Abstract
NBU1 is an integrated 10.3-kbp Bacteroides element, which can excise and transfer to Bacteroides or Escherichia coli recipients, where it integrates into the recipient genome. NBU1 relies on large, >60-kbp, conjugative transposons for factors that trigger excision and for mobilization of the circular form to recipients. Previously, we showed that a single integrase gene, intN1, was necessary and sufficient for integration of NBU1 into its target site on the Bacteroides or E. coli genome. We now show that an unexpectedly large region of NBU1 is required for excision. This region includes, in addition to intN1, four open reading frames plus a large region downstream of the fourth gene, prmN1. This downstream sequence was designated XRS, for “excision-required sequence.” XRS contains the oriT of the circular form of NBU1 and about two-thirds of the adjacent mobilization gene, mobN1. This is the first time an oriT, which is involved in conjugal transfer of the circular form, has been implicated in excision. Disruption of the gene immediately downstream of intN1, orf2, completely abolished excision. The next open reading frame, orf2x, was too small to be disrupted, so we still do not know whether it plays a role in the excision reaction. Deletions were made in each of two open reading frames downstream of orf2x, orf3 and prmN1. Both of these deletions abolished excision, indicating that these genes are also essential for excision. Attempts to complement various mutations in the excision region led us to realize that a portion of the excision region carrying prmN1 and part of the XRS (XRSHIII) inhibited excision when provided in trans on a multicopy plasmid (8 to 10 copies per cell). However, a fragment carrying prmN1, XRS, and the entire mobilization gene, mobN1, did not have this effect. The smaller fragment may be interfering with excision by attracting proteins made by the intact NBU1 and thus removing them from the excision complex. Our results show clearly that excision is a complex process that involves several proteins and a cis-acting region (XRS) which includes the oriT. We suggest that this complex excision machinery may be necessary to allow NBU1 to coordinate nicking at the ends during excision and nicking at the oriT during conjugal transfer, to prevent premature nicking at the oriT before NBU1 has excised and circularized.
NBUs (nonreplicating Bacteroides units) are 10- to 12-kbp integrated elements that can be excised and mobilized in trans by tetracycline-inducible Bacteroides conjugative transposons. Two regions of NBU1, the best studied of the NBUs, have been characterized. The NBU1 integrase gene, intN1, is located near one end of the element and is transcribed away from the end. This gene and the upstream NBU1 integration region, attN1, are necessary and sufficient for integration. IntN1 is a member of the phage lambda family of site-specific integrases, although it is only distantly related to the phage lambda integrase (26). In Bacteroides species, NBU1 integration is site specific and the primary target site contains a 14-bp sequence that is located in the 3′ end of the Leu-tRNA gene. NBU1 also integrates in Escherichia coli, but the integration is less specific and the target site sequences have only partial identity to the Bacteroides 14-bp target site sequence (26, 27). In both Bacteroides and E. coli, the integration of NBU1 is independent of RecA (6).
A second region of NBU1 has been characterized previously, a 2-kbp region near the center of the element which is necessary for mobilization of the circular form. This region contains the transfer origin (oriT) and the mobN1 gene, which encodes the protein that nicks at the oriT during mobilization (15, 16). MobN1 is a distant relative of the IncP TraI (16, 30). Genes similar to mobN1 have also been found on the mobilizable Bacteroides transposon Tn4555, the mobilizable Bacteroides plasmids pBI143 and pIP421, and the mobilizable gram-positive bacterial plasmid pMV158 (9, 30, 31, 33, 40). All of the small Bacteroides plasmids and the 10- to 12-kbp integrated elements are mobilized not only by Bacteroides conjugative transposons but also by the IncP plasmids of the enterics (15–17, 24, 28, 30, 40, 41). Smith and Parker (31) have located the nic site in the oriT on Tn4555. Since the DNA sequence of NBU1 is 86% identical to Tn4555 in this region, the NBU1 nic site is probably in the same place. Although NBU1 and Tn4555 have high DNA sequence identity in the oriT-mob region (78%), they appear to be quite different outside this region (16, 26, 39).
We report here the complete sequence of NBU1 and define the region of NBU1 that is necessary for its excision and for formation of the circular transfer intermediate. This region proved to be unexpectedly large and contains six open reading frames. By contrast, phage lambda needs only its integrase and one small basic protein, Xis, for excision (1). However, lambda does not have an oriT. The gram-positive bacterial conjugative transposon Tn916 does have an internal oriT, but the promoter for the transfer functions and the operon for the transfer genes are separated when the element is integrated. The transfer functions that nick at the oriT of Tn916 are not made until the element has excised and circularized (4). We propose that the more complex excision system of NBU1 may be needed because the transfer functions are provided in trans by the conjugative transposons. The efficient excision of NBU1 requires the coordination of excision (nicking at the ends) with the nicking at the internal oriT, the step that initiates the transfer of the circular intermediate.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. E. coli DH5αMCR (Gibco BRL) was used for most of the cloning and vector construction. E. coli strains S17-1 (29) and DH5αMCR were used as host strains for the donors, and DH5αMCR was the E. coli recipient in Bacteroides-to-E. coli matings. These strains were grown aerobically on Luria-Bertani broth or agar. The following antibiotic concentrations were used unless otherwise noted: ampicillin, 100 μg/ml; chloramphenicol, 20 μg/ml; and kanamycin, 50 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Comments and references |
|---|---|---|
| E. coli | ||
| DH5αMCR | RecA | GibcoBRL |
| S17-1 | RecA, RP4 Tra | Contains the transfer functions of RP4 integrated in the chromosome (29) |
| B. thetaiotaomicron BT5482 strains | ||
| BT4100N1-S1 | Thy Tpmr | Spontaneous thymidine-requiring (Thy−) strain that results in resistance to trimethoprim (Tpmr); contains an integrated NBU1 (28) |
| BT4104 | Thy−TpmrTcr | Spontaneous thymidine-requiring strain that contains CTnERL |
| BT4104N1-3 | Thy−TpmrTcr | BT4104 containing NBU1 integrated into primary insertion site (28) |
| Plasmids | ||
| pNLY1 | (AprCmr) Cmr | 2.5-kbp PstI-KpnI fragment containing IS4351-cat from pFD308 blunted and cloned into blunted AatII site of pFD160 (32, 33; this study) |
| pAMS9 | (Knr Cmr) Cmr Tcr | tetQ rteA rteB operon cloned on pNJR24 which is compatible with pFD160 vectors (34) |
| pLYL05 | (Apr) Cfxr | Similar to pNLY1 except contains the cfxA gene (15, 32) |
| pLYL7 | (Apr Mob−) Cfxr Mob− | oriT vector constructed to study oriT/MobN1 of NBU1 (15, 16) |
| pY5, pY11, and pY17 | (Apr Tcr) Emr Rep+ | pEG920 mobilization-deficient shuttle vector integrated into NBU1 in orf3, prmN1, and mobN1, respectively (25, 28) |
| pY5D and Y11D | (Apr Tcr) Emr Rep− Int+ Exc+/− | pB8-51 deleted from pY5 and pY11; integrate in Bacteroides; NBU1 excision defective (28). |
| pY17D | (Apr Tcr Mob+/−) | pEG920 integrated in mobN1 and pB8–51 deleted; does not transfer to Bacteroides recipients; used to subclone regions of NBU1 into pNV19 (15, 25, 28) |
| pNV19 | (Apr) Emr | Insertional vector consisting of ermF region and pB8–51 mobilization region from pVAL1 cloned into pUC19 (26, 41) |
| pNW17 | (Apr) Emr Int+ Exc+ | NBU1 from PvuII to ClaI of pY17D blunted and cloned into SmaI on pNV19 (6) |
| pNW18 | (Apr) Emr Int+ Exc+ | 7.7-kbp SphI fragment of pNW17 cloned into SphI site of pNV19 (this study) |
| pNW18ScaI | (Apr) Emr Int+ Exc+ | 6.6-kbp ScaI-SphI fragment of pNW17 blunted and cloned into SmaI site of pNV19; unstable in E. coli (this study) |
| pCQW1 | (Apr) Emr Rep− GUS | Insertional promoter vector containing E. coli GUS (uidA) to detect transcription when integrated in an ORF (8) |
| pMJF2 | (Apr) Emr Rep+ GUS | Replicative GUS (uidA) vector used to detect promoters on clones (8) |
| pChuR | (Apr) Emr Rep+ GUS | 443-bp SphI fragment containing PChuR cloned into SphI site of pMJF2; genes cloned into the SmaI site are expressed from PChuR. (5; J. D'Elia, this study) |
| pGERM | (Apr) Emr Rep− | pUC19 with 782-bp HaeII RK2oriT (L27758) fragment cloned into SapI and 1.3-kbp ermG (L42817) cloned into SspI; Mobilized by IncPα plasmids and is an insertional vector in Bacteroides spp. (this lab) |
| pG-Sph18 | (Apr) Emr Int+ Exc+ | 7.7-kbp SphI fragment of pNW18 cloned into SphI site of pGERM (this study) |
| pG-Sph18ΔPrm | (Apr) Emr Int+ Exc− | 377-bp deletion (bp7699–8073) of prmN1 in pG-Sph18 (this study) |
| pG-Sph18ΔOrf3 | (Apr) Emr Int+ Exc− | 925-bp deletion (bp 6228–7003) of orf3 in pG-Sph18 (this study) |
Phenotypes of E. coli strains containing the plasmids are shown in parentheses, and phenotypes of the Bacteroides strains containing the plasmids are shown outside the parentheses.
The Bacteroides strains were derivatives of Bacteroides thetaiotaomicron 5482. These strains were grown in prereduced Trypticase-yeast extract-glucose broth (13) or supplemented brain heart infusion broth (6) or agar plates incubated in BBL GasPak jars. The following antibiotic concentrations were used: tetracycline for induction of NBU1 excision, 1 to 2 μg/ml; chloramphenicol, 15 μg/ml; erythromycin, 10 μg/ml; cefoxitin, 20 μg/ml; rifampin, 10 μg/ml; gentamicin, 200 μg/ml. Thymidine at 100 μg/ml and trimethoprim at 200 μg/ml were added to thymidine-requiring (Thy−) spontaneous mutants.
Bacterial conjugations.
The procedures for filters matings between E. coli and Bacteroides strains have been previously described (6, 24, 41). Mating conditions were used which favored the donor: aerobic for E. coli donors and anaerobic for Bacteroides donors. Insertional and replicative shuttle vectors were mobilized from E. coli donors either by one of the IncP plasmids, R751 or RP4, or by transfer functions of RP4 integrated in the chromosome of S17-1 (29). The transfer functions of the conjugative transposon CTnERL were used to mobilize vectors out of Bacteroides donors to either Bacteroides or E. coli recipients.
DNA isolation and Southern blot analysis.
Plasmids were isolated from E. coli and Bacteroides strains by using the Ish Horowitz modification of the alkaline lysis prodedure (21). Total DNA was isolated by a modification of the method described by Saito and Miura (20). Following the phenol extraction step, 0.8 volume of isopropanol was added all at once instead of gradually. After allowing at least 1 h for precipitation at room temperature, the precipitated nucleic acids were centrifuged. The pellet was washed with cold 70% ethanol, dried, and resuspended in TE (0.01M Tris, 0.001 M EDTA [pH 8]) containing 50 μg of RNase per ml. The preparation contained chromosomal DNA (usually observed as a clump in the isopropanol step), plasmids, and the tetracycline-induced excised closed circular forms of NBU1.
The DNA to be analyzed by Southern blotting was digested with restriction enzymes and run on 0.8% agarose gels in Tris-acetate buffer (21). The 1.7-kbp HincII fragment containing the joined ends of the excised circular form of NBU1 (Fig. 1) was labeled and used to detect the excision of NBU1. The excised circular form of NBU1 produces a 1.7-kbp HincII fragment in addition to two chromosome-NBU1 junction bands on the Southern blots. All hybridization probes were labeled with fluorescein-dUTP by using random primers as specified in the NEN Life Sciences Renaissance kit protocol. The Southern blots were developed using a chemiluminescent substrate and exposure of film.
FIG. 1.
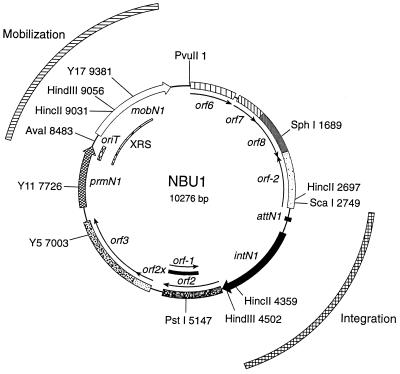
Excised circular form of NBU1. When NBU1 is induced to excise out of the Bacteroides chromosomal target site, it forms the double-stranded circular form shown in the map. The circular form is the transfer intermediate of the element. The regions required for integration, attN1 and intN1, and mobilization, oriT and mobN1, are indicated by the arcs. The possible ORFs and the locations of the known genes are labeled and are described in Table 2. The sites are numbered relative to the PvuII site. Y5, Y11, and Y17 are the sites where the mobilization-defective vector, pEG920, integrated into the circular form of NBU1. The excision and formation of the circular form of NBU1 are detected on Southern blots by using the 1.7-kbp HincII fragment (bp 2697 to 4359) that contains the NBU1 joined ends as the probe. XRS is the extended region between prmN1 and Y17 that is necessary in cis for the excision of NBU1.
GUS assays of transcriptional fusions.
The β-glucuronidase (GUS) gene (uidA) from E. coli was cloned into insertional or replicative vectors to detect transcription of the genes in NBU1. The NBU1 open reading frames (ORFs) determined from the sequencing results were checked for transcription strength by using an internal fragment cloned in the insertional vector, pCQW1 (8), and by cloning the upstream region or possible promoter region into the replicative vector pMJF2 (8). The assays were done as previously described (8).
PCR analysis of NBU1 excision.
PCR was used to determine if the target site of NBU1 following excision was intact or whether a copy of NBU1 remained in the site. The target was re-formed, and the PCR product was sequenced. The primers used were FT1-5′ (TCTAAATACAGAAGCCTTTGGA) and RT1-5′ (TCGAAAACCTTCTGGTAGTGCA), and they produced a 295-bp product. A 2-μl volume of a DNA preparation from a tetracycline-induced B. thetaiotaomicron strain containing a derivative of NBU1 integrated in the chromosome and CTnERL was used as the template for the PCR. The cycling conditions were as follows: (i) 5 min at 95°C followed by addition of Taq polymerase; (ii) 25 to 30 cycles of 1 min at 94°C, 1 min at 60°C, and 2 min at 72°C; and (iii) final extension of 10 min at 72°C. The PCR products were sequenced directly following purification using the Promega PCR cleanup kit or were cloned into the Promega PCR cloning vector, p-GEM-T, for sequencing.
Cloning of the minimal region of NBU1 required for insertion and excision.
The insertional vector, pNV19 (26), which was constructed previously for use in studies to determine the minimal region of NBU1 required for integration, was used to clone additional regions of NBU1 to determine the sequences required for both integration and excision. This vector contains the mobilization region of pB8-51 that is recognized by the IncP plasmids and by CTnERL for mobilization of the vector. The pNV19::NBU1 constructs (Table 1) were mobilized from E. coli to Bacteroides recipients, and excised forms of the vector could be mobilized by CTnERL from Bacteroides to E. coli. The integration of the constructs (ΩpNV19::NBU1) into the primary target site was verified by Southern blots. The possible excision of the ΩpNV19::NBU1 constructs was determined by tetracycline induction of the regulatory region of CTnERL followed by Southern blot or PCR analysis as described above. When the oriT-mobN1 region was shown to be included in the region required for excision, a second vector with no mobilization region recognized by Bacteroides was used and several of the NBU1 fragments were retested for excision. The vector, pGERM, is pUC19 with the RK2 oriT 782-bp HaeII fragment (L27758; bp 50590 to 51377), which allows mobilization by IncPα plasmids in E. coli hosts, and the ermG of CTn7853 (L42817), which provides a selectable erythromycin resistance marker in Bacteroides spp. The 7.7-kbp Sph1 fragment from pNW18 containing the NBU1 sequences was cloned into pGERM(pG-Sph18) and was shown by Southern blotting and PCR analysis to excise normally. Various deletions of the 7.7-kbp SphI fragment were made, cloned into pGERM, and used to determine the minimal region required for excision. Internal deletions of prmN1 and orf3 were also constructed and tested for their effect on excision (Table 1; also see Fig. 2).
FIG. 2.
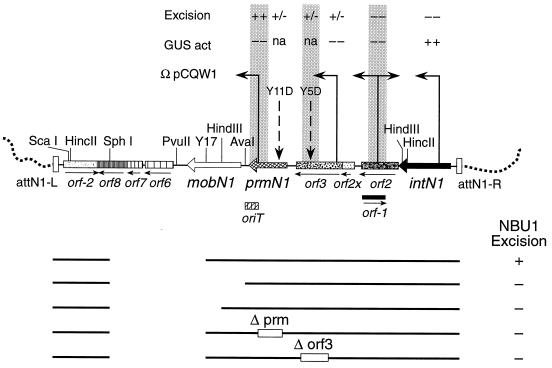
Regions of NBU1 required for excision. NBU1 is shown integrated in the Bacteroides target site. The chromosomal Leu-tRNA gene in the chromosomal target site is located upstream of attN-L. The possible ORFs and known genes are labeled, and the locations of the integrated forms of pEG920 insertion vectors, pY5D in orf3, pY11D in prmN1, and pY17 in mobN1, are indicated. The insertion sites and orientations of the uidA reporter vector, pCQW1, are indicated by the arrows above the map. The insertions were tested for their effect on the excision of NBU1 and the level of transcription of the ORFs as measured by the production of GUS from the pCQW1 insertions. Excision was determined by the detection of the NBU1 joined ends on Southern blots, and the results are expressed relative to the intensity observed for wild-type NBU1: wild excision (++) and weak but detectable excision (+/−). Transcription levels of the various pCQW insertions are relative to the GUS activity for the insertion in intN1: 7 U/mg of protein (++) and <0.2 U/mg of protein (−). Transcription could not be measured for the pY5D and pY11D insertions (not applicable [na]). The subcloned region of NBU1 required for both integration and excision on pNW18 and subcloned on a 7.7-kbp SphI fragment into pGERM, pG-Sph18, is indicated below the map. None of the ORFs between PvuII and attN-L are required for either integration or for excision. Other NBU1 subclones of the 7.7-kbp SphI fragment in pGERM used to determine the region required for excision are also shown: SphI-AvaI, Sph-HindIII, and pG-Sph18 with deletions in prmN1 (Δprm, pG-Sph18Δprm) and orf3 (Δorf3, pG-Sph18Δorf3). The 220-bp oriT, required in cis for mobilization, is also part of the XRS between prmN1 and Y17 that is required in cis for NBU1 excision. The excision (+) or lack of excision (−) of each subclone is shown to the right.
DNA sequencing and analysis.
Various regions of NBU1 were cloned into pUC19 derivatives. The DNA was sequenced at the University of Illinois Biotechnology Facility using the Applied Biosystems model 373A version 2.0.1S dye terminator sequencing system. The sequencing was completed by primer walking and by sequencing of PCR products across restriction sites. The resulting nucleotide and amino acid sequences were used to search the various databases by using Gapped Blast and Psi-Blast programs (2). The GenBank accession numbers for the oriT-mobN1 region (15) and the attN1-intN1 (26) are L13840 and U51917, respectively. The entire NBU1 sequence has been submitted.
RESULTS AND DISCUSSION
Sequence analysis of NBU1.
Previously, about 3.5 kbp of NBU1 had been sequenced. We have now completed the entire NBU1 sequence. The analysis of the sequence revealed that NBU1 is 10,276 bp in size and that it contains 12 possible ORFs (Figs. 1 and 2). There were no Sau3A (GATC) sites in the entire element. The lack of GATC sites in Bacteroides sequences has been noted before and may be one of the reasons why large clones of Bacteroides DNA are difficult to maintain in E. coli hosts such as cosmid clones (23, 33; unpublished data). The lack of GATC sites in NBU1 suggests that NBU1 originated in Bacteroides or has been in Bacteroides spp. long enough to acquire the trait. This is consistent with the G+C content of most of the ORFs on NBU1, which were close to the range of 40 to 43% seen in Bacteroides spp. genomes. Some exceptions were orf6, orf7, and orf8, which had lower G+C contents and could thus have come from outside the Bacteroides spp. As will be evident from a later section, these ORFs play no role in excision or transfer.
Table 2 lists the ORFs, their locations relative to the PvuII site on the circular map of NBU1, and the nearest homolog(s) in the protein databases to their deduced amino acid sequences. The most significant similarities were Orf2X, which had high amino acid sequence similarity (62%) to the C-terminal region of the proposed integrase of the mobilizable transposon Tn4555 (accession no. U75371; C. J. Smith et al., unpublished data), and PrmN1, which had significant amino acid similarity in its N-terminal half to the N-terminal portion of a number of bacterial DNA primases. The published sequence from the NBU1-like mobilizable transposon Tn4399 (10, 17) includes a portion of a gene that has high sequence identity to prmN1. Genes like prmN1 may prove to be common components of NBU1-type elements. It is not yet known if Tn4399 carries the entire gene, and it is not yet known if Tn4555 (31, 39) includes such a gene.
TABLE 2.
Location and possible gene products of NBU1 ORFs
| ORF or region | Locationa (5′ to 3′) | No. of bp | No. of amino acidsb | %G + C | Search results (accession no.) and comments |
|---|---|---|---|---|---|
| attN1 region (14 bp and IRs) | 2697–3216c (2960–3027) | 500c | |||
| intN1 | 3232–4569 | 1,338 | 445 | 40.6 | C-terminal similarity to lambda family of site-specific integrases |
| orf2 | 4577–5518 | 942 | 313 | 43.2 | |
| orf −1 | 5487–4905 | 582 | 194 | 43.2 | |
| orf2x | 5688–6002 | 316 | 104 | 41 | 62% similarity to C-terminal 53 aa of tnpA of Tn4555 (U75371) |
| orf3 | 6007–7197 (Y5 bp 7003) | 1,191 | 396 | 46.6 | Helicase-like protein in Sulfolobus islandicus sequence (U93082). |
| prmN1 | 7429–8385 (Y11 bp 7726) | 957 | 318 | 47.2 | >80% sequence identity to prmN2 of NBU2 and a sequence contained on Tn4399 (10, 16); N-terminal amino acid similarity to bacterial DNA primases, e.g., Listeria monocytogenes (55% [U13165]), Legionella pneumophila (60% [U63641]), and dnaG of E. coli (38% [U28379]) |
| oriTd | 8213–8433 (nic at 8385)d | 220 | >90% sequence identity to NBU2 and Tn4555 (16, 31) | ||
| mobN1 | 8592–9995 (Y17 bp 9381) | 1,404 | 467 | 44.3 | 83% identity to NBU2 mobN2 (L42370) (16), 73% identity to mobATn Tn4555 (U38243), and 27–31% identity to Bacteroides plasmids pBI143 and pIP421, respectively (30, 33, 40) |
| orf6 | 36–755 | 720 | 239 | 31.7 | |
| orf7 | 811–1254 | 444 | 147 | 28.3 | |
| orf8 | 1264–2004 | 741 | 246 | 34.6 | 67% identity and 83% similarity to C-terminal 160 aa of DNA topoisomerase I (topA) from Methanococcus jannaschii (U67605) and lower but significant similarities to DNA topoisomerases from Helicobacter pylori (Y10747) and Methanobacterium thermoautotrophicum (AE000921) |
| orf −2 | 2839–2030 | 810 | 269 | 40.1 | None |
From previous studies, it is clear that PrmN1 is not required for and does not enhance the transfer of plasmids containing the NBU1 mobilization region (15, 16). Nor does the circular form of NBU1 (Fig. 1) replicate in any known hosts. Thus, it is unlikely that PrmN1 is a primase. As will be evident from later sections, this protein instead plays a role in the excision process. In the oriT-mob region, NBU1 has over 78% sequence identity to the mobilizable transposon, Tn4555, and to NBU2. The oriT nic site as identified by Smith and Parker (31) is located at the end of prmN1 at bp 8385 on our map (Table 2). We assume that this is probably the nic site on NBU1 due to its high sequence identity to Tn4555 in this region. Previous studies of the mobilization region of NBU1 had placed the oriT nic site upstream of this region of sequence identity to Tn4555 within prmN1 (16).
Genes essential for excision.
Previously we showed that the region consisting of the NBU1 closed ends (attN1) and the intN1 gene were sufficient for integration into the primary NBU1 target site but were not sufficient for excision (Fig. 1) (26). IntN1 is a member of the lambda integrase family, although the amino acid similarity is relatively low and is confined to the C-terminal end. The integrases of the gram-positive bacterial conjugative transposons Tn916 and Tn5276 are also members of the lambda integrase family (18, 38). Like lambda, both Tn916 and Tn5276 have a small gene downstream of the integrase gene that has characteristics similar to those of lambda Xis, a protein essential for excision of phage lambda from the chromosome. The function of the cognate gene on Tn916 has been shown in in vitro assays to facilitate the excision of Tn916 (19). Accordingly, we expected a similar int-xis gene organization on NBU1. This proved not to be the case.
To obtain clones of a larger region of NBU1 in a plasmid that replicated in E. coli and could be transferred to Bacteroides but did not replicate in Bacteroides strains, we took advantage of some cointegrates of NBU1 and a plasmid, pEG920, that we had isolated inadvertently in connection with another study (pY5, pY11, and pY17 [25, 28]). In all of the NBU1::pEG920 hybrids, exactly the same sequence of pEG920 was involved in the insertion but the insertions had occurred at different sites on NBU1 (Fig. 1). We used these hybrids to help determine which genes were necessary for excision, for three reasons. First, the integrated pEG920 sequences provided convenient cloning sites. Second, by deleting the portion of pEG920 that contained the Bacteroides replication origin, pB8–51, to create pY5D and pY11D, we produced insertional vectors with disruptions in NBU1 genes. Third, since the insertions of pEG920 into NBU1 occurred by a process that did not create direct repeats at the ends of the insertion, the pEG920 insertions at Y5 and Y11 were nonrevertible disruptions. pY5D and pY11D integrated into the Bacteroides chromosome via the ends of NBU1. pY5D and pY11D contained NBU1 with pEG920 inserted in orf3 and prmN1, respectively (Fig. 1). pY17D could not be mobilized by IncP plasmids, and this was determined to be due to the pEG920 insertion site being in the C-terminal end of mobN1 (15).
At first, the phenotype of these pY5D and pY11D disruptions was confusing because although both disruptions decreased excision, they did not eliminate it completely (Fig. 1) (28). Since the N-terminal portion of these genes might be sufficient for excision, we also constructed deletions in each of these genes (Table 1; Fig. 2). To avoid possible polarity effects, we made an in-frame deletion in orf3. Both deletions eliminated most of the gene, and both abolished excision completely. Thus, orf3 and prmN1 are essential for excision. Moreover, the partial-excision phenotype of the disruption mutants suggests that the N-terminal portion of the proteins encoded by these genes is important for their function. A single-crossover disruption in orf2 completely abolished excision (Fig. 1). This disruption might have had a polar effect on expression of orf3, which is essential for excision, but this seems unlikely in view of the size of the region between these two genes (170 bp) (Table 2). We attempted to construct an in-frame deletion in orf2 to be certain of this, but the deletion clone was so unstable in E. coli that the construct could not be introduced into Bacteroides.
Minimum region required for NBU1 excision.
To determine whether any DNA other than intN1, orf2, orf3, and prmN1 was required for excision, subcloning was used to determine the minimum size of an excision-proficient element. A 9.3-kbp region of NBU1 was cloned to produce pNW17 (Table 1). pNW17 replicates in E. coli but not in Bacteroides spp. and can be mobilized by both IncP plasmids and Bacteroides conjugative transposons (CTns). pNW17 was transferred into B. thetaiotaomicron BT4104, where it integrated into the primary target site of NBU1 via the ends of NBU1 to produce BT4104ΩpNW17. When BT4104ΩpNW17, which contained a copy of CTnERL as well as integrated pNW17, was grown in the presence of tetracycline, ΩpNW17 excised at levels similar to that seen for wild-type NBU1. Since excised pNW17 could be mobilized back to E. coli by CTnERL, the level of excision could be semiquantitated by a mating-out assay. The excision and transfer of ΩpNW17 to E. coli and Bacteroides recipients occurred at frequencies of 10−5 to 10−6 per recipient, frequencies similar to that estimated previously for wild-type NBU1 (6, 28). The excision of ΩpNW17 could also be demonstrated directly by Southern blot analysis using a probe containing the joined ends of the circular-form NBU1 similar to that seen in Fig. 3B below. The fact that the joined ends of NBU1 could be detected in the circular form also confirmed that ΩpNW17 was excising like NBU1. This was further confirmed by the DNA sequence of the PCR amplified product of the joined ends. Using the mating-out assay, we found that the excision and transfer frequencies of both ΩpY5D and ΩpY11D (Fig. 2) from BT4104 to E. coli recipients were 50- to 100-fold lower than that observed for ΩpNW17, which correlated to the decreased excision observed by Southern blot analysis.
FIG. 3.
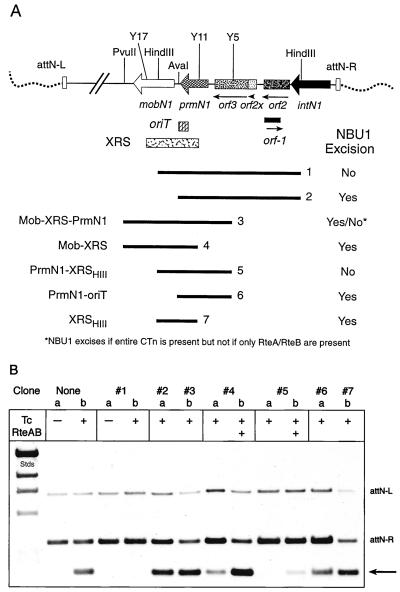
Effects of fragments cloned in trans on NBU1 excision. Regions of NBU1 (A) were cloned onto shuttle vectors and transferred into Bacteroides strain BT4104N1-3, which contained the conjugative transposon, CTn-ERL, and an integrated copy of NBU1. The effect of the cloned fragments on the excision of the wild-type copy of NBU1 was determined by Southern blotting (B) and indicated as Yes for excision and No for no excision at the right. (B) Southern blot of the HincII-digested DNA preparations of the strains containing the various cloned fragments shown in panel A. Wild-type NBU1 excision is shown in the strain that contained vector with no insert (None). The probe was the 1.7-kbp HincII fragment of NBU1 containing the joined ends (Fig. 1). Excision of NBU1 was observed as the appearance of the 1.7-kbp HincII fragment containing the joined ends of the NBU1 circular form, indicated by the arrow. The positions of the attN-L and attN-R junctions are indicated. The HindIII size standards of lambda are in the left lane (stds). No excision was detected without induction of the CTnERL regulatory operon containing rteA and rteB by growth of the strains in tetracycline (Tc + versus Tc −). The effect of additional RteA and RteB provided by the coresident plasmid, pAMS9, is shown for fragments 4 and 5 in lanes 4b and 5b, respectively. Note that fragment 3 allowed NBU1 excision in BT4104N1-3 at wild-type levels but later experiments showed that it completely inhibited NBU1 excision like fragment 5 if only the regulatory region of the CTn was present in strain BT4100N1-S1(pAMS9).
When the 1.7-kbp region between PvuII and SphI was deleted from pNW17 to produce pNW18 (Table 1), there was no reduction in the excision of the construct (data not shown). Thus, orf6 to orf8 were not required for excision. A further SphI-to-ScaI deletion (1.1 kbp) of pNW18 (pNW18ScaI), which removed part of orf −2, also did not affect excision as measured by Southern blotting. However, this deletion destabilized the plasmid in E. coli, making it difficult to quantitate the level of excision. Because of its instability, this construct was not used in further experiments. The SphI-AvaI (6.8-kbp) and SphI-HindIII (7.4-kbp) fragments of NBU1 in pY17D were first cloned into pNV19 and later cloned into pGERM (Table 1) and mobilized into B. thetaiotaomicron BT4104. In BT4104, all of these constructs integrated site specifically into the chromosome via the ends of NBU1. None of these integrated constructs excised (Fig. 2). Thus, the minimal region required for NBU1 excision includes attN1, intN1, orf2, orf2x, orf3, prmN1, the oriT, and about two-thirds of the coding region of mobN1 to the Y17 site. Since deleting into the oriT abolished excision, the region downstream of prmN1 must have some essential cis function. Accordingly, this region has been designated the excision-required sequence (XRS). At this point we cannot rule out the possibility that a partially functional MobN1 is being produced from the truncated mobN1 gene and that it is one of the factors required for excision.
Attempt to detect transcription of orf2, orf3, and prmN1.
In an attempt to detect transcription of the ORFs downstream of intN1, disruptions were made in each of the ORFs in this region by using pCQW1 (Table 1) (6), a suicide vector that has the promoterless E. coli GUS uidA gene downstream of the cloning site. The results are summarized in Figure 2. Although expression of intN1 was easily detectable, no GUS activity was detected in any of the other fusions. In our experience, GUS is not a very sensitive indicator of gene expression, and so expression could well have been below the level of detection. Clearly, however, if the intN1 promoter is running downstream genes, there is a significant shutdown of transcripts after the end of intN1. An interesting feature of the pCQW1 insertion in the middle of the prmN1 (made with a 377-bp internal fragment, bp 7712 to 8089) was that this disruption mutant excised as efficiently as the wild type. This disruption cut about 300 bp off the 3′ end of the gene. This is further evidence that the N-terminal part of PrmN1 is responsible for most or all of its activity. Another conclusion from this experiment is that prmN1 does not have to be immediately adjacent to the oriT in order to function, because inserting a large (7.8-kbp) DNA segment in this region can be done without impairing excision.
We also tried cloning the regions upstream of each of the ORFs in the GUS fusion vector, pMJF2, which replicates in Bacteroides spp. and which has a copy number of about 8 to 10 per cell (8). No transcription was detected from any of these constructs in any of the B. thetaiotaomicron hosts tested. The host strains used included strains containing or lacking a copy of CTnERL or an intact NBU1 in the chromosome.
Excision is conservative and restores the integration site.
Although evidence cited in previous sections suggested that PrmN1 was not functioning as a DNA primase, there was one remaining possible role for a primase. Previously, on the basis of Southern blot analysis, we assumed that excision was a conservative rather than a replicative process, which completely removed the NBU1 from its integration site and restored the integration site. This assumption had not, however, been tested directly. To test it, PCR primers were used to amplify the integration site after NBU1 had been induced to excise by exposing the cells to tetracycline. When wild-type NBU1, ΩpNW17, ΩpNW18, and ΩpG-Sph18 were induced for excision, PCR products of the regenerated target sites were observed (data not shown). The sequences of these target site PCR products were identical to the sequence of the site before NBU1 integrated. Thus, excision is conservative rather than replicative and restores the integration site. Taken together with other evidence, this suggests strongly that PrmN1 is not functioning as a primase.
Some segments of NBU1 inhibit excision.
Attempts to complement some of the insertion and deletion mutants were unsuccessful. That is, no excision was detected when the cloned region was provided in trans. It was possible that the apparent failure to complement mutations in NBU1, especially the clones that contained the region downstream of intN1, resulted from inhibition of NBU1 excision due to the presence of the cloned regions in multiple copies. DNA sequences that bind regulatory proteins or other factors made in limiting concentrations in the cell can titrate such factors when cloned on multicopy vectors. This was observed for regulated promoter regions in the starch utilization operon of B. thetaiotaomicron 5482 (7). Overproduction of gene products could also interfere with carefully regulated operations, for example factors necessary for the excision of NBU1, by changing the stoichiometry of the excision complex. Several of the pNLY1, pLYL05, and pLYL7 vectors (Table 1) containing cloned regions of NBU1 were tested for their effect on the excision of a wild-type NBU1 in BT4104N1-3 (Fig. 3). Fragments 1 (4.5-kbp HindIII fragment, bp 4502 to 9056) and 5 (2-kbp fragment, SstI on pY5D to HindIII bp 9056) completely inhibited the excision of wild-type NBU1. Fragment 5 was separated into two overlapping clones: fragment 6, containing prmN1-oriT (SstI pY5D to AvaI), and fragment 7, containing XRSHIII (HindIII of pY11D to HindIII bp 9056). Neither fragment 6 nor fragment 7 had any effect on the excision of NBU1 (Fig. 3B, lanes 6 and 7). Thus, PrmN1 plus the XRSHIII were required for the inhibition of excision observed with fragment 5. Fragment 4 (mobN1-XRS) reduced excision by about 70% but did not entirely eliminate it (lane 4a). A deletion of the oriT portion of XRS on fragment 4, leaving the XRS through mobN1 region from AvaI to PvuII, no longer reduced the NBU1 excision (data not shown). This suggests that interaction of MobN1 with the oriT part of XRS was involved in the partial inhibition of excision by fragment 4. Fragment 3 contained all three of the regions, prmN1-XRS-mobN1, on pLYL7 (15) (Table 1), but this clone did not reduce excision like fragment 4 and did not inhibit excision like fragment 5. Thus, the inclusion of mobN1 on fragment 3 prevented the inhibition of NBU1 excision observed with fragment 5. Functions on CTn that must interact with MobN1 and oriT for transfer of the circular intermediate are probably not required for excision. However, the interactions of CTn functions with MobN1 and/or oriT may be contributing to the differences observed between fragment 3 and fragment 5 on NBU1 excision.
We had established previously that two putative regulatory proteins provided by the conjugative transposons RteA and RteB were essential for NBU1 excision (34–36). We had also noted that providing these proteins in trans seemed to enhance transfer of the conjugative transposon, indicating that the concentration of RteA and RteB was limiting. Accordingly, we introduced pAMS9, a plasmid that carries rteA, rteB, and a third possible regulatory gene, rteC, which has no apparent effect on NBU1 excision (34), into the strains carrying CTn- ERL, NBU1, and vectors containing fragment 4 or 5. pAMS9 restored the decreased excision seen in the strain carrying fragment 4 (XRS-mobN1) to wild-type levels (Fig. 3B, lanes 4) but relieved the suppression of NBU1 excision by fragment 5 (prmN1-XRSHIII) only slightly (Figure 3B, lanes 5). Thus, the effect of having multiple copies of prmN1-XRSHIII in trans is more likely to be due to some interaction between PrmN1 and either DNA sequences or proteins encoded on NBU1 than to an interaction with proteins provided by the conjugative transposon.
In all of these experiments, the Bacteroides host strain contained a copy of the conjugative transposon, CTnERL. This conjugative transposon was needed to provide in trans functions to trigger NBU1 excision. CTnERL is about 70 kbp in size and could thus contain a number of genes that influence excision. To create a simpler system for triggering NBU1 excision, we replaced the conjugative transposon with pAMS9 to determine if the regulatory region containing rteA and rteB was sufficient to induce NBU1 excision (34, 35). pAMS9 was transferred to the B. thetaiotaomicron strain BT4100S1N1, which contained a copy of NBU1 in the chromosome but no copy of the conjugative transposon. When this strain was exposed to tetracycline to allow expression of rteA and rteB on pAMS9, the circular form of NBU1 was detectable at the wild-type levels as seen in Fig. 3B. Thus, the rteA-rteB region is sufficient to trigger NBU1 excision. We then introduced pLYL7::fragment 3 into BT4100N1-S1(pAMS9). In this strain, NBU1 excision was completely inhibited, whereas in BT4104N1-3 which contained CTnERL, excision of NBU1 was not affected by pLYL7:: fragment 3. Thus, although rteA and rteB appeared at first to be the only CTn genes involved in NBU1 excision, there is at least one other trans-acting function on CTnERL that prevents inhibition of excision by fragment 3, which encodes MobN1, but has no effect on fragment 5. This is the first indication that proteins other than RteA and RteB encoded by the CTn might be interacting with the NBU1 excision complex.
Preliminary model for CTn-regulated NBU1 excision and transfer.
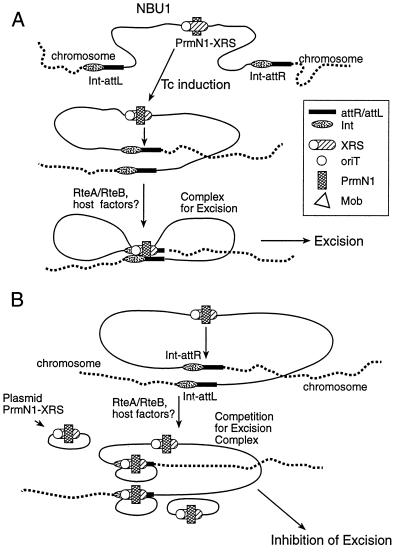
A model that accounts for all of the data provided here is shown in Fig. 4. The basic premise of the model is that for efficient transfer of an intact NBU1 to a recipient, it is important that the MobN1 is prevented from nicking at the internal oriT prior to the completion of the excision and circularization of NBU1. If nicking at the oriT and strand transfer begins before excision is completed, the element could function like an F-mediated Hfr and only part of the element would be transferred. Conversely, after the circular intermediate has been formed, it is equally important that the oriT then become available for interaction with the MobN1 so that transfer functions furnished by the conjugative transposon can interact with the MobN1-oriT complex for conjugal transfer of the element. The model in Fig. 4A posits that the NBU1 monitors its excision status by the protein-protein complex that forms on the integrated form and on the circular form. The protein complex that forms on the integrated NBU1 both excises the element and blocks oriT until excision is complete. Once the NBU1 has circularized, the protein complex disassociates so that no nicking occurs at the joined ends of the circular form and nicking by Mob can occur at oriT.
FIG. 4.
Model for the NBU1 excision process. (A) The normal process for NBU1 excision. The regulatory factors RteA and RteB on the conjugative transposon are induced by growth of the strain in tetracycline. The mechanism of the interaction(s) between RteA and RteB and NBU1 is not known. IntN1 is shown forming complexes with the junctions of NBU1, attN-L, and attN-R. Orf2 is also required for excision and may be involved in the complexes. Host factors such as integration host factor (IHF) are also suspected to be involved. PrmN1 is shown binding to the XRS region. The extent of the XRS which includes both the oriT region and the N-terminal region of mobN1 strongly suggests the possibility that MobN1 is also involved in this complex. The PrmN1-XRS complex interacts with the junction complexes, possibly sequentially, and aligns the ends in a conformation required for excision of NBU1. (B) Effect of multiple copies of the PrmN1-XRS region produced from fragment 5 on the excision of NBU1. The extra PrmN1-XRS complexes produced by the plasmid copies interact with the two IntN1-junction complexes and prevent the correct alignment of the ends of the element dictated by the single copy of the PrmN1-XRS in cis. Disruption of the stoichiometry of the reaction prevents NBU1 excision.
At this time, we do not know what gene products are required for the nicking and rejoining of DNA strands at attN-R and attN-L. IntN1 is a member of the lambda integrase family and is probably a component of the excision complex. The orf2 product was required for excision, but it is not a small basic protein like the excision enhancer proteins (Xis) of known lambda-type systems or of conjugative transposons such as Tn916 and Tn5276 (18, 19, 38) or the integrative Streptomyces plasmids pSAM2 and SLP1 (3, 22).
Figure 4B explains how providing the prmN1-oriT segment of the excision region in multiple copies might block excision. If multiple copies of this complex are present on coresident plasmids, they could prevent the ends from coming together by binding separately to the junction complexes. If excision requires one PrmN1-XRS complex interacting with two IntN1-attN junction complexes, the plasmid copies of PrmN1-XRS would interfere with the stoichiometry of the required excision complex. The excision inhibition caused by multiple copies of PrmN1-XRS supports both a sequential model for the three looped excision complex shown in Fig. 4A and a model where the ends come together first and then interact with PrmN1-XRS. However, at this point we favor the model shown in Fig. 4A, with PrmN1-XRS facilitating the proper alignment of the three complexes. The requirement for the XRS sequence in cis for excision resembles the requirement for enhancer sequences for the excision of Mu (14, 37), and the Hin (11, 12) and Gin (14) invertase systems but appears to be more complicated. The Mu enhancer region does not function in trans for excision in vivo, but it could do so in the in vitro assays. The Mu enhancer is important only for the complex formation, not for the excision (37).
Although the precise details of how NBU1 excision works have not yet been resolved, it is clear from the data presented here that excision is more complex than any excision process previously studied. This could be due to the need for NBU1 to control nicking at the ends and at the oriT so that they do not occur simultaneously. This is a problem that other excising elements such as Mu, Hin, and Gin do not have, since they are not transferred by conjugation following excision. It will be interesting to learn whether the conjugative transposons, such as CTnERL, which should have the same coordination problem, also have similarly complex excision systems.
ACKNOWLEDGMENTS
We thank John D'Elia for the construction of the PChuR shuttle vector and Jeff Smith for generously sharing his vectors.
This work was supported by grant AI22383 from the National Institutes of Health.
REFERENCES
- 1.Abremski K, Gottesman S. Site specific recombination: Xis-independent excisive recombination of bacteriophage lambda. J Mol Biol. 1981;153:67–78. doi: 10.1016/0022-2836(81)90527-1. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schaffler A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped Blast and Psi-Blast: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brasch M A, Cohen S N. Excisive recombination of the SLP1 element in Streptomyces lividans is mediated by Int and enhanced by Xis. J Bacteriol. 1993;175:3075–3082. doi: 10.1128/jb.175.10.3075-3082.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celli J, Trieu-Cuot P. Circularization of Tn916 is required for expression of the transposon-encoded transfer functions: characterization of long tetracycline-inducible transcripts reading through the attachment site. Mol Microbiol. 1998;28:103–117. doi: 10.1046/j.1365-2958.1998.00778.x. [DOI] [PubMed] [Google Scholar]
- 5.Cheng Q, Hwa V, Salyers A A. A locus that contributes to colonization of the intestinal tract by Bacteroides thetaiotaomicron contains a single regulatory gene (chuR) that links two polysaccharide utilization pathways. J Bacteriol. 1992;174:7185–7193. doi: 10.1128/jb.174.22.7185-7193.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper A J, Kalinowski A P, Shoemaker N B, Salyers A A. Construction and characterization of a Bacteroides thetaiotaomicron recA mutant: transfer of Bacteroides integrated conjugative elements is RecA independent. J Bacteriol. 1997;179:6221–6227. doi: 10.1128/jb.179.20.6221-6227.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Elia J N, Salyers A A. Effect of regulatory protein levels on utilization of starch by Bacteroides thetaiotaomicron. J Bacteriol. 1996;178:7180–7186. doi: 10.1128/jb.178.24.7180-7186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldhaus M J, Hwa V, Cheng Q, Salyers A A. Use of an Escherichia coli β-glucuronidase gene as a reporter gene for investigation of Bacteroides promoters. J Bacteriol. 1991;173:4540–4543. doi: 10.1128/jb.173.14.4540-4543.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guzman L M, Espinosa M. The mobilization protein, MobM, of the streptococcal plasmid pMV158 specifically cleaves supercoiled DNA at the plasmid oriT. J Mol Biol. 1997;266:688–702. doi: 10.1006/jmbi.1996.0824. [DOI] [PubMed] [Google Scholar]
- 10.Hecht D W, Thompson J S, Malamy M H. Characterization of the termini and transposition products of Tn4399, a conjugal mobilizing transposon of Bacteroides fragilis. Proc Natl Acad Sci USA. 1989;86:5340–5344. doi: 10.1073/pnas.86.14.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heichman K A, Johnson R C. The Hin invertasome: protein-mediated joining of distant recombination sites at the enhancer. Science. 1990;249:511–517. doi: 10.1126/science.2166334. [DOI] [PubMed] [Google Scholar]
- 12.Heichman K A, Moskowitz I P G, Johnson R C. Configuration of DNA strands and mechanism of strand exchange in the Hin invertasome as revealed by analysis of recombinant knots. Genes Dev. 1991;5:1622–1634. doi: 10.1101/gad.5.9.1622. [DOI] [PubMed] [Google Scholar]
- 13.Holdeman L V, Cato E P, Moore W E C. Anaerobe laboratory manual. 4th ed. Blacksburg: Virginia Polytechnical Institute and State University; 1977. [Google Scholar]
- 14.Kanaar R, Klippel A, Shekhtman E, Dungan J M, Kahmann R, Cozzarelli N R. Processive recombination by the phage Mu Gin system: implications for the mechanisms of DNA strand exchange, DNA site alignment, and enhancer action. Cell. 1990;62:353–366. doi: 10.1016/0092-8674(90)90372-l. [DOI] [PubMed] [Google Scholar]
- 15.Li L-Y, Shoemaker N B, Salyers A A. Characterization of the mobilization region of a Bacteroides insertion element (NBU1) that is excised and transferred by Bacteroides conjugative transposons. J Bacteriol. 1993;175:6588–6598. doi: 10.1128/jb.175.20.6588-6598.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L-Y, Shoemaker N B, Wang G-R, Cole S P, Hashimoto M K, Wang J, Salyers A A. The mobilization regions of two integrated Bacteroides elements, NBU1 and NBU2, have only a single mobilization protein and may be on a cassette. J Bacteriol. 1995;177:3940–3945. doi: 10.1128/jb.177.14.3940-3945.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy C G, Malamy M H. Requirements for strand- and site-specific cleavage within the oriT region of Tn4399, a mobilizing transposon from Bacteroides fragilis. J Bacteriol. 1995;177:3158–3165. doi: 10.1128/jb.177.11.3158-3165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rauch P J G, deVos W M. Identification and characterization of genes involved in excision of the Lactococcus lactis conjugative transposon Tn5276. J Bacteriol. 1994;176:2165–2171. doi: 10.1128/jb.176.8.2165-2171.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudy C K, Taylor K L, Hinerfeld D, Scott J R, Churchward G. Excision of a conjugative transposon in vitro by the Int and Xis proteins of Tn916. Nucleic Acids Res. 1997;25:4061–4066. doi: 10.1093/nar/25.20.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saito H, Miura K I. Preparation of transforming deoxy-ribonucleic acid by phenol treatment. Biochim Biophys Acta. 1963;72:619–629. [PubMed] [Google Scholar]
- 21.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1989. [Google Scholar]
- 22.Sezonov G, Duchene A-M, Friedmann A, Guerineau M, Pernodet J-L. Replicase, excisionase and integrase genes of the Streptomyces element pSAM2 constitute an operon positively regulated by the pra gene. J Bacteriol. 1998;180:3056–3061. doi: 10.1128/jb.180.12.3056-3061.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shoemaker N B, Barber R D, Salyers A A. Cloning and characterization of a Bacteroides conjugal tetracycline resistance element by using a shuttle cosmid vector. J Bacteriol. 1989;171:1294–1302. doi: 10.1128/jb.171.3.1294-1302.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shoemaker N B, Getty C E, Guthrie E P, Salyers A A. Regions of Bacteroides plasmids pBFTM10 and pB8-51 that allow Escherichia coli-Bacteroides shuttle vectors to be mobilized by IncP plasmids and by a conjugative Bacteroides tetracycline resistance element. J Bacteriol. 1986;166:959–965. doi: 10.1128/jb.166.3.959-965.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shoemaker N B, Li L-Y, Salyers A A. An unusual type of cointegrate formation between a Bacteroides plasmid and the excised circular form of an integrated element (NBU1) Plasmid. 1994;32:312–317. doi: 10.1006/plas.1994.1070. [DOI] [PubMed] [Google Scholar]
- 26.Shoemaker N B, Wang G-R, Salyers A A. The Bacteroides mobilizable insertion element, NBU1, integrates into the 3′ end of a Leu-tRNA gene and has an integrase that is a member of the lambda integrase family. J Bacteriol. 1996;178:3594–3600. doi: 10.1128/jb.178.12.3594-3600.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shoemaker N B, Wang G-R, Salyers A A. NBU1 a mobilizable site specific integrated element from Bacteroides spp., can integrate non-specifically in Escherichia coli. J Bacteriol. 1996b;178:3601–3607. doi: 10.1128/jb.178.12.3601-3607.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shoemaker N B, Wang G-R, Stevens A M, Salyers A A. Excision, transfer, and integration of NBU1, a mobilizable site-selective insertion element. J Bacteriol. 1993;175:6578–6587. doi: 10.1128/jb.175.20.6578-6587.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon R, Priefer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 30.Smith C J, Parker A C. A gene product related to TraI is required for the mobilization of Bacteroides mobilizable transposons and plasmids. Mol Microbiol. 1996;20:741–750. doi: 10.1111/j.1365-2958.1996.tb02513.x. [DOI] [PubMed] [Google Scholar]
- 31.Smith C J, Parker A C. The transfer origin for Bacteroides mobilizable transposon Tn4555 is related to a plasmid family from gram-positive bacteria. J Bacteriol. 1998;180:435–439. doi: 10.1128/jb.180.2.435-439.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith C J, Rogers M B, McKee M L. Heterologous gene expression in Bacteroides fragilis. Plasmid. 1992;27:141–154. doi: 10.1016/0147-619x(92)90014-2. [DOI] [PubMed] [Google Scholar]
- 33.Smith C J, Rollins L A, Parker A C. Nucleotide sequence determination and genetic analysis of the Bacteroides plasmid, pBI143. Plasmid. 1995;34:211–222. doi: 10.1006/plas.1995.0007. [DOI] [PubMed] [Google Scholar]
- 34.Stevens A M, Shoemaker N B, Li L-Y, Salyers A A. Tetracycline regulation of genes on Bacteroides conjugative transposons. J Bacteriol. 1993;175:6134–6141. doi: 10.1128/jb.175.19.6134-6141.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stevens A M, Sanders J M, Shoemaker N B, Salyers A A. Genes involved in production of plasmidlike forms by a Bacteroides conjugal chromosomal element share amino acid homology with two-component regulatory systems. J Bacteriol. 1992;174:2935–2942. doi: 10.1128/jb.174.9.2935-2942.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevens A M, Shoemaker N B, Salyers A A. The region of a Bacteroides conjugal chromosomal tetracycline resistance element that is responsible for production of plasmidlike forms from unlinked chromosomal DNA might also be involved in transfer of the element. J Bacteriol. 1990;172:4271–4279. doi: 10.1128/jb.172.8.4271-4279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Surette M G, Chaconas G. The Mu transpositional enhancer can function in trans: requirement for the enhancer for synapsis but not strand cleavage. Cell. 1992;68:1101–1108. doi: 10.1016/0092-8674(92)90081-m. [DOI] [PubMed] [Google Scholar]
- 38.Taylor K L, Churchwood G. Specific DNA cleavage mediated by the integrase of the conjugative transposon Tn916. J Bacteriol. 1997;179:1117–1125. doi: 10.1128/jb.179.4.1117-1125.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tribble G D, Parker A C, Smith C J. The Bacteroides mobilizable transposon Tn4555 integrates by a site-specific recombination mechanism similar to that of the gram-positive bacterial element Tn916. J Bacteriol. 1997;179:2731–2739. doi: 10.1128/jb.179.8.2731-2739.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trinh S, Reysset G. Identification and DNA sequence of the mobilization region of the 5-Nitroimidazole resistance plasmid pIP421 from Bacteroides fragilis. J Bacteriol. 1997;179:4071–4074. doi: 10.1128/jb.179.12.4071-4074.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valentine P J, Shoemaker N B, Salyers A A. Mobilization of Bacteroides plasmids by Bacteroides conjugal elements. J Bacteriol. 1988;170:1319–1324. doi: 10.1128/jb.170.3.1319-1324.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]