–83 [DOI] [PubMed] [Google Scholar]
- Pierce H, Zhang D, Magnon C, Lucas D, Christin JR, et al. 2017. Cholinergic signals from the CNS regulate G-CSF-mediated HSC mobilization from bone marrow via a glucocorticoid signaling relay. Cell Stem Cell 20(5):648–58.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho-Ribeiro FA, Baddal B, Haarsma R, O’Seaghdha M, Yang NJ, et al. 2018. Blocking neuronal signaling to immune cells treats Streptococcal invasive infection. Cell 173(5):1083–97.e22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirzgalska RM, Seixas E, Seidman JS, Link VM, Sánchez NM, et al. 2017. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat. Med. 23(11):1309–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter P, Susarla SC, Polikepahad S, Qian Y, Hampton J, et al. 2009. Link between allergic asthma and airway mucosal infection suggested by proteinase-secreting household fungi. Mucosal Immunol. 2(6):504–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riol-Blanco L, Ordovas-Montanes J, Perro M, Naval E, Thiriot A, et al. 2014. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature 510(7503):157–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts LB, Schnoeller C, Berkachy R, Darby M, Pillaye J, et al. 2021. Acetylcholine production by group 2 innate lymphoid cells promotes mucosal immunity to helminths. Sci. Immunol. 6(57):eabd0359. [DOI] [PubMed] [Google Scholar]
- Schiller M, Ben-Shaanan TL, Rolls A. 2021. Neuronal regulation of immunity: Why, how and where? Nat. Rev. Immunol. 21(1):20–36 [DOI] [PubMed] [Google Scholar]
- Serhan N, Basso L, Sibilano R, Petitfils C, Meixiong J, et al. 2019. House dust mites activate nociceptor-mast cell clusters to drive type 2 skin inflammation. Nat. Immunol. 20(11):1435–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurin MR, Shurin GV, Zlotnikov SB, Bunimovich YL. 2020. The neuroimmune axis in the tumor microenvironment. J. Immunol. 204(2):280–85 [DOI] [PubMed] [Google Scholar]
- Sokol CL, Barton GM, Farr AG, Medzhitov R. 2008. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat. Immunol. 9(3):310–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot S, Abdulnour R-EE, Burkett PR, Lee S, Cronin SJF, et al. 2015. Silencing nociceptor neurons reduces allergic airway inflammation. Neuron 87(2):341–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamari M, Ver Heul AM, Kim BS. 2021. Immunosensation: neuroimmune cross talk in the skin. Annu. Rev. Immunol. 39:369–93 [DOI] [PubMed] [Google Scholar]
- Tracey KJ. 2002. The inflammatory reflex. Nature 420(6917):853–59 [DOI] [PubMed] [Google Scholar]
- Tränkner D, Hahne N, Sugino K, Hoon MA, Zuker C. 2014. Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. PNAS 111(31):11515–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undem BJ, Riccio MM, Weinreich D, Ellis JL, Myers AC. 1995. Neurophysiology of mast cell-nerve interactions in the airways. Int. Arch. Allergy Immunol. 107(1–3):199–201 [DOI] [PubMed] [Google Scholar]
- van Diest SA, Stanisor OI, Boeckxstaens GE, de Jonge WJ, van den Wijngaard RM. 2012. Relevance of mast cell-nerve interactions in intestinal nociception. Biochim. Biophys. Acta Mol. Basis Dis 1822(1):74–84 [DOI] [PubMed] [Google Scholar]
- Van Dyken SJ, Locksley RM. 2018. Chitins and chitinase activity in airway diseases. J. Allergy Clin. Immunol. 142(2):364–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Fernandes H, Artis D. 2018. Neuronal-immune system cross-talk in homeostasis. Science 359(6383):1465–66 [DOI] [PubMed] [Google Scholar]
- Veiga-Fernandes H, Coles MC, Foster KE, Patel A, Williams A, et al. 2007. Tyrosine kinase receptor RET is a key regulator of Peyer’s patch organogenesis. Nature 446(7135):547–51 [DOI] [PubMed] [Google Scholar]
- Veiga-Fernandes H, Pachnis V. 2017. Neuroimmune regulation during intestinal development and homeostasis. Nat. Immunol. 18:116–22 [DOI] [PubMed] [Google Scholar]
- Wallrapp A, Riesenfeld SJ, Burkett PR, Abdulnour R-EE, Nyman J, et al. 2017. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature 549(7672):351–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters N, Trunkle T, Sura M, Pascual DW. 2005. Enhanced immunoglobulin A response and protection against Salmonella enterica serovar typhimurium in the absence of the substance P receptor. Infect. Immun. 73(1):317–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr MR, Pietras EM, Passegué E. 2011. Mechanisms controlling hematopoietic stem cell functions during normal hematopoiesis and hematological malignancies. Wiley Interdiscip. Rev. Syst. Biol. Med. 3(6):681–701 [DOI] [PubMed] [Google Scholar]
- Webster JI, Tonelli L, Sternberg EM. 2002. Neuroendocrine regulation of immunity. Annu. Rev. Immunol. 20:125–63 [DOI] [PubMed] [Google Scholar]
- Weigand LA, Myers AC, Meeker S, Undem BJ. 2009. Mast cell-cholinergic nerve interaction in mouse airways. J. Physiol. 587(Pt. 13):3355–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe J 2001. Airway receptors. Respir. Physiol. 125(1–2):3–15 [DOI] [PubMed] [Google Scholar]
- Williams RM, Berthoud H-R, Stead RH. 1997. Vagal afferent nerve fibres contact mast cells in rat small intestinal mucosa. Neuroimmunomodulation 4(5–6):266–70 [DOI] [PubMed] [Google Scholar]
- Wolf Y, Boura-Halfon S, Cortese N, Haimon Z, Sar Shalom H, et al. 2017. Brown-adipose-tissue macrophages control tissue innervation and homeostatic energy expenditure. Nat. Immunol. 18(6):665–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C-M, Colomb DG, Akbarali HI, Qiao L-Y. 2011. Prolonged sympathetic innervation of sensory neurons in rat thoracolumbar dorsal root ganglia during chronic colitis. Neurogastroenterol. Motil. 23(8):801–e339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z-Z, Kim YH, Bang S, Zhang Y, Berta T, et al. 2015. Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nat. Med. 21(11):1326–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, et al. 2011. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 147(5):1146–58 [DOI] [PubMed] [Google Scholar]
- Yang X, Zhao C, Gao Z, Su X. 2014. A novel regulator of lung inflammation and immunity: pulmonary parasympathetic inflammatory reflex. QJM 107(10):789–92 [DOI] [PubMed] [Google Scholar]
- Zanos TP, Silverman HA, Levy T, Tsaava T, Battinelli E, et al. 2018. Identification of cytokine-specific sensory neural signals by decoding murine vagus nerve activity. PNAS 115(21):E4843–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Edwards TN, Chaudhri VK, Wu J, Cohen JA, et al. 2021. Nonpeptidergic neurons suppress mast cells via glutamate to maintain skin homeostasis. Cell 184(8):2151–66.e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Lei B, Yuan Y, Zhang L, Hu L, et al. 2020. Brain control of humoral immune responses amenable to behavioural modulation. Nature 581(7807):204–8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.