Abstract
The next robotics frontier will be led by biohybrids. Capable biohybrid robots require microfluidics to sustain, improve, and scale the architectural complexity of their core ingredient: biological tissues. Advances in microfluidics have already revolutionized disease modeling and drug development, and are positioned to impact regenerative medicine but have yet to apply to biohybrids. Fusing microfluidics with living materials will improve tissue perfusion and maturation, and enable precise patterning of sensing, processing, and control elements. This perspective suggests future developments in advanced biohybrids.
Keywords: biohybrid robotics, microfluidics, bioactuators, tissue engineering, soft robotics
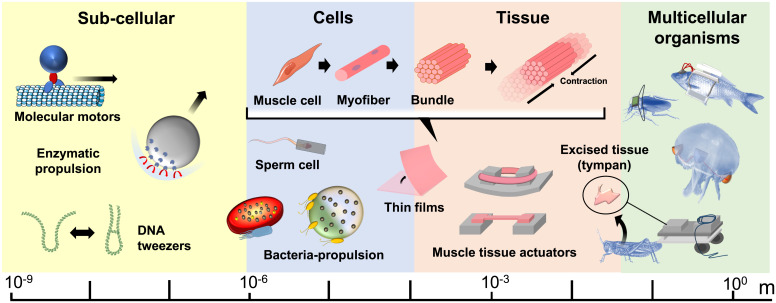
Scientists no longer perceive cells and tissues as pure biological models but also as materials for construction and engineering (1). In robotics, advances in manufacturing and biological sciences support the idea of abandoning an imitating approach (bio-inspiration) in favor of incorporating biological materials into robots (bio-integration). A biological functionality can be implemented in controllable machines at various scales, in which biohybridization ranges from biomolecular processes to whole-body multicellular organisms (Fig. 1) (2, 3). At one end of this range, subcellular actuation is realized through biological linear macromolecules, motor proteins, and enzymes, which generate subnanodevices or nanodevices capable of rotational motion, propulsion, stretching, pulling, and so forth (2). One example is the origami robots based on folded DNA, which move along predefined pathways or work as miniaturized gears or tweezers. These actions are enabled by the activity of specific enzymes, as well as the molecular recognition and mechanical properties of single- and double-DNA strands (4, 5). Another example is the nanosystems that are propelled by biocompatible reactions catalyzed by associated enzymes (6). At the opposite end of the bio-integration range are machines fused with intact multicellular living beings (7, 8). In this domain, physiological interactions between individual animals and artificial devices can be exploited in a bidirectional way: animals (e.g., insects) can be used to control machines (e.g., mobile robots) (8, 9), but also integrated artificial control systems can serve to steer the animal behavior (10–12).
Fig. 1.
Multiscale biohybridization. Subcellular biohybrid systems are actuated by dynamic molecular interactions and the catalytic activity of enzymes. Microrobots can be obtained from individual motile cells (e.g., bacteria, algae, protozoa, sperm cells). Contractile cells can be assembled in vitro to form micro- and milli-scale actuators consisting of functional muscle-tissue constructs. Tissue can be explanted from animals and integrated into machines for various functions (e.g., actuation or auditory sensing). Finally, multicellular organisms can either be used to command the machines or controlled by integrated artificial devices.
Biohybrid robotics has, however, been dominated by biofunctions realized at cellular and tissue levels (2). In fact, the use of cells allows us to capture the properties that are unique to living materials (e.g., their autonomous, multifunctional, and adaptive nature; their proliferative and self-healing abilities) within a compact, multifunctional unit. Cells are modules that can individually generate robots at a small scale (e.g., microrobots based on unicellular organisms) or be assembled into large constructs with tailored configurations (e.g., muscle tissue–based actuators) (13).
Over the last 15 y, different cell types and biomaterials have been combined to engineer biohybrid robots with various motion abilities (e.g., swimming, bending, rotating) (14, 15). These robots were intended for a multitude of applications, including drug loading, delivery, and screening, as well as fluid actuation, bioimaging, cardiac tissue repair, and object manipulation. In biohybrid robots, cells can be used for sensing, communication, integration, or as a power supply, but, thus far, they have been predominantly used for actuation (2, 16–19). In cell-based actuation, two main approaches are possible. The first one is to use single cells, which are endowed with fast motility, to generate robots at the microscale level (microrobots) (20). The second approach uses multicellular constructs organized by self-assembly into tissues that are capable of force generation and deformation due to the intrinsic cell contractility (2, 21).
The growing interest in biohybrid robots is explained by the desirable properties found in cell-based materials, such as their intrinsic softness and degradability, which render them promising in terms of environmental safety and compatibility with dynamic environments (22). Moreover, cells can efficiently extract the energy stored in natural organic molecules and other essential nutrients directly from their surroundings. Thus, they have great potential for use in robots with renewable and autonomous power systems. In addition, cells and cell clusters are adaptive materials that can intelligently react to complex and adverse surroundings. Finally, another intriguing aspect of using living cells for robotics is that a single biological module can have multiple functions (e.g., sensing and actuation), given that these abilities are already present and highly integrated at the single- or multicell level (23). The tight functional integration observed in living systems drives two important considerations regarding engineering with living materials. On the one hand, due to the intrinsic multifunctionality of cells (e.g., actuation, sensing, power supply, signaling, control), biohybrid robots offer a new approach toward device miniaturization. On the other hand, bottom-up tissue engineering can assemble cells into spatially tailorable three-dimensional (3D) configurations and create biorobots with controlled macroscale designs and autonomous multifunctionality. The future evolution of biological machines in both these opposite tendencies (i.e., miniaturization and scaling-up) still requires considerable efforts in technological innovation. One of the core innovations needed will be the ability to finely control the fluids that perfuse the biological tissues. The emerging biomedical discipline of microfluidics has the potential to greatly improve the perfusion of biohybrid robots (24).
This Perspective article describes how living tissues can be combined with microfluidic devices and what benefits are gained from this combination, particularly concerning tissue fabrication, development, and functional control. We will highlight how impactful microfluidic technologies could be in supporting perfusion and vascularization of large tissues, as well as in functionally integrating tissues for use in robotics. Therefore, we will describe how constructs can be assembled from contractile cells to serve as robotic bioactuators and discuss how microfluidics will improve the generation, maturation, and actuation of these constructs. Through this perspective, we will lay out one of the most promising directions for future biorobotic research.
2. Robotic Functions Enabled by Cells
Scientists are growing increasingly curious about biohybrid robots, making efforts to unlock the potential of cells in realizing more performant machines (2, 3). Living cells have demonstrated utility in sensing, control, and power supply, but especially actuation of robots (2). In the following paragraphs, we briefly retrace the historical milestones in using cells for actuation and other functionalities, while delineating the most promising directions for improvement.
2.1. Cells for Actuation.
It was the year 2005 when individual cardiomyocytes were developed into muscle bundles and integrated with silicon micromechanical structures to be used as systems for force transduction and locomotion (25). Since then, bioactuators based on contractile muscle cells have actuated robots for various functions (2, 3). Cardiomuscular thin films could generate micropumps (26) but also adopt functional, 3D conformations for motility tasks (i.e., gripping, walking, and swimming) with a high spatial and temporal control and output forces as high as 4 mN/cm2 (27). Through the years, progress in biofabrication led to larger, more complex, and more performant biohybrid robots, including object manipulators and fast swimmers (28–30). Readers are referred to the SI Appendix for a more detailed description of achievements in bioactuation (24).
The major motivation for advancing muscle-based actuators is to be found in the following unique properties of the natural muscle as an actuator: silent operation; intrinsic softness; biodegradability; self-healing ability; and the use of glucose as an energy-dense, cheap, and eco-friendly fuel (2, 3). Importantly, the intrinsic modular structure of the muscle cells confers inherent scalability to biohybrid actuators, which might extend from the submillimeter to the meter range. As the contraction force generated by a single skeletal myotube and cardiomyocyte are ∼1 and 10 µN, respectively, muscle cells are an intriguing option for actuation with onboarded propulsion systems, as they efficiently produce detectable forces at small scales (i.e., microdomain). In comparison, the smallest conventional actuators (piezoelectric actuators) can only be efficiently scaled in the minidomain (>1 mm) (2, 3). At the same time, biohybrid actuators are attractive for their versatile configurability via bottom-up assembly of cells, which will lead to biomachines with tailored, application-specific designs and integrated multifunctionality. As such, bioactuators are associated with a unique potential in both scale tendencies: on the one hand, contractile cells will enable microscopic bioactuators that are promising for miniaturized biomedical robotics; on the other hand, macroscale bioactuators will enable lifelike movements, self-healing, and soft touch in robots for close contact and direct interaction with humans and other species. Finally, bioactuators could maintain a low mass to generated power ratio over time, which is a unique feature of such systems otherwise precluded in any other type of artificial actuator (2, 3). In fact, a potential power supply for living muscle–based actuators (e.g., a small volume of high-glucose solution) would have a smaller mass than that of the powering systems of conventional actuators. In combination with the high energy efficiency of the muscle (>50%), such a compact setting would allow the biohybrid actuators to minimize the power to mass ratio over long periods of operation (>100 s). In a hypothetical muscle tissue endowed with an autonomous feeding system, this estimated ratio is around 5 kg/kW, which could be kept constant over long time ranges (106 s) (2). In the future, a perfect bioactuator could therefore outperform all other synthetic technologies in long-term tasks. As such, biohybrid actuators represent an attractive option for robots involved in monitoring applications (e.g., for environmental surveillance) that require a long operative lifetime (and therefore an efficient energy economy), in addition to ecological compliance.
Despite the considerable progress achieved in the past decade in manufacturing contractile tissue in vitro, current bioactuators based on engineered muscle tissue are not as performant as native muscle or any other available actuation technology (SI Appendix) (2). As fabricated with volumes ranging from ∼0.1 cm3 to 0.01 m3, synthetic actuators generate forces varying between 102 and 105 N, which are several orders of magnitude higher than those produced by current bioactuators (falling instead in the 10−6 to 10−3 N domain). Moreover, even if keeping the mass to power ratio low over time is promising for the long-lasting operativity of biorobots (2), engineered muscle tissue still suffers from poor medium- and long-term functionality, due to the decay of cell viability and constructs’ stability in vitro over time. Intriguingly, cells from insects and self-stimulatory robot designs can extend the durability of bioactuators to a few months (SI Appendix) (2, 32).
To leverage the performance of bioactuators, we need a deeper understanding of muscle-tissue biology, as well as tailored engineering strategies (2). First, muscle-tissue maturation in vitro must become more efficient, biomimetic, and controllable. In this context, genetic engineering of muscle cells might augment their contractility by modulating the expression of the cytoskeleton and contraction-related proteins (e.g., actin, myosin, troponin, titin), as well as the regulators of the myogenic differentiation and muscle-tissue development. Second, the force dispersion caused by the limited coherence between the synthetic skeleton structure and the muscle tissue has to be reduced by implementing interfaces that maximize the force transfer from the contractile unit to the skeleton (2). Third, to expand their operational versatility, bioactuators need systems that support cell viability over time and in “out-of-the-lab” spaces. Fourth, advanced modeling based on multiscale and multiphysics simulation is warranted to rapidly optimize bioactuators' performance. Finally, we lack fabrication strategies to generate macroscale constructs while controlling the microstructure and maintaining muscle functionality. For example, we need to develop 3D tissue architectures that combine efficient systems for guaranteeing tissue perfusion and scaffold microstructuring for enabling cell alignment and myofiber orientation. Scaling-up bioactuators is essential to achieve output forces that compete with those generated by synthetic actuators, but it poses challenges concerning the survival and reactivity of cells over time, which are: 1) the delivery of nutrients and oxygen through vascularization strategies; 2) an effective interface between the living and nonliving materials; 3) appropriate biomaterials’ properties enabling 3D biofabrication; and 4) efficient methods to expand cells (especially those of primary origin) while retaining their functionality (31).
2.2. Cells for Sensing, Control, and Power Supply.
Cells can perceive and process external stimuli, as well as efficiently extract chemical energy from nutrients found in the environment and convert it into mechanical energy. Bacteria, specialized cells from multicellular organisms, and other organisms can be exploited to fulfill robotic functions of sensing, control, and power supply. In the future, these biological functions will be studied as tightly associated with robotic motor functions.
Chemical biosensing has been demonstrated through many examples of biosensors, typically realized with bacteria (32–35). Biohybrid technologies to recognize and sense mechanical stimuli have been shown through cells specialized in tactile or auditory perception (16, 36), while the optical reactivity of certain cell types and organisms has opened perspectives on biological vision and the control of robots (37–39). At the beginning of the millennium, retina prostheses composed of living cells and engineered materials have started research in biohybrid technologies to restore vision (40–42), and these have been strongly fostered by micro- and nano-technological advancements. Optogenetic genome modification imposes photosensitivity to cells. This acquired, unnatural ability can be exploited to control the native functionality of the cells (e.g., electrochemical signaling or physical contraction for neurons and muscle cells, respectively) (37, 43). Moreover, the light refractivity of certain organisms could be used to sense optical signals in the environment: for example, the plastic and adaptable responses of a plasmodium to light exposure generated integrated information processors for robotic control (38). Overcoming these difficult challenges requires information processing, a task that future biohybrid robots might solve using the computation and learning abilities of cells. More than a decade ago, neurons from humans and other species were already cultured on artificial chips to drive robot locomotion or carry out other functions (44–46), whereas more recently, research on the biological control of robots has focused on using neurons to regulate contraction of muscle-based bioactuators (47–49).
Another possible use of living cells in robotics concerns power sourcing and the creation of bioelectricity. By interfacing spontaneously beating cardiomyocytes with materials that generate voltage via the piezoelectric effect, electric energy could be produced and used to power microelectronic devices (50, 51). Even if not yet demonstrated in robots, emergent biohybrid technologies based on microorganisms are also promising for power supply and chemosynthetic functions to support robots. For example, future machines could work thanks to microbial fuel-cell technology, which exploits bacteria to convert the chemical energy of organic compounds into electricity (52). Alternatively, whole-cell–based photosynthetic biohybrid systems that mediate sustainable solar to chemical conversion (e.g., for light-facilitated carbon dioxide reduction and biohydrogen production) could serve for energy production and storage, supporting future robotics in environmental applications (53).
Cells provide biohybrid robots the potential not only to develop intelligence, understand their environment, and modulate their behavior accordingly, but also to autoregulate their morphology through the proliferative, self-assembly, and regenerative abilities of cells (22). Even if unique cellular functions (e.g., self-healing and adaptation) have huge potential for robotics, they have not been thoroughly investigated yet (54). Studies on embodied intelligence, adaptation, repair, and other advanced properties of robotic living materials are expected to exponentially increase in the next decade.
3. Microfluidics to Bridge Robotics and Tissue Engineering
Stemming from the technological combination of fluid mechanics and microelectromechanical systems, microfluidics is a versatile tool that has made tremendous contributions to several research areas, such as inkjet printing, air and water quality control, fundamental cell biology, biomolecular analysis, and personalized medicine (55). Microfluidics enables one to handle the full range from nano- to milliliter volumes of fluids by using micropneumatic systems (e.g., liquid pumps, gas-driven valves) and microfluidic structures (chips) to direct off-chip and on-chip fluids, respectively. By understanding and controlling fluid behavior in microsystems, we can embrace the philosophy of reducing the size of complex macroscale systems while retaining or improving certain original features. This miniaturization potential has attracted the attention of scientists from other research domains (i.e., robotics and tissue engineering).
3.1. Microfluidics for Robotics.
For instance, microfluidics could valuably synergize with dynamical systems like robots (56). In particular, the ability to master fluids is extremely relevant in soft robotics, in which actuation is often driven by fluidic pressure. As the material deformation in soft robots is typically caused by fluid pressure, microfluidic technology could help achieve a fine distribution of active actuation sites. Nevertheless, there have only been a few publications on the interface between robotics and microfluidics (56–59). This sparsity of research is due to soft robotics being a young discipline that emerged about a decade ago and microfluidics having evolved over the past two decades with a strong focus on biological sciences.
3.2. Microfluidics for Tissue Engineering.
Microfluidics is at the foundation of many applications that contain cells. In particular, microfluidic technologies have been useful in studies on cellular processes (e.g., growth, aging) (60, 61), properties (e.g., adherence, confinement) (62), and microenvironments (63). In recent years, microfluidics and tissue engineering have increasingly converged. Microfluidics allows us to implement fluid perfusion and flow in micrometer- to millimeter-sized channels; culture multiple cell types in one system; tune biochemical gradients; and stimulate cells by mechanical force (64, 65).
In the future, microfluidics will further contribute to tissue engineering by allowing scientists to replicate and control the conditions found in cellular microenvironments and leverage the emerging field of organoids (i.e., organ-like multicellular clusters generated from pluripotent cells). Moreover, microfluidic systems will help us efficiently fabricate tissue-mimicking structures, as they can be used as production lines of micro-engineered units to form larger tissues with architectural and cellular complexity. Finally, microfluidics might allow us to culture large tissue constructs that comprise perfusable channel systems mimicking natural tissue vascularization (66–68). Microfluidics could regulate the applied fluid flows by finely distributing the fluid pressures within the vessel-like channels (66). One could envision that mastering fluid microdynamics in large tissue volumes will ensure fine control over cell survival, thus leading to long-term construct viability in vitro (69).
The growing importance of microfluidics in medicine suggests that its combination with soft robotics might not be limited to the sole actuation of deformable, inert materials (56); it might also be possible to apply the concepts learned in microfluidics to regulate the growth, function, and long-term viability of the living materials that are used in soft biohybrid technologies. Thus far, microfluidics and biohybrid robotics have overlapped in the development of contractile tissues for bioactuation (70). Some of these contractile tissues developed in microfluidic devices have been used as bioactuation modules and perform motion in robots (2, 47, 49, 71). Nevertheless, the future contribution of microfluidics to biohybrid robotics is potentially much broader.
3.3. Potential of Microfluidics for Biohybrid Robotics.
In Fig. 2, we illustrate the potential of microfluidics in the future evolution of biohybrid robots—a combination of tissue engineering and robotics. Even if most biohybrid robotic research has thus far focused on bioactuation, the interest in other biofunctionalities is increasing, as demonstrated by recent publications concerning the sensing of the environment and control of movements (16, 18, 19, 47). Microfluidics will play a crucial role in well-established biofunctionalities, as well as in the emergent ones.
Fig. 2.
Future contribution of microfluidics to biohybrid robots. Microfluidic biohybrid robots will combine different cell types, such as muscle and neural cells. Fine networks of innervation will enable the selective control of specific bioactuators within multiple arrays. Microfluidics allows one to tune the cell microenvironment conditions and microfabricate cell-laden biomaterials. These applications will lead to increasingly versatile and performant biohybrids. Finally, microfluidic tissue perfusion and bioreactor systems will generate biohybrids of larger size and capable of durable and autonomous functions.
The agility of microfluidic platforms in hosting heterocellular culture will facilitate the integration of specific cell types (e.g., neural cells) with muscle tissues that can confer actuation-associated functions (e.g., motor control or proprioception). Fluidic technologies enable us to safely manipulate fragile microtissue units and morphologically control their formation during large-scale production. In microfluidic chips, the cells are cultured in chambers that not only protect them from external environments but also interface them with other functional components of the microfluidic devices. For instance, through microfluidic chips, cells could be implemented in robots for environmental applications, such as detecting water contamination (72). These cells form microfluidic biosensors, which are built within microfluidic chips that can integrate analytical components and be assembled onto swimming robots. In this configuration, cells can be exposed to liquid collected from the environment and then analyzed. Alterations of the cell viability are correlated with the presence of known or unknown contaminants through a transduced signal (typically an impedance- or a chromatophore-based response), thus allowing the researchers to evaluate the quality of aqueous samples. One example is the Envirobot, a compartmented autonomous robot with multiple biosensors made of different vertebrate cells and microorganisms, that was developed for large-scale multiparametric water biosensing (73, 74). Since microfluidics allows us to control the cell exposure to external fluids for biosensing, this technology expands the scope of potential applications of biohybrid machines. In addition to environmental analysis and exploration, microfluidic biosensors could be applied to medical robots that can examine biological samples from patients in situ.
Recognizing biochemicals in the surroundings, whether they are liquid media or air, is a typical use for biosensors, and it can be exploited to create experiences of “taste” or “olfaction” in robots, in which the biochemical detection communicates with the robot control system to affect motion (18). Nevertheless, the perception of other types of stimuli remains widely unexplored. Intriguingly, in recent work, the tympanal organ of a locust was used as an auditory sensor to control the movements of a robotic platform (16). The extracted tissue included the intact auditory nerve and was interfaced with the robot through a microphysiological system creating modular tissue support capable of recording neural activity triggered by sound. This acoustic biosensor was realized in a microfluidic chip that was positioned on top of the robotic platform and equipped with electrodes to transmit the electrophysiological response of the auditory nerve to the robot. While all the electronics necessary to process the signal and run the algorithms for robot control were mounted on the robotic structure, the microfluidic chip offered a chamber to preserve the biosensor’s viability and perform electrophysiological recordings, proving the integrability of complex tissue within robots and its use for innovative functions, such as perceiving auditory stimuli. Soon, microfluidics might unlock the technological implementation of auditory and tactile perception based on cells’ understanding of mechanical waves and loading. Importantly, the next generation of microfluidic biosensors could avoid the use of preformed tissues directly extracted from animals and instead use cell aggregates or tissues built via bottom-up tissue engineering. This way, sensory cells could be patterned according to predefined designs that better interface with the synthetic components of the microchips. Through organ-on-a-chip and biofabrication technologies, microfluidics will therefore enhance the performance of contractile tissue and also enable novel forms and applications of biohybrid robots.
One major trend in biohybrid robotics is scaling up the biological component to realize large-scale capable robots that have a high energy-conversion efficiency. Microfluidics-based microphysiologies will likely enable large, durable, and autonomous biological machines. In such a scenario, microfluidic control might provide sufficient tissue perfusion to efficiently distribute oxygen and nutrients to cells within engineered tissues of large size. Also, portable microfluidic systems could be developed, which would allow cells to be supported within mobile robots: protected and perfused within microfluidic bioreactors, cells could then explore environments outside of the laboratory space. One example of such a portable system is the current program on Chips in Space (75). How and to what extent microfluidics will improve biohybrid robots remain to be seen, but it will most likely begin with an improved assembly of cells and then touch on the realization of more sophisticated robotic biomodules, like bioactuators.
4. Microfluidics for Bioactuators
Actuators made of cells allow biohybrid machines to move and interact with their environment (2, 21, 76). Thus far, bioactuators have efficiently powered only micro- and minimachines (2, 21). Microrobots can be actuated by just one or a few clustered living motile cells (20, 21, 77), but millimeter-sized actuators consisting of tissues require large numbers of contractile cells such as skeletal or cardiac muscle cells (14). Microfluidic platforms can replicate the chemical and biophysical factors of the cell microenvironment and can promote the desired organization of engineered muscles. Specifically, microfluidic platforms can tune cell-to-cell interactions, cell recruitment, and tissue maturation (72), and are promising for the future evolution of bioactuators, in regard to the fabrication of small-scale bioactuators, as well as the generation, vascularization, and control of large-muscle tissue.
4.1. Microscale Bioactuators.
Microfluidics contributes to the fabrication of small bioactuators by: 1) enabling biocompatible and efficient manufacturing methods, and 2) facilitating the integration of multiple cell types into controlled cocultured systems. From a manufacturing perspective, microfluidic flows can produce bubbles to increase the porosity of biomaterials used as scaffolds for muscle cell culture (78). Alternatively, droplet-based microfluidic techniques can continuously generate cell-loaded microgels for tissue regeneration and achieve microscale precision, high monodispersity, and control over geometrical features (79). Various formulations of microgels encapsulating cardiomyocytes have been presented, including mini-heart tissues capable of beating at a high frequency (80). From a coculture perspective, microfluidics will substantially advance small-scale bioactuators by facilitating the creation and study of heterocellular assemblies. In the future, such bioactuators will be characterized by high biomimetism in terms of cellular complexity and functionality. Importantly, coculturing the myogenic cells with other cell types (e.g., neurons, fibroblasts) enhances muscle formation and function (81–84). Furthermore, microfluidic platforms will permit tissue microenvironmental niches that contain regenerative cells with an undifferentiated profile, namely stem cells. For instance, these niches can serve as reservoirs of muscle progenitor cells (i.e., satellite cells), which can be recruited to repair possible tissue damage. Thanks to microfluidics, it is to be expected that microscale bioactuators will further evolve toward controllable geometries with high cellular heterogeneity and biofidel replication of complex tissue functions, such as repair and regeneration.
4.2. Macroscale Bioactuators.
The survival of artificial tissues beyond the millimeter scale can only be sustained by dynamic cell culture (85–87). Microfluidics improves the dynamic cell culture of muscle tissues, specifically the formation of myofibers with tuned biophysical and functional properties (e.g., nuclei density, size, and force generation) (88). After maturation, macroscale bioactuators need to be integrated with fluidic channels running through their whole volume, so that biofactors (e.g., nutrients and signaling inputs) can be homogeneously delivered to all cells. Here, we will show how microfluidics contributes to the development, vascularization, and control of muscle constructs beyond the millimeter scale.
4.2.1. Creation and maturation of macroscale bioactuators.
Microfluidics can synthesize microscale hydrogels that contain myogenic cells; these hydrogels become the building blocks of large contractile tissues (89). The contractile functional units of muscles are myofibers. The cell alignment and the myofiber formation are guided by biomaterials with fibrous micro- or nanostructures, which create an anisotropic topography (90, 91). Microfibers for cell encapsulation are fabricated through microfluidic fiber spinning using various natural and synthetic polymers, including alginate, one of the most popular biomaterials in muscle-tissue engineering (91–93). Recently, Zhao et al. (94) developed a flow-focusing chip to tune the geometries of the formed microfibers and create interpenetrating networks, where skeletal muscle cells are oriented into fiber-like microstructures. Microfluidics can therefore create fibrous elements and anisotropic scaffolds with complex architectures for muscle-tissue growth. In the future, such fine control over the cells and biomaterials will allow us to place contractile cells in specific positions within the bioactuators to achieve fine-grain actuation in soft biohybrid robots. By controlling the morphology and position of the contractile actuation units, we will realize complex actuation schemes and programmable deformations of large-muscle constructs.
Bioactuators are typically fabricated from muscle progenitor cells. Once the constructs are built up, they then have to mature to reach full functionality. Microfluidics enhances muscle-tissue development and maturation by easily implementing coculture systems between muscle cells and other cell types. For example, the presence of fibroblasts can promote the differentiation of myogenic cells, while the presence of adipocytes can alter the availability of metabolites and affect the metabolic processes in muscle cells (95). Endothelial cells and neurons can generate integrated networks essential for the functioning of tissue; these networks of endothelial cells and neurons perfuse tissue (vascularization) or convey signals (innervation), respectively. In summary, future macroscale bioactuators will likely benefit from microfluidics when generating microunits for bottom-up assembly; encapsulating and aligning cells through orientable microfibers; maturing myocytes through hetero-cellular systems; and integrating functional tissue.
4.2.2. Survival and functional control of macroscale bioactuators.
Vascularization aims to distribute oxygen and nutrients across the tissue, as these are essential for cell survival and functionality. Engineered muscles can contain living vessels made of endothelial cells that form a vascularization system. Alternatively, bioactuators can also be vascularized by sculpting networks of perfusable channels in the scaffolds when sacrificial templates are removed from the microfluidic chips (66, 67, 96). These microvessel networks could then be perfused while maintaining fluidic control. Viable tissue constructs at the mesoscale (i.e., centimeter size) might become possible thanks to microfluidic technologies and hierarchical vascular systems mimicking in vivo networks.
While cell survival relies on spread-out fluidic networks for liquid mass transfer, actuation depends on integrated circuits that transmit the stimuli to contract or relax fibers. The contractility and actuation performance of bioactuators can be controlled not only by treating cells with biochemicals but also by controlling them via electrical, optical, and mechanical stimulation (2). Some materials used in microfluidic platforms are compatible with electrical current and light transmission; therefore, these platforms can implement tools to stimulate muscle cells both electrically and optically (71, 97). Moreover, mechanical stimulation in the form of fluid flow can provide additional control over cell function within microchips (64). For instance, 3D cardiomyocyte constructs can be placed into a microfluidic setting to get mechanically strained and electrically paced while receiving pharmacological treatment (98). Microfluidic chips are therefore synthetic biocompatible settings for the control of tissue contractions. These chips represent a step forward in the bio-integration challenge, suggesting that the translation of microfluidic bioactuators to robotics might occur soon due to their ability to interface with both cells and stimulation technologies for the remote control of cell activities.
In addition to biophysical methods, biological effectors, such as neurons, can be used to electrochemically control myocytic activity. Neurons represent a safe and sustainable strategy to regulate muscle contraction, as this stimulation approach protects muscle cells from direct exposure to electrodes, preserves viability, and reduces muscle fatigue (99). As such, muscle neurotization has the potential to extend the functional duration of bioactuators. Several works have shown that skeletal muscle cells and motor neurons can be effectively combined within microfluidic platforms to model the neuromuscular junction (49, 71, 97, 100). In particular, microfluidic chips enable the functional coupling of the two cell types, while spatially controlling the cells through the compartmentalized geometry of the microchips.
In 2019, Aydin et al. (47) demonstrated the neuromuscular actuation of a biohybrid swimmer developed on a millimeter-scale platform in a static culture environment. This work showcased a robot embodying multiple living functions (i.e., actuation and control) through a precise organization of neurons and muscle tissue, opening up new perspectives in biohybrid robotics. While combining multiple tissues is already possible on the small tissue scale within static culture, scaling up such systems for large robots will require dynamical fluidics. Tailorable designs of microfluidic platforms will enable complex configurations of neural networks for motor control of muscle with the benefits of a dynamic culture environment. Importantly, microchannels and microfluidic platforms are used to stretch axons and guide the growth of neurite projections for the bottom-up construction of neural circuits that can be easily combined with microelectrode arrays (101, 102). Neural cells controlling the bioactuators could be stimulated via electrodes patterned into the microfluidic chambers or via microchannels that deliver stimulatory or inhibitory biochemicals (e.g., neurotransmitters) (Fig. 3). Evoking skeletal-muscle responses through neural-cell mediation will extend muscle responsiveness to stimuli and prolong the robot’s activity. In the near future, sympathetic innervation of engineered cardiac muscles might be also achieved, which will enable better control of cardiomyocytes that typically undergo spontaneous contraction and are, therefore, not optimal for controllable robotic actuation. When muscle constructs are endowed with precise innervation patterns and cultured within microfluidic systems, they will power, actuate, and intelligently command durable biohybrid robots.
Fig. 3.
Future microfluidic bioactuation. Microfluidic platforms made of biocompatible and soft materials will contain arrays of muscle-tissue actuators. The platform will be compartmented to organize the interactions among different cell types. Each actuator will be controlled by one neural motor unit (e.g., a neurosphere). The neural units will be stimulated through electrode lines inscribed within the platforms, or channels that deliver functional biochemicals (e.g., neurotransmitters). Pillars and channels will enable us to control the orientation of and guide neurite projections. Microfluidic chambers containing patterned arrays of neural actuators could be fabricated with various morphologies: for instance, sheet-like chambers will deform according to actuators’ arrangement and enable locomotion of medusoid robots mimicking the swimming of jellyfish.
5. Challenges and Future Perspectives
Biohybrid robotics is only in its infancy: much territory is yet to explore, and many challenges are yet to be solved. To transform biohybrids into capable platforms that can operate in real-world settings, several challenges will have to be solved concerning cell longevity, tissue fabrication, and robotic functionality. One of the main issues is that living materials need precise environmental conditions to survive; in particular, the cell environment should enable efficient biochemical and gaseous exchange. Accurately controlling fluidic behavior will likely advance biohybrid robotics on various levels. Precise microfluidic regulation will improve the construction and development of biohybrid systems. First, microfluidics will allow us to generate assemblable building blocks as a foundation for more complex and biomimetic biomodules. Second, microfluidic platforms will enable us to biomechanically stimulate cells to enhance bioactuators’ tissue maturation. Even more important is effective tissue perfusion enabled by microfluidics. Efficient perfusion will pave the way toward engineered macroscale tissue, which will not only revolutionize biohybrid robotics but have drastic repercussions on the overall tissue engineering field. We expect that only microfluidic circuits will enable tissue over the centimeter scale, as they only can guarantee perfusion with fine (i.e., microscale resolution) liquid control and distribution in 3D tissue configurations (92). Moreover, microfluidic tissue engineering can safely fabricate precisely microdesigned tissue architectures, which are crucial for future biorobotic development. Microfluidics can print shapes and assemble building blocks with a specific morphology at microlevel resolution (e.g., microfibers for muscle-tissue formation), all while operating in wet conditions with reduced cellular stress as compared with other biofabrication methods (e.g., extrusion bioprinting) (92). In addition to these major contributions, microfluidics will advance additional aspects of biohybrid robotics, such as the fluid control for cocultivation and improved tissue maturation, as well as the technical implementation of control and monitoring tools (e.g., microchips integrated with technologies for optical stimulation, electrophysiological recording, and mechanostimulation of cells).
Despite this huge potential, to complete the transition of microfluidic technologies to biohybrid robots, future research has to solve one major issue that is common in many biohybrid systems. The hydrophobicity of microfluidic chip constituents reduces their compatibility with the hydrophilic matter of the engineered tissues, made of cells and scaffolding biomaterials (93, 103). To successfully integrate living and nonliving materials, the biological and synthetic phases must become more physicochemically compatible. Investigating medical-grade materials for microfluidic prototyping and production involves more and more scientists who try to establish appropriate bio-interfaces for organ-on-a-chip systems.
Although bioactuation has been the most investigated area thus far, biohybrid robots will certainly evolve to acquire other biological functionalities. One exciting direction that biohybrid robotics might take is to implement different types of neurons, which could provide future machines with computational, plastic, or adaptive abilities (48, 104–106). Implementing cells with a demanding metabolism like neurons renders the microfluidic solution even more meaningful, as it can regulate the nutrient delivery to fulfill precise requirements in terms of glucose and oxygen consumption. The possibility of building microfluidic chips with compartmental designs supports scientists in providing specific nutrients to different cell types within heterocellular systems, as well as in controlling their interaction and functions.
In the next decade, we will hopefully see biohybrid robots that will integrate bioreactor systems and microfluidic control to eventually leave the laboratory space and conquer other environments while keeping their biomodules alive and functional for a long time. Autonomous nutrition and functional sustainability will become possible, solving the limitations that biohybrid machines currently face in environments other than tightly controlled and stationary cell-culture dishes. Perspectives on bioreactor technologies for future autonomous biohybrid robots can be found in the SI Appendix (107–114).
Finally, we have thus far discussed how microfluidics will advance biohybrid robotics in the view of improved tissue fabrication, maturation, and control. However, the opposite relation—will biohybrid robotics advance microfluidics?—also deserves space for consideration. Converging fluids and soft dynamic microbodies can improve our understanding of the fluid dynamics during interaction with compliant materials. In particular, the dynamism, softness, and complex morphologies of bioactuators could be used to advance flow prediction within inertial microfluidic devices (SI Appendix). Biohybrid robotics will raise novel questions in fundamental microfluidics (e.g., concerning inertial focusing and viscous streaming) (115–119) and amplify the spectrum of opportunities in applied microfluidics.
We hope the present Perspective will guide scientists in finding effective bio-integration strategies and inspire their fantasy to implement these solutions into robotic applications. Hopefully, we will see autonomous, untethered, sizable biohybrid robots working efficiently and conquering new territories in the next decade.
Supplementary Material
Acknowledgments
No funding was received relative to this article.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2200741119/-/DCSupplemental.
Data Availability
All study data are included in the article and SI Appendix.
References
- 1.Nguyen P. Q., Courchesne N. D., Duraj-Thatte A., Praveschotinunt P., Joshi N. S., Engineered living materials: Prospects and challenges for using biological systems to direct the assembly of smart materials. Adv. Mater. 30, e1704847 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ricotti L., et al. , Biohybrid actuators for robotics: A review of devices actuated by living cells. Sci. Robot. 2, eaaq0495 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Gao L., et al. , Recent progress in engineering functional biohybrid robots actuated by living cells. Acta Biomater. 121, 29–40 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Endo M., Sugiyama H., DNA origami nanomachines. Molecules 23, E1766 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elbaz J., Moshe M., Willner I., Coherent activation of DNA tweezers: A “SET-RESET” logic system. Angew. Chem. Int. Ed. Engl. 48, 3834–3837 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Arqué X., et al. , Intrinsic enzymatic properties modulate the self-propulsion of micromotors. Nat. Commun. 10, 2826 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y., Sato H., Insect-computer hybrid robot. Mol. Front. J. 2, 30–42 (2018). [Google Scholar]
- 8.Ando N., Emoto S., Kanzaki R., Odour-tracking capability of a silkmoth driving a mobile robot with turning bias and time delay. Bioinspir. Biomim. 8, 016008 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Ando N., Kanzaki R., Using insects to drive mobile robots - hybrid robots bridge the gap between biological and artificial systems. Arthropod Struct. Dev. 46, 723–735 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Erickson J. C., Herrera M., Bustamante M., Shingiro A., Bowen T., Effective stimulus parameters for directed locomotion in Madagascar hissing cockroach biobot. PLoS One 10, e0134348 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi N., Yoshida M., Matsumoto N., Uematsu K., Artificial control of swimming in goldfish by brain stimulation: Confirmation of the midbrain nuclei as the swimming center. Neurosci. Lett. 452, 42–46 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Xu N. W., et al. , Developing biohybrid robotic jellyfish (Aurelia aurita) for free-swimming tests in the laboratory and in the field. Bio Protoc. 11, e3974 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bedau M. A., McCaskill J. S., Packard N. H., Rasmussen S., Living technology: Exploiting life’s principles in technology. Artif. Life 16, 89–97 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Ricotti L., Menciassi A., Bio-hybrid muscle cell-based actuators. Biomed. Microdevices 14, 987–998 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Raman R., Bashir R., Biomimicry, biofabrication, and biohybrid systems: The emergence and evolution of biological design. Adv. Healthc. Mater. 6, 1700496 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Fishel I., et al. , Ear-bot: Locust ear-on-a-chip bio-hybrid platform. Sensors (Basel) 21, 228 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Justus K. B., et al. , A biosensing soft robot: Autonomous parsing of chemical signals through integrated organic and inorganic interfaces. Sci. Robot. 4, eaax0765 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Anderson M. J., Sullivan J. G., Horiuchi T. K., Fuller S. B., Daniel T. L., A bio-hybrid odor-guided autonomous palm-sized air vehicle. Bioinspir. Biomim. 16, 026002 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Huang J. V., Wei Y., Krapp H. G., A biohybrid fly-robot interface system that performs active collision avoidance. Bioinspir. Biomim. 14, 065001 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Patino T., Mestre R., Sánchez S., Miniaturized soft bio-hybrid robotics: A step forward into healthcare applications. Lab Chip 16, 3626–3630 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Carlsen R. W., Sitti M., Bio-hybrid cell-based actuators for microsystems. Small 10, 3831–3851 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Mazzolai B., Laschi C., A vision for future bioinspired and biohybrid robots. Sci. Robot. 5, eaba6893 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Kothari P., Johnson C., Sandone C., Iglesias P. A., Robinson D. N., How the mechanobiome drives cell behavior, viewed through the lens of control theory. J. Cell Sci. 132, jcs234476 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filippi M., Buchner T., Yasa O., Weirich S., Katzschmann R. K., Microfluidic tissue engineering and bio-actuation. Adv. Mater. 34, 2108427 (2022). [DOI] [PubMed] [Google Scholar]
- 25.Xi J., Schmidt J. J., Montemagno C. D., Self-assembled microdevices driven by muscle. Nat. Mater. 4, 180–184 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Tanaka Y., et al. , An actuated pump on-chip powered by cultured cardiomyocytes. Lab Chip 6, 362–368 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Feinberg A. W., et al. , Muscular thin films for building actuators and powering devices. Science 317, 1366–1370 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Morimoto Y., Onoe H., Takeuchi S., Biohybrid robot powered by an antagonistic pair of skeletal muscle tissues. Sci. Robot. 3, eaat4440 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Park S.-J., et al. , Phototactic guidance of a tissue-engineered soft-robotic ray. Science 353, 158–162 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee K. Y., et al. , An autonomously swimming biohybrid fish designed with human cardiac biophysics. Science 375, 639–647 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Won P., Ko S. H., Majidi C., Feinberg A. W., Webster-Wood V. A., Biohybrid actuators for soft robotics: Challenges in scaling up. Actuators 9, 96 (2020). [Google Scholar]
- 32.Belkin S., et al. , Remote detection of buried landmines using a bacterial sensor. Nat. Biotechnol. 35, 308–310 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Borghol N., et al. , Monitoring of E. coli immobilization on modified gold electrode: A new bacteria-based glucose sensor. Biotechnol. Bioprocess Eng.; BBE 15, 220–228 (2010). [Google Scholar]
- 34.Rantala A., et al. , Luminescent bacteria-based sensing method for methylmercury specific determination. Anal. Bioanal. Chem. 400, 1041–1049 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Ivask A., Hakkila K., Virta M., Detection of organomercurials with sensor bacteria. Anal. Chem. 73, 5168–5171 (2001). [DOI] [PubMed] [Google Scholar]
- 36.Salgarella A. R., et al. , A bio-hybrid mechanotransduction system based on ciliate cells. Microelectron. Eng. 144, 51–56 (2015). [Google Scholar]
- 37.Vurro V., Venturino I., Lanzani G., A perspective on the use of light as a driving element for bio-hybrid actuation. Appl. Phys. Lett. 120, 080502 (2022). [Google Scholar]
- 38.Tsuda S., Zauner K.-P., Gunji Y.-P., Robot control with biological cells. Biosystems 87, 215–223 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Raman R., et al. , Optogenetic skeletal muscle-powered adaptive biological machines. Proc. Natl. Acad. Sci. U.S.A. 113, 3497–3502 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer J. S., et al. , Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc. Natl. Acad. Sci. U.S.A. 106, 16698–16703 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marcos L. F., Wilson S. L., Roach P., Tissue engineering of the retina: From organoids to microfluidic chips. J. Tissue Eng. 12, 20417314211059876 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yagi T., Watanabe M., Ohnishi Y., Okuma S., Mukai T., “Biohybrid retinal implant: research and development update in 2005” in Conference Proceedings. 2nd International IEEE EMBS Conference on Neural Engineering, 2005 (Piscataway, NJ, IEEE, 2005), pp. 248–251. DOI: 10.1109/CNE.2005.1419603. [DOI]
- 43.Mahmoudi P., Veladi H., Pakdel F. G., Optogenetics, tools and applications in neurobiology. J. Med. Signals Sens. 7, 71–79 (2017). [PMC free article] [PubMed] [Google Scholar]
- 44.Macis E., Tedesco M., Massobrio P., Raiteri R., Martinoia S., An automated microdrop delivery system for neuronal network patterning on microelectrode arrays. J. Neurosci. Methods 161, 88–95 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Buccelli S., et al. , A neuromorphic prosthesis to restore communication in neuronal networks. iScience 19, 402–414 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pizzi R. M. R., et al. , A cultured human neural network operates a robotic actuator. Biosystems 95, 137–144 (2009). [DOI] [PubMed] [Google Scholar]
- 47.Aydin O., et al. , Neuromuscular actuation of biohybrid motile bots. Proc. Natl. Acad. Sci. U.S.A. 116, 19841–19847 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aydin O., et al. , Development of 3D neuromuscular bioactuators. APL Bioeng. 4, 016107 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cvetkovic C., Rich M. H., Raman R., Kong H., Bashir R., A 3D-printed platform for modular neuromuscular motor units. Microsyst. Nanoeng. 3, 17015 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishisaka T., Sato H., Akiyama Y., Furukawa Y., Morishima K., Muscle-actuated power generator using cultured cardiomyocytes and PZT fiber. Conf. Proc. IEEE Eng. Med. Biol. Soc. (Suppl.), 6685–6688 (2006). [DOI] [PubMed] [Google Scholar]
- 51.Xi J., Dy E., Hung M.-T., Montemagno C.. Development of a self-assembled muscle-powered piezoelectric microgenerator. NSTI-Nanotech. 1, 3–6 ( 2004). [Google Scholar]
- 52.Chaturvedi V., Verma P., Microbial fuel cell: A green approach for the utilization of waste for the generation of bioelectricity. Bioresour. Bioprocess. 3, 38 (2016). [Google Scholar]
- 53.Li L., Xu Z., Huang X., Whole-cell-based photosynthetic biohybrid systems for energy and environmental applications. ChemPlusChem 86, 1021–1036 (2021). [DOI] [PubMed] [Google Scholar]
- 54.Raman R., et al. , Damage, healing, and remodeling in optogenetic skeletal muscle bioactuators. Adv. Healthc. Mater. 6, 1700030 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whitesides G. M., The origins and the future of microfluidics. Nature 442, 368–373 (2006). [DOI] [PubMed] [Google Scholar]
- 56.Gao R. Z., Ren C. L., Synergizing microfluidics with soft robotics: A perspective on miniaturization and future directions. Biomicrofluidics 15, 011302 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Konda A., et al. , Reversible mechanical deformations of soft microchannel networks for sensing in soft robotic systems. Adv. Intell. Syst. 1, 1900027 (2019). [Google Scholar]
- 58.Wehner M., et al. , An integrated design and fabrication strategy for entirely soft, autonomous robots. Nature 536, 451–455 (2016). [DOI] [PubMed] [Google Scholar]
- 59.McDonald K., Ranzani T., Hardware methods for onboard control of fluidically actuated soft robots. Front. Robot. AI 8, 720702 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Samal P., van Blitterswijk C., Truckenmüller R., Giselbrecht S., Grow with the flow: when morphogenesis meets microfluidics. Adv. Mater. 31, e1805764 (2019). [DOI] [PubMed] [Google Scholar]
- 61.Chokkalingam V., et al. , Probing cellular heterogeneity in cytokine-secreting immune cells using droplet-based microfluidics. Lab Chip 13, 4740–4744 (2013). [DOI] [PubMed] [Google Scholar]
- 62.Pelletier J., et al. , Physical manipulation of the Escherichia coli chromosome reveals its soft nature. Proc. Natl. Acad. Sci. U.S.A. 109, E2649–E2656 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Manbachi A., et al. , Microcirculation within grooved substrates regulates cell positioning and cell docking inside microfluidic channels. Lab Chip 8, 747–754 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Polacheck W. J., Li R., Uzel S. G. M., Kamm R. D., Microfluidic platforms for mechanobiology. Lab Chip 13, 2252–2267 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Joseph X., Akhil V., Arathi A., Mohanan P. V., Comprehensive development in organ-on-a-chip technology. J. Pharm. Sci. 111, 18–31 (2022). [DOI] [PubMed] [Google Scholar]
- 66.Tronolone J. J., Jain A., Engineering new microvascular networks on-chip: Ingredients, assembly, and best practices. Adv. Funct. Mater. 31, 2007199 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yue T., et al. , A modular microfluidic system based on a multilayered configuration to generate large-scale perfusable microvascular networks. Microsyst. Nanoeng. 7, 4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dellaquila A., Le Bao C., Letourneur D., Simon-Yarza T., In vitro strategies to vascularize 3D physiologically relevant models. Adv. Sci. (Weinh.) 8, e2100798 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei L., Li W., Entcheva E., Li Z., Microfluidics-enabled 96-well perfusion system for high-throughput tissue engineering and long-term all-optical electrophysiology. Lab Chip 20, 4031–4042 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Visone R., et al. , Cardiac meets skeletal: What’s new in microfluidic models for muscle tissue engineering. Molecules 21, 1128 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vila O. F., et al. , Quantification of human neuromuscular function through optogenetics. Theranostics 9, 1232–1246 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tan L., Schirmer K., Cell culture-based biosensing techniques for detecting toxicity in water. Curr. Opin. Biotechnol. 45, 59–68 (2017). [DOI] [PubMed] [Google Scholar]
- 73.van der Meer J. R., Towards improved biomonitoring tools for an intensified sustainable multi-use environment. Microb. Biotechnol. 9, 658–665 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nano-Tera.ch, Nano-Tera [homepage]. http://www.nanotera.ch/#desc. Accessed 11 August 2022.
- 75.National Center for Advancing Translational Sciences, Tissue chips in space. 2016. https://ncats.nih.gov/tissuechip/projects/space. Accessed 11 August 2022.
- 76.Romano D., Donati E., Benelli G., Stefanini C., A review on animal-robot interaction: From bio-hybrid organisms to mixed societies. Biol. Cybern. 113, 201–225 (2019). [DOI] [PubMed] [Google Scholar]
- 77.Stanton M. M., Trichet-Paredes C., Sánchez S., Applications of three-dimensional (3D) printing for microswimmers and bio-hybrid robotics. Lab Chip 15, 1634–1637 (2015). [DOI] [PubMed] [Google Scholar]
- 78.Chung K. Y., Mishra N. C., Wang C. C., Lin F. H., Lin K. H., Fabricating scaffolds by microfluidics. Biomicrofluidics 3, 22403 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rossow T., Lienemann P. S., Mooney D. J., Cell microencapsulation by droplet microfluidic templating. Macromol. Chem. Phys. 218, 1600380 (2017). [Google Scholar]
- 80.Guerzoni L. P. B., et al. , A layer-by-layer single-cell coating technique to produce injectable beating mini heart tissues via microfluidics. Biomacromolecules 20, 3746–3754 (2019). [DOI] [PubMed] [Google Scholar]
- 81.Uzel S. G. M., Pavesi A., Kamm R. D., Microfabrication and microfluidics for muscle tissue models. Prog. Biophys. Mol. Biol. 115, 279–293 (2014). [DOI] [PubMed] [Google Scholar]
- 82.Krieger J., Park B.-W., Lambert C. R., Malcuit C., 3D skeletal muscle fascicle engineering is improved with TGF-β1 treatment of myogenic cells and their co-culture with myofibroblasts. PeerJ 6, e4939 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thummarati P., Kino-Oka M., Effect of co-culturing fibroblasts in human skeletal muscle cell sheet on angiogenic cytokine balance and angiogenesis. Front. Bioeng. Biotechnol. 8, 578140 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shahin-Shamsabadi A., Selvaganapathy P. R., A 3D self-assembled in vitro model to simulate direct and indirect interactions between adipocytes and skeletal muscle cells. Adv. Biosyst. 4, e2000034 (2020). [DOI] [PubMed] [Google Scholar]
- 85.Stottlemire B. J., Chakravarti A. R., Whitlow J. W., Berkland C. J., He M., Remote-controlled 3D porous magnetic interface toward high-throughput dynamic 3D cell culture. ACS Biomater. Sci. Eng. 7, 4535–4544 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gardel L. S., Serra L. A., Reis R. L., Gomes M. E., Use of perfusion bioreactors and large animal models for long bone tissue engineering. Tissue Eng. Part B Rev. 20, 126–146 (2014). [DOI] [PubMed] [Google Scholar]
- 87.Nguyen B.-N. B., Ko H., Moriarty R. A., Etheridge J. M., Fisher J. P., Dynamic bioreactor culture of high volume engineered bone tissue. Tissue Eng. Part A 22, 263–271 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Juhas M., Bursac N., Roles of adherent myogenic cells and dynamic culture in engineered muscle function and maintenance of satellite cells. Biomaterials 35, 9438–9446 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hosseinzadeh S., Rezayat S. M., Giaseddin A., Aliyan A., Soleimani M., Microfluidic system for synthesis of nanofibrous conductive hydrogel and muscle differentiation. J. Biomater. Appl. 32, 853–861 (2018). [DOI] [PubMed] [Google Scholar]
- 90.Jana S., Levengood S. K., Zhang M., Anisotropic materials for skeletal muscle tissue engineering. Adv. Mater. 28, 10588–10612 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Filippi M., Born G., Felder-Flesch D., Scherberich A., Use of nanoparticles in skeletal tissue regeneration and engineering. Histol. Histopathol. 35, 331–350 (2020). [DOI] [PubMed] [Google Scholar]
- 92.Daniele M. A., Boyd D. A., Adams A. A., Ligler F. S., Microfluidic strategies for design and assembly of microfibers and nanofibers with tissue engineering and regenerative medicine applications. Adv. Healthc. Mater. 4, 11–28 (2015). [DOI] [PubMed] [Google Scholar]
- 93.Filippi M., Born G., Chaaban M., Scherberich A., Natural polymeric scaffolds in bone regeneration. Front. Bioeng. Biotechnol. 8, 474 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao M., et al. , A flexible microfluidic strategy to generate grooved microfibers for guiding cell alignment. Biomater. Sci. 9, 4880–4890 (2021). [DOI] [PubMed] [Google Scholar]
- 95.Isu G., et al. , Fatty acid-based monolayer culture to promote in vitro neonatal rat cardiomyocyte maturation. Biochim. Biophys. Acta Mol. Cell Res. 1867, 118561 (2020). [DOI] [PubMed] [Google Scholar]
- 96.Wan L., Flegle J., Ozdoganlar B., LeDuc P. R., Toward vasculature in skeletal muscle-on-a-chip through thermo-responsive sacrificial templates. Micromachines (Basel) 11, 907 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Osaki T., Uzel S. G. M., Kamm R. D., Microphysiological 3D model of amyotrophic lateral sclerosis (ALS) from human iPS-derived muscle cells and optogenetic motor neurons. Sci. Adv. 4, eaat5847 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marsano A., et al. , Beating heart on a chip: A novel microfluidic platform to generate functional 3D cardiac microtissues. Lab Chip 16, 599–610 (2016). [DOI] [PubMed] [Google Scholar]
- 99.Wan J. J., Qin Z., Wang P. Y., Sun Y., Liu X., Muscle fatigue: General understanding and treatment. Exp. Mol. Med. 49, e384 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yamamoto K., et al. , Development of a human neuromuscular tissue-on-a-chip model on a 24-well-plate-format compartmentalized microfluidic device. Lab Chip 21, 1897–1907 (2021). [DOI] [PubMed] [Google Scholar]
- 101.Forró C., et al. , Modular microstructure design to build neuronal networks of defined functional connectivity. Biosens. Bioelectron. 122, 75–87 (2018). [DOI] [PubMed] [Google Scholar]
- 102.Morin F., et al. , Constraining the connectivity of neuronal networks cultured on microelectrode arrays with microfluidic techniques: A step towards neuron-based functional chips. Biosens. Bioelectron. 21, 1093–1100 (2006). [DOI] [PubMed] [Google Scholar]
- 103.Berthier E., Young E. W. K., Beebe D., Engineers are from PDMS-land, biologists are from Polystyrenia. Lab Chip 12, 1224–1237 (2012). [DOI] [PubMed] [Google Scholar]
- 104.George R., et al. , Plasticity and adaptation in neuromorphic biohybrid systems. iScience 23, 101589 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Keren H., Partzsch J., Marom S., Mayr C. G., A biohybrid setup for coupling biological and neuromorphic neural networks. Front. Neurosci. 13, 432 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Menciassi A., Takeuchi S., Kamm R. D., Biohybrid systems: Borrowing from nature to make better machines. APL Bioeng. 4, 020401 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Haessler U., Kalinin Y., Swartz M. A., Wu M., An agarose-based microfluidic platform with a gradient buffer for 3D chemotaxis studies. Biomed. Microdevices 11, 827–835 (2009). [DOI] [PubMed] [Google Scholar]
- 108.Bayir E., Sahinler M., Celtikoglu M. M., Sendemir A., “Bioreactors in tissue engineering: Mimicking the microenvironment” in Biomaterials for Organ and Tissue Regeneration, Vrana N. E., Knopf-Marques H., Barthes J., Eds. (Sawston, UK, Woodhead Publishing, 2020), chap. 27, pp. 709–752. 10.1016/B978-0-08-102906-0.00018-0. [DOI] [Google Scholar]
- 109.Eghbali H., Nava M. M., Mohebbi-Kalhori D., Raimondi M. T., Hollow fiber bioreactor technology for tissue engineering applications. Int. J. Artif. Organs 39, 1–15 (2016). [DOI] [PubMed] [Google Scholar]
- 110.Bettinger C. J., et al. , Three-dimensional microfluidic tissue-engineering scaffolds using a flexible biodegradable polymer. Adv. Mater. 18, 165–169 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Amador G. J., et al. , Temperature gradients drive bulk flow within microchannel lined by fluid-fluid interfaces. Small 15, e1900472 (2019). [DOI] [PubMed] [Google Scholar]
- 112.Cacucciolo V., et al. , Stretchable pumps for soft machines. Nature 572, 516–519 (2019). [DOI] [PubMed] [Google Scholar]
- 113.Diban N., Stamatialis D., Polymeric hollow fiber membranes for bioartificial organs and tissue engineering applications. J. Chem. Technol. Biotechnol. 89, 633–643 (2014). [Google Scholar]
- 114.Acome E., et al. , Hydraulically amplified self-healing electrostatic actuators with muscle-like performance. Science 359, 61–65 (2018). [DOI] [PubMed] [Google Scholar]
- 115.Agarwal S., Chan F. K., Rallabandi B., Gazzola M., Hilgenfeldt S., An unrecognized inertial force induced by flow curvature in microfluidics. Proc. Natl. Acad. Sci. U.S.A. 118, e2103822118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bhosale Y., Vishwanathan G., Parthasarathy T., Juarez G., Gazzola M., Multi-curvature viscous streaming: Flow topology and particle manipulation. ArXiv [Preprint] (2021). https://arxiv.org/abs/2111.07184 [DOI] [PMC free article] [PubMed]
- 117.Di Carlo D., Inertial microfluidics. Lab Chip 9, 3038–3046 (2009). [DOI] [PubMed] [Google Scholar]
- 118.Zhang J., et al. , Fundamentals and applications of inertial microfluidics: A review. Lab Chip 16, 10–34 (2016). [DOI] [PubMed] [Google Scholar]
- 119.Martel J. M., Toner M., Inertial focusing in microfluidics. Annu. Rev. Biomed. Eng. 16, 371–396 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.