Significance
The ubiquitin ligase APC/C (anaphase-promoting complex/cyclosome) is essential for the control of mitosis, and its action is tightly regulated. The phosphorylation of APC/C by mitotic protein kinase Cdk1-cyclin B facilitates its association with its co-activator protein Cdc20, while the phosphorylation of Cdc20 inhibits it. Trying to explain this apparent paradox and to gain insight into the mode of regulation of APC/C, we found that following the binding of Cdc20 to APC/C, it became protected against phosphorylation. We propose a model according to which a pool of unphosphorylated Cdc20, originating either from initial stages of mitosis or by the action of protein phosphatases, combines with phosphorylated APC/C and thus allows the regulation of APC/C activity at different stages of mitosis.
Keywords: ubiquitin, cell cycle, mitosis
Abstract
The ubiquitin ligase APC/C (anaphase-promoting complex/cyclosome) is essential for the control of mitosis, and its activity is subject to tight regulation. In early mitosis, APC/C is inhibited by the mitotic checkpoint system, but subsequently it regains activity and promotes metaphase-anaphase transition by targeting cyclin B and securin for degradation. The phosphorylation of APC/C by the mitotic protein kinase Cdk1-cyclin B facilitates its interaction with its coactivator Cdc20, while the phosphorylation of Cdc20 inhibits its binding to APC/C. This raises the question of how Cdc20 binds to APC/C under conditions of high Cdk1 activity. It seemed possible that the opposing action of protein phosphatases produces a fraction of unphosphorylated Cdc20 that binds to APC/C. We found, however, that while inhibitors of protein phosphatases PP2A and PP1 increased the overall phosphorylation of Cdc20 in anaphase extracts from Xenopus eggs, they did not decrease the levels of Cdc20 bound to APC/C. Searching for alternative mechanisms, we found that following the binding of Cdc20 to APC/C, it became significantly protected against phosphorylation by Cdk1. Protection was mainly at threonine sites at the N-terminal region of Cdc20, known to affect its interaction with APC/C. A model is proposed according to which a pool of unphosphorylated Cdc20, originating from initial stages of mitosis or from phosphatase action, combines with phosphorylated APC/C and thus becomes stabilized against further phosphorylation, possibly by steric hindrance of Cdk1 action. This pool of APCCdc20 appears to be required for the regulation of APC/C activity at different stages of mitosis.
A large, multisubunit ubiquitin ligase complex, called the anaphase-promoting complex/cyclosome (APC/C), is essential for the control of mitosis in eukaryotic cells. It targets for degradation cell-cycle regulatory proteins such as cyclin B, the activating subunit of protein kinase Cdk1, and securin, an inhibitor of the separation of sister chromatids. The activity of APC/C itself is tightly regulated by phosphorylation and by the action of coactivators and inhibitors that act at different stages of the cell cycle [reviewed by Barford (1), Watson et al. (2), and Yamano (3)]. In entry into mitosis, rising levels of Cdk1-cyclin B phosphorylate some subunits of APC/C. The phosphorylation of APC/C allows its association with the coactivator Cdc20 (4–7) by relieving auto-inhibition of its APC1 subunit (8–10). In prometaphase, APC/C is kept inactive, and thus premature separation of sister chromatids is prevented by the mitotic (or spindle assembly) checkpoint system, which ensures correct chromosome segregation by monitoring the bipolar attachment of chromatids to the mitotic spindle [reviewed by Musacchio and Salmon (11), Lara-Gonzalez et al. (12), and London and Biggins (13)]. The mitotic checkpoint system acts by the assembly of a mitotic checkpoint complex (MCC), an inhibitor of APC/C composed of BubR1, Bub3, Cdc20, and Mad2. When the mitotic checkpoint is satisfied, MCC is disassembled by several pathways (14–17) and APC/CCdc20 regains activity and promotes the metaphase-to-anaphase transition.
In contrast to the activation of APC/C by phosphorylation, the binding of Cdc20 to APC/C and its capacity to stimulate APC/C activity are inhibited by its phosphorylation by Cdk1-cyclin B (18, 19). This has been shown to be mainly due to the phosphorylation of three conserved threonine residues at the N-terminal region of Cdc20 (T64, T78, and T79 in Xenopus and T55, T59, and T70 in human Cdc20), close to its C-box APC/C-binding site (19). The phosphorylation of these T residues of Cdc20 has also been shown to increase the efficiency of the mitotic checkpoint system (20–22).
The opposing effects of the phosphorylation of APC/C and of Cdc20 raise the question of how Cdc20 binds to APC/C under conditions of high Cdk1 activity. The binding of Cdc20 to APC/C is required at two stages of mitosis. First, during active mitotic checkpoint, the binding of MCC to APC/C, and thus the inhibition of APC/C, requires the presence of APC/C-bound Cdc20 (23–25). Thus, the APC/C-MCC complex contains two molecules of Cdc20, one that is bound to APC/C and another that is an integral part of the MCC. Second, subsequent to the inactivation of the mitotic checkpoint, Cdc20 must be bound to APC/C to activate it for the initiation of the degradation of cyclin B, while levels of Cdk1-cyclin B are still high.
To solve this problem, it has been proposed that dephosphorylation of Cdc20 by protein phosphatases may allow its association with the APC/C. Thus, Labit and coworkers (19) reported that in extracts of Xenopus eggs, a species of protein phosphatase 2A is active in the anaphase, and that in anaphase extracts, only a small fraction of APC/C-bound Cdc20 is phosphorylated on T79. The authors proposed that during anaphase, Cdc20 is at a dynamic state of phosphorylation-dephosphorylation, and that only the fraction that is dephosphorylated binds to mitotic APC/C (19). Subsequently, Fujimitsu and Yamano (26) reported that phosphatase PP2A-B56 binds to the APC1 subunit of APC/C, and that this association stimulates the dephosphorylation of Cdc20 and its loading onto APC/C. PP2A-B56 has also been shown to bind to the BubR1 component of MCC and was proposed to promote Cdc20 dephosphorylation (22). Another phosphatase, PP1, has been reported to dephosphorylate threonine sites at the N-terminal region of Cdc20 in Caenorhabditis elegans (27) and in human cells (28). The preferential action of protein phosphatases on N-terminal threonines of Cdc20 may be accounted for by the faster dephosphorylation of pThr than of pSer residues by these enzymes (29). However, we are not aware of any report on a regulatory mechanism that stimulates the activity of a specific protein phosphatase in mitosis.
While the action of protein phosphatases may be involved in the association of Cdc20 with APC/C in mitosis [reviewed by Kataria and Yamano (30)], it is not clear whether the magnitude of their action is sufficient to account for all or only a part of this process—in other words, whether the rate of phosphatase action is sufficient to counteract the strong action of protein kinase Cdk1 in mitosis. We therefore examined the possibility that following the binding of unphosphorylated Cdc20 to APC/C, it may be converted to a form protected against phosphorylation.
Results
Effects of Inhibitors of Protein Phosphatases on the Phosphorylation of Cdc20 and on Its Binding to APC/C in Anaphase Extracts of Xenopus Eggs.
To explain how Cdc20 binds to APC/C in mitosis despite its inhibitory phosphorylation by Cdk1, it was proposed that in anaphase, Cdc20 is at a dynamic state between Cdk1-catalyzed phosphorylation and phosphatase-catalyzed dephosphorylation, and that the fraction of Cdc20 that is dephosphorylated binds to and activates mitotic APC/C (19). In such case, it would be expected that the inhibition of phosphatase activity would decrease the proportion of dephosphorylated Cdc20 and thus would inhibit its binding to APC/C.
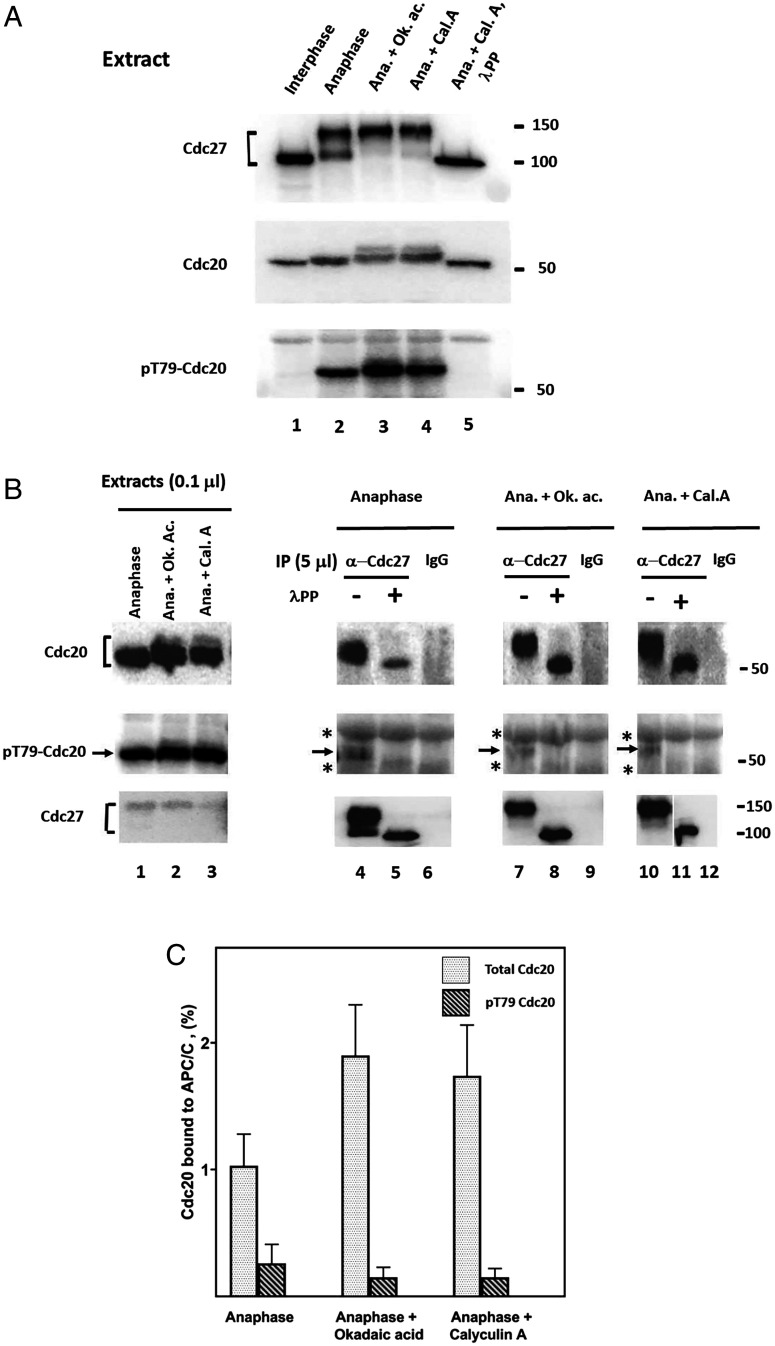
We tested this possibility by monitoring the effects of phosphatase inhibitors in extracts of Xenopus eggs in the anaphase. We used two protein phosphatase inhibitors: okadaic acid, which inhibits PP2A-type protein phosphatases at low nanomolar concentrations, but is less effective in inhibiting PP1 phosphatases, and calyculin A, which strongly inhibits both PP2A and PP1 phosphatases at low nanomolar concentrations (31). While other types of protein phosphatases may be also present in Xenopus egg extracts, it seems that PP2A and PP1 classes are predominant, since treatment of anaphase extracts by both inhibitors caused the accumulation of highly phosphorylated forms of Cdc27 and Cdc20, as indicated by their retarded migration on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 1A, lanes 3 and 4 versus lane 2). These electrophoretic migration shifts were reversed by the treatment of samples with lambda phosphatase (Fig. 1A, lane 5). Both phosphatase inhibitors also increased the phosphorylation of T79 residue of Cdc20 in anaphase extracts (Fig. 1A, Bottom, lanes 3 and 4 versus 2).
Fig. 1.
Influence of phosphatase inhibitors on the binding of Cdc20 to APC/C in anaphase extracts from Xenopus eggs. (A) Effects of okadaic acid and calyculin A on the phosphorylation state of Cdc27 and Cdc20 in anaphase extracts. Interphase or anaphase extracts from Xenopus eggs were prepared as described under Materials and Methods. Anaphase extracts were incubated at 23 °C for 30 min with okadaic acid (Calbiochem #MBS459620) or calyculin A (Cell Signaling #CST-9902S) (1 mM, each), as indicated. DMSO solvent was at a final concentration of 0.5% (v/v) in all incubations. Where indicated, the sample was treated with lambda phosphatase (see Materials and Methods). Samples of 0.1 mL of extracts were subjected to SDS-PAGE and were immunoblotted as indicated. Numbers on the right indicate the position of molecular mass marker proteins. Ana., anaphase; Ok. ac, okadaic acid; Cal. A., calyculin A; lPP, lambda phosphatase. (B) Effects of phosphatase inhibitors on the binding to APC/C of total and T79-phosphorylated Cdc20. Anaphase extracts from Xenopus eggs were incubated with or without phosphatase inhibitors as in A, and then were subjected to immunoprecipitation with anti-Cdc27 antibodies or with nonimmune IgG, as indicated. Immunoprecipitation was carried out as described under Materials and Methods, except that beads were washed four times with buffer A that contained 0.3 M NaCl and 0.1% (vol/vol) NP-40 and then twice with buffer A. Where indicated, samples were treated with lambda phosphatase (“lPP”), as described under Materials and Methods. Samples of immunoprecipitates derived from 5 mL extracts (lanes 4–12) or 0.1 mL of unfractionated extracts (lanes 1–3) were subjected to immunoblotting with antibody directed against Xenopus Cdc20 (Upper) or against its T79-phosphorylated derivative (Center). (Lower) Immunoblot form Cdc27 bait in same immunoprecipitates. Asterisks, cross-reacting material; arrows, position of pT79-Cdc20. (C) Quantitation of data similar to B. Data from three experiments similar to B were quantified. APC/C-bound total Cdc20 or pT79-Cdc20 were expressed as the percentage of each in unfractionated extracts.
Since the treatment of anaphase extracts by the phosphatase inhibitors increased the phosphorylation of Cdc20, it could be expected that the binding of Cdc20 to APC/C would be decreased by these treatments. However, we found that the phosphatase inhibitors did not decrease, but even slightly increased, the levels of Cdc20 associated with APC/C, as estimated following immunoprecipitation with antibody directed against the Cdc27 subunit of APC/C (Fig. 1B, Upper, lanes 7 to 12 versus 4 to 6). Cdc20 associated with APC/C was extensively phosphorylated, as indicated by the pronounced effect of lambda phosphatase treatment on its electrophoretic migration.
How is phosphorylated Cdc20 bound to APC/C? We examined the possibility that while many sites of APC/C-associated Cdc20 are phosphorylated, some other sites at its N-terminal portion, involved in its interaction with APC/C, escape phosphorylation, even when phosphatases PP2A and PP1 are inhibited. Probing immunoprecipitated APC/C with antibody specific for pT79 of Cdc20, we first confirmed previous results (19) that in anaphase, only a small part of Cdc20 bound to APC/C is phosphorylated on T79, as opposed to strong T79 phosphorylation of free Cdc20 in the same extract (Fig. 1B, Center, lanes 4 to 6 versus 1). We furthermore found that upon incubation of anaphase extracts with phosphatase inhibitors, also very low fractions of APC/C-bound Cdc20 were phosphorylated on T79 (Fig. 1B, Center, lanes 7 to 12). In these experiments, the signal detected by the pT79-Cdc20 antibody was small but specific, since it was absent in samples treated with lambda phosphatase or in sham precipitation with nonimmune immunoglobulin G (IgG) (Fig. 1B, Center, arrows). Quantitation of results from similar experiments (Fig. 1C) indicated an 8- to 10-fold decrease in the amounts of pT79-Cdc20 versus total Cdc20 associated with APC/C in samples immunopurified from anaphase extracts incubated with phosphatase inhibitors. It thus seems that the T79 residue of APC/C-bound Cdc20, and possibly other Cdk1 phosphorylation sites at the N-terminal region of Cdc20, escape phosphorylation even when phosphatases PP2A and PP1 are inhibited and free Cdc20 is hyperphosphorylated. Seeking explanations for these initial observations, we continued to examine this problem in a well-defined, purified system.
Different Effects of Protein Kinase Cdk1 on Free versus APC/C-Bound Cdc20.
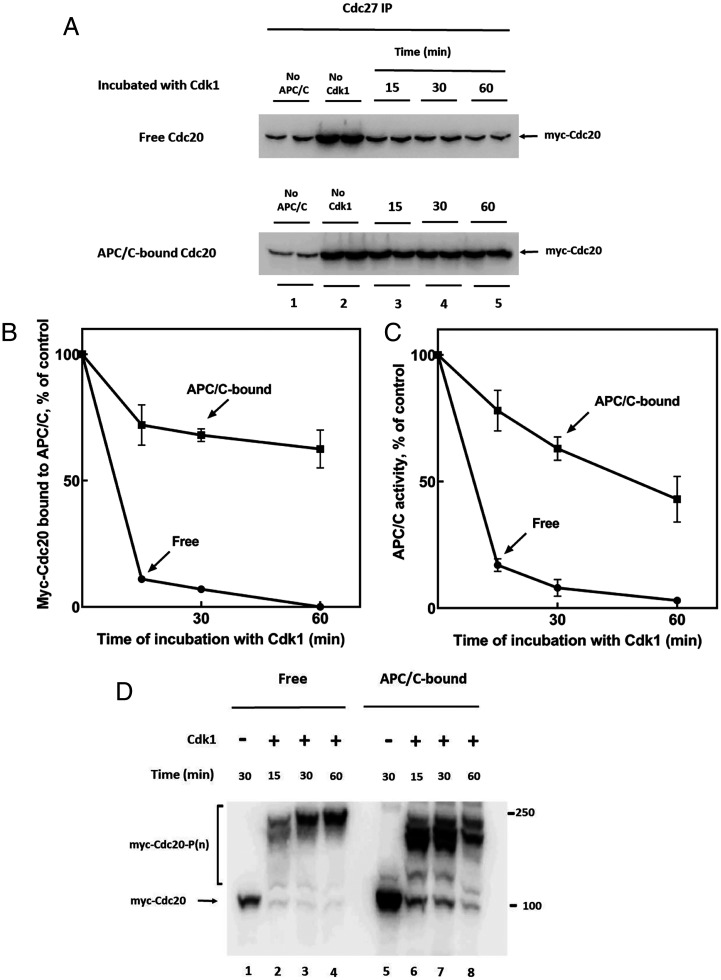
A possible explanation for the problem of how Cdc20 binds to APC/C under conditions of high protein kinase activity is that following the binding of unphosphorylated Cdc20 to APC/C, it becomes less susceptible to protein kinase action, possibly due to steric hindrance. We therefore compared the effects of Cdk1-catalyzed phosphorylation of free and APC/C-bound Cdc20 in a system composed of purified components. For this purpose, we used highly phosphorylated APC/C, immunopurified from Xenopus anaphase extracts incubated with okadaic acid (see Materials and Methods) and purified recombinant Myc-tagged human Cdc20. For phosphorylation reactions, we used Cdk1 activated by a nondegradable derivative of cyclin B (hereafter referred to as “Cdk1”) (32). In the experiment shown in Fig. 2A, Upper, free myc-Cdc20 was first incubated for various times with Cdk1 and adenosine triphosphate (ATP) and then Cdk1 action was stopped with excess p27Kip1. Subsequently, samples were mixed with APC/C and the binding of myc-Cdc20 to APC/C (immunoprecipitated with anti-Cdc27 beads) was estimated by immunoblotting. Samples were treated with lambda phosphatase, to prevent interference by phosphorylation of estimation of Cdc20 levels. As expected, Cdk1-catalyzed phosphorylation of free Cdc20 rapidly inhibited its subsequent binding to APC/C (Fig. 2A, Upper panel, lanes 3 to 5 versus 2). By contrast, when myc-Cdc20 was first bound to APC/C and then was subjected to phosphorylation by Cdk1, the action of Cdk1 to the release of Myc-Cdc20 from APC/C was much slower (Fig. 2A, Lower panel). Quantitation of immunoblots (Fig. 2B) showed that while incubation of free Myc-Cdc20 with Cdk1 for 15 min was sufficient to prevent most of its binding to APC/C, more than half of APC/C-bound myc-Cdc20 remained associated even following 60 min of phosphorylation. In parallel experiments, we also determined the effects of Cdk1-promoted phosphorylation on the capacity of myc-Cdc20 to stimulate the activity of APC/C, as measured by the ubiquitylation of 125I-cyclin, in the presence of E1, UbcH10, and ubiquitin. As shown in SI Appendix, Fig. S1A and quantified in Fig. 2C, a short incubation of free myc-Cdc20 with Cdk1 was sufficient to block most of its capacity to stimulate APC/C activity. Contrarily, prolonged incubation of Cdk1 with myc-Cdc20 bound to APC/C caused only a partial decline in myc-Cdc20-stimulated APC/C activity (SI Appendix, Fig. S1B, quantified in Fig. 2C). Thus, the effects of phosphorylation of free or APC/C-bound myc-Cdc20 on APC/C activity reflected the influence of these treatments on the binding of myc-Cdc20 to APC/C. It should be noted that in experiments with APC/C-bound myc-Cdc20, both APC/C and myc-Cdc20 could be subject to the action of Cdk1, but since we used strongly phosphorylated APC/C, isolated from anaphase extracts that had been treated with okadaic acid, we assume that most of the observed effects were due to the phosphorylation of Cdc20. One possible explanation for these results is that the binding of Cdc20 to APC/C converts it to a form less susceptible to phosphorylation by Cdk1. The slow Cdk1-dependent release of Cdc20 from APC/C suggests that the protection of Cdc20 against phosphorylation is not absolute.
Fig. 2.
Differences in effects of Cdk1 on free and APC/C-bound Cdc20. (A) Effects of Cdk1-catalyzed phosphorylation of free or APC/C-bound Cdc20 on its association with APC/C. (Upper) Free myc-Cdc20 (50 nM) was phosphorylated in a reaction mixture that contained in a volume of 50 mL: 40 mM Tris-HCl (pH 7.6), 3 mM MgCl2, 1 mM ATP, 10 mM phosphocreatine, 0.1 mg/mL creatine phosphokinase, 2 mg/mL BSA and 40 units/mL Cdk1-cyclin B (referred to as “Cdk1”). Following incubation at 23 °C for the time periods indicated, the binding of myc-Cdc20 to immunopurified APC/C (on 4 mL anti-Cdc27 beads) was assayed following washing of beads as described under Materials and Methods. (Lower) myc-Cdc20 pre-bound to immunopurified APC/C (see Materials and Methods) was incubated with Cdk1 under conditions similar to those described above and then beads were washed and the amount myc-Cdc20 that remained associated with APC/C was estimated by immunoblotting. All samples had been subjected to treatment with lambda phosphatase. “No APC/C,” nonspecific absorption of myc-Cdc20 to anti-Cdc27 beads without APC; “No Cdk1,” samples incubated without Cdk1 for 30 min. (B) Quantitation of data from A. Background values (“No APC/C”) were subtracted and myc-Cdc20 bound to APC/C were expressed as the percentage of the control sample incubated without Cdk1. (C) Effects of phosphorylation by Cdk1 of free or APC/C-bound myc-Cdc20 on the activity of APC/C. Quantitation of APC/C activity data from SI Appendix, Figs. S1 A and B. Background values if ubiquitylated 125I-cyclin obtained without added myc-Cdc20 (lanes 2 in SI Appendix, Figs. S1 A and B, “No Cdc20”) were subtracted and results were expressed as the percentage of sample incubated without Cdk1 for 30 min (lane 3 in SI Appendix, Fig. S1A and lane 4 in SI Appendix, Fig. S1B). (D) Phos-tag-SDS-PAGE analysis of patterns of phosphorylation by Cdk1 of free and APC/C-bound myc-Cdc20. The experiment was similar to that described in A, except free myc-Cdc20 was not applied to APC/C, samples were not treated with lambda phosphatase and were subjected to Phos-tag-SDS-PAGE (see Materials and Methods).
Another possible explanation for these observations is that a protein phosphatase that is strongly associated with immunopurified APC/C opposes the action of Cdk. Since it was reported that phosphatase PP2A-B56 binds to APC/C (26), we have re-examined the effects of phosphatase inhibitors in the purified system. In the experiment shown in SI Appendix, Fig. S2A, myc-Cdc20 was bound to APC/C and then subjected to the action of Cdk1, in the absence or presence of okadaic acid or calyculin A. The effects of these treatments on APC/C activity were determined. If a type 2A phosphatase, such as PP2A-B56, is present and active in the purified system, it would be expected that upon its inhibition, increased phosphorylation would lead to the release of Cdc20 from APC/C and thus to the decrease of APC/C activity. However, we could not detect any decrease in APC/C activity following incubation with phosphatase inhibitors as compared to incubation with Cdk1 alone (SI Appendix, Fig. S2A, lanes 5 and 6 versus lane 4).
Since the above-described experiments were performed with APC/C from Xenopus eggs, we also examined whether the association of Cdc20 with APC/C from another source affects similarly its susceptibility to the action of Cdk1. As shown in SI Appendix, Fig. S2B, prior binding of Cdc20 to APC/C from HeLa cells similarly slowed down its inactivation by Cdk1.
Influence of Association of Cdc20 with APC/C on the Pattern of Its Phosphorylation by Cdk1.
We next examined whether the above-described differences in the action of Cdk1 on free versus APC/C-bound Cdc20 are indeed due to different patterns of phosphorylation in these states. For this purpose, we used Phos-tag reagent (33), which binds to phosphorylated proteins and strongly retards their migration in SDS-PAGE. In the experiment shown in Fig. 2D, free or APC/C-bound myc-Cdc20 was incubated with Cdk1 under conditions similar to those in Fig. 2A, and then were subjected to Phos-tag-SDS-PAGE and immunoblotting for myc-Cdc20. The incubation of free myc-Cdc20 with Cdk1 rapidly converted it to multiphosphorylated forms that had strongly retarded electrophoretic migration. APC/C-bound myc-Cdc20 was also rapidly phosphorylated, but the predominant phosphorylated species migrated faster in Phos-tag-SDS-PAGE than those of phosphorylated free Cdc20 (Fig. 2D, lanes 6 to 8 versus 2 to 4). These results suggest that Cdc20 bound to APC/C is not completely phosphorylated at all of its sites. It is notable that although only a very small fraction of APC/C-bound myc-Cdc20 remained unphosphorylated following incubation with Cdk1 (Fig. 2D, lanes 6 to 8), much of APC/C remained active under these conditions (Fig. 2C). This indicates that some forms of partially phosphorylated Cdc20 can still activate APC/C. We therefore asked whether some specific sites of APC/C-bound Cdc20 escape phosphorylation, and whether these species, phosphorylated on other sites, could still bind to and activate APC/C.
Sites at N-Terminal Region of Cdc20 Are Protected from Phosphorylation by Interaction with APC/C.
We next examined which phosphorylation sites of Cdc20 are protected against Cdk1-catalyzed phosphorylation when Cdc20 is bound to APC/C. Cdc20 has 8 potential Cdk1 phosphorylation sites (S/T-P); the phosphorylation of three T sites at its N-terminal region was shown to inhibit its binding to APC/C (19, 29). It seemed possible that the phosphorylation of these three T sites, which are in proximity to the APC/C-binding C-box of Cdc20 (34), are protected by their interaction with APC/C. To examine this possibility, we expressed and purified a mutant of myc-Cdc20 (human) in which S/T residues in all its Cdk phosphorylation sites, except for the three N-terminal T sites, have been converted to nonphosphorylatable A or V residues (Cdc20-5AV). For control experiments, we also expressed a Cdc20 mutant in which only the three N-terminal region T residues have been converted to V (N3V), and another in which all 8 sites were mutated (8AV). We first examined the effects of Cdk1-catalyzed phosphorylation of the different free Cdc20 mutants on their action to stimulate APC/C (SI Appendix, Fig. S2). As expected, phosphorylation of wild-type Cdc20 markedly inhibited stimulation of APC/C activity, while incubation of Cdc20-8AV with Cdk1 did not inhibit it. Also as expected, treatment of the 5AV mutant with Cdk1, in which the 3 N-terminal region threonines are available for phosphorylation, also strongly inhibited its action to stimulate APC/C. We noticed, however, that the Cdc20-5AV mutant was inhibited by Cdk1 to a lesser degree than wild-type Cdc20 (SI Appendix, Fig. S2, lane 7 versus lane 3). These results suggested that while the phosphorylation of the three T residues at the N-terminal region of Cdc20 has a predominant influence on its interaction with APC/C, some additional, as yet unidentified, phosphorylation site(s) also affect this interaction. This conclusion was supported by the additional finding that the N3V mutant of Cdc20, in which phosphorylation of the three T sites is prevented, is not completely resistant to inhibition by Cdk1, as is the 8AV mutant (SI Appendix, Fig. S2, lane 5 versus lane 9).
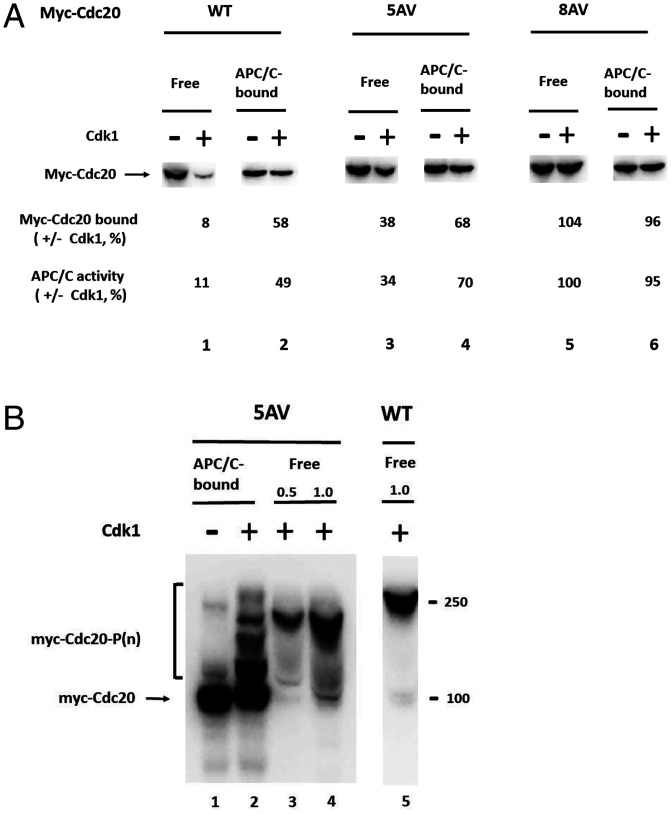
We next examined whether the three T residues at the N-terminal region of Cdc20 are protected from phosphorylation by interaction with Cdc20. The 5AV mutant was bound to APC/C, subjected to the action of Cdk1, and then the amount that remained bound to APC/C, as well as the residual APC/C activity, were determined in comparison to similar treatments of free Cdc20-5AV. As shown in Fig. 3A, lane 4 versus 3, following binding to APC/C and phosphorylation by Cdk1, there was an approximately twofold increase in the amount of APC/C-bound Cdc20-5AV and in the activity of APC/C, as compared to values obtained following phosphorylation of free Cdc20-5AV mutant. These results indicated significant protection of the three N-terminal threonine residues of Cdc20 by binding to APC/C, since these are the only sites available for phosphorylation in the 5AV mutant.
Fig. 3.
Protection of sites affecting the binding of Cdc20 to APC/C against phosphorylation by Cdk1. (A) Protection against Cdk1 action of a mutant of Cdc20 (5AV) that can be phosphorylated only on three T residues at its N-terminal region. Free or APC/C-bound forms of the indicated wild-type or mutant myc-Cdc20s were incubated (at 23 °C for 30 min) with or without Cdk1, as described for Fig. 2A. Myc-Cdc20 bound to APC/C was detected by immunoblotting and was expressed as the percentage of the control incubated without Cdk1. In separate samples of immunoprecipitates, not treated by lambda phosphatase, the activity of APC/C was determined as described for SI Appendix, Figs. S1 A and B and results were expressed as described for Fig. 2C. (B) Patterns of phosphorylation of free and APC/C-bound 5AV mutant of Cdc20, analyzed by Phos-tag-SDS-PAGE. Free of APC/C-bound mutant of myc-Cdc20 were subjected to phosphorylation by Cdk1, as described in Fig. 2 A and D. Samples were resolved by Phos-tag-SDS-PAGE and were immunoblotted with Myc-tag. Phosphorylated free myc-Cdc20 5AV was applied at one-half (lane 3, “0.5”) or at equal amount (lane 4, “1.0”) to that of wild-type myc-Cdc20 (lane 5).
The suggestion that the N-terminal threonine residues of Cdc20 are protected from phosphorylation by interaction with APC/C was further examined by the pattern of phosphorylation of APC/C-bound versus free Cdc20-5AV mutant, analyzed by Phos-tag gel electrophoresis. As shown in Fig. 3B, phosphorylation by Cdk1 of free Cdc20-5AV strongly retarded its migration in Phos-tag-SDS-PAGE, although retardation of migration was less than that of wild-type multiphosphorylated Cdc20 (Fig. 3B, lanes 3 and 4 versus 5). Binding of Cdc20-5AV mutant to APC/C before phosphorylation by Cdk1 resulted in a pronounced shift to faster migrating, less phosphorylated derivatives of Cdc20-5AV. This shift was more marked than that observed for wild-type Cdc20 (Fig. 2D). This indicates that at least some of the three threonine residues at the N-terminal region of Cdc20 are strongly protected from phosphorylation by interaction with APC/C.
Discussion
In this investigation, we wished to gain insight into the problem of how the unphosphorylated form of Cdc20, which can bind to phosphorylated APC/C, is maintained despite the high activity of protein kinase Cdk-cyclin B in mitosis. This association of Cdc20 with APC/C is necessary for APC/C regulation at different stages on mitosis (see Introduction). Our results indicate that the binding of Cdc20 to APC/C does not simply reflect the fraction of nonphosphorylated Cdc20, due to the balance between the action of protein kinases and phosphatases in the extract, since inhibitors of protein phosphatases 2A and 1 increase the overall phosphorylation of free Cdc20 in anaphase extracts (Fig. 1A), but they do not decrease the binding of Cdc20 to APC/C (Fig. 1B). These results suggest that the actions of protein phosphatases PP2A (19, 26) and PP1 (27, 28) are not sufficient to account by themselves for the fraction of unphosphorylated Cdc20 that binds to APC/C, since this fraction is decreased by the phosphatase inhibitors. However, the action of protein phosphatases may be important to provide a continued flux of unphosphorylated Cdc20 that is subsequently stabilized by interaction with APC/C, as described below.
Using a system composed of purified components, we found that while phosphorylation of free Cdc20 prevented its binding to APC/C, following the binding of Cdc20 to APC/C, it became protected, at least partially, from Cdk1-catalyzed phosphorylation (Fig. 2). By the use of phosphorylation site mutants of Cdc20, we showed that protection from phosphorylation took place at sites of Cdc20 that influence its interaction with APC/C, such as three T phosphorylation sites at its N-terminal region (Fig. 3). By contrast, other sites of Cdc20 were phosphorylated by Cdk1 under these conditions, without influencing its binding to and stimulation of APC/C activity. Protection against phosphorylation may be due to the steric hindrance of protein kinase action on the N-terminal domain of Cdc20 containing the C-box that is closely associated with phosphorylated APC/C (9).
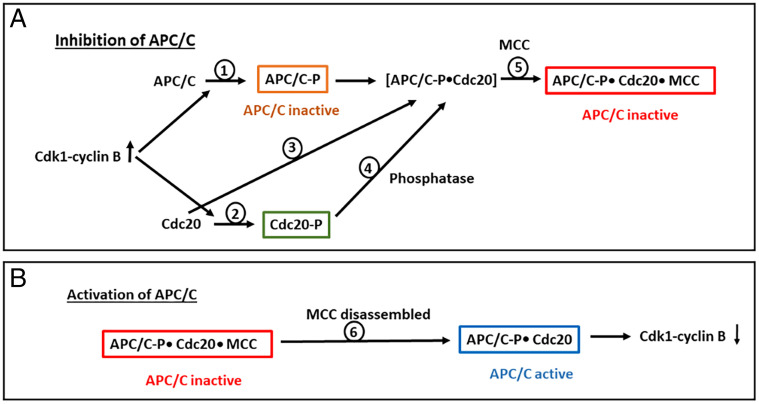
Our results are compatible with a possible sequence of events shown in Fig. 4. In this scheme, phosphorylation of Cdc20 refers only to sites that affect its binding to APC/C, such as the three threonines at its N-terminal region. The association of Cdc20 with APC/C is required both during active mitotic checkpoint, for its binding to and inhibition by MCC (23–25) (Fig. 4A), and following the disassembly of MCC, for the activation of APC/C in exit from the mitotic checkpoint (Fig. 4B). We assume that in both cases, Cdc20 must remain unphosphorylated for binding to APC/C. It is possible that part of the Cdc20 molecules remain unphosphorylated at the onset of mitosis, when the increase in the activity of Cdk1-cyclin B begins to phosphorylate both APC/C (Fig. 4A, step 1) and Cdc20 (step 2). At this stage, a mixture of phosphorylated and unphosphorylated forms of both APC/C and Cdc20 may exist, and thus a population of unphosphorylated Cdc20 may bind to the population of phosphorylated APC/C (step 3). Alternatively or in addition, part of the pool of phosphorylated Cdc20 may be subject to the action of protein phosphatases, including phosphatases that are not inhibited by okadaic acid and calyculin A, to provide a steady flux of unphosphorylated Cdc20 for binding to APC/C (Fig. 4A, step 4). In both cases, unphosphorylated Cdc20 bound to APC/C becomes protected against phosphorylation by Cdk1, and APC/CCdc20 can combine with MCC for its effective inhibition during the mitotic checkpoint (step 5). Following disassembly of MCC in exit from the mitotic checkpoint (Fig. 4B, step 6), unphosphorylated Cdc20 bound to phosphorylated APC/C now stimulates APC/C to regain its activity and to initiate the degradation of cyclin B (step 7). Even though only a limited subpopulation of APC/C is associated with unphosphorylated Cdc20 at this stage, it may cause a sufficient decrease in the activity of Cdk1-cyclin B to tip the phosphatase/kinase balance toward the dephosphorylation of Cdc20, thus further increasing APC/C activity.
Fig. 4.
Possible roles of Cdc20 phosphorylation in the regulation of APC/C activity in mitosis. See the Discussion. (A) Inhibition of APC/C in active mitotic checkpoint. (B) Release of APC/C from mitotic checkpoint inhibition. Phosphorylation of Cdc20 refers to sites known to affect its interaction with APC/C, such as the three T residues at its N-terminal region. APC/C-P, Cdc20-P, phosphorylated forms of APC/C or Cdc20.
The above scheme may also provide an explanation for the observations that the phosphorylation of Cdc20 at its N-terminal threonines is required for efficient action of the mitotic checkpoint system to inhibit metaphase-anaphase transition (20–22). While at first glance this may seem to contradict the expectation that the association of unphosphorylated Cdc20 with APC/C is required for binding to and inhibition by MCC (step 5), it may reflect the effects of the existence of different pools of Cdc20 and APC/C in mitosis. Under conditions of high Cdk1 activity, a large part of the population of Cdc20 molecules is phosphorylated in an inaccessible pool not available for binding to APC/C (Fig. 4A, green box). Consequently, a sizable portion of APC/C molecules remains unbound to Cdc20, and is thus segregated into an inactive pool (Fig. 4A, brown box). This would ensure that all APC/C molecules are inactive during mitotic checkpoint, including those not bound to MCC. In addition, as suggested by Bancroft et al. (28), the large pool of phosphorylated Cdc20 in mitosis, while not accessible for binding to APC/C, may be accessible for incorporation into MCC, thus increasing the absolute concentration of MCC. It is obvious that much further investigation is necessary, including precise estimation of the different pools of Cdc20, APC/C, MCC, and their combinations, as well as their phosphorylation at specific sites at different stages of mitosis, to critically examine the possible mechanisms discussed above for the roles of Cdc20 phosphorylation in the regulation of APC/C.
Materials and Methods
Antibodies.
Rabbit polyclonal antibodies used for the immunoprecipitation of APC/C were as follows: for Xenopus APC/C, antibody raised against 17-amino acid C-terminal peptide of Cdc27; for human APC/C, antibody raised against 14- amino acid C-terminal peptide of APC4. Both antibodies were purified by affinity chromatography with the corresponding antigens. Antibodies were bound to Affi-Prep-Protein A beads (Bio-Rad) at a concentration of 0.5 mg/mL packed beads. For control immunoprecipitation, nonimmune rabbit IgG (Invitrogen no. 31235) was bound to Protein A beads at a similar concentration. The following mouse monoclonal antibodies were used for immunoblotting: for Xenopus Cdc20, Abcam no. 18217, 1:500; for pT79 of Xenopus Cdc20, monoclonal antibody BT2 (generously provided by Dr. Julian Gannon, Cancer Research UK, Clare Hall Laboratories, South Mimms, UK, and described in ref. 19), 2 μg/mL; anti-Cdc27 (AF3.1) Santa Cruz no. 9972, 1:200; anti-Myc-tag, Cell Signaling no. 2276, 1:1,000.
Preparations.
The nondegradable derivative of human cyclin B (GST-Δ88-cyclin B) and his6-p27Kip1-T178A were expressed in bacteria and purified by their affinity tags. Extracts from cytostatic factor (CSF)–arrested Xenopus eggs were prepared as described (35). They were first converted to interphase extracts by incubation with 0.5 mM CaCl2 (23 °C, 40 min) and then to anaphase extracts by a further incubation with GST-Δ88-cyclin B (0.1 mg/mL) for 40 min.
For immunopurification of extensively phosphorylated Xenopus APC/C, anaphase extracts were prepared as above, but with the further addition of 1 μM okadaic acid, 1 mM ATP, 10 mM phosphocreatine, and 0.1 mg/mL creatine phosphokinase. Following incubation at 23 °C for 40 min, extracts were mixed with anti-Cdc27 beads at a ratio of 5:1 (extract:packed beads) and were rotated at 4 °C for 2 h. Subsequently, beads were washed 4 times with ∼50 vol of buffer A (50 mM Tris ⋅ HCl, pH 7.2, 20% [vol/vol] glycerol, 1 mg/mL bovine serum albumin [BSA], and 0.5 mM dithiothreitol [DTT]) that contained 0.3 M NaCl, and then twice with buffer A. Washed beads were suspended in 4 vol of buffer A, stored in small samples at −70 °C and used only once. (This preparation is referred to as “APC/C beads” in this article.) APC/C from HeLa cells was purified by a similar procedure, except that extracts from mitotic HeLa cells (36) were incubated with okadaic acid and ATP regeneration mixture at 30 °C for 1 h, and APC/C was purified on anti-APC4 beads.
Recombinant human myc3-his6-Cdc20 was expressed in baculovirus-infected SF9 insect cells as described (37). Its phosphorylation site mutants were produced by QuikChange Multi Site Directed Mutagenesis (Agilent Technologies), as follows: 8AV, S or T residues in all 8 potential Cdk1 phosphorylation sites of Cdc20 concerted to A or V (S41V, T55V, T59V, T70V, T106A, T157A, S408A, S452A); and 3V, 3 T residues close to N terminus converted to V; 5AV, all sites, except for the 3 T residues close to N terminus converted to A or V. All of the constructs were transferred to bacmids, and bacmid DNA was transfected to SF9 cells to generate recombinant baculoviruses (Bac-to-Bac Baculovirus Expression System, Invitrogen). All of the constructs were expressed in SF9 insect cells and purified by chromatography on Ni-NTA agarose (Quiagen).
Protein kinase Cdk1- GST-Δ88-cyclin B (referred to as Cdk1) was prepared by the incubation of interphase extracts from clam oocytes with GST-Δ88-cyclin B and ATP, followed by purification, as described (32). Units of H1 histone kinase activity of Cdk1 were as defined previously (32).
Assay of APC/C Activity.
The activity of APC/C to ubiquitylate cyclin B was determined as described previously (32), with minor modifications as follows: reaction mixtures (referred to as cyclin ubiquitylation mixture) contained in a volume of 10 μL:40 mM Tris ⋅ HCl (pH 7.6), 5 mM MgCl2, 1 mM DTT, 1 mg/mL BSA, 50 μM ubiquitin, 100 nM recombinant E1 (Enzo no. BML-UW9410), 200 nM UbcH10, 2 mM AMP-PNP (adenosine-5′-[β−γ-imido]triphosphate) and ∼20 ng of 125I-labeled (13-91)-cyclin B-protein A (32) (125I-cyclin). APC/C and myc-Cdc20 were added as specified in the figure legends. Unless otherwise indicated, mixtures were incubated at 15 °C for 10 min with shaking at 1,400 rpm; samples were quenched with SDS sample buffer and subjected to SDS-PAGE and phosphorimager analysis. Results were calculated as the percentage of 125I-cyclin converted to higher molecular-sized ubiquitylated derivatives.
Assay of Binding of Myc-Cdc20 to APC/C.
Pre-assay, APC/C bound to anti-cdc27 beads (4 μL packed beads) was pretreated to reduce nonspecific adsorption of myc-Cdc20 to beads, by mixing with 0.8 mL of buffer A that contained 10 mg/mL BSA and 0.1 M NaCl, at 4 °C for 1 h. Subsequently, beads were collected by centrifugation (2,000 rpm for 3 min at 4 °C), resuspended in 50 μL of the above buffer and mixed with myc-Cdc20 (50 nM) that had been previously subjected to incubation with or without Cdk1, as specified in the figure legends. Samples were mixed at 4 °C for 90 min to allow for the binding of myc-Cdc20 to APC/C. Nonspecific binding to beads was estimated in parallel samples in which myc-Cdc20 was incubated with anti-Cdc27 beads without APC/C. Subsequently, beads were washed twice with 1-mL portions of buffer A that contained 0.1 M NaCl and 0.1% Nonidet P-40, once with buffer A and then resuspended in a small volume of buffer A. A sample of bead suspension was subjected to treatment with lambda phosphatase, by incubation with 1,200 U lambda phosphatase (New England Biolabs no. P0753) and 2 mM MnCl2, for 1 h at 30 °C with shaking. Samples were subjected to SDS-PAGE, and the amounts of myc-Cdc20 were estimated by immunoblotting with anti-Myc tag antibody. Other samples that were not treated with lambda phosphatase were subjected to Phos-tag-SDS-PAGE, as described in ref. (28), except that gels were run at 10 mA per gel for 3 h.
To determine the effect of Cdk1 on the release of myc-Cdc20 from APC/C, nonphosphorylated myc-Cdc20 was first bound to APC/C beads, as described above. Subsequently, beads were washed twice with buffer A and resuspended in 50 μL of a mixture that contained ATP, as described in the Fig. 2A legend. Following incubation with or without Cdk1, as specified in the figures, samples were washed, treated with lambda phosphatase, and subjected to immunoblotting, as described above.
Supplementary Material
Acknowledgments
We thank Dr. Julian Gannon, Cancer Research UK, Clare Hall Laboratories, South Mimms, UK, for mouse monoclonal antibody BT2 directed against pT79 of Xenopus Cdc20. We are grateful to Dr. Michael Fry for insightful comments on the manuscript. This work was supported by grants from the Israel Science Foundation, the Israel Cancer Research Fund, the Gitta and Saul Kurlat Foundation, and the RICBAC Foundation.
Footnotes
Reviewers: M.P., New York University Cancer Institute; and A.V., California Institute of Technology.
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2210367119/-/DCSupplemental.
Data Availability
All study data are included in the article or in SI Appendix.
References
- 1.Barford D., Structural interconversions of the anaphase-promoting complex/cyclosome (APC/C) regulate cell cycle transitions. Curr. Opin. Struct. Biol. 61, 86–97 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Watson E. R., Brown N. G., Peters J. M., Stark H., Schulman B. A., Posing the APC/C E3 ubiquitin ligase to orchestrate cell division. Trends Cell Biol. 29, 117–134 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamano H., APC/C: Current understanding and future perspectives. F1000 Res. 8, F1000 Faculty Rev-725 (2019). [Google Scholar]
- 4.Lahav-Baratz S., Sudakin V., Ruderman J. V., Hershko A., Reversible phosphorylation controls the activity of cyclosome-associated cyclin-ubiquitin ligase. Proc. Natl. Acad. Sci. U.S.A. 92, 9303–9307 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shteinberg M., Protopopov Y., Listovsky T., Brandeis M., Hershko A., Phosphorylation of the cyclosome is required for its stimulation by Fizzy/cdc20. Biochem. Biophys. Res. Commun. 260, 193–198 (1999). [DOI] [PubMed] [Google Scholar]
- 6.Rudner A. D., Murray A. W., Phosphorylation by Cdc28 activates the Cdc20-dependent activity of the anaphase-promoting complex. J. Cell Biol. 149, 1377–1390 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kraft C., et al. , Mitotic regulation of the human anaphase-promoting complex by phosphorylation. EMBO J. 22, 6598–6609 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang S., et al. , Molecular mechanism of APC/C activation by mitotic phosphorylation. Nature 533, 260–264 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiao R., et al. , Mechanism of APC/CCDC20 activation by mitotic phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 113, E2570–E2578 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujimitsu K., Grimaldi M., Yamano H., Cyclin-dependent kinase 1-dependent activation of APC/C ubiquitin ligase. Science 352, 1121–1124 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Musacchio A., Salmon E. D., The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 8, 379–393 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Lara-Gonzalez P., Westhorpe F. G., Taylor S. S., The spindle assembly checkpoint. Curr. Biol. 22, R966–R980 (2012). [DOI] [PubMed] [Google Scholar]
- 13.London N., Biggins S., Signalling dynamics in the spindle checkpoint response. Nat. Rev. Mol. Cell Biol. 15, 736–747 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reddy S. K., Rape M., Margansky W. A., Kirschner M. W., Ubiquitination by the anaphase-promoting complex drives spindle checkpoint inactivation. Nature 446, 921–925 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Eytan E., Sitry-Shevah D., Teichner A., Hershko A., Roles of different pools of the mitotic checkpoint complex and the mechanisms of their disassembly. Proc. Natl. Acad. Sci. U.S.A. 110, 10568–10573 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eytan E., et al. , Disassembly of mitotic checkpoint complexes by the joint action of the AAA-ATPase TRIP13 and p31(comet). Proc. Natl. Acad. Sci. U.S.A. 111, 12019–12024 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sitry-Shevah D., Kaisari S., Teichner A., Miniowitz-Shemtov S., Hershko A., Role of ubiquitylation of components of mitotic checkpoint complex in their dissociation from anaphase-promoting complex/cyclosome. Proc. Natl. Acad. Sci. U.S.A. 115, 1777–1782 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yudkovsky Y., Shteinberg M., Listovsky T., Brandeis M., Hershko A., Phosphorylation of Cdc20/fizzy negatively regulates the mammalian cyclosome/APC in the mitotic checkpoint. Biochem. Biophys. Res. Commun. 271, 299–304 (2000). [DOI] [PubMed] [Google Scholar]
- 19.Labit H., et al. , Dephosphorylation of Cdc20 is required for its C-box-dependent activation of the APC/C. EMBO J. 31, 3351–3362 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung E., Chen R. H., Phosphorylation of Cdc20 is required for its inhibition by the spindle checkpoint. Nat. Cell Biol. 5, 748–753 (2003). [DOI] [PubMed] [Google Scholar]
- 21.D’Angiolella V., Mari C., Nocera D., Rametti L., Grieco D., The spindle checkpoint requires cyclin-dependent kinase activity. Genes Dev. 17, 2520–2525 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hein J. B., Garvanska D. H., Nasa I., Kettenbach A. N., Nilsson J., Coupling of Cdc20 inhibition and activation by BubR1. J. Cell Biol. 220, e202012081 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Izawa D., Pines J., The mitotic checkpoint complex binds a second CDC20 to inhibit active APC/C. Nature 517, 631–634 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alfieri C., et al. , Molecular basis of APC/C regulation by the spindle assembly checkpoint. Nature 536, 431–436 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamaguchi M., et al. , Cryo-EM of mitotic checkpoint complex-bound APC/C reveals reciprocal and conformational regulation of ubiquitin ligation. Mol. Cell 63, 593–607 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujimitsu K., Yamano H., PP2A-B56 binds to Apc1 and promotes Cdc20 association with the APC/C ubiquitin ligase in mitosis. EMBO Rep. 21, e48503 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim T., et al. , Kinetochores accelerate or delay APC/C activation by directing Cdc20 to opposing fates. Genes Dev. 31, 1089–1094 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bancroft J., et al. , PP1 promotes cyclin B destruction and the metaphase-anaphase transition by dephosphorylating CDC20. Mol. Biol. Cell 31, 2315–2330 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hein J. B., Hertz E. P. T., Garvanska D. H., Kruse T., Nilsson J., Distinct kinetics of serine and threonine dephosphorylation are essential for mitosis. Nat. Cell Biol. 19, 1433–1440 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Kataria M., Yamano H., Interplay between phosphatases and the anaphase-promoting complex/cyclosome in mitosis. Cells 8, E814 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishihara H., et al. , Calyculin A and okadaic acid: Inhibitors of protein phosphatase activity. Biochem. Biophys. Res. Commun. 159, 871–877 (1989). [DOI] [PubMed] [Google Scholar]
- 32.Sudakin V., Shteinberg M., Ganoth D., Hershko J., Hershko A., Binding of activated cyclosome to p13(suc1). Use for affinity purification. J. Biol. Chem. 272, 18051–18059 (1997). [DOI] [PubMed] [Google Scholar]
- 33.Kinoshita E., Kinoshita-Kikuta E., Takiyama K., Koike T., Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol. Cell. Proteomics 5, 749–757 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Schwab M., Neutzner M., Möcker D., Seufert W., Yeast Hct1 recognizes the mitotic cyclin Clb2 and other substrates of the ubiquitin ligase APC. EMBO J. 20, 5165–5175 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray A. W., Cell cycle extracts. Methods Cell Biol. 36, 581–605 (1991). [PubMed] [Google Scholar]
- 36.Braunstein I., Miniowitz S., Moshe Y., Hershko A., Inhibitory factors associated with anaphase-promoting complex/cylosome in mitotic checkpoint. Proc. Natl. Acad. Sci. U.S.A. 104, 4870–4875 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaisari S., Sitry-Shevah D., Miniowitz-Shemtov S., Hershko A., Intermediates in the assembly of mitotic checkpoint complexes and their role in the regulation of the anaphase-promoting complex. Proc. Natl. Acad. Sci. U.S.A. 113, 966–971 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article or in SI Appendix.