Abstract
Background and Aims
Pregnancy‐induced hypertension is one of the top three ranked diseases during pregnancy that cause maternal, fetal, and neonatal morbidity and mortality worldwide. To provide adequate information to clinicians and researchers who are striving for potential interventions, biochemical profiling of such patients is required.
Methods
A hospital‐based case‐control study design was conducted from August 2020 to May 2021 to evaluate serum lipid profile, uric acid, and high sensitivity C‐reactive protein (hs‐CRP) among women with pregnancy‐induced hypertension compared to normotensive pregnant women. Data were entered and analyzed using SPSS version 25. Independent t‐test and χ 2 were used to compare the relationship of variables between the two groups. A p‐value less than 0.05 was used to test statistical significance.
Results
The result of this study showed that while the levels (mean ± SD) of serum total cholesterol (TC), triglyceride (TG), low‐density lipoprotein‐cholesterol (LDL‐C), TC/high‐density lipoprotein‐cholesterol (HDL‐C), TG/HDL‐C, LDL‐C/HDL‐C were significantly elevated, HDL‐C was decreased among women with pregnancy‐induced hypertension than normotensive pregnant women (p < 0.0001). The levels (mean ± SD) of uric acid and hs‐CRP were significantly higher among women with pregnancy‐induced hypertension compared to normotensive pregnant women (p < 0.0001).
Conclusion
This study indicated that pregnancy‐induced hypertension women have lipid abnormalities, increased systemic inflammatory markers, and hyperuricemia compared to normotensive pregnant women. Thus, women with PIH showing high dyslipidemia, hyperuricemia, and inflammation are likely to develop hypertension. Therefore, evaluation of these potential biomarkers during early antenatal care services may help seek interventions in PIH.
Keywords: Ethiopia, inflammation, lipid profiles, pregnancy‐induced hypertension, uric acid
1. INTRODUCTION
Hypertension (HTN) in pregnancy is an elevation of systolic (BP ≥ 140 mmHg) or diastolic (BP ≥ 90 mmHg) blood pressures. Pregnancy‐induced hypertension (PIH) is characterized by high blood pressure, with/without protein in the urine, and pathological edema during pregnancy. 1 Elevated diastolic or systolic blood pressure is an important marker of PIH and its reduction helps reduce the risk of HTN. 2 The American society of hypertension guidelines classified hypertensive disorder of pregnancy as follows 3 : Pre‐eclampsia–eclampsia, chronic HTN (of any cause), gestational HTN, and chronic HTN with superimposed pre‐eclampsia.
PIH is one of the top three leading causes of maternal, fetal, and neonatal morbidity and mortality. 4 The World Health Organization (WHO) states that severe HTN in pregnancy will increase both the mother's and fetus' risks 5 , 6 such as poor placenta transfer, growth restrictions, preterm birth, placenta abortion, and neonatal death.
Even though PIH has multifarious risk factors, its pathophysiology and etiology are not well understood. Placental implantation with abnormal tissue layer invasion of uterine vessels and immunologic intolerance between maternal, placental, and fetal tissues are a number of the etiological factors for PIH. 7
Reduction in placenta blood flow results in ischemia and hypoxia, which releases ischemic factors and dysregulates immune cells. Placental ischemic factors released on the maternal endothelial system cause vascular endothelial dysfunction that enhances the formation of vasoconstrictors (endothelin‐1 and thromboxane), increased vascular sensitivity to angiotensin II, increased free‐radical (superoxide), and decreased formation of vasodilators (nitric oxide and prostacyclin). 6
Metabolic disturbances of lipids are believed as the risk of PIH by inducing endothelial cell dysfunction next to oxidative stress and insulin resistance. Insulin resistance reduces lipoprotein lipase activity resulting in decreasing the hydrolysis of triglycerides from lipoproteins. 8 Thus, women with PIH are at greater risk of developing HTN, cardiovascular disease (CVD), diabetes mellitus (DM), and kidney diseases in later life. 9 , 10 , 11
Uric acid is one of the foremost consistent and earliest notable changes in PIH and has been indicated as a higher predictor of maternal and fetal risk than blood pressure level. 12 The potential origin of uric acid is renal dysfunction, excessive tissue breakdown, acidosis, and excessive activity of the xanthine oxidase enzyme. Elevated serum uric acid is not a straightforward marker for the disease's severity but rather contributes directly to the pathological process of the disorder. 12 In pre‐eclamptic women, uric acid damages the placenta vascular structure and enters the smooth muscle via an organic ion transporter then it activates the intracellular mitogen‐activated protein (p38) and nuclear transcription factors (NFK‐B). This stimulates overproduction and expression of platelet‐derived growth factors, thromboxane, angiotensin II, C‐reactive protein, and pro‐inflammatory cytokines resulting in the loss of the endothelial cell structure and function. 13 , 14
Acute‐phase proteins (APPs) are used as diagnostic markers and C‐reactive proteins are observed to increase more than 50% in APPS. 15 High sensitivity C‐reactive protein (hs‐CRP) is an acute‐phase reactant protein produced in the liver by stimulation of interleukin‐6 (IL‐6) and can be a marker for tissue damage and systematic inflammation. An increased level of inflammatory markers including IL‐6, tumor necrosis factor‐α (TNF‐α), and C‐reactive proteins play a key role in vascular inflammation in hypertensive patients in the early and advanced stages. 16 C‐reactive protein activates inflammatory immune cells like monocytes and macrophages, which, in turn, promotes low‐density lipoprotein (LDL) scavenging to form foam cells in the intima of endothelial cells. Moreover, C‐reactive protein lowers the production of vasodilating substances including nitric oxide and activates vascular smooth muscle cells to become highly proliferative. 17
PIH is often diagnosed in late pregnancy when few clinical manifestations such as high blood pressure, urinary protein, and edema are presented. 4 Early detection and intervention reduce the hypertensive crisis of the mother and fetal complications, 18 which is possible through an understanding of its risk factors, pathological process, and clinical presentations. A study done by Parmar et al. 1 has proved that metabolic modification and biochemical alterations occur in the early phase of pregnancy or precede clinical manifestations of PIH.
Assessing metabolic abnormalities and inflammatory markers for pregnant women starting from the early phase of antenatal care (ANC) services suggested that women are at risk making the assessment and potential intervention challenging. 3 For better PH management and to prevent its complication, early detection of the case is the most crucial approach. Assessing these biomarkers for pregnant women during ANC service follow‐up is helpful for the early detection of diseases and to take timely intervention for hypertensive disorders that complicate pregnancy. Therefore, to overcome the challenges of PIH and its complications, and most importantly to take our part in support of Ethiopia's campaign “No Woman or Child should Die of Pregnancy,” we evaluate serum lipid profile, uric acid, and hs‐CRP levels among women with PIH compared to NTP women who attended Ambo University Referral Hospital, Ambo, Ethiopia.
2. METHODS
2.1. Study design, areas, and periods
A hospital‐based case‐control study was conducted from August 2020 to May 2021 at Ambo University Referral Hospital (AURH), Ambo, Ethiopia.
2.2. Study population and sampling techniques
The study populations were women who attended ANC clinics at AURH during the study period. By using a convenient sampling technique, 140 eligible study participants (70 cases and 70 controls) who visited ANC clinics at AURH hospital during the data collection period were selected.
2.3. Inclusion criteria
PIH group (cases): Pregnant women diagnosed with HTN (BP≥ 140/90 mmHg) by an obstetrician.
Normotensive pregnant (NTP) group (control): Pregnant women diagnosed with no HTN (˂140/90 mmHg) by an obstetrician.
Both groups are age and gestation (20 weeks) matched.
2.4. Exclusion criteria
Based on the investigation information (patient medical history, clinical data, ultrasound, and laboratory investigations), we excluded pregnant women before 20 gestational weeks and who were on antihypertensive drugs, khat chewing, smoking, and alcohol abuse. We also excluded pregnant women with a history of renal and CVDs, gestational diabetes, gout, obesity, systemic lupus erythematosus, dyslipidemia, rheumatoid arthritis, thyroid disorders, multiple pregnancies, cervical and breast cancers, asthma, and infection (like hepatitis B virus [HBV], human immunodeficiency virus [HIV], and Urinary tract infection [UTI]).
2.5. Sample size
The sample size was calculated using the double population formula by considering the mean ± standard deviation (SD) of serum high‐density lipoprotein‐cholesterol (HDL‐C). While it was 39.68 ± 7.5 mg/dl for the cases, it was 43.72 ± 7.35 mg/dl for healthy controls. We took 95% of a confidence interval, 80% of power, 1:1 ratio, and the critical value, α = 5% (α = 0.05). About 10% of the nonrespondents rate was considered. Using the G*Power statistical software for Windows (version 3.1.2.9), the sample size was calculated as 122. Having added a 15% nonrespondent rate, the final sample size was 140, in which we enrolled each 70 PIH and 70 NTP women.
2.6. Variables
Lipid profiles and their ratios, uric acid, and hs‐CRP were the dependent variables whereas gestational age, age, gravidity, parity, family history of HTN, history of multipartners, and blood pressure were the independent variables.
3. MEASUREMENTS AND DATA COLLECTION
3.1. Data collection procedures
Written informed consent was obtained from the study participants involved in this study. General practitioners and obstetricians have checked the detailed medical history of the study participants (whether they have renal and CVDs, gout, known dyslipidemia, DM, rheumatoid arthritis, and infection like HBV, HIV, and UTI) and did physical examinations as well. Further, laboratory and ultrasound investigations were used to confirm the above medical complications and if found, were excluded from the study. The information related to obstetrics and medical status was obtained by data collectors using a pretested semistructured questionnaire through face‐to‐face interviews. Gestational age was estimated by ultrasound.
3.2. Blood pressure measurement
The blood pressure was measured with a mercury sphygmomanometer. After the study, participants were allowed to rest for about 5 min, and a cuff of an Accoson mercury sphygmomanometer was applied around the upper left arm at the level of the heart. The systolic blood pressure was accepted as the first sound heard (Korotkoff sound 1) and the diastolic blood pressure was the disappearance of sounds completely (korotkoff sound 5). 19 Elevated blood pressure was repeated after at least 4 h to take the average. The cut‐off points for elevated systolic and diastolic blood pressure were equal and above 140 and 90 mmHg, respectively. 20
3.3. Blood sample collection, processing, and analysis
About 3–5 milliliter (ml) volume of blood was withdrawn from the antecubital vein by well‐experienced and trained medical laboratory technologists who followed aseptic techniques.
The collected blood sample was transferred into a serum separator tube (SST) and allowed to keep for 30 min to form complete clotting and clot retraction. The serum sample was separated by centrifugation at 4000 rpm for 5 min by centrifuge Humax 4K (Human; 2017). The serum sample was stored in a refrigerator 0593 (Techno Diagnostics; 2017) at −20°C temperature until the day of use. Meanwhile, lipid profiles (triglyceride [TG], total cholesterol [TC], HDL‐C, and LDL‐C) and uric acid levels were measured by Cobas C‐311 analyzer for Clinical Chemistry (Roche, 2020), and serum hs‐CRP was analyzed by Fluorescence Immunoassay based Finecare™ FIA meter (Wondfo; 2018) at AURH Laboratory. Finally, the serum lipid profile ratios (TC/HDL‐C, TG/HDL‐C, and LDL‐C/HDL‐C) were calculated.
3.4. Interpretation of the results
The results for lipid profiles were interpreted by using the cut‐off value which is established by the National Cholesterol Education Program (TC < 200 mg/dl, TG < 150 mg/dl, LDL‐C < 130 mg/dl, and HDL‐C < 40 mg/dl) (NIH; 2001). The TC/HDL‐C ratio ˃ 5, 21 TG/HDL‐C ratio ˃ 4.5, and LDL‐C/HDL‐C ratio ˃ 3.5 22 were taken as baseline values for interpretation of the lipid profile ratios. Uric acid's normal reference value was 2.6–6 mg/dl and its ≥6 mg/dl was considered an abnormal value. 23 While hsCRP below 1 mg/L was considered normal, 1–3 mg/L was considered a moderate increase, and ˃3 mg/L was considered a high value. 24 Therefore, after the tests have been analyzed the results were interpreted based on the normal reference values established.
4. DATA QUALITY ASSURANCE
4.1. Data collection quality control measures
Data were collected using semistructured questionnaires. The training was provided to the data collectors on the study objectives, procedures, confidentiality, respondent's right, and informed consent. In addition, questionnaires were pretested on the hospital which was not selected for the study area and some modifications were made. The data collection process was supervised intensively.
4.2. Pre‐analytical phase
Participants were well‐prepared and the SST was coded with the collection date, a medical record number (MRN), and a unique identification number. After processing, the serum sample was stored at below −20°C under daily temperature monitoring.
4.3. Analytic phase
Laboratory analysis was done at AURH Laboratory, clinical chemistry section. According to the laboratory quality management system policy, calibration and daily maintenance were performed. Internal laboratory quality control was run to ensure the accuracy and reliability of laboratory testing before running patient samples. The control result was interpreted using a Levey‐Jennings chart. The control result fell within the acceptable ranges (mean ± 2 SD). After the sample was towed and mixed throughout, it was run by senior laboratory technologists.
4.4. Post analytical phase
The printed results were checked for the unit of reporting, the correctness of the MRN, and the unique identification card number. Then, the printed out or recorded (in the absence of printer paper) results were approved by the laboratory quality officer. Lipid profile ratios were calculated and recorded in the resulting form provided.
4.5. Data processing and analysis
The data were coded and entered into Epi‐data statistical software (version 3.1, 2008), and then it was exported to SPSS software (version 25.0, 2013) for analysis. While the difference between the two groups was compared using the independent student's two‐tailed t‐test, the difference between categorical variables between the two groups was compared using the χ 2 for independence and Fisher's exact t‐test. The percentage (%) distribution was done for both continuous and categorical variables. Then the results were presented in tables and figures. The “p < 0.05” at a 95% of confidence level was considered statistically significant.
5. RESULTS
5.1. General characteristics of the study participants
One hundred and forty (70 of each with PIH and NTP) pregnant women, ages between 18 and 39 years old, participated in this study. The age (mean ± SD) of women with PIH and NTP was found 27.56 ± 5.25 and 26.73 ± 5.23 years, respectively. Also, the majority of women with PIH (82.9%) and NTP (87.2%) were between the ages of 19 and 35 years. About 82.9% and 91.4% of PIH and NTP women, respectively, were preterm and 17.1% and 8.6%, respectively, of them, were term. The mean gestational age distribution (in weeks) was 34.23 ± 4.25 for women with PIH and 30.66 ± 4.78 for women with NTP. The mean age distribution difference between the groups was not statistically significant, but the mean difference in gestational age (in weeks) between groups was statistically significant. The SBP and DBP of women with PIH were 151.86 ± 10.20 and 101.67 ± 7.67 mmHg, respectively, whereas the SBP and DBP of the NTP women were 106.97 ± 6.35 and 78.79 ± 7.58 mmHg, respectively. In both cases, the data distribution was shown statistical significance (“p < 0.05”; Table 1).
Table 1.
General characteristics of study participants in both PIH and NTP women
| Variables | PIH (n = 70) | NTP (n = 70) | T value | p Value (two‐tailed) | 95% CI |
|---|---|---|---|---|---|
| Mean Age (years) | 27.56 ± 5.23 | 26.73 ± 4.94 | −0.979 | 0.329 | −2.54, 0.859 |
| Mean GA (weeks) | 34.23 ± 4.25 | 30.66 + 4.78 | −4.669 | 0.000* | −5.08, −2.06 |
| SBP (mmHg) | 151.86 ± 10.20 | 106.97 ± 6.35 | −31.269 | 0.000* | −47.73, −42.04 |
| DBP (mmHg) | 101.67 ± 7.67 | 78.79 ± 7.58 | −22.285 | 0.000* | −26.07, −21.81 |
Abbreviations: CI, confidence interval; DBP, diastolic blood pressure; mmHg, millimeter mercury; NTP, normotensive pregnant; PIH, pregnancy‐induced hypertension; SBP, systolic blood pressure.
*p < 0.001.
5.2. Comparison of obstetric history between PIH and NTP women
About 37.1% of women with PIH were nulliparous, 25.7% were primipara and the remaining 37.1% were multipara. In contrast, 40% of NTP women were nulliparous, 27.1% were primipara, and 32.9% were multipara. Gravidity status of women with PIH versus NTP women was: primigravida 60% versus 44.3%, 55.7%, and multigravida 40% versus 55.7%, respectively. Only 5.7% of women with PIH had a history of multipartner and the remaining had not. Similarly, only 2.9% of the NTP women had a history of multipartner and 97.1% had not. The difference in parity, gravidity, and history of multipartner between the two groups were not statistically significant. Concerning family history, our study showed that only 8.6% of PIH and 2.9% of NTP women had a family history of high blood pressure (“p < 0.05”; Table 2).
Table 2.
Obstetric history distribution of PIH and NTP women
| Variables | Category | PIH (n = 70) (%) | NTP (n = 70) (%) | p Value (two‐tailed) | 95% CI |
|---|---|---|---|---|---|
| Parity | Nulliparous | 37.1 | 40.0 | 0.54 | 0.58, 2.85 |
| Primipara | 25.7 | 27.1 | |||
| Multipara | 37.1 | 32.9 | |||
| Gravidity | Primigravida | 60.0 | 44.3 | 0.27 | 0.27, 1.04 |
| Multigravida | 40.0 | 55.7 | |||
| History of multipartner | Yes | 5.70 | 2.90 | 0.36 | 0.07, 2.56 |
| No | 94.3 | 97.1 | |||
| Family history of high BP | Yes | 8.60 | 2.90 | 0.03* | 0.056, 1.57 |
| No | 91.4 | 90.0 | |||
| Do not know | 0.00 | 7.10 |
Abbreviations: BP, blood pressure; CI, confidence interval; NTP, normotensive pregnant; PIH, pregnancy‐induced hypertension.
*p < 0.001.
5.3. Biochemical parameters
5.3.1. Comparison of serum lipid profiles, uric acid, and hs‐CRP between PIH and NTP women
The current study result showed that the levels (mean ± SD) of TC, TG, LDL‐C, hs‐CRP, and uric acid were found significantly higher in PIH compared to NTP women (“p < 0.001”). However, the level (mean ± SD) of HDL‐C was found significantly lower in PIH compared to NTP (“p < 0.05”) as tabulated in Table 3.
Table 3.
Comparison of serum lipid profiles, uric acid, and hs‐CRP levels between PIH and NTP women
| Parameters (mg/dl) | Groups | Mean ± SD | Minimum | Maximum | Range | p Value (two‐tailed) | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| TC | PIH | 184.80 ± 54.07 | 105.0 | 453.7 | 348.7 | 0.000* | −47.40, −16.13 | |
| NTP | 153.03 ± 38.12 | 78.40 | 245.0 | 166.6 | ||||
| TG | PIH | 236.25 ± 71.76 | 108.5 | 413.00 | 304.5 | 0.000* | −80.51, −32.60 | |
| NTP | 179.68 ± 71.57 | 80.50 | 389.5 | 309.0 | ||||
| HDL‐C | PIH | 35.52 ± 11.53 | 12.00 | 61.20 | 49.20 | 0.000* | 2.72, 9.44 | |
| NTP | 41.60 ± 8.29 | 26.00 | 65.00 | 39.00 | ||||
| LDL‐C | PIH | 130.20 ± 50.31 | 58.80 | 300.0 | 241.2 | 0.000* | −59.06, −31.45 | |
| NTP | 84.94 ± 29.46 | 19.60 | 171.0 | 151.4 | ||||
| Uric Acid | PIH | 6.65 ± 1.97 | 2.900 | 11.90 | 9.000 | 0.000* | −3.40, −2.30 | |
| NTP | 3.80 ± 1.20 | 1.900 | 8.600 | 6.700 | ||||
| hs‐CRP (mg/L) | PIH | 12.25 ± 14.12 | 0.400 | 70.90 | 70.50 | 0.000* | −14.47, −7.71 | |
| NTP | 1.16 ± 1.36 | 0.100 | 7.200 | 7.100 | ||||
Abbreviations: CI, confidence interval; HDL‐C, high‐density lipoprotein‐cholesterol; hs‐CRP, high sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein‐cholesterol; mg/L, milligram per liter; mg/dl, milligram per deciliter; NTP, normotensive pregnant; PIH, pregnancy‐induced hypertension; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; VLDL‐C, very‐low‐density lipoprotein‐cholesterol.
*p < 0.001.
5.3.2. Categorical values of serum lipid profiles and uric acid between PIH and NTP women as compared to the baseline value
Having compared PIH versus NTP, we found that whereas TC (31.% vs. 14.3%), TG (88.6% vs. 57.1%), LDL‐C (37.1% vs. 32.9%), HDL‐C (67.1% vs. 42.9%), and uric acid (75.7% vs. 7.1%) were found above the baseline values (Figure 1).
Figure 1.
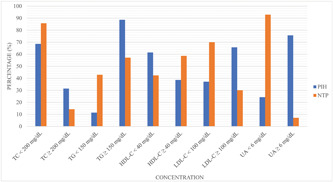
Categorical values of serum lipid profiles and uric acid between PIH and NTP groups. HDL‐C, high‐density lipoprotein‐cholesterol; LDL‐C, low‐density lipoprotein‐cholesterol; mg/dl, milligram per deciliter; NTP, normotensive pregnant; PIH, pregnancy‐induced hypertension; TC, Total cholesterol; TG, triglyceride; UA, uric acid.
5.3.3. Comparison of serum lipid profile ratios between PIH and NTP women
The ratio of lipid profile is vital in predicting cardiovascular risk. These ratios reflect the proportion of atherogenic to anti‐atherogenic lipids and lipoproteins. The ratio of TC/HDL‐C, TG/HDL‐C, and LDL‐C/HDL‐C values were found significantly higher in women with PIH compared to NTP women (“p < 0.001”; Table 4).
Table 4.
Comparison of serum lipid profile ratios between PIH and NTP women groups
| Parameters | Groups | Mean ± SD | Minimum | Maximum | Range | p Value (two‐tailed) | 95% CI |
|---|---|---|---|---|---|---|---|
| TC/HDL‐C | PIH | 5.92 ± 3.13 | 2.60 | 20.0 | 17.4 | 0.000* | −2.77, −1.21 |
| NTP | 3.93 ± 1.03 | 1.20 | 7.80 | 5.60 | |||
| TG/HDL‐C | PIH | 7.43 ± 3.79 | 2.30 | 20.0 | 17.7 | 0.000* | −4.06, −2.06 |
| NTP | 4.37 ± 1.84 | 1.26 | 9.00 | 7.74 | |||
| LDL‐C/HDL‐C | PIH | 4.37 ± 2.53 | 1.10 | 13.7 | 12.6 | 0.000* | −2.45, −1.15 |
| NTP | 2.57 ± 1.08 | 0.50 | 6.10 | 5.60 |
Abbreviations: CI, confidence interval; LDL‐C/HDL‐C, the ratio of low‐density lipoprotein‐cholesterol to high‐density lipoprotein‐cholesterol; NTP, normotensive pregnant; PIH, pregnancy‐induced hypertension; TC/HDL‐C, the ratio of total cholesterol to high‐density lipoprotein‐cholesterol; TG/HDL‐C, the ratio of triglyceride to high‐density lipoprotein‐cholesterol.
* p < 0.001.
5.3.4. Categorical values of lipid profile ratios for both PIH and NTP women as compared to the baseline value
For PIH cases, the ratios were found higher than the baseline: TC/HDL‐C by 51.4%; TG/HLD‐C by 74.3%; and LDL‐C/HDL‐C by 67.1%. On the other hand, for the NTP cases, the ratios were found higher than the baseline: TC/HDL‐C by 11.4%; TG/HDL‐C by 29%; and LDL‐C/HDL‐C by 28.3%. For each group, the remaining study participants were below the baseline value for the listed parameters.
5.3.5. Categorical value of serum hs‐CRP for both PIH and NTP women as compared to the baseline value
The levels of hs‐CRP were classified as low, moderate, and high risk to evaluate the inflammatory status of the study participants. Among PIH women in this study, 11.4%, 35.7%, and 52.9% fall in <1 mg/dL, 1–3 mg/dL, and >3 mg/L levels of hs‐CRP, respectively. The NTP women showed that 72.9%, 14.3%, and 12.9% were found in <1, 1–3, and >3 mg/L levels of hs‐CRP, respectively (Figure 2).
Figure 2.
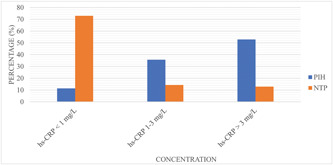
Categorical levels of serum hs‐CRP between PIH and NTP women group. hs‐CRP, high sensitivity C‐reactive protein; mg/L, milligram per liter; NTP, normotensive pregnant; PIH, pregnancy‐induced hypertension.
6. DISCUSSION
PIH is a common complication of pregnancy that contributes to maternal and perinatal morbidity and mortality. 10 Various risk factors are known in the event of PIH but etiology and pathophysiology are not yet fully understood. Abnormal placental implantation and immunologic intolerance between maternal, placenta, and fetus are some famed etiologic factors. 25 Dyslipidemia adversely affects the functional and structural walls of arteries and promotes atherosclerosis. This change impairs blood pressure regulation, which, in turn, leads to HTN and CVD events. 26 , 27
The key findings of the current study are that the levels of serum TC, TG, LDL‐C, UA, and hs‐CRP were significantly increased and HDL‐C was significantly decreased in women who were diagnosed with PIH compared to NTP women which, in both cases, are known to be associated with major risk factors for HTN. 28 Similarly, an increased level of uric acid (hyperuricemia) damages placenta vascular structures and enters smooth muscles cells, then it activates gene expression of platelet‐derived growth factors, pro‐inflammatory cytokines, and vasoconstrictor (thromboxane) that results in loss of the endothelial cell structure and function. 13 , 14 Because pre‐eclampsia is characterized by an intensive inflammation response, an elevated level of CRP and cytokines including IL‐6 and TNF‐α role a major play in causing vascular endothelial cell damage. 28
During normal pregnancy, lipid metabolism is under hormonal control. 29 Maternal estrogen hormone 29 and hyperinsulinemia 27 modulate this metabolic adaption. However, in HTN conditions, levels of estrogen decline and insulin resistance onset lead to an increase in several folds of blood lipids and can become atherogenic dyslipidemia in women with PIH. 27 This change in lipid metabolism in pre‐eclampsia causes endothelial dysfunction that reduces vasodilator molecules and rises vasoconstriction molecules as well as will increase pro‐inflammatory molecules and oxidative stress. 30
In this study, TC was significantly elevated in women with PIH compared to NTP women. About 31.4% of women with PIH were found above the baseline value. This finding is in agreement with the study done by a few researchers. 31 , 32 , 33 This may associate with insulin resistance in HTN disorder in pregnancy that suppresses lipoprotein lipase activity and will increase free fatty acid mobilization from visceral adipocytes. 34 This could highlight hypercholesterolemia in HTN disorders during pregnancy.
The mean value of TG level was significantly higher in women with PIH than in NTP women. About 88.6% of women with PIH have shown that serum TG concentration levels are above the baseline value. This result is in line with the previous study. 6 , 35 The elevation of serum TG may be due to a decrease in hepatic lipase activity responsible for the endogenous biosynthesis of TG and diminished lipoprotein lipase activity that keeps TG in adipocyte tissue. 27 This contributes to an endothelial pathology directly predisposed in uterine vessels or indirectly via generating small size dense LDL‐C (sLDL‐C) and/or it should be related to hypercoagulability. 6
Nearly 65.7% of women with PIH have shown higher LDL‐C above the baseline value and the mean value of LDL‐C was found to be higher in PIH when compared to NTP women. This study is in agreement with the reports. 6 , 33 , 36 LDL is principally synthesized within the liver and its main function is providing cholesterol to peripheral tissues. sLDL particles are more atherogenic because they have an increased ability to infiltrate tissues. 37 The sLDL‐C is more vulnerable to oxidation than the traditional LDL‐C. Its oxidation due to oxidative stress in endothelial cells causes a reduction of prostacyclin to thromboxane A‐2 ratio and scales to back other vasodilator molecules. Additionally, it upregulates pro‐inflammatory cytokines and intracellular vascular adhesion molecules. Moreover, sLDL‐C peroxidation is involved in the foam cell formation in the intima of an endothelial cell. This results in an endothelial cell dysfunction that leads to HTN. 38
In this study, we have also evaluated whether serum HDL‐C is a risk factor for PIH. The mean value of HDL‐C was significantly lower in women with PIH than in NTP women. When compared with the baseline value, around two per three (67.1%) of women in PIH had serum HDL‐C value below the baseline value. This finding is comparable to the studies done by Singh and colleagues. 36 , 39 Significantly lowered serum HDL‐C is because of the reverse impact of atherogenic lipoprotein (LDL‐C) and inflammatory burden. HDL‐C plays a vital role in protecting from endothelial cell damage by scavenging cholesterol discharged from macrophages. 40 Decreasing the HDL‐C concentration in women with PIH reduces the stimulation of nitric oxide (NO) that is resulting in placental endothelial cell dysfunction. Impairment of an endothelial cell could be a general features of pre‐eclampsia and eclampsia. The reduced level of HDL‐C is not only due to hypoestrogenemia but also due to insulin resistance. 27
The lipid profile ratios which reflect the balance between the risks and protecting lipoprotein capability have been calculated. These ratios indicate the proportion of atherogenic to anti‐atherogenic lipids. 41 In our study, the ratios of TC/HDL‐C, TG/HDL‐C, and LDL‐C/HDL‐C were significantly higher in PIH than in NTP women. Meanwhile, more than half (51.4%) of PIH women have shown a TC/HDL‐C value above the cut‐off point, and about 67% of them have shown LDL‐C/HDL‐C value above the cut‐off point. This result is in agreement with the study of Priyanka and colleagues. 33 , 42 The study done by Sniderman et al., indicated that the ratios of TC/HDL‐C and LDL‐C/HDL‐C can be used as a biomarker to predict future cardiovascular risk and as a prognostic biomarker to assess the severity of the case. Therefore, lipid ratios are better predictors of cardiovascular risk assessment than isolated lipid parameters. 43
In the present study, 75.7% of PIH women have been shown serum uric acid levels above the cut‐off point. This indicates a significant difference between women with PIH and NTP women. This finding supports previous studies. 44 , 45 An experimental study suggests that raising uric acid impairs the renin‐angiotensin system and induces oxidative stress via reducing vasodilators (nitric oxide) molecules. This links hyperuricemia with the pathological process of HTN. 46
Placental/maternal tissue ischemia activates xanthine oxidase which results in the overproduction of uric acid with free radicals including superoxide (O2 −) and is considered a contributor to oxidative stress. 47 In the reduced state of ant‐oxidants (vitamin C), an increased level of uric acid becomes pro‐oxidant (urate radicals). This hyperuricemia activates the immune cells to release pro‐inflammatory cytokines and chemo‐attractant molecules that play a role in endothelial cell dysfunction. 48 A previous study on pre‐eclampsia has indicated that uric acid damages placenta tube structures and it enters smooth muscles resulting in the production of vasoconstrictor molecules, platelet‐derived growth factors and inflammatory marker (C‐reactive protein) leads to functional and structural loss of endothelial cells. 14 The elevation of uric acid can precede clinical manifestations in PIH by several weeks. Thus, its evaluation is a better indicator of the onset of pre‐eclampsia and predicts the development of eclampsia. 49
The level of the known inflammatory marker, hs‐CRP, was significantly higher in women with PIH compared to NTP women and comparable results were reported by Deveci et al. 50 The elevation of hs‐CRP in women with PIH reveals the presence of systemic inflammation. An experimental study result has shown that a significantly higher hs‐CRP level has been associated with PIH women compared to NTP women and it has also been highly pronounced with aggravation of PIH. 51 A study done by Veerbeek et al. indicated that immune imbalance and cytokines, particularly inflammatory factors, play a vital role in immune regulation as they are associated with PIH. The extent of hs‐CRP directly reflects the state of the body's inflammatory response. 51 With the severity of pre‐eclampsia, hs‐CRP levels in peripheral blood will increase. Therefore, assessing serum hs‐CRP will be used as a biomarker to monitor hypertensive disorder during pregnancy. 52 Inflammation shares a pathophysiologic role in PIH. Therefore, as a component of inflammatory mediators, an elevated level of C‐reactive protein raises blood pressure by lowering nitric oxide and increasing endothelin‐1 production inflicting constriction that, in turn, is causing vascular endothelial cell damage. 53
Therefore, based on our result, we deduce that antenatal screening for serum lipids levels, uric acid, and hs‐CRP is indispensable for the early detection and monitoring of the disease activity of women with PIH. To our best knowledge, this is the first‐ever study in Ethiopia that assessed the lipids profile and markers of inflammation between pregnant women with PIH and NTP women. This may fill the gap in seeking potential biomarkers for possible intervention.
7. STRENGTHS AND LIMITATIONS OF THE STUDY
The comprehensive evaluations of serum lipid profile, uric acid, and hs‐CRP in women PIH compared to NTP women were attempted for the first time in Ethiopia. Even though the data collection period overlapped with the outbreak of the COVID‐19 pandemic, we managed to overcome the challenge of this pandemic by strict adherence to the recommended preventive measures to protect ourselves and study participants during sample collection and laboratory works. We also recommend a multibiomarker approach for early diagnosis and intervention. Though the above‐mentioned strengths, the study also has limitations, such as a small sample size and a lack of assessing dietary habits, which the latter is associated with dyslipidemia.
7.1. Recommendation
The findings obtained from this study have shown that raising serum lipid profiles (except lowering HDL‐C), uric acid, and hs‐CRP were associated with PIH. Early evaluation of these biomarkers for pregnant women starting from the first visit during ANC services could help for the early detection of PIH cases or estimate whether they are at risk or not. We strongly recommend that early screening of dyslipidemia, hyperuricemia, and inflammation for pregnant women should be given attention to prevent PIH and its future complications. It is better to implement an assessment of the serum lipid profile, uric acid, and hs‐CRP as a component of routine laboratory tests during ANC service in this study area in particular and in Ethiopia in general for a better pregnancy outcome.
8. CONCLUSION
Serum TC, TG, LDL‐C, TC/HDL‐C, TG/HDL‐C, and LDL‐C/HDL‐C were significantly elevated and HDL‐C was decreased in PIH than in NTP women (p < 0.0001). Moreover, our findings confirmed that hyperuricemia indicator (uric acid), and a potential inflammatory marker (hs‐CRP) were highly pronounced in women with PIH compared to NTP women (p < 0.0001). Therefore, in addition to being potential biomarkers to understand the pathophysiology of the PIH, these biomarkers will be used as potential diagnostic/screening biomarkers in early detection and timely intervention of PIH to reduce the risk of women during their pregnancy. We also believe this study benefits clinicians, researchers, and policy‐makers who are striving to protect against death and morbidity due to PIH worldwide, particularly in Ethiopia. Finally, the outcome of this study provides an insight for those at the forefront of the mission of “No Woman or Child should Die of Pregnancy in Ethiopia.”
AUTHOR CONTRIBUTIONS
Bilisuma G. Areda: Conceptualization; data curation; formal analysis; methodology; writing—original draft; writing—review & editing. Solomon T. Gizaw: Conceptualization; data curation; formal analysis; methodology; supervision; writing—original draft; writing—review & editing. Delesa H. Berdida: Data curation; formal analysis; methodology. Abbul H. Kebalo: Writing—original draft; writing—review & editing. All authors have read and approved the final draft of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The proposal was approved by the Department Research and Ethics Review Committee (number SOM/BCHM/091/2021) and the Institute Review Committee (IRB) of Addis Ababa University. The official letter (SOM/BCHM/102/2020) was written by the university to the AURH administrator and the hospital granted permission for sample collection. The study was performed by ethical standards of the Declaration of Helsinki and informed consent was obtained from all patients. The results of the study were communicated to the responsible bodies for any beneficiary or corrective measures. Written informed consent for data collection and publication was already obtained (Supporting Information).
TRANSPARENCY STATEMENT
Solomon T. Gizaw (PhD) affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
We acknowledge the staff of AURH, the Department of Medical Biochemistry of AAU ethical review committee for the ethical clearance, and AAU for the financial support. The research budget is partly funded by Addis Ababa University.
Areda BG, Gizaw ST, Berdida DH, Kebalo AH. Evaluation of serum lipid profiles, uric acid, and high sensitivity C‐reactive protein levels between pregnancy‐induced hypertension and normotensive pregnant women attending Ambo University Referral Hospital, Ambo, Ethiopia, 2020: a case‐control study. Health Sci Rep. 2022;5:e806. 10.1002/hsr2.806
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author (solomon.tebeje@aau.edu.et). The data are not publicly available due to ethical restrictions.
REFERENCES
- 1. Parmar MT, Solanki HM, Gosalia VV. Study of risk factors of perinatal death in pregnancy induced hypertension (PIH). Natl J Community Med. 2012;3(04):703‐707. [Google Scholar]
- 2. Kacica M, Dennison B, Aubrey R. Hypertensive Disorders in Pregnancy guideline summary. New York State Department of Health; 2013. [Google Scholar]
- 3. American College of Gynecologists . Task force on hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' task force on hypertension in pregnancy. Obstet Gynecol. 2013;122(5):1122‐1131. [DOI] [PubMed] [Google Scholar]
- 4. National Collaborating Centre for Women's and Children's Health (UK) . Hypertension in pregnancy: the management of hypertensive disorders during pregnancy. [PubMed]
- 5. World Health Organization . WHO recommendations for prevention and treatment of pre‐eclampsia and eclampsia. 2011. [PubMed]
- 6. Latha P, Ganesan S. Evaluation of serum uric acid and lipid profile in gestational hypertension. Int J Pharm Bio Sci. 2013;4:496‐502. [Google Scholar]
- 7. Payne BA, Hutcheon JA, Ansermino JM, et al. A risk prediction model for the assessment and triage of women with hypertensive disorders of pregnancy in low‐resourced settings: the miniPIERS (pre‐eclampsia integrated estimate of RiSk) multi‐country prospective cohort study. PLoS Med. 2014;11(1):1001589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grimes SB, Wild R. Effect of pregnancy on lipid metabolism and lipoprotein levels. Endotext [Internet]. 2018.
- 9. Mitka M. Any hypertension during pregnancy raises risk for several chronic diseases. JAMA. 2013;309(10):971‐972. [DOI] [PubMed] [Google Scholar]
- 10. Kintiraki E, Papakatsika S, Kotronis G, Goulis D, Kotsis V. Pregnancy‐induced hypertension. Hormones. 2015;14(2):211‐223. [DOI] [PubMed] [Google Scholar]
- 11. Männistö T, Mendola P, Vääräsmäki M, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127(6):681‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hawkins TL, Roberts JM, Mangos GJ, Davis GK, Roberts LM, Brown MA. Plasma uric acid remains a marker of poor outcome in hypertensive pregnancy: a retrospective cohort study. BJOG. 2012;119(4):484‐492. [DOI] [PubMed] [Google Scholar]
- 13. Al‐Jameil N, Aziz Khan F, Fareed Khan M, Tabassum H. A brief overview of preeclampsia. J Clin Med Res. 2014;6(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Masoura S, Makedou K, Theodoridis T, Kourtis A, Zepiridis L, Athanasiadis A. The involvement of uric acid in the pathogenesis of preeclampsia. Curr Hypertens Rev. 2015;11(2):110‐115. [DOI] [PubMed] [Google Scholar]
- 15. Jain S, Gautam V, Naseem S. Acute‐phase proteins: as diagnostic tool. J Pharm Bioallied Sci. 2011;3(1):118‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dawri S, Padwal M, Melinkeri R. Evaluation of high sensitivity C‐reactive protein and serum lipid profile in prehypertension and essential hypertension. Natl J Integr Res Med. 2014;5(1):1‐5. [Google Scholar]
- 17. Hage FG. C‐reactive protein and hypertension. J Hum Hypertens. 2014;28(7):410‐415. [DOI] [PubMed] [Google Scholar]
- 18. Tagetti A, Fava C. Diagnosis of hypertensive disorders in pregnancy: an update. J Lab Precis Med. 2020;5(8):8. [Google Scholar]
- 19. Lowe SA, Bowyer L, Lust K, et al. SOMANZ guidelines for the management of hypertensive disorders of pregnancy 2014. Aust N Z J Obstet Gynaecol. 2015;55(5):1‐29. [DOI] [PubMed] [Google Scholar]
- 20. James PA, Oparil S, Carter BL, et al. Evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507‐520. [DOI] [PubMed] [Google Scholar]
- 21. Emerson JF. Desirable components of a screening lipid profile. Lab Med. 2003;34(6):476‐480. [Google Scholar]
- 22. Salazar MR, Carbajal HA, Espeche WG, et al. Relation among the plasma triglyceride/high‐density lipoprotein cholesterol concentration ratio, insulin resistance, and associated cardio‐metabolic risk factors in men and women. Am J Cardiol. 2012;109(12):1749‐1753. [DOI] [PubMed] [Google Scholar]
- 23. Desideri G, Castaldo G, Lombardi A, et al. Is it time to revise the normal range of serum uric acid levels? Eur Rev Med Pharmacol Sci. 2014;18(9):1295‐1306. [PubMed] [Google Scholar]
- 24. Mazurek K, Zmijewski P, Czajkowska A, Lutosławska G. High‐sensitivity C‐reactive protein (hsCRP) in young adults: relation to aerobic capacity, physical activity and risk factors for cardiovascular diseases. Biol Sport. 2011;28(4):227‐232. [Google Scholar]
- 25. Sava RI, March KL, Pepine CJ. Hypertension in pregnancy: taking cues from pathophysiology for clinical practice. Clin Cardiol. 2018;41(2):220‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Otsuka T, Takada H, Nishiyama Y, et al. Dyslipidemia and the risk of developing hypertension in a working‐age male population. J Am Heart Assoc. 2016;5(3):003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wild R, Weedin EA, Wilson D. Dyslipidemia in pregnancy. Cardiol Clin. 2015;33(2):209‐215. [DOI] [PubMed] [Google Scholar]
- 28. Farzadnia M, Ayatollahi H, Hasan‐Zade M, Rahimi HR. A comparative study of serum level of vascular cell adhesion molecule‐1 (sVCAM‐1), intercellular adhesion molecule‐1 (ICAM‐1) and high sensitive C‐reactive protein (hs‐CRP) in normal and pre‐eclamptic pregnancies. Iran J Basic Med Sci. 2013;16(5):689‐693. [PMC free article] [PubMed] [Google Scholar]
- 29. Paradisi G, Ianniello F, Tomei C, et al. Longitudinal changes of adiponectin, carbohydrate and lipid metabolism in pregnant women at high risk for gestational diabetes. Gynecol Endocrinol. 2010;26(7):539‐545. [DOI] [PubMed] [Google Scholar]
- 30. Mcelwain CJ, Tuboly E, Mccarthy FP, McCarthy CM. Mechanisms of endothelial dysfunction in pre‐eclampsia and gestational diabetes mellitus: windows into future cardio‐metabolic health? Front Endocrinol (Lausanne). 2020;11:655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thathagari V, Veerendra KC. Evaluation of serum lipids in preeclampsia: a comparative study. Int J Reprod Contracept Obstet Gynecol. 2018;7(4):1372‐1376. [Google Scholar]
- 32. Gohil JT, Patel PK, Gupta P. Estimation of lipid profile in subjects of preeclampsia. J Obstet Gynaecol India. 2011;61(4):399‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Priyanka BP, Vijayasree AP, Devraj JP, Santosh Kumar SK, Mummadi MK, Boiroju NK. A study of serum lipid profile in normal pregnancy and pregnancy induced hypertensive disorders: a case‐control study. Int J Reprod Contracept Obstet Gynecol. 2019;8(5):2071‐2076. [Google Scholar]
- 34. Ghooshchi G, Masoomian M, Sarafraz Yazdi M, et al. Evaluation of the lipid profile of hypertensive patients compared to non‐hypertensive individuals. J Patient Saf Qual Improv. 2014;2(3):120‐122. [Google Scholar]
- 35. Rizal S, Joshi BR, Dhakal A, Sagtani RA. Association of serum uric acid and serum lipid profile in pre‐eclampsia—a hospital based case‐control study. Birat J Health Sci. 2019;4(3):831‐834. [Google Scholar]
- 36. Singh DU. Serum lipid profile in early pregnancy as a predictor of preeclampsia. Int J Med Res Rev. 2013;1(2):56‐62. [Google Scholar]
- 37. Jairam V, Uchida K, Narayanaswami V. Pathophysiology of lipoprotein oxidation. In: Kostner G, Frank S, eds., eds.Lipoproteins: role in health and diseases. InTech;;2012:383‐408. [Google Scholar]
- 38. Harvey RA, Ferrier DR. Globular Proteins. Biochemistry. 5th ed. Lippincott Williams and Wilkins, a Walters Kluwer business Co.; 2011:33‐34. [Google Scholar]
- 39. Rathore V, Priyadarshini TD, Rathore MS, et al. A cross sectional study to assess serum lipid profile among pregnant women suffering with pregnancy induced hypertension. Sch. J. App. Med. Sci. 2016;4(6D):2095‐2101. [Google Scholar]
- 40. Feingold KR, Grunfeld C. Introduction to lipids and lipoproteins. Endotext [Internet]. MDText. Com, Inc. 2018.
- 41. Millán J, Pintó X, Muñoz A, et al. Lipoprotein ratios: physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag. 2009;5:757. [PMC free article] [PubMed] [Google Scholar]
- 42. Sridhara SK. A comparative analysis of serum lipid profile between normotensive and hypertensive pregnant women. Int J Reprod Contracept Obstet Gynecol. 2019;8(5):2060‐2065. [Google Scholar]
- 43. Sniderman AD, Williams K, Contois JH, et al. A meta‐analysis of low‐density lipoprotein cholesterol, non‐high‐density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4(3):337‐345. [DOI] [PubMed] [Google Scholar]
- 44. Agarwal V, Gupta BK, Vishnu A, et al. Association of lipid profile and uric acid with pre‐eclampsia of third trimester in nullipara women. J Clin Diagn Res. 2014;8(7):CC04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sarmah J. A comparative study of serum uric acid in gestational hypertension, preeclampsia and normal pregnancy. IOSR J Dent Med Sci. 2015;14(8):4‐6. [Google Scholar]
- 46. Feig DI, Madero M, Jalal DI, Sanchez‐Lozada LG, Johnson RJ. Uric acid and the origins of hypertension. J Pediatr. 2013;162(5):896‐902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Santillan AA, Garduño JC, De Leon Ponce MA. Uric acid in pregnancy: new concepts. Contrib Nephrol. 2018;192:110‐115. [DOI] [PubMed] [Google Scholar]
- 48. Mishra PK, Yadav MK, Yadav KP, et al. Evaluation of serum uric acid and lipid profile in pre‐eclamptic women: a hospital based study. J Med Sci and Clin Res. 2016;4:11314‐11320. [Google Scholar]
- 49. Sahijwani D, Desai A, Oza H, et al. Serum uric acid as a prognostic marker of pregnancy induced hypertension. J South Asian Fed Obstet Gynaecol. 2012;4(3):130‐133. [Google Scholar]
- 50. Deveci K, Sogut E, Evliyaoglu O, Duras N. Pregnancy‐associated plasma protein‐A and C‐reactive protein levels in pre‐eclamptic and normotensive pregnant women at third trimester. J Obstet Gynaecol Res. 2009;35(1):94‐98. [DOI] [PubMed] [Google Scholar]
- 51. Veerbeek JHW, Hermes W, Breimer AY, et al. Cardiovascular disease risk factors after early‐onset preeclampsia, late‐onset preeclampsia, and pregnancy‐induced hypertension. Hypertension. 2015;65(3):600‐606. [DOI] [PubMed] [Google Scholar]
- 52. Kong D, Wang H, Liu Y, Li H, Wang H, Zhu P. Correlation between the expression of inflammatory cytokines IL‐6, TNF‐α and hs‐CRP and unfavorable fetal outcomes in patients with pregnancy‐induced hypertension. Exp Ther Med. 2018;16(3):1982‐1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Harmon AC, Cornelius DC, Amaral LM, et al. The role of inflammation in the pathology of preeclampsia. Clin Sci. 2016;130(6):409‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author (solomon.tebeje@aau.edu.et). The data are not publicly available due to ethical restrictions.


