Abstract
The amygdala is a hub subcortical region that is crucial in a wide array of affective and motivation related behaviors. While early research contributed significantly to our understanding of this region’s extensive connections to other subcortical and cortical regions, recent methodological advances have enabled researchers to better understand the details of these circuits and their behavioral contributions. Much of this work has focused specifically on investigating the role of amygdala circuits in social cognition. In this chapter, we review both longstanding knowledge and novel research on the amygdala’s structure, function, and involvement in social cognition. We focus specifically on the amygdala’s circuits with the medial prefrontal cortex, the orbitofrontal cortex, and the hippocampus, as these regions share extensive anatomic and functional connections with the amygdala. Furthermore, we discuss how dysfunction in the amygdala may contribute to social deficits in clinical disorders including Autism Spectrum Disorder, Social Anxiety Disorder, and Williams Syndrome. We conclude that social functions mediated by the amygdala is orchestrated through multiple intricate interactions between the amygdala and its interconnected brain regions, endorsing the importance of understanding the amygdala from network perspectives.
Keywords: Amygdala, social behavior, network, non-human primates, social dysfunction, anatomic connectivity, functional connectivity
Introduction
The amygdala is a limbic structure consisting of a collection of nuclei and located in the temporal lobe. It has extensive and reciprocal connections with a variety of cortical and subcortical regions (Amaral and Price, 1984; Mcdonald, 1998; Sah et al., 2003) and is thus involved in a wide diversity of behavioral processes.
Across species, amygdala subregions each receive projections from and send projections to distinct combinations of cortical and subcortical regions (Sah et al., 2003). The amygdala consists of groups of nuclei, which are often clustered into two large subregions. These subregions are the centromedial amygdala (CM), a more superficial cluster consisting of the cortical, medial, and central nuclei, and the basolateral amygdala (BLA), a deeper cluster consisting of the basal, lateral, and accessory basal nuclei (Mcdonald, 2014). Each of these nuclei have distinct anatomic connections and, subsequently, distinct functions. For instance, the central nucleus (Ce) is heavily connected to the periaqueductal gray and other brainstem regions enabling it to play a large role in associative learning behaviors such as fear conditioning (Cassell et al., 1986; Rizvi et al., 1991). By contrast, the BLA sends far-reaching projections to a wide array of cortical and subcortical regions including the prefrontal, ventral hippocampus, and nucleus accumbens (NAcc) (Mcdonald, 1998). This structural connectivity enables this subdivision to coordinate adaptive behaviors in response to both internal and external stimuli. Through its extensive connections with cortical and subcortical regions, the BLA contributes to a variety of higher-level cognitive processes, and thus is heavily studied in non-human primate research.
Much of the anatomic detail of the amygdala’s interareal connectivity was mapped out in early anatomic studies using anterograde and retrograde tracers in rats, macaques, and other mammals. However, the ability to uncover details of these structural connections was often limited due to methodological constraints. Methodological advances including increased efficacy and precision of tracer studies in animals and vastly improved neuroimaging techniques in humans have allowed for better understanding of the anatomic connections to and from the amygdala. An example of improved neuroimaging techniques is the recent work conducted with high strength 7 Tesla MRI scans, which has been used to demonstrate unique relationships between distinct amygdala nuclei volumes and Major Depressive Disorder symptoms. Thus, in using this high- powered MRI scanner, the authors demonstrated its suitability for conducting research of this type and also the importance of appreciating the amygdala as a unit comprised of distinct and functionally different subnuclei (Brown et al., 2019). In this chapter, we will discuss recent advances in our understanding of the amygdala’s anatomic connections to select cortical and subcortical regions.
Our understanding of the amygdala’s functional role in behavior was greatly informed by early lesion studies in animal models. Lesions of the temporal lobes, including the amygdala, brown (Brown and Sharpey-Schafer, 1888) resulted in changes in social behavior in macaques. Further lesions, this time selectively of the amygdala, resulted in behavioral changes including increased tameness in monkeys and alterations to association learning (Weiskrantz, 1956). Investigations in rodents implicated the amygdala in fear conditioning, a form of association learning in which an unconditioned stimulus, often a painful foot shock in rodent studies, is paired with a conditioned stimulus such as a tone or light (Blanchard and Blanchard, 1972; LeDoux et al., 1990). Studies of humans with damage to this area found deficits in emotion perception and processing (Adolphs et al., 1994; Anderson and Phelps, 2001). These early studies provided a foundational understanding of the amygdala’s role in association learning and affective processes.
This early foundational research enabled scientists to determine broad behavioral attributes of the amygdala, yet difficulties in controlling the size and precision of lesions as well as relatively limited knowledge about the subregions of the amygdala and connected structures restricted the conclusions that could be drawn from these studies. Advances in methodological techniques, such as optogenetic and chemogenetic manipulations, have progressed research into the amygdala and extended our understanding of its functional roles. These advancements have enabled a more specific understanding of the roles of the distinct amygdala subregions and networks in processing stimuli and producing behaviors. In this chapter, we will highlight early insights as well as recent advances in amygdala structure and function in relation to key cortical and subcortical regions.
Recent research has also focused on better understanding the amygdala’s role in social cognition. Initial lesion studies found that, across a variety of species, both neonatal and adult amygdala lesions led to deficits in the development and expression of social behaviors, demonstrating that the amygdala makes profound contributions to social cognition (Adolphs et al., 1994; Diergaarde et al., 2004; Machado and Bachevalier, 2006; Prather et al., 2001). Additionally, there has also been a focus on understanding the contributions of specific amygdala circuits to social cognition. In particular, prefrontal-amygdala circuits have been implicated in a variety of social behaviors including social perception, learning, and decision-making (Gangopadhyay et al., 2021), and amygdala-hippocampal projections have been linked to social memory and interaction behaviors (Hitti and Siegelbaum, 2014; Okuyama et al., 2016; Ortiz et al., 2019). Thus, in this chapter we will highlight recent advances in our understanding of the amygdala-mPFC, amygdala OFC, and amygdala-hippocampus circuits in social cognition.
As stated above, the amygdala is ideally suited to play a role in a wide variety of social processes, given its extensive and reciprocal connections across the brain (Amaral and Price, 1984; Mcdonald, 1998; Sah et al., 2003). Furthermore, it has been implicated in a wide variety of behavioral processes, including fear-related processes (Cassell et al., 1986; Rizvi et al., 1991) and social cognition (Adolphs et al., 1994; Diergaarde et al., 2004; Machado and Bachevalier, 2006; Prather et al., 2001). It is therefore unsurprising that disruption of the amygdala or the amygdala circuitry results in a variety of neuropsychiatric disorders.
Our initial understanding of the role of the amygdala came from patients with lesions of their amygdala. These patients, often presenting with bilateral ablations of the amygdala, showed impairments in social behavior that encompassed difficulties in recognizing and responding appropriately to social stimuli (Adolphs et al., 1994; Broks et al., 1998; Calder, 1996; Young et al., 1995). Interestingly, there were also indications that these individuals had some trouble with “theory of mind” when these lesions were acquired early in development; that is, they showed impairments in ascribing or understanding the emotional states and beliefs of others (Shaw et al., 2004). However, recent research in understanding the clinical significance of the amygdala and its connectivity has moved away from lesion studies. We will discuss several neuroimaging studies and what they tell us about the role of the amygdala in certain neuropsychiatric disorders.
Amygdala-Cortical Circuits
The extensive reach of the amygdala in a wide range of affective behavioral processes is rooted in its vast structural connections to a variety of cortical and subcortical regions. When examining amygdalo-cortical connections, the amygdala shows widespread connections to the frontal, insular, temporal, and occipital cortices (Amaral and Price, 1984). However, by far the densest projections exist between the amygdala and both the medial prefrontal cortex (mPFC) and the orbitofrontal cortex (OFC) (Amaral and Price, 1984). In particular, the BLA supplies dense projections to the prefrontal cortex (Carmichael and Price, 1995a). These connections, conserved across mammalian evolution (Öngür and Price, 2000), are bidirectional and show distinct organizational patterns (Carmichael and Price, 1995b; Ghashghaei and Barbas, 2002), suggesting that amygdala-mPFC and amygdala-OFC networks make unique contributions to affective processing. This is further supported by retrograde and anterograde tracer studies of macaque prefrontal cortex networks demonstrating that the OFC and mPFC regions show distinct intra- and inter-areal connectivity patterns from one another (Carmichael and Price, 1996).
Medial Prefrontal Cortex
Amygdala-mPFC Structure and Function
The mPFC is the cortical area with the most pronounced amygdala and general limbic connections (Carmichael and Price, 1995a; Kondo et al., 2005). Abundant evidence supports the functions of this region in decision-making and affective processing (Etkin et al., 2011; Euston et al., 2012). Due to its extensive outputs to regions critical to autonomic or visceral responses, including the hypothalamus and periaqueductal gray, the mPFC has long been considered to have a role in modulating autonomic responses related to emotions (Öngür et al., 1998; Price and Drevets, 2010). This is supported by vmPFC lesion studies in humans that result in deficits in typical autonomic responses to emotional stimuli (Damasio et al., 1990).
This region’s coordinated interplay with the amygdala has more recently been implicated in an array of social processes across humans, monkeys, and rodents (Gangopadhyay et al., 2021). Structural connections from the mPFC to the amygdala project primarily to the BLA (Mcdonald et al., 1996), and these BLA-mPFC projections have been implicated in fear (Senn et al., 2014) and anxiety (Felix-Ortiz et al., 2016). Interestingly, recent advances have helped delineate the topographical organization of these projections, some of which remained unclear due to methodological constraints of the earlier studies.
Advances in Amygdala-mPFC Structural Connectivity
Recent improvements in tracing methods have supplied researchers with more precise tools to dissect amygdala-mPFC connectivity. In one 2016 study, researchers used smaller, simultaneous retrograde tracer injections in rats to investigate the details of the structural organization of amygdalo-cortical networks, such as the amygdala-mPFC-lateral hypothalamus (LHA) network that is involved in cognitive control of motivated behaviors (Reppucci and Petrovich, 2016). They injected retrograde tracers in the dorsal anterior cingulate, prelimbic (PL), infralimbic area (ILA), or rostromedial orbital mPFC to determine the brain regions that projected to them. In addition to confirming prior studies showing that the mPFC received projections from the BLA but not the CeA, they found that these projections are topographically organized such that anterior BLA projected more strongly to the dorsal mPFC and posterior BLA projected to the ventral mPFC. These findings provided novel information about the topographical organization of these projections. Further, they found that the PL and ILA subregions of the mPFC showed the densest projections to the amygdala, also supporting prior studies (Hoover and Vertes, 2007). Importantly, these structural findings are consistent with functional research demonstrating that BLA inputs to pyramidal mPFC neurons in the PL and ILA influence reward and aversion based associative learning (Laviolette et al., 2005; Sotres-Bayon et al., 2012) as well as research demonstrating that BLA influences mPFC activity via inhibitory interneurons in associative learning (Chefer et al., 2011; Garcia et al., 1999). The topographic specificity of the projections between the BLA and the mPFC (Reppucci and Petrovich, 2016) implies the existence of distinct subnetworks driven by distinct neuronal populations and, thus, reveal the need for further investigation into the organization of the amygdala-mPFC projections and their associated functional roles.
Advances in Amygdala-mPFC Functional Connectivity
Much of the research investigating the amygdala-mPFC network in humans has focused on the directional dynamics of this network. Typically in adults, the extensive connections from the mPFC exert inhibitory control on the amygdala serving to regulate emotional expression (Motzkin et al., 2015; Quirk et al., 2003). However, as demonstrated in both humans and rodents, in negative states such as under stress or anxiety, the regulatory mechanisms of the mPFC become disrupted and amygdala output increases resulting in a shift towards a directional influence from the amygdala to the mPFC, providing a potential mechanism for stress-related increases in fear and anxiety behaviors (Correll et al., 2005; Rauch et al., 2006; Shin et al., 2004).
The amygdala-mPFC circuit undergoes both structural and functional connectivity (FC) changes during adolescence, at which point it matures into its adult-like state (Decety et al., 2012; Swartz et al., 2014), suggesting that various stages of pre-adolescent and adolescent development may be sensitive periods for the maturation of this circuit. This is bolstered by work demonstrating that this circuit is particularly sensitive to early life stressors (Gee et al., 2013; Yan et al., 2017). A recent study (Guadagno et al., 2018a) used disrupted maternal care to investigate the effects of early life stress on the development of amygdala-mPFC circuit FC in rats. Researchers limited mothers’ and their litter’s access to bedding, a paradigm that has previously been demonstrated to disrupt maternal care and has been validated as a chronic stressor (Guadagno et al., 2018b). When resting-state fMRI scans on preweaning and adult rats exposed to this stressor were performed, they found that chronic early life stress exerted distinct, lateralized effects on posterior and anterior BLA networks. Both chronically stressed preweaning and adult rats showed greater FC changes in the right than in the left BLA with the right anterior BLA showing reduced connectivity to the ipsilateral and contralateral IL and PL mPFC. In preweaning pups, the posterior BLA showed reduced FC to all of the mPFC except for increased FC to the contralateral IL. In adults, the posterior BLA showed increased FC to ipsilateral IL and PL but decreased FC to contralateral IL and PL. Chronically stressed adult rats also showed altered BLA FC to a wide array of other brain regions including midbrain, deep gray, hypothalamic, and hippocampal regions. Behaviorally, chronically stressed pups and adults displayed enhanced fear conditioning and reduced fear extinction. In summary, this study demonstrated that chronic early-life stress results in lateralized reductions of anterior BLA FC with the mPFC that appear in preweaning pups and persist into adulthood. These findings suggest that reduced anterior BLA-mPFC resting-state functional connectivity may underlie increased fear behaviors that result from exposure to chronic, early-life stress.
Amygdala-mPFC in Social Cognition
Recent research has focused on systems-level investigations of the networks involved in social cognition and has largely implicated various prefrontal-amygdala circuits (Gangopadhyay et al., 2021). In particular, the amygdala-mPFC circuit has been connected to a wide range of social behaviors including, social learning and decision-making, and specific subregions have been shown to contribute uniquely to these behaviors (Allsop et al., 2018; Dal Monte et al., 2020; Gangopadhyay et al., 2021). One such subregion, the anterior cingulate cortex (ACC), has been demonstrated to contribute to a broad array of cognitive processes and social behavior, perhaps by estimating motivations of others and updating those estimations based on incoming behavioral evidence (Apps et al., 2016). It should also be noted that the rostral ACC exhibits unique cytoarchitectonic and functional connectivity patterns compared to caudal ACC that supports more affective and social functions (Apps et al., 2016).
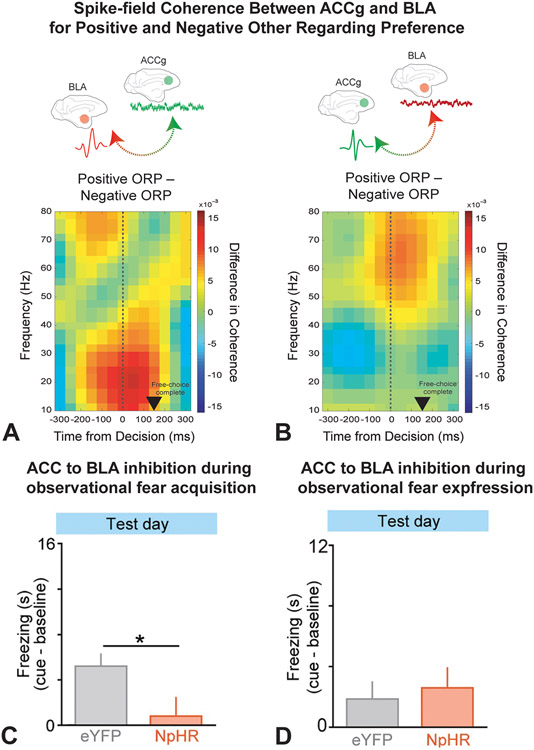
Recently, researchers recorded neurons from the BLA and ACC gyrus (ACCg) simultaneously in macaques as they performed a social reward allocation task with a partner monkey (Dal Monte et al., 2020). The macaques were presented with two trial types: 1) “self-reward” trials in which monkeys preferred to choose to deliver a reward to just to themselves over both themselves and the other monkey at the same time and 2) “other-reward” trials in which they preferred to choose to deliver a reward to the other monkey over disposing of the reward by having it dispensed into an empty bottle. The researchers analyzed spike-field coherence between the ACCg and BLA as well as directionality of information flow to investigate if there were specific interareal synchronization patterns associated with either positive or negative other regarding preferences (ORP). The results showed that positive ORP decisions – when the monkeys chose to deliver reward to the other monkey – and negative ORP decisions – when monkeys chose to give themselves but not the other monkey a reward – were associated with different interareal synchrony patterns that were specific to certain frequency bands. In the beta frequency range, BLA spikes and ACCg local field potentials (LFPs) showed enhanced coherence during positive ORP but decreased coherence during negative ORP (Fig. 1A). Similarly, in the gamma frequency range, ACCg spikes and BLA LFPs showed enhanced coherence during positive ORP and suppressed coherence during negative ORP (Fig. 1B). Additionally, a partial directed coherence analysis, undertaken to investigate the direction of information flow between the ACCg and BLA, revealed that the BLA generally influenced the ACCg for positive ORP outcomes for both beta and gamma bands while the ACCg generally influenced the BLA for negative ORP outcomes. These results suggest that the ACCg and BLA influence social decision-making behaviors through frequency-specific and direction-selective coordination, with the BLA engaging the ACCg specifically for positive ORP decisions. Further, these results suggest that communication directionality in the amygdala-mPFC circuit may show differential behavioral effects across multiple domains including social, as shown here, and stress-related, as previously discussed, processes.
Figure 1. ACC-BLA Circuits in Social Cognition.
A–B: Dal Monte et al., 2020. A: Differences in BLA spike to ACCg LFP coherence between positive and negative ORP over time. BLA spikes and ACCg LFPs showed increased coherence in the beta frequency range for positive ORP and decreased coherence in the beta frequency band for negative ORP. B: Differences in ACCg spike to BLA LFP coherence between positive and negative ORP over time. ACCg spikes and BLA LFPs showed increased coherence in the gamma frequency range for positive ORP and decreased coherence in the gamma frequency range for negative ORP. C–D: Allsop et al., 2018. ACC→ BLA inhibition during observational fear acquisition. C: When ACC→ BLA neurons were inhibited with halorhodopsin (NpHR) during observational acquisition, NpHR mice showed significantly decreased freezing to the cue compared to control mice (eYFP) on test day. D: When ACC→ BLA neurons were inhibited with NpHR during observational fear expression, there was no significant difference between freezing behavior in control (eYFP) and NpHR mice on test day.
Complementing the above results, Allsop and colleagues demonstrated that ACC neurons projecting to the BLA are critical for observational social learning. They performed single-unit electrophysiology recordings in the ACC or BLA of mice undergoing an observational learning paradigm (Allsop et al., 2018) in which a mouse acquired fear conditioning by only being exposed to a conspecific receiving a series of foot shocks paired with a predictive cue. Not only did both ACC and BLA neurons respond to observational fear acquisition, but ACC neurons that projected to the BLA showed a greater activity response to the predictive cue. Further, inhibition of these ACC neurons affected the BLA neurons’ responses to the predictive cue and impaired the mice’s ability to learn from observation but not their ability to express behaviors related to formerly observed associations (Fig. 1C-D). Thus, the BLA-projecting ACC neurons appear to play a crucial role in the acquisition of observation-based learned associations by encoding and transmitting socially observed cue information. Lastly, the researchers demonstrated that ACC inputs to the BLA are involved in more ethologically relevant observation-based social behaviors thus strengthening the results. These results are consistent with, and extend, former research implicating the ACC and amygdala in both observational learning as well as broader social cognition (Basile et al., 2020; Chang et al., 2015; Jeon et al., 2010; Olsson et al., 2007).
Orbitofrontal Cortex:
Amygdala-OFC Structure and Function
The OFC is well known to be involved in computations for reward prediction error (Schultz et al., 1997), encoding value (Padoa-Schioppa and Assad, 2006), and reinforcement learning (Rudebeck and Murray, 2008). The OFC possesses dense connections with the amygdala (Amaral and Price, 1984) and the OFC-amygdala circuit is believed to be critical for goal directed behaviors (Sharpe and Schoenbaum, 2016). Macaque studies have demonstrated bidirectional projections between the amygdala and the OFC (Ghashghaei and Barbas, 2002) with connections from the amygdala to the medial, lateral, and posterior regions of the OFC (Carmichael and Price, 1995a). Additionally, the amygdala-OFC circuit shows distinct connectivity patterns between subregions. While the medial amygdala sends dense projections to the posterior OFC, the basolateral amygdala sends its densest projections to both the posterior and medial OFC (Carmichael and Price, 1995a). Studies demonstrating the involvement of different amygdala nuclei in distinct behaviors suggest that the subregion-specific connectivity may contribute uniquely to different behaviors yang (Yang and Wang, 2017).
Moreover, the existence of a the uncinate fasciculus (UF), a white matter tract connecting the OFC and the medial temporal lobe, supports the importance of efficient communication between these regions. While there has been disagreement regarding whether this tract actually projects to the amygdala specifically, more recent studies in macaques have demonstrated substantial connections between the OFC and the amygdala that are considered to be part of the UF (Von Der Heide et al., 2013). Further, recent studies in macaques (Abivardi and Bach, 2017) have demonstrated that the dense projections previously found between the OFC and BLA (Ghashghaei and Barbas, 2002) are part of the UF, providing additional support for the existence of direct connections between these two regions. Additionally, there is research showing that the UF white matter tract is highly conserved across macaques and humans (Thiebaut de Schotten et al., 2012). FC studies in humans have shown distinct activity correlation patterns between the medial and lateral OFC and the amygdala (Zald et al., 2014), supporting the existence of similar amygdala-OFC connectivity as in macaques.
The amygdala-OFC circuit is involved in processes critical for processing sensory stimuli, predicting outcomes, and conducting goal directed behaviors (Padoa-Schioppa and Assad, 2006; Rudebeck and Murray, 2008; Schultz et al., 1997; Sharpe and Schoenbaum, 2016). The BLA and OFC both encode the relationship between cue and outcome during associative learning, but they also make unique contributions to the process. BLA neurons are tuned to the magnitude of reward or punishment predicted by a cue (Belova et al., 2008), and to cue-predicted outcomes relative to background reward rates (Bermudez and Schultz, 2010). Furthermore, amygdala neurons show rapid reversal of cue preference related activity in response to reward contingency reversal (Belova et al., 2008), suggesting that amygdala encodes outcome value. OFC neurons encode a broad range of outcome-related variables, including the predictive values of cues, the value of the offered options, and value that is independent of the type and amount of reward (Padoa-Schioppa and Assad, 2006). These studies and subsequent research in macaques and humans (Elliott et al., 2008; Hosokawa et al., 2007) suggest that OFC neurons encode the relative rather than absolute values of rewards. After reversal, the subset of OFC neurons that responded to the old contingencies is replaced by a new populations of OFC neurons that respond to the new contingencies (Sharpe and Schoenbaum, 2016). Additional research has also demonstrated that OFC neurons strongly represent chosen option value, reward prediction error, past choices, and outcomes across multiple trials (Sul et al., 2010). Thus, researchers have proposed that the amygdala is critically involved in encoding and updating associations between predictive cues and outcomes while the OFC, after receiving this information from the amygdala, is able to form a broad network of associations based on experiences and under different conditions (Sharpe and Schoenbaum, 2016). Building upon these findings, a recent focus has been on better understanding the specific functional contributions of distinct OFC circuits, including bidirectional projections between the amygdala and OFC.
Advances in Amygdala-OFC Structural Connectivity
As previously discussed, research in animals has demonstrated that the distinct amygdala subregions show unique structural connectivity patterns (Carmichael and Price, 1995a). As seen for the amygdala-mPFC circuit, researchers have capitalized on methodological and technological advances to conduct more precise investigations on the anatomic connectivity of the amygdala-OFC circuit.
A recent study used probabilistic tractography to more precisely delineate reciprocal connections between the OFC and the distinct subregions of the amygdala (Matyi and Spielberg, 2020). Probabilistic tractography is a technique that enables researchers to infer the orientation and distributions of white matter tracts by estimating their paths from voxels as well as the probability that they pass through other voxels from neuroimaging. They investigated connectivity between each OFC voxel and the four largest amygdala subregions: the lateral, basal, accessory basal, and cortico-amygdaloid transition area nuclei. They also directly compared the connectivity patterns between the OFC and each of the four amygdala nuclei to determine if any OFC subregions showed preferential connectivity to a certain amygdala nucleus. The researchers found that all amygdala nuclei have structural connections that spanned extensively across all regions of the OFC. Furthermore, the amygdala nuclei had distinct OFC structural connectivity patterns: 1) the lateral nucleus showed extensive connectivity in the right hemisphere and restricted connectivity to middle and posterior OFC in the left hemisphere, 2) the cortico-amygdaloid transition area and accessory basal nuclei were primarily connected to the anterior and lateral OFC, and 3) the basal nucleus’ connections were restricted to the posterior-middle OFC (Matyi and Spielberg, 2020).
Advances in Amygdala-OFC Functional Connectivity
A recent methodological advance using viral ablation of the amygdala has enabled researchers to examine of the functional roles of distinct OFC circuits in reinforcement learning. Researchers selectively ablated amygdala-projecting OFC neurons, OFC-projecting amygdala neurons, and nucleus accumbens (NAcc)-projecting OFC neurons, and looked at the effects of these ablations on rats’ performance in a probabilistic reversal learning task (Groman et al., 2019). While ablation of both NAcc-projecting OFC neurons and OFC-projecting amygdala neurons reduced the number of correct choices following reversal, ablation of amygdala-projecting OFC neurons increased the number of correct choices following reversal. By characterizing the effects of the amygdala-projecting OFC neuronal ablation on decision-making, the team determined that increased correct choices were driven by an impaired ability to retain information about the values of prior unchosen outcomes. This suggests that amygdala-projecting OFC neurons might reduce the influence of previous trial outcomes on subsequent decisions thus increasing the rats’ ability to update preferences following a reward contingency reversal. Further, while both OFC→NAcc and amygdala→OFC neuronal ablation resulted in reduced performance following reversal, the researchers determined that there were different underlying mechanisms in each situation. Ablating OFC→NAcc neurons impaired rats’ ability to use negative outcomes to guide behavior, but ablating amygdala→OFC neurons impaired rats’ ability to use positive outcomes to guide behaviors. In summary, these three circuits demonstrate distinct functions: the OFC→amygdala neurons maintain action values across previous choices, amygdala→OFC neurons update action values in response to positive outcomes, and OFC→NAcc neurons update action values in response to aversive outcomes. Future work investigating the function of analogous circuits in non-human primates will help to create a more complete understanding of the role of bidirectional projections between the OFC and amygdala.
Amygdala-OFC in Social Cognition
Research in humans has demonstrated that both amygdala and OFC volume are positively correlated with social network size (Bickart et al., 2011; Powell et al., 2012), and both of these areas have direct anatomic connections to the superior temporal sulcus (STS) (Seltzer and Pandya, 1989; Stefanacci and Amaral, 2002), a key region in face processing in primates (Allison et al., 2000; Freiwald et al., 2016; Tsao et al., 2008). Taken together, both these regions may be implicated in encoding and/or integrating important social features needed for social interactions, such as the identities of conspecifics and values and emotions associated with individual conspecifics. Much of the recent work in social cognition has focused on asking: is social cognition driven by the “social brain,” or are these computations carried out by more domain-general processes (Lockwood et al., 2020)? As a potential solution to this question, Lockwood and colleagues proposed a need to examine the social specificity from the unique perspectives of computational, algorithmic, and implementational levels. Previous research has demonstrated that the OFC and ventromedial PFC (vmPFC), two regions implicated in reward and decision-making, show increased BOLD activity when human subjects were shown pictures of faces, suggesting that domain-general mechanisms may enable these regions to contribute similarly to social and general reward-based situations levy (Levy and Glimcher, 2012). However, follow-up work in macaques identified unique subpopulations of OFC neurons that responded to social, but not non-social, rewards (Watson and Platt, 2012). In rhesus macaques, OFC neural activity to reward cues differed based on the presence of a conspecific, suggesting that social context modulates OFC value representation (Azzi et al., 2012). Further, they also found that motivation to work for reward was affected by the presence of a conspecific suggesting that the OFC may play a role in integrating social context and motivational value. Further research is required to better understand the relationships between social and non-social ensembles in the OFC. One hypothesis is that the two ensembles might be in a regulatory relationship to promote one type of behavior over another (Gangopadhyay et al., 2021; Jennings et al., 2019). Extending the role of the OFC in value processing and the amygdala in affective processing, it is likely that the amygdala-OFC circuit is involved in integrating emotional and value information to guide social functions.
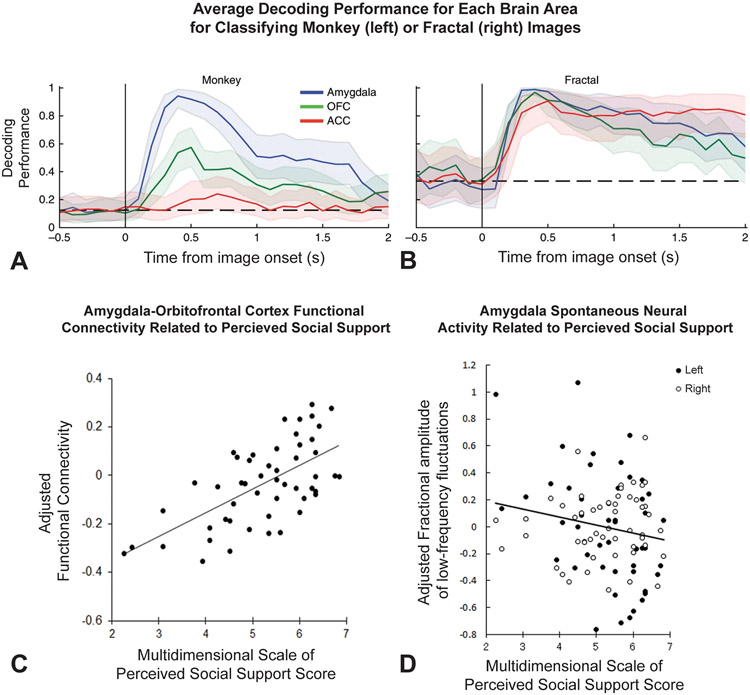
The ability to learn and assess social hierarchies is fundamental to primate social lives, and it is a process that involves associating individuals with various values, similar to the reinforcement learning processes that rely on the OFC in non-social situations. Therefore, researchers set out to investigate the putative roles of the OFC and amygdala, among other regions, in the coding of reward values associated with social hierarchy in macaque monkeys (Munuera et al., 2018). To do so, they recorded from the amygdala, OFC, and ACC neurons in macaques as they freely viewed images of either faces of in-group conspecifics or non-social control fractals that were associated with specific reward amounts. They found social rank-responsive neurons in all three regions with the proportion being highest in the amygdala. 30.6% of amygdala neurons strongly represented hierarchical rank of individuals in their social group. OFC and ACC neurons also showed neuronal selectivity for hierarchical rank but at a much lower rate of 16.7% and 6.3% of neurons, respectively. Importantly, given that neurons in all three regions represented reward values associated with non-social stimuli, they then investigated whether neural representations of rewards associated with both social and non-social images were encoded by overlapping or distinct neuronal populations and found that the same amygdala neurons, but not the same OFC or ACC neurons, that represented social rank also represented rewards associated with non-social images (Fig. 2A-B). These results suggest that, rather than having neural ensembles dedicated to social processing, the amygdala instead represents social and non-social information through a shared neural coding mechanism. The results from this study therefore informatively constrains the manner in which the amygdala contributes to the “social brain” at the algorithmic and implementational levels of processing (Lockwood et al., 2020). Future work is needed to further investigate the specific contributions of amygdala, OFC, and amygdala-OFC interactions in social valuation.
Figure 2. OFC-Amygdala Circuits in Social Cognition.
A–B: Munuera et al., 2018. To determine if neural representations of hierarchical rank and rewards associated with fractal images were encoded by overlapping neuronal populations, the researchers trained a linear decoder to distinguish between fractal trials with either a large reward or no reward. The decoder was then tested on held-out trials of the same type and trials in which monkeys viewed two images of monkey faces. A: Depicts the average decoding performance for Amygdala, OFC, and ACC for distinguishing between trials in which hierarchical rank of the two monkey face images presented differed. Decoding performance on monkey face trials was greater in the amygdala than in the OFC or ACC. B: Depicts the average decoding performance for Amygdala, OFC, and ACC for classifying fractal images. Decoding performance on held-out fractal trials was high for all three regions. C–D: Sato et al., 2020 C: Left amygdala to right OFC functional connectivity was positively correlated with Multidimensional Scale of Perceived Social Support (MSPSS) scores. D: Bilateral amygdala fractional amplitude of low-frequency fluctuations (fALFF) values were negatively correlated with MSPSS scores.
In humans, researchers have demonstrated a strong link between amygdala-OFC functional and structural connectivity and social measures such as perceived social support and social network size (Hampton et al., 2016; Sato et al., 2016; Sato et al., 2020). Following a study demonstrating that left amygdala volume was positively correlated with perceived social support (Sato et al., 2016) researchers sought to investigate the underlying neural mechanisms of this link (Sato et al., 2020). To do so, they performed resting-state fMRI scans and evaluated perceived social support using the Multidimensional Scale of Perceived Social Support (MSPSS) in healthy subjects. They found that stronger left amygdala to right OFC FC was associated with higher reported levels of perceived social support (Fig. 2C). Additionally, they investigated associations between fractional amplitude of low-frequency fluctuations (fALFF), a measure though to be indicative of spontaneous neural activity levels, in the amygdala and MSPSS scores. They found that lower fALFF in the bilateral amygdala was associated with higher MSPSS scores (Fig. 2D), and this relationship was mediated by amygdala volume. Thus, it appears that resting-state amygdala activity and amygdala-OFC network activity play a crucial role in perceived social support and may thus contribute to downstream positive social behaviors. Conversely, FC between the amygdala and OFC was shown to be reduced after stress and in patients with depression (Cheng et al., 2018; Clewett et al., 2013). Taken together, the amygdala-OFC circuit seems to be important for social function, and dysfunction in this circuit may be an underlying mechanism/contributor to disorders in which positive social functions are disrupted.
Amygdala-Hippocampal Circuits
In addition to its dense connections to the mPFC and OFC, the amygdala also shows extensive connections with other subcortical structures including hypothalamic, brain stem, and other medial temporal lobe regions (Sah et al., 2003). Many of these amygdala-subcortical connections facilitate coordinated autonomic, endocrine, and behavioral responses related to basic biological drives such as motivational, social, and reproductive behaviors (Mcdonald, 2014).
Hippocampus
Amygdala-Hippocampus Structure and Function
One such subcortical structure that has been well studied in relation to the amygdala is the hippocampus. The hippocampus (HPC), divided into the dentate gyrus, subiculum, and CA1-4 regions, is a core region for episodic memory. Spatial subdivisions of the HPC also show distinct functions with the dorsal HPC, or posterior in primates, being more involved in cognitive processes, and the ventral HPC, or anterior in primates, being more involved in stress, emotion, and affect processing (Fanselow and Dong, 2010). In both primates and rats, the amygdala is one of only four structures that has direct, strong and reciprocal connections with the HPC (Mcdonald, 1998; Pitkänen et al., 2000; Saunders et al., 1988). As demonstrated in rodents, hippocampal projections to the amygdala largely originate from the subiculum and adjacent CA1 region and most strongly terminate in the posterior basomedial nucleus and more weakly in other nuclei (Canteras and Swanson, 1992; Mcdonald, 1998; Pitkänen et al., 2000). In the opposite direction, studies in rats show that all amygdala nuclei, except the central, project across all subregions of the HPC (Pitkänen et al., 2000). While the BLA sends strong projections to the medial temporal lobe, including the HPC (Petrovich et al., 2001; Pitkänen et al., 2000), it does have very strong monosynaptic connections with the ventral CA1 (Felix-Ortiz et al., 2013; Yang et al., 2016). Compared to rats, macaques show some anatomic differences in amygdala-hippocampal circuitry with the accessory basal, medial basal, and cortical nuclei sending discrete projections to HPC’s CA fields and subiculum (Saunders et al., 1988). It is important to bear in mind this distinction between rodent and primate amygdala-hippocampal structural connectivity when investigating the function of the amygdala-HPC network.
While the HPC is the key region for episodic memory, the amygdala is strongly linked to fear memory. In both humans and rodents, subjects with amygdala lesions do not show behavioral responses in fear learning paradigms while those with hippocampal lesions show behavioral reactivity to learned fear cues but no signs of declarative memory of the association (Bechara et al., 1995; Desmedt et al., 1998; LaBar et al., 1995). Although this early work suggests a dissociation of the two memory systems, research shows that these two regions interact in significant ways during both the encoding and consolidation of memories related to emotional events. For example, work showing increased amygdala activity during presentation of emotional stimuli suggests that the amygdala may enhance emotional memory encoding by increasing attention to these events (Phelps et al., 2004; Vuilleumier et al., 2001). Additionally, the amygdala, specifically the BLA, has been shown to modulate memory consolidation by mediating the effects of stress hormones on memory and through projections to other brain regions including the caudate nucleus, nucleus accumbens, and cortical regions (McGaugh, 2004).
Advances in Amygdala-Hippocampus Structural Connectivity
As seen with amygdalo-cortical projections, early tracer studies identified broad amygdala-hippocampal structural connectivity, but many details of this anatomy, particularly in primates, have remained relatively unexplored. More specifically, where amygdala projections terminate in the HPC has not been well studied. Because hippocampal subregions are functionally distinct (Strange et al., 2014), a more precise understanding of the amygdala terminal locations is critical for researchers’ ability to investigate amygdala-hippocampal circuit functions. Recently, researchers sought to map out inhibitory neurons in the macaque HPC as well as their innervation by the amygdala (Wang and Barbas, 2018). To do so, they first labeled hippocampal neurons in macaques with calretinin, parvalbumin or calbindin, three calcium-binding proteins that have been used to label neurochemical groups of inhibitory neurons in rodents (DeFelipe, 1997). They found that calretinin and parvalbumin showed laminated, regional labeling in the HPC. Next, they systematically injected tracers into the amygdala and investigated the specific locations these neurons terminated in the HPC. They showed that amygdala projections terminated along the longitudinal axis of the HPC but most strongly innervated the anterior HPC and more weakly innervated the posterior HPC. They also found distinct patterns of innervation in CA1 and CA3 with amygdala projections innervating calretinin inhibitory neurons in CA1 but both calretinin and parvalbumin inhibitory neurons in CA3. These results suggest that the amygdala projections to the HPC may comprise distinct subcircuits that differentially influence behavioral processes specific to these regions. Such detailed understanding of the neuronal and projection characteristics of the amygdala-hippocampal circuit may be a critical component of improving our understanding of disorders related to dysregulation in emotional fear memories processes such as post-traumatic stress disorder.
Amygdala-Hippocampus Functional Connectivity Advances
Both the amygdala and ventral hippocampus (vHPC) have long been implicated in fear and anxiety behaviors (Kjelstrup et al., 2002; LaBar et al., 1995), and recent methodological advances have enabled researchers to investigate the details of this functional relationship. To investigate the functional projections of the ACC and vHPC to the amygdala during fear responses, researchers used designer receptors exclusively activated by designer drugs or DREADDs – a chemogenetic tool using a receptor exclusively activated by a synthetic ligand to target particular populations of neurons – to inhibit glutamatergic projections from the ACC or vHPC to the BLA in mice soon or long after they underwent fear conditioning (Ortiz et al., 2019). They found that inhibiting either of these projections resulted in a reduction of fear to fear-inducing novel situations but had no effect on fear responses in a fear conditioning context. This suggests that ACC and vHPC projections to the BLA play a specific role in expressing fear related to novel contexts and thus dysfunction in this circuit could contribute to generalized fear related to anxiety disorders. Further work on amygdala-HPC circuits related to novelty-based fear and anxiety behaviors sought to determine if there was functional specificity of the subregional projections in this network (Pi et al., 2020), since cells in the vCA1 specifically have recently been shown to be involved in anxiety behaviors (Jimenez et al., 2018). Therefore, researchers, using optogenetics, probed the functional distinctions between posterior BLA-ventral CA1 and anterior BLA-ventral CA1 circuits in mediating anxiety behaviors in an exploratory task in mice. They found that stimulating the posterior BLA-ventral CA1 projections resulted in increased approaches (suggestive of reduced anxiety) whereas stimulating anterior BLA-ventral CA1 resulted in decreased approaches (increased anxiety) (Pi et al., 2020). These results elucidate both structural and functional distinctions of amygdala-hippocampus sub-circuits which will be integral to gaining a better understanding of networks related to anxiety across a variety of disorders. Further, this work highlights the importance of moving beyond simple attributions of whole brain regions to certain behaviors, as these regions often consist of both anatomically and functionally distinct sub-regions and -circuits.
Amygdala-Hippocampus in Social Cognition
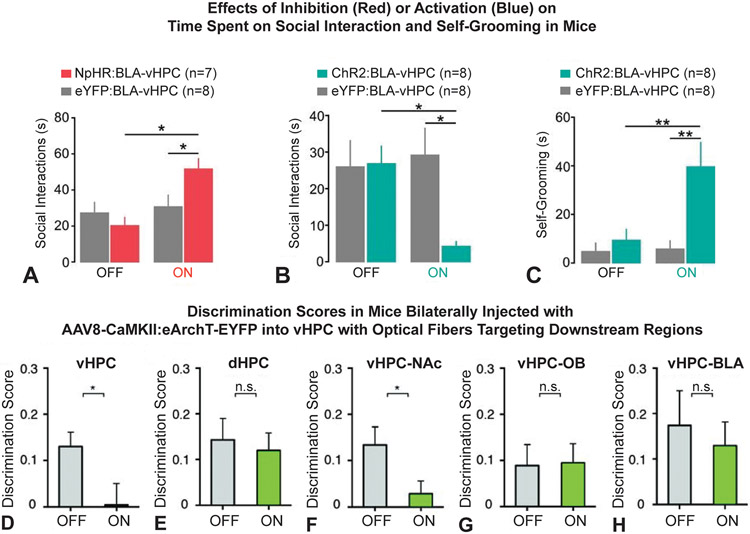
While the amygdala has long been considered an essential region for social behaviors, only recently has the hippocampus or the amygdala-hippocampus network been shown to be important for social behaviors. In fact, early work showed that vHPC lesions in adult rats did not lead to social deficits in adulthood (Becker et al., 1999) indicating that this region was not necessary for the expression of normal social behavior. However, recently, researchers have suggested that these lesions may not have been specific to the vHPC, possibly affecting other hippocampal regions that might have had opposite effects, thus challenging the effect of the lesion on behavior. The lesions were also performed 2 weeks before the behavioral tests, which may have allowed time for compensatory mechanisms to take hold (Felix-Ortiz and Tye, 2014). Furthermore, Felix-Ortiz and Tye recently found that, in mice, BLA inputs to the ventral hippocampus modulate social behavior such that optogenetic inhibition of BLA-vHPC projections led to increased social interactions in the home cage intruder test (a test commonly used to probe fear and anxiety-like behaviors) as demonstrated by increased social exploration of the intruder (Fig. 3A). Conversely, activation of the BLA-vHPC projections resulted in a decrease in time spent exploring the intruder (Fig. 3B). Additionally, this BLA-vHPC activation was also linked to increased self-grooming (Fig. 3C), indicative of anxiety-like behaviors. These findings were the first to demonstrate a causal relationship between BLA projections to the vHPC and social behaviors. Moreover, this circuit’s dual effect on both social and anxiety behaviors may give insight into mechanistic explanations for the high comorbidity rates seen between social deficit and anxiety disorders (Zaboski and Storch, 2018) and also shed light on the regulation of social and non-social behaviors in this circuit (Gangopadhyay et al., 2021).
Figure 3. Hippocampus-Amygdala Circuits in Social Cognition.
A–C: Felix-Ortiz & Tye, 2014 A: Inactivation of basolateral amygdala (BLA)→ventral hippocampus (vHPC) neurons with halorhodopsin (NpHR) increased social interactions in the resident intruder task in comparison to control (eYFP) mice. B: Activation of BLA→vHPC neurons with channelrhodopsin-2 (ChR2) decreased social interactions in the resident intruder task in comparison to control (eYFP) mice. C: Activation of BLA→vHPC neurons with ChR2 also increased self-grooming behaviors in comparison to eYFP control mice. D–H: Okyuama et al., 2016. Comparisons of social discrimination scores when investigated brain regions were optogenetically inhibited (laser ON) versus when they were not (laser OFF). D: Inhibition of vHPC neurons significantly decreased social discrimination scores. E: Inhibition of dorsal hippocampus (dHPC) neurons did not affect social discrimination scores. F: Inhibition of vHPC→Nucleus Accumbens (NAcc) projections significantly decreased social discrimination scores. G: Inhibition of vHPC→Olfactory Bulb (OB) projections did not affect social discrimination scores. H: Inhibition of vHPC→BLA projections did not affect social discrimination scores.
Building upon this work demonstrating the vHPC’s role in social behaviors, further research has focused on understanding finer-level segmentations of hippocampal circuit functions. To investigate the role of CA1 neurons in social behaviors, researchers first used optogenetics to inhibit ventral CA1 or dorsal CA1 cell bodies (Okuyama et al., 2016). Inhibition of the ventral CA1, but not dorsal CA1, resulted in social discrimination test deficits as seen by increased sniffing of familiar conspecifics versus novel conspecifics in the resident-intruder test (Fig. 3D-E). They then identified brain regions downstream of ventral CA1 that were involved in social memory by labeling neurons activated by social interaction. With this method, they identified that the BLA, along with the NAcc shell and olfactory bulb, were the main targets of the social interaction specific ventral CA1 projections. However, when they inhibited ventral CA1 terminals in these three regions as mice completed social discrimination tasks, they determined that only vHPC-NAcc projections were essential for social memory related behaviors (Fig. 3F-H) (Okuyama et al., 2016). In addition to demonstrating a key role for ventral CA1-NAcc projections in social discrimination, these results implicate ventral CA1-BLA projections in social interaction behaviors and highlight a need for further investigation of the specific functional contribution of this circuit to social cognition.
In addition to the CA1, CA2 neurons have also been implicated in social behaviors. Using a transgenic mouse line, researchers demonstrated that selective inactivation of CA2 pyramidal neurons in adult mice resulted in social memory impairments as indicated by reduced social recognition of familiar conspecifics in both the three-chamber social novelty test and the direct interaction test, as well as lack of habituation during repeated exposure to the same conspecific (Hitti and Siegelbaum, 2014). Inactivation of CA2 pyramidal neurons did not affect the mice’s ability to detect and discriminate social and non-social odors, indicating that this is a deficit in social memory. Additionally, the inactivation of CA2 pyramidal neuron did not cause spatial memory deficits, as measured by the Morris water maze, nor did it cause contextual memory deficits, as measured by fear conditioning (Hitti and Siegelbaum, 2014). While researchers have yet to investigate CA2-amygdala circuits in relation to social cognition, these results, along with the strong reciprocal connections between the HPC and the amygdala, demonstrate the need to examine the role of the amygdala-HPC circuit in mediating social behavior.
Amygdala Networks in Social Disorders
The amygdala has been consistently implicated in many neuropsychiatric disorders. Of particular relevance to this chapter, human patients, presenting with lesions of the amygdala, were found to display significant impairment in social behavior, including the recognition and matching of face expression and gaze direction (Adolphs et al., 1994; Broks et al., 1998; Calder, 1996; Young et al., 1995). Furthermore, lesions of the amygdala in rhesus macaques similarly were shown to disrupt, though in a more muted fashion, social behavior. Bilateral aspiration lesions, that encompass the amygdala and surrounding regions, including parts of the piriform cortex and entorhinal cortex, were shown to result in monkeys that show reduced initiation of social behaviors compared to controls (Bachevalier et al., 1999). However, these animals also showed a more generalized reduction in activity, and, coupled with the non-selective nature of the lesion, drawing a conclusion about amygdala function is challenging. A more selective lesioning of the amygdala in neonatal animals (Bauman et al., 2004) did produce deficits in fear processing, with animals showing increased fear of conspecifics but decreased fear to normally aversive objects like snakes. A study with more nuanced behavioral measures using eye-tracking showed impaired attention capture by threatening face stimuli and reduced exploration of the eye region in lesioned animals compared to control animals (Dal Monte et al., 2015). Taken together, these lesion studies implicate the amygdala in social behavior and processing and attending to social information.
It should be noted that there exist drawbacks to the use of lesion studies in trying to delineate amygdala function. Unlike excitotoxic lesions, aspiration methods, which were used in most earlier lesion studies, impact neighboring regions and white matter tracts, thus throwing into question the specificity of the amygdala in the observed behavior (Murray and Rudebeck, 2018). Furthermore, in human patients, lesion studies often occur years after the initial damage, allowing for possible compensatory mechanisms to counteract the initial loss of function. The small number of subjects further diminishes the utility of these studies. However, these studies do suggest that the amygdala plays a causal role in social behavior, and that the dysfunction of the amygdala would result in a variety of behavioral and social deficits.
Autism Spectrum Disorder
Autism spectrum disorder (ASD) is a neurodevelopmental disorder presenting with a heterogenous mixture of behavioral abnormalities of varying severity, first described by Kanner (Kanner, 1943). The behavioral deficits are characterized by 1) deficits in social interaction, communication and reciprocity, and by 2) the presence of restricted, repetitive patterns of activity or movements (American Psychiatric Association, 2013). However, ASD is considered a “spectrum” since the symptoms seen in people with ASD vary widely in specificity and severity. This diversity in how ASD manifests also hints at the heterogeneity in the underlying causes of ASD.
About 2% of children are diagnosed with ASD (Baio et al., 2018). The development of ASD is also heterogenous, with evidence to show the existence of an early-onset and a late-onset ‘regressive’ phenotype (Werner and Dawson, 2005). Infants with the early-onset phenotype displayed marked changes in social behavior, namely reduced joint attention and babbling communicative behaviors, as early as 12 months of age. In contrast, infants with regressive ASD demonstrated normal development until 18-24 months, at which point they regressed developmentally to resemble the early-onset infants. Given the heterogeneity that exists in ASD, in terms of its symptomatology, etiology, and ontogeny, it is unsurprising that dissecting the neurobiological underpinnings of ASD has proven to be challenging.
Studies in infants with autism have found a larger volume and greater neuronal numbers in the amygdala in ASD compared to neurotypically-developing controls (Avino et al., 2018; Mosconi et al., 2009; Schumann et al., 2009; Schumann et al., 2004; Sparks et al., 2002). However, in older adolescents and in adults with ASD, the amygdala is similar (Haznedar et al., 2000) or reduced in volume (Nacewicz et al., 2006; Pierce et al., 2001; Schumann and Amaral, 2006) and neuronal numbers (Avino et al., 2018). Given that the amygdala in neurotypical individuals continues to grow in size throughout adolescence and into adulthood, the initial increase in amygdala size followed by the subsequent reduction seen in ASD would imply that it is the trajectory of amygdala development that might be critically affected in ASD (Schumann et al., 2011; Schumann et al., 2004).
Beyond changes in morphology and development, there is also differential activation of the amygdala in response to social stimuli seen in ASD. When shown faces of unfamiliar individuals in a face perception task, individuals with ASD showed reduced activation in the amygdala to these faces as compared to neurotypical individuals (Pierce et al., 2001). Interestingly, Pierce and colleagues further extended this study (Pierce et al., 2004) by showing individuals with ASD faces of personally relevant individuals (for example, a mother or a coworker). In contrast to unfamiliar faces, these familiar faces did elicit an activation of the amygdala in a manner similar to controls. Furthermore, in a study that tracked the eye gaze position while individuals were presented with face stimuli, greater activation of the right amygdala was seen in response to familiar and unfamiliar faces in individuals with ASD compared to controls, while a greater activation of the left amygdala was seen in response to emotional faces in ASD as compared to controls (Dalton et al., 2005). Moreover, looking at the eye region of the presented faces elicited a proportionate increase in amygdala activation in ASD individuals. In a task which relied on looking at the eyes to make judgements about the gender or expression of the person in the presented stimuli, individuals with ASD not only performed less accurately than neurotypical controls, but also showed reduced activation in the amygdala during task performance (Baron-Cohen et al., 1999). While the reasons underlying this pattern of amygdala activation are unclear, some possibilities are that individuals with ASD find social stimuli aversive or that they are less motivated to look at social stimuli (Chevallier et al., 2012; Schumann et al., 2011; Sigman et al., 2006).
In addition to changes in amygdala activation to social stimuli, amygdala connectivity is also disrupted in ASD. However, given the recency of this research, functional connectivity studies exploring the amygdala circuitry in ASD remain few in number (Nomi and Uddin, 2015). Furthermore, the different ASD profiles of the subject pools used, and the difference in tasks and methodologies employed in these studies, make the results challenging to interpret. However, there is research that demonstrates the importance of amygdala-cortical circuits in ASD. For instance, studies indicate the disruption of the amygdala-mPFC connectivity is important for ASD, as discussed below.
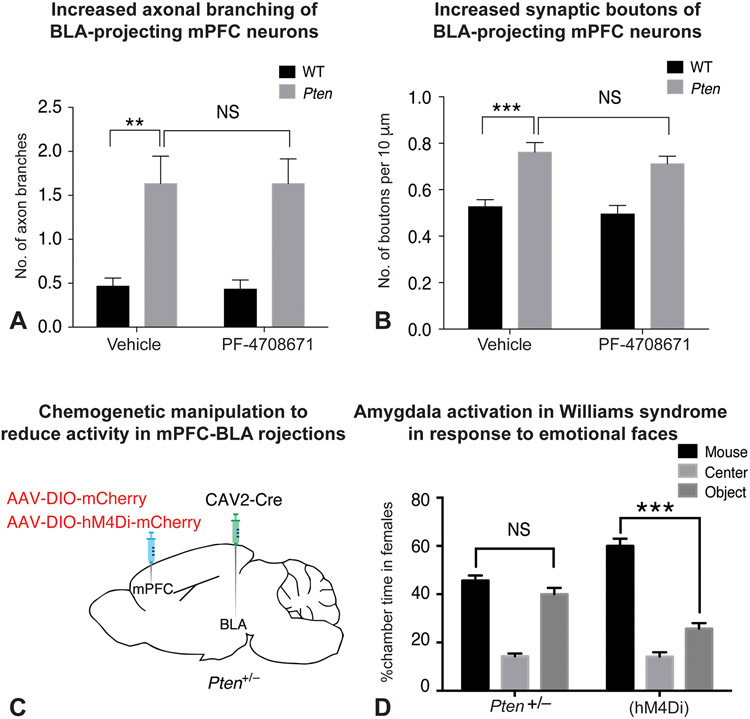
In preschool children diagnosed with ASD, with a mean age of 3.5 years, a decreased resting state connectivity is seen between the amygdala and the frontal, including the mPFC, and temporal lobes compared to typically-developing controls (Shen et al., 2016), and the strength of this connectivity predicted the severity of observed symptoms. Similarly, in older children of 7-12 years of age diagnosed with ASD (Li et al., 2020), the resting state FC of the amygdala-mPFC appears to be weaker than in age-matched controls. These children also had significantly weaker positive causal influence from the mPFC to the amygdala (Granger Causality analysis) and the raw values of the Granger Causality strength between the two also predicted the severity of observed symptoms. Interestingly, a similar dysregulation of the amygdala-mPFC circuit was seen in a mouse model of autism, wherein the researchers (Huang et al., 2016) used pten+/− heterozygous mice as a model for ASD. PTEN (for phosphatase and tensin homolog), through its inhibitory actions on Akt signaling, is important in regulating cell growth and survival, and a mutation of this gene has been found in a subset of individuals with ASD (Busch et al., 2019). Huang and colleagues observed a hypertrophic connectivity between the mPFC and the amygdala, specifically the BLA, in these mice. The projections from the mPFC that terminate in the BLA showed excessive synaptic branching (Fig. 4A) and boutons (Fig. 4B). Furthermore, along with this increased connectivity, there was a concomitant hyperactivity in response to novel social stimuli seen in the BLA, mPFC and in the BLA-projecting mPFC cells specifically, as seen by cfos+ reactivity. Furthermore, inhibiting the activity in these BLA-projecting mPFC cells, using the chemogenetic DREADDs system (Fig. 4C), reduced activity within the mPFC and the BLA, and also corrected behavioral deficits seen in these mice in the three-chamber test of sociability (Fig. 4D). Thus, there appears to exist a dysregulation of the amygdala-cortical circuit in ASD, and data from an animal model of ASD suggests that attenuating the dysregulation also normalizes social behavior.
Figure 4: Amygdala-cortical circuit in animal model of ASD.
A-D: Huang et al., 2016. A: Quantification of increased branching of mPFC axon terminals on the BLA in pten+/− mice (vehicle), and this phenotype is not reversed by inhibiting S6K1 in adults (PF-4708671) B: Quantification of increase in synaptic boutons on mPFC axon terminals in the BLA in pten+/− mice (vehicle), and this phenotype is not reversed by inhibiting S6K1 in adults (PF-4708671) C: Details of chemogenetic system used to reduce activity in the mPFC projections to the BLA D: Reducing activity of the mPFC-BLA projections rescues behavioral deficits in a three-chamber sociability task, pten+/− mice show no preference (pten+/−) for social stimuli over non-social stimuli, and this phenotype is rescued on inhibiting activity (hM4Di).
Given what we now know of the abnormal developmental trajectory of the amygdala in ASD, and the presence of distinct patterns of projections from the amygdala nuclei (see preceding section on amygdala structural and functional connectivity), it behooves us to carefully examine the development and disruption of these individual amygdala nuclei and subregions. Perhaps doing so will lead to a better understanding of the neurobiology of ASD and explain some of its heterogeneity.
Social Anxiety Disorder
The Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 2013) characterizes social anxiety disorder (SAD) as a “marked fear or anxiety about one or more social situations in which the individual is exposed to possible scrutiny by others”. While apprehension of a social situation, for example public speaking, is normal, SAD causes distress or anxiety in an amount that is disproportionate to the situation and leads to a clinically-relevant impairment in behavior and in the quality of life for an individual. Furthermore, the symptoms are persistent, often lasting for many months and beyond. In fact, this disorder has been called “a disorder of lost opportunities” (Stein and Gorman, 2001), since individuals suffering from SAD often make decisions to minimize exposure to social situations including school, work and personal relationships. It is a fairly prevalent disorder with a lifetime prevalence of around 12-16% (Magee et al., 1996; Ruscio et al., 2008), often presenting early in life (Kessler et al., 2012) and is more common in women than men (Talepasand and Nokani, 2010). Similar to ASD, the etiology of SAD is not well understood, though ASD and SAD often present as comorbid conditions (Zaboski and Storch, 2018). However, given the role the amygdala plays in fear and anxiety associated behaviors (Davis, 1992; LaBar et al., 1995), it makes for a highly-relevant region of interest while evaluating the circuitry underlying SAD.
The amygdala is activated by negative facial expressions in humans (Morris et al., 1998; Morris et al., 1996), even when the faces are not consciously perceived (Monk et al., 2008; Whalen et al., 1998). Furthermore, patients with bilateral amygdala lesions were found to display deficits in recognizing facial expressions for fearful (Adolphs et al., 1994; Calder, 1996) or “blended” expressions – expressions that consist of multiple distinct emotions in a single facial expression (Adolphs et al., 1998). The amygdala is also important for fear conditioning (Blanchard and Blanchard, 1972; Cassell et al., 1986; Rizvi et al., 1991; LeDoux et al., 1990). Thus, given its central role in perception of fearful social stimuli and in the acquisition of fear, it is unsurprisingly to find amygdala dysfunction in SAD.
When shown human facial stimuli, patients with SAD showed significantly greater BOLD activation in the left allocortex, including the amygdala, for contemptuous and angry faces compared to happy faces (Phan et al., 2006; Stein et al., 2002) and to emotional faces (Hahn et al., 2011). Patients with SAD were shown to exhibit hyperactivation of the amygdala to “fear-relevant stimuli” (Birbaumer et al., 1998); they showed greater activation in the amygdala to neutral faces compared to controls when the presentation of faces was interspersed with presentation of an aversive odor. In a study that paired aversive odor with neutral faces in a fear conditioning paradigm, hyperactivation of the amygdala and hippocampus was seen in patients with SAD compared to healthy controls (Schneider et al., 1999). Similarly, pairing neutral faces with a painful pressure stimulus produced increased activity to the faces in the amygdala and the OFC (Veit et al., 2002). Patients were also found to display increased activation in several subcortical and limbic regions, including the amygdala and the parahippocampus, prior to a socially challenging event (Lorberbaum et al., 2004). Furthermore, they showed a decrease in activation of several cortical regions, including the OFC and the PFC.
Beyond a hyperactivation to face expression stimuli, disrupted amygdala-cortical circuits are also observed in SAD. The use of resting-state FC allows us to look at interactions between brain regions when a task is not being performed. Resting-state FC is also indicative of structural connectivity between the brain regions involved (Greicius et al., 2009). Alterations in the resting FC between several cortical regions has been suggested in SAD (Liao et al., 2010). Hahn and colleagues (Hahn et al., 2011) found reduced FC between the left amygdala and the left mOFC, while no changes were observed for the right amygdala. Furthermore, changes in amygdala FC have also been observed in SAD in anticipation of a social challenge. When looking at FC prior to a fearful social event (public speaking), patients with SAD reported greater levels of stress compared to controls (Fig. 5A) along with a transient decrease in negative FC between the amygdala and cortical regions compared to controls, who showed a transient increase in negative FC (Fig. 5B), and these changes correlated with the severity of observed symptoms (Cremers et al., 2015). Additional evidence for altered amygdala-cortical FC underlying SAD comes from studies looking at cognitive behavioral therapy (CBT) in moderating the symptoms of SAD. Bilateral amygdala-pregenual ACC connectivity (Klumpp et al., 2014) and left amygdala-left dmPFC and dACC connectivity (Yuan et al., 2016) served as predictors for the degree to which CBT could alleviate symptoms in patients, suggesting a dysregulation of amygdala-cortical connectivity in SAD.
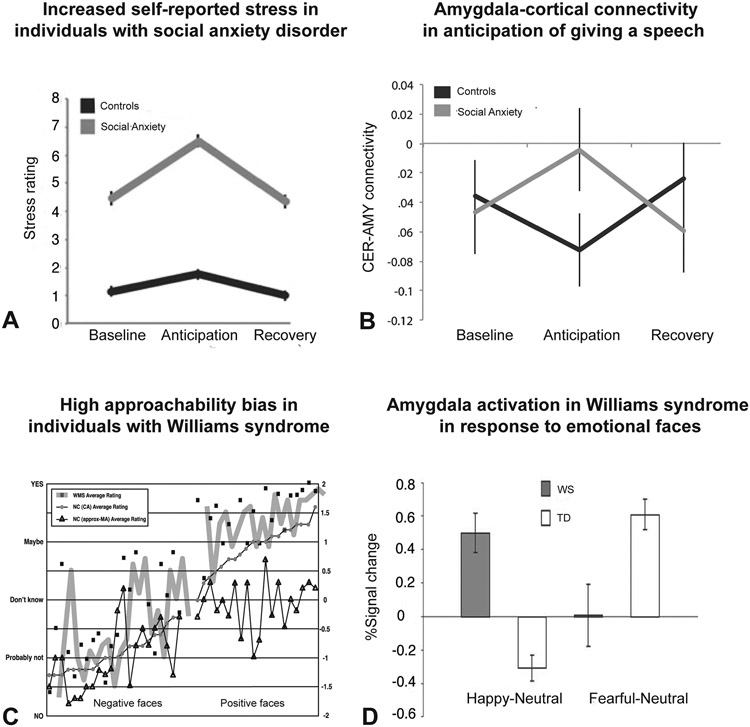
Figure 5: Amygdala activation in Social Anxiety Disorder and Williams Syndrome.
A–B: Cremers et al., 2015. A: Self-stress reported on 11-point Likert-scale by individuals with social anxiety and controls during: baseline, when instructed they would be giving a public speech (anticipation) and when they were informed that no speech would have to be given (recovery) B: Amygdala-cortical connectivity during baseline, anticipation and recovery periods showing transient decrease in negative functional connectivity in individuals with social anxiety C: Jones et al., 2000. Individuals with Williams syndrome (WMS) are more likely to approach unfamiliar individuals than age-matched normal controls (NC (CA)) and approximately mental age-matched normal controls (NC (approx-MA)). D: Haas et al., 2009. Amygdala activation to happy (left) and fearful (right) faces in William syndrome (WS) and typically developing controls (TD)
The challenge of studying the neural circuitry underlying SAD is further compounded by the lack of a suitable animal model. The behaviors that characterize SAD are largely human in nature, and most animal models show more generalized behavioral deficits (Reus et al., 2014). However, a recent animal model developed by Toth and colleagues (Toth et al., 2013) uses “social fear conditioning” in rats to induce social fear of a novel conspecific in a manner that is specific to the social domain over the more generalized anxiety and novelty aversion seen in other animal models. Based on operant conditioning, the researchers delivered a mild electrical shock to animals whenever they investigated a conspecific. This yielded a “socially fearful” phenotype with animals displaying a selective aversion to social stimuli. It would be interesting to further dissect the amygdala circuitry in these animals, given the various methodological advances that are currently available in rodents (and as discussed in the preceding sections).
While research into the neural underpinnings of SAD is ongoing, it is clear that a dysregulated amygdala is central to its neuropathology. Further research will not only help elucidate the role of the amygdala and its network connections in SAD, but also suggest potential therapeutic interventions to control or mitigate the deleterious effects of this disorder.
Williams Syndrome
While the previous two disorders discussed involve an increase in anxiety or aversion to social stimuli and situations, another fascinating disorder – Williams syndrome – offers an alternative perspective on the dysregulation of social behavior. Williams syndrome is caused by a deletion of about 26–28 genes in chromosome band 7q11.23 (Ewart et al., 1993; Peoples et al., 2000) and is characterized by craniofacial deformities, general learning disabilities, and visuospatial impairments (Bellugi et al., 2000; Martens et al., 2008; Morris and Mervis, 2000). Individuals with Williams syndrome demonstrate remarkable affiliative social behaviors (Bellugi et al., 1999). They are often hyperverbal, with their language being more expressive and complex than age-matched controls, and are more likely to give highly positive ratings on an approachability rating task that measures bias towards or against unfamiliar individuals (Fig. 5C) (Bellugi et al., 1999; Jones et al., 2000). Given this hypersociability, Williams syndrome could offer remarkable insight into what role the amygdala and the amygdala-cortical circuits play in social behavior.
Unfortunately, given low prevalence of this disorder, there exist only a handful of studies looking at the neurobiological basis of Williams syndrome. One postmortem study did find reduced amygdala volume in a single patient with Williams syndrome, with the most pronounced difference in the lateral nucleus (Galaburda and Bellugi, 2000). Individuals with Williams syndrome were also found to show a general reduction in cerebral volume (Jones et al., 2002; Martens et al., 2008; Reiss et al., 2004; Reiss et al., 2000). However, an MRI study has demonstrated that certain regions are spared from volume reduction. These regions include the amygdala, OFC, mPFC and ACC (Reiss et al., 2004), which are important for social behaviors as discussed in the earlier sections, and their volume appears to be disproportionately increased compared to controls. However, these morphological results have been inconsistently reported in the literature. For instance, Chiang and colleagues (Chiang et al., 2007) found that the volume of the amygdala was preserved, but not significantly larger, in Williams syndrome relative to controls.
There are also a few studies looking at abnormal amygdala activation in these patients. There is reduced amygdala activation to faces but increased activation to threatening scenes seen in Williams syndrome (Meyer-Lindenberg et al., 2005). Haas and colleagues (Haas et al., 2009) have further demonstrated that the amygdala in patients with Williams syndrome responds differently to positive and negative expressions, with increased activation being observed for happy faces and diminished activity in response to fearful ones (Fig. 5D).
Given the paucity of studies looking at the amygdala in Williams syndrome, it is difficult to theorize about the role for the amygdala and the amygdala-cortical circuits in driving the unique social behaviors seen in Williams syndrome. However, it is clear that amygdala dysregulation is observed in these patients, and that this dysregulation occurs in a manner consistent with the hypersociability seen in these individuals. Further studies, elaborating on the changes in amygdala function and connectivity, should be useful in predicting and managing symptom severity in Williams syndrome.
Conclusion
In this chapter, we have highlighted both early and contemporary work on amygdala functional and structural connectivity that have been fundamental to our understanding of this structure. We have featured recent work demonstrating advances in our understanding of the structural and functional connectivity of the amygdala, the critical role these amygdala circuits have in social behavior, and their implications for social disorders. The recent advances discussed here are by no means exhaustive. For example, the amygdala is highly connected to a broad range of cortical and subcortical regions beyond those discussed here, and these other circuits also make significant contributions to a wide array of affective and motivational behaviors. However, we believe we have covered noteworthy discoveries and trends in the field with discussing the amygdala circuits with respect to the mPFC, OFC and HPC. As demonstrated by structural findings, technological advances in tracing and neuroimaging methods have enabled us to shift towards a better understanding of the specific anatomic connections of detailed amygdala subregions. Similarly, as seen in research investigating amygdala’s role in affective and social behaviors, methods such as optogenetics and DREADDs have enabled researchers to determine behavioral contributions of specific subcircuits of the amygdala. Overall, our chapter emphasizes the importance of understanding the network perspectives of amygdala function in order to better understand its role in regulating complex behaviors, including those that are central to social interactions, and to advance our knowledge toward implementing promising therapeutics for individuals affected by social behavioral disorders.
Acknowledgements
This work was supported by Science Fellowship from the Gruber Foundation (O.C.M.) and the National Institute of Mental Health (R01 MH120081, R01MH110750) (S.W.C.C and A.M.).
Footnotes
Competing Financial Interests
The authors declare no competing financial interests
References
- Abivardi A & Bach DR (2017). Deconstructing white matter connectivity of human amygdala nuclei with thalamus and cortex subdivisions in vivo. Human Brain Mapping 38: 3927–3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D & Damasio AR (1998). The human amygdala in social judgment. Nature 393: 470–474. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, et al. (1994). Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372: 669–672. [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A & McCarthy G (2000). Social perception from visual cues: Role of the STS region. Trends in Cognitive Sciences. [DOI] [PubMed] [Google Scholar]
- Allsop SA, Wichmann R, Mills F, et al. (2018). Corticoamygdala Transfer of Socially Derived Information Gates Observational Learning. Cell 173: 1329–1342 e1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG & Price JL (1984). Amygdalo-cortical projections in the monkey (Macaca fascicularis). Journal of Comparative Neurology 230: 465–496. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (5th ed.). [Google Scholar]
- Anderson AK & Phelps EA (2001). Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature 411: 305–309. [DOI] [PubMed] [Google Scholar]
- Apps MAJ, Rushworth MFS & Chang SWC (2016). The Anterior Cingulate Gyrus and Social Cognition: Tracking the Motivation of Others. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avino TA, Barger N, Vargas MV, et al. (2018). Neuron numbers increase in the human amygdala from birth to adulthood, but not in autism. Proc Natl Acad Sci U S A 115: 3710–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi JCB, Sirigu A & Duhamel JR (2012). Modulation of value representation by social context in the primate orbitofrontal cortex. Proceedings of the National Academy of Sciences of the United States of America 109: 2126–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J, Beauregard M & Alvarado MC (1999). Long-term effects of neonatal damage to the hippocampal formation and amygdaloid complex on object discrimination and object recognition in rhesus monkeys (Macaca mulatta). Behav Neurosci 113: 1127–1151. [DOI] [PubMed] [Google Scholar]
- Baio J, Wiggins L, Christensen DL, et al. (2018). Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill Summ 67: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, et al. (1999). Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci 11: 1891–1898. [DOI] [PubMed] [Google Scholar]
- Basile BM, Schafroth JL, Karaskiewicz CL, et al. (2020). The anterior cingulate cortex is necessary for forming prosocial preferences from vicarious reinforcement in monkeys. PLoS Biology 18: e3000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, et al. (2004). The development of social behavior following neonatal amygdala lesions in rhesus monkeys. J Cogn Neurosci 16: 1388–1411. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, et al. (1995). Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science 269: 1115–1118. [DOI] [PubMed] [Google Scholar]
- Becker A, Grecksch G, Bernstein HG, et al. (1999). Social behaviour in rats lesioned with ibotenic acid in the hippocampus: Quantitative and qualitative analysis. Psychopharmacology 144: 333–338. [DOI] [PubMed] [Google Scholar]
- Bellugi U, Adolphs R, Cassady C, et al. (1999). Towards the neural basis for hypersociability in a genetic syndrome. Neuroreport 10: 1653–1657. [DOI] [PubMed] [Google Scholar]
- Bellugi U, Lichtenberger L, Jones W, et al. (2000). The neurocognitive profile of Williams syndrome: A complex pattern of strengths and weaknesses. Journal of Cognitive Neuroscience 12: 7–29. [DOI] [PubMed] [Google Scholar]
- Belova MA, Paton JJ & Salzman CD (2008). Moment-to-moment tracking of state value in the amygdala. Journal of Neuroscience 28: 10023–10030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez MA & Schultz W (2010). Responses of amygdala neurons to positive reward-predicting stimuli depend on background reward (contingency) rather than stimulus-reward pairing (contiguity). Journal of Neurophysiology 103: 1158–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickart KC, Wright CI, Dautoff RJ, et al. (2011). Amygdala volume and social network size in humans. Nature Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbaumer N, Grodd W, Diedrich O, et al. (1998). fMRI reveals amygdala activation to human faces in social phobics. Neuroreport 9: 1223–1226. [DOI] [PubMed] [Google Scholar]
- Blanchard DC & Blanchard RJ (1972). Innate and conditioned reactions to threat in rats with amygdaloid lesions. Journal of Comparative and Physiological Psychology 81: 281–290. [DOI] [PubMed] [Google Scholar]
- Broks P, Young AW, Maratos EJ, et al. (1998). Face processing impairments after encephalitis: amygdala damage and recognition of fear. Neuropsychologia 36: 59–70. [DOI] [PubMed] [Google Scholar]
- Brown S & Sharpey-Schafer EA (1888). An investigation into the functions of the occipital and temporal lobes of the monkey's brain. Philosophical Transactions of The Royal Society B 179. [Google Scholar]
- Brown SSG, Rutland JW, Verma G, et al. (2019). Structural MRI at 7T reveals amygdala nuclei and hippocampal subfield volumetric association with Major Depressive Disorder symptom severity. Scientific Reports 9: 10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch RM, Srivastava S, Hogue O, et al. (2019). Neurobehavioral phenotype of autism spectrum disorder associated with germline heterozygous mutations in PTEN. Transl Psychiatry 9: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder AJ (1996). Facial Emotion Recognition after Bilateral Amygdala Damage: Differentially Severe Impairment of Fear. Cognitive Neuropsychology 13: 699–745. [Google Scholar]
- Canteras NS & Swanson LW (1992). Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: A PHAL anterograde tract-tracing study in the rat. Journal of Comparative Neurology 324: 180–194. [DOI] [PubMed] [Google Scholar]
- Carmichael ST & Price JL (1995a). Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. Journal of Comparative Neurology 363: 615–641. [DOI] [PubMed] [Google Scholar]
- Carmichael ST & Price JL (1995b). Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. Journal of Comparative Neurology 363: 642–664. [DOI] [PubMed] [Google Scholar]
- Carmichael ST & Price JL (1996). Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. Journal of Comparative Neurology 371: 179–207. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Gray TS & Kiss JZ (1986). Neuronal architecture in the rat central nucleus of the amygdala: A cytological, hodological, and immunocytochemical study. Journal of Comparative Neurology 246: 478–499. [DOI] [PubMed] [Google Scholar]
- Chang SWC, Fagan NA, Toda K, et al. (2015). Neural mechanisms of social decision-making in the primate amygdala. Proceedings of the National Academy of Sciences of the United States of America 112: 16012–16017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Wang R & Shippenberg TS (2011). Basolateral amygdala-driven augmentation of medial prefrontal cortex gabaergic neurotransmission in response to environmental stimuli associated with cocaine administration. Neuropsychopharmacology 36: 2018–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W, Rolls ET, Qiu J, et al. (2018). Functional connectivity of the human amygdala in health and in depression. Social Cognitive and Affective Neuroscience 13: 557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier C, Kohls G, Troiani V, et al. (2012). The social motivation theory of autism. Trends Cogn Sci 16: 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang MC, Reiss AL, Lee AD, et al. (2007). 3D pattern of brain abnormalities in Williams syndrome visualized using tensor-based morphometry. Neuroimage 36: 1096–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewett D, Schoeke A & Mather M (2013). Amygdala functional connectivity is reduced after the cold pressor task. Cognitive, Affective and Behavioral Neuroscience 13: 501–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CM, Rosenkranz JA & Grace AA (2005). Chronic cold stress alters prefrontal cortical modulation of amygdala neuronal activity in rats. Biological Psychiatry 58: 382–391. [DOI] [PubMed] [Google Scholar]
- Cremers HR, Veer IM, Spinhoven P, et al. (2015). Altered cortical-amygdala coupling in social anxiety disorder during the anticipation of giving a public speech. Psychol Med 45: 1521–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Monte O, Chu CCJ, Fagan NA, et al. (2020). Specialized medial prefrontal–amygdala coordination in other-regarding decision preference. Nature Neuroscience 23: 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Monte O, Costa VD, Noble PL, et al. (2015). Amygdala lesions in rhesus macaques decrease attention to threat. Nat Commun 6: 10161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, et al. (2005). Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci 8: 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR, Tranel D & Damasio H (1990). Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behavioural Brain Research 41: 81–94. [DOI] [PubMed] [Google Scholar]
- Davis M (1992). The role of the amygdala in fear-potentiated startle: implications for animal models of anxiety. Trends Pharmacol Sci 13: 35–41. [DOI] [PubMed] [Google Scholar]
- Decety J, Michalska KJ & Kinzler KD (2012). The contribution of emotion and cognition to moral sensitivity: A neurodevelopmental study. Cerebral Cortex 22: 209–220. [DOI] [PubMed] [Google Scholar]
- DeFelipe J (1997). Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex. Journal of Chemical Neuroanatomy. [DOI] [PubMed] [Google Scholar]
- Desmedt A, Garcia R & Jaffard R (1998). Differential modulation of changes in hippocampal-septal synaptic excitability by the amygdala as a function of either elemental or contextual fear conditioning in mice. Journal of Neuroscience 18: 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diergaarde L, Gerrits MAFM, Stuy A, et al. (2004). Neonatal Amygdala Lesions and Juvenile Isolation in the Rat: Differential Effects on Locomotor and Social Behavior Later in Life. Behavioral Neuroscience 118: 298–305. [DOI] [PubMed] [Google Scholar]
- Elliott R, Agnew Z & Deakin JFW (2008). Medial orbitofrontal cortex codes relative rather than absolute value of financial rewards in humans. European Journal of Neuroscience 27: 2213–2218. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T & Kalisch R (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euston DR, Gruber AJ & McNaughton BL (2012). The Role of Medial Prefrontal Cortex in Memory and Decision Making. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewart AK, Morris CA, Atkinson D, et al. (1993). Hemizygosity at the Elastin Locus in a Developmental Disorder, Williams-Syndrome. Nature Genetics 5: 11–16. [DOI] [PubMed] [Google Scholar]
- Fanselow MS & Dong HW (2010). Are the Dorsal and Ventral Hippocampus Functionally Distinct Structures? Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Beyeler A, Seo C, et al. (2013). BLA to vHPC inputs modulate anxiety-related behaviors. Neuron 79: 658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, et al. (2016). Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience 321: 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC & Tye KM (2014). Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. Journal of Neuroscience 34: 586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiwald W, Duchaine B & Yovel G (2016). Face Processing Systems: From Neurons to Real-World Social Perception. Annual Review of Neuroscience 39: 325–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaburda AM & Bellugi U (2000). V. Multi-level analysis of cortical neuroanatomy in Williams syndrome. J Cogn Neurosci 12 Suppl 1: 74–88. [DOI] [PubMed] [Google Scholar]
- Gangopadhyay P, Chawla M, Dal Monte O, et al. (2021). Prefrontal–amygdala circuits in social decision-making. Nature Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R, Vouimba RM, Baudry M, et al. (1999). The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature 402: 294–296. [DOI] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, et al. (2013). Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences of the United States of America 110: 15638–15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT & Barbas H (2002). Pathways for emotion: Interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience 115: 1261–1279. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, et al. (2009). Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 19: 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, Keistler C, Keip AJ, et al. (2019). Orbitofrontal Circuits Control Multiple Reinforcement-Learning Processes. Neuron 103: 734–746 e733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadagno A, Kang MS, Devenyi GA, et al. (2018a). Reduced resting-state functional connectivity of the basolateral amygdala to the medial prefrontal cortex in preweaning rats exposed to chronic early-life stress. Brain Structure and Function 223: 3711–3729. [DOI] [PubMed] [Google Scholar]
- Guadagno A, Wong TP & Walker CD (2018b). Morphological and functional changes in the preweaning basolateral amygdala induced by early chronic stress associate with anxiety and fear behavior in adult male, but not female rats. Progress in Neuro-Psychopharmacology and Biological Psychiatry 81: 25–37. [DOI] [PubMed] [Google Scholar]
- Haas BW, Mills D, Yam A, et al. (2009). Genetic influences on sociability: heightened amygdala reactivity and event-related responses to positive social stimuli in Williams syndrome. J Neurosci 29: 1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Stein P, Windischberger C, et al. (2011). Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage 56: 881–889. [DOI] [PubMed] [Google Scholar]
- Hampton WH, Unger A, Von Der Heide RJ, et al. (2016). Neural connections foster social connections: A diffusion-weighted imaging study of social networks. Social Cognitive and Affective Neuroscience 11: 721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haznedar MM, Buchsbaum MS, Wei TC, et al. (2000). Limbic circuitry in patients with autism spectrum disorders studied with positron emission tomography and magnetic resonance imaging. Am J Psychiatry 157: 1994–2001. [DOI] [PubMed] [Google Scholar]
- Hitti FL & Siegelbaum SA (2014). The hippocampal CA2 region is essential for social memory. Nature 508: 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB & Vertes RP (2007). Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Structure and Function 212: 149–179. [DOI] [PubMed] [Google Scholar]
- Hosokawa T, Kato K, Inoue M, et al. (2007). Neurons in the macaque orbitofrontal cortex code relative preference of both rewarding and aversive outcomes. Neuroscience Research 57: 434–445. [DOI] [PubMed] [Google Scholar]
- Huang WC, Chen Y & Page DT (2016). Hyperconnectivity of prefrontal cortex to amygdala projections in a mouse model of macrocephaly/autism syndrome. Nat Commun 7: 13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Kim CK, Marshel JH, et al. (2019). Interacting neural ensembles in orbitofrontal cortex for social and feeding behaviour. Nature 565: 645–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon D, Kim S, Chetana M, et al. (2010). Observational fear learning involves affective pain system and Ca v 1.2 Ca 2+ channels in ACC. Nature Neuroscience 13: 482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez JC, Su K, Goldberg AR, et al. (2018). Anxiety Cells in a Hippocampal-Hypothalamic Circuit. Neuron 97: 670–683 e676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W, Bellugi U, Lai Z, et al. (2000). II. Hypersociability in Williams Syndrome. J Cogn Neurosci 12 Suppl 1: 30–46. [DOI] [PubMed] [Google Scholar]
- Jones W, Hesselink J, Courchesne E, et al. (2002). Cerebellar abnormalities in infants and toddlers with Williams syndrome. Dev Med Child Neurol 44: 688–694. [DOI] [PubMed] [Google Scholar]
- Kanner L (1943). Autistic disturbances of affective contact. Nervous Child 2: 217–250. [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, et al. (2012). Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res 21: 169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, et al. (2002). Reduced fear expression after lesions of the ventral hippocampus. Proceedings of the National Academy of Sciences of the United States of America 99: 10825–10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Fitzgerald DA, Angstadt M, et al. (2014). Neural response during attentional control and emotion processing predicts improvement after cognitive behavioral therapy in generalized social anxiety disorder. Psychol Med 44: 3109–3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H, Saleem KS & Price JL (2005). Differential connections of the perirhinal and parahippocampal cortex with the orbital and medial prefrontal networks in macaque monkeys. Journal of Comparative Neurology 493: 479–509. [DOI] [PubMed] [Google Scholar]
- LaBar KS, LeDoux JE, Spencer DD, et al. (1995). Impaired fear conditioning following unilateral temporal lobectomy in humans. Journal of Neuroscience 15: 6846–6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Lipski WJ & Grace AA (2005). A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D 4 receptor-dependent basolateral amygdala input. Journal of Neuroscience 25: 6066–6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, et al. (1990). The lateral amygdaloid nucleus: Sensory interface of the amygdala in fear conditioning. Journal of Neuroscience 10: 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DJ & Glimcher PW (2012). The root of all value: A neural common currency for choice. Current Opinion in Neurobiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, He C, Jian T, et al. (2020). Attenuated link between the medial prefrontal cortex and the amygdala in children with autism spectrum disorder: Evidence from effective connectivity within the "social brain". Progress in Neuropsychology and Biological Psychiatry Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Liao W, Qiu C, Gentili C, et al. (2010). Altered effective connectivity network of the amygdala in social anxiety disorder: a resting-state FMRI study. PLoS One 5: e15238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood PL, Apps MAJ & Chang SWC (2020). Is There a ‘Social’ Brain? Implementations and Algorithms. Trends in Cognitive Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorberbaum JP, Kose S, Johnson MR, et al. (2004). Neural correlates of speech anticipatory anxiety in generalized social phobia. Neuroreport 15: 2701–2705. [PubMed] [Google Scholar]
- Machado CJ & Bachevalier J (2006). The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta). Behavioral Neuroscience 120: 761–786. [DOI] [PubMed] [Google Scholar]
- Magee WJ, Eaton WW, Wittchen HU, et al. (1996). Agoraphobia, simple phobia, and social phobia in the National Comorbidity Survey. Arch Gen Psychiatry 53: 159–168. [DOI] [PubMed] [Google Scholar]
- Martens MA, Wilson SJ & Reutens DC (2008). Research Review: Williams syndrome: a critical review of the cognitive, behavioral, and neuroanatomical phenotype. Journal of Child Psychology and Psychiatry 49: 576–608. [DOI] [PubMed] [Google Scholar]
- Matyi MA & Spielberg JM (2020). Differential spatial patterns of structural connectivity of amygdala nuclei with orbitofrontal cortex. Human Brain Mapping 42: 1391–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdonald AJ (1998). Cortical pathways to the mammalian amygdala. Progress in Neurobiology 55: 257–332. [DOI] [PubMed] [Google Scholar]
- Mcdonald AJ (2014). Amygdala. Encyclopedia of the Neurological Sciences. Second Edi ed.: Academic Press. [Google Scholar]
- Mcdonald AJ, Mascagni F & Guo L (1996). Projections of the medial and lateral prefrontal cortices to the amygdala: A Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience 71: 55–75. [DOI] [PubMed] [Google Scholar]
- McGaugh JL (2004). The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Hariri AR, Munoz KE, et al. (2005). Neural correlates of genetically abnormal social cognition in Williams syndrome. Nat Neurosci 8: 991–993. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, et al. (2008). Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry 65: 568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CA & Mervis CB (2000). Williams syndrome and related disorders. Annu Rev Genomics Hum Genet 1: 461–484. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Buchel C, et al. (1998). A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain 121 (Pt 1): 47–57. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, et al. (1996). A differential neural response in the human amygdala to fearful and happy facial expressions. Nature 383: 812–815. [DOI] [PubMed] [Google Scholar]
- Mosconi MW, Cody-Hazlett H, Poe MD, et al. (2009). Longitudinal study of amygdala volume and joint attention in 2- to 4-year-old children with autism. Arch Gen Psychiatry 66: 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin JC, Philippi CL, Wolf RC, et al. (2015). Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biological Psychiatry 77: 276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munuera J, Rigotti M & Salzman CD (2018). Shared neural coding for social hierarchy and reward value in primate amygdala. Nature Neuroscience 21: 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA & Rudebeck PH (2018). Specializations for reward-guided decision-making in the primate ventral prefrontal cortex. Nat Rev Neurosci 19: 404–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacewicz BM, Dalton KM, Johnstone T, et al. (2006). Amygdala volume and nonverbal social impairment in adolescent and adult males with autism. Arch Gen Psychiatry 63: 1417–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomi JS & Uddin LQ (2015). Face processing in autism spectrum disorders: From brain regions to brain networks. Neuropsychologia 71: 201–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama T, Kitamura T, Roy DS, et al. (2016). Ventral CA1 neurons store social memory. Science 353: 1536–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A, Nearing KI & Phelps EA (2007). Learning fears by observing others: The neural systems of social fear transmission. Social Cognitive and Affective Neuroscience 2: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öngür D, An X & Price JL (1998). Prefrontal cortical projections to the hypothalamus in macaque monkeys. Journal of Comparative Neurology 401: 480–505. [PubMed] [Google Scholar]
- Öngür D & Price JL (2000). The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. [DOI] [PubMed] [Google Scholar]
- Ortiz S, Latsko MS, Fouty JL, et al. (2019). Anterior Cingulate Cortex and Ventral Hippocampal Inputs to the Basolateral Amygdala Selectively Control Generalized Fear. The Journal of neuroscience : the official journal of the Society for Neuroscience 39: 6526–6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoa-Schioppa C & Assad JA (2006). Neurons in the orbitofrontal cortex encode economic value. Nature 441: 223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples R, Franke Y, Wang YK, et al. (2000). A physical map, including a BAC/PAC clone contig, of the Williams-Beuren syndrome-deletion region at 7q11.23. American Journal of Human Genetics 66: 47–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS & Swanson LW (2001). Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Research Reviews. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, et al. (2006). Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biological Psychiatry 59: 424–429. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, et al. (2004). Extinction learning in humans: Role of the amygdala and vmPFC. Neuron 43: 897–905. [DOI] [PubMed] [Google Scholar]
- Pi G, Gao D, Wu D, et al. (2020). Posterior basolateral amygdala to ventral hippocampal CA1 drives approach behaviour to exert an anxiolytic effect. Nature Communications 11: 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Haist F, Sedaghat F, et al. (2004). The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain 127: 2703–2716. [DOI] [PubMed] [Google Scholar]
- Pierce K, Muller RA, Ambrose J, et al. (2001). Face processing occurs outside the fusiform 'face area' in autism: evidence from functional MRI. Brain 124: 2059–2073. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Pikkarainen M, Nurminen N, et al. (2000). Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. Annals of the New York Academy of Sciences. [DOI] [PubMed] [Google Scholar]
- Powell J, Lewis PA, Roberts N, et al. (2012). Orbital prefrontal cortex volume predicts social network size: An imaging study of individual differences in humans. Proceedings of the Royal Society B: Biological Sciences 279: 2157–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather MD, Lavenex P, Mauldin-Jourdain ML, et al. (2001). Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience 106: 653–658. [DOI] [PubMed] [Google Scholar]
- Price JL & Drevets WC (2010). Neurocircuitry of mood disorders. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, et al. (2003). Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci 23: 8800–8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM & Phelps EA (2006). Neurocircuitry Models of Posttraumatic Stress Disorder and Extinction: Human Neuroimaging Research-Past, Present, and Future. Biological Psychiatry. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Eckert MA, Rose FE, et al. (2004). An experiment of nature: brain anatomy parallels cognition and behavior in Williams syndrome. J Neurosci 24: 5009–5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss AL, Eliez S, Schmitt JE, et al. (2000). Neuroanatomy of Williams syndrome: A high-resolution MRI study. Journal of Cognitive Neuroscience 12: 65–73. [DOI] [PubMed] [Google Scholar]
- Reppucci CJ & Petrovich GD (2016). Organization of connections between the amygdala, medial prefrontal cortex, and lateral hypothalamus: a single and double retrograde tracing study in rats. Brain Structure and Function 221: 2937–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reus GZ, dos Santos MAB, Abelaira HM, et al. (2014). Animal models of social anxiety disorder and their validity criteria. Life Sciences 114: 1–3. [DOI] [PubMed] [Google Scholar]
- Rizvi TA, Ennis M, Behbehani MM, et al. (1991). Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: Topography and reciprocity. Journal of Comparative Neurology 303: 121–131. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH & Murray EA (2008). Amygdala and orbitofrontal cortex lesions differentially influence choices during object reversal learning. Journal of Neuroscience 28: 8338–8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscio AM, Brown TA, Chiu WT, et al. (2008). Social fears and social phobia in the USA: results from the National Comorbidity Survey Replication. Psychol Med 38: 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber ESL, De Armentia ML, et al. (2003). The amygdaloid complex: Anatomy and physiology. Physiological Reviews. [DOI] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Kubota Y, et al. (2016). The association between perceived social support and amygdala structure. Neuropsychologia 85: 237–244. [DOI] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Uono S, et al. (2020). Amygdala activity related to perceived social support. Scientific Reports 10: 2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders RC, Rosene DL & Van Hoesen GW (1988). Comparison of the efferents of the amygdala and the hippocampal formation in the rhesus monkey: II. Reciprocal and non-reciprocal connections. Journal of Comparative Neurology 271: 185–207. [DOI] [PubMed] [Google Scholar]
- Schneider F, Weiss U, Kessler C, et al. (1999). Subcortical correlates of differential classical conditioning of aversive emotional reactions in social phobia. Biol Psychiatry 45: 863–871. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P & Montague PR (1997). A neural substrate of prediction and reward. Science 275: 1593–1599. [DOI] [PubMed] [Google Scholar]
- Schumann CM & Amaral DG (2006). Stereological analysis of amygdala neuron number in autism. J Neurosci 26: 7674–7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Barnes CC, Lord C, et al. (2009). Amygdala enlargement in toddlers with autism related to severity of social and communication impairments. Biol Psychiatry 66: 942–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Bauman MD & Amaral DG (2011). Abnormal structure or function of the amygdala is a common component of neurodevelopmental disorders. Neuropsychologia 49: 745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, et al. (2004). The amygdala is enlarged in children but not adolescents with autism; The hippocampus is enlarged at all ages. Journal of Neuroscience 24: 6392–6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer B & Pandya DN (1989). Frontal lobe connections of the superior temporal sulcus in the rhesus monkey. Journal of Comparative Neurology 281: 97–113. [DOI] [PubMed] [Google Scholar]
- Senn V, Wolff SBE, Herry C, et al. (2014). Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron 81: 428–437. [DOI] [PubMed] [Google Scholar]
- Sharpe MJ & Schoenbaum G (2016). Back to basics: Making predictions in the orbitofrontal-amygdala circuit. Neurobiology of Learning and Memory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Lawrence EJ, Radbourne C, et al. (2004). The impact of early and late damage to the human amygdala on 'theory of mind' reasoning. Brain 127: 1535–1548. [DOI] [PubMed] [Google Scholar]
- Shen MD, Li DD, Keown CL, et al. (2016). Functional Connectivity of the Amygdala Is Disrupted in Preschool-Aged Children With Autism Spectrum Disorder. Journal of the American Academy of Child and Adolescent Psychiatry 55: 817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, et al. (2004). Regional Cerebral Blood Flow in the Amygdala and Medial Prefrontal Cortex during Traumatic Imagery in Male and Female Vietnam Veterans with PTSD. Archives of General Psychiatry 61: 168–176. [DOI] [PubMed] [Google Scholar]
- Sigman M, Spence SJ & Wang AT (2006). Autism from developmental and neuropsychological perspectives. Annu Rev Clin Psychol 2: 327–355. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, et al. (2012). Gating of Fear in Prelimbic Cortex by Hippocampal and Amygdala Inputs. Neuron 76: 804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks BF, Friedman SD, Shaw DW, et al. (2002). Brain structural abnormalities in young children with autism spectrum disorder. Neurology 59: 184–192. [DOI] [PubMed] [Google Scholar]
- Stefanacci L & Amaral DG (2002). Some observations on cortical inputs to the macaque monkey amygdala: An anterograde tracing study. Journal of Comparative Neurology 451: 301–323. [DOI] [PubMed] [Google Scholar]
- Stein MB, Goldin PR, Sareen J, et al. (2002). Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry 59: 1027–1034. [DOI] [PubMed] [Google Scholar]
- Stein MB & Gorman JM (2001). Unmasking social anxiety disorder. J Psychiatry Neurosci 26: 185–189. [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, et al. (2014). Functional organization of the hippocampal longitudinal axis. Nature Reviews Neuroscience. [DOI] [PubMed] [Google Scholar]
- Sul JH, Kim H, Huh N, et al. (2010). Distinct roles of rodent orbitofrontal and medial prefrontal cortex in decision making. Neuron 66: 449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Carrasco M, Wiggins JL, et al. (2014). Age-related changes in the structure and function of prefrontal cortex-amygdala circuitry in children and adolescents: A multi-modal imaging approach. NeuroImage 86: 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talepasand S & Nokani M (2010). Social phobia symptoms: prevalence and sociodemographic correlates. Arch Iran Med 13: 522–527. [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell'Acqua F, Valabregue R, et al. (2012). Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex 48: 82–96. [DOI] [PubMed] [Google Scholar]
- Toth I, Neumann ID & Slattery DA (2013). Social fear conditioning as an animal model of social anxiety disorder. Curr Protoc Neurosci Chapter 9: Unit9 42. [DOI] [PubMed] [Google Scholar]
- Tsao DY, Moeller S & Freiwald WA (2008). Comparing face patch systems in macaques and humans. Proceedings of the National Academy of Sciences of the United States of America 105: 19514–19519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit R, Flor H, Erb M, et al. (2002). Brain circuits involved in emotional learning in antisocial behavior and social phobia in humans. Neurosci Lett 328: 233–236. [DOI] [PubMed] [Google Scholar]
- Von Der Heide RJ, Skipper LM, Klobusicky E, et al. (2013). Dissecting the uncinate fasciculus: Disorders, controversies and a hypothesis. Brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, et al. (2001). Effects of attention and emotion on face processing in the human brain: An event-related fMRI study. Neuron 30: 829–841. [DOI] [PubMed] [Google Scholar]
- Wang J & Barbas H (2018). Specificity of primate amygdalar pathways to hippocampus. Journal of Neuroscience 38: 10019–10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson KK & Platt ML (2012). Social signals in primate orbitofrontal cortex. Current Biology 22: 2268–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskrantz L (1956). Behavioral changes associated with ablation of the amygdaloid complex in monkeys. J Comp Physiol Psychol 49: 381–391. [DOI] [PubMed] [Google Scholar]
- Werner E & Dawson G (2005). Validation of the phenomenon of autistic regression using home videotapes. Arch Gen Psychiatry 62: 889–895. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, et al. (1998). Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience 18: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan CG, Rincón-Cortés M, Raineki C, et al. (2017). Aberrant development of intrinsic brain activity in a rat model of caregiver maltreatment of offspring. Translational Psychiatry 7: e1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y & Wang JZ (2017). From structure to behavior in basolateral amygdala-hippocampus circuits. Frontiers in Neural Circuits. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wang ZH, Jin S, et al. (2016). Opposite monosynaptic scaling of BLP-vCA1 inputs governs hopefulness-and helplessness-modulated spatial learning and memory. Nature Communications 7: 11935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AW, Aggleton JP, Hellawell DJ, et al. (1995). Face processing impairments after amygdalotomy. Brain 118 (Pt 1): 15–24. [DOI] [PubMed] [Google Scholar]
- Yuan M, Zhu H, Qiu C, et al. (2016). Group cognitive behavioral therapy modulates the resting-state functional connectivity of amygdala-related network in patients with generalized social anxiety disorder. BMC Psychiatry 16: 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaboski BA & Storch EA (2018). Comorbid autism spectrum disorder and anxiety disorders: a brief review. Future Neurol 13: 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, McHugo M, Ray KL, et al. (2014). Meta-Analytic Connectivity Modeling Reveals Differential Functional Connectivity of the Medial and Lateral Orbitofrontal Cortex. Cerebral Cortex 24: 232–248. [DOI] [PMC free article] [PubMed] [Google Scholar]