Abstract
Purpose
The best pattern of neoadjuvant therapy for resectable locoregional esophageal cancer has not been determined. Our study evaluated the efficacy and postoperative events of different treatments using the Bayesian network meta‐analysis.
Methods
We systematically tracked randomized clinical trials from the Medline, EMBASE, and Cochrane Library databases. The following treatments were included: neoadjuvant chemoradiation followed by surgery (NCRT + S), neoadjuvant chemotherapy followed by surgery (NCT + S), neoadjuvant radiotherapy followed by surgery (NRT + S), and surgery alone (S). The Revised Cochrane risk‐of‐bias tools were used to assess the quality of included trials. Overall survival (OS) and progression‐free survival or disease‐free survival (PFS/DFS) were assessed through hazard ratios (HR). Locoregional recurrence, distant metastasis, postoperative mortality, and postoperative morbidity were assessed through odds ratios (OR). These outcomes were compared between different treatments through Bayesian network meta‐analysis.
Results
Twenty trials with 4384 patients were included. Compared with S, only NCRT + S could significantly improve OS for patients with esophageal cancer (HR = 0.78, 95% confidence interval [CI] 0.68–0.88). NCRT + S and NCT + S significantly improved PFS/DFS compared with S (NCRT + S vs. S, HR = 0.72, 95% CI 0.63–0.81; NCT + S vs. S, HR = 0.81, 95% CI 0.69–0.97). NCRT + S significantly reduced both locoregional recurrence (OR = 0.67, 95% CI 0.51–0.88) and distant metastasis (OR = 0.63, 95% CI 0.45–0.90) compared with S. There were no differences in postoperative morbidity between the four treatments. However, NCRT + S also increased postoperative mortality compared with S (OR = 1.77, 95% CI 1.09–2.82) and NCT + S (OR = 1.96, 95% CI 1.11–3.51).
Conclusion
NCRT + S is the most efficient neoadjuvant treatment for resectable locoregional esophageal cancer. However, NCRT + S increases the risk of postoperative mortality but not morbidity.
Keywords: esophagus cancer, neoadjuvant chemotherapy, neoadjuvant radiotherapy, network meta‐analysis
Our study evaluated the efficacy and postoperative events of different treatments by using the Bayesian net‐work meta‐analysis. Randomized clinical trials from Medline, EMBASE, and Cochrane Library database were systematically tracked. The following treatments were included: neoadjuvant chemoradiation followed by surgery (NCRT+S), neoadjuvant chemotherapy followed by surgery (NCT+S), neoadjuvant radiotherapy followed by surgery (NRT+S), and surgery alone (S). Twenty trials with 4384 patients were included. Compared with S, only NCRT+S could significantly improve OS for patients with esophageal cancer (HR=0.78, 95% CI: 0.68‐0.88). NCRT+S and NCT+S significantly improved PFS/DFS compared with S (NCRT+S vs. S, HR=0.72, 95% CI: 0.63‐0.81; NCT+S vs. S, HR=0.81, 95%CI: 0.69‐0.97). NCRT+S significantly reduced both locoregional recurrence (OR=0.67, 95%CI: 0.51‐0.88) and distant metastasis (OR=0.63, 95%CI: 0.45‐0.90) compared with S. There were no differences in postoperative morbidity between the four treatments. However, NCRT+S also increased postoperative mortality compared with S (OR=1.77, 95%CI: 1.09‐2.82) and NCT+S (OR=1.96, 95%CI: 1.11‐3.51).
INTRODUCTION
Esophageal cancer leads to a poor prognosis and is the sixth most common cause of cancer‐related deaths in the world. 1 Surgery is an important treatment for resectable esophageal cancer, but the survival of patients receiving surgery alone is unfavorable, with a 5‐year overall survival (OS) of 15–25%. 2 Neoadjuvant therapy has been a vital pattern before surgery to eliminate micro‐metastatic disease, reduce tumor burden, and improve surgery compliance, finally leading to better postoperative locoregional control, distant control, and OS.
However, the best pattern of neoadjuvant therapy has not been determined. Many randomized control trials have shown the significant advantage of neoadjuvant chemoradiotherapy followed by surgery (NCRT + S) compared with surgery alone, 3 , 4 , 5 including a large phase III study from China. 6 In addition, other research has shown the benefits of neoadjuvant chemotherapy followed by surgery (NCT + S) and neoadjuvant radiotherapy followed by surgery (NRT + S) compared to surgery alone. 7 , 8 , 9 However, few studies have compared different neoadjuvant treatments to distinguish the best neoadjuvant therapies. Four previous randomized trials compared NCRT+S and NCT + S, and only one obsolete trial demonstrated the benefit of NCRT + S beyond NCT + S with just a positive p value 10 , 11 , 12 , 13 ; no trials have directly compared NRT + S with other neoadjuvant treatments.
Previous network meta‐analysis (NMA) suggested that NCRT + S might improve OS more than the other treatments, with some defects and insufficient evidence. 14 , 15 Several randomized trials have reported their results in recent years, 6 , 11 , 12 so the NMA needed to be updated.
The optimal choice among NCRT + S, NCT + S, and NRT + S remained unclear, and high‐quality evidence is needed to direct clinicians. Our study evaluates the effect of neoadjuvant treatments for esophageal cancer and determines the best treatment pattern using the Bayesian NMA.
PATIENTS AND METHODS
Systematic review
Searching strategy
This study was performed following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses 2020 (PRISMA 2020) statement for network meta‐analysis (Supplement 1 in Appendix S1). This NMA was registered in the Prospective Register of Systematic Reviews (Identification code: CRD42020170734).
Eligible data from the Medline, EMBASE, and Cochrane Library databases were systematically searched until April 30, 2021. The search terms are shown in Supplement 2 in Appendix S1.
Inclusion and exclusion criteria
The inclusion criteria were as follows:
Randomized clinical trials (RCTs).
Studies only including patients with esophagus cancer or esophagogastric cancer.
Full text published in English.
Studies comparing different neoadjuvant treatments or surgery alone.
The exclusion criteria were as follows:
- Poor‐qualities studies were defined by:
- Studies reporting Kaplan–Meier curves withoutcensor, whether reporting censor or not in addition.
- Studies not reporting Kaplan–Meier curves or estimated hazard ratios (HR).
- Studies including sample size of less than 50.
Studies with nonstandard and heterogeneous neoadjuvant treatments such as hyperthermia and radiation at low doses (below 30 Gy).
Studies on perioperative and intraoperative treatments.
If several of the same trial publications were retrieved, the most recent information was extracted from these publications.
Data extraction and calculation
Two reviewers independently perused the full text of references meeting the aforestated criteria. The discrepancies of the two reviewers were resolved through discussion, including a third reviewer. Outcomes assessed from the trials included OS, progression‐free survival or disease‐free survival (PFS/DFS), postoperative mortality, postoperative morbidity, locoregional recurrence (LRR), and distant metastases (DM). Postoperative mortality was defined as mortality within 1 or 3 months, or mortality during hospitalization after surgery. Postoperative morbidity was defined as surgery complications during hospitalization such as hemorrhage, heart failure, and fistula, and was calculated according to the number of incurred patients.
The OS and PFS/DFS were assessed by HRs and their standard error, which was extracted or calculated through the following approaches:
For studies directly reporting the HR and its 95% confidential interval (CI), the HR was collected and its standard error (SE) was calculated from the 95% CI.
For studies not reporting the HR but having a survival curve with an at‐risk table, the HR and its SE were calculated using methods outlined by Parmar et al. 16 The number of events and censors was extracted from the survival curve for every interval through WebPlotDigitizer version 4.2 software. Finally, the HR and its SE were calculated by combining all time intervals.
For studies not reporting the HR or at‐risk tables but containing survival curves and follow‐up information, the estimate of censor was approximated based on a linear pattern. Then the HR was calculated as the second approach.
The other outcomes were assessed by odd ratios (ORs) and their standard error, calculated from the number of events and sample size.
Quality assessment
Two reviewers independently assessed the quality of included trials through the revised Cochrane risk‐of‐bias tool for randomized trials (RoB 2). 17 The discrepancies were resolved through discussion, including a third reviewer. The assessment scale was as follows: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, selection of the reported result, and overall bias, with each domain judged as any of the three levels: low, high, or unclear.
Data synthesis and statistical analysis
Bayesian NMA was carried out to synthesize all therapeutic options within a mixed treatment comparison framework. The random‐effects model was prioritized to address the trial‐specific effects, which were components of the overarching distribution. Uninformative prior distribution was given to all parameters. The node‐split method was used to assess the inconsistency. The estimates of relative effects and 95% CI were reported. The surface under the cumulative ranking (SUCRA) scores was also calculated. All statistical analysis was conducted through R 3.6.0.
RESULTS
Trial characteristics
In total 20 trials with 4384 patients were included in the final quantitative NMA. The search and selection process are displayed in a PRISMA 2020 flow diagram in Figure 1. The basic characteristics of the trials included are shown in Table 1. All trials were comparable in terms of clinical features, and the assumption of transitivity was acceptable. The network plot for treatment comparison of OS is shown in Figure 2, and the plots for other outcomes are shown in Supplement 4 in Appendix S1.
FIGURE 1.

Literature search and selection progress
TABLE 1.
Trial characteristics
| Trials | Year | Designs | N | Histology | Location | Preoperative stage | RT schedule | CT schedule | CT cycles |
|---|---|---|---|---|---|---|---|---|---|
| Gignoux 7 | 1987 | NRT + S vs. S | 208 | SCC | Thoracic esophagus | T1‐3N0‐1M0 | 33 Gy/3.3 Gy/10f | — | — |
| Nygaard 13 | 1992 | NCRT + S vs. NCT + S vs. NRT + S vs. S | 186 | SCC | Thoracic esophagus (below 21 cm) | T1‐2N0‐1M0 | 35 Gy/1.75 Gy/20f | PB | 2 |
| Walsh 18 | 1996 | NCRT + S vs. S | 113 | AC | Esophagus (excluding cervical esophagus) | NA | 40 Gy/2.67 Gy/15f | PF | 2 |
| Bosset 19 | 1997 | NCRT + S vs. S | 282 | SCC | Thoracic esophagus | I‐II | 37 Gy/3.7 Gy/10f | P | 2 |
| Law 8 | 1997 | NCT + S vs. S | 147 | SCC | Thoracic esophagus | NA | — | PF | 2 |
| Ancona 20 | 2001 | NCT + S vs. S | 94 | SCC | Esophagus | IIA‐III | — | PF | 2 |
| Urba 21 | 2001 | NCRT + S vs. S | 100 | AC, SCC | Esophagus | NA | 45 Gy/1.5 Gy/30f | PFV | 2 |
| Lee 22 | 2004 | NCRT + S vs. S | 101 | SCC | Thoracic esophagus | IIA‐III | 45.6 Gy/1.2 Gy/38f | PF | 2 |
| Burmeister 5 | 2005 | NCRT + S vs. S | 256 | AC, SCC | Thoracic esophagus | T1‐3N0‐1M0 | 35 Gy/2.3 Gy/15f | PF | 1 |
| Kelsen 23 | 2007 | NCT + S vs. S | 243 | AC, SCC | Esophagus or GEJ | I‐III | — | PF | 3 |
| Tepper 24 | 2008 | NCRT + S vs. S | 56 | AC, SCC | Thoracic esophagus (below 20 cm) or GEJ | T1‐3N0‐1M0 | 50.4 Gy/1.8 Gy/28f | PF | 2 |
| Allum 25 | 2009 | NCT + S vs. S | 802 | AC, SCC, UC | Esophagus or GEJ | NA | — | PF | 2 |
| Lv 26 | 2010 | NCRT + S vs. S | 238 | SCC | Thoracic esophagus | IIA‐III | 40 Gy/2 Gy/20f | TP | 2 |
| Bonstra 9 | 2011 | NCT + S vs. S | 169 | SCC | Thoracic esophagus | I‐III or M1a | — | EP | 2–4 |
| Burmeister 10 | 2011 | NCT + S vs. NCRT + S | 75 | AC | Esophagus or GEJ | IIA‐III | 35 Gy/2.3 Gy/15f | PF | 2 |
| Marittte 4 | 2014 | NCRT + S vs. S | 195 | AC, SCC | Thoracic esophagus | I‐II | 45 Gy/1.8 Gy/25f | PF | 2 |
| Shapiro 3 | 2015 | NCRT + S vs. S | 368 | AC, SCC, UC | Esophagus or GEJ | T1N1M0 or T2–3N0–1M0 | 41.4 Gy/1.8 Gy/23f | TP | 5 a |
| Stahl 11 | 2017 | NCRT + S vs. NCT + S | 119 | AC | GEJ | T3‐4NxM0 | 30 Gy/2 Gy/15f | PFFo/EP | 5 a |
| Yang 6 | 2018 | NCRT + S vs. S | 451 | SCC | Thoracic esophagus | T1‐4N1M0 or T4N0M0 | 40 Gy/2 Gy/20f | NP | 2 |
| VonDobeln 12 | 2019 | NCRT + S vs. NCT + S | 181 | AC, SCC | Esophagus or GEJ | T1N1, T2‐3N0‐1 or M0‐M1a | 40 Gy/2 Gy/20f | PF | 3 |
Abbreviations: AC, adenocarcinoma; CT, chemotherapy; EP, cisplatin and etoposide; f, fractions; GEJ, gastroesophageal junction; NA, not available; NP, cisplatin and vinorelbine; P, single drug cisplatin; PB, cisplatin and bleomycin; PF, cisplatin and fluorouracil; PFFo, cisplatin, fluorouracil, and folinic acid; PFV, cisplatin, fluorouracil, and vinblastine; RT, radiotherapy; S, surgery; SCC, squamous cell carcinoma; TP, cisplatin and paclitaxel; UC, undifferentiated carcinoma.
The chemotherapy was given weekly.
FIGURE 2.
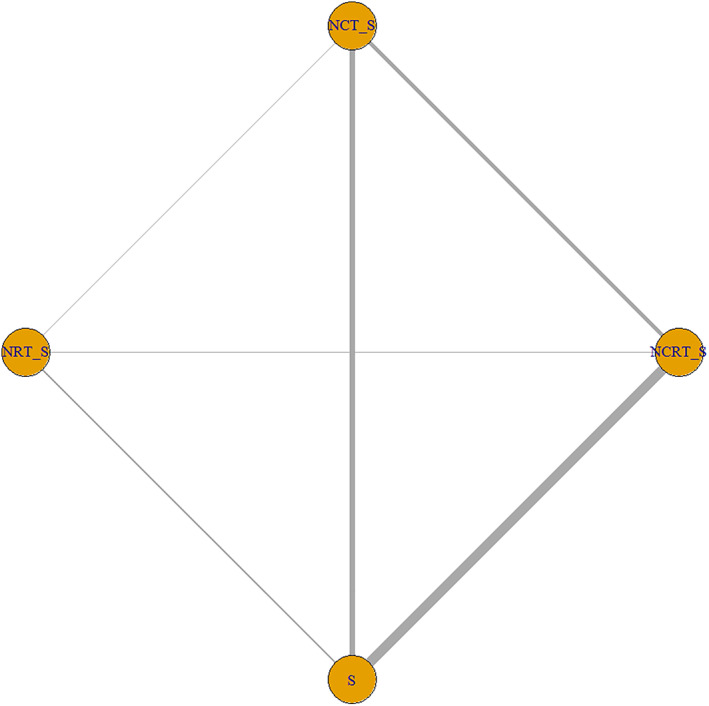
The network plot for treatment comparison of overall survival. Each line represents a type of head‐to‐head comparison. The width of lines is proportional to the number of trials comparing the connected treatments. S, surgery; NCT_S, neoadjuvant chemotherapy followed by surgery; NRT_S, neoadjuvant radiotherapy followed by surgery; NCRT_S, neoadjuvant chemoradiotherapy followed by surgery.
Quality assessment
The summary of the risk‐of‐bias assessment is shown in Supplement 3 in Appendix S1. No RCT had a high risk of bias. As expected, the blinding of participants was not explicitly indicated in most studies, especially in those involved in radiotherapy. We believe that it was unlikely that deviations would arise. However, the selection bias might exist, as the allocation concealment was not explicitly mentioned in most studies.
Inconsistency assessment
Four arms of different treatments were categorized to make comparisons. The node‐split analyses for OS and other outcomes are shown in Supplement 5 in Appendix S1. There were no significant differences between direct and indirect evidence in all comparations for all outcomes (p > 0.05), except for NRT + S versus NCT + S (p = 0.047) in the subgroup analysis of squamous cell carcinoma (SCC) for OS.
Results of NMA
Survivals
The pooled treatment effects of OS for all the evaluated treatment options are shown in Table 2a. Compared with S, NCRT + S could significantly improve the OS for patients with esophageal cancer (HR = 0.78, 95% CI 0.68–0.88), while NCT + S and NRT + S failed to significantly improve the OS (NCT + S vs. S, HR = 0.90, 95% CI 0.78–1.02; NRT + S vs. S, HR = 0.86, 95% CI 0.65–1.10). Compared with NCT + S, NCRT + S failed to improve the OS with statistical significance (HR = 0.91, 95% CI 0.69–1.21). The ranking analysis based on SUCRA scores showed that NCRT + S was the most likely to be the best option in terms of OS benefit (Supplement 6a in Appendix S1).
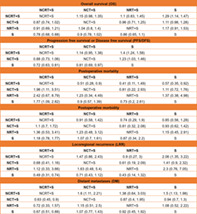
TABLE 2.
The treatment effect for all the evaluated treatment options
| a. Overall survival | ||||
|---|---|---|---|---|
| NCRT + S | NCT + S | NRT + S | S | |
| NCRT + S | NCRT + S | 1.15 (0.98, 1.35) | 1.1 (0.83, 1.45) | 1.29 (1.14, 1.47) |
| NCT + S | 0.87 (0.74, 1.02) | NCT + S | 0.96 (0.71, 1.25) | 1.11 (0.98, 1.28) |
| NRT + S | 0.91 (0.69, 1.21) | 1.04 (0.8, 1.4) | NRT + S | 1.17 (0.91, 1.53) |
| S | 0.78 (0.68, 0.88) | 0.9 (0.78, 1.02) | 0.86 (0.65, 1.1) | S |
| b. Progression‐free survival or disease‐free survival | ||||
|---|---|---|---|---|
| NCRT + S | NCT + S | S | ||
| NCRT + S | NCRT + S | 1.14 (0.95, 1.38) | 1.4 (1.24, 1.58) | |
| NCT + S | 0.88 (0.73, 1.06) | NCT + S | 1.23 (1.03, 1.46) | |
| S | 0.72 (0.63, 0.81) | 0.81 (0.69, 0.97) | S | |
| c. Postoperative mortality | ||||
|---|---|---|---|---|
| NCRT + S | NCT + S | NRT + S | S | |
| NCRT + S | NCRT + S | 0.51 (0.28, 0.9) | 0.41 (0.11, 1.49) | 0.57 (0.35, 0.92) |
| NCT + S | 1.96 (1.11, 3.51) | NCT + S | 0.81 (0.22, 2.93) | 1.11 (0.72, 1.76) |
| NRT + S | 2.42 (0.67, 8.79) | 1.23 (0.34, 4.49) | NRT + S | 1.37 (0.38, 4.98) |
| S | 1.77 (1.09, 2.82) | 0.9 (0.57, 1.39) | 0.73 (0.2, 2.61) | S |
| d. Postoperative morbidity | ||||
|---|---|---|---|---|
| NCRT + S | NCT + S | NRT + S | S | |
| NCRT + S | NCRT + S | 0.91 (0.58, 1.42) | 0.74 (0.29, 1.9) | 0.85 (0.56, 1.28) |
| NCT + S | 1.1 (0.7, 1.72) | NCT + S | 0.81 (0.32, 2.08) | 0.93 (0.62, 1.42) |
| NRT + S | 1.36 (0.53, 3.41) | 1.23 (0.48, 3.12) | NRT + S | 1.15 (0.45, 2.91) |
| S | 1.18 (0.78, 1.77) | 1.07 (0.7, 1.61) | 0.87 (0.34, 2.2) | S |
| e. Locoregional recurrence | ||||
|---|---|---|---|---|
| NCRT + S | NCT + S | NRT + S | S | |
| NCRT + S | NCRT + S | 1.47 (0.86, 2.43) | 0.9 (0.27, 3) | 2.06 (1.35, 3.22) |
| NCT + S | 0.68 (0.41, 1.16) | NCT + S | 0.61 (0.19, 2.09) | 1.41 (0.9, 2.32) |
| NRT + S | 1.12 (0.33, 3.66) | 1.63 (0.48, 5.4) | NRT + S | 2.3 (0.76, 7.05) |
| S | 0.49 (0.31, 0.74) | 0.71 (0.43, 1.12) | 0.43 (0.14, 1.32) | S |
| f. Distant metastases | ||||
|---|---|---|---|---|
| NCRT + S | NCT + S | NRT + S | S | |
| NCRT + S | NCRT + S | 1.6 (1.11, 2.21) | 1.38 (0.64, 3.03) | 1.5 (1.13, 1.98) |
| NCT + S | 0.63 (0.45, 0.9) | NCT + S | 0.87 (0.4, 1.95) | 0.94 (0.7, 1.3) |
| NRT + S | 0.72 (0.33, 1.57) | 1.15 (0.51, 2.5) | NRT + S | 1.08 (0.52, 2.22) |
| S | 0.67 (0.51, 0.88) | 1.07 (0.77, 1.43) | 0.92 (0.45, 1.92) | S |
Note: Statistically confident results are in bold.
Abbreviations: NCRT + S, neoadjuvant chemoradiotherapy followed by surgery; NCT + S, neoadjuvant chemotherapy followed by surgery; NRT + S, neoadjuvant radiotherapy followed by surgery; S, surgery.
In total 14 studies and three arms (NCRT + S, NCT + S, and S) were available for PFS/DFS analysis. The treatment effects of PFS/DFS are shown in Table 2b. Compared with S, NCRT + S and NCT + S significantly improved the PFS/DFS for patients with esophageal cancer (NCRT + S vs. S, HR = 0.72, 95% CI 0.63–0.81; NCT + S vs. S, HR = 0.81, 95% CI 0.69–0.97). NCRT + S improved the PFS/DFS from NCT + S without statistical significance (HR = 0.88, 95% CI 0.73–1.06). NCRT + S also reached the top of SUCRA scores in all treatments (Supplement 6b in Appendix S1).
Postoperative events
Eighteen studies were available for postoperative mortality and eight for postoperative morbidity. The treatment effects are shown in Table 2c,d. NCRT + S significantly increased postoperative mortality compared to S (OR = 1.77, 95% CI 1.09–2.82) and NCT + S (OR = 1.96, 95% CI 1.11–3.51). However, no significant difference in postoperative morbidity was detected between the four treatments. NCRT + S reached the lowest SUCRA scores of postoperative mortality and morbidity in all treatments (Supplement 6c,d in Appendix S1).
Failure patterns
Fourteen studies were available for LRR and DM. The treatment effects are shown in Table 2e,f. LRR was significantly lower in the NCRT + S group than in the S group (OR = 0.49, 95% CI 0.31–0.74). Compared with the S group, DM was significantly reduced in both the NCRT + S group and the NCT + S group (NCRT + S vs. S, OR = 0.67, 95% CI 0.51–0.88; NCRT + S vs. S, OR = 0.63, 95% CI 0.45–0.90). No significant difference was detected between the other groups. NCRT + S reached the top of the SUCRA scores of both LRR and DM in all treatments (Supplement 6e,f in Appendix S1). Although NRT + S failed to reach significant differences in treatment effect analyses, it was the second group in ranking analysis for LRR and DM.
Subgroup analyses of pathological types
Fourteen studies were available for SCC and eight for adenocarcinoma (AC). The treatment effects of OS are shown in Table 3. For patients with SCC, both NCRT + S and NCT + S could significantly improve OS compared with S (NCRT + S vs. NCT + S, HR = 0.76, 95% CI 0.64–0.90; NCT + S vs. S, HR = 0.83, 95% CI 0.71–0.99). No significant difference was detected between the other groups. For patients with AC, no significant difference in OS was detected between the treatment groups, but both NCRT + S and NCT + S were apparently associated with better OS over S (NCRT + S vs. NCT + S, HR = 0.79, 95% CI 0.60–1.04; NCT + S vs. S, HR = 0.88, 95% CI 0.62–1.28). NCRT + S reached the top of the SUCRA scores in all treatments for both SCC and AC (Supplement 6g,h in Appendix S1).
TABLE 3.
Subgroup analyses of pathological types for OS effects
| a. Squamous cell carcinoma | ||||
|---|---|---|---|---|
| NCRT + S | NCT + S | NRT + S | S | |
| NCRT + S | NCRT + S | 1.09 (0.88, 1.37) | 1.12 (0.81, 1.49) | 1.31 (1.11, 1.55) |
| NCT + S | 0.91 (0.73, 1.13) | NCT + S | 1.03 (0.74, 1.35) | 1.2 (1.01, 1.41) |
| NRT + S | 0.89 (0.67, 1.23) | 0.97 (0.74, 1.36) | NRT + S | 1.17 (0.92, 1.56) |
| S | 0.76 (0.64, 0.9) | 0.83 (0.71, 0.99) | 0.85 (0.64, 1.09) | S |
| b. Adenocarcinoma | ||||
|---|---|---|---|---|
| NCRT + S | NCT + S | S | ||
| NCRT + S | NCRT + S | 1.11 (0.82, 1.56) | 1.27 (0.96, 1.68) | |
| NCT + S | 0.9 (0.64, 1.22) | NCT + S | 1.14 (0.78, 1.61) | |
| S | 0.79 (0.6, 1.04) | 0.88 (0.62, 1.28) | S | |
Note: Statistically confident results are in bold.
Abbreviations: NCRT + S, neoadjuvant chemoradiotherapy followed by surgery; NCT + S, neoadjuvant chemotherapy followed by surgery; NRT + S, neoadjuvant radiotherapy followed by surgery; S, surgery.
DISCUSSION
This NMA of 4384 patients in 20 RCTs demonstrated that NCRT + S was the most effective neoadjuvant therapy pattern for resectable esophageal cancer. NCRT + S increased OS and PFS/DFS, and decreased LRR and DM compared with the other treatments without expanding the probability of postoperative morbidity. NCRT + S was the most effective treatment for both SCC and AC, although the OS effect over S was not statistically significant for AC. This NMA provides the best available evidence for the use of neoadjuvant CRT in esophageal cancer.
Several high‐quality RCTs reported the efficiency and postoperative events of NCRT + S compared with S. The NEOCRTEC5010 study published in 2018 reported the OS and DFS benefits by NCRT + S compared with surgery alone in squamous esophageal cancer. 6 The classical CROSS study also demonstrated the efficiency of NCRT + S over surgery alone. 3 Our study confirms the role of NCRT in the neoadjuvant treatments of esophageal cancer.
While the survival benefit of NCRT over surgery alone has been established, the relative merits of radiotherapy and chemotherapy have not been determined. The long‐term results of the POET study showed a borderline OS advantage for NCRT + S over NCT + S (p = 0.055). Another two phase II studies also failed to reach a statistically significant survival advantage for NCRT over NCT. 10 , 12 Our study and previous meta‐analyses did not find survival differences between NCRT + S and NCT + S. The possible reason for this is that only a few trials directly compared NCRT + S and NCT + S (four studies with 472 patients in our study; Table 1). Further studies still need to be conducted to compare the efficiency of NCRT and NCT.
Interestingly, our study found that NCRT + S significantly increased the postoperative mortality of esophageal cancer compared with NCT + S or S alone. This result was consistent with a previously published NMA. 14 The total postoperative deaths were 64 in the NCRT + S group and 35 in the S group. The main reasons for postoperative death in the NCRT + S group were respiratory complications (41% of all cases where specific complications were reported), severe infection and sepsis (23%), and anastomotic leak (17%). However, NCRT + S did not increase the postoperative morbidity compared with either NCT + S or S. Among the included 13 trials of NCRT + S, only one trial published in 1997 reported that the postoperative complications of NCRT + S were significantly higher than for S. The other 12 trials (including the CROSS and NEOCRTEC5010 studies) reported no significant difference between NCRT + S and other treatments. We further recalculated the postoperative morbidity as the number of events instead of incurred patients. We found no significant differences between the four treatments, consistent with postoperative morbidity calculated with the number of incurred patients. The possible reason for this could be that NCRT + S increased the severity of postoperative complications instead of the number. Postoperative complications should therefore be monitored and treated promptly when the patient has been treated with NCRT. Another interesting result of our study is that NCRT + S could significantly reduce DM compared with NCT + S. This could be because of the synergistic effect of radiotherapy and chemotherapy. Radiation could reduce tumor volume and eliminate tumor cells in local vessels, which could help concurrent chemotherapy to reduce DM.
Chan et al. reported another NMA of neoadjuvant therapy in 2018. 14 The results also showed that NCRT + S was the most efficient treatment for esophageal cancer. However, this NMA had some drawbacks. The radiation dose of some included trials was 20Gy, which was insufficient. In addition, several exploratory and small‐sized trials were included in the final analysis, reducing the homogeneity and credibility of results. Finally, this study did not analyze postoperative morbidity, and the results of postoperative mortality were uncertain (NCRT + S might increase the risk of postoperative mortality in esophageal cancer patients). Our NMA avoided these points, and added one published trial and two updated trials to expand the evidence base. Studies with nonstandard treatments or lower quality were excluded as the exclusion criteria mentioned above. Our results confirmed that NCRT + S increased postoperative mortality compared with NCT + S or surgery alone. We further analyzed postoperative morbidity.
There are a few limitations of this NMA. First, this analysis used published data instead of individual patient data, where some uncontrolled confounding factors could affect the results. Second, the sample sizes for NRT + S arm were limited with two studies (published in 1987 and 1992) of 150 patients and the radiotherapy techniques in the two studies were outdated traditional two‐fields radiation. However, we could not find a trial with NRT + S using modern techniques, so we had to include the outdated studies. Thus, the results of NRT + S need to be treated with caution because of uncertainties and the wide 95% CIs. Third, the variability of included trials in patient populations, treatments, and procedures should not be ignored, even though the tests for inconsistency were negative. One of the important confounding factors was the location of esophageal cancer (gastroesophageal junction, EGJ or thoracic esophagus), which might induce a different response to neoadjuvant treatments. Our study could not estimate the effect of location on different neoadjuvant treatments because of insufficient evidence. Fourth, our analysis did not distinguish between chemotherapy regimens or radiation doses. In most trials, platinum‐based chemotherapy and a total radiation dose of 40–50 Gy were used. Finally, neoadjuvant immunotherapy was not included in this study. Although studies on neoadjuvant immunotherapy were in full flow, most were nonrandomized controlled studies and had not yet reported mature survival data.
In conclusion, NCRT + S could be the most efficient neoadjuvant treatment for resectable locoregional esophageal cancer. However, NCRT + S could increase the risk of postoperative mortality but not postoperative morbidity, and clinicians must be cautious in postoperative management.
AUTHOR CONTRIBUTIONS
Y.B., Z.M., M.Y., and Z.H. contributed to the conception and design of the study. Y.B., Z.M., and M.Y. performed the searches and collected raw data. Y.W. performed the statistical analysis. Y.B. wrote the main part of the manuscript. M.Y. and Z.M. wrote part of the manuscript. Y.M. and Z.H. reviewed and edited the manuscript. All authors contributed to the manuscript revision and approved the submitted version.
FUNDING INFORMATION
This work was supported by the National Key Research and Development Program (2017YFC1311000 and 1311002) and the CAMS Innovation Fund for Medical Sciences (No. 2016‐I2M‐1‐011).
Supporting information
Appendix S1 Supporting Information
Bao Y, Ma Z, Yuan M, Wang Y, Men Y, Hui Z. Comparison of different neoadjuvant treatments for resectable locoregional esophageal cancer: A systematic review and network meta‐analysis . Thorac Cancer. 2022;13(17):2515–2523. 10.1111/1759-7714.14588
Yongxing Bao, Zeliang Ma, and Meng Yuan have contributed equally to this work.
Funding information CAMS Innovation Fund for Medical Sciences, Grant/Award Number: 2016‐I2M‐1‐011; National Key Research and Development Program, Grant/Award Numbers: 2017YFC1311000, 2017YFC1311002
DATA AVAILABILITY STATEMENT
All data were extracted from published articles.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381(9864):400–12. 10.1016/s0140-6736(12)60643-6 [DOI] [PubMed] [Google Scholar]
- 3. Shapiro J, van Lanschot JJB, Hulshof M, van Hagen P, van Berge Henegouwen M, Wijnhoven BPL, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long‐term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–8. 10.1016/s1470-2045(15)00040-6 [DOI] [PubMed] [Google Scholar]
- 4. Mariette C, Dahan L, Mornex F, Maillard E, Thomas PA, Meunier B, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol. 2014;32(23):2416–22. 10.1200/JCO.2013.53.6532 [DOI] [PubMed] [Google Scholar]
- 5. Burmeister BH, Smithers BM, Gebski V, Fitzgerald L, Simes RJ, Devitt P, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6(9):659–68. 10.1016/s1470-2045(05)70288-6 [DOI] [PubMed] [Google Scholar]
- 6. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open‐label clinical trial. J Clin Oncol. 2018;36(27):2796–803. 10.1200/jco.2018.79.1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gignoux M, Roussel A, Paillot B, Gillet M, Schlag P, Favre JP, Dalesio O, Buyse M, Duez N (1987) The value of preoperative radiotherapy in esophageal cancer: results of a study of the E.O.R.T.C. World J Surg 11 (4):426‐432. doi: 10.1007/bf01655805 [DOI] [PubMed] [Google Scholar]
- 8. Law S, Fok M, Chow S, et al. Preoperative chemotherapy versus surgical therapy alone for squamous cell carcinoma of the esophagus: a prospective randomized trial. J Thorac Cardiovasc Surg. 1997;114(2):210–7. 10.1016/s0022-5223(97)70147-8 [DOI] [PubMed] [Google Scholar]
- 9. Boonstra JJ, Kok TC, Wijnhoven BP, et al. Chemotherapy followed by surgery versus surgery alone in patients with resectable oesophageal squamous cell carcinoma: long‐term results of a randomized controlled trial. BMC Cancer. 2011;11:181. 10.1186/1471-2407-11-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burmeister BH, Thomas JM, Burmeister EA, Walpole ET, Harvey JA, Thomson DB, et al. Is concurrent radiation therapy required in patients receiving preoperative chemotherapy for adenocarcinoma of the oesophagus? A randomised phase II trial. Eur J Cancer. 2011;47(3):354–60. 10.1016/j.ejca.2010.09.009 [DOI] [PubMed] [Google Scholar]
- 11. Stahl M, Walz MK, Riera‐Knorrenschild J, et al. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): long‐term results of a controlled randomised trial. Eur J Cancer. 2017;81:183–90. 10.1016/j.ejca.2017.04.027 [DOI] [PubMed] [Google Scholar]
- 12. von Dobeln GA, Klevebro F, Jacobsen AB, et al. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or gastroesophageal junction: long‐term results of a randomized clinical trial. Dis Esophagus. 2019;32(2):1–11. 10.1093/dote/doy078 [DOI] [PubMed] [Google Scholar]
- 13. Nygaard K, Hagen S, Hansen HS, Hatlevoll R, Hultborn R, Jakobsen A, et al. Pre‐operative radiotherapy prolongs survival in operable esophageal carcinoma: a randomized, multicenter study of pre‐operative radiotherapy and chemotherapy. The second Scandinavian trial in esophageal cancer. World J Surg. 1992;16 (6):1104‐1109; discussion 1110:1104–9. 10.1007/bf02067069 [DOI] [PubMed] [Google Scholar]
- 14. Chan KKW, Saluja R, Delos Santos K, Lien K, Shah K, Cramarossa G, et al. Neoadjuvant treatments for locally advanced, resectable esophageal cancer: a network meta‐analysis. Int J Cancer. 2018;143(2):430–7. 10.1002/ijc.31312 [DOI] [PubMed] [Google Scholar]
- 15. Huang Y, Wang H, Luo G, Zhang Y, Wang L, Li K. A systematic review and network meta‐analysis of neoadjuvant therapy combined with surgery for patients with resectable esophageal squamous cell carcinoma. Int J Surg. 2017;38:41–7. 10.1016/j.ijsu.2016.12.035 [DOI] [PubMed] [Google Scholar]
- 16. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815–34. [DOI] [PubMed] [Google Scholar]
- 17. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 18. Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy TPJ. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335(7):462–7. 10.1056/nejm199608153350702 [DOI] [PubMed] [Google Scholar]
- 19. Bosset JF, Gignoux M, Triboulet JP, Tiret E, Mantion G, Elias D, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous‐cell cancer of the esophagus. N Engl J Med. 1997;337(3):161–7. 10.1056/NEJM199707173370304 [DOI] [PubMed] [Google Scholar]
- 20. Ancona E, Ruol A, Santi S, Merigliano S, Sileni VC, Koussis H, et al. Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long term survival of patients with resectable esophageal squamous cell carcinoma: final report of a randomized, controlled trial of preoperative chemotherapy versus surgery alone. Cancer. 2001;91(11):2165–74. [PubMed] [Google Scholar]
- 21. Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19(2):305–13. 10.1200/jco.2001.19.2.305 [DOI] [PubMed] [Google Scholar]
- 22. Lee JL, Park SI, Kim SB, Jung HY, Lee GH, Kim JH, et al. A single institutional phase III trial of preoperative chemotherapy with hyperfractionation radiotherapy plus surgery versus surgery alone for resectable esophageal squamous cell carcinoma. Ann Oncol. 2004;15(6):947–54. 10.1093/annonc/mdh219 [DOI] [PubMed] [Google Scholar]
- 23. Kelsen DP, Winter KA, Gunderson LL, Mortimer J, Estes NC, Haller DG, et al. Long‐term results of RTOG trial 8911 (USA intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol. 2007;25(24):3719–25. 10.1200/JCO.2006.10.4760 [DOI] [PubMed] [Google Scholar]
- 24. Tepper J, Krasna MJ, Niedzwiecki D, Hollis D, Reed CE, Goldberg R, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26(7):1086–92. 10.1200/jco.2007.12.9593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long‐term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27(30):5062–7. 10.1200/JCO.2009.22.2083 [DOI] [PubMed] [Google Scholar]
- 26. Lv J, Cao XF, Zhu B, Ji L, Tao L, Wang DD. Long‐term efficacy of perioperative chemoradiotherapy on esophageal squamous cell carcinoma. World J Gastroenterol. 2010;16(13):1649–54. 10.3748/wjg.v16.i13.1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information
Data Availability Statement
All data were extracted from published articles.


