Abstract
Pain is complex and is a unique experience for individuals in that no two people will have exactly the same physiological and emotional response to the same noxious stimulus or injury. Pain is composed of two essential processes: a sensory component that allows for discrimination of the intensity and location of a painful stimulus and an emotional component that underlies the affective, motivational, unpleasant, and aversive response to a painful stimulus. Kappa opioid receptor (KOR) activation in the periphery and throughout the neuroaxis modulates both of these components of the pain experience. In this chapter we focus on recent findings that KORs contribute to the emotional, aversive nature of chronic pain, including how expression in the limbic circuitry contributes to anhedonic states and components of opioid misuse disorder. While the primary focus is on preclinical pain models, we also highlight clinical or human research where there is strong evidence for KOR involvement in negative affective states associated with chronic pain and opioid misuse.
Keywords: Amygdala, Analgesia, Anhedonia, Antinociception, Biased agonist, Chronic pain, Dopamine, Dynorphin, Emotional component of pain, Endogenous opioid, Inflammatory pain, Kappa opioid receptor, Mesolimbic circuitry, Negative affect, Neuropathic pain, Nociception, Nucleus accumbens, Opioid misuse, Opioid use disorder, Pain, Pain aversion, Peripherally-restricted, Ventral tegmental area
1. Introduction
The family of opioid receptors includes four homologous 7 transmembrane spanning G protein-coupled receptors (GPCRs) denoted kappa, mu, delta, (KOR, MOR, DOR, respectively), and the opioid receptor-like/nociceptin receptor (ORL1). Opioids are also involved in a myriad of other physiological, defensive, and behavioral processes, including autonomic regulation, control of breathing, immune function, gut transit, and itch (Bodnar 2018). In the early years of opioid receptor exploration, researchers understood the addiction liability associated with MOR agonists, and hoped that the identification of compounds targeting the alternate opioid receptors, including KOR, might lead to non-addictive alternatives for pain relief. Unfortunately, while acute activation of DOR and KOR does result in analgesia in preclinical models, KOR agonists produce strongly aversive emotional states. Subsequent research has implicated both dynorphin peptides and KOR activation in the acute and chronic impact of pain, stress, and addiction, though their contribution to each of these issues is complex and likely changes over time. There is now consensus that KOR agonists can produce dysphoria, depressive-like symptoms, and psychotomimetic effects in humans (Kumor et al. 1986; Pfeiffer et al. 1986; Wadenberg 2003) and elicit place aversion and depressive-like behaviors (Shippenberg and Herz 1986; Shippenberg et al. 1993; Bruchas et al. 2010; Knoll and Carlezon 2010; Chavkin and Koob 2016) as well as stimulate drug-seeking (Valdez et al. 2007; Grella et al. 2014; Nygard et al. 2016; Lê et al. 2018) in rodents. Activation of the KOR system also elicits signs of anxiety and fear in animals and humans (Chartoff and Mavrikaki 2015; Chavkin and Koob 2016; Darcq and Kieffer 2018); however, spinal administration of KOR agonists produce antinociceptive effects in various preclinical pain models. The characterization of the crystal structures of inactive and active states of KOR has provided insights for drug–receptor interactions allowing new concepts for novel drug design (Wu et al. 2012; Che et al. 2018). This structural characterization together with identification of the signaling events that elicit antinociceptive versus dysphoric and psychotomimetic effects has provided extensive advancement in novel chemical entities that hold promise as new pain treatments with minimal aversive effects including depressive or addictive properties. In addition, peripherally restricted compounds, low efficacy ligands, and compounds with mixed mechanisms of action have shown promising results in early clinical trials. In this chapter, we will discuss (1) the involvement of KOR systems in the brain that contribute to pain and negative affective states associated with ongoing persistent pain and (2) the potential involvement of KOR systems in opioid medication misuse and opioid use disorder in the context of concomitant occurrence with chronic pain, given the recent evidence that KOR agonists can trigger drug relapse and that a systematic review confirm that chronic pain patients have high rates (13–38%) of opioid misuse (defined as opioid use contrary to the directed or prescribed pattern of use (Vowles et al. 2015).
2. Functional Upregulation of KOR Systems in Chronic Pain States
One of the first studies to report that dynorphin expression is increased in chronic pain states used a model of polyarthritis produced by intradermal tail injection of Mycobacterium butyricum that caused a gradual development of arthritic limbs, hypophagia (reduction of food intake), hypodipsia (reduced thirst), and reductions in thermal and mechanical sensory thresholds. This arthritic model was associated with a significant upregulation of immunoreactive dynorphin in the spinal cord that correlated with both the intensity and time course of mechanical hyperalgesia (Fig. 1) (Millan et al. 1985) and KOR antagonism potentiated this hyperalgesic response (Millan et al. 1987). KOR antagonism also enhanced pain hypersensitivities in a model of neuropathic pain (Xu et al. 2004). Together these data suggest that the dynamic endogenous tone of dynorphin at KORs is an adaptive process to suppress nociceptive transmission associated with tissue injury. Constitutive (global) KOR knockout produced enhanced pain hypersensitivity in a model of chronic inflammatory pain, but interestingly this enhancement was modality specific where mechanical, but not thermal, hypersensitivity was exaggerated in mice lacking KORs (Gavériaux-Ruff et al. 2008). Constitutive KOR knockout mice also exhibited enhanced nociceptive responses in a model of chemical visceral pain (intraperitoneal acidic acid) where abdominal constrictions were significantly greater, although there was no effect of KOR deletion on formalin-induced nocifensive (licking, flinching, guarding) behaviors (Simonin et al. 1998).
Fig. 1.
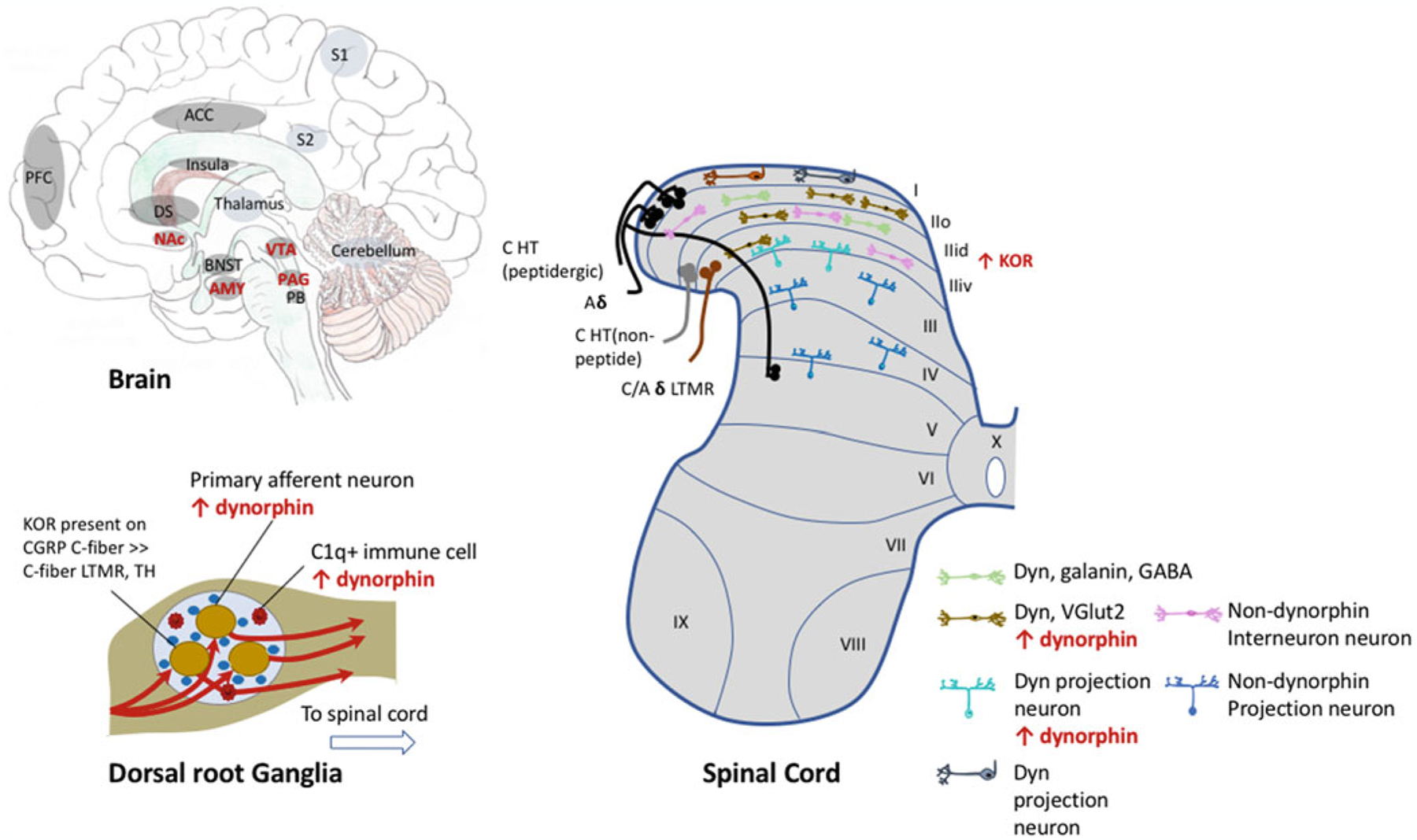
Evidence for increased dynorphin or KOR function or expression in various peripheral or central nervous system brain regions in chronic pain states. Dorsal Root Ganglia: Following nerve injury, dynorphin is present in primary afferent neurons, where it is not expressed under pain-naïve conditions (Sapio et al. 2020), is produced by C1q+ immune cells (Mika et al. 2010). Spinal Cord: Neurons in the spinal cord are organized into laminae, where ascending projection neurons are enriched in lamina I and throughout lamina II-V, whereas lamina II primarily consists of interneurons, including dynorphin containing inhibitory or excitatory neurons. Projection neurons make up 5 distinct circuits that relay sensory information to the brain. The spinothalamic pathway is known for transmitting pain information to the thalamus, and subsequently to the somatosensory cortex (S1) to provide information about pain intensity and location. Nociceptive projection neurons in lamina I relay pain information to specific brainstem (e.g., lateral parabrachial nucleus) and thalamic nuclei, which further project to areas involved in the affective, emotional component of pain such as the amygdala and hypothalamus. Primary afferent nociceptors including C-fiber and A-delta HT (high threshold) project to lamina I and outer lamina II (IIo), while low-threshold mechanoreceptor (LTMR) neurons project to inner lamina II. Prodynorphin immunoreactivity is present in galanin-positive, GABAergic interneurons in lamina I/II (Sardella et al. 2011) and glutamatergic (VGlut2+) (Nahin et al. 1992) interneurons that synapse with projection neurons in lamina III, that also contain dynorphin, where a recent study showed that the dynorphin containing neurons was 88% GABA to 12% glutamatergic (Duan et al. 2014). Dynorphin is upregulated following tissue or nerve injury in excitatory dynorphin-containing interneurons and dynorphin-containing projection neurons (Iadarola et al. 1988a; Ruda et al. 1988; Sapio et al. 2020). It is unclear whether dynorphin expression within dynorphin projection neurons in lamina I is modified in chronic pain states. There is an increase in KOR expression and function within the spinal cord have also been described. Brain: Many brain regions show increased KOR function in chronic pain states (highlighted in red text). ACC Anterior cingulate cortex, AMY amygdala, BNST bed nucleus of the stria terminalis, DS dorsal striatum, NAc nucleus accumbens, PAG periaqueductal gray, PB parabrachial nucleus, PFC prefrontal cortex, S1 somatosensory cortex, S2 secondary somatosensory cortex, VTA ventral tegmental area. A detailed description of spinal neurons in nociceptive transmission has been described (Todd 2010; Peirs and Seal 2016)
Many inflammatory agents (carrageenan, phorbol ester, yeast, and complete Freund’s adjuvant) that rapidly induce edema and thermal hyperalgesia increased dynorphin mRNA within 8h and dynorphin A (1–8) peptide in the spinal cord within 48h following tissue injury (Iadarola et al. 1988a). Spinal dynorphin is upregulated in models of preclinical models of neuropathic and inflammatory chronic pain (Fig. 1) (Millan et al. 1985, 1986, 1988; Iadarola et al. 1988a, b; Kajander et al. 1990; Draisci et al. 1991; Malan et al. 2000; Rosén et al. 2000; Wang et al. 2001), as well as elevated in the cerebrospinal fluid of chronic pain patients (Vaerøy et al. 1991; Samuelsson et al. 1993). Moreover, dynorphin peptides can remain elevated for several months concurrent with persistent pain (Iadarola et al. 1988a; Kajander et al. 1990; Malan et al. 2000). Dynorphin is also present in primary afferent nociceptors and dynorphin neuropeptides were shown to be upregulated in dorsal root ganglia neurons in models of chronic pain (Fig. 1) (Calzà et al. 1998; Mika et al. 2010).
KOR agonists have been shown to produce antinociceptive effects in various visceral, inflammatory, and neuropathic pain models, although KOR agonists were found to have no effect on nociceptive responses in models of chronic musculoskeletal pain such as intramuscular acid injection (Sluka et al. 2002). Considering KOR agonists produce motor impairment and hypo-locomotion (Castellano et al. 1988; Bakshi et al. 1990), it is important to dissociate changes in reflexive nociceptive behaviors from potential confounds of motor impairment. The KOR agonist SA14867 (more than 31,000 and 2,200-fold higher affinity for KOR compared to mu and delta opioid receptors) elicited a large therapeutic window of antinociceptive effects relative to sedative/motor effects (as measured by rotarod) compared to other KOR agonists (Tsukahara-Ohsumi et al. 2011), demonstrating that indeed changes in threshold evoked sensory responses are not solely attributed to changes in motor performance.
2.1. Dynorphin Peptides Can Exacerbate Nociceptive Transmission via Non-opioid Mechanisms
Spinal dynorphin peptides have also been associated with exacerbation of pain outcomes. Spinal administration of KOR agonists produce significant changes in the excitability of some superficial nociceptive neurons, where both facilitation and inhibition of excitability and expansion of receptive fields were reported (Hylden et al. 1991). Later studies concluded that inhibitory effects of spinal dynorphins and KOR agonists on C-fiber excitability were mediated via KOR activation, whereas the facilitation induced by dynorphin peptides was not opioid receptor-mediated (Knox and Dickenson 1987). The prodynorphin precursor can be cleaved into various dynorphin peptides, where some peptides (e.g., dynorphin (1–17)) were reported to facilitate NMDA-mediated currents via direct excitatory effects on this ionotropic receptor (Caudle and Dubner 1998). Spinal dynorphin A (2–13) peptide also interacts with bradykinin receptors to promote hyperalgesia (Lee et al. 2015), where blockade of spinal bradykinin receptors also reverses neuropathic pain, but only when there was elevated spinal dynorphin A (2–13) peptides (Lai et al. 2006). Similarly, spinal dynorphin peptides dynorphin A (1–17), dynorphin A (2–17), dynorphin A (2–13), and dynorphin (11–17) produced long-lasting allodynia (painful response to a stimulus that is not normally painful), where effects persisted up to 70 days after a single injection (Vanderah et al. 1996; Laughlin et al. 1997). In these studies naloxone did not reverse the pronociceptive effects of dynorphin peptides, but rather were blocked by NMDA receptor antagonists, and the longer duration of action after a single injection suggests that dynorphin peptides can produce the phenomenon of spinal wind-up that contributes to central sensitization. Additionally, pain hypersensitivity associated with nerve injury recovered in prodynorphin knockout mice, consistent with the report that intrathecal dynorphin antiserum reversed neuropathic pain (Wang et al. 2001). Similarly, neutralization of dynorphin peptides with antibodies was also shown to attenuate pain hypersensitivities associated with neuropathic pain (Malan et al. 2000; Gardell et al. 2004). These data suggest that the injury-induced upregulation of dynorphin produces pronociceptive effects and is required for the maintenance of persistent neuropathic pain. Indeed, a review argued that a dynorphin targeted gene silencing strategy to block dynorphin upregulation is a rational drug approach for treatment of chronic pain (Podvin et al. 2016). However, generation of mice that specifically ablated dynorphin containing inhibitory (but not excitatory) interneurons enhanced static and dynamic mechanical sensory thresholds in the absence of injury, demonstrating that these neurons normally act to prevent low-threshold mechanical stimuli from activating pain transmission neurons (Duan et al. 2014).
There is the possibility that KORs can also facilitate nociception, as this receptor is expressed on astrocytes, whereby KOR activation in these glial cells triggers their hypertrophy (Xu et al. 2007). Hence, in a model of neuropathic pain, KOR activation on astrocytes was shown to produce proliferation of spinal cord astrocytes via activation of p38 MAP kinase (Xu et al. 2007). The activation and hypertrophy of spinal astrocytes contributes to the maintenance of persistent pain states as well as MOR analgesic tolerance (Eidson and Murphy 2019; Ji et al. 2019; Donnelly et al. 2020). Taken together, spinal dynorphin peptides can either inhibit or facilitate nociceptive transmission; however, the facilitating effects of dynorphins are not mediated by neuronal KORs. Whether they engage astrocyte KORs that contributes to enhanced nociceptor excitability remains unknown.
2.2. Chronic Pain Changes KOR Function and Expression in Supra-Spinal Sites
While early research focused on dynorphin peptides and KORs in peripheral nociceptors and the spinal cord, chronic or persistent pain also increases KOR function in various brain structures. In an arthritic pain model, KOR binding increased in the dorsomedial and dorsolateral periaqueductal gray (PAG) (Millan et al. 1987). However, it is unknown what the functional relevance is of the chronic pain-induced KOR upregulation in the dorsal PAG. The PAG is a key structure in descending modulation (both facilitation and inhibition) of nociception, risk assessment triggering sympathetic and emotional responses as well as the learning and action of defensive and aversive behaviors (Lefler et al. 2020; Mokhtar and Singh 2020). Activation of the dorsolateral sub-region of the PAG produces emotional arousal and a stress response (increased heart rate, blood pressure, and alertness), whereas the ventrolateral sub-region is known for its involvement in modulating nociceptive transmission and opioid-mediated antinociception (Bagley and Ingram 2020). Given that KOR activation in the dorsal PAG causes anxiogenic effects and escape behavior (Maraschin et al. 2017), it would be of interest to determine the extent KOR activation in the ventrolateral region may contribute to aspects of negative affective states associated with chronic pain. Kappa ORs present in the ventrolateral PAG and are partially responsible for oxytocin-induced analgesia (Ge et al. 2002). An elegant study recently demonstrated that KOR activation in the ventrolateral PAG reduced GABAergic transmission onto PAG dopamine neurons, suggesting that KOR activation leads to disinhibition of these neurons to modulate pain transmission (Li and Kash 2019). These ventrolateral PAG dopamine neurons project to the extended amygdala (BNST and CeA), areas known to regulate stress and anxiety where they contribute to Pavlovian fear conditioning (Matthews et al. 2016) and were proposed to be responsible for aberrant fear memory formation in PTSD patients (Torrisi et al. 2019). In addition, they also contribute to wakefulness where they have reciprocal connections with the sleep–wake regulatory system (Lu et al. 2006). Hence, there is a possibility that KOR modulation of PAG neurons in both the dorsal and ventral regions may contribute to the emotional, affective dimension of the pain response. The subsequent sections in this chapter will focus on how chronic pain alters KOR expression and function in mesolimbic circuitry and the functional consequences of this enhanced KOR system.
It was identified that a group of GABAergic prodynorphin positive neurons (tyrosine hydroxylase negative) in the brain stem (present in both humans and rodents) are poised to regulate pain transmission. LJA5 (lateral pons, juxta A5) projects to the lateral and ventrolateral PAG, the lateral parabrachial nucleus and to lamina I of the spinal cord, and receives input from many stress and sensory areas including the somatosensory and insula cortices, the paraventricular nucleus of the hypothalamus, the dorsomedial nucleus and lateral hypothalamus, central nucleus of the amygdala, periaqueductal gray and lateral parabrachial nucleus (Agostinelli et al. 2021). Whether chronic pain changes the expression of prodynorphin transcript in this specific brain region has yet to be assessed, but these neurons are the only known inhibitory neurons that project directly and selectively to innervate lamina I of the spinal cord, and appose dynorphin neurons in lamina I that project to the parabrachial nucleus (Standaert et al. 1986).
An important component of the pain experience is the negative, affective, emotional component of pain. Narita and colleagues were the first to report the occurrence of an anxiogenic phenotype in mice with chronic pain and that the time line of resolution of negative affect correlated with recovery of sensory pain hyper-sensitivity (Narita et al. 2006). Subsequent studies by Yalcin and colleagues elegantly reported the timeline history/development of various anxiogenic and depressive-like behaviors associated with neuropathic pain in mice (Yalcin et al. 2011). Chronic inflammatory, but not neuropathic pain, produced a significant increase in KOR agonist stimulated [35S]GTPγS binding in membranes prepared from the amygdala at 4 weeks post-injury. In the amygdala, KOR is thought to contribute to anxiety-like behavior, as intracerebral amygdala injection of dynorphin A precipitated an anxiogenic-like phenotype (Narita et al. 2006). Given the overlap in circuitry is involved in pain processing, emotional learning, and fear, it is perhaps not surprising that chronic pain causes an upregulation of KOR systems in limbic brain structures. We recently demonstrated that KOR and dynorphin mRNA transcript were upregulated in the nucleus accumbens (NAc) and ventral tegmental area (VTA) of chronic pain mice, compared to sham controls (Liu et al. 2019), while others also report an upregulation of KOR mRNA in the locus coeruleus (Llorca-Torralba et al. 2020) and prefrontal cortex (Palmisano et al. 2018).
To confirm to what extent the upregulation in transcript expression within mesolimbic circuitry translates into an increased function of the receptor, we showed using ex vivo autoradiography that KOR agonist-induced [35S]GTPγS autoradio-graphic binding was increased in the NAc and VTA of chronic pain mice (Liu et al. 2019). Enhanced KOR agonist stimulated [35S]GTPγS was also reported in the prefrontal cortex and somatosensory cortex one month after induction of neuropathic pain (Llorca-Torralba et al. 2020). The enhanced KOR activity was also evidenced by an increase in the phosphorylated state of the KOR in the NAc in the absence of exogenous agonist administration (Liu et al. 2019). Prodynorphin mRNA was increased in the NAc, the prefrontal cortex and anterior cingulate cortex 2 weeks after nerve injury model of neuropathic pain (Palmisano et al. 2018; Liu et al. 2019). Interestingly, which peptide is cleaved from the prodynorphin peptide was suggested to be brain region specific. Hence, bioconversion of dynorphin B produced DYN B (1–7) in cortical areas, whereas it produced dynorphin B (2–13) in the striatum (Bivehed et al. 2017). These data suggest that prodynorphin cleavage to various peptides within specific brain regions can influence whether KOR or non-opioid mediated effects are produced and would presumably influence various aspects of the pain experience.
2.3. Kappa OR Agonist-Induced Place Aversion Is Enhanced in Chronic Pain States
Kappa OR-mediated dysphoric states are evaluated in rodent models using agonist-induced conditioned place aversion (CPA). CPA is evident in [pain-naïve] rodents when conditioned to KOR (unbiased) agonists, non-specific opioid antagonists such as naloxone, or lithium chloride (LiCl, which produces gastrointestinal distress, emesis, and vomiting in humans). KOR agonist-induced CPA can be elicited following systemic administration or microinjection directly into the VTA, NAc, prefrontal cortex, and lateral hypothalamus, but not the dorsal striatum or substantia nigra (Fig. 2a) (Bals-Kubik et al. 1993; Tejeda and Bonci 2018). Microinjection of KOR antagonists into the prefrontal cortex or dorsal raphe nucleus blocked CPA produced by systemic administration of a KOR agonist, suggesting a critical role for these brain regions in the aversive state (Land et al. 2009; Tejeda et al. 2013). Using conditional knockout mice, KORs on dopamine neurons were shown to be sufficient to produce a place aversion (Chefer et al. 2013; Ehrich et al. 2015b) and re-expression of KOR in dopamine neurons using a viral approach recovered KOR-mediated place aversion suggesting that KOR on dopamine neurons is sufficient for KOR-mediated place aversion (Chefer et al. 2013). The activation of KORs on mesolimbic dopamine neurons that are responsible for KOR agonist-induced aversion was subsequently identified to require activation of p38 MAP kinase (Ehrich et al. 2015b), although there was a dissociation between aversion and changes in dopamine release within the ventral striatum.
Fig. 2.
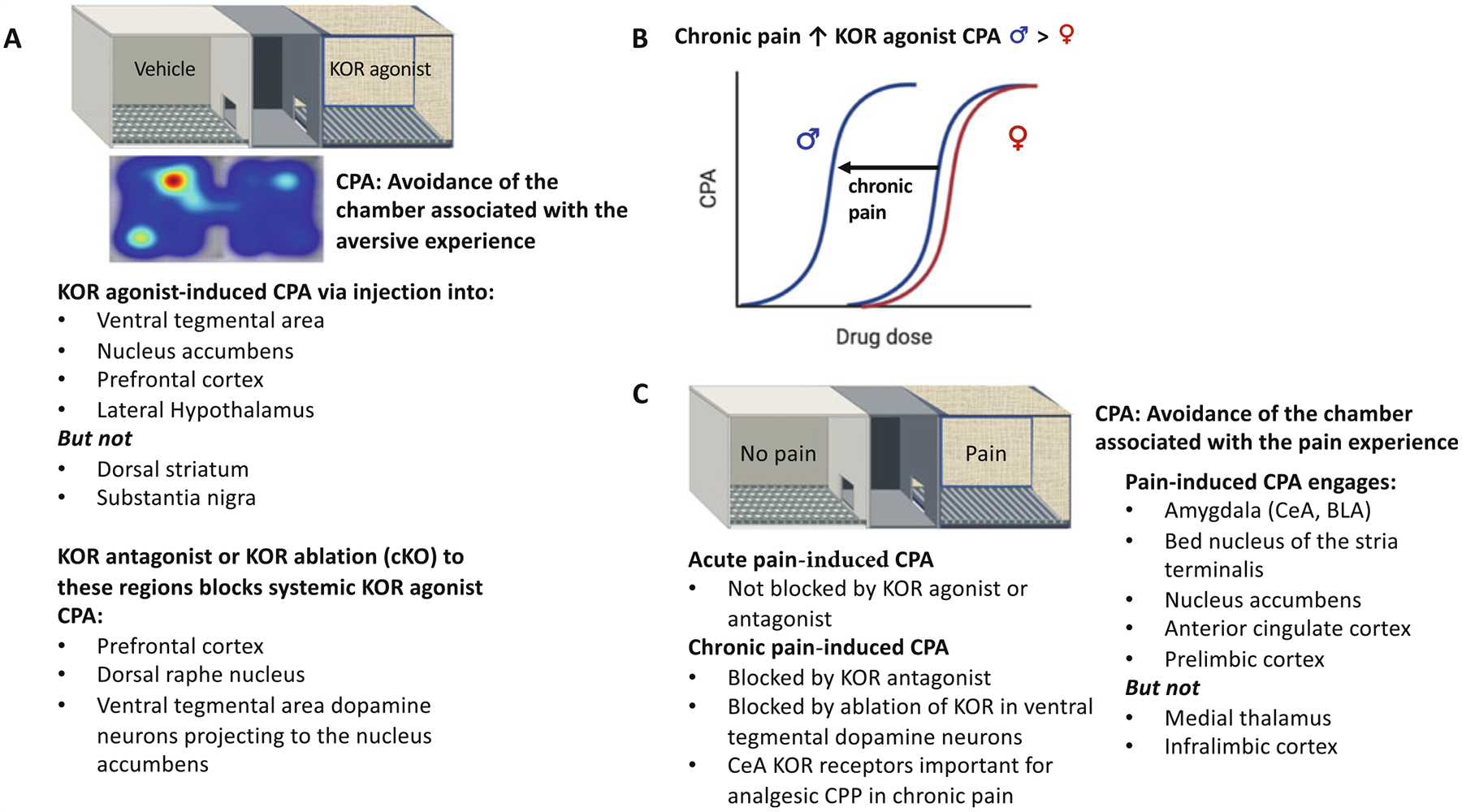
Use of conditioned place preference protocols to measure kappa opioid receptor agonist mediated aversion and pain-induced aversion. (a) KOR agonist-induced place aversion (CPA) can be generated by intracerebral injection into specific brain regions, whereas genetic ablation of KORs from specific brain regions eliminates place aversion following systemic agonist administration. (b) Chronic pain enhances KOR agonist-induced place aversion in male, but not female, mice. (c) Pain-induced aversion where an acute pain stimulus is used for conditioning is not blocked by either KOR agonists or antagonists, however, KOR antagonists block CPA in chronic pain states. Brain regions important for the expression of pain-induced place aversion include the amygdala, bed nucleus of the stria terminalis, nucleus accumbens, anterior cingulate cortex, and prelimbic cortex
Despite this strong evidence for KOR on dopamine neurons projecting to the NAc being responsible for KOR-mediated aversion, re-expression of KORs in the dorsal raphe using lentivirus approaches in the KOR knockout mouse also restored KOR agonist-induced aversion, whereas re-expression of a mutant receptor that failed to activate p38 MAP kinase did not restore aversion (Land et al. 2009). Indeed, subsequent studies also reported that KOR activation in serotoninergic dorsal raphe neurons is both necessary and sufficient to mediate KOR agonist aversive behavioral effects as well (Land et al. 2009; Ehrich et al. 2015b). It remains unclear whether the dopamine and serotonin systems integrate to produce the aversive state associated with KOR activation or if there are multiple brain regions that are sufficient to elicit this aversive state. Considering that KORs are expressed on terminals of dopamine neurons projecting to various targets including (but not limited to) the basolateral amygdala (BLA), NAc, and prefrontal cortex, it is not unreasonable to question whether the inhibitory effects on dopamine release to other targets may also contribute to KOR-mediated aversion, although there are conflicting reports of KOR regulation of dopamine neurons within each of these brain regions (Tejeda and Bonci 2018; Margolis and Karkhanis 2019). KOR agonists also appear to modulate other circuits (independent of monoamines such as dopamine) to produce aversion. Inhibition of glutamate and GABA synaptic transmission in the NAc shell by KOR agonists (Hjelmstad and Fields 2001) was also proposed to contribute to KOR agonist-induced aversion.
Considering chronic pain is a stressor in itself, it is unknown if the associated KOR upregulation is generalizable to all stressors. Stress induced by maternal separation and social isolation increased KOR expression in the amygdala (Nakamoto et al. 2020). However, chronic stress induced by social defeat decreased dynorphin transcript in the NAc (Donahue et al. 2015), although acute social defeat stress increased it in this brain region and additionally induced KOR-mediated stress-induced analgesia. Despite the lack of change in prodynorphin transcript in the chronic stress state, this latter study reported that ablating KORs from dopamine neurons using a conditional knockout approach delayed the development of chronic stress-induced anhedonia, as measured by intracranial self-stimulation (ICSS, an operant behavior sensitive to increases and decreases in the reinforcing efficacy of rewarding brain stimulation) of monopolar electrodes within the lateral hypothalamus. This study concluded that the KOR expression on dopamine neurons contributed to an increase in stress resilience. Nevertheless, the findings from this study argue that the dynorphin and KOR upregulation in chronic pain states is not generalizable across all types of chronic stress, but the dial up in function appears to be generalizable to withdrawal associated with chronic drug use (Koob 2020).
To determine the extent KOR aversion may be enhanced in chronic pain, we used the CPA assay. The KOR agonist-induced dose-dependent CPA was shifted to the left in male, but not female, chronic pain mice, suggesting a sex-dependent enhancement of KOR-mediated effects in chronic pain states (Fig. 2b) (Liu et al. 2019). This sex difference is consistent with human imaging data showing that KOR binding is greater in men than women in multiple brain regions, including the anterior cingulate cortex, which is involved in the emotional affective dimension of pain (Vijay et al. 2016). Furthermore, females were shown to be less sensitive than males to the KOR agonist-induced deficits in motivation as measured by ICSS of the medial forebrain bundle and less effective in suppressing evoked NAc dopamine release measured via fast scanning cyclic voltammetry (Conway et al. 2019). Nevertheless, there are conflicting reports of the effects of ongoing pain on KOR-mediated aversion, where the place aversion to a KOR agonist was absent in male rats with persistent ongoing pain induced by complete Freund’s adjuvant (Shippenberg et al. 1988). Although it is unclear if the pain may have interfered with the essential cue learning required to elicit a CPA as a positive control was not included in the study. Taken together, chronic pain causes a sex-dependent enhancement of KOR-mediated aversion, although it is unknown if the same circuits are engaged as those in the absence of pain.
3. Kappa OR Modulation of the Affective, Emotional Dimension of Pain
Similar to the use of a CPA protocol to detect a drug-induced aversive state, pairing a painful event (rather than a drug) with contextual cues allows animals to learn the association of an environment with a painful stimulus. Painful stimuli such as intraplantar formalin or carrageenan, or intraperitoneal injection of acetic acid can produce a pain evoked CPA (Fig. 2c). Optogenetic activation or inhibition of nociceptive neurons using real-time place conditioning paradigms also shows place conditioning to painful stimuli (Daou et al. 2013; Iyer et al. 2014; Park et al. 2015; Beaudry et al. 2017). The aversive component of pain has been captured by other place avoidance paradigms to try to capture pain affect in chronic pain states. LaBuda and Fuchs (2000) reported a place avoidance escape assay whereby the rodent has a conflict between staying in a dark compartment associated with a mechanical stimulus or entering a light compartment with no, or non-painful, sensory stimuli. A CPA can also be produced by combining experimenter-provoked mechanical allodynia during the place conditioning, whereby von Frey filaments are applied to the injured hind paw through a grid floor in the place conditioning apparatus (Hummel et al. 2008). The CPA induced by sensory stimulation in either neuropathic or inflammatory chronic pain models was maintained for at least one month in the absence of further conditioning and non-rewarding doses of morphine given during the pain-paired conditioning sessions attenuated the CPA. But what, if any role does the KOR system have in modifying the affective emotional aversive component of this pain experience?
The role of KORs in mediating the affective dimension of pain states most likely depends on the duration of pain (acute vs. chronic) or the conditioning protocol used to assess the aversive painful state or more likely the co-occurrence of a negative affective state associated with chronic pain. The CPA produced by intraperitoneal injection of acetic acid was not blocked by pretreatment with a KOR antagonist (or KOR agonist) (Bagdas et al. 2016). Electroacupuncture prevented the expression of a CPA to intraplantar injection of complete Freund’s adjuvant and this effect was absent in rodents pretreated with a MOR, but not KOR, antagonist injected into the rostral anterior cingulate cortex (Zhang et al. 2012). Indeed, in rats with postsurgical or neuropathic pain, endogenous MOR signaling in the anterior cingulate cortex was necessary for the expression of a conditioned place preference (CPP) to non-opioid pain relieving treatments such as a peripheral nerve block or spinal clonidine (an alpha2-adrenergic agonist) and this receptor activation was also associated with dopamine release in the NAc (Navratilova et al. 2015).
3.1. Kappa OR Ligands Do Not Alter Acute Pain-Induced Aversion or Depression of ICSS
The affective dimension of pain can be captured using ICSS, an operant procedure in which subjects emit a learned response such as a lever press to earn pulses of electrical stimulation to brain reward areas and is reliant on dopamine release in the ventral striatum. Studies have shown that acute visceral pain (Leitl et al. 2014a) and different models of inflammatory pain (formalin or complete Freund’s adjuvant) (Leitl et al. 2014b) depressed ICSS. Pain-induced (complete Freund’s adjuvant, formalin or lactic acid) depression of ICSS was not prevented by pretreatment with a KOR antagonist (Leitl et al. 2014a, b). Despite the lack of involvement in KOR systems for the expression of acute pain-induced aversion, it does not rule out the possibility that activation of KORs contributes to negative affective states associated with chronic pain states. Indeed, chronic pain is highly co-morbid with mood disorders in clinical populations. KOR agonists activate the HPA axis, and produce pro-depressive and anxiogenic-like effects, whereas KOR antagonists cause opposing effects in producing anxiolytic-like and anti-depressant effects (Van’t Veer and Carlezon 2013). Ablation of KORs from dopamine neurons (Van’t Veer and Carlezon 2013) or KORs on BLA glutamatergic neurons that project to the medial prefrontal cortex (Tejeda et al. 2015) results in an anxiolytic phenotype, suggesting that KOR modulation of these circuits is critical to the expression of negative affect. KOR activation within the BLA was necessary for the anxiogenic effect of corticotropin releasing factor (Bruchas et al. 2009) and eliminating KOR from BLA glutamatergic neurons projecting to the BNST also has an anxiolytic phenotype (Crowley et al. 2016). Considerable evidence suggests that the KOR system within the NAc also underlies negative affective states and heightens stress reactivity in various psychiatric disorders. For example, dynorphin expression is increased in the ventral striatum of suicidal individuals (Hurd et al. 1997) and in animal models of depression (Shirayama et al. 2004; Carlezon and Krystal 2016; Tejeda and Bonci 2018).
3.2. Kappa OR Systems Contribute to Ongoing Persistent Pain States
The lack of KOR involvement in acute pain aversion or ICSS depression may be due to the temporal relationship between the onset of pain and development of negative affective-like behaviors. Anxiety and depressive behaviors that accompany chronic pain states in rodents do not typically begin to manifest until weeks 4–8 following injury (Yalcin et al. 2011). Thus, the KOR system may only be engaged following tissue or nerve injury that induces the negative affective-like behaviors (anxiety and depression) versus the initial associated with an acute painful event. This idea is supported by the finding that, in a model of joint pain, KOR knockout mice showed reduced anxiety (elevated plus maze) (Negrete et al. 2016), where global KOR knockout does not produce an anxiolytic phenotype in the absence of ongoing pain (pain-naïve animals) (Kieffer 1999). Thus, there are contradictory findings reported for the involvement of KOR in pain-induced negative affect and pain aversion, but if one considers the temporal relationship with development of co-morbid affective states, the delayed KOR system involvement more closely correlates with negative affective states.
While the CPP paradigm is useful for studying reward and aversion, the interpretation of data in chronic pain states can be somewhat difficult; the effect size of this CPP may be exaggerated if the rodent was experiencing an aversive state in the non-drug paired chamber. To circumvent this confound, we used a one-sided conditioning protocol (Bechara and van der Kooy 1992) to determine whether chronic pain could evoke a CPA in the absence of stimulating the dermatome affected by an injury. Both mice and rats, independent of sex, produced a CPA to ongoing persistent inflammatory and neuropathic pain, whereas sham control animals had no preference to either chamber (Liu et al. 2019). We interpreted these data to suggest that one can capture the ongoing aversive component of chronic pain. In separate cohorts of mice, KOR antagonism with JDTic prevented place aversion in male, but not female, chronic neuropathic pain mice, demonstrating a sex-specific engagement of KORs in pain aversive states (Fig. 2c), which was generalizable to chronic inflammatory pain. It is noteworthy that KOR blockade had no effect on mechanical withdrawal thresholds in sham or chronic pain mice demonstrating that KOR involvement in pain circuitry is restricted to the affective, but not sensory, dimension of chronic pain. This study demonstrated that KOR expression on dopamine neurons was sufficient to produce the chronic pain-induced CPA. In addition to capturing this ongoing pain response, other studies have shown that the negative reinforcement produced by gabapentin and morphine in chronic pain models was absent in rodents that received KOR antagonists (Liu et al. 2019; Navratilova et al. 2019). One interpretation of these data is that blocking the negative affect by KOR antagonism eliminated the motivation and drive for pain relief produced by gabapentin and morphine. One could even suggest that the alleviation of the negative affective state was the only motivation to seek morphine or gabapentin.
In addition to the contribution of KORs within mesolimbic dopamine neurons to the tonic aversive component of ongoing chronic pain, KORs within the amygdala (CeA and BLA) have also been implicated in modulating the pain experience including pain-induced aversion (Corder et al. 2019; Navratilova et al. 2019; Phelps et al. 2019). In un-injured pain-naïve animals, stress produced allodynia (painful response to something not normally painful) via a KOR-dependent mechanism in the CeA (Xie et al. 2017). The CeA receives both nociceptive sensory input from the spinal cord via the parabrachial nucleus (Chiang et al. 2020) and noradrenergic innervation from the locus coeruleus (also implicated in reinstatement of drug-seeking behavior) (España et al. 2016), making it a central brain region for integrating pain and stress. The lateral CeA has been termed the “nociceptive amygdala” given that neurons in this brain region respond exclusively to noxious stimulation (Neugebauer et al. 2020). Intra-CeA administration of a KOR antagonist had no effect on mechanical hypersensitivity in a rat model of neuropathic pain, but prevented the CPP to intravenous gabapentin, suggesting that KOR blockade in this brain region eliminated the aversiveness of ongoing pain (Navratilova et al. 2019). This latter study also demonstrated that KOR blockade also reduced synaptically evoked CeA neuronal spiking in chronic pain, but not sham control, animals, leading the authors to hypothesize that increased KOR activity produces a tonic disinhibition of CeA output neurons that promotes ongoing aversive aspects of neuropathic pain. While KORs in the CeA do not modulate pain hypersensitivity, they may be involved in the loss of diffuse noxious inhibitor control (descending circuits such as the PAG, locus coeruleus and dorsal raphe nucleus that regulate nociceptive transmission) that occurs in various chronic pain states (Phelps et al. 2019). Another recent study reported that optogenetic activation of CeA neurons suppressed both pain-elicited reflexive and self-recuperating behaviors across sensory modalities and abolished neuropathic pain hypersensitivities, whereas inhibiting CeA neuronal activity exacerbated pain and produced a strong aversion (Hua et al. 2020). Future studies are needed to understand to what extent KOR systems in the extended amygdala also contribute to the pain experience. Figure 3 highlights the anatomical expression and contribution of the dynorphin-KOR system in the sensory and affective components of chronic, persistent pain.
Fig. 3.
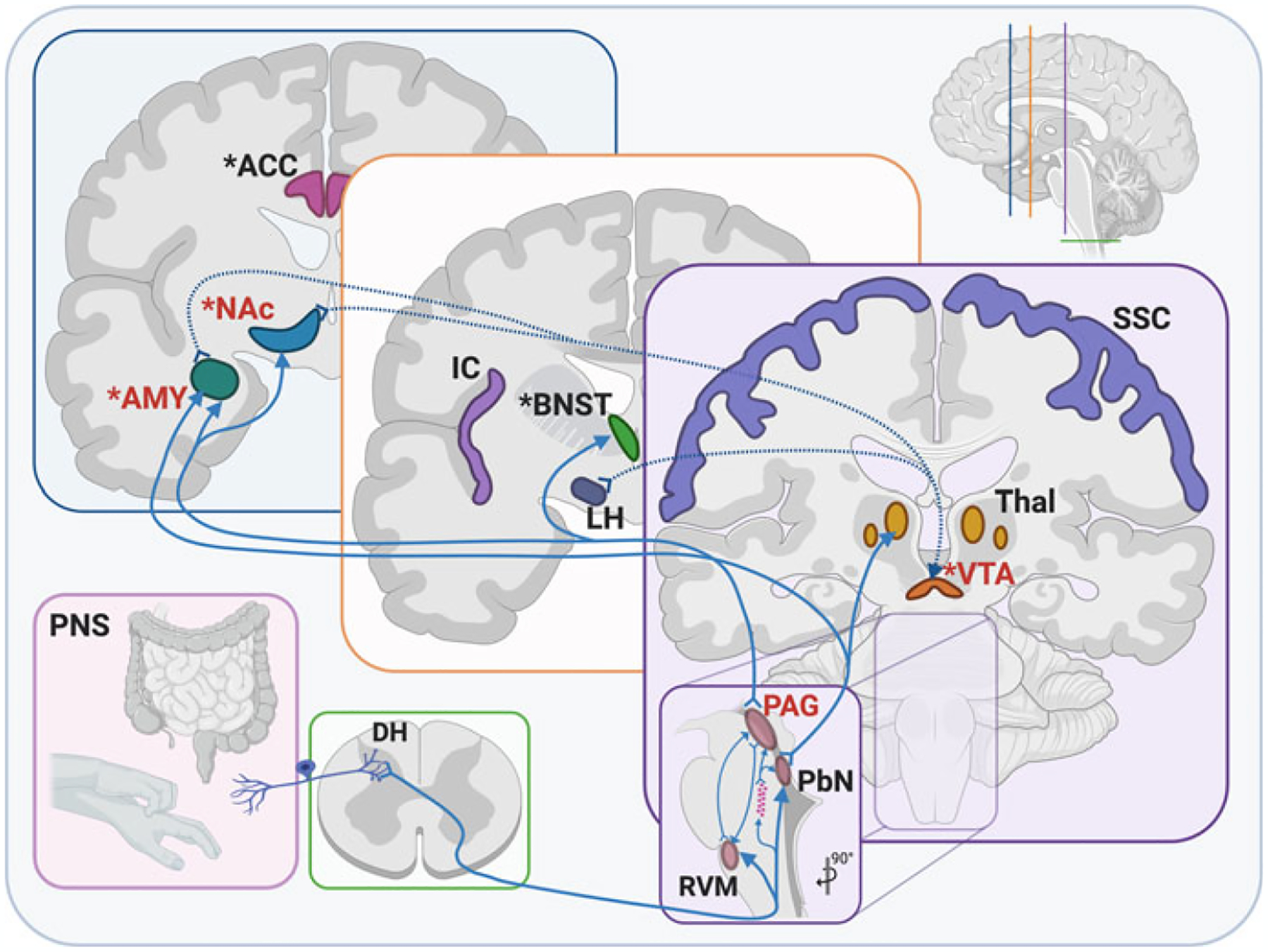
Anatomical expression and contribution of the dynorphin-KOR system in the sensory and affective components of chronic, persistent pain. KORs are present on peripheral sensory inputs from skin and visceral targets, where they synapse onto the DH of the spinal cord. Within the spinal cord, KORs are concentrated in the lamina II of the superficial DH. From here, sensory information is sent via ascending connections into numerous brain stem nuclei, including the RVM, PbN, and a cluster of LJA5 cells. From there, sensory information is relayed into midbrain regions, including PAG and VTA, where KORs are concentrated and shown to be upregulated following chronic pain. Connections from the brain stem and midbrain are further relayed to numerous forebrain areas, including PbN to Thal and AMY, as well as PAG to AMY, BNST, and NAc, all regions shown to contribute to the affective dimension of pain. KOR is also increased in NAc and AMY following chronic pain. Other regions involved in the affective and/or sensory dimensions of pain are the ACC, IC, and SSC, though the connectivity and specific contribution of KOR is not yet fully understood. *areas known to be involved in affective pain; red text: areas where KOR activity is increased following chronic pain; dotted arrows: known dynorphin connections; pink stars: LJA5 cells. ACC anterior cingulate cortex, AMY amygdala, BNST bed nucleus of the stria terminalis, DH dorsal horn, IC insular cortex, LH lateral hypothalamus, NAc nucleus accumbens, PAG periaqueductal gray, PbN parabrachial nucleus, PNS peripheral nervous system (sensory components), RVM rostral ventromedial medulla, SSC somatosensory cortex, Thal thalamic nuclei, VTA ventral tegmental area
4. Is There Potential for KOR Antagonism as a Management Strategy for Chronic Pain-Associated Negative Affect?
The dysphoric and negative affective states induced by KOR activation generated promise for KOR antagonists as a novel treatment strategy for mood disorders and pharmacotherapy for substance use disorders. A review is available summarizing the completed clinical trials that evaluated safety and effectiveness of KOR antagonists for treatments of substance use and mood disorders (Carlezon and Krystal 2016). Pharmacokinetic studies and potential drug interactions with ethanol were evaluated for the KOR antagonist LY2456302 in healthy subjects (Lowe et al. 2014) as was safety, tolerability, and pharmacokinetics of oral doses of JDTic, which was prematurely stopped due to cardiac adverse events (Buda et al. 2015). One mechanism implicated in KOR-mediated aversion and negative effects on mood is the modulation of mesolimbic dopamine circuitry (Van’t Veer and Carlezon 2013). This circuitry plays a central role in motivational processes and incorporates dopaminergic neurons in the VTA that project to the NAc (Berridge and Kringelbach 2013, 2015; Zarrindast and Khakpai 2015). KORs are known to modulate this circuitry by inhibiting dopamine release at dopaminergic nerve terminals within the NAc (Spanagel et al. 1992; Ebner et al. 2010; Cahill et al. 2014; Ehrich et al. 2015b; Chartoff et al. 2016). Considering that systems involved in affective aspects of pain processing and other affective and motivational systems interact extensively (Jarcho et al. 2012; Elman et al. 2013), there is potential that KOR blockade may prove to be a novel pain management strategy.
Epidemiological evidence shows that chronic pain is second only to bipolar disorder as the major cause of suicide among all medical illnesses (Asmundson and Katz 2009; Elman et al. 2013) and that mood disorders are highly co-morbid in chronic pain patients, where the prevalence of depression ranges between 30 and 80%, depending on the pain etiology (Bair et al. 2003; Howe and Sullivan 2014). It is consistently reported that co-morbid psychopathology in patients with chronic pain exhibit increased pain intensity and increased pain-related disability (Jamison and Edwards 2013; Martel et al. 2014), thus managing negative affect holds promise in reducing pain ratings in patients with chronic pain. We, and others, identified that mesolimbic circuitry dysfunction (including dopamine neurotransmission) precipitates mood disorders, impairs motivated behavior, and likely contributes to chronic pain (Narita et al. 2005; Cahill et al. 2014; Hipólito et al. 2015; Taylor et al. 2015; Borsook et al. 2016; Evans and Cahill 2016; Cahill and Taylor 2017; Liu et al. 2019; Massaly et al. 2019). There is compelling evidence that KORs within the ventral striatum underlies negative affective states and heightens stress reactivity in psychiatric diseases (Ebner et al. 2010; Knoll and Carlezon 2010). Supporting this thesis is the report that prodynorphin mRNA was increased in the ventral striatum of victims of suicide (Hurd et al. 1997), although no difference in dynorphin levels in cerebral spinal fluid was found in patients with non-suicide self-injurious behavior, yet this group did have lower levels of other endogenous opioids (Stanley et al. 2010).
Preclinical and human subject research have identified strong correlations between dysfunction of this mesolimbic circuitry and chronic pain (Jarcho et al. 2012; Elman et al. 2013; Borsook et al. 2016; Evans and Cahill 2016; Taylor et al. 2016; Cahill and Taylor 2017). The increase in extracellular dopamine following intra-VTA or systemic administration of MOR agonist is absent in chronic neuropathic pain (Ozaki et al. 2002; Taylor et al. 2015) and inflammatory pain states (Narita et al. 2005; Hipólito et al. 2015), which correlates with the loss of opioid reward as measured by CPP following intra-VTA or systemic administration of MOR agonists (Ozaki et al. 2002; Taylor et al. 2015). Using in vivo microdialysis in awake, freely moving animals, we demonstrated that blocking KORs recovered the loss of opioid-induced dopamine release in the NAc associated with neuropathic pain (Liu et al. 2019). This finding is consistent with previous reports that blocking KORs recovered opioid-evoked dopamine release in the formalin model of inflammatory pain (Narita et al. 2005). Interestingly, the morphine-evoked increases in NAc extracellular dopamine can be blocked by administration of a KOR agonist into the NAc but not the VTA (Spanagel et al. 1992), implying that KORs within the ventral striatum are important in the negative modulation of mesolimbic dopamine dependent motivation produced by MOR agonists such as morphine. Given that hypo-dopaminergic states contribute to chronic pain (Borsook et al. 2016; Taylor et al. 2016) and mood disorders co-morbid with chronic pain (Elman et al. 2013), recovering rewarding effects is an important component of alleviating chronic pain. While KOR antagonism had no effect on opioid reward in pain-naive cohorts, it restored DAMGO-induced CPP in chronic pain animals (Liu et al. 2019). This study further highlights the involvement of NAc KORs in chronic pain-induced negative affective states. Using an inflammatory model of chronic pain induced by complete Freund’s adjuvant, Massaly and colleagues showed that the reduced motivation for sucrose self-administration (via progressive ratio testing) required the engaged dynorphin neurons in the NAc and was prevented by KOR antagonism (Massaly et al. 2019). Taken together, KORs contribute to the hypo-dopaminergic tone and reward-related behaviors in chronic pain without altering sensory pain hypersensitivities, suggesting that KOR antagonists may be an effective treatment in pain management for chronic pain patients.
5. Evidence That KOR Systems May Contribute to Drug-Seeking Behavior in Chronic Pain States
As noted above, mood disorders are highly co-morbid in chronic pain patients (Bair et al. 2003; Howe and Sullivan 2014) and are a risk factor for the development of opioid use disorder (OUD) (Evans and Cahill 2016). A systematic review reported that chronic pain patients have high rates (13–38%) of opioid misuse (defined as opioid use contrary to the directed or prescribed pattern of use, regardless of the presence or absence of harm or adverse effects) (Vowles et al. 2015). This is consistent with reports where electronic health records of >5,000 patients with opioid use disorder revealed the majority of patients reported chronic pain preceded their opioid use disorder, and 85% of this cohort had a co-morbid mental disorder (Hser et al. 2017), a finding recently duplicated by another study (Higgins et al. 2020). The high abuse rates of therapeutic opioids have fueled a strong debate on the treatment practices of using prescription opioids and whether pain is a major factor in addiction susceptibility. With the overwhelming evidence that negative affective states drive opioid use disorder (Evans and Cahill 2016), including in chronic pain patients, it is expected that chronic pain states lead to activation of KOR systems that are involved in stress-induced reinstatement of opioid drug-seeking behavior. However, how KOR systems modulate drug-seeking behavior in chronic pain states has not been explored, but there are various clinical trials that have reported the effectiveness of KOR partial agonism or antagonism in modulating substance use disorders (Table 1).
Table 1.
Clinical trials that KOR partial agonism or antagonism improves substance use disorder (pain is noted). Nalmefene (Selincro®), a mu opioid receptor inverse agonist with weak partial KOR agonist properties, is used in the treatment of alcohol use disorder. Buprenorphine (partial mu opioid agonist, ORL-1 agonist, KOR antagonist/partial KOR agonist, and delta opioid antagonist) and naloxone (Zubsolv®, Suboxone®) combination has been used for treating opioid use disorder (Heo and Scott 2018) and this combination was reported to reduce pain. Buprenorphine in combination with samidorphan (MOR antagonist) showed beneficial effects over placebo to reduce symptoms in major depressive disorder patients who had inadequate responses to antidepressants, although this drug combination was recently rejected by the US Food and Drug Administration. Polymorphisms in the KOR gene are associated with opioid dependence. Previous reviews have discussed the safety and efficacy of buprenorphine for the treatment for chronic pain (Davis et al. 2018; Pergolizzi and Raffa 2019) or opioid use disorder (Parida et al. 2019)
| Species | Sex | Protocol | KOR ligand | Brain region | Model | Outcome | Ref |
|---|---|---|---|---|---|---|---|
| Human | M/F | Phase IV clinical trial | Nalmefene | na | EtOH-dependent outpatients | ↓ Heavy drinking days ↓ Total EtOH consumption |
(Barrio et al. 2018) |
| Human | M/F | Double -blind Multicenter Randomized | Nalmefene | na | AUD (>60 g/day M >40 g/day F) | ↓ Heavy drinking days ↓ Total EtOH consumption |
(Miyata et al. 2019) |
| Human | M/F | FMRI Placebo controlled, double-blind | Nalmefene | Putamen, angular gyrus, supramarginal gyrus, (↔amygdala) | AUD | ↑ BOLD to emotional faces in areas responsible for empathy and social cognition, attentional shift happy > fearful | (Vollstädt-Klein et al. 2019) |
| Human | M/F | Double-blind, placebo control trial | Nalmefene | na | AUD | ↓ Heavy drinking days | (Mason et al. 1999) |
| Human | Male | FMRI with i.v. EtOH (6% v/v to achieve 80 mg/dL) | Nalmefene | Striatum | Heavy drinkers | ↓ BOLD during reward anticipation | (Quelch et al. 2017) |
| Human | ORPK1 gene polymorphism (rsl0958350-rs7016778-rsl2675595) | na | Methadone maintenance for OUD | Polymorphism was associated with opioid withdrawal | (Wang et al. 2014) | ||
| Human | ORPK1 gene polymorphism (rs997917, rs6985606) | na | Methadone maintenance for OUD | Polymorphism was associated with opioid dependence | (Albonaim et al. 2017) | ||
| Human | ORPK1 gene polymorphism (KOR 36G > T SNP, rs6473797, rsl6918842, rs3802279) | na | Heroin-dependence | Polymorphism was associated with heroin dependence | (Yuferov et al. 2004; Gerra et al. 2007; Levran et al. 2008; Yuanyuan et al. 2018) | ||
| Human | Male | Suicide ideation for inpatients | Buprenorphine (sublingual) | na | OUD and major depressive disorder | ↓ Suicide ideation | (Ahmadi et al. 2018) |
| Human | M/F | Multicenter double-blind placebo controlled trial | Buprenorphine/samidorphan (not approved by FDA) | na | Major depressive disorder | ↓ Depression (HAM-D, 17-item scale, Montgomery-Åsberg depression rating scale and the clinical global impressions severity scale) | (Ehrich et al. 2015a; Fava et al. 2016) |
| Human | M/F | OUD | Buprenorphine-naloxone | na | OUD | ↓ Pain intensity (in non-chronic pain cohorts) | (Becker et al. 2015) |
| Human | M/F | In patient oxycodone selfadministration | Buprenorphine-naloxone | na | OUD/chronic pain transitioning from opioid to Bup/Nx | ↓ Pain ratings when switched to Bup/Nx ↔ between placebo and oxycodone preference, those that showed oxycodone preference had lower Bup/Nx dose, more withdrawal and more pain | (Roux et al. 2013) |
| Human | M/F | Pilot clinical trial | Buprenorphine-naloxone | na | OUD/chronic pain transitioning from opioid to Bup/Nx | ↓ Average and worse pain after switching to Bup/Nx | (Rosenblum et al. 2012) |
| Human | M/F | Postsecondary analysis of randomized trials | Buprenorphine-naloxone | na | OUD | >50% reported pain at baseline Improvement in pain correlated with increased retention of treatment | (Shulman et al. 2020) |
| Human | M/F | Open-label randomized | Buprenorphine-naloxone | na | OUD | ↔Pain intensity, affective pain or sensory pain but women had greater affective pain than men | (Latif et al. 2019) |
| Human | M/F | Postsecondary analysis of randomized trials | Buprenorphine-naloxone | na | OUD | Patients with flare-up pain were at higher risk of relapse | (Griffin et al. 2016) |
| Human | M/F | Postsecondary analysis of randomized trial | Buprenorphine-naloxone | na | OUD with chronic pain | ↓ Pain over course of 12 week treatment, those with high pail volatility were more likely to relapse | (Worley et al. 2015, 2017) |
| Human | Male | Case study (40 year+ with chronic pain) | Buprenorphine-naloxone | na | Chronic pain with long-term opioid use | ↓ Pain, improved function and quality of life | |
| Human | Male | Randomized trial | Buprenorphine-naloxone vs. methadone | na | Chronic pain and OUD | ↓ Pain at 6 months | (Neumann et al. 2013) |
As noted above, there is overlapping expression of the KOR and its endogenous ligands dynorphins within reward and stress pathways, which contributes to the ability of this system to alter stress- and reward-related signaling in the brain (Bruchas et al. 2010; Wee and Koob 2010; Crowley and Kash 2015). Drug relapse can be produced by a stressful event, presentations of cues associated with drug taking, as well as the drug itself (drug-priming). A number of studies have implicated KORs in stress-induced relapse/reinstatement of drug-seeking behavior (Table 2). KOR antagonists block stress-induced reinstatement, while KOR agonists, such as U50,488, induce reinstatement of cocaine (Beardsley et al. 2005; McLaughlin et al. 2006; Redila and Chavkin 2008; Polter et al. 2014; Heinsbroek et al. 2018), nicotine (Jackson et al. 2013; Nygard et al. 2016), and alcohol (Funk et al. 2014, 2019a, b; Lê et al. 2018) seeking behavior. Stress caused by injection of yohimbine (Zhou et al. 2013) or food deprivation (Sedki et al. 2015) precipitated reinstatement of heroin drug-seeking, is also blocked by pretreatment with the KOR antagonist nor-BNI.
Table 2.
Evidence that KOR activation contributes to stress-induced reinstatement of opioid seeking behavior in preclinical models
| KOR ligand | Species | Sex | Drug of abuse/protocol | Stressor/pain | Main outcome | Reference | Pain component |
|---|---|---|---|---|---|---|---|
| Nor-BNI | Sprague Dawley rats | Male | Heroin IVSA and stress-induced reinstatement | Yohimbine (YOH) | KOR blockade reduced reinstatement | (Zhou et al. 2013) | No |
| Nor-BNI | Sprague Dawley rats | Male | Heroin IVSA and stress-induced reinstatement | Food deprivation | KOR blockade reduced reinstatement | (Sedki et al. 2015) | No |
| Nor-BNI | Mouse (C57B1/6) | Male | Morphine CPP and drug-prime reinstatement | Incisional postsurgical pain model | KOR blockade enhanced drug-primed reinstatement | (Nwaneshiudu et al. 2020) | Yes |
| LY2456302 | Sprague Dawley rats | Male | Oxycodone IVSA and cue-induced reinstatement | None | KOR blockade had no effect on reinstatement | (Bossert et al. 2019) | No |
| CJ-15208 (Cyclo[Pro-Sar-Phe-D-Phe]) or Nor-BNI | Mouse (C57B1/6) | Male | Morphine CPP and stress-induced reinstatement | Forced swim stress | Both drug interventions prevented reinstatement | (Brice-Tutt et al. 2020; Ferracane et al. 2020) | No |
| Buprenorphine + naltrexone | Sprague Dawley rats | Male | Morphine CPP and drug-prime reinstatement | None | The combination blocked drug-primed reinstatement | (Cordery et al. 2014) | No |
| Dezocine (partial MOR agonist, KOR antagonist) or buprenorphine | Sprague Dawley rats | Male | Morphine CPP and drug-prime reinstatement | None | Both drug interventions prevented reinstatement | (Wu et al. 2019) | No |
| Nor-BNI | Sprague Dawley rats | Male | Morphine CPP and drug-prime reinstatement | None | KOR blockade did not alter drug-primed reinstatement | (He et al. 2019) | No |
While there is significant promise in the potential for KOR antagonists to attenuate drug relapse, distinct mechanisms have been shown to underlie reinstatement due to either cue, drug-priming or stress. For example, context-induced reinstatement of oxycodone was blocked by MOR, but not KOR antagonists (Bossert et al. 2019), albeit low dose naloxone (0.03 mg/kg) increased heroin drug intake and elevated ICSS thresholds (above already elevated baseline levels) when stimuli was paired with the naloxone treatment (Kenny et al. 2006). Additionally, social defeat-induced stress produced KOR-mediated anhedonia, as measured by ICSS (Donahue et al. 2015). Other studies show that KOR antagonism did not affect morphine (Glick et al. 1995) or heroin (Negus et al. 1993) self-administration or context-induced reinstatement of oxycodone self-administration (Bossert et al. 2019). Furthermore, KOR agonists decreased cocaine intake and cocaine drug-seeking (Glick et al. 1995; Schenk et al. 1999; Morani et al. 2009), as well as cocaine or amphetamine-induced reinstatement in mice (Schenk and Partridge 2001) and non-human primate (Negus et al. 1997; Mello and Negus 2000; Rüedi-Bettschen et al. 2010). Similarly, KOR agonists reduced oxycodone self-administration in non-human primates (Zamarripa et al. 2020a), decreased morphine self-administration in mice (Glick et al. 1995) and blocked oxycodone CPP in male rats (Zamarripa et al. 2020b). Considering ongoing pain is a stressor in itself, it is unknown to what extent KOR systems contribute to stress- and drug-priming-induced reinstatement under conditions of chronic pain. However, one study reported that post-operative pain (incisional model) suppressed morphine-primed reinstatement in self-administration studies and KOR antagonism reversed this inhibition (Table 2) (Nwaneshiudu et al. 2020).
6. Conclusions
The dynorphin-KOR system is a near ubiquitously expressed receptor system found throughout the brain and body that has been implicated in a wide array of physiology and behaviors, including nociceptive processing, chronic pain states, and emotional regulation/dysregulation. It remains a prime target for the potential development of novel therapeutics for the treatment of acute and chronic pain, in the absence of abuse liability. However, likely due to the widespread expression of both the endogenous dynorphin peptides and KOR, designing appropriate pharmacotherapies has proved challenging, due to many unwanted physical and affective side effects. Over the past few decades, researchers have developed a deeper understanding of dynamic signaling capabilities involved with both dynorphin and KOR under various physiological conditions, as well as within and between various tissues and brain regions. This knowledge has resulted in the potential development of both biased agonists and peripherally restricted compounds targeting KOR for the alleviation of pain. Furthermore, while KOR agonists have long been considered the ultimate goal for drug development, due to the upregulation of KOR observed in the chronic pain states, researchers have found some success in using KOR antagonists to decrease the affective dimension of pain. This has opened another avenue of research into the role of dynorphin and KOR in emotional dysregulation following chronic pain and/or opioid use.
Acknowledgements
Funding:
The Shirley Hatos Foundation supports CMC, CJE, and LL. NIH Grant numbers R01DA041781 (CMC), 1UG3TR003148-01 (CMC), and 2P50 DA005010 (CMC, CJE), and the Department of Defense Grant number W81XWH-15-1-0435 (CMC).
Abbreviations
- ACTH
Adrenocorticotropic hormone
- BLA
Basolateral amygdala
- BNST
Bed nucleus of the stria terminalis
- CeA
Central nucleus of the amygdala
- CPA
Conditioned place aversion
- CPP
Conditioned place preference
- CRF
Corticotropin-releasing hormone
- DOR
Delta opioid receptor
- GABA
Gamma-aminobutyric acid
- GPCRs
G-protein coupled receptors
- ICSS
Intracranial self-stimulation
- JDTic
C28H39N3O3 kappa opioid antagonist
- KOR
Kappa opioid receptor
- LiCl
Lithium chloride
- MAP
Mitogen-activated protein
- MK801
Dizocilpine, NMDA antagonist
- MOR
Mu opioid receptor
- nor-BNI
Nor-binaltorphimine, kappa opioid antagonist
- NMDA
Nucleus accumbens NAc
- ORL1
Opioid receptor-like/nociceptin receptor
- PAG
Periaqueductal gray
- VTA
Ventral tegmental area
Contributor Information
Catherine M. Cahill, Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, CA, USA Shirley and Stefan Hatos Center for Neuropharmacology, University of California Los Angeles, Los Angeles, CA, USA; Semel Institute for Neuroscience and Human Behavior, University of California Los Angeles, Los Angeles, CA, USA; David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA.
Lindsay Lueptow, Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, CA, USA; Shirley and Stefan Hatos Center for Neuropharmacology, University of California Los Angeles, Los Angeles, CA, USA; Department of Psychology, University of California Los Angeles, Los Angeles, CA, USA.
Hannah Kim, Shirley and Stefan Hatos Center for Neuropharmacology, University of California Los Angeles, Los Angeles, CA, USA.
Raj Shusharla, Shirley and Stefan Hatos Center for Neuropharmacology, University of California Los Angeles, Los Angeles, CA, USA.
Amy Bishop, Shirley and Stefan Hatos Center for Neuropharmacology, University of California Los Angeles, Los Angeles, CA, USA.
Christopher J. Evans, Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, CA, USA Shirley and Stefan Hatos Center for Neuropharmacology, University of California Los Angeles, Los Angeles, CA, USA; Semel Institute for Neuroscience and Human Behavior, University of California Los Angeles, Los Angeles, CA, USA; David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA; Department of Psychology, University of California Los Angeles, Los Angeles, CA, USA.
References
- Agostinelli LJ, Mix MR, Hefti MM, Scammell TE, Bassuk AG (2021) Input-output connections of LJA5 prodynorphin neurons. J Comp Neurol 529:635–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi J, Jahromi MS, Ehsaei Z (2018) The effectiveness of different singly administered high doses of buprenorphine in reducing suicidal ideation in acutely depressed people with co-morbid opiate dependence: a randomized, double-blind, clinical trial. Trials 19:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albonaim A, Fazel H, Sharafshah A, Omarmeli V, Rezaei S, Ajamian F, Keshavarz P (2017) Association of OPRK1 gene polymorphisms with opioid dependence in addicted men undergoing methadone treatment in an Iranian population. J Addict Dis 36:227–235 [DOI] [PubMed] [Google Scholar]
- Asmundson GJG, Katz J (2009) Understanding the co-occurrence of anxiety disorders and chronic pain: state-of-the-art. Depress Anxiety 26:888–901 [DOI] [PubMed] [Google Scholar]
- Bagdas D, Muldoon PP, Alsharari S, Carroll FI, Negus SS, Damaj MI (2016) Expression and pharmacological modulation of visceral pain-induced conditioned place aversion in mice. Neuropharmacology 102:236–243. 10.1016/j.neuropharm.2015.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley EE, Ingram SL (2020) Endogenous opioid peptides in the descending pain modulatory circuit. Neuropharmacology 173:108131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bair MJ, Robinson RL, Katon W, Kroenke K (2003) Depression and pain comorbidity: a literature review. Arch Intern Med 163:2433–2445 [DOI] [PubMed] [Google Scholar]
- Bakshi R, Newman AH, Faden AI (1990) Dynorphin A-(1–17) induces alterations in free fatty acids, excitatory amino acids, and motor function through an opiate-receptor-mediated mechanism. J Neurosci 10:3793–3800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS (1993) Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther 264:489–495 [PubMed] [Google Scholar]
- Barrio P, Ortega L, Guardia J, Roncero C, Yuguero L, Gual A (2018) Who receives nalmefene and how does it work in the real world? A single-arm, phase IV study of nalmefene in alcohol dependent outpatients: baseline and 1-month results. Clin Drug Investig 38:147–155 [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Howard JL, Shelton KL, Carroll FI (2005) Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berl) 183:118–126 [DOI] [PubMed] [Google Scholar]
- Beaudry H, Daou I, Ase AR, Ribeiro-da-Silva A, Seguela P (2017) Distinct behavioral responses evoked by selective optogenetic stimulation of the major TRPV1+ and MrgD+ subsets of C-fibers. Pain 158:2329–2339 [DOI] [PubMed] [Google Scholar]
- Bechara A, van der Kooy D (1992) A single brain stem substrate mediates the motivational effects of both opiates and food in nondeprived rats but not in deprived rats. Behav Neurosci 106:351–363 [DOI] [PubMed] [Google Scholar]
- Becker WC, Ganoczy D, Fiellin DA, Bohnert ASB (2015) Buprenorphine/naloxone dose and pain intensity among individuals initiating treatment for opioid use disorder. J Subst Abuse Treat 48:128–131 [DOI] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML (2013) Neuroscience of affect: brain mechanisms of pleasure and displeasure. Curr Opin Neurobiol 23:294–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML (2015) Pleasure systems in the brain. Neuron 86:646–664. 10.1016/j.neuron.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivehed E, Strömvall R, Bergquist J, Bakalkin G, Andersson M (2017) Region-specific bioconversion of dynorphin neuropeptide detected by in situ histochemistry and MALDI imaging mass spectrometry. Peptides 87:20–27 [DOI] [PubMed] [Google Scholar]
- Bodnar RJ (2018) Endogenous opiates and behavior: 2016. Peptides 101:167–212 [DOI] [PubMed] [Google Scholar]
- Borsook D, Linnman C, Faria V, Strassman AM, Becerra L, Elman I (2016) Reward deficiency and anti-reward in pain chronification. Neurosci Biobehav Rev 68:282–297 [DOI] [PubMed] [Google Scholar]
- Bossert JM, Hoots JK, Fredriksson I, Adhikary S, Zhang M, Venniro M, Shaham Y (2019) Role of mu, but not delta or kappa, opioid receptors in context-induced reinstatement of oxycodone seeking. Eur J Neurosci 50:2075–2085 [DOI] [PubMed] [Google Scholar]
- Brice-Tutt AC, Wilson LL, Eans SO, Stacy HM, Simons CA, Simpson GG, Coleman JS, Ferracane MJ, Aldrich JV, McLaughlin JP (2020) Multifunctional opioid receptor agonism and antagonism by a novel macrocyclic tetrapeptide prevents reinstatement of morphine-seeking behaviour. Br J Pharmacol 177:4209–4222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Lemos JC, Chavkin C (2009) CRF1-R activation of the dynorphin/kappa opioid system in the mouse basolateral amygdala mediates anxiety-like behavior. PLoS One 4: e8528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C (2010) The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res 1314:44–55. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2819621&tool=pmcentrez&rendertype=abstract. Accessed 16 Sept 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buda JJ, Carroll FI, Kosten TR, Swearingen D, Walters BB (2015) A double-blind, placebo-controlled trial to evaluate the safety, tolerability, and pharmacokinetics of single, escalating oral doses of JDTic. Neuropsychopharmacology 40:2059–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill CM, Taylor AM (2017) Neuroinflammation – a co-occurring phenomenon linking chronic pain and opioid dependence. Curr Opin Behav Sci 13:171–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill CM, Taylor AMW, Cook C, Ong E, Morón JA, Evans CJ (2014) Does the kappa opioid receptor system contribute to pain aversion? Front Pharmacol 5:253. http://journal.frontiersin.org/article/10.3389/fphar.2014.00253/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzà L, Pozza M, Zanni M, Manzini CU, Manzini E, Hökfelt T (1998) Peptide plasticity in primary sensory neurons and spinal cord during adjuvant-induced arthritis in the rat: an immunocyto-chemical and in situ hybridization study. Neuroscience 82:575–589 [DOI] [PubMed] [Google Scholar]
- Carlezon WAJ, Krystal AD (2016) Kappa-opioid antagonists for psychiatric disorders: from bench to clinical trials. Depress Anxiety 33:895–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano C, Ammassari-Teule M, Libri V, Pavone F (1988) Effects of kappa-opioid receptor agonists on locomotor activity and memory processes in mice. Pol J Pharmacol Pharm 40:507–513 [PubMed] [Google Scholar]
- Caudle RM, Dubner R (1998) Ifenprodil blocks the excitatory effects of the opioid peptide dynorphin 1–17 on NMDA receptor-mediated currents in the CA3 region of the guinea pig hippocampus. Neuropeptides 32:87–95 [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Mavrikaki M (2015) Sex differences in kappa opioid receptor function and their potential impact on addiction. Front Neurosci 9:466. http://journal.frontiersin.org/article/10.3389/fnins.2015.00466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff EH, Ebner SR, Sparrow A, Potter D, Baker PM, Ragozzino ME, Roitman MF (2016) Relative timing between kappa opioid receptor activation and cocaine determines the impact on reward and dopamine release. Neuropsychopharmacology 41:989–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, Koob GF (2016) Dynorphin, dysphoria, and dependence: the stress of addiction. Neuropsychopharmacology 41:373–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che T et al. (2018) Structure of the nanobody-stabilized active state of the kappa opioid receptor. Cell 172:55–67.e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Backman CM, Gigante ED, Shippenberg TS (2013) Kappa opioid receptors on dopaminergic neurons are necessary for kappa-mediated place aversion. Neuropsychopharmacology 38:2623–2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang MC, Nguyen EK, Canto-Bustos M, Papale AE, Oswald A-MM, Ross SE (2020) Divergent neural pathways emanating from the lateral parabrachial nucleus mediate distinct components of the pain response. Neuron 106:927–939.e5 [DOI] [PubMed] [Google Scholar]
- Conway SM, Puttick D, Russell S, Potter D, Roitman MF, Chartoff EH (2019) Females are less sensitive than males to the motivational- and dopamine-suppressing effects of kappa opioid receptor activation. Neuropharmacology 146:231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder G, Ahanonu B, Grewe BF, Wang D, Schnitzer MJ, Scherrer G (2019) An amygdalar neural ensemble that encodes the unpleasantness of pain. Science 363:276–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordery SF, Taverner A, Ridzwan IE, Guy RH, Delgado-Charro MB, Husbands SM, Bailey CP (2014) A non-rewarding, non-aversive buprenorphine/naltrexone combination attenuates drug-primed reinstatement to cocaine and morphine in rats in a conditioned place preference paradigm. Addict Biol 19:575–586 [DOI] [PubMed] [Google Scholar]
- Crowley NA, Kash TL (2015) Kappa opioid receptor signaling in the brain: circuitry and implications for treatment. Prog Neuropsychopharmacol Biol Psychiatry 62:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley NA, Bloodgood DW, Hardaway JA, Kendra AM, McCall JG, Al-Hasani R, McCall NM, Yu W, Schools ZL, Krashes MJ, Lowell BB, Whistler JL, Bruchas MR, Kash TL (2016) Dynorphin controls the gain of an amygdalar anxiety circuit. Cell Rep 14:2774–2783. http://linkinghub.elsevier.com/retrieve/pii/S2211124716302042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daou I, Tuttle AH, Longo G, Wieskopf JS, Bonin RP, Ase AR, Wood JN, De Koninck Y, Ribeiroda-Silva A, Mogil JS, Seguela P (2013) Remote optogenetic activation and sensitization of pain pathways in freely moving mice. J Neurosci 33:18631–18640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcq E, Kieffer BL (2018) Opioid receptors: drivers to addiction? Nat Rev Neurosci 19:499–514 [DOI] [PubMed] [Google Scholar]
- Davis MP, Pasternak G, Behm B (2018) Treating chronic pain: an overview of clinical studies centered on the buprenorphine option. Drugs 78:1211–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue RJ, Landino SM, Golden SA, Carroll FI, Russo SJ, Carlezon WAJ (2015) Effects of acute and chronic social defeat stress are differentially mediated by the dynorphin/kappa-opioid receptor system. Behav Pharmacol 26:654–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly CR, Andriessen AS, Chen G, Wang K, Jiang C, Maixner W, Ji R-R (2020) Central nervous system targets: glial cell mechanisms in chronic pain. Neurotherapeutics 17:846–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draisci G, Kajander KC, Dubner R, Bennett GJ, Iadarola MJ (1991) Up-regulation of opioid gene expression in spinal cord evoked by experimental nerve injuries and inflammation. Brain Res 560:186–192 [DOI] [PubMed] [Google Scholar]
- Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia-Campmany L, Krashes M, Knowlton W, Velasquez T, Ren X, Ross S, Lowell BB, Wang Y, Goulding M, Ma Q (2014) Identification of spinal circuits transmitting and gating mechanical pain. Cell 159:1417–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner SR, Roitman MF, Potter DN, Rachlin AB, Chartoff EH (2010) Depressive-like effects of the kappa opioid receptor agonist salvinorin A are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharmacology (Berl) 210:241–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrich E, Turncliff R, Du Y, Leigh-Pemberton R, Fernandez E, Jones R, Fava M (2015a) Evaluation of opioid modulation in major depressive disorder. Neuropsychopharmacology 40:1448–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrich JM, Messinger DI, Knakal CR, Kuhar JR, Schattauer SS, Bruchas MR, Zweifel LS, Kieffer BL, Phillips PEM, Chavkin C (2015b) Kappa opioid receptor-induced aversion requires p38 MAPK activation in VTA dopamine neurons. J Neurosci 35:12917–12931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidson LN, Murphy AZ (2019) Inflammatory mediators of opioid tolerance: implications for dependency and addiction. Peptides 115:51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Borsook D, Volkow ND (2013) Pain and suicidality: insights from reward and addiction neuroscience. Prog Neurobiol 109:1–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Schmeichel BE, Berridge CW (2016) Norepinephrine at the nexus of arousal, motivation and relapse. Brain Res 1641:207–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CJ, Cahill CM (2016) Neurobiology of opioid dependence in creating addiction vulnerability [version 1; referees: 3 approved]. F1000Research 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Memisoglu A, Thase ME, Bodkin JA, Trivedi MH, de Somer M, Du Y, Leigh-Pemberton- R, DiPetrillo L, Silverman B, Ehrich E (2016) Opioid modulation with buprenorphine/samidorphan as adjunctive treatment for inadequate response to antidepressants: a randomized double-blind placebo-controlled trial. Am J Psychiatry 173:499–508 [DOI] [PubMed] [Google Scholar]
- Ferracane MJ, Brice-Tutt AC, Coleman JS, Simpson GG, Wilson LL, Eans SO, Stacy HM, Murray TF, McLaughlin JP, Aldrich JV (2020) Design, synthesis, and characterization of the macrocyclic tetrapeptide cyclo[Pro-Sar-Phe-d-Phe]: a mixed opioid receptor agonist-antagonist following oral administration. ACS Chem Nerosci 11:1324–1336 [DOI] [PubMed] [Google Scholar]
- Funk D, Coen K, Lê AD (2014) The role of kappa opioid receptors in stress-induced reinstatement of alcohol seeking in rats. Brain Behav 4:356–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Coen K, Tamadon S, Lê AD (2019a) Effects of the alpha-1 antagonist prazosin on KOR agonist-induced reinstatement of alcohol seeking. Int J Neuropsychopharmacol 22:724–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Coen K, Tamadon S, Lê AD (2019b) Effect of chronic alcohol vapor exposure on reinstatement of alcohol seeking induced by U50,488. Neuropharmacology 148:210–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardell LR, Ibrahim M, Wang R, Wang Z, Ossipov MH, Malan TPJ, Porreca F, Lai J (2004) Mouse strains that lack spinal dynorphin upregulation after peripheral nerve injury do not develop neuropathic pain. Neuroscience 123:43–52 [DOI] [PubMed] [Google Scholar]
- Gavériaux-Ruff C, Karchewski LA, Hever X, Matifas A, Kieffer BL (2008) Inflammatory pain is enhanced in delta opioid receptor-knockout mice. Eur J Neurosci 27:2558–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Lundeberg T, Yu L-C (2002) Blockade effect of mu and kappa opioid antagonists on the anti-nociception induced by intra-periaqueductal grey injection of oxytocin in rats. Brain Res 927:204–207 [DOI] [PubMed] [Google Scholar]
- Gerra G, Leonardi C, Cortese E, D’Amore A, Lucchini A, Strepparola G, Serio G, Farina G, Magnelli F, Zaimovic A, Mancini A, Turci M, Manfredini M, Donnini C (2007) Human kappa opioid receptor gene (OPRK1) polymorphism is associated with opiate addiction. Am J Med Genet B Neuropsychiatr Genet 144B:771–775 [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Raucci J, Archer S (1995) Kappa opioid inhibition of morphine and cocaine self-administration in rats. Brain Res 681:147–152 [DOI] [PubMed] [Google Scholar]
- Grella SL, Funk D, Coen K, Li Z, Lê AD (2014) Role of the kappa-opioid receptor system in stress-induced reinstatement of nicotine seeking in rats. Behav Brain Res 265:188–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin ML, McDermott KA, McHugh RK, Fitzmaurice GM, Jamison RN, Weiss RD (2016) Longitudinal association between pain severity and subsequent opioid use in prescription opioid dependent patients with chronic pain. Drug Alcohol Depend 163:216–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Lu Y, Shen Y, Wu F, Xu X, Kong E, Huang Z, Sun Y, Yu W (2019) Transgenic increase in the β-endorphin concentration in cerebrospinal fluid alleviates morphine-primed relapse behavior through the μ opioid receptor in rats. J Med Virol 91:1158–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinsbroek JA, Furbish AB, Peters J (2018) A single, extinction-based treatment with a kappa opioid receptor agonist elicits a long-term reduction in cocaine relapse. Neuropsychopharmacology 43:1492–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo Y-A, Scott LJ (2018) Buprenorphine/naloxone (Zubsolv(®)): a review in opioid dependence. CNS Drugs 32:875–882 [DOI] [PubMed] [Google Scholar]
- Higgins C, Smith BH, Matthews K (2020) Comparison of psychiatric comorbidity in treatment-seeking, opioid-dependent patients with versus without chronic pain. Addiction 115:249–258 [DOI] [PubMed] [Google Scholar]
- Hipólito L, Wilson-Poe A, Campos-Jurado Y, Zhong E, Gonzalez-Romero J, Virag L, Whittington R, Comer SD, Carlton SM, Walker BM, Bruchas MR, Morón JA (2015) Inflammatory pain promotes increased opioid self-administration: role of dysregulated ventral tegmental area μ opioid receptors. J Neurosci 35:12217–12231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmstad GO, Fields HL (2001) Kappa opioid receptor inhibition of glutamatergic transmission in the nucleus accumbens shell. J Neurophysiol 85:1153–1158 [DOI] [PubMed] [Google Scholar]
- Howe CQ, Sullivan MD (2014) The missing “P” in pain management: how the current opioid epidemic highlights the need for psychiatric services in chronic pain care. Gen Hosp Psychiatry 36:99–104 [DOI] [PubMed] [Google Scholar]
- Hser Y-I, Mooney LJ, Saxon AJ, Miotto K, Bell DS, Huang D (2017) Chronic pain among patients with opioid use disorder: results from electronic health records data. J Subst Abuse Treat 77:26–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua T, Chen B, Lu D, Sakurai K, Zhao S, Han B-X, Kim J, Yin L, Chen Y, Lu J, Wang F (2020) General anesthetics activate a potent central pain-suppression circuit in the amygdala. Nat Neurosci 23:854–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel M, Lu P, Cummons TA, Whiteside GT (2008) The persistence of a long-term negative affective state following the induction of either acute or chronic pain. Pain 140:436–445. 10.1016/j.pain.2008.09.020 [DOI] [PubMed] [Google Scholar]
- Hurd YL, Herman MM, Hyde TM, Bigelow LB, Weinberger DR, Kleinman JE (1997) Prodynorphin mRNA expression is increased in the patch vs matrix compartment of the caudate nucleus in suicide subjects. Mol Psychiatry 2:495–500 [DOI] [PubMed] [Google Scholar]
- Hylden JL, Nahin RL, Traub RJ, Dubner R (1991) Effects of spinal kappa-opioid receptor agonists on the responsiveness of nociceptive superficial dorsal horn neurons. Pain 44:187–193 [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Brady LS, Draisci G, Dubner R (1988a) Enhancement of dynorphin gene expression in spinal cord following experimental inflammation: stimulus specificity, behavioral parameters and opioid receptor binding. Pain 35:313–326. http://www.ncbi.nlm.nih.gov/pubmed/2906426. Accessed 1 Oct 2014 [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Douglass J, Civelli O, Naranjo JR (1988b) Differential activation of spinal cord dynorphin and enkephalin neurons during hyperalgesia: evidence using cDNA hybridization. Brain Res 455:205–212 [DOI] [PubMed] [Google Scholar]
- Iyer SM, Montgomery KL, Towne C, Lee SY, Ramakrishnan C, Deisseroth K, Delp SL (2014) Virally mediated optogenetic excitation and inhibition of pain in freely moving nontransgenic mice. Nat Biotechnol 32:274–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, McLaughlin JP, Carroll FI, Damaj MI (2013) Effects of the kappa opioid receptor antagonist, norbinaltorphimine, on stress and drug-induced reinstatement of nicotine-conditioned place preference in mice. Psychopharmacology (Berl) 226:763–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison RN, Edwards RR (2013) Risk factor assessment for problematic use of opioids for chronic pain. Clin Neuropsychol 27:60–80 [DOI] [PubMed] [Google Scholar]
- Jarcho JM, Mayer EA, Jiang ZK, Feier NA, London ED (2012) Pain, affective symptoms, and cognitive deficits in patients with cerebral dopamine dysfunction. Pain 153:744–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji R-R, Donnelly CR, Nedergaard M (2019) Astrocytes in chronic pain and itch. Nat Rev Neurosci 20:667–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajander KC, Sahara Y, Iadarola MJ, Bennett GJ (1990) Dynorphin increases in the dorsal spinal cord in rats with a painful peripheral neuropathy. Peptides 11:719–728 [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF (2006) Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci 26:5894–5900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer BL (1999) Opioids: first lessons from knockout mice. Trends Pharmacol Sci 20:19–26 [DOI] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA (2010) Dynorphin, stress, and depression. Brain Res 1314:56–73. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2819644&tool=pmcentrez&rendertype=abstract. Accessed 5 Sept 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox RJ, Dickenson AH (1987) Effects of selective and non-selective kappa-opioid receptor agonists on cutaneous C-fibre-evoked responses of rat dorsal horn neurones. Brain Res 415:21–29 [DOI] [PubMed] [Google Scholar]
- Koob GF (2020) Neurobiology of opioid addiction: opponent process, hyperkatifeia, and negative reinforcement. Biol Psychiatry 87:44–53 [DOI] [PubMed] [Google Scholar]
- Kumor KM, Haertzen CA, Johnson RE, Kocher T, Jasinski D (1986) Human psychopharmacology of ketocyclazocine as compared with cyclazocine, morphine and placebo. J Pharmacol Exp Ther 238:960–968 [PubMed] [Google Scholar]
- LaBuda CJ, Fuchs PN (2000) A behavioral test paradigm to measure the aversive quality of inflammatory and neuropathic pain in rats. Exp Neurol 163:490–494 [DOI] [PubMed] [Google Scholar]
- Lai J, Luo M-C, Chen Q, Ma S, Gardell LR, Ossipov MH, Porreca F (2006) Dynorphin A activates bradykinin receptors to maintain neuropathic pain. Nat Neurosci 9:1534–1540 [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, Hnasko TS, Palmiter RD, Chavkin C (2009) Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci U S A 106:19168–19173. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2776420&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latif Z-E-H, Solli KK, Opheim A, Kunoe N, Benth JŠ, Krajci P, Sharma-Haase K, Tanum L (2019) No increased pain among opioid-dependent individuals treated with extended-release naltrexone or buprenorphine-naloxone: a 3-month randomized study and 9-month open-treatment follow-up study. Am J Addict 28:77–85 [DOI] [PubMed] [Google Scholar]
- Laughlin TM, Vanderah TW, Lashbrook J, Nichols ML, Ossipov M, Porreca F, Wilcox GL (1997) Spinally administered dynorphin A produces long-lasting allodynia: involvement of NMDA but not opioid receptors. Pain 72:253–260 [DOI] [PubMed] [Google Scholar]
- Lê AD, Funk D, Coen K, Tamadon S, Shaham Y (2018) Role of κ-opioid receptors in the bed nucleus of stria terminalis in reinstatement of alcohol seeking. Neuropsychopharmacology 43:838–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Hall SM, Ramos-Colon C, Remesic M, Rankin D, Vanderah TW, Porreca F, Lai J, Hruby VJ (2015) Blockade of non-opioid excitatory effects of spinal dynorphin A at bradykinin receptors. Recept Clin Investig 2(1):517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefler Y, Campagner D, Branco T (2020) The role of the periaqueductal gray in escape behavior. Curr Opin Neurobiol 60:115–121 [DOI] [PubMed] [Google Scholar]
- Leitl MD, Onvani S, Bowers MS, Cheng K, Rice KC, Carlezon WA, Banks ML, Negus SS (2014a) Pain-related depression of the mesolimbic dopamine system in rats: expression, blockade by analgesics, and role of endogenous κ-opioids. Neuropsychopharmacology 39:614–624. http://www.ncbi.nlm.nih.gov/pubmed/24008352. Accessed 1 Oct 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitl MD, Potter DN, Cheng K, Rice KC, Carlezon WA, Negus SS (2014b) Sustained pain-related depression of behavior: effects of intraplantar formalin and complete freund’s adjuvant on intracranial self-stimulation (ICSS) and endogenous kappa opioid biomarkers in rats. Mol Pain 10:62. http://www.ncbi.nlm.nih.gov/pubmed/25245060. Accessed 3 Oct 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O, Londono D, O’Hara K, Nielsen DA, Peles E, Rotrosen J, Casadonte P, Linzy S, Randesi M, Ott J, Adelson M, Kreek MJ (2008) Genetic susceptibility to heroin addiction: a candidate gene association study. Genes Brain Behav 7:720–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Kash TL (2019) κ-opioid receptor modulation of GABAergic inputs onto ventrolateral periaqueductal gray dopamine neurons. Mol Neuropsychiatry 5:190–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SS et al. (2019) Kappa opioid receptors drive a tonic aversive component of chronic pain. J Neurosci 39(21):4162–4178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorca-Torralba M, Pilar-Cuéllar F, da Silva BG, Mico JA, Berrocoso E (2020) Opioid receptors mRNAs expression and opioids agonist-dependent G-protein activation in the rat brain following neuropathy. Prog Neuropsychopharmacol Biol Psychiatry 99:109857. [DOI] [PubMed] [Google Scholar]
- Lowe SL, Wong CJ, Witcher J, Gonzales CR, Dickinson GL, Bell RL, Rorick-Kehn L, Weller M, Stoltz RR, Royalty J, Tauscher-Wisniewski S (2014) Safety, tolerability, and pharmacokinetic evaluation of single- and multiple-ascending doses of a novel kappa opioid receptor antagonist LY2456302 and drug interaction with ethanol in healthy subjects. J Clin Pharmacol 54:968–978 [DOI] [PubMed] [Google Scholar]
- Lu J, Jhou TC, Saper CB (2006) Identification of wake-active dopaminergic neurons in the ventral periaqueductal gray matter. J Neurosci 26:193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malan TP, Ossipov MH, Gardell LR, Ibrahim M, Bian D, Lai J, Porreca F (2000) Extraterritorial neuropathic pain correlates with multisegmental elevation of spinal dynorphin in nerve-injured rats. Pain 86:185–194 [DOI] [PubMed] [Google Scholar]
- Maraschin JC, Almeida CB, Rangel MP, Roncon CM, Sestile CC, Zangrossi HJ, Graeff FG, Audi EA (2017) Participation of dorsal periaqueductal gray 5-HT1A receptors in the panicolytic-like effect of the κ-opioid receptor antagonist Nor-BNI. Behav Brain Res 327:75–82 [DOI] [PubMed] [Google Scholar]
- Margolis EB, Karkhanis AN (2019) Dopaminergic cellular and circuit contributions to kappa opioid receptor mediated aversion. Neurochem Int 129:104504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MO, Jamison RN, Wasan AD, Edwards RR (2014) The association between catastrophizing and craving in patients with chronic pain prescribed opioid therapy: a preliminary analysis. Pain Med 15:1757–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BJ, Salvato FR, Williams LD, Ritvo EC, Cutler RB (1999) A double-blind, placebo-controlled study of oral nalmefene for alcohol dependence. Arch Gen Psychiatry 56:719–724 [DOI] [PubMed] [Google Scholar]
- Massaly N et al. (2019) Pain-induced negative affect is mediated via recruitment of the nucleus accumbens kappa opioid system. Neuron 102(3):564–573.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews GA, Nieh EH, Vander Weele CM, Halbert SA, Pradhan RV, Yosafat AS, Glober GF, Izadmehr EM, Thomas RE, Lacy GD, Wildes CP, Ungless MA, Tye KM (2016) Dorsal raphe dopamine neurons represent the experience of social isolation. Cell 164:617–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C (2006) Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology 31:787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Negus SS (2000) Interactions between kappa opioid agonists and cocaine. Preclinical studies. Ann N Y Acad Sci 909:104–132 [DOI] [PubMed] [Google Scholar]
- Mika J, Rojewska E, Makuch W, Przewlocka B (2010) Minocycline reduces the injury-induced expression of prodynorphin and pronociceptin in the dorsal root ganglion in a rat model of neuropathic pain. Neuroscience 165:1420–1428 [DOI] [PubMed] [Google Scholar]
- Millan MJ, Millan MH, Pilcher CW, Członkowski A, Herz A, Colpaert FC (1985) Spinal cord dynorphin may modulate nociception via a kappa-opioid receptor in chronic arthritic rats. Brain Res 340:156–159 [DOI] [PubMed] [Google Scholar]
- Millan MJ, Millan MH, Członkowski A, Höllt V, Pilcher CW, Herz A, Colpaert FC (1986) A model of chronic pain in the rat: response of multiple opioid systems to adjuvant-induced arthritis. J Neurosci 6:899–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Członkowski A, Pilcher CW, Almeida OF, Millan MH, Colpaert FC, Herz A (1987) A model of chronic pain in the rat: functional correlates of alterations in the activity of opioid systems. J Neurosci 7:77–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Członkowski A, Morris B, Stein C, Arendt R, Huber A, Höllt V, Herz A (1988) Inflammation of the hind limb as a model of unilateral, localized pain: influence on multiple opioid systems in the spinal cord of the rat. Pain 35:299–312 [DOI] [PubMed] [Google Scholar]
- Miyata H, Takahashi M, Murai Y, Tsuneyoshi K, Hayashi T, Meulien D, Sørensen P, Higuchi S (2019) Nalmefene in alcohol-dependent patients with a high drinking risk: randomized controlled trial. Psychiatry Clin Neurosci 73:697–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokhtar M, Singh P (2020) Neuroanatomy, periaqueductal gray. In: StatPearls [Internet]. StatPearls Publishing, Treasure Island: [PubMed] [Google Scholar]
- Morani AS, Kivell B, Prisinzano TE, Schenk S (2009) Effect of kappa-opioid receptor agonists U69593, U50488H, spiradoline and salvinorin A on cocaine-induced drug-seeking in rats. Pharmacol Biochem Behav 94:244–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahin RL, Hylden JL, Humphrey E (1992) Demonstration of dynorphin A 1–8 immunoreactive axons contacting spinal cord projection neurons in a rat model of peripheral inflammation and hyperalgesia. Pain 51:135–143 [DOI] [PubMed] [Google Scholar]
- Nakamoto K, Taniguchi A, Tokuyama S (2020) Changes in opioid receptors, opioid peptides and morphine antinociception in mice subjected to early life stress. Eur J Pharmacol 881:173173. [DOI] [PubMed] [Google Scholar]
- Narita M, Kishimoto Y, Ise Y, Yajima Y, Misawa K, Suzuki T (2005) Direct evidence for the involvement of the mesolimbic kappa-opioid system in the morphine-induced rewarding effect under an inflammatory pain-like state. Neuropsychopharmacology 30:111–118. http://www.ncbi.nlm.nih.gov/pubmed/15257306. Accessed 1 Oct 2014 [DOI] [PubMed] [Google Scholar]
- Narita M, Kaneko C, Miyoshi K, Nagumo Y, Kuzumaki N, Nakajima M, Nanjo K, Matsuzawa K, Yamazaki M, Suzuki T (2006) Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology 31:739–750 [DOI] [PubMed] [Google Scholar]
- Navratilova E, Xie JY, Meske D, Qu C, Morimura K, Okun A, Arakawa N, Ossipov M, Fields HL, Porreca F (2015) Endogenous opioid activity in the anterior cingulate cortex is required for relief of pain. J Neurosci 35:7264–7271. http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.3862-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E, Ji G, Phelps C, Qu C, Hein M, Yakhnitsa V, Neugebauer V, Porreca F (2019) Kappa opioid signaling in the central nucleus of the amygdala promotes disinhibition and aversiveness of chronic neuropathic pain. Pain 160:824–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrete R, Garcia Gutierrez MS, Manzanares J, Maldonado R (2016) Involvement of the dynorphin/KOR system on the nociceptive, emotional and cognitive manifestations of joint pain in mice. Neuropharmacology 116:315–327 [DOI] [PubMed] [Google Scholar]
- Negus SS, Henriksen SJ, Mattox A, Pasternak GW, Portoghese PS, Takemori AE, Weinger MB, Koob GF (1993) Effect of antagonists selective for mu, delta and kappa opioid receptors on the reinforcing effects of heroin in rats. J Pharmacol Exp Ther 265:1245–1252 [PubMed] [Google Scholar]
- Negus SS, Mello NK, Portoghese PS, Lin CE (1997) Effects of kappa opioids on cocaine self-administration by rhesus monkeys. J Pharmacol Exp Ther 282:44–55 [PubMed] [Google Scholar]
- Neugebauer V, Mazzitelli M, Cragg B, Ji G, Navratilova E, Porreca F (2020) Amygdala, neuropeptides, and chronic pain-related affective behaviors. Neuropharmacology 170:108052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann AM, Blondell RD, Jaanimägi U, Giambrone AK, Homish GG, Lozano JR, Kowalik U, Azadfard M (2013) A preliminary study comparing methadone and buprenorphine in patients with chronic pain and coexistent opioid addiction. J Addict Dis 32:68–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwaneshiudu CA, Shi X-Y, Clark JD (2020) Incisional injury modulates morphine reward and morphine-primed reinstatement: a role of kappa opioid receptor activation. Anesth Analg 130:248–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygard SK, Hourguettes NJ, Sobczak GG, Carlezon WA, Bruchas MR (2016) Stress-induced reinstatement of nicotine preference requires dynorphin/kappa opioid activity in the basolateral amygdala. J Neurosci 36:9937–9948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki S, Narita M, Narita M, Iino M, Sugita J, Matsumura Y, Suzuki T (2002) Suppression of the morphine-induced rewarding effect in the rat with neuropathic pain: implication of the reduction in mu-opioid receptor functions in the ventral tegmental area. J Neurochem 82:1192–1198 [DOI] [PubMed] [Google Scholar]
- Palmisano M, Caputi FF, Mercatelli D, Romualdi P, Candeletti S (2018) Dynorphinergic system alterations in the corticostriatal circuitry of neuropathic mice support its role in the negative affective component of pain. Genes Brain Behav:e12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parida S, Carroll KM, Petrakis IL, Sofuoglu M (2019) Buprenorphine treatment for opioid use disorder: recent progress. Expert Rev Clin Pharmacol 12:791–803 [DOI] [PubMed] [Google Scholar]
- Park S II et al. (2015) Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nat Biotechnol 33:1280–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirs C, Seal RP (2016) Neural circuits for pain: recent advances and current views. Science 354:578–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergolizzi JVJ, Raffa RB (2019) Safety and efficacy of the unique opioid buprenorphine for the treatment of chronic pain. J Pain Res 12:3299–3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM (1986) Psychotomimesis mediated by kappa opiate receptors. Science 233:774–776. http://www.ncbi.nlm.nih.gov/pubmed/3016896. Accessed 25 Sept 2014 [DOI] [PubMed] [Google Scholar]
- Phelps CE, Navratilova E, Dickenson AH, Porreca F, Bannister K (2019) Kappa opioid signaling in the right central amygdala causes hind paw specific loss of diffuse noxious inhibitory controls in experimental neuropathic pain. Pain 160:1614–1621 [DOI] [PubMed] [Google Scholar]
- Podvin S, Yaksh T, Hook V (2016) The emerging role of spinal dynorphin in chronic pain: a therapeutic perspective. Annu Rev Pharmacol Toxicol 56:511–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polter AM, Bishop RA, Briand LA, Graziane NM, Pierce RC, Kauer JA (2014) Poststress block of kappa opioid receptors rescues long-term potentiation of inhibitory synapses and prevents reinstatement of cocaine seeking. Biol Psychiatry 76:785–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quelch DR, Mick I, McGonigle J, Ramos AC, Flechais RSA, Bolstridge M, Rabiner E, Wall MB, Newbould RD, Steiniger-Brach B, van den Berg F, Boyce M, Østergaard Nilausen D, Breuning Sluth L, Meulien D, von der Goltz C, Nutt D, Lingford-Hughes A (2017) Nalmefene reduces reward anticipation in alcohol dependence: an experimental functional magnetic resonance imaging study. Biol Psychiatry 81:941–948 [DOI] [PubMed] [Google Scholar]
- Redila VA, Chavkin C (2008) Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology (Berl) 200:59–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosén A, Lundeberg T, Bytner B, Nylander I (2000) Central changes in nociceptin dynorphin B and Met-enkephalin-Arg-Phe in different models of nociception. Brain Res 857:212–218 [DOI] [PubMed] [Google Scholar]
- Rosenblum A, Cruciani RA, Strain EC, Cleland CM, Joseph H, Magura S, Marsch LA, McNicholas LF, Savage SR, Sundaram A, Portenoy RK (2012) Sublingual buprenorphine/naloxone for chronic pain in at-risk patients: development and pilot test of a clinical protocol. J Opioid Manag 8:369–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux P, Sullivan MA, Cohen J, Fugon L, Jones JD, Vosburg SK, Cooper ZD, Manubay JM, Mogali S, Comer SD (2013) Buprenorphine/naloxone as a promising therapeutic option for opioid abusing patients with chronic pain: reduction of pain, opioid withdrawal symptoms, and abuse liability of oral oxycodone. Pain 154:1442–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruda MA, Iadarola MJ, Cohen LV, Young WS 3rd (1988) In situ hybridization histochemistry and immunocytochemistry reveal an increase in spinal dynorphin biosynthesis in a rat model of peripheral inflammation and hyperalgesia. Proc Natl Acad Sci U S A 85:622–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüedi-Bettschen D, Rowlett JK, Spealman RD, Platt DM (2010) Attenuation of cocaine-induced reinstatement of drug seeking in squirrel monkeys: kappa opioid and serotonergic mechanisms. Psychopharmacology (Berl) 210:169–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson H, Ekman R, Hedner T (1993) CSF neuropeptides in cancer pain: effects of spinal opioid therapy. Acta Anaesthesiol Scand 37:502–508 [DOI] [PubMed] [Google Scholar]
- Sapio MR, Iadarola MJ, Loydpierson AJ, Kim JJ, Thierry-Mieg D, Thierry-Mieg J, Maric D, Mannes AJ (2020) Dynorphin and enkephalin opioid peptides and transcripts in spinal cord and dorsal root ganglion during peripheral inflammatory hyperalgesia and allodynia. J Pain 21 (9–10):988–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardella TCP, Polgár E, Garzillo F, Furuta T, Kaneko T, Watanabe M, Todd AJ (2011) Dynorphin is expressed primarily by GABAergic neurons that contain galanin in the rat dorsal horn. Mol Pain 7:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Partridge B (2001) Effect of the kappa-opioid receptor agonist, U69593, on reinstatement of extinguished amphetamine self-administration behavior. Pharmacol Biochem Behav 68:629–634 [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B, Shippenberg TS (1999) U69593, a kappa-opioid agonist, decreases cocaine self-administration and decreases cocaine-produced drug-seeking. Psychopharmacology (Berl) 144:339–346 [DOI] [PubMed] [Google Scholar]
- Sedki F, Eigenmann K, Gelinas J, Schouela N, Courchesne S, Shalev U (2015) A role for kappa-, but not mu-opioid, receptor activation in acute food deprivation-induced reinstatement of heroin seeking in rats. Addict Biol 20:423–432 [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Herz A (1986) Differential effects of mu and kappa opioid systems on motivational processes. NIDA Res Monogr 75:563–566 [PubMed] [Google Scholar]
- Shippenberg TS, Stein C, Huber A, Millan MJ, Herz A (1988) Motivational effects of opioids in an animal model of prolonged inflammatory pain: alteration in the effects of kappa- but not of mu-receptor agonists. Pain 35:179–186 [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Bals-Kubik R, Herz A (1993) Examination of the neurochemical substrates mediating the motivational effects of opioids: role of the mesolimbic dopamine system and D-1 vs. D-2 dopamine receptors. J Pharmacol Exp Ther 265:53–59. http://www.ncbi.nlm.nih.gov/pubmed/8386244. Accessed 3 Oct 2014 [PubMed] [Google Scholar]
- Shirayama Y, Ishida H, Iwata M, Hazama G-I, Kawahara R, Duman RS (2004) Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem 90:1258–1268 [DOI] [PubMed] [Google Scholar]
- Shulman M, Luo S, Campbell ANC, Scodes J, Pavlicova M, Broffman A, Saxon AJ, Nunes EV (2020) Secondary analysis of pain outcomes in a large pragmatic randomized trial of buprenorphine/naloxone versus methadone for opioid use disorder. J Addict Med 14(5):e188–e194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonin F, Valverde O, Smadja C, Slowe S, Kitchen I, Dierich A, Le Meur M, Roques BP, Maldonado R, Kieffer BL (1998) Disruption of the kappa-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective kappa-agonist U-50,488H and attenuates morphine withdrawal. EMBO J 17:886–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka KA, Rohlwing JJ, Bussey RA, Eikenberry SA, Wilken JM (2002) Chronic muscle pain induced by repeated acid Injection is reversed by spinally administered mu- and delta-, but not kappa-, opioid receptor agonists. J Pharmacol Exp Ther 302:1146–1150 [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS (1992) Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A 89:2046–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert DG, Watson SJ, Houghten RA, Saper CB (1986) Opioid peptide immunoreactivity in spinal and trigeminal dorsal horn neurons projecting to the parabrachial nucleus in the rat. J Neurosci 6:1220–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley B, Sher L, Wilson S, Ekman R, Huang Y, Mann JJ (2010) Non-suicidal self-injurious behavior, endogenous opioids and monoamine neurotransmitters. J Affect Disord 124:134–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AMW, Castonguay A, Taylor AJ, Murphy NP, Ghogha A, Cook C, Xue L, Olmstead MC, de Koninck Y, Evans CJ, Cahill CM (2015) Microglia disrupt mesolimbic reward circuitry in chronic pain. J Neurosci 35:8442–8450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AMW, Becker S, Schweinhardt P, Cahill C (2016) Mesolimbic dopamine signaling in acute and chronic pain: implications for motivation, analgesia, and addiction. Pain 157(6):1194–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda HA, Bonci A (2018) Dynorphin/kappa-opioid receptor control of dopamine dynamics: implications for negative affective states and psychiatric disorders. Brain Res [DOI] [PubMed] [Google Scholar]
- Tejeda HA, Counotte DS, Oh E, Ramamoorthy S, Schultz-Kuszak KN, Bäckman CM, Chefer V, O’Donnell P, Shippenberg TS (2013) Prefrontal cortical kappa-opioid receptor modulation of local neurotransmission and conditioned place aversion. Neuropsychopharmacology 38:1770–1779. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3717537&tool=pmcentrez&rendertype=abstract. Accessed 1 Oct 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda HA, Hanks AN, Scott L, Mejias-Aponte C, Hughes ZA, O’Donnell P (2015) Prefrontal cortical kappa opioid receptors attenuate responses to amygdala inputs. Neuropsychopharmacology 40:2856–2864. 10.1038/npp.2015.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ (2010) Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci 11:823–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi SA, Leggio GM, Drago F, Salomone S (2019) Therapeutic challenges of post-traumatic stress disorder: focus on the dopaminergic system. Front Pharmacol 10:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara-Ohsumi Y, Tsuji F, Niwa M, Hata T, Narita M, Suzuki T, Sasano M, Aono H (2011) The kappa opioid receptor agonist SA14867 has antinociceptive and weak sedative effects in models of acute and chronic pain. Eur J Pharmacol 671:53–60 [DOI] [PubMed] [Google Scholar]
- Vaerøy H, Nyberg F, Terenius L (1991) No evidence for endorphin deficiency in fibromyalgia following investigation of cerebrospinal fluid (CSF) dynorphin A and Met-enkephalin-Arg6-Phe7. Pain 46:139–143 [DOI] [PubMed] [Google Scholar]
- Valdez GR, Platt DM, Rowlett JK, Rüedi-Bettschen D, Spealman RD (2007) Kappa agonist-induced reinstatement of cocaine seeking in squirrel monkeys: a role for opioid and stress-related mechanisms. J Pharmacol Exp Ther 323:525–533 [DOI] [PubMed] [Google Scholar]
- Van’t Veer A, Carlezon WAJ (2013) Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology (Berl) 229:435–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderah TW, Laughlin T, Lashbrook JM, Nichols ML, Wilcox GL, Ossipov MH, Malan TPJ, Porreca F (1996) Single intrathecal injections of dynorphin A or des-Tyr-dynorphins produce long-lasting allodynia in rats: blockade by MK-801 but not naloxone. Pain 68:275–281 [DOI] [PubMed] [Google Scholar]
- Vijay A, Wang S, Worhunsky P, Zheng M-Q, Nabulsi N, Ropchan J, Krishnan-Sarin S, Huang Y, Morris ED (2016) PET imaging reveals sex differences in kappa opioid receptor availability in humans, in vivo. Am J Nucl Med Mol Imaging 6:205–214 [PMC free article] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Bumb JM, Otto A, Dinter C, Karl D, Koopmann A, Hermann D, Mann K, Kiefer F (2019) The effects of nalmefene on emotion processing in alcohol use disorder – a randomized, controlled fMRI study. Eur Neuropsychopharmacol 29:1442–1452 [DOI] [PubMed] [Google Scholar]
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN (2015) Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 156:569–576 [DOI] [PubMed] [Google Scholar]
- Wadenberg M-LG (2003) A review of the properties of spiradoline: a potent and selective kappa-opioid receptor agonist. CNS Drug Rev 9:187–198. http://www.ncbi.nlm.nih.gov/pubmed/12847558. Accessed 1 Oct 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Gardell LR, Ossipov MH, Vanderah TW, Brennan MB, Hochgeschwender U, Hruby VJ, Malan TP, Lai J, Porreca F (2001) Pronociceptive actions of dynorphin maintain chronic neuropathic pain. J Neurosci 21:1779–1786. http://www.ncbi.nlm.nih.gov/pubmed/11222667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S-C, Tsou H-H, Chung R-H, Chang Y-S, Fang C-P, Chen C-H, Ho I-K, Kuo H-W, Liu SC, Shih Y-H, Wu H-Y, Huang B-H, Lin K-M, Chen ACH, Hsiao C-F, Liu Y-L (2014) The association of genetic polymorphisms in the κ-opioid receptor 1 gene with body weight, alcohol use, and withdrawal symptoms in patients with methadone maintenance. J Clin Psychopharmacol 34:205–211 [DOI] [PubMed] [Google Scholar]
- Wee S, Koob GF (2010) The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl) 210:121–135. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2879894&tool=pmcentrez&rendertype=abstract. Accessed 16 Sept 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley MJ, Heinzerling KG, Shoptaw S, Ling W (2015) Pain volatility and prescription opioid addiction treatment outcomes in patients with chronic pain. Exp Clin Psychopharmacol 23:428–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley MJ, Heinzerling KG, Shoptaw S, Ling W (2017) Volatility and change in chronic pain severity predict outcomes of treatment for prescription opioid addiction. Addiction 112:1202–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Wacker D, Mileni M, Katritch V, Han GW, Vardy E, Liu W, Thompson AA, Huang X-P, Carroll FI, Mascarella SW, Westkaemper RB, Mosier PD, Roth BL, Cherezov V, Stevens RC (2012) Structure of the human kappa-opioid receptor in complex with JDTic. Nature 485:327–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F-X, Babazada H, Gao H, Huang X-P, Xi C-H, Chen C-H, Xi J, Yu W-F, Liu R (2019) Dezocine alleviates morphine-induced dependence in rats. Anesth Analg 128:1328–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie JY, De Felice M, Kopruszinski CM, Eyde N, LaVigne J, Remeniuk B, Hernandez P, Yue X, Goshima N, Ossipov M, King T, Streicher JM, Navratilova E, Dodick D, Rosen H, Roberts E, Porreca F (2017) Kappa opioid receptor antagonists: a possible new class of therapeutics for migraine prevention. Cephalalgia 37:780–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Petraschka M, McLaughlin JP, Westenbroek RE, Caron MG, Lefkowitz RJ, Czyzyk TA, Pintar JE, Terman GW, Chavkin C (2004) Neuropathic pain activates the endogenous kappa opioid system in mouse spinal cord and induces opioid receptor tolerance. J Neurosci 24:4576–4584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Bruchas MR, Ippolito DL, Gendron L, Chavkin C (2007) Sciatic nerve ligation-induced proliferation of spinal cord astrocytes is mediated by kappa opioid activation of p38 mitogen-activated protein kinase. J Neurosci 27:2570–2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin I, Bohren Y, Waltisperger E, Sage-Ciocca D, Yin JC, Freund-Mercier M-J, Barrot M (2011) A time-dependent history of mood disorders in a murine model of neuropathic pain. Biol Psychiatry 70:946–953 [DOI] [PubMed] [Google Scholar]
- Yuanyuan J, Rui S, Hua T, Jingjing C, Cuola D, Yuhui S, Shuguang W (2018) Genetic association analyses and meta-analysis of Dynorphin-Kappa Opioid system potential functional variants with heroin dependence. Neurosci Lett 685:75–82 [DOI] [PubMed] [Google Scholar]
- Yuferov V, Fussell D, LaForge KS, Nielsen DA, Gordon D, Ho A, Leal SM, Ott J, Kreek MJ (2004) Redefinition of the human kappa opioid receptor gene (OPRK1) structure and association of haplotypes with opiate addiction. Pharmacogenetics 14:793–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamarripa CA, Naylor JE, Huskinson SL, Townsend EA, Prisinzano TE, Freeman KB (2020a) Kappa opioid agonists reduce oxycodone self-administration in male rhesus monkeys. Psycho-pharmacology (Berl) 237:1471–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamarripa CA, Patel TR, Williams BC, Pareek T, Schrock HM, Prisinzano TE, Freeman KB (2020b) The kappa-opioid receptor agonist, nalfurafine, blocks acquisition of oxycodone self-administration and oxycodone’s conditioned rewarding effects in male rats. Behav Pharmacol 31(8):792–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrindast M-R, Khakpai F (2015) The modulatory role of dopamine in anxiety-like behavior. Arch Iran Med 18:591–603 [PubMed] [Google Scholar]
- Zhang Y, Meng X, Li A, Xin J, Berman BM, Lao L, Tan M, Ren K, Zhang R-X (2012) Electroacupuncture alleviates affective pain in an inflammatory pain rat model. Eur J Pain 16:170–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Leri F, Grella SL, Aldrich JV, Kreek MJ (2013) Involvement of dynorphin and kappa opioid receptor in yohimbine-induced reinstatement of heroin seeking in rats. Synapse 67:358–361 [DOI] [PMC free article] [PubMed] [Google Scholar]


