Abstract
Background
The coronavirus disease 2019 pandemic has had a devastating impact on the everyday lives of the world's population and to this end, the development of curative vaccines was upheld as a welcome panacea. Despite the undeniable negative impact of the disease on human beings, lower than expected proportions of people have taken up the vaccines, particularly in the developing non-Western world. Ethiopia represents an interesting case example, of a nation where COVID-19 vaccine acceptance levels have not been well investigated and a need exists to assess the overall level of vaccine acceptance.
Methods
A systematic multidatabase search for relevant articles was carried out across Google Scholar, Web of Science, Science Direct, Hinari, EMBASE, Boolean operator, and PubMed. Two reviewers independently selected, reviewed, screened, and extracted data by using a Microsoft Excel spreadsheet. The Joanna Briggs Institute prevalence critical appraisal tools and the modified NewcastleOttawa Scale (NOS) were used to assess the quality of evidence. All studies conducted in Ethiopia, reporting vaccine acceptance rates were incorporated. The extracted data were imported into the comprehensive meta-analysis version 3.0 for further analysis. Heterogeneity was confirmed using Higgins's method, and publication bias was checked by using Beggs and Eggers tests. A random-effects meta-analysis model with a 95% confidence interval was computed to estimate the pooled prevalence. Furthermore, subgroup analysis based on the study area and sample size was done. Results and Conclusion. After reviewing 67 sources, 18 articles fulfilled the inclusion criteria and were included in the meta-analysis. The pooled prevalence of COVID-19 vaccine acceptance in Ethiopia was 57.8% (95% CI: 47.2%–67.8%). The level of COVID-19 vaccine acceptance in Ethiopia was at a lower rate than necessary to achieve herd immunity. The highest level of vaccine acceptance rate was reported via online or telephone surveys followed by the southern region of Ethiopia. The lowest vaccine acceptance patterns were reported in Addis Ababa.
1. Introduction
The coronavirus (COVID)-19 disease is caused by a highly contagious acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and for several months after its emergence, no proven vaccine was available. Globally, the fatality rate of COVID-19 infection was estimated at 0.5% to 1% [1]. Its negative impact reached the everyday lives of all human beings globally [2–5] to the extent of disrupting the normal economic and social activities of the world's population [6–10]. In response, global communities took different measures to contain its spread, and these included lockdowns and border closures [11–13].
There has been an implementation of various public health measures that included hand hygiene, lockdowns, and social distancing in most parts of the world. Even so, the overall impact of these COVID-19 disease prevention measures has varied from one set to the other [14]. Medically, vaccines have been separated from other preventative measures on the basis of their superior prevention and disease control profiles [15, 16]. Even so, their uptake remains an issue of contention.
Barriers to vaccine acceptance are complex; moreover, they are context-specific and fluctuate across the place, time, and vaccine type [17]. However, there has been a continuous distribution of COVID-19 vaccines across the world population including Ethiopia. That said, hesitation against vaccines represents the single most notable obstacle to having adequate coverage across various populations. Vaccine hesitation was identified by the World Health Organization as one of the top global health threats as of the year 2019 [18].
The corpus of evidence related to COVID-19 vaccine acceptance suggests that a considerable portion of people are opposed to the vaccine. A global report on COVID-19 vaccine acceptance reported the acceptance rate to be under 67% [19].
Notably, most reports in Ethiopia show lower vaccine coverage. Additionally, the lack of specified investigation of the above strongly points to an acknowledgment that, the overall level of COVID-19 vaccine acceptance in Ethiopia represents a poorly investigated phenomenon. Guided by this, this meta-analysis offers an assessment of the overall level of vaccine acceptance in Ethiopia.
1.1. Question
What is the overall level of COVID-19 vaccine acceptance in Ethiopia?
2. Methods
2.1. Reporting
The preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline [20] was used as a reporting framework within this meta-analysis (additional file 1 S1).
2.2. Searching Strategies
The PRISMA systematic review protocol was followed as a reporting guideline and eligible studies for the analysis were selected in terms of abstracts, titles, and then for full articles on the basis of the inclusion criteria. EMBASE, PubMed, Hinari, Google Scholar, Web of Science, Science Direct, and African Journals Online were systematically searched to identify articles that included Medical Subject Headings and free-text languages. These databases were searched by using both controlled and free-text languages. In terms of free-text searches, the keywords included the following combination of terms: (Willingness, OR COVID-19 Vaccine OR Acceptance) AND Ethiopia. The controlled searches included the following Medical Subject Headings (MeSH) terms: “COVID-19 Vaccine Acceptance” and “Ethiopia” as recommended for each database. Search terms were used individually and in combination using “AND” and “OR” Boolean operators. The search was guided by PICO, a population that was intended to take the vaccine.
2.3. Inclusion and Exclusion Criteria
The following types of studies from 2019 to 2022 were included; study populations comprised any age group, study outcome was “willingness or intention to take vaccine or vaccine acceptance,” study design is cross-sectional and studies written in English were included. However, in this systematic review and meta-analysis; qualitative studies and data on those who took the vaccine were excluded.
2.4. Outcome of Interest: PICO
The population of the study was any age group and the outcome of interest was vaccine acceptance which thus was reported as “Are you willing to take COVID-19 Vaccine if available to you?” and measured as yes and no or willing versus unwilling. The level of vaccine acceptance was presented as a frequency and percentage.
2.5. Screening and Data Extraction
Screening for titles and abstracts against the inclusion was conducted by the two reviewers (SAT and GGD). Furthermore, an independent assessment was made for the full-text articles based on the predetermined inclusion criteria. Inconsistencies across the reviewers were dealt with via a discussion and consensus-seeking engagements involving all the investigators. Data extraction was made by three authors (TM, GZM, and BGE) independently from a random sample of 20% of the papers to check consistency and cross variation.
2.6. Study Quality Assessment
A structured data abstraction form was constructed in Microsoft Excel. Attention was given to clarity of data, objective, study design, population, sample size, and proportion of vaccine acceptance (Table 1). The modified version of the NewcastleOttawa Scale for the cross-sectional study [39] was used for the methodological qualities of each article. Additionally, studies were critically appraised with the Joanna Briggs Institute prevalence critical appraisal tool [40].
Table 1.
Characteristics of included studies, their area, sample size, and outcome.
| Author | Year | Region | Sample size | Prevalence (%) |
|---|---|---|---|---|
| Abebe et al. [21] | 2021 | Southern, (Gurage) | 492 | 62.60 |
| Mohammed et al. [22] | 2021 | Addis Ababa | 614 | 39.70 |
| Tadele Admasu [23] | 2021 | Addis Ababa | 422 | 42.30 |
| Sahile et al. [24] | 2022 | Addis Ababa | 407 | 39.80 |
| Tsegaw et al. [25] | 2021 | Debre Berhan | 423 | 69.30 |
| Handebo et al. [26] | 2021 | Gondar | 301 | 54.80 |
| Mose and Yeshaneh [27] | 2021 | Southwest (Wolkite) | 396 | 70.70 |
| Mose [28] | 2021 | Southern | 630 | 61.0 |
| Hailemariam et al. [29] | 2021 | Southwest | 412 | 31.30 |
| Aemro et al. [30] | 2021 | Northwest | 440 | 54.10 |
| Mose et al. [31] | 2021 | Southwest | 420 | 58.80 |
| Belsti et al. [32] | 2021 | Online | 1184 | 31.40 |
| Zeleke and Bayeh [33] | 2021 | Northwest | 538 | 29.00 |
| Rikitu Terefa et al. [34] | 2021 | Online | 522 | 62.10 |
| Oyekale [35] | 2021 | Telephone | 2178 | 92.30 |
| Dereje et al. [36] | 2021 | Addis Ababa | 422 | 80.90 |
| Adane et al. [37] | 2021 | Northeast (Dessie) | 404 | 64.0 |
| Bereket et al. [38] | 2021 | Online | 668 | 72.20 |
2.7. Data Synthesis and Statistical Analysis
Data were extracted using a Microsoft Excel spreadsheet and imported to comprehensive meta-analysis version3.0 software for further analysis. The pooled effect size with a 95% confidence interval of national COVID-19 vaccine acceptance; a rate that was determined using a weighted inverse variance random-effects model. The I2 statistic; 25, 50, and 75% representing a low, moderate, and high heterogeneity consecutively assessed the heterogeneity across the studies [41], whereas the publication bias was evaluated by funnel plot and Eggers and Beggs test [42].
3. Result
3.1. Selection of the Studies
A comprehensive literature search of the databases yielded a total of 67 published articles, of which 20 articles were retrieved from Google Scholar, 13 articles from PubMed, 12 articles from African Journals online, 7 articles from Hinari, and 15 articles from EMBASE, Web of Science, and Science Direct. Thirty-one articles were excluded for duplication and scope. The other 18 articles were excluded for failing to offer reports on the outcome. A total of 18 full-text articles that fulfilled the eligibility criteria with a total sample size of 10873 were included in the final analysis for the systematic review and meta-analysis (Figure 1).
Figure 1.
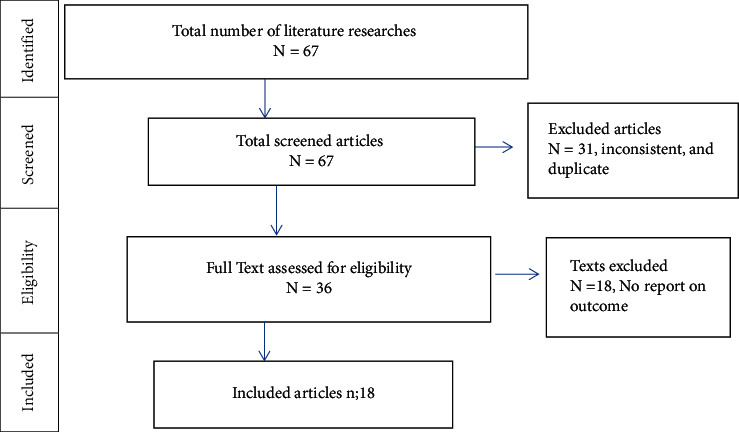
PRISMA flow chart for showing the screening and selection process of studies.
3.2. Characteristics of the Included Studies
Pertinent data relating to “year of publication,” authors, the outcome, and sample sizes with other main findings from the selected articles were extracted and presented in Table 1. All articles were cross-sectional and conducted in Ethiopia. The studies were distributed in southern region [21, 27–29, 31], Addis Ababa [22–24, 36], Amhara region [25, 26, 30, 33, 37], and online or telephone. [32, 34, 35, 38]. The sample size of the selected studies ranged from 301 to 2178 (Table 1).
3.3. Subgroup Analysis
According to the subgroup analysis report, the highest level of vaccine acceptance (68.7%; 95% CI: 34.1%–90.3%) was reported in online or telephone surveys whilst the lowest level of vaccine acceptance (51.8%; 95% CI: 33.3%–69.8%) was reported in Addis Ababa. Regarding the sample size, the highest level of vaccine acceptance (74.0%; 95% CI: 23.5%–96.4%) was reported in studies with a sample size of larger than 800 (Table 2).
Table 2.
Level of COVID-19 vaccine acceptance by study area and sample size.
| Variables | Characteristics | Included studies | Sample size | Prevalence (95% CI) |
|---|---|---|---|---|
| Area | Addis Ababa | 4 | 1865 | 51.8% (33.3%–69.8%) |
| Amhara | 5 | 2106 | 54.2% (39.4%–68.3%) | |
| Southern | 5 | 2350 | 57.0% (44.4%–68.7%) | |
| Other (online/telephone) | 4 | 4552 | 68.7% (34.1%–90.3%) | |
|
| ||||
| Sample size | <400 | 2 | 697 | 63.5% (59.8%–67.0%) |
| 400–800 | 13 | 6814 | 55.2% (46.7%–63.4%) | |
| >800 | 3 | 3362 | 74.0% (23.5%–96.4%) | |
3.4. COVID-19 Vaccine Acceptance
In this systematic review and meta-analysis, the pooled estimate of the COVID-19 vaccine acceptance rate was illustrated via a forest plot. The pooled prevalence of vaccine acceptance in Ethiopia was 57.8% (95% CI: 47.2%–67.8%) (Figure 2).
Figure 2.
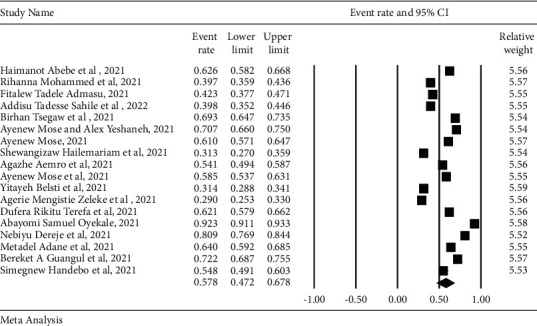
Forest plot for the pooled prevalence of COVID-19 vaccine acceptance.
3.5. Assessment of Publication Bias
A symmetrical funnel plot was observed. Begg's and Egger's tests showed the absence of significant publication bias at a p value of >0.05 (Figure 3).
Figure 3.
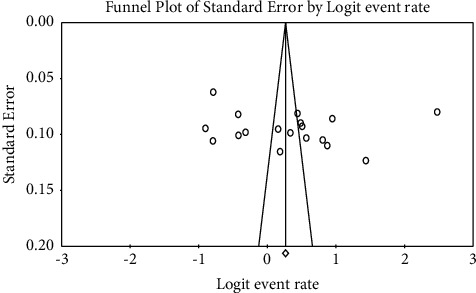
Funnel plot of the included studies.
3.6. Heterogeneity
For the identification of the possible causes of variation across different studies, meta-regression analysis was conducted using sample size and study area. The result showed that there was no significant heterogeneity across the studies (p > 0.05) (Table 3).
Table 3.
Sources of heterogeneity across the studies.
| Source of heterogeneity | Coefficient | Standard error | t 2 (%) | p value |
|---|---|---|---|---|
| Study area | 0.0731 | 0.4951 | 99.10 | 0.8827 |
| Sample size | 0.529 | 0.5709 | 99.08 | 0.3535 |
4. Discussion
The current systematic review and meta-analysis provided critical evidence on the level of COVID-19 vaccine acceptance in Ethiopia. This study found the overall level of vaccine acceptance in Ethiopia to be at 58.7%. This was consistent with the findings of 48.93% in Africa [43], 58.5% in low and middle income countries [44], 20.0% to 58.2% in Nigeria [45], 48% among adults in Saudi Arabia [46], 67% among adults in Kuwait [47], 62.6% in Jordan [48], 63% in Hong Kong [49], 65.75% in Japan [50], 53% in Pakistan [51], 61.7% in Iraq [52], 65.6% in Qatar [53], and 51.6% in Turkey [54]. The finding from this study was also consistent with the results from 64% in the UK (56), 63.5% in Kenya [55], and 50.2% in Nigeria [56].
A higher level of vaccine acceptance was reported in different countries. These included Malawi with an overall prevalence of 82.7% [57], 71.4% in Mozambique [58], 84.9% in Rwanda [59], 71.0% in Côte d'Ivoire [60], 75.3% in China [61], 74.65% among adults in Bangladesh [62], 85% in Israel [63], 91.3% of adults in China [15], 94.3% in Malaysia [64], and 91.5% in Italy [65]. This variation might be due to variation in the availability of vaccine type and population characteristics.
By contrast, the level of vaccine acceptance in the current study was higher than the findings of 34% in Liberia [66], 21.4% in Lebanon [67], and 27.7% in the Democratic Republic of Congo [68]. Such variation might be due to variations in sample size and level of awareness among the study participants.
The vaccine acceptance level was higher in different countries including 78% in Scotland [69], 78.5% in Greece [70], 75% in Portugal [71], 77.65% in France [72], 68.5% in the United States [73], 80% in Canada [74], 90.1% in South Africa [75], and 80.9% in Uganda [76]. This difference might be due to population characteristics and the availability of vaccine options.
5. Conclusion
The level of COVID-19 vaccine acceptance in Ethiopia was at a lower rate than necessary to achieve herd immunity. The highest level of vaccine acceptance was reported in online or telephone surveys followed by the Southern region of Ethiopia whereas a lower level of vaccine acceptance was reported in Addis Ababa. With regards to the sample size, the highest level of vaccine acceptance was reported in studies with a sample size larger than 800. Concerned bodies in Ethiopia including the government should work on scaling up the vaccine coverage for the Ethiopian people.
Data Availability
All the data are contained within the article.
Disclosure
The preprinted form of this manuscript was posted at research square in the form preprint accessed from https://www.researchsquare.com/article/rs-1332473/v1 DOI: 10.21203/rs.3.rs-1332473/v1.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
SAT and TM were involved in the development of the protocol, design, selection of the study, data extraction, statistical analysis, and development of the initial draft of the manuscript. GGD, GZM, and BGE got involved in the preparation and editing of the final draft of the manuscript. All authors read and approved the final draft of the manuscript.
References
- 1.Russell T. W., Hellewell J., Jarvis C. I., et al. Estimating the infection and case fatality ratio for coronavirus disease (COVID-19) using age-adjusted data from the outbreak on the diamond princess cruise ship, February 2020. Euro Surveillance . 2020;25(12) doi: 10.2807/1560-7917.es.2020.25.12.2000256.2000256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patrick S. W., Henkhaus L. E., Zickafoose J. S., et al. Well-being of parents and children during the COVID-19 pandemic: a national survey. Pediatrics . 2020;146(4) doi: 10.1542/peds.2020-016824.e2020016824 [DOI] [PubMed] [Google Scholar]
- 3.Aslam F., Awan T. M., Syed J. H., Kashif A., Parveen M. Sentiments and emotions evoked by news headlines of coronavirus disease (COVID-19) outbreak. Humanities and Social Sciences Communications . 2020;7(1):23–29. doi: 10.1057/s41599-020-0523-3. [DOI] [Google Scholar]
- 4.Pišot S., Milovanović I., Šimunič B., et al. Maintaining everyday life praxis in the time of COVID-19 pandemic measures (ELP-COVID-19 survey) The European Journal of Public Health . 2020;30(6):1181–1186. doi: 10.1093/eurpub/ckaa157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lades L. K., Laffan K., Daly M., Delaney L. Daily emotional well‐being during the COVID‐19 pandemic. British Journal of Health Psychology . 2020;25(4):902–911. doi: 10.1111/bjhp.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKibbin W., Fernando R. Economics in the Time of COVID-19 . Washington, DC, USA: CEPR Press; 2020. The economic impact of COVID-19. [Google Scholar]
- 7.Yu Z., Razzaq A., Rehman A., Shah A., Jameel K., Mor R. S. Disruption in global supply chain and socio-economic shocks: a lesson from COVID-19 for sustainable production and consumption. Operations Management Research . 2021;14:1–16. doi: 10.1007/s12063-021-00179-y. [DOI] [Google Scholar]
- 8.Das K. Impact of COVID-19 pandemic into solar energy generation sector. 2020. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3580341 .
- 9.Mofijur M., Fattah I. R., Alam M. A., et al. Impact of COVID-19 on the social, economic, environmental and energy domains: lessons learnt from a global pandemic. Sustainable Production and Consumption . 2020;26 doi: 10.1016/j.spc.2020.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiao S., Friedman D. B., Tam C. C., Zeng C., Li X. Vaccine acceptance among college students in South Carolina: do information sources and trust in information make a difference? medRxiv . 2020;12 doi: 10.1101/2020.12.02.20242982.20242982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koh D. COVID-19 lockdowns throughout the world. Occupational Medicine . 2020;70(5):p. 322. doi: 10.1093/occmed/kqaa073. [DOI] [Google Scholar]
- 12.Lake M. A. What we know so far: COVID-19 current clinical knowledge and research. Clinical Medicine . 2020;20(2):124–127. doi: 10.7861/clinmed.2019-coron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dariya B., Nagaraju G. P. Understanding novel COVID-19: its impact on organ failure and risk assessment for diabetic and cancer patients. Cytokine & Growth Factor Reviews . 2020;53:43–52. doi: 10.1016/j.cytogfr.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Looi M.-K. Covid-19: is a second wave hitting Europe? BMJ . 2020;371 doi: 10.1136/bmj.m4113. [DOI] [PubMed] [Google Scholar]
- 15.Wang J., Jing R., Lai X., et al. Acceptance of COVID-19 vaccination during the COVID-19 pandemic in China. Vaccines . 2020;8(3):p. 482. doi: 10.3390/vaccines8030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harapan H., Wagner A. L., Yufika A., et al. Acceptance of a COVID-19 vaccine in Southeast Asia: a cross-sectional study in Indonesia. Frontiers in Public Health . 2020;8:p. 381. doi: 10.3389/fpubh.2020.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omer S. B., Salmon D. A., Orenstein W. A., Dehart M. P., Halsey N. Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. New England Journal of Medicine . 2009;360(19):1981–1988. doi: 10.1056/nejmsa0806477. [DOI] [PubMed] [Google Scholar]
- 18.Organization W. H. Ethiopia introduces COVID-19 vaccine in a national launching ceremony. 2021. https://www.afro.who.int/news/ethiopia-introduces-covid-19-vaccine-national-launching-ceremony .
- 19.Feleszko W., Lewulis P., Czarnecki A., Waszkiewicz P. Flattening the curve of COVID-19 vaccine rejection—a global overview. Vaccines . 2020;9 doi: 10.3390/vaccines9010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D., Liberati A., Tetzlaff J., Altman D. G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine . 2009;6(7) doi: 10.1371/journal.pmed.1000097.e1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abebe H., Shitu S., Mose A. Understanding of COVID-19 vaccine knowledge, attitude, acceptance, and determinates of COVID-19 vaccine acceptance among adult population in Ethiopia. Infection and Drug Resistance . 2021;14:2015–2025. doi: 10.2147/idr.s312116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohammed R., Nguse T. M., Habte B. M., Fentie A. M., Gebretekle G. B. COVID-19 vaccine hesitancy among Ethiopian healthcare workers. PLoS One . 2021;16(12) doi: 10.1371/journal.pone.0261125.e0261125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tadele Admasu F. Knowledge and proportion of COVID-19 vaccination and associated factors among cancer patients attending public hospitals of Addis Ababa, Ethiopia, 2021: a multicenter study. Infection and Drug Resistance . 2021;14:4865–4876. doi: 10.2147/idr.s340324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahile A. T., Mulugeta B., Hadush S., Fikre E. M. COVID-19 vaccine acceptance and its predictors among college students in Addis Ababa, Ethiopia, 2021: a cross-sectional survey. Patient Preference and Adherence . 2022;16:255–263. doi: 10.2147/ppa.s348132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsegaw B. T., Amogne F. K., Demisse T. L., et al. Coronavirus disease 2019 vaccine acceptance and perceived barriers among university students in northeast Ethiopia: a cross-sectional study. Clinical epidemiology and global health . 2021;12 doi: 10.1016/j.cegh.2021.100848.100848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Handebo S., Wolde M., Shitu K., Kassie A. Determinant of intention to receive COVID-19 vaccine among school teachers in Gondar city, Northwest Ethiopia. PLoS One . 2021;16(6) doi: 10.1371/journal.pone.0253499.e0253499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mose A., Yeshaneh A. COVID-19 vaccine acceptance and its associated factors among pregnant women attending antenatal care clinic in southwest Ethiopia: institutional-based cross-sectional study. International Journal of General Medicine . 2021;14:2385–2395. doi: 10.2147/ijgm.s314346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mose A. Willingness to receive COVID-19 vaccine and its determinant factors among lactating mothers in Ethiopia: a cross-sectional study. Infection and Drug Resistance . 2021;14:4249–4259. doi: 10.2147/idr.s336486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hailemariam S., Mekonnen B., Shifera N., et al. Predictors of pregnant women’s intention to vaccinate against coronavirus disease 2019: a facility-based cross-sectional study in southwest Ethiopia. SAGE open medicine . 2021;9 doi: 10.1177/20503121211038454.205031212110384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aemro A., Amare N. S., Shetie B., Chekol B., Wassie M. Determinants of COVID-19 vaccine hesitancy among health care workers in Amhara region referral hospitals, Northwest Ethiopia: a cross-sectional study. Epidemiology and Infection . 2021;149 doi: 10.1017/S0950268821002259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mose A., Haile K., Timerga A. COVID-19 vaccine hesitancy among medical and health science students attending Wolkite university in Ethiopia. PLoS One . 2022;17(1) doi: 10.1371/journal.pone.0263081.e0263081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belsti Y., Gela Y. Y., Akalu Y., et al. Willingness of Ethiopian population to receive COVID-19 vaccine. Journal of Multidisciplinary Healthcare . 2021;14:1233–1243. doi: 10.2147/jmdh.s312637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeleke A. M., Bayeh G. M. Knowledge, attitude and practice towards COVID-19 and associated factors among pregnant women at debark town northwest Ethiopia: an institutional-based cross-sectional study. World Journal of Advanced Science and Technology . 2022;1 [Google Scholar]
- 34.Rikitu Terefa D., Shama A. T., Feyisa B. R., et al. COVID-19 vaccine uptake and associated factors among health professionals in Ethiopia. Infection and Drug Resistance . 2021;14:5531–5541. doi: 10.2147/idr.s344647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oyekale A. S. Willingness to take COVID-19 vaccines in Ethiopia: an instrumental variable probit approach. International Journal of Environmental Research and Public Health . 2021;18(17):p. 8892. doi: 10.3390/ijerph18178892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dereje N., Tesfaye A., Tamene B., et al. COVID-19 vaccine hesitancy in Addis Ababa, Ethiopia: a mixed-methods study. medRxiv . 2021;12 doi: 10.1136/bmjopen-2021-052432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adane M., Ademas A., Kloos H. Knowledge, attitudes, and perceptions of COVID-19 vaccine and refusal to receive COVID-19 vaccine among healthcare workers in northeastern Ethiopia. BMC Public Health . 2022;22(1):128–214. doi: 10.1186/s12889-021-12362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bereket A G., Georgiana G., Mensur O., et al. Healthcare workers attitude towards SARS-COVID-2 vaccine, Ethiopia. Global Journal of Infectious Diseases and Clinical Research . 2021;7(1):043–048. doi: 10.17352/2455-5363.000045. [DOI] [Google Scholar]
- 39.Modesti P. A., Reboldi G., Cappuccio F. P., et al. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS One . 2016;11(1) doi: 10.1371/journal.pone.0147601.e0147601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munn Z., Moola S., Riitano D., Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. International Journal of Health Policy and Management . 2014;3(3):123–128. doi: 10.15171/ijhpm.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higgins J. P. T., Thompson S. G., Deeks J. J., Altman D. G. Measuring inconsistency in meta-analyses. BMJ . 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peters J. L., Sutton A. J., Jones D. R., Abrams K. R., Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA . 2006;295(6):676–680. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 43.Wake A. D. The acceptance rate toward COVID-19 vaccine in Africa: a systematic review and meta-analysis. Global Pediatric Health . 2021;8 doi: 10.1177/2333794x211048738.2333794X2110487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patwary M. M., Alam M. A., Bardhan M., et al. COVID-19 vaccine acceptance among low-and lower-middle-income countries: a rapid systematic review and meta-analysis. Vaccines . 2022;10(3):p. 427. doi: 10.3390/vaccines10030427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olu-Abiodun O., Abiodun O., Okafor N. COVID-19 vaccination in Nigeria: a rapid review of vaccine acceptance rate and the associated factors. PLoS One . 2022;17(5) doi: 10.1371/journal.pone.0267691.e0267691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alfageeh E. I., Alshareef N., Angawi K., Alhazmi F., Chirwa G. C. Acceptability of a COVID-19 vaccine among the Saudi population. Vaccines . 2021;9(3):p. 226. doi: 10.3390/vaccines9030226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shakeel C. S., Mujeeb A. A., Mirza M. S., Chaudhry B., Khan S. J. Global COVID-19 vaccine acceptance: a systematic review of associated social and behavioral factors. Vaccines . 2022;10(1):p. 110. doi: 10.3390/vaccines10010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Elimat T., AbuAlSamen M. M., Almomani B. A., Al-Sawalha N. A., Alali F. Q. Acceptance and attitudes toward COVID-19 vaccines: a cross-sectional study from Jordan. PLoS One . 2021;16(4) doi: 10.1371/journal.pone.0250555.e0250555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwok K. O., Li K.-K., Wei W. I., Tang A., Wong S. Y. S., Lee S. S. Influenza vaccine uptake, COVID-19 vaccination intention and vaccine hesitancy among nurses: a survey. International Journal of Nursing Studies . 2021;114 doi: 10.1016/j.ijnurstu.2020.103854.103854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoda T., Katsuyama H. Willingness to receive COVID-19 vaccination in Japan. Vaccines . 2021;9(1):p. 48. doi: 10.3390/vaccines9010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chaudhary F. A., Ahmad B., Khalid M. D., Fazal A., Javaid M. M., Butt D. Q. Factors influencing COVID-19 vaccine hesitancy and acceptance among the Pakistani population. Human Vaccines & Immunotherapeutics . 2021;17(10):3365–3370. doi: 10.1080/21645515.2021.1944743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al‐Metwali B. Z., Al‐Jumaili A. A., Al‐Alag Z. A., Sorofman B. Exploring the acceptance of COVID‐19 vaccine among healthcare workers and general population using health belief model. Journal of Evaluation in Clinical Practice . 2021;27(5):1112–1122. doi: 10.1111/jep.13581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Al-Mulla R., Abu-Madi M., Talafha Q. M., Tayyem R. F., Abdallah A. M. COVID-19 vaccine hesitancy in a representative education sector population in Qatar. Vaccines . 2021;9(6):p. 665. doi: 10.3390/vaccines9060665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.İkiışık H., Akif Sezerol M., Taşçı Y., Maral I. COVID‐19 vaccine hesitancy: a community‐based research in Turkey. International Journal of Clinical Practice . 2021;75(8) doi: 10.1111/ijcp.14336.e14336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Orangi S., Pinchoff J., Mwanga D., et al. Assessing the level and determinants of COVID-19 vaccine confidence in Kenya. Vaccines . 2021;9(8):p. 936. doi: 10.3390/vaccines9080936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alice Tobin E., Okonofua M., Adeke A., Obi A. Willingness to accept a COVID-19 vaccine in Nigeria: a population-based cross-sectional study. Central African Journal of Public Health . 2021;7(2):p. 53. doi: 10.11648/j.cajph.20210702.12. [DOI] [Google Scholar]
- 57.Kanyanda S., Markhof Y., Wollburg P., Zezza A. Acceptance of COVID-19 vaccines in sub-Saharan Africa: evidence from six national phone surveys. BMJ Open . 2021;11(12) doi: 10.1136/bmjopen-2021-055159.e055159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dula J., Mulhanga A., Nhanombe A., et al. COVID-19 vaccine acceptability and its determinants in Mozambique: an online survey. Vaccines . 2021;9(8):p. 828. doi: 10.3390/vaccines9080828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arce J. S. S., Warren S., Meriggi N., et al. COVID-19 vaccine acceptance and hesitancy in low and middle income countries. Nature Medicine . 2021;27 doi: 10.1038/s41591-021-01454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Africa C. COVID 19 vaccine perceptions: A 15 country study. 2021. https://africacdc.org/download/covid-19-vaccine-perceptions-a-15-country-study/
- 61.Wang Q., Xiu S., Zhao S., et al. Vaccine hesitancy: COVID-19 and influenza vaccine willingness among parents in wuxi, China—a cross-sectional study. Vaccines . 2021;9(4):p. 342. doi: 10.3390/vaccines9040342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abedin M., Islam M. A., Rahman F. N., et al. Willingness to vaccinate against COVID-19 among Bangladeshi adults: understanding the strategies to optimize vaccination coverage. PLoS One . 2021;16(4) doi: 10.1371/journal.pone.0250495.e0250495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zigron A., Dror A. A., Morozov N. G., et al. COVID-19 vaccine acceptance among dental professionals based on employment status during the pandemic. Frontiers of Medicine . 2021;8 doi: 10.3389/fmed.2021.618403.618403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wong L. P., Alias H., Wong P.-F., Lee H. Y., AbuBakar S. The use of the health belief model to assess predictors of intent to receive the COVID-19 vaccine and willingness to pay. Human Vaccines & Immunotherapeutics . 2020;16(9):2204–2214. doi: 10.1080/21645515.2020.1790279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trabucco Aurilio M., Mennini F. S., Gazzillo S., et al. Intention to be vaccinated for COVID-19 among Italian nurses during the pandemic. Vaccines . 2021;9(5):p. 500. doi: 10.3390/vaccines9050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seydou A. Who Wants COVID-19 Vaccination? In 5 West African Countries, Hesitancy Is High, Trust Low . AfricaPortal.org, Africa; 2021. [Google Scholar]
- 67.Al Ahdab S. A cross-sectional survey of knowledge, attitude and practice (KAP) towards COVID-19 pandemic among the Syrian residents. BMC Public Health . 2021;21:296–297. doi: 10.1186/s12889-021-10353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kabamba Nzaji M., Kabamba Ngombe L., Ngoie Mwamba G., et al. Acceptability of vaccination against COVID-19 among healthcare workers in the democratic republic of the Congo. Pragmatic and Observational Research . 2020;11:103–109. doi: 10.2147/por.s271096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Williams L., Flowers P., McLeod J., Young D., Rollins L. Social patterning and stability of intention to accept a COVID-19 vaccine in Scotland: will those most at risk accept a vaccine? Vaccines . 2021;9(1):p. 17. doi: 10.3390/vaccines9010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Papagiannis D., Rachiotis G., Malli F., et al. Acceptability of COVID-19 vaccination among Greek health professionals. Vaccines . 2021;9(3):p. 200. doi: 10.3390/vaccines9030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Neumann-Böhme S., Varghese N. E., Sabat I., et al. Once we have it, will we use it? a European survey on willingness to be vaccinated against COVID-19. European journal of health economics . 2020;21:977–982. doi: 10.1007/s10198-020-01208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Detoc M., Bruel S., Frappe P., Tardy B., Botelho-Nevers E., Gagneux-Brunon A. Intention to participate in a COVID-19 vaccine clinical trial and to get vaccinated against COVID-19 in France during the pandemic. Vaccine . 2020;38(45):7002–7006. doi: 10.1016/j.vaccine.2020.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reiter P. L., Pennell M. L., Katz M. L. Acceptability of a COVID-19 vaccine among adults in the United States: how many people would get vaccinated? Vaccine . 2020;38(42):6500–6507. doi: 10.1016/j.vaccine.2020.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taylor S., Landry C. A., Paluszek M. M., Groenewoud R., Rachor G. S., Asmundson G. J. G. A proactive approach for managing COVID-19: the importance of understanding the motivational roots of vaccination hesitancy for SARS-CoV2. Frontiers in Psychology . 2020;11 doi: 10.3389/fpsyg.2020.575950.575950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adeniyi O. V., Stead D., Singata-Madliki M., et al. Acceptance of COVID-19 vaccine among the healthcare workers in the Eastern Cape, South Africa: a cross sectional study. Vaccines . 2021;9(6):p. 666. doi: 10.3390/vaccines9060666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dzieciolowska S., Hamel D., Gadio S., et al. Covid-19 vaccine acceptance, hesitancy, and refusal among Canadian healthcare workers: a multicenter survey. American Journal of Infection Control . 2021;49(9):1152–1157. doi: 10.1016/j.ajic.2021.04.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data are contained within the article.


