Significance
Sedentary behaviors (SBs), like watching television (TV) or using a computer, take up a large portion of adult leisure time and are linked to increased risk of chronic disease and mortality. We investigate whether SBs are associated with all-cause dementia regardless of physical activity (PA). In this prospective cohort study using data from the UK Biobank, high levels of cognitively passive SB (TV) were associated with increased risk of dementia, while high levels of cognitively active SB (computer) were associated with reduced risk of dementia. These relationships remained strong regardless of PA levels. Reducing cognitively passive TV watching and increasing more cognitively active SBs are promising targets for reducing risk of neurodegenerative disease regardless of levels of PA engagement.
Keywords: sitting, exercise, brain health, Alzheimer’s disease, television
Abstract
Sedentary behavior (SB) is associated with cardiometabolic disease and mortality, but its association with dementia is currently unclear. This study investigates whether SB is associated with incident dementia regardless of engagement in physical activity (PA). A total of 146,651 participants from the UK Biobank who were 60 years or older and did not have a diagnosis of dementia (mean [SD] age: 64.59 [2.84] years) were included. Self-reported leisure-time SBs were divided into two domains: time spent watching television (TV) or time spent using a computer. A total of 3,507 individuals were diagnosed with all-cause dementia over a mean follow-up of 11.87 (±1.17) years. In models adjusted for a wide range of covariates, including time spent in PA, time spent watching TV was associated with increased risk of incident dementia (HR [95% CI] = 1.24 [1.15 to 1.32]) and time spent using a computer was associated with decreased risk of incident dementia (HR [95% CI] = 0.85 [0.81 to 0.90]). In joint associations with PA, TV time and computer time remained significantly associated with dementia risk at all PA levels. Reducing time spent in cognitively passive SB (i.e., TV time) and increasing time spent in cognitively active SB (i.e., computer time) may be effective behavioral modification targets for reducing risk of dementia regardless of engagement in PA.
Exercise and physical activity (PA) have shown promise in reducing rates of cognitive decline, structural brain atrophy, and dementia risk in older adults (1–4). However, despite decades of work, and recommendations to increase exercise participation and levels of PA, engagement in purposeful exercise has not substantially increased (5, 6). In contrast to PA, the effects of sedentary behavior (SB) or time spent sitting on the risk of developing dementia have received less attention, though recent work suggests that SB may have detrimental effects on cognition, brain health, and the development of neurodegenerative disease (7–12). It is critical to understand how SB impacts brain aging since more than half of adults in Europe and the United States engage in high levels of SB and prevalence of high SB has increased over the last 20 years (13, 14). The goal of this study is to determine the association between leisure-time SB and incidence of all-cause dementia and to examine these associations within the context of differing levels of PA engagement.
SB is defined as “any waking behavior characterized by an energy expenditure ≤ 1.5 METs [metabolic equivalent units] while in a sitting or reclining posture” (15). Previous work has shown that SB is associated with increased risk of mortality and the development of cardiometabolic disease (16, 17), and there is an ongoing debate over whether the physiological effects of SBs are fully ameliorated by complementary PA (18, 19). In prospective cohorts and meta-analyses, the associations between SB and mortality are highest in individuals with low levels of PA, and these risks are generally attenuated toward the null in individuals engaged in high levels of PA (20–22). SB occurs in a variety of contexts or domains, and in addition to total sitting time, researchers have often used television (TV) watching as a marker of SB since this is a common behavior done while sitting (23). Unlike total sitting time, TV watching time remains associated with increased risk of mortality, despite significant attenuation, in individuals who engage in high levels of PA (20). Thus, taking types or context of SB into account may be essential to understanding the effects of SB on health outcomes.
SB is also associated with cognitive and structural brain aging (7, 11, 12, 24–26), although associations with cognition, and modification of these associations by PA, lack clarity in the literature (27, 28). Distinctive SB domains may have different effects on cognition, with cognitively passive SBs like watching TV demonstrating negative associations with cognition and cognitively active SBs like computer use or reading showing positive associations with cognition (7, 24, 29–31). In addition, previous work has linked SBs with dementia and Alzheimer’s disease in case-control studies (11). However, a recent prospective study that combined TV viewing with radio listening as one SB exposure found no association with incident dementia (32). Despite continued focus on SB and aspects of cardiometabolic health and mortality, to our knowledge, no prospective studies have examined the association between different SB domains and incident dementia, and none have examined these relationships in relation to PA engagement (33). Here, we use the UK Biobank, the largest prospective cohort of community-dwelling adults to include measures of self-reported SB, PA, and follow-up diagnoses of dementia to test the hypothesis that different leisure-time SB domains (TV watching vs. computer use) are associated with incident dementia and that these associations are not modified by engagement in PA.
Results
There were a total of 146,651 participants after excluding participants younger than 60 years old (n = 284,891), those with prevalent dementia at the start of follow-up (n = 163), and those that did not have complete covariate data (n = 70,709). Baseline characteristics of participants included in this study are shown in Table 1. Over 1,741,333 person years of follow-up (mean follow-up [SD] = 11.87 [1.17] years), there were 3,507 cases of incident dementia.
Table 1.
Baseline characteristics of study participants
| No incident dementia (n = 143,144) | Incident dementia (n = 3,507) | |
|---|---|---|
| Variables | Mean (SD) or n (%) | Mean (SD) or n (%) |
| Age (y) | 64.55 (2.84) | 66.17 (2.7) |
| Sex (female) | 71,147 (49.7) | 1,511 (43.09) |
| Education (college or higher) | 42,227 (29.5) | 805 (22.95) |
| Townsend deprivation index | −1.71 (2.87) | −1.21 (3.13) |
| Ethnicity (white) | 139,889 (97.73) | 3,411 (97.26) |
| Apoe status | ||
| 1 ε4 allele | 33,628 (23.49) | 1,443 (41.15) |
| 2 ε4 alleles | 3,058 (2.14) | 415 (11.83) |
| BMI (kg/m2) | 27.42 (4.4) | 27.51 (4.7) |
| Smoking status | ||
| Never | 71,406 (49.88) | 1,553 (44.28) |
| Former | 60,661 (42.38) | 1,627 (46.39) |
| Current | 11,077 (7.74) | 327 (9.32) |
| Alcohol | ||
| Never | 19,296 (15.46) | 602 (20.38) |
| Moderate | 64,134 (51.37) | 1,447 (48.98) |
| Excessive | 41,415 (33.17) | 905 (30.64) |
| Any chronic condition (present) | 67,952 (47.47) | 2,056 (58.63) |
| Depression (yes) | 51,000 (35.63) | 1,403 (40.01) |
| Healthy diet score (yes) | 77,272 (53.98) | 1,804 (51.44) |
| Social contact (yes) | 139,765 (97.64) | 3,383 (96.46) |
| TV watching time (h/day) | 3.01 (1.64) | 3.39 (1.86) |
| Computer time (h/day) | 1.03 (1.24) | 0.93 (1.31) |
| PA (MET h/day) | 0.94 (0.91) | 0.95 (0.98) |
In minimally adjusted models that included all exposures (SBs and PA) and with age and sex as covariates, PA was associated with reduced risk of incident dementia (Hazard Ratio (HR) [95% CI] = 0.96 [0.93 to 0.99]), TV watching time was associated with increased risk of incident dementia (HR [95% CI] = 1.31 [1.23 to 1.40]), and computer use was associated with reduced risk of incident dementia HR [95% CI] = 0.80 [0.76 to 0.85]). When analyzed by tertile of behavior, individuals in both the medium and the highest PA tertiles showed reduced risk of incident dementia relative to the lowest PA tertile (HRmediumPA [95% CI] = 0.92 [0.85 to 0.99] and HRhighPA [95% CI] = 0.87 [0.81 to 0.95]), the highest tertile of TV viewing time was associated with increased dementia risk relative to low TV time (HR [95% CI] = 1.28 [1.18 to 1.39]; Table 2), and both medium and high computer time were associated with reduced risk of incident dementia (HRmediumComputer [95% CI] = 0.63 [0.58 to 0.68] and HRhighComputer [95% CI] = 0.70 [0.65 to 0.76]; Table 2). Results were generally unchanged in fully adjusted models that included a range of demographic and health covariates (Methods and Table 2). When analyzed as continuous variables, PA (HR [95% CI] = 0.96 [0.94 to 0.99]) and computer use (HR [95% CI] = 0.85 [0.81 to 0.90]) remained associated with reduced risk of dementia, while TV time was associated with increased risk of incident dementia (HR [95% CI] = 1.24 [1.15 to 1.32]). In analyses by tertile for PA or SB, the highest PA tertile was associated with reduced dementia risk relative to the lowest PA tertile (HRhighPA [95% CI] = 0.88 [0.81 to 0.95]), the highest tertile of TV viewing time was associated with increased dementia risk relative to low TV time (HR [95% CI] = 1.21 [1.11 to 1.32]; Table 2), and both medium and high computer time were associated with reduced risk of incident dementia (HRmediumComputer [95% CI] = 0.70 [0.64 to 0.76] and HRhighComputer [95% CI] = 0.76 [0.70 to 0.83]; Table 2). In sensitivity analyses, results were unchanged when including all participants aged 40 years and older or when excluding participants who developed incident dementia within 5 years of baseline (SI Appendix, Table S1). In additional sensitivity analyses, results were similar when missing values were imputed (SI Appendix, Table S1), suggesting missing data did not introduce bias into our results. Finally, results were similar when analyzed using a competing risks regression model that takes into account the competing risk of nondementia mortality in this population and when including employment type at baseline in models (SI Appendix, Table S1).
Table 2.
Risk of incident dementia (n = 3,507) according to SB domain in the UK Biobank
| Model | Variable | HR | Lower | Upper | P value | FDR-corrected P value | p-trend | FDR-corrected p-trend |
|---|---|---|---|---|---|---|---|---|
| Model 1a | PA (MET h/day) | 0.96 | 0.93 | 0.99 | 6.76E-03 | 8.54E-03 | ||
| TV (h/day) | 1.31 | 1.23 | 1.40 | 1.20E-15 | 5.75E-15 | |||
| Computer (h/day) | 0.80 | 0.76 | 0.85 | 1.48E-15 | 5.90E-15 | |||
| PA lowest tertile | 1.00 | |||||||
| PA medium tertile | 0.92 | 0.85 | 0.99 | 3.54E-02 | 4.04E-02 | |||
| PA highest tertile | 0.87 | 0.81 | 0.95 | 1.04E-03 | 1.56E-03 | 5.26E-04 | 8.42E-04 | |
| TV lowest tertile | 1.00 | |||||||
| TV medium tertile | 1.05 | 0.96 | 1.14 | 2.91E-01 | 3.04E-01 | |||
| TV highest tertile | 1.28 | 1.18 | 1.39 | 3.69E-09 | 8.04E-09 | 1.05E-11 | 3.15E-11 | |
| Computer lowest tertile | 1.00 | |||||||
| Computer medium tertile | 0.63 | 0.58 | 0.68 | 1.35E-27 | 3.25E-26 | |||
| Computer highest tertile | 0.70 | 0.65 | 0.76 | 6.40E-18 | 5.12E-17 | 2.14E-20 | 2.57E-19 | |
| Model 2b | PA (MET h/day) | 0.96 | 0.94 | 0.99 | 1.25E-02 | 1.50E-02 | ||
| TV (h/day) | 1.24 | 1.15 | 1.32 | 1.36E-09 | 3.28E-09 | |||
| Computer (h/day) | 0.85 | 0.81 | 0.90 | 1.93E-08 | 3.86E-08 | |||
| PA lowest tertile | 1.00 | |||||||
| PA medium tertile | 0.93 | 0.85 | 1.01 | 6.96E-02 | 7.60E-02 | |||
| PA highest tertile | 0.88 | 0.81 | 0.95 | 1.82E-03 | 2.43E-03 | 1.42E-03 | 2.01E-03 | |
| TV lowest tertile | 1.00 | |||||||
| TV medium tertile | 1.04 | 0.95 | 1.13 | 3.92E-01 | 3.92E-01 | |||
| TV highest tertile | 1.21 | 1.11 | 1.32 | 1.75E-05 | 3.00E-05 | 1.61E-06 | 2.97E-06 | |
| Computer lowest tertile | 1.00 | |||||||
| Computer medium tertile | 0.70 | 0.64 | 0.76 | 1.93E-16 | 1.16E-15 | |||
| Computer highest tertile | 0.76 | 0.70 | 0.83 | 8.99E-11 | 2.40E-10 | 7.97E-12 | 2.73E-11 |
aModel 1: Cox proportional hazards regression was adjusted for age and sex, and all models included TV, computer use, and PA.
bModel 2: Cox proportional hazards regression was adjusted for model 1, education, Townsend deprivation index, ethnicity, APOE ε4 genotype, alcohol consumption, smoking status, chronic disease, depression, healthy diet score, BMI, social contact, and sleep.
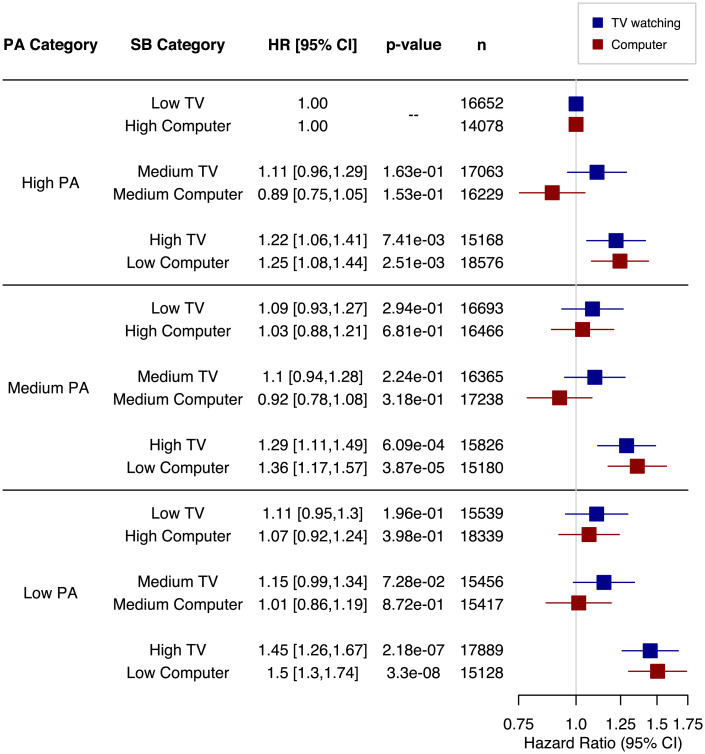
We also investigated whether associations between SB types and engagement in PA are related and whether PA may enhance or attenuate these relationships by including interaction terms in the models. When analyzed as continuous variables, there were significant interactions between PA and both TV time (HRmodel 1 [95% CI] = 1.03 [1.02 to 1.04] and HRmodel 2 [95% CI] = 1.02 [1.00 to 1.03]) and computer time (HRmodel 1 [95% CI] = 0.91 [0.89 to 0.93] and HRmodel 2 [95% CI] = 0.93 [0.91 to 0.96]). To better interpret these interactions, we examined joint associations of PA and SB tertiles (Fig. 1). In analyses of these joint associations, with high PA and low TV watching time as the reference group, there is a clear pattern in which the highest TV viewing times were significantly associated with increased dementia risk across all levels of PA, though there was some minor attenuation of risk with high PA. Similarly, for all PA levels, relative to high PA and high computer time, low computer use time was associated with increased risk of incident dementia despite some attenuation of risk in higher levels of PA (Fig. 1).
Fig. 1.
Joint association of PA and SBs with risk of incident dementia. Models are fully adjusted (Table 2 gives full list of covariates). Reference categories are the lowest risk for each SB (i.e., high PA and low TV or high PA and high computer time), and results show that at all levels of PA, high TV time and low computer time are both associated with increased risk of dementia.
Discussion
In this study, we show that SBs are associated with incident dementia, however SB domain and context determine the direction of the associations. Time spent watching TV, a commonly used marker of SB, is associated with increased risk of all-cause dementia, while leisure-time spent using a computer is associated with reduced risk of incident dementia. These results remain significant following adjustment for demographic, health, and lifestyle variables, including time spent in PA and sleep, as well as in sensitivity analyses.
The associations between SBs and incident dementia are not strongly altered by the level of PA engagement. While there were significant interactions between PA and SBs for incident dementia, both TV time and computer use remain significantly associated with risk of incident dementia, with some attenuation across PA levels. Thus, unlike in studies of cardiovascular disease or all-cause mortality (20–22), the association between leisure-time SB and brain health is not meaningfully related to time spent in PA in this sample. Our results help clarify associations of SB with brain health and suggest that it is not time spent sitting per se but the type or context of leisure-time SB that is associated with dementia risk. While PA is associated with a beneficial reduction in dementia risk, this relationship does not strongly impact associations between leisure-time SB and dementia risk, suggesting two key and potentially separate behavioral pathways for altering risk of incident dementia.
Our results are consistent with previous work showing that the domain or type of SB plays an important role in the health impacts of inactivity. For example, several studies have shown that TV time is associated with mortality and poor cardiometabolic biomarkers, whereas computer time is not (34–36), and compared with total sitting time, TV viewing time may be associated with mortality in those with very high levels of PA, despite significant attenuation relative to low PA (20). Our results are also consistent with studies showing inverse relationships of TV time and computer time with brain outcomes. For example, Bakrania and colleagues (7) showed that TV viewing time was negatively associated with cognition, while computer time was positively related to cognitive performance at baseline in the UK Biobank. In addition, follow-up cognitive testing in this sample showed that TV watching at baseline was associated with increased odds of cognitive decline and computer use at baseline was associated with reduced odds of cognitive decline (7). Others have also found these divergent effects of cognitively active (computer use, playing board games, or reading) and cognitively passive (TV watching) SBs on cognition (37–39). In addition, Nemoto et al. (30) and Wanders et al. (31) reported that TV viewing time had a less consistent relationship with cognition than more cognitively active SBs (computer use or reading), which showed more consistently positive relationships with aspects of cognition in older adults. Overall, previous literature focused on cognitive outcomes underscores the importance of assessing the leisure-time SB domain and is consistent with our findings that cognitively passive and cognitively active SB domains have different associations with risk of incident dementia.
There are several potential explanations for the difference in associations between TV time and computer time on brain health. First, previous links between SB and health have focused on reduced muscle activity during sitting and associated detrimental physiological effects (16, 18, 19). TV viewing time, in particular, has been associated with cardiovascular and cardiometabolic disease risk, and links between cardiovascular health and dementia risk are well established (40, 41). In support of a metabolic physiological hypothesis, sitting while watching TV is associated with uniquely low levels of muscle activity and energy expenditure compared with sitting while using a computer (42, 43). Thus, there may be physiological mechanisms linking TV watching time and brain health, a hypothesis supported by several studies showing that uninterrupted sitting for long periods of time is linked with reduced cerebrovascular hemodynamics (44–46). In this scenario, relatively greater cognitive stimulation during computer use may overcome the detrimental physiological effects of sitting (7). Recent work has shown that cognitively challenging activities, including computer use, can beneficially impact cognition and brain health and have been associated with reduced dementia risk (47, 48). This work fits into a larger literature that supports a positive association between intellectually challenging activities throughout life and reduced late-life dementia risk (49). For example, Staff et al. (50) showed that intellectual engagement, assessed using a wide-ranging questionnaire, was associated with cognitive performance late in life. Thus, cognitively passive SB that typically occurs with TV time may be particularly detrimental for brain health, linked with the physiological effects of sitting, while cognitively active SBs like computer use may benefit brain health due to the enhanced cognitive challenges. Alternatively, these domains of SB may be associated with other behavioral differences that are linked with brain health. For example, it is possible that energy intake during TV viewing differs from other forms of SB, leading to poor cardiometabolic outcomes that may influence brain health (51, 52). However, our results were unchanged following adjustment for diet, alcohol consumption, and obesity, reducing the likelihood of this explanation. In addition, TV viewing often occurs in the evening, and postprandial sedentary time may be detrimental for cardiometabolic physiology (20), which may in turn impact brain health (53). Finally, while we include a range of covariates, we cannot fully rule out that some form of residual confounding may play a role in our results.
Although understanding the underlying mechanisms relating TV time and computer time to incident dementia is important, our results highlight a key behavioral characteristic for public health messaging and modification. Reducing cognitively passive SBs like TV watching and increasing cognitively active SBs like computer use, by even a small amount, may have an important impact on dementia risk in individuals regardless of their engagement in PA.
Strengths and Limitations.
Our study has several strengths, including the size of the UK Biobank cohort, with the largest prospective sample analyzed to date. A second strength is the inclusion of a wide range of covariates linked with increased risk of developing dementia. While our study has notable strengths, there are limitations that may form the foundation for future studies of SB and brain health. Our study uses an observational design, which despite the large sample size and the wide range of covariates available, may still allow for residual or unmeasured confounding. We cannot fully rule out reverse causality in this type of design, however our sensitivity analyses excluded disease diagnoses within 5 years of baseline, and we found no attenuation of incident dementia risk with TV watching or computer use, increasing our confidence that we are not measuring reverse causality in these relationships. SB and PA exposures were measured at only a single time point, and these behaviors may change over time. Future studies examining change in SB and PA behaviors will help us understand the role of long-term SB in brain health. Behaviors reported here were measured using self-reported questionnaire-based data, which may lead to measurement error. For example, Salthouse (54) noted that questionnaires assessing intellectual activities may suffer from positive self-presentation or desirability bias or that participants may have trouble accurately remembering time spent in these activities. Thus, the use of objective methods for measuring both SB and PA are needed in future studies. The reliance on hospital records and death registry for dementia diagnosis is a limitation of this study since it may underestimate cases with dementia in this cohort. However, expert adjudication of cases is not possible in a cohort this large, and a previous validation study supported these methods for determining all-cause dementia and demonstrated agreement with primary care records (55). Finally, the UK Biobank is ethnically and racially homogeneous, which limits generalizability of our findings to more diverse populations, though researchers have argued that generally, results from this cohort could be externally valid for linking exposures with health outcomes (56). Future studies that include a more diverse sample would enhance the generalizability of these results.
Conclusions
TV watching time, a prevalent SB domain, is associated with increased risk of developing all-cause dementia, while computer use is associated with reduced risk of developing dementia. Unlike other health outcomes, associations between SBs and dementia are not strongly attenuated with high levels of PA. A focus on reducing leisure time spent in cognitively passive TV watching and increasing time spent in more cognitively active SBs creates promising targets for reducing risk of neurodegenerative disease regardless of levels of PA engagement.
Methods
The UK Biobank, funded by the Wellcome Trust and other charity agencies, collected extensive baseline and follow-up data from a large community-dwelling study cohort (n ≈ 500,000) between 2006 and 2010 (56–59). Individuals were invited to participate in the study if they were aged 40 to 69 years; lived within 40 km of 1 of 22 assessment centers in England, Scotland, and Wales; and were registered with the National Health Service (56, 58). For the original data collection by the UK Biobank, there were no exclusion criteria based on health, although volunteers were required to attend an assessment center (56, 58). Participants for our analyses were excluded from the proposed study if they reported a diagnosis of Alzheimer’s disease or other age-related dementias at the baseline visit.
Self-Reported SB and PA.
Self-reported levels of SB were measured via a touchscreen questionnaire in all participants at the baseline examination in 2006 to 2010. Two types of leisure-time SB were assessed in this questionnaire: watching TV (“In a typical day, how many hours do you spend watching TV?”) and using a computer (“In a typical day, how many hours do you spend using the computer? [Do not include using a computer at work]”). Participants were allowed to answer less than 1 h or any number of hours between 0 and 24 h (in whole numbers). Participants who answered greater than 8 h for TV watching and greater than 6 h for computer use were asked to confirm their response. For individuals who responded with less than 1 h per day (but greater than 0 h), we recoded their value to 0.5 h/day. Self-reported moderate PA (MPA), vigorous PA (VPA), and walking were measured via the touchscreen questionnaire in all participants at the baseline visit, with questions based on the International Physical Activity Questionnaire (IPAQ) (60). We used IPAQ summary data from Cassidy et al. (61), whose analysis followed IPAQ Research Committee recommendations (62). Time spent in each activity was weighted by energy expended in these activities (3.3 METs for walking, 4.0 METs for MPA, and 8.0 METs for VPA) to calculate a sum of MET minutes per week of PA and were converted to MET hours per day for analyses. For analyses here, PA and SB exposures were both assessed as continuous variables (after square root transformation) and grouped into tertiles of SB and PA. For TV watching time, the lower boundary of the second tertile was 2 h and the lower boundary of the third tertile was 4 h. For computer use time, the lower boundary of the second tertile was 0.5 h and the lower boundary of the third tertile was 1 h. For PA, the lower boundary of the second tertile was 2.81 MET h/day and the lower boundary of the third tertile was 7.10 MET h/day.
Dementia Diagnosis.
Hospital inpatient records were used to determine all-cause dementia diagnoses (58, 63). Hospital records were drawn from the Hospital Episode Statistics for England, the Scottish Morbidity Record data for Scotland, and the Patient Episode Database for Wales, which contained hospital admission dates and diagnoses. Additional cases were derived from linkages with death register datasets for England, Scotland, and Wales (58, 63). The International Classification of Diseases (ICD) coding system was used to determine primary or secondary diagnoses of all-cause dementia and Alzheimer’s disease from hospital records or as an underlying or contributing cause of death from death register linkages [see Lourida et al. (63) for ICD9 and ICD10 codes used for diagnoses].
Statistical Analyses.
We conducted complete case analyses and restricted analyses to participants 60 years and older at the start of follow-up with complete covariate, exposure, and outcome data (58). We used Cox proportional hazard regression models to examine the associations of different SB measures with incident all-cause dementia. Participants were followed up from their baseline visit until their first dementia diagnosis, death, loss to follow-up, or last date of hospital admission from the respective database (England: March 31, 2021; Wales: March 6, 2018; and Scotland: May 7, 2021) (58). We tested the proportionality of hazards assumption using Schoenfeld residuals (64) (P > 0.05 for all models).
Models were adjusted for a range of covariates, all measured at the baseline visit, that accounted for demographic, lifestyle, and health characteristics. First, model 1 was mutually adjusted for TV, computer use, and PA and was further adjusted for age at the start of follow-up and sex. The fully adjusted model (model 2) was then evaluated, which included the following additional covariates: education (higher education or no higher education), socioeconomic status using the Townsend deprivation index (a higher score is associated with higher deprivation), the presence of the APOE ε4 allele (genetic risk for dementia; 0, 1, or 2 ε4 alleles), ethnicity (white or nonwhite), chronic conditions (received a diagnosis of cardiovascular disease, diabetes, or cancer), smoking status (never smoker, former smoker, or current smoker), alcohol use [none, moderate alcohol consumption, or high alcohol consumption following Lourida et al. (63)], body mass index (BMI), depression (self-report or doctor diagnosed), adherence to a healthy diet [derived from Lourida et al. (63)], hours of sleep (self-reported from the question: “About how many hours sleep do you get in every 24 hours? [Please include naps]”), and social contact (whether or not an individual had at least two of the following each week: a visit from a family member, someone to confide in, or engagement in a leisure activity [sports club or gym, pub or social club, religious group, adult education class, or other group activity]) (65). Correction for multiple testing was implemented for primary outcomes using the Benjamini-Hochberg false discovery rate (FDR) method (66), with FDR < 0.05 considered as statistically significant. In sensitivity analyses, we excluded participants who developed dementia within 5 years of the start of follow-up and included all participants aged 40 years and older in Cox proportional hazards models. In addition, we carried out further sensitivity analyses to examine the impact of missing exposure and covariate data. Missing data were imputed using multiple imputation by chained equations (MICE), with 40 imputations using the mice package in R statistical computing software (67). Rubin’s rules (68) were followed to compute hazard ratios. We assessed risk in the presence of competing events to examine whether competing risk of death from causes other than dementia impacts the associations between SB, PA, and dementia using the cmprsk package in R. These analyses take into account participants who die without experiencing the event of interest (i.e., diagnosis of dementia), treating these deaths as competing risks, which provides a more conservative estimate of associations among SB, PA, and dementia compared with treating these participants as censored (69). Finally, because complexity of job requirements is associated with cognitive performance (70), we include a categorization of job type at baseline (we excluded participants who were retired and unemployed at baseline) in models, where job complexity was divided into two categories [managers and skilled positions vs. unskilled positions following Howe et al. (65)].
To determine whether associations between incident dementia and SB domains were altered by engagement in PA, we included PA or PA tertile as a covariate in our models as described above. In secondary evaluations, we examined the interactions of SBs and PA.
Supplementary Material
Acknowledgments
This work was conducted using the UK Biobank dataset under application 21259. Study authors are supported by the NIH (P30AG072980, P30AG019610, R56AG067200, R01AG049464, and R01AG72445), the State of Arizona and Arizona Department of Health Services, and the McKnight Brain Research Foundation.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2206931119/-/DCSupplemental.
Data, Materials, and Software Availability
Data used in this study are available by application to the UK Biobank (71). All other study data are included in the article and/or SI Appendix.
References
- 1.Norton S., Matthews F. E., Barnes D. E., Yaffe K., Brayne C., Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol. 13, 788–794 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Raichlen D. A., Alexander G. E., Adaptive capacity: An evolutionary neuroscience model linking exercise, cognition, and brain health. Trends Neurosci. 40, 408–421 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erickson K. I., Leckie R. L., Weinstein A. M., Physical activity, fitness, and gray matter volume. Neurobiol. Aging 35 (suppl. 2), S20–S28 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erickson K. I., et al. , Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U.S.A. 108, 3017–3022 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keadle S. K., McKinnon R., Graubard B. I., Troiano R. P., Prevalence and trends in physical activity among older adults in the United States: A comparison across three national surveys. Prev. Med. 89, 37–43 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitfield G. P., et al. , Trends in meeting physical activity guidelines among urban and rural dwelling adults—United States, 2008–2017. MMWR Morb. Mortal. Wkly. Rep. 68, 513–518 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakrania K., et al. , Associations between sedentary behaviors and cognitive function: Cross-sectional and prospective findings from the UK biobank. Am. J. Epidemiol. 187, 441–454 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Felez-Nobrega M., Hillman C. H., Dowd K. P., Cirera E., Puig-Ribera A., ActivPAL™ determined sedentary behaviour, physical activity and academic achievement in college students. J. Sports Sci. 36, 2311–2316 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Heisz J. J., Vandermorris S., Wu J., McIntosh A. R., Ryan J. D., Age differences in the association of physical activity, sociocognitive engagement, and TV viewing on face memory. Health Psychol. 34, 83–88 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Ku P.-W., Liu Y.-T., Lo M.-K., Chen L.-J., Stubbs B., Higher levels of objectively measured sedentary behavior is associated with worse cognitive ability: Two-year follow-up study in community-dwelling older adults. Exp. Gerontol. 99, 110–114 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Lindstrom H. A., et al. , The relationships between television viewing in midlife and the development of Alzheimer’s disease in a case-control study. Brain Cogn. 58, 157–165 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Siddarth P., Burggren A. C., Eyre H. A., Small G. W., Merrill D. A., Sedentary behavior associated with reduced medial temporal lobe thickness in middle-aged and older adults. PLoS One 13, e0195549 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.López-Valenciano A., et al. , Changes in sedentary behaviour in European Union adults between 2002 and 2017. BMC Public Health 20, 1206 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuna J. M. Jr., Johnson W. D., Tudor-Locke C., Adult self-reported and objectively monitored physical activity and sedentary behavior: NHANES 2005-2006. Int. J. Behav. Nutr. Phys. Act. 10, 126 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tremblay M. S., et al. ; SBRN Terminology Consensus Project Participants, Sedentary behavior research network (SBRN)–terminology consensus project process and outcome. Int. J. Behav. Nutr. Phys. Act. 14, 75 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamilton M. T., The role of skeletal muscle contractile duration throughout the whole day: Reducing sedentary time and promoting universal physical activity in all people. J. Physiol. 596, 1331–1340 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Healy G. N., Matthews C. E., Dunstan D. W., Winkler E. A., Owen N., Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003-06. Eur. Heart J. 32, 590–597 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton M. T., Healy G. N., Dunstan D. W., Zderic T. W., Owen N., Too little exercise and too much sitting: Inactivity physiology and the need for new recommendations on sedentary behavior. Curr. Cardiovasc. Risk Rep. 2, 292–298 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raichlen D. A., et al. , Sitting, squatting, and the evolutionary biology of human inactivity. Proc. Natl. Acad. Sci. U.S.A. 117, 7115–7121 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ekelund U., et al. ; Lancet Physical Activity Series 2 Executive Committe; Lancet Sedentary Behaviour Working Group, Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet 388, 1302–1310 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Ekelund U., et al. , Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: Systematic review and harmonised meta-analysis. BMJ 366, l4570 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stamatakis E., et al. , Sitting time, physical activity, and risk of mortality in adults. J. Am. Coll. Cardiol. 73, 2062–2072 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Patterson F., et al. , Towards a demographic risk profile for sedentary behaviours in middle-aged British adults: A cross-sectional population study. BMJ Open 8, e019639 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Copeland J. L., et al. , Sedentary time in older adults: A critical review of measurement, associations with health, and interventions. Br. J. Sports Med. 51, 1539 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Falck R. S., Davis J. C., Liu-Ambrose T., What is the association between sedentary behaviour and cognitive function? A systematic review. Br. J. Sports Med. 51, 800–811 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Fancourt D., Steptoe A., Television viewing and cognitive decline in older age: Findings from the English Longitudinal Study of Ageing. Sci. Rep. 9, 2851 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loprinzi P., The effects of sedentary behavior on memory and markers of memory function: A systematic review. Phys. Sportsmed. 47, 387–394 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Olanrewaju O., Stockwell S., Stubbs B., Smith L., Sedentary behaviours, cognitive function, and possible mechanisms in older adults: A systematic review. Aging Clin. Exp. Res. 32, 969–984 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Anatürk M., Suri S., Smith S. M., Ebmeier K. P., Sexton C. E., Leisure activities and their relationship with MRI measures of brain structure, functional connectivity, and cognition in the UK Biobank cohort. Front. Aging Neurosci. 13, 734866 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nemoto Y., et al. , The association of single and combined factors of sedentary behavior and physical activity with subjective cognitive complaints among community-dwelling older adults: Cross-sectional study. PLoS One 13, e0195384 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wanders L., et al. , Association between sedentary time and cognitive function: A focus on different domains of sedentary behavior. Prev. Med. 153, 106731 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Wang H.-X., Karp A., Winblad B., Fratiglioni L., Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: A longitudinal study from the Kungsholmen project. Am. J. Epidemiol. 155, 1081–1087 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Dillon K., Gardiner P. A., Association between sedentary behaviour and risk of dementia: An evidence gap. Transl. Psychiatry 11, 195 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altenburg T. M., de Kroon M. L., Renders C. M., Hirasing R., Chinapaw M. J., TV time but not computer time is associated with cardiometabolic risk in Dutch young adults. PLoS One 8, e57749 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basterra-Gortari F. J., et al. , Television viewing, computer use, time driving and all-cause mortality: The SUN cohort. J. Am. Heart Assoc. 3, e000864 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nang E. E. K., et al. , Television screen time, but not computer use and reading time, is associated with cardio-metabolic biomarkers in a multiethnic Asian population: A cross-sectional study. Int. J. Behav. Nutr. Phys. Act. 10, 70 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamer M., Stamatakis E., Prospective study of sedentary behavior, risk of depression, and cognitive impairment. Med. Sci. Sports Exerc. 46, 718–723 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kesse-Guyot E., et al. , Cross-sectional and longitudinal associations of different sedentary behaviors with cognitive performance in older adults. PLoS One 7, e47831 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J. Y., et al. , Leisure activity and risk of cognitive impairment: The Chongqing aging study. Neurology 66, 911–913 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Newman A. B., et al. , Dementia and Alzheimer’s disease incidence in relationship to cardiovascular disease in the Cardiovascular Health Study cohort. J. Am. Geriatr. Soc. 53, 1101–1107 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Wolters F. J., et al. , Coronary heart disease, heart failure, and the risk of dementia: A systematic review and meta-analysis. Alzheimers Dement. 14, 1493–1504 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Barnett R. K., Greever C., Yagi K., Rhoan B., Keadle S. K., Reexamining the energy cost of sedentary behaviors from the 2011 adult compendium. J. Phys. Act. Health 18, 206–211 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Mansoubi M., et al. , Energy expenditure during common sitting and standing tasks: Examining the 1.5 MET definition of sedentary behaviour. BMC Public Health 15, 516 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carter S. E., et al. , Regular walking breaks prevent the decline in cerebral blood flow associated with prolonged sitting. J. Appl. Physiol. (1985) 125, 790–798 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Perdomo S. J., Gibbs B. B., Kowalsky R. J., Taormina J. M., Balzer J. R., Effects of alternating standing and sitting compared to prolonged sitting on cerebrovascular hemodynamics. Sport Sci. Health 15, 375–383 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wheeler M. J., et al. , Morning exercise mitigates the impact of prolonged sitting on cerebral blood flow in older adults. J. Appl. Physiol. (1985) 126, 1049–1055 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Almeida O. P., et al. , Older men who use computers have lower risk of dementia. PLoS One 7, e44239 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yates L. A., Ziser S., Spector A., Orrell M., Cognitive leisure activities and future risk of cognitive impairment and dementia: Systematic review and meta-analysis. Int. Psychogeriatr. 28, 1791–1806 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Hertzog C., Kramer A. F., Wilson R. S., Lindenberger U., Enrichment effects on adult cognitive development: Can the functional capacity of older adults be preserved and enhanced? Psychol. Sci. Public Interest 9, 1–65 (2008). [DOI] [PubMed] [Google Scholar]
- 50.Staff R. T., Hogan M. J., Williams D. S., Whalley L. J., Intellectual engagement and cognitive ability in later life (the “use it or lose it” conjecture): Longitudinal, prospective study. BMJ 363, k4925 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kivimäki M., et al. ; IPD-Work consortium, Physical inactivity, cardiometabolic disease, and risk of dementia: An individual-participant meta-analysis. BMJ 365, l1495 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wagner M., et al. , Evaluation of the concurrent trajectories of cardiometabolic risk factors in the 14 years before dementia. JAMA Psychiatry 75, 1033–1042 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohara T., et al. , Glucose tolerance status and risk of dementia in the community: The Hisayama study. Neurology 77, 1126–1134 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Salthouse T. A., Mental exercise and mental aging: Evaluating the validity of the “use it or lose it” hypothesis. Perspect. Psychol. Sci. 1, 68–87 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Wilkinson T., et al. ; Dementias Platform UK and UK Biobank, Identifying dementia outcomes in UK Biobank: A validation study of primary care, hospital admissions and mortality data. Eur. J. Epidemiol. 34, 557–565 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fry A., et al. , Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am. J. Epidemiol. 186, 1026–1034 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Collins R., What makes UK Biobank special? Lancet 379, 1173–1174 (2012). [DOI] [PubMed] [Google Scholar]
- 58.Raichlen D. A., et al. , Association of physical activity with incidence of dementia is attenuated by air pollution. Med. Sci. Sports Exerc. 54, 1131–1138 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sudlow C., et al. , UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12, e1001779 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Craig C. L., et al. , International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 35, 1381–1395 (2003). [DOI] [PubMed] [Google Scholar]
- 61.Cassidy S., Chau J. Y., Catt M., Bauman A., Trenell M. I., Cross-sectional study of diet, physical activity, television viewing and sleep duration in 233,110 adults from the UK Biobank; the behavioural phenotype of cardiovascular disease and type 2 diabetes. BMJ Open 6, e010038 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.IPAQ Research Committee, Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ)—Short and Long Forms. https://sites.google.com/site/theipaq/scoring-protocol (2005). Accessed 2 August 2022.
- 63.Lourida I., et al. , Association of lifestyle and genetic risk with incidence of dementia. JAMA 322, 430–437 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schoenfeld D., Partial residuals for the proportional hazards regression model. Biometrika 69, 239–241 (1982). [Google Scholar]
- 65.Howe L. D., et al. , Effects of body mass index on relationship status, social contact and socio-economic position: Mendelian randomization and within-sibling study in UK Biobank. Int. J. Epidemiol. 49, 1173–1184 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benjamini Y., Hochberg Y., Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol. 57, 289–300 (1995). [Google Scholar]
- 67.Van Buuren S., Groothuis-Oudshoorn K., mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 45, 1–67 (2011). [Google Scholar]
- 68.Rubin D. B., Multiple Imputation for Nonresponse in Surveys (John Wiley & Sons, 2004), vol. 81. [Google Scholar]
- 69.Berry S. D., Ngo L., Samelson E. J., Kiel D. P., Competing risk of death: An important consideration in studies of older adults. J. Am. Geriatr. Soc. 58, 783–787 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schooler C., Use it-and keep it, longer, probably: A reply to Salthouse (2006). Perspect. Psychol. Sci. 2, 24–29 (2007). [DOI] [PubMed] [Google Scholar]
- 71.Raichlen D. A., et al. , Data from “Leisure-time sedentary behaviors are differentially associated with all-cause dementia regardless of engagement in physical activity. UK Biobank. https://www.ukbiobank.ac.uk. Accessed 8 August 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in this study are available by application to the UK Biobank (71). All other study data are included in the article and/or SI Appendix.