Abstract
Here, we report a case of metastatic Epstein–Barr virus (EBV)‐related primary pulmonary lymphoepithelioma‐like carcinoma (PPLELC) in a young, nonsmoking female who responded well to treatment with two types of immune checkpoint inhibitors (ICIs). This is the first case report of a favorable outcome to ICIs in the late‐line treatment of advanced PPLELC patients with programmed cell death‐ligand 1 (PD‐L1) expression.
Keywords: immune checkpoint inhibitor, PPLELC, primary pulmonary lymphoepithelioma‐like carcinoma
Primary pulmonary lymphoepithelioma‐like carcinoma (PPLELC) is a rare subtype of non–small‐cell lung cancer. Here, is the first reported case of a favorable outcome to two types of immune checkpoint inhibitors in advanced PPLELC patients with PD‐L1 expression.
INTRODUCTION
Primary pulmonary lymphoepithelioma‐like carcinoma (PPLELC) or lymphoepithelial carcinoma (LEC) 1 is a rare subtype of non–small‐cell lung cancer (NSCLC), which accounts for 0.7% of all lung malignancies. 2 Although the prognosis in the early stage appears to be better than for other forms of NSCLC, it remains dismal in the late stage. 3
Cancer immunotherapy has recently shown benefits in survival over standard chemotherapy for advanced NSCLC. 4 , 5 , 6 , 7 However, treatment strategies for PPLELC have not been fully defined. Here, we presented a case of metastatic Epstein–Barr virus (EBV)‐related PPLELC that had achieved tumor control with immune checkpoint inhibitors (ICIs).
CASE REPORT
A 39‐year‐old nonsmoking Thai woman was diagnosed with advanced‐stage PPLELC with intrapulmonary and non‐regional lymph node metastases. Transbronchial biopsy revealed nonkeratinizing (undifferentiated) carcinoma that resembled a primary lung lymphoepithelioma (Figure 1(a)). Immunohistochemical staining was positive for AE1/AE3, p40, and p63 (Figure 1(b)). The tumor tested positive for Epstein–Barr virus (EBV)‐encoded small RNA in situ hybridization (EBER‐ISH) (Figure 1(c)). Ear, nose, and throat examination showed a normal nasopharynx. A 400‐gene next‐generation sequencing identified no actionable gene mutations. The programmed cell death‐ligand 1 (PD‐L1) expression's tumor proportion score (TPS) was 10% (Figure 1(d)). There was no evidence of microsatellite instability. The tumor mutational burden was low.
FIGURE 1.
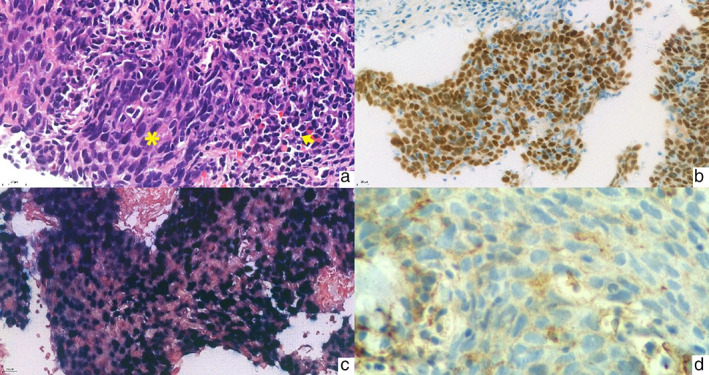
(a) Lymphoepithelial carcinoma (LEC) shows the sheet of poorly differentiated squamous cell carcinoma. The tumor shows a sheet of spindle‐shaped cells with eosinophilic cytoplasm and basophilic nuclei (asterisk) with some lymphocyte infiltrate accompanied by surrounding lymphoplasmacytic stroma (arrow) (hematoxylin–eosin, original magnification ×400). (b) p40 immunohistochemistry in LEC. The tumor cells show diffuse and strong nuclear p40 staining indicating squamous differentiation (original magnification ×400). (c) Epstein–Barr virus (EBV)‐encoded small RNA (EBER) in situ hybridization in LEC. EBER is diffusely positive in the tumor cells (original magnification ×400). (d) Low expression of PD‐L1 (22C3) immunohistochemistry in LEC. The tumor cells show faint incomplete membrane staining of the PD‐L1 staining (original magnification ×600)
The patient was started first‐line treatment with carboplatin and gemcitabine. After six cycles, she achieved a partial response. Her post‐treatment scan revealed improvement of the lung lesion. However, progression of the non‐regional lymph nodes was found. She, then, received pembrolizumab, a PD‐1 inhibitor with the best response being stable disease (SD). The primary tumor progressed after 12 cycles; treatment was changed to docetaxel.
After six cycles, the patient again demonstrated progressive lung disease, and a bone scan revealed hypertrophic pulmonary osteoarthropathy. Accordingly, the patient was given capecitabine, on which new liver metastases developed after three cycles. The patient was then put on pemetrexed. After two cycles, the primary lung lesion had progressed, with right pleural effusion and multiple new bone metastases. Medical pleurodesis was successfully done. Palliative radiation was administered to the left humerus and both hips. She received atezolizumab, a PD‐L1 inhibitor, bevacizumab, carboplatin, and paclitaxel (ABCP), as implemented in the IMpower150 study. Interestingly, the primary tumor size appeared to shrink; even after many preceding treatments, she managed to handle this difficult‐to‐tolerate regimen well. The best overall response was SD.
Her disease, unfortunately, deteriorated after completing six cycles of ABCP. She died in June 2020 because of rapid disease progression following the first dose of vinorelbine. Her overall survival (OS) was 28 months.
DISCUSSION
PPLELC, initially characterized in 1987 by Bégin, 2 has similar morphology to undifferentiated nasopharyngeal carcinoma. 2 PPLELC is linked to EBV and is mostly observed in East Asian populations. 2 Available treatments include surgery, chemotherapy, and radiotherapy, according to the tumor stages. Because most PPLELC patients lack mutations in known driver genes, targeted therapies are not promising treatment strategies. 8 , 9 To our knowledge, no clinical research trials have assessed the best treatment regimens for PPLELC and no treatment guidelines have been explicitly defined.
ICI activity has been observed in virus‐associated cancers. For instance, pembrolizumab has been reported to receive a higher objective response rate (ORR) in human papillomavirus (HPV)‐positive head and neck squamous cell carcinoma (25% ORR) compared to HPV‐negative tumors (14% ORR). 10 Similar results were seen in Merkel cell carcinoma (linked to the polyomavirus) with an ORR of 56%. 11 These studies suggested that virus‐associated cancers are more likely to respond to a PD‐1/PD‐L1 blockade. Accordingly, PD‐L1 expression on tumor cells was predictive of favorable treatment outcomes in patients with NSCLC. 12 , 13 These findings provide a possible rationale for treating PPLELC patients with ICIs.
Recent research has validated PD‐L1 labeling on tumor cells in PPLELC. Chan et al. 14 reported that of 29 PPLELC cases, 41% had >50% PD‐L1‐positivity, and 93% had >1% PD‐L1 positivity. Additionally, Jiang et al. 15 showed that PPLELC patients who had positive PD‐L1 treated with anti‐PD‐1/L1 demonstrated superior progression‐free survival (PFS) and OS than those with negative PD‐L1. In the latest review, Wu et al. 16 retrieved the data for 128 PPLELC cases, five of whom received ICIs in the second‐line treatment, whose median PD‐L1 expression was 40% (range, 30%–80%). Unexpectedly, three of these responded well, with the median OS being 25.6 months (range, 10.2–50.2 months). 16 This is consistent with our patient, who had positive PD‐L1 with TPS of 10% and demonstrated a favorable durable tumor control to pembrolizumab in the second line and atezolizumab in combination with chemotherapy even in the sixth line of treatment. Although the effectiveness of atezolizumab could not solely be validated because of its combination with chemotherapy, using chemotherapy alone in a later line before ABCP regimen showed a shorter time to disease progression. This may imply the efficacy of ICI therapy in this case. Therefore, our experience agrees with the suggestion that PD‐L1 expression is a biomarker for improved survival and potential immunotherapy effectiveness in advanced PPLELC.
This case is the first report to show the potential advantage of using ICIs in the late line therapy, using PD‐1/PD‐L1 inhibitors one after another, for advanced PPLELC. This patient achieved a final OS of 28 months, which is in line with the median OS of 24 months, as previously reported. 16 Although the efficacy of ICIs in PPLELC is difficult to quantify because of the low incidence, our findings suggest that EBV‐associated PPLELC patients with positive PD‐L1 expression may benefit from PD‐1/PD‐L1 inhibition, providing a feasible treatment approach. Further large clinical trials are warranted to obtain more insight into the usefulness of such treatment regimens for this rare subtype of lung cancer.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ACKNOWLEDGMENT
The authors thank the family of the patient whose case is presented here for granting permission to publish this case report and Siriraj Hospital for permission to disclose this information.
Archwamety A, Ruangchira‐urai R, Akewanlop C, Korphaisarn K. Primary pulmonary lymphoepithelioma‐like carcinoma treated with immunotherapy: A case report and literature review. Thorac Cancer. 2022;13(17):2539–2541. 10.1111/1759-7714.14580
DATA AVAILABILITY STATEMENT
The original contributions presented in this work are included in the supplementary material; further inquiries can be directed to the corresponding author.
REFERENCES
- 1. Nicholson AG, Tsao MS, Beasley MB, Borczuk AC, Brambilla E, Cooper WA, et al. The 2021 WHO classification of lung tumors: impact of advances since 2015. J Thorac Oncol. 2022;17(3):362–87. [DOI] [PubMed] [Google Scholar]
- 2. Bégin LR, Eskandari J, Joncas J, Panasci L. Epstein‐Barr virus related lymphoepithelioma‐like carcinoma of lung. J Surg Oncol. 1987;36(4):280–3. [DOI] [PubMed] [Google Scholar]
- 3. Chan AT, Teo PM, Lam KC, Chan WY, Chow JH, Yim AP, et al. Multimodality treatment of primary lymphoepithelioma‐like carcinoma of the lung. Cancer. 1998;83(5):925–9. [DOI] [PubMed] [Google Scholar]
- 4. Lin Z, Fu S, Zhou Y, Zhang X, Chen C, He LN, et al. First‐line platinum‐based chemotherapy and survival outcomes in locally advanced or metastatic pulmonary lymphoepithelioma‐like carcinoma. Lung Cancer. 2019;137:100–7. [DOI] [PubMed] [Google Scholar]
- 5. Reck M, Rodríguez‐Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med. 2016;375(19):1823–33. [DOI] [PubMed] [Google Scholar]
- 6. Gandhi L, Rodríguez‐Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med. 2018;378(22):2078–92. [DOI] [PubMed] [Google Scholar]
- 7. Borghaei H, Paz‐Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med. 2015;373(17):1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen B, Zhang Y, Dai S, Zhou P, Luo W, Wang Z, et al. Molecular characteristics of primary pulmonary lymphoepithelioma‐like carcinoma based on integrated genomic analyses. Signal Transduct Target Ther. 2021;6(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xie Z, Liu L, Lin X, Xie X, Gu Y, Liu M, et al. A multicenter analysis of genomic profiles and PD‐L1 expression of primary lymphoepithelioma‐like carcinoma of the lung. Mod Pathol. 2020;33(4):626–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mehra R, Seiwert TY, Gupta S, Weiss J, Gluck I, Eder JP, et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long‐term follow‐up in KEYNOTE‐012. Br J Cancer. 2018;119(2):153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, et al. PD‐1 blockade with pembrolizumab in advanced merkel‐cell carcinoma. N Engl J Med. 2016;374(26):2542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim C, Rajan A, DeBrito PA, Giaccone G. Metastatic lymphoepithelioma‐like carcinoma of the lung treated with nivolumab: a case report and focused review of literature. Transl Lung Cancer Res. 2016;5(6):720–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McLaughlin J, Han G, Schalper KA, Carvajal‐Hausdorf D, Pelekanou V, Rehman J, et al. Quantitative assessment of the heterogeneity of PD‐L1 expression in non‐small‐cell lung cancer. JAMA Oncol. 2016;2(1):46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chan AWH, Tong JHM, Kwan JSH, Chow C, Chung LY, Chau SL, et al. Assessment of programmed cell death ligand‐1 expression by 4 diagnostic assays and its clinicopathological correlation in a large cohort of surgical resected non‐small cell lung carcinoma. Mod Pathol. 2018;31(9):1381–90. [DOI] [PubMed] [Google Scholar]
- 15. Jiang L, Wang L, Li PF, Zhang XK, Chen JW, Qiu HJ, et al. Positive expression of programmed death ligand‐1 correlates with superior outcomes and might be a therapeutic target in primary pulmonary lymphoepithelioma‐like carcinoma. Onco Targets Ther. 2015;8:1451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu Z, Xian X, Wang K, Cheng D, Li W, Chen B. Immune checkpoint blockade therapy may be a feasible option for primary pulmonary lymphoepithelioma‐like carcinoma. Front Oncol. 2021;11:626566. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in this work are included in the supplementary material; further inquiries can be directed to the corresponding author.


