Abstract
High‐frequency oscillations (HFO) in scalp EEG are a new and promising noninvasive epilepsy biomarker, providing added prognostic value, particularly in pediatric lesional epilepsy. However, it is unclear if lesion characteristics, such as lesion volume, depth, type, and localization, impact scalp HFO rates. We analyzed scalp EEG from 13 children and adolescents with focal epilepsy associated with focal cortical dysplasia (FCD), low‐grade tumors, or hippocampal sclerosis. We applied a validated automated detector to determine HFO rates in bipolar channels. We identified the lesion characteristics in MRI. Larger lesions defined by MRI volumetric analysis corresponded to higher cumulative scalp HFO rates (P = .01) that were detectable in a higher number of channels (P = .05). Both superficial and deep lesions generated HFO detectable in the scalp EEG. Lesion type (FCD vs tumor) and lobar localization (temporal vs extratemporal) did not affect scalp HFO rates in our study. Our observations support that all lesions may generate HFO detectable in scalp EEG, irrespective of their characteristics, whereas larger epileptogenic lesions generate higher scalp HFO rates over larger areas that are thus more accessible to detection. Our study provides crucial insight into scalp HFO detectability in pediatric lesional epilepsy, facilitating their implementation as an epilepsy biomarker in a clinical setting.
Keywords: children, focal epilepsy, HFO, high frequency oscillations, lesion volume, scalp EEG
1. INTRODUCTION
In pediatric epilepsy, a focal brain lesion, commonly a focal cortical dysplasia or a glioneuronal tumor, strongly correlates with anti‐seizure drug (ASD) failure. 1 , 2 For children with drug‐resistant focal lesional epilepsy, epilepsy surgery is the treatment of choice, 3 achieving seizure freedom in two‐thirds of cases. 4 , 5 , 6 , 7 Following epilepsy onset, prediction of drug resistance in focal lesional epilepsy is crucial for timely referral for presurgical evaluation (and, potentially, epilepsy surgery) since early intervention may prevent cognitive decline, particularly in young children. 4 Following epilepsy surgery, the prediction of seizure freedom is crucial for the timely initiation of ASD withdrawal as ASD may negatively impact the developing brain at a susceptible time window. 8 Prognostication in both scenarios, if attainable, would improve the treatment management and, thus, both the seizure outcome and the cognitive development of affected children.
High‐frequency oscillations (HFO) in scalp EEG are a new and promising noninvasive epilepsy biomarker providing added prognostic value, particularly in the pediatric population. 9 , 10 , 11 , 12 , 13 , 14 Beyond their initial use for demarcating the seizure onset zone in focal lesional epilepsy and thus tailoring epilepsy surgery, 15 , 16 , 17 , 18 , 19 , 20 scalp HFO are currently investigated as potential biomarkers of epileptogenesis and treatment response. 21 , 22 The utility of scalp HFO as a biomarker in pediatric focal lesional epilepsy has been investigated in recent studies that corroborated a positive correlation of scalp HFO rates with (a) seizure risk at the presence of focal lesions, as in tuberous sclerosis, 23 and (b) seizure frequency, as a measure of disease severity, decreasing following successful surgical treatment. 9 , 10 , 24 Scalp HFO detectability has been shown to correlate with (a) patient age, with higher HFO rates in younger children, 25 (b) sleep stage, with higher HFO rates in N3 sleep. 26 However, the impact of lesion characteristics, such as lesion volume, depth, type, and localization on scalp HFO detectability, potentially of crucial importance for further studies, is still unclear.
To assess the effect of lesion characteristics on scalp HFO detectability and thus decode the individual variability of scalp HFO, we retrospectively analyzed scalp EEG from children and adolescents with focal lesional epilepsy. We compared HFO rates with the volume of the epileptogenic lesion and between the subgroups of FCD vs tumors, superficial vs deep, and temporal vs extratemporal lesions.
2. PATIENTS AND METHODS
2.1. Patient recruitment
We considered children and adolescents with focal lesional epilepsy who fulfilled the following inclusion criteria: (a) scalp EEG recorded at a high sampling frequency (>1000 Hz), containing ≥10 minutes of NREM sleep, recorded at >2 hours from the most recent seizure, (b) high‐resolution brain MRI supporting the diagnosis of a focal circumscribed epileptogenic lesion. We determined the lesion type based on radiological criteria and verified by histopathology in patients who underwent epilepsy surgery. We classified lesions according to (a) their sublobar localization, based on anatomical landmarks, and (b) their depth into superficial, involving the lateral neocortex, and deep, involving the medial and/or basal but not the lateral aspects of the frontal, parietal, temporal, and occipital lobes.
The collection of patient data and the scientific analysis were approved by and performed according to the guidelines and regulations of the local ethics committee (Kantonale Ethikkommission Zürich, KEK‐ZH PB‐2021‐01246). All parents and patients, where applicable, have given written informed consent.
2.2. Scalp EEG recording and data selection
Patients underwent afternoon nap or whole‐night video‐EEG with 21 electrodes according to the 10–20 system. Impedances were typically ≤5 kΩ. Recordings were performed at a 1024 Hz sampling rate by the Deltamed® EEG system for afternoon nap and the Micromed® EEG system for whole‐night recordings. For whole‐night video‐EEG, we only considered the first 3 hours of sleep. 26 We identified sleep stages 27 and selected the NREM sleep stage N3 for analysis as the most sensitive time window for scalp HFO detection, 26 reverting to N2 otherwise.
2.3. Automated scalp HFO detection in EEG
We re‐referenced to a bipolar montage using all combinations of neighboring electrodes, thus obtaining 52 bipolar channels. 10 , 25 , 26 We conducted scalp HFO detection in the 80–250 Hz frequency band with a clinically validated, automated HFO detector applied to each bipolar channel within each 5‐minutes data interval. 9 , 10 , 25 , 26 To address artifact pollution, we selected only NREM sleep segments for analysis, as these are less contaminated by muscle artifacts. Our automated detector then rejected candidate events co‐occurring bilaterally or presenting a peak‐to‐peak amplitude ≥40 µV or signal‐to‐noise ratio <9. Finally, we calculated a z‐score from the 250–500 Hz band‐pass‐filtered data overtime and rejected events occurring at high z‐score timepoints, exceeding the median z‐score by 1.5 times the interquartile range for each patient. 10 Although our automated detector uses a finite impulse response (FIR) filter that is expected to reduce induced oscillations, 28 this study did not further distinguish between false ripples and ripples observable without a high‐pass filter. 29 , 30 We calculated the HFO rate for each bipolar channel by dividing the number of detected HFO by the duration of the analyzed EEG, resulting in the unit HFO/min. We identified the HFO area as an area delineated by bipolar channels with consistently high HFO rates across scalp EEG channels for each patient that were defined as HFO area channels. 10 , 31 First, we calculated the rate threshold (93th percentile of the HFO rate distribution) from the EEG across data intervals and channels. Then, we counted the number of intervals with an above‐threshold HFO rate for each channel. Finally, we calculated the 95th percentile of these occurrence values. Channels with higher occurrence values constituted the HFO area. We calculated the cumulative HFO rate by adding the HFO rates of all HFO area channels (Figure 1).
FIGURE 1.
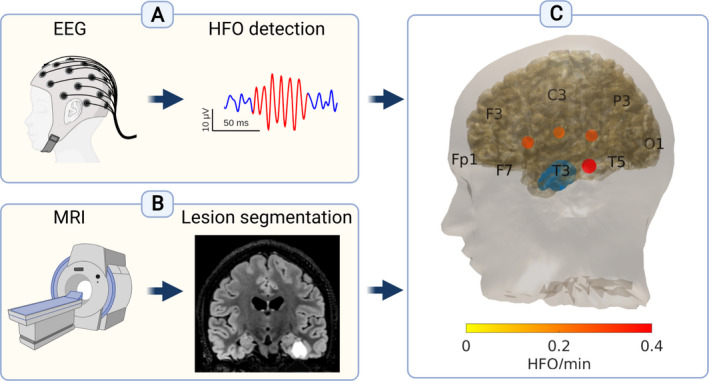
Scalp high frequency oscillations (HFO) detection from the EEG and lesion volume estimation from the MRI of each patient. Our analysis pipeline is demonstrated in the exemplary case of patient 11 with focal lesional epilepsy associated with a ganglioglioma in the left inferior/basal temporal region. (A) We analyzed scalp EEG with high sampling frequency (>1000 Hz) and detected HFO. (B) We analyzed MRI scans, delineated the epileptogenic lesion, and calculated the lesion volume. (C) 3D reconstruction of the cortical surface generated from the MRI. The lesion is depicted in blue. Blobs in different shades of red depict the scalp HFO rate in HFO area channels. Different color intensity corresponds to different HFO rates. Created with BioRender.com
2.4. Lesion volume calculation in MRI
MRI scans were performed on a 3‐Tesla scanner (Discovery 750®, General Electric Medical System) with an 8‐channel head coil, including a volumetric, whole‐brain, T1‐weighted sequence. Lesions were manually segmented by an experienced neuroradiologist (AG) using 3D Slicer, and lesion volumes were calculated based on the segmented area 10 (Figure 1). We opted for the manual segmentation of brain lesions as a well‐established method for defining lesion volumes that remains the gold standard for testing and training automated methods. 32 , 33 We decided against semi‐automated, fully automated, or deep learning‐based methods as lesion heterogeneity in our study may have affected algorithm performance. Although no inter‐ or intra‐rater reliability estimates were calculated for the lesion volumes in our study, it should be noted that previous studies involving the manual segmentation of brain lesions have reported intraclass correlation and dice similarity coefficients of 0.97 and 0.66, respectively. 34
2.5. Statistics
To investigate the impact of lesion volume on the cumulative HFO rates and the number of HFO area channels, we performed Spearman's rank correlation. To compare the HFO rates between dichotomized groups for lesion depth (superficial vs deep), type (FCD vs tumor), and lobar localization (temporal vs extratemporal), we used the Wilcoxon rank‐sum test. We reported continuous data either by mean and range or by mean and standard deviation (mean ± SD). Statistical analysis was performed with Matlab R2020a. Significance was established at P ≤ .05. We did not employ a correction for multiple comparisons.
3. RESULTS
3.1. Patient characteristics and HFO rates
We included 13 patients (five female) with a mean age of 8.4 years (range 1.5–16.0) at the scalp EEG recording. Six patients underwent afternoon nap EEG and seven whole‐night video‐EEG. Lesion type included FCD in six patients, low‐grade tumors in five, and hippocampal sclerosis in two. Radiological diagnosis was verified by histopathology in 8 cases. Eight of the patients had a deep‐seated lesion. The lobar localization was temporal in seven, extending to the occipital lobe in one, and extratemporal in six patients. The mean lesion volume was 3638 ± 2064 mm3 (Table 1).
TABLE 1.
Clinical features and high frequency oscillations (HFO) rates of our patients
| PAT. NR. |
Age (y) |
Sex | Lesion characteristics | Cumulative HFO rate (HFO/min) | ||||
|---|---|---|---|---|---|---|---|---|
| Type | Lateralization and lobar localization | Sublobar localization | Depth | Volume (mm3) | ||||
| 1 | 1.5 | F | Ganglioglioma* | R temporal | Fusiform gyrus | Deep | 7062 | 2.13 |
| 2 | 1.5 | F | FCD | L frontal | Pars opercularis | Superficial | 3935 | 1.83 |
| 3 | 2.8 | F | FCD 2a* | R frontal | Superior frontal sulcus | Superficial | 4780 | 2.40 |
| 4 | 3.9 | M | FCD | L frontal | Superior frontal sulcus | Superficial | 4799 | 0.08 |
| 5 | 4.9 | M | Hippocampal sclerosis* | L temporal | Hippocampus | Deep | 1032 | 0.25 |
| 6 | 7.6 | M | Diffuse glioma | R temporal | Temporal pole | Deep | 1600 | 0.03 |
| 7 | 8.9 | M | FCD | L temporo‐occipital | Posterior middle temporal sulcus | Superficial | 2969 | 0.47 |
| 8 | 9.5 | M | Ganglioglioma* | L medial temporal | Parahippocampal gryrus | Deep | 3901 | 1.16 |
| 9 | 11.4 | F | Angiocentric glioma* | R parietal | Post‐central gyrus | Deep | 5190 | 0.84 |
| 10 | 11.5 | M | FCD | L parietal | Post‐central gyrus | Superficial | 5986 | 4.12 |
| 11 | 13.3 | M | FCD* | R fronto‐basal | Anterior orbital gyrus | Deep | 595 | 0.00 |
| 12 | 15.8 | M | Hippocampal sclerosis* | L medio‐temporal | Hippocampus | Deep | 944 | 0.31 |
| 13 | 16.0 | F | Ganglioglioma* | L inferior/basal temporal | Fusiform gyrus | Deep | 4504 | 1.20 |
Clinical features include the lesion type (according to radiological criteria in all patients and verified by histopathology in surgical patients), the lesion lateralization and (sub‐)lobar localization, the lesion depth, the lesion volume, and the HFO rates.
Abbreviations: *, surgical patients; f, female; FCD, focal cortical dysplasia; L, left; m, male; R, right; y, year(s).
We analyzed 360 minutes of scalp EEG data, including 350 minutes of N3 (all but one patient) and 10 minutes of N2 sleep (patient 3). We detected 1024 HFO, all but 51 in N3 sleep. The mean duration of analyzed data per patient was 27.7 ± 12.2 minutes, with a mean of 78.8 ± 97.1 detected HFO per patient (Table 1).
3.2. Scalp HFO rates are higher for larger lesions
The cumulative HFO rate over the HFO area channels increased with lesion volume (Spearman's r: .70, P = .01, Figure 2). Similarly, the number of HFO area channels increased with lesion volume (Spearman's r: .55, P = .05). In addition, both superficial and deep lesions generated HFO detectable in scalp EEG. However, while superficial lesions generated higher mean cumulative HFO rates than deep lesions (1.78 ± 1.62 HFO/min vs 0.74 ± 0.74 HFO/min), this difference did not reach statistical significance in our cohort (P = .22) due to high standard deviation. Interestingly, among small‐volume (<1000 mm3) and deep‐seated lesions, the larger (944 mm3) but not the smaller one (595 mm3) generated HFO detectable on the scalp. Furthermore, neither lesion type (FCD,1.48 ± 1.62 HFO/min vs low‐grade tumors, 1.07 ± 0.76 HFO/min, P = .93) nor lobar localization (temporal, 0.79 ± 0.74 HFO/min vs extratemporal, 1.55 ± 1.58 HFO/min, P = .63) affected the mean cumulative HFO rate. However, it should be noted that the use of uncorrected P‐values poses a limitation to the generalizability of our results.
FIGURE 2.
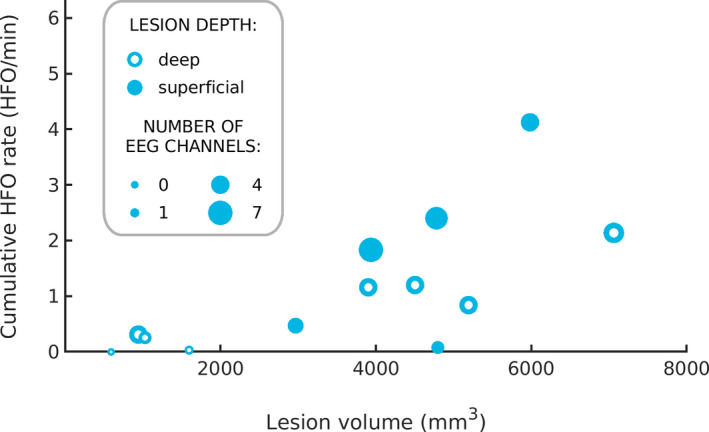
Larger lesions generate higher scalp HFO rates. The size of each circle reflects the number of HFO area channels that contributed to the cumulative HFO rate. Superficial lesions are depicted by full circles and deep lesions by empty circles. Both the cumulative HFO rate and the number of HFO area channels increased with lesion volume
4. DISCUSSION
The main finding of our study is that both the cumulative scalp HFO rate and the number of HFO area channels increased with lesion volume. It should be noted that a higher number of HFO area channels is more likely to result in a higher cumulative HFO rate. Our study suggests that larger volume epileptogenic lesions correspond to larger neuronal populations that may extend over larger areas and exhibit a higher degree of synchrony when generating scalp HFO. This observation is in line with a previous report supporting that the detectability of scalp HFO correlates with the strength of underlying cortical generators, as demonstrated in an experimental setting during neurosurgical interventions. 35 Furthermore, a simulation study of cortical generators suggested that several factors besides their extent, such as their distance from the skull, and their orientation and localization relative to the gyri and sulci, may impact the detectability of HFO in scalp EEG. 17 Our finding that lesions <1000 mm3 can generate scalp‐detectable HFO may serve as a reference for future simulation studies.
Superficial lesions, rather than deep‐seated lesions, have been reported to generate HFO and epileptic spikes that are better detectable in the scalp EEG in an adult cohort. 36 However, this past study included one‐third of patients with hippocampal abnormalities and was thus representative for the adult but not for the pediatric population with lesional focal epilepsy, 37 challenging the broad applicability of its findings. In the pediatric population, we have previously reported two patients with medial temporal lesions that showed much lower scalp HFO rates than those with superficial lesions. 9 In our present study, including mainly patients with FCD and low‐grade tumors, the most common epilepsy‐associated lesions in the pediatric age group, 37 mean HFO rates were higher for superficial than deep lesions (Figure 2), although this difference did not reach statistical significance. Future studies in larger cohorts may strengthen our results and corroborate the intuitive finding that HFO generated by superficial lesions are more easily detected on the scalp. Conversely, our findings support that even deep lesions may generate scalp‐detectable HFO. Furthermore, scalp HFO were equally well detected regardless of lobar localization (temporal vs extratemporal) in our study, in contrast to spikes that may escape detection when generated in frontal or other extratemporal regions. 38 , 39 This observation extends the applicability of scalp HFO as a biomarker to lesional epilepsy arising from the medial temporal structures, the basal frontal, temporal, and occipital regions, and the sulcal depth of parietal regions.
The type of the epileptogenic lesion (FCD vs low‐grade tumor) was not associated with differences in the scalp HFO rate in our cohort. This finding is in line with an adult lesional epilepsy study that found no differences in the scalp HFO rate when comparing cortical malformations with hippocampal abnormalities. 36 However, this finding is in contrast to an intracranial EEG study that showed higher HFO rates in FCD, medial temporal sclerosis, and nodular heterotopia than atrophy, polymicrogyria, and tuberous sclerosis, 40 suggesting that different lesion types feature different degrees of intrinsic epileptogenicity that impact intracranial HFO rates. 40 , 41 While larger studies in more homogeneous cohorts may help resolve controversies surrounding the specific impact of lesion type on HFO rates, our observations suggest that this impact is relatively small, thus supporting the broad applicability of scalp HFO as an EEG biomarker in focal lesional epilepsy.
5. CONCLUSIONS
Our findings support that epileptogenic lesions are generally accessible to assessment through the scalp HFO that they generate, irrespective of their characteristics. However, larger epileptogenic lesions generate higher scalp HFO rates over larger areas that are thus more accessible to detection. Our study provides crucial insight into scalp HFO detectability in pediatric lesional epilepsy, paving the way for scalp HFO implementation as a biomarker of seizure propensity and treatment response in a clinical setting.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
CODE AVAILABILITY
The HFO detection software is freely available at the GitHub repository (https://github.com/ZurichNCH/Automatic‐High‐Frequency‐Oscillation‐Detector).
ACKNOWLEDGMENTS
We thank the neurophysiology technicians, B. Alessandri, C. Carosio, and D. Gonçalves Carvalho, for their assistance with EEG recordings and data analysis. We thank the Swiss National Science Foundation (CRSK‐3_190895 to GR and JS), the Anna Mueller Grocholski Foundation and the Vontobel Foundation (to GR) for funding. The funders had no role in the design or analysis of the study.
Cserpan D, Gennari A, Gaito L, et al. Scalp HFO rates are higher for larger lesions. Epilepsia Open. 2022;7:496–503. 10.1002/epi4.12596
DATA AVAILABILITY STATEMENT
The raw EEG data and the HFO markings will be published upon acceptance of the article.
REFERENCES
- 1. Wirrell E, Wong‐Kisiel L, Mandrekar J, Nickels K. Predictors and course of medically intractable epilepsy in young children presenting before 36 months of age: a retrospective, population‐based study. Epilepsia. 2012;53(9):1563–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wirrell EC. Predicting pharmacoresistance in pediatric epilepsy. Epilepsia. 2013;54(Suppl 2):19–22. [DOI] [PubMed] [Google Scholar]
- 3. Dwivedi R, Ramanujam B, Chandra PS, Sapra S, Gulati S, Kalaivani M, et al. Surgery for drug‐resistant epilepsy in children. N Engl J Med. 2017;377(17):1639–47. [DOI] [PubMed] [Google Scholar]
- 4. Kadish NE, Bast T, Reuner G, Wagner K, Mayer H, Schubert‐Bast S, et al. Epilepsy surgery in the first 3 years of life: predictors of seizure freedom and cognitive development. Clin Neurosurgery. 2019;84(6):e368–77. [DOI] [PubMed] [Google Scholar]
- 5. Kogias E, Bast T, Schubert‐Bast S, Wiegand G, Brandt A, Strobl K, et al. Multilobar epilepsy surgery in childhood and adolescence: predictors of long‐term seizure freedom. Neurosurgery. 2020;88(1):174–82. [DOI] [PubMed] [Google Scholar]
- 6. Ramantani G, Kadish NE, Mayer H, Anastasopoulos C, Wagner K, Reuner G, et al. Frontal lobe epilepsy surgery in childhood and adolescence: predictors of long‐term seizure freedom, overall cognitive and adaptive functioning. Clin Neurosurgery. 2018;83(1):93–102. [DOI] [PubMed] [Google Scholar]
- 7. Ramantani G, Stathi A, Brandt A, Strobl K, Schubert‐Bast S, Wiegand G, et al. Posterior cortex epilepsy surgery in childhood and adolescence: predictors of long‐term seizure outcome. Epilepsia. 2017;58(3):412–9. [DOI] [PubMed] [Google Scholar]
- 8. Boshuisen K, van Schooneveld MMJ, Uiterwaal CSPM, Cross JH, Harrison S, Polster T, et al. Intelligence quotient improves after antiepileptic drug withdrawal following pediatric epilepsy surgery. Ann Neurol. 2015;78(1):104–14. [DOI] [PubMed] [Google Scholar]
- 9. Boran E, Sarnthein J, Krayenbühl N, Ramantani G, Fedele T. High‐frequency oscillations in scalp EEG mirror seizure frequency in pediatric focal epilepsy. Sci Rep. 2019;9(1):16560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cserpan D, Gennari A, Gaito L, Lo Biundo SP, Tuura R, Sarnthein J, et al. Scalp HFO rates decrease after successful epilepsy surgery and are not impacted by the skull defect resulting from craniotomy. Sci Rep. 2022;12(1):1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nariai H, Hussain SA, Bernardo D, Motoi H, Sonoda M, Kuroda N, et al. Scalp EEG interictal high frequency oscillations as an objective biomarker of infantile spasms. Clin Neurophysiol. 2020;131(11):2527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kramer MA, Ostrowski LM, Song DY, Thorn EL, Stoyell SM, Parnes M, et al. Scalp recorded spike ripples predict seizure risk in childhood epilepsy better than spikes. Brain. 2019;142(5):1296–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yan L, Li L, Chen J, Wang L, Jiang L, Hu Y. Application of high‐frequency oscillations on scalp eeg in infant spasm: a prospective controlled study. Front Hum Neurosci. 2021;15:682011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qian P, Li H, Xue J, Yang Z. Scalp‐recorded high‐frequency oscillations in atypical benign partial epilepsy. Clin Neurophysiol. 2016;127(10):3306–13. [DOI] [PubMed] [Google Scholar]
- 15. Andrade‐Valenca LP, Dubeau F, Mari F, Zelmann R, Gotman J. Interictal scalp fast oscillations as a marker of the seizure onset zone. Neurology. 2011;77(6):524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Melani F, Zelmann R, Dubeau F, Gotman J. Occurrence of scalp‐fast oscillations among patients with different spiking rate and their role as epileptogenicity marker. Epilepsy Res. 2013;106(3):345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zelmann R, Lina JM, Schulze‐Bonhage A, Gotman J, Jacobs J. Scalp EEG is not a blur: it can see high frequency oscillations although their generators are small. Brain Topogr. 2014;27(5):683–704. [DOI] [PubMed] [Google Scholar]
- 18. Pizzo F, Frauscher B, Ferrari‐Marinho T, Amiri M, Dubeau F, Gotman J. Detectability of fast ripples (>250 Hz) on the scalp EEG: a proof‐of‐principle study with subdermal electrodes. Brain Topogr. 2016;29(3):358–67. [DOI] [PubMed] [Google Scholar]
- 19. van Klink N, Mol A, Ferrier C, Hillebrand A, Huiskamp G, Zijlmans M. Beamforming applied to surface EEG improves ripple visibility. Clin Neurophysiol. 2018;129(1):101–11. [DOI] [PubMed] [Google Scholar]
- 20. Kuhnke N, Klus C, Dümpelmann M, Schulze‐Bonhage A, Jacobs J. Simultaneously recorded intracranial and scalp high frequency oscillations help identify patients with poor postsurgical seizure outcome. Clin Neurophysiol. 2019;130(1):128–37. [DOI] [PubMed] [Google Scholar]
- 21. Santana‐Gomez CE, Engel J, Staba R. Drug‐resistant epilepsy and the hypothesis of intrinsic severity: what about the high‐frequency oscillations? Epilepsia Open. 2021. 10.1002/epi4.12565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fan Y, Dong L, Liu X, Wang H, Liu Y. Recent advances in the non‐invasive detection of high‐frequency oscillations in the human brain. Rev Neurosci. 2021;32(3):305–21. [DOI] [PubMed] [Google Scholar]
- 23. Bernardo D, Nariai H, Hussain SA, Sankar R, Salamon N, Krueger DA, et al. Visual and semi‐automatic non‐invasive detection of interictal fast ripples: a potential biomarker of epilepsy in children with tuberous sclerosis complex. Clin Neurophysiol. 2018;129(7):1458–66. [DOI] [PubMed] [Google Scholar]
- 24. Burelo K, Ramantani G, Indiveri G, Sarnthein J. A neuromorphic spiking neural network detects epileptic high frequency oscillations in the scalp EEG. Sci Rep. 2022;12(1):1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cserpan D, Boran E, Lo Biundo SP, Rosch R, Sarnthein J, Ramantani G. Scalp high‐frequency oscillation rates are higher in younger children. Brain Commun. 2021;3(2):fcab052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cserpan D, Rosch R, Lo Biundo SP, Sarnthein J, Ramantani G. Variation of scalp EEG high frequency oscillation rate with sleep stage and time spent in sleep in patients with pediatric epilepsy. Clin Neurophysiol. 2022;135:117–25. [DOI] [PubMed] [Google Scholar]
- 27. Berry RB, Brooks R, Gamaldo C, Harding SM, Lloyd RM, Quan SF, et al. AASM scoring manual updates for 2017 (Version 2.4). J Clin Sleep Med. 2017;13(5):665–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park CJ, Hong SB. High frequency oscillations in epilepsy: detection methods and considerations in clinical application. J Epilepsy Res. 2019;9(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bénar CG, Chauvière L, Bartolomei F, Wendling F. Pitfalls of high‐pass filtering for detecting epileptic oscillations: a technical note on “false” ripples. Clin Neurophysiol. 2010;121(3):301–10. [DOI] [PubMed] [Google Scholar]
- 30. Kobayashi K, Shibata T, Tsuchiya H, Akiyama T. Exclusion of the possibility of “false ripples” from ripple band high‐frequency oscillations recorded from scalp electroencephalogram in children with epilepsy. Front Hum Neurosci. 2021;15:696882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fedele T, Burnos S, Boran E, Krayenbühl N, Hilfiker P, Grunwald T, et al. Resection of high frequency oscillations predicts seizure outcome in the individual patient. Sci Rep. 2017;7(1):13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kulaseharan S, Aminpour A, Ebrahimi M, Widjaja E. Identifying lesions in paediatric epilepsy using morphometric and textural analysis of magnetic resonance images. Neuroimage Clin. 2019;21:101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gau K, Schmidt CSM, Urbach H, Zentner J, Schulze‐Bonhage A, Kaller CP, et al. Accuracy and practical aspects of semi‐ and fully automatic segmentation methods for resected brain areas. Neuroradiology. 2020;62(12):1637–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Egger C, Opfer R, Wang C, Kepp T, Sormani MP, Spies L, et al. MRI FLAIR lesion segmentation in multiple sclerosis: does automated segmentation hold up with manual annotation? Neuroimage Clin. 2017;13:264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Burnos S, Fedele T, Schmid O, Krayenbühl N, Sarnthein J. Detectability of the somatosensory evoked high frequency oscillation (HFO) co‐recorded by scalp EEG and ECoG under propofol. Neuroimage Clin. 2016;10:318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cuello‐Oderiz C, von Ellenrieder N, Dubeau F, Gotman J. Influence of the location and type of epileptogenic lesion on scalp interictal epileptiform discharges and high‐frequency oscillations. Epilepsia. 2017;58(12):2153–63. [DOI] [PubMed] [Google Scholar]
- 37. Blumcke I, Spreafico R, Haaker G, Coras R, Kobow K, Bien CG, et al. Histopathological findings in brain tissue obtained during epilepsy surgery. N Engl J Med. 2017;377(17):1648–56. [DOI] [PubMed] [Google Scholar]
- 38. Ramantani G, Dümpelmann M, Koessler L, Brandt A, Cosandier‐Rimélé D, Zentner J, et al. Simultaneous subdural and scalp EEG correlates of frontal lobe epileptic sources. Epilepsia. 2014;55(2):278–88. [DOI] [PubMed] [Google Scholar]
- 39. Rémi J, Vollmar C, de Marinis A, Heinlin J, Peraud A, Noachtar S. Congruence and discrepancy of interictal and ictal EEG with MRI lesions in focal epilepsies. Neurology. 2011;77(14):1383–90. [DOI] [PubMed] [Google Scholar]
- 40. Ferrari‐Marinho T, Perucca P, Mok K, Olivier A, Hall J, Dubeau F, et al. Pathologic substrates of focal epilepsy influence the generation of high‐frequency oscillations. Epilepsia. 2015;56(4):592–8. [DOI] [PubMed] [Google Scholar]
- 41. Kerber K, LeVan P, Dümpelmann M, Fauser S, Korinthenberg R, Schulze‐Bonhage A, et al. High frequency oscillations mirror disease activity in patients with focal cortical dysplasia. Epilepsia. 2013;54(8):1428–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw EEG data and the HFO markings will be published upon acceptance of the article.


