Abstract
Sequence analysis in Pseudomonas putida DOT-T1E revealed a second toluene efflux system for toluene metabolism encoded by the ttgDEF genes, which are adjacent to the tod genes. The ttgDEF genes were expressed in response to the presence of aromatic hydrocarbons such as toluene and styrene in the culture medium. To characterize the contribution of the TtgDEF system to toluene tolerance in P. putida, site-directed mutagenesis was used to knock out the gene in the wild-type DOT-T1E strain and in a mutant derivative, DOT-T1E-18. This mutant carried a Tn5 insertion in the ttgABC gene cluster, which encodes a toluene efflux pump that is synthesized constitutively. For site-directed mutagenesis, a cassette to knock out the ttgD gene and encoding resistance to tellurite was constructed in vitro and transferred to the corresponding host chromosome via the suicide plasmid pKNG101. Successful replacement of the wild-type sequences with the mutant cassette was confirmed by Southern hybridization. A single ttgD mutant, DOT-T1E-1, and a double mutant with knock outs in the ttgD and ttgA genes, DOT-T1E-82, were obtained and characterized for toluene tolerance. This was assayed by the sudden addition of toluene (0.3% [vol/vol]) to the liquid culture medium of cells growing on Luria-Bertani (LB) medium (noninduced) or on LB medium with toluene supplied via the gas phase (induced). Induced cells of the single ttgD mutant were more sensitive to sudden toluene shock than were the wild-type cells; however, noninduced wild-type and ttgD mutant cells were equally tolerant to toluene shock. Noninduced cells of the double DOT-T1E-82 mutant did not survive upon sudden toluene shock; however, they still remained viable upon sudden toluene shock if they had been previously induced. These results are discussed in the context of the use of multiple efflux pumps involved in solvent tolerance in P. putida DOT-T1E.
Organic solvents with a logPOW value (i.e., the logarithm of the partition coefficient of the target compound in a mixture of octanol and water) between 1.5 and 3 are extremely toxic to microorganisms, a characteristic that has been well documented for toluene (logPOW 2.5) (5, 8, 10, 38, 39). De Smet et al. (8) demonstrated that toluene destabilizes the inner membrane of gram-negative bacteria, causing a transition from a lamellar bilayer state to a hexagonal state. This in turn gives rise to the leakage of proteins, lipids, and ions, as well as disrupting the cell membrane potential. The consequent collapse of ATP synthesis, together with other lesions, leads to cell death (39).
Pseudomonas putida strains have been isolated that are able to grow in culture medium with toluene or related aromatic hydrocarbons added to the liquid medium (7, 12, 19, 32, 40). The key element involved in the tolerance to organic solvents in these P. putida strains is a series of energy-dependent pumps that actively remove the organic solvent from cell membranes (17–19, 23, 33, 34). This conclusion was based on the following findings: (i) P. putida strains treated with the uncoupler carbonyl cyanide p-trifluoromethoxyphenyl hydrazone accumulated higher levels of solvents in cell membranes than did untreated cells (14, 34), and (ii) transposon mutants of P. putida that were sensitive to toluene and other chemicals accumulated 5- to 20-fold higher levels of solvents in cell membranes than did the wild-type strain (17, 19, 33, 34).
Constitutive and inducible efflux pumps seem to be involved in solvent tolerance. These efflux pumps, which belong to the resistance-nodulation-division (RND) family of pumps, consist of three components: an inner membrane transporter (component B), an outer membrane protein (component C), and a periplasmic protein (component A). Together, these components coordinate the efflux of solvents from the cytoplasmatic membrane across the outer membrane, although the mechanism by which this occurs is still unknown (5, 28, 38). Recently, Ramos et al. (33) described a constitutive efflux pump encoded by the ttgABC genes that makes the P. putida DOT-T1E cells tolerant to toluene; Kieboom et al. (17, 18) have suggested that the SrpABC pump involved in solvent tolerance in P. putida S12 is inducible. In both cases all three proteins are encoded by genes that seem to be organized in a single operon (17, 18, 33).
In this study we report the identification in P. putida DOT-T1E of a second efflux pump for toluene, which is induced in response to certain aromatic hydrocarbons and which is also made up of three proteins encoded by the ttgDEF genes. These genes are linked to the chromosomal tod genes for toluene metabolism.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture media.
P. putida DOT-T1E was grown at 30°C in Luria-Bertani (LB) medium or minimal medium M9 (1) supplemented with toluene (vapor phase), benzoate (10 mM), or succinate (20 mM) as the sole carbon source. Mutant strains of P. putida DOT-T1E generated previously and those constructed in this study are shown in Table 1. Escherichia coli DH5αF′ was used for cloning experiments and was grown at 37°C in LB medium. E. coli CC118λpir was used to replicate plasmids based on the R6K replicon (11). Competent E. coli cells were prepared according to the method of Inoue et al. (14).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| P. putida DOT-T1E | Apr Rifr Tolr Tol+ | 32 |
| P. putida DOT-T1E-18 | Apr KmrttgB∇ Tn5 Tol+ | 33 |
| P. putida DOT-T1-B6m1 | AprttgD∇Telr Tol+ | This study |
| P. putida DOT-T1E-82 | AprttgD∇TelrttgB∇Tn5 Tol+ | This study |
| E. coli DH5αF′ | recA1 | Stratagene, Inc. |
| E. coli CC118λpir | Rifr, host for replication of plasmids based in R6K replicon | 11 |
| Plasmids | ||
| pBluescript II KS(−) | Apr, cloning vector | Stratagene, Inc. |
| pT1-125 | AprtodT | 27 |
| pKNG101 | Smr mob+sacBR, R6K replicon | 16 |
| pKNG6-11 | pKNG101 bearing dut::telAB:: ‘ttgD ttgE’ genes | This study |
| pRK600 | Cmr mob+ tra+, ColE1 replicon | 11 |
| pT1-B6 | AprtodT-ttgDEF | This study |
| pT1-B611 | Apr Telr, dut::kilA telAB:: ‘ttgD ttgE’ | This study |
| pUC19 | Apr, cloning vector | 41 |
| pUT/tel | Apr TelroriR6K mob+ | 37 |
Apr, Cmr, Rifr, Smr, Telr, and Tolr represent resistance to ampicillin, chloramphenicol, rifampin, streptomycin, telurite, and toluene, respectively. Tol+ indicates the ability to use toluene as the sole carbon source.
pUC19 (41) and pBS(SK−) (Stratagene, Inc.) were used for cloning experiments. The helper plasmid pRK600 was used to mobilize tra-lacking mob+ plasmids (11). The R6K-based pKNG101 plasmid was used for in vivo allelic replacements as described before (16). The plasmids constructed in this study are shown in Table 1, and their relevant properties are described in the Results section.
Potassium tellurite was used at a concentration of 15 μg/ml for P. putida DOT-T1E and 5 μg/ml for E. coli DH5αF′. The antibiotics used were as follows: ampicillin (Ap), 100 μg/ml; kanamycin (Km), 50 μg/ml; streptomycin (Sm), 100 μg/ml; and rifampin (Rif), 20 μg/ml.
Construction of a gene bank of P. putida DOT-T1E.
DNA from P. putida DOT-T1E was isolated by the CTAB (cetyltrimethylammonium bromide) method as described before (4). To construct a gene bank, P. putida DOT-T1E DNA was partially digested with Sau3AI, and DNA fragments were separated through a sucrose gradient (10 to 40% [wt/vol]) for 20 h at 24,000 rpm in a 50 Ti Sorvall rotor (36). Aliquots of 0.5 ml were collected and analyzed by agarose gel electrophoresis and then visualized after ethidium bromide staining. Fractions containing fragments of longer than 6 kb were pooled, dialyzed against sterile and deionized water, concentrated for further ligation to pUC19 digested with BamHI, and dephosphorylated with calf intestinal phosphatase. More than 3,000 white colonies were obtained after transformation into E. coli DH5αF′ and selection on LB solid medium supplemented with 100 μg of ampicillin, 20 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside, and 20 μg of isopropyl-β-d-thiogalactopyranoside per ml. Colony screening hybridization was performed according to the method of Sambrook et al. (36) to find clones with the genes of interest. Probes were obtained by PCR using appropriate primers, and labeling was done with dUTP-digoxigenin (Boehringer Mannheim).
Plasmid isolation and DNA sequence.
Plasmids were isolated with a Qiagen kit (Qiagen GmbH). Plasmid DNA was sequenced in both strands with universal, reverse, or specifically designed primers in an automatic DNA sequencer (model 377; Perkin-Elmer, Inc.). Sequences were analyzed and compared with the Blastx programs (2), which are available from the National Institute for Biotechnology Information server.
PCR.
DNA amplification reactions were done in a GeneAmp PCR system 2400 by using the appropriate primers. Internal primers for amplification of ttgD (5′-CATGGCATGAACGGCTGTTTC-3′ and 5′-CTGACTTGAGCCTGATTATCCC-3′), ttgE (5′-GTGGTCCAGGTTATCGAGCAGC-3′ and 5′-CGGCGCAAGTGCAGGCAGTCAGCACTCCATT-3′), and ttgF (5′-GCAGATAACGATGGTGACAGCGAAC-3′ and 5′-CAGATAATTGTCCACGCCCTCGTCG-3′) were used. The cycling conditions were as follows: 68°C for 2 min and 94°C for 2 min, followed by 30 cycles of 94°C for 30 s, 52°C for 30 s, and 68°C for 1 min.
Primer extension analysis.
P. putida DOT-T1E was grown overnight in M9 minimal medium with succinate as the sole carbon source. Cells were then pelleted and resuspended in fresh medium at a turbidity of 0.4 at 660 nm. Aliquots of the culture were incubated in the absence or in the presence of different aromatic hydrocarbons supplied via the gas phase, at 200 rpm and at 30°C until the culture reached a turbidity of 1.0 at 660 nm.
Samples (3 ml) were collected into chilled tubes, and cells were pelleted and processed for RNA isolation according to the method of Marqués et al. (26). RNA was treated with DNase I-RNase-free and RNase inhibitor (Boehringer Mannheim) to ensure complete removal of DNA and to maintain the integrity of mRNA. The sequence of the primer used for primer extension (5′-CTCTACGAACATGCGTTTCTGCAG-3′) was complementary to the ttgD gene. This primer was labeled at its 5′-end with [γ-32P]ATP and T4 polynucleotide kinase. About 105 cpm of labeled primer was hybridized to 30 μg of total RNA, and extension was carried out with an avian myeloblastosis virus reverse transcriptase (RT) as described earlier (26). Electrophoresis of cDNA products was done in a urea-polyacrylamide sequencing gel to separate the reaction products, and dry gels were exposed to X-ray film and visualized (26).
RT-PCR.
RNA was prepared as described above. RT-PCR was carried out with the Titan One Tube RT-PCR system (RT-PCR) by using the appropriate primers. Positive and negative controls were included in each experiment. The cycling conditions were as follows: 50°C for 3 min and 94°C for 2 min, followed by 10 cycles of 94°C for 30 s, 60°C for 30 s, 68°C for 1 min, and further cooling to 4°C. PCR products were separated in agarose gels in TAE buffer (40 mM Tris-acetate, 1 mM EDTA; pH 8.0) and visualized after staining with ethidium bromide.
Accession number.
Nucleotide sequences of efflux system genes described here have been submitted to the GenBank-EMBL data bank under accession number AFY19106.
RESULTS
Genes homologous to efflux pumps for solvents are located downstream from the tod operon of P. putida DOT-T1E.
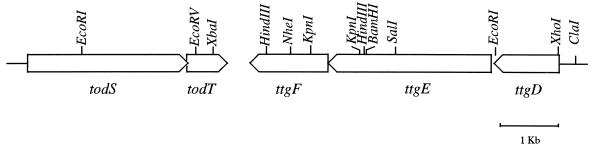
P. putida DOT-T1E metabolizes toluene via the toluene dioxygenase pathway (27). The genes of this pathway are organized in two adjacent transcriptional units, todXFC1C2BADEGIH and todST, which are transcribed in the same direction (27). About 400 bp downstream from todT, a partial open reading frame (ORF) in the opposite direction to todT was found. It showed high sequence identity with the oprM, ttgC, and srpC genes of several Pseudomonas strains. This partial sequence could not be completed with the available subclone (pT1-125) because no more adjacent DNA was available in this plasmid. Given that the genes encode the outer membrane element of RND pumps involved in the efflux of toxic compounds from the cell, including antibiotics and solvents (OprM and TtgC) or solvents only (SrpC), and because the genes form part of the operons (mexAB oprM, ttgABC, and srpABC), we decided to determine whether the identified ORF was part of a similar type of gene cluster. To this end, we screened a genomic P. putida DOT-T1E library against a PCR probe generated by using primers based on the todT gene (5′-CTGGTTCGAGTAACTGAGCGGCTCAAGATAGCCT-3′) and the DNA of the partial ORF we had identified (5′-CGGCGCAAGTGCAGGCAGTCAGCACTCCATT-3′). This yielded a 2-kb fragment which was labeled with dUTP digoxigenin during amplification. Four positive clones were found, all of which were identical in size after digestion with different restriction enzymes (not shown). One random clone, pT1-B6 (Table 1), was retained for further studies. The clone was characterized and was found to contain the 3′ end of the todT gene and about 8 kb of the adjacent DNA (Fig. 1). This DNA was sequenced on both strands (DNA sequence deposited at GenBank under accession number AFY19106). Analysis of the DNA sequence revealed the existence of three ORFs of 1,147, 3,143, and 1,439 bp which were organized like an operon and whose transcriptional direction was opposite to that of the todT gene (Fig. 1). Because the ATG of the third ORF overlaps the stop codon of the second ORF, and a stretch of only 14 bp bridges the first and the second ORFs, we deduced that the three ORFs might form part of an operon. We confirmed the operon's structure by performing RT-PCR with RNA isolated from cells growing in the presence of toluene in the gas phase. Using oligonucleotide primers based on ORF1 (5′-GCGTATCAACATGCAGTACAC-3′) and ORF2 (5′-CGGTCAATGAAGAAGCGAGACATG-3′), ORF2 (5′-CGGCGCAAGTGCAGGCAGTCAGCACTCCATT-3′), and ORF3 (5′-CAGATAATTGTCCACGCCCTCGTCG-3′), we obtained amplification products of 730 and 1,455 bp. These sizes were plausible based on the DNA sequence we determined. This confirmed the operon structure of the genes. Upstream of the first ORF, a stretch of about 1,500 bp was sequenced, but no evidence for the presence of other ORFs was found (we called this piece of DNA dut, for DNA upstream ttgD genes).
FIG. 1.
Physical map of the todST operon and the ttgDEF gene cluster in the solvent-tolerant P. putida DOT-T1E strain. Restriction sites for XhoI, HindIII, EcoRI, ClaI, KpnI, EcoRV, NheI, and XbaI are shown. The arrows indicate the direction of transcription.
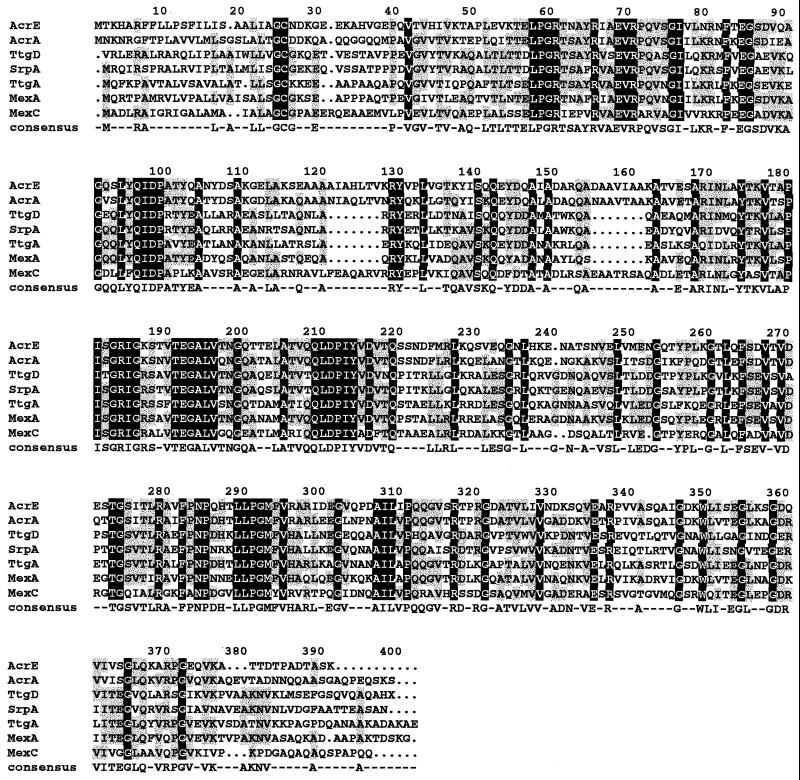
The three ORFs were translated in the corresponding polypeptides sequences, yielding putative peptides of 382, 1,048, and 480 amino acids. The polypeptide sequences were compared against sequences deposited in the data bank, and we found that the first, second, and third peptides exhibited homology to the periplasmic fusion protein, to the pump element of RND efflux pumps, and to the porin component of RND efflux pumps. The highest homologies were with the SrpABC system of P. putida S12 (75% identity, 88% similarity) (17) and with the TtgABC pump of P. putida DOT-T1E (59% identity, 77% similarity) (33); for this reason the putative new pump of P. putida DOT-T1E was named TtgDEF, and the genes were named ttgDEF. Lower homology was found with the efflux pumps of P. aeruginosa mexEFoprN (44% identity) (21) and the of E. coli acrAB (45% identity) and acrEF (43% identity) (3, 9, 24). The homology between these efflux pumps was further confirmed when multiple alignments of the pump elements were performed. As an example, an alignment of the periplasmic fusion TtgD protein with homologous proteins (SrpA, TtgA, MexA, AcrE, AcrA, and MexC) is shown in Fig. 2.
FIG. 2.
Sequence alignment of TtgD protein with homologues. The sources of protein sequences were as follows: P. putida SrpA (17), P. putida TtgA (33), P. aeruginosa MexA (30, 31), E. coli AcrE (20), E. coli AcrA (24), and P. aeruginosa MexC (29). The ALIGN program was used (2). If the residue was identical to all the aligned proteins it appears against a black background. If the residue was identical to at least 51% of the aligned proteins, it appears against a gray background. A residue was chosen for the consensus if it appeared in at least four of the seven aligned proteins.
Transcription of the ttgDEF cluster takes place in response to solvents in the culture medium.
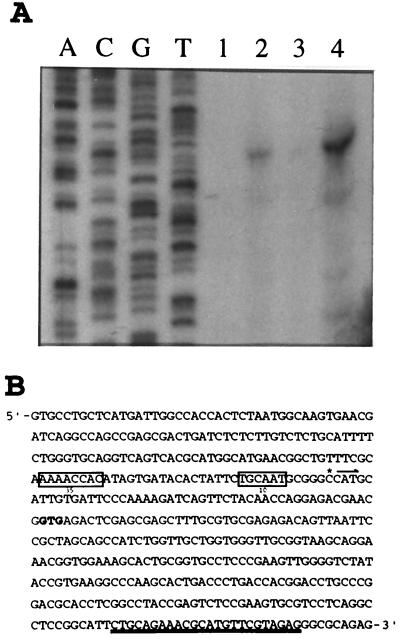
To determine the pattern of expression of the ttgDEF genes in response to solvents, we isolated total RNA from P. putida DOT-T1E cultures growing exponentially in the absence and in the presence of the aromatic hydrocarbons toluene, styrene, and m-xylene. Equal amounts of mRNA were used in primer extension analyses, which revealed that transcription occurred only in the presence of toluene and styrene. The transcription initiation point in both cases was the same and was located 50 bp upstream from the G of the first GTG—the start codon—of ttgD (Fig. 3).
FIG. 3.
Determination of the transcription initiation site of the ttgDEF operon. (A) Total RNA was isolated from P. putida DOT-T1E cells grown on succinic acid (lane 1) or with succinic acid in the presence the aromatic hydrocarbon (supplied via the gas phase toluene [lane 2], m-xylene [lane 3], or styrene [lane 4]). Then primer extension was done as described in Materials and Methods. The figure shows the cDNA (296 nucleotides) obtained after reverse transcription of 20 μg of total RNA with an oligonucleotide complementary to ttgD. A DNA sequencinqg ladder is also shown. (B) DNA sequence of the ttgD promoter region. The transcription initiation point is indicated by an asterisk followed by an arrow which shows the direction of transcription: the −10 and −35 sequences are boxed; the first GTG of ttgD is shown in boldface, and the complementary sequence of the oligonucleotide used for primer extension of ttgD is double underlined.
Construction of a ttgDEF null mutant of P. putida DOT-T1E by gene replacement.
To assign the TtgDEF proteins a possible role in solvent tolerance, we decided to inactivate the cluster via site-directed mutagenesis with the mobilizable suicide plasmid pKNG6-11 (Table 1). Plasmid pT1-B6 was digested with XhoI and a 2.5-kb fragment comprising part of the ttgD gene, and its upstream region was removed and replaced with a 3.0-kb cassette encoding tellurite resistance (37). The resulting plasmid was called pT1-B611. Then, a 7-kb BamHI fragment of pT1-B611 bearing the tellurite cassette flanked on one side by “ttgD and ttgE” and on the other side by ca. 2 kb of P. putida DNA, which we have called dut, was cloned into the single BamHI site of pKNG101 to yield pKNG6-11. Plasmid pKNG6-11 was used to deliver the dut::telAB::'ttgD ttgE' mutation to the host chromosome via homologous recombination. This plasmid has the advantage of containing the streptomycin resistance gene (Sm) as a selectable marker for the cointegration event and the Bacillus subtilis sacB gene as a counterselectable marker to enhance the second step, i.e., allelic exchange (16, 35).
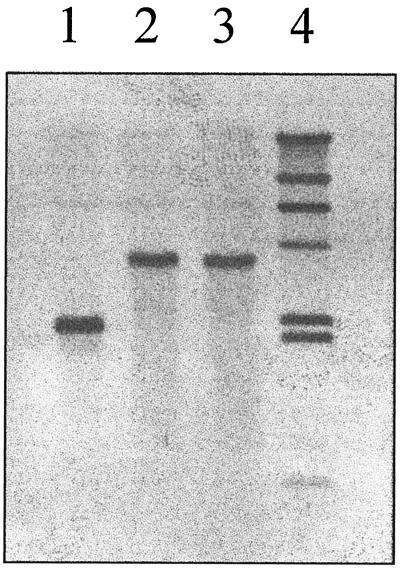
After triparental mating as described in Materials and Methods, transconjugants of P. putida DOT-T1E that were Sm appeared at a rate of 10−5 per recipient cell. A sucrose-sensitive clone was retained for further study. Upon repetitive growth of the merodiploid at 30°C in LB medium, cells were spread on LB plates with 5% (wt/vol) sucrose. The second crossover event was expected to result in the acquisition of tolerance to sucrose and in the loss of the Sm character. We searched for Sm clones among sucrose-tolerant colonies; one of these clones was retained for further study. The second crossover event was confirmed by hybridization (Fig. 4). This mutant strain was called P. putida DOT-T1E-1.
FIG. 4.
Replacement of the chromosomal ttgD by a knockout ttgD::telAB cassette. Total DNA was digested with EcoRI. Lane 4, lambda HindIII markers; lane 1, wild-type P. putida DOT-T1E; lane 2, DNA from a resolve clone of DOT-T1E-18 that was Sm Suc Tel; lane 3, DNA from a resolve clone of DOT-T1E-18 that was Sms, Sucr, Telr. The DNA probe was the ttgD gene randomly labeled with digoxigenin-dUTP and the digoxigenin-dUTP hybrid DNA in the Southern membrane was detected by using an enzyme immunoassay according to manufacturer's instructions (Boehringer Mannheim).
Phenotypic analysis of the ttgDEF null mutant of P. putida DOT-T1E.
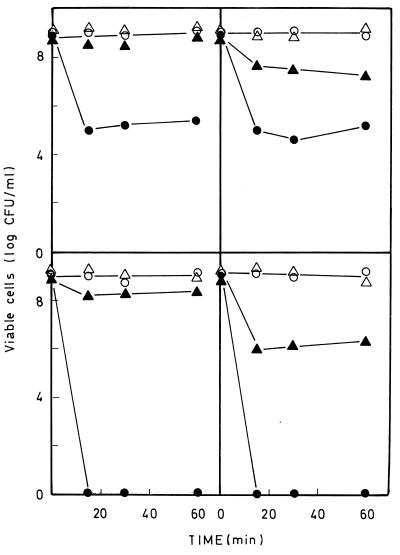
To determine the possible role of the ttgDEF gene products in solvent tolerance, wild-type DOT-T1E and mutant DOT-T1E-1 bacterial cells were grown overnight on LB medium with or without toluene supplied via the gas phase. Cells were then diluted and allowed to grow exponentially under the same culture conditions. When the turbidity of the cultures at 660 nm was ca. 1, the cultures were challenged by adding toluene to a 0.3% (vol/vol) concentration in the liquid medium. The results obtained are shown in Fig. 5. Upon the addition of toluene, a similar fraction (ca. 10−4) of the wild-type and mutant cells survived the toluene shock. In cultures preinduced with toluene through the gas phase, almost 100% of the wild-type cells survived the toluene shock, whereas only about 1% of the mutant cells remained viable (Fig. 5).
FIG. 5.
Survival in response to toluene shocks of the wild-type P. putida DOT-T1E and mutant derivatives lacking toluene efflux pumps. Cells were grown in 30 ml of LB medium alone (circles) or LB medium with toluene in the gas phase (triangles) until the culture reached a turbidity of about 1 at 660 nm. These cultures contained about 109 CFU/ml. The cultures were divided in two halves; to one we added (0.3% [vol/vol]) toluene (solid symbols), and the other was kept as a control (open symbols). The number of viable cells was kept as a control (open symbols). The number of viable cells was determined before toluene was added and 15, 30, and 60 min later. Top left panel, P. putida DOT-T1E; top right panel, P. putida DOT-T1E-1; bottom left panel, P. putida DOT-T1E-18; bottom right panel, P. putida DOT-T1E-82.
Because a number of efflux pumps are involved in antibiotic efflux, we studied the behavior of the wild-type and mutant DOT-T1E-1 on LB plates with toluene in the gas phase to induce the ttgDEF pump. The plates were supplemented with different antibiotics supplied in a disk (cefotoxime, 30 μg, ciprofloxamine, 15 μg; gentamicin, 10 μg; kanamycin, 30 μg; nalidixic acid, 30 μg; neomicin, 30 μg; piperacillin, 100 μg; and tetracycline, 30 μg), and the halo of growth inhibition was measured. The ttgD mutant strain was as sensitive as the wild type to the antibiotics, suggesting that this efflux pump is probably not involved in the efflux of these antibiotics.
Construction of a double ttgABC ttgDEF null mutant and its phenotypic analysis.
To further elucidate the role of the different solvent efflux pumps in tolerance to toluene in P. putida DOT-T1E, we decided to explore the phenotype of a mutant lacking both the constitutive ttgABC pump genes and the ttgDEF pump genes. To this end, we took advantage of the fact that after random Tn5 mutagenesis we had isolated a mutant that exhibited an insertion within the ttgABC genes (33). This mutant was more sensitive than the wild type to toluene shocks under both noninduced and induced conditions (33; see also Fig. 5). We transferred the dut::telAB::'ttgD ttgE' mutation into the chromosome of the ttgABC mutant as described above for the wild-type strain. A mutant clone called P. putida DOT-T1E-82 was obtained and challenged with toluene. It was found that under noninduced conditions, mutant DOT-T1E-82 cells were nonviable when exposed to a toluene shock of 0.3% (vol/vol) (Fig. 5). When DOT-T1E-82 cells preexposed to toluene were subjected to toluene shock, a significant fraction of the mutant cells (0.01 to 0.1%) survived (Fig. 5).
Location of the ttgDEF genes in Pseudomonas strains bearing the tod pathway.
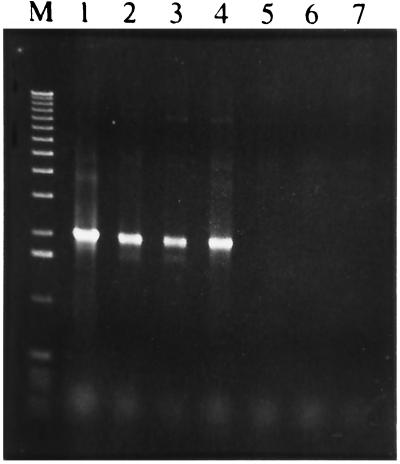
Huertas et al. (M. J. Huertas, E. Duque, R. Roselló-Mora, G. Mosqueda, P. Godoy, B. Christensen, S. Molin, and J. L. Ramos, submitted for publication) have characterized a number of Pseudomonas strains that grow on toluene via the toluene dioxygenase pathway encoded by the tod genes. They found that some of these strains (e.g., P. putida SMO116) were toluene sensitive, whereas others were moderately tolerant to toluene (e.g., P. putida F1) or able to tolerate high toluene concentrations (e.g., P. putida MTB6). We used oligonucleotide primers designed based on the 3′ end of todT (5′-CTGGTTCGAGTAACTGAGCGGCTCAAGATAGC CT-3′) and on the end of the 3′ end of ttgF (5′-CGGCGCAAGTGCAGGCAGTCAGCACTCCATT-3′) (note that the genes are divergently organized; see Fig. 1) to see if we could amplify the intervening DNA fragment by using total DNA from the strains referred to above. As a control we used P. putida DOT-T1E. We always found a 2.0-kb amplified DNA fragment (Fig. 6), which suggests that the todST operon and the ttgDEF operon had the same organization in these strains. When the same amplification was done with strains lacking the tod pathway (e.g., P. putida KT2440, P. aeruginosa PAO1, P. mendocina KR1, and E. coli), no amplification was found. Because these strains lack the todT gene, the absence of amplification may reflect not only the absence of the ttgDEF genes but also the lack of sequences homologous to todT near ttgF. For this reason we designed primers based on the ttgD, ttgE, and ttgF sequences (see Materials and Methods), which were expected to result in DNA fragments of 0.8, 1.2, and 1.4 kb. No amplification products were found when DNA prepared from P. putida KT2440, P. aeruginosa PAO1, or P. mendocina KR1 was used, whereas amplification of P. putida DOT-T1E, P. putida F1, and P. putida SMO116 yielded the expected fragments (not shown).
FIG. 6.
PCR amplification of chromosomal DNA of Pseudomonas strains that use toluene as the sole carbon source. The primers used for amplification and the conditions used are given in the text. Lane 1, P. putida DOT-T1E; lane 2, P. putida F1; lane 3, P. putida SMO116; lane 4, P. putida MTB6; lane 5, P. putida KT2440; lane 6, P. aeruginosa PAO1; lane 7, P. mendocina KR1; lane M, Boehringer Mannheim DNA marker X.
DISCUSSION
Operon structure of the ttgDEF genes and linkage to the tod genes.
Our results show that adjacent to the tod genes for toluene degradation is a set of toluene efflux genes, which we have called ttgDEF. The physical organization of the tod and the ttgDEF genes seems to be maintained in a number of strains isolated in different countries around the world. It is possible that in Pseudomonas spp. the tod genes for the degradation of toluene via the toluene dioxygenase pathway and the ttgDEF genes, which encode toluene efflux system, have coevolved to confer increased tolerance to toluene. Nonetheless, it should be noted that among tod-proficient strains different levels of tolerance to toluene have been found, and no explanation for this differential behavior has been offered to date.
The ttgDEF genes in P. putida DOT-T1E seem to be organized as an operon. This conclusion is based on (i) the identification of a single transcription initiation point upstream from ttgD, (ii) the overlapping structure of the ttgE and ttgF genes, and (iii) the positive results in RT-PCR based on primers designed on the basis of the ttgD and ttgE DNA sequences and the ttgE and ttgF DNA sequences.
ttgD promoter.
Expression of the ttgDEF genes seems to be under positive regulation in response to the presence of aromatic hydrocarbons e.g., toluene and styrene in the culture medium (see Fig. 3). The main transcription initiation point was identified by primer extension analysis. The sequence upstream from the transcription initiation point of ttgD was analyzed for similarity with consensus promoter sequences recognized by RNA-polymerase with different sigma factors. In general, no significant homology in the −10 and the −35 regions of this promoter was found with regard to consensus sequences (25). The todXFC1C2BADEGIH genes form an operon whose transcription is mediated by the TodT protein in response to aromatic hydrocarbons (22). The PtodX promoter is made up of three regions, the −10/−35 region, an AT-rich region upstream from −40 (about 66% AT), and an inverted ATAAAGTTTAT motif at around −110 that represents the TodT binding site (22). Sequence alignment of PtodX and PttgD revealed no significant sequence conservation. This suggests that the regulators of these two promoters are likely distinct.
Distinct profile for antibiotic efflux of TtgABC and TtgDEF pumps.
The TtgABC efflux pump of P. putida, the MexAB-OprM pump of P. aeruginosa and the AcrAB-TolC pump of E. coli all remove antibiotics and aromatic hydrocarbons (3, 23, 33). The TtgDEF pump seems not to remove antibiotics such as cefotoxime, ciprofloxamine, gentamicin, kanamycin, nalidixic acid, piperacillin, and tetracycline because the wild type and the ttgD mutant were equally sensitive to these antibiotics. This suggests that the TtgDEF efflux system is more restricted in substrate specificity than the TtgABC, MexAB-OprM and AcrAB-TolC pumps. According to Isken and de Bont (15), the SrpABC pump of P. putida S12, which shows the greatest homology with the TtgDEF pump, expels aromatic hydrocarbons but not antibiotics. It thus seems that within the RND family of solvent efflux pumps, two subfamilies can be distinguished based on their ability to efflux certain antibiotics.
Role of the TtgDEF pump in toluene tolerance.
We have shown that the TtgDEF pump is inducible by toluene and styrene. The mutant strain P. putida DOT-T1E-1, which lacks this pump as a result of a knockout by gene replacement, is as tolerant as the wild-type strain to sudden toluene shock (Fig. 5). This is probably because the constitutive TtgABC pump is functional in the mutant strain. However, the ttgDEF mutant strain DOT-T1E-1 is less tolerant than the wild type to this aromatic hydrocarbon under induced conditions, where only 1% of the mutant bacterial cell population survived sudden toluene shock, in contrast to the almost 100% survival of P. putida DOT-T1E wild-type cells (Fig. 5). This is unequivocal evidence that the TtgDEF pump plays a key role in solvent tolerance.
The number of DOT-T1E-1 cells that tolerate sudden toluene shock once they have been exposed to toluene via the gas phase is higher than the number of cells that survive this shock if they have not been exposed to toluene before (Fig. 5). We interpret this to mean that another inducible pump in addition to TtgDEF may still operate in solvent extrusion. A homologous nonidentical inducible pump, called SrpABC, has been described in P. putida S12 (17, 18). If such a pump is also present in P. putida DOT-T1E, it would further explain how this strain finds ways to escape death when exposed to saturating concentrations of highly toxic organic solvents. It should also be noted that in P. aeruginosa, up to three efflux pumps for antibiotic removal (MexAB-OprM, MexCD-OprJ, and MexEF-OprN) have been described, with all three of them contributing to solvent tolerance (23).
A double mutant lacking the TtgABC and TtgDEF pumps has a phenotype worthy of detailed analysis: noninduced cells of the double mutant were nonviable when subjected to sudden toluene shock, as expected since the parental DOT-T1E-18 mutant lacking the TtgABC system was also unable to tolerate sudden toluene shock (Fig. 5). However, the double mutant was more tolerant to toluene shock under induced conditions than we had initially anticipated, with about 0.1 to 0.01% cells surviving the shock. This again suggests that another inducible pump may also operate in P. putida DOT-T1E to ensure toluene tolerance.
In summary, P. putida DOT-T1E is an unusual microorganism in that it is able to tolerate shocks of highly toxic compounds such as toluene. This seems to be achieved through the controlled expression of a number of energy-dependent efflux pumps, two of which have been characterized so far.
ACKNOWLEDGMENTS
This study was supported by grants from the Comisión Interministerial de Ciencia y Tecnología (BIO97-0641) and the European Commission (BIO4-CT98-0283). Gilberto Mosqueda was the recipient of a grant from the Agencia de Cooperación Iberoamericana of the Ministerio de Educación in Spain.
We thank Estrella Duque for assistance with solvent shock assays. We thank Maribel Ramos-González for critical reading of the manuscript.
REFERENCES
- 1.Abril M A, Michán C, Ramos J L. Regulator and enzyme specificities of the TOL plasmid-encoded upper pathway for degradation of aromatic hydrocarbons and expansion of the substrate range of the pathway. J Bacteriol. 1989;171:6782–6790. doi: 10.1128/jb.171.12.6782-6790.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Aono R, Tsukagoshi N, Yamamoto M. Involvement of outer membrane protein TolC, a possible member of the mar-sox regulon, in maintenance and improvement of organic solvent tolerance of Escherichia coli K-12. J Bacteriol. 1998;180:938–944. doi: 10.1128/jb.180.4.938-944.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1991. [Google Scholar]
- 5.Bolhuis H, van Veen H W, Poolman B, Driessen A J M, Konings W N. Mechanisms of multidrug transporters. FEMS Microbiol Rev. 1997;21:55–84. doi: 10.1111/j.1574-6976.1997.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen Q, Janssen D B, Witholt B. Growth on octane alters the membrane lipid fatty acids of Pseudomonas oleovorans due to the induction of alkB and synthesis of octanol. J Bacteriol. 1995;177:6894–6901. doi: 10.1128/jb.177.23.6894-6901.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruden D L, Wolfram J H, Rogers R D, Gibson D T. Physiological properties of a Pseudomonas strain which grows with p-xylene in a two-phase (organic-aqueous) medium. Appl Environ Microbiol. 1992;58:2723–2729. doi: 10.1128/aem.58.9.2723-2729.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Smet M J, Kingma J, Witholt B. The effect of toluene on the structure and permeability of the outer and cytoplasmic membranes of Escherichia coli. Biochim Biophys Acta. 1978;506:64–80. doi: 10.1016/0005-2736(78)90435-2. [DOI] [PubMed] [Google Scholar]
- 9.Fralick J A. Evidence that TolC is required for functioning of the Mar/AcrAB efflux pump of Escherichia coli. J Bacteriol. 1996;178:5803–5805. doi: 10.1128/jb.178.19.5803-5805.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heipieper H-J, Diefenbach R, Keweloh H. Conversion of cis unsaturated fatty acids to trans, a possible mechanism for the protection of phenol-degrading Pseudomonas putida P8 from substrate toxicity. Appl Environ Microbiol. 1992;58:1847–1852. doi: 10.1128/aem.58.6.1847-1852.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrero M, de Lorenzo V, Timmis K N. Transposon vectors containing non-antibiotic selection markers for cloning and stable chromosomal insertion of foreign DNA in gram-negative bacteria. J Bacteriol. 1990;172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inoue A, Horikoshi K. A Pseudomonas thrives in high concentration of toluene. Nature. 1989;338:264–265. [Google Scholar]
- 13.Inoue A, Nojima H, Okayama H. High-efficiency transformation of Escherichia coli with plasmid. Gene. 1990;96:23–28. doi: 10.1016/0378-1119(90)90336-p. [DOI] [PubMed] [Google Scholar]
- 14.Isken S, de Bont J A M. Active efflux of toluene in a solvent-resistant bacterium. J Bacteriol. 1996;178:6056–6058. doi: 10.1128/jb.178.20.6056-6058.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isken S, de Bont J A M. Bacteria tolerant to organic solvents. Extremophiles. 1998;3:229–238. doi: 10.1007/s007920050065. [DOI] [PubMed] [Google Scholar]
- 16.Kaniga K, Delor I, Cornerlis G R. A wide-host-range suicide vector for improving reverse genetics in gram-negative: inactivation of the blaA gene of Yersinia criteoli. Gene. 1991;109:137–141. doi: 10.1016/0378-1119(91)90599-7. [DOI] [PubMed] [Google Scholar]
- 17.Kieboom J, Dennis J J, de Bont J A M, Zylstra G J. Identification and molecular characterization of an efflux pump involved in Pseudomonas putida S12 solvent tolerance. J Biol Chem. 1998;273:85–91. doi: 10.1074/jbc.273.1.85. [DOI] [PubMed] [Google Scholar]
- 18.Kieboom J, Dennis J J, Zylstra G J, de Bont J A M. Active efflux of organic solvents by Pseudomonas putida S12 is induced by solvents. J Bacteriol. 1998;180:6769–6772. doi: 10.1128/jb.180.24.6769-6772.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim K, Lee S, Lee K, Lim D. Isolation and characterization of toluene-sensitive mutants from toluene-resistant bacterium Pseudomonas putida GM73. J Bacteriol. 1998;180:3692–3696. doi: 10.1128/jb.180.14.3692-3696.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein J, Henrich B, Plapp R. Molecular cloning of the envC gene of Escherichia coli. Curr Microbiol. 1990;21:341–347. [Google Scholar]
- 21.Köhler T, Michéa-Hamzehpour M, Henze U, Gotoh N, Curty L K, Pechère J-C. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol Microbiol. 1997;23:343–354. doi: 10.1046/j.1365-2958.1997.2281594.x. [DOI] [PubMed] [Google Scholar]
- 22.Lau P C K, Wang Y, Patel A, Labbé D, Bergeron H, Brousseau R, Konishi K, Pawlings M. The bacterial basic region leucine zipper histidine kinase regulating toluene degradation. Proc Natl Acad Sci USA. 1997;94:1453–1458. doi: 10.1073/pnas.94.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X-Z, Poole K. Organic solvent-tolerant mutants of Pseudomonas aeruginosa display multiple antibiotic resistance. J Bacteriol. 1998;180:2987–2991. doi: 10.1139/cjm-45-1-18. [DOI] [PubMed] [Google Scholar]
- 24.Ma D, Cook D N, Alberti M, Pon N G, Nikaido H, Hearst J E. Molecular cloning and characterization of acrA and acrE genes of Escherichia coli. J Bacteriol. 1993;175:6299–6313. doi: 10.1128/jb.175.19.6299-6313.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marqués S, Manzanera M, González-Pérez M M, Gallegos M T, Ramos J L. The XylS-dependent Pm promoter is transcribed in vivo by RNA polymerase with ς54 or ς38 depending on the rowth phase. Mol Microbiol. 1999;31:1105–1113. doi: 10.1046/j.1365-2958.1999.01249.x. [DOI] [PubMed] [Google Scholar]
- 26.Marqués S, Ramos J L, Timmis K N. Analysis of the mRNA structure of the Pseudomonas putida TOL meta fission pathway operon around the transcription initiation point, the xylTE and the xylFJ region. Biochim Biophys Acta. 1993;1216:227–236. doi: 10.1016/0167-4781(93)90149-8. [DOI] [PubMed] [Google Scholar]
- 27.Mosqueda G, Ramos-González M I, Ramos J L. Toluene metabolism by the solvent-tolerant Pseudomonas putida DOT-T1 strain, and its role in solvent impermeabilization. Gene. 1999;232:69–76. doi: 10.1016/s0378-1119(99)00113-4. [DOI] [PubMed] [Google Scholar]
- 28.Paulsen I T, Brown M H, Skurray R A. Proton-dependent multidrug efflux systems. Microbiol Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poole K, Gotoh N, Tsujimoto H, Zhao Q, Wada A, Yamasaki T, Neshat S, Yamagishi J, Li X Z, Nishino T. Overexpression of the mexC-mexD-oprJ efflux operon in nfxB-type multidrug-resistant strains of Pseudomonas aeruginosa. Mol Microbiol. 1996;21:713–724. doi: 10.1046/j.1365-2958.1996.281397.x. [DOI] [PubMed] [Google Scholar]
- 30.Poole K, Heinrichs D E, Neshat S. Cloning and sequence analysis of an EnvCD homologue in Pseudomonas aeruginosa: regulation by iron and possible involvement in the secretion of the siderophore pyoverdine. Mol Microbiol. 1993;10:529–554. doi: 10.1111/j.1365-2958.1993.tb00925.x. [DOI] [PubMed] [Google Scholar]
- 31.Poole K, Krebes K, McNally C, Neshat S. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J Bacteriol. 1993;175:7363–7372. doi: 10.1128/jb.175.22.7363-7372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramos J L, Duque E, Huertas M J, Haïdour A. Isolation and expansion of the catabolic potential of a Pseudomonas putida strain able to grow in the presence of high concentrations of aromatic hydrocarbons. J Bacteriol. 1995;177:3911–3916. doi: 10.1128/jb.177.14.3911-3916.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramos J L, Duque E, Godoy P, Segura A. Efflux pumps involved in toluene tolerance in Pseudomonas putida DOT-T1E. J Bacteriol. 1998;180:3323–3329. doi: 10.1128/jb.180.13.3323-3329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramos J L, Duque E, Rodríguez-Herva J J, Godoy P, Haïdour A, Reyes F, Fernández-Barrero A. Mechanisms for solvent tolerance in bacteria. J Biol Chem. 1997;272:3887–3890. doi: 10.1074/jbc.272.7.3887. [DOI] [PubMed] [Google Scholar]
- 35.Rodríguez-Herva J J, Ramos J L. Characterization of an OprL null mutant of Pseudomonas putida. J Bacteriol. 1996;178:5836–5840. doi: 10.1128/jb.178.19.5836-5840.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 37.Sánchez-Romero J M, Díaz-Orejas R, de Lorenzo V. Resistance to tellurite as a selection marker for genetic manipulation of Pseudomonas strains. Appl Environ Microbiol. 1998;64:4010–4046. doi: 10.1128/aem.64.10.4040-4046.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Segura A, Duque E, Mosqueda G, Ramos J L, Junker F. Multiple responses of gram-negative bacteria to organic solvents. Environ Microbiol. 1999;1:191–198. doi: 10.1046/j.1462-2920.1999.00033.x. [DOI] [PubMed] [Google Scholar]
- 39.Sikkema J, de Bont J A M, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev. 1995;59:201–222. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weber F J, Ooijkaas L P, Schemen R M W, Hartmans S, de Bont J A M. Adaptation of Pseudomonas putida S12 to high concentrations of styrene and other organic solvents. Appl Environ Microbiol. 1993;59:3502–3504. doi: 10.1128/aem.59.10.3502-3504.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequence of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]