Abstract
Filoviruses cause severe hemorrhagic fever with case fatality rates as high as 90%. Filovirus-specific monoclonal antibodies (mAbs) confer protection in nonhuman primates as late as 5 days after challenge, and FDA-approved mAbs REGN-EB3 and mAb114 have demonstrated efficacy against Ebola virus (EBOV) infection in humans. Vectorized antibody expression mediated by adeno-associated virus (AAV) can generate protective and sustained concentrations of therapeutic mAbs in animal models for a variety of infectious diseases, including EBOV. Here we demonstrate that AAV6.2FF-mediated expression of murine IgG2a EBOV mAbs, 2G4 and 5D2, protects from mouse-adapted (MA)-EBOV infection with none of the surviving mice developing anti-VP40 antibodies above background. Protective serum concentrations of AAV6.2FF-2G4/AAV6.2FF-5D2 did not alter endogenous antibody responses to heterologous virus infection. AAV-mediated expression of EBOV mAbs 100 and 114, and pan-ebolavirus mAbs, FVM04, ADI-15878, and CA45, as human IgG1 antibodies conferred protection against MA-EBOV at low serum concentrations, with minimum protective serum levels as low as 2 μg/mL. Vectorized expression of murine IgG2a or human IgG1 mAbs led to sustained expression in the serum of mice for >400 days or for the lifetime of the animal, respectively. AAV6.2FF-mediated mAb expression offers an alternative to recombinant antibody administration in scenarios where long-term protection is preferable to passive immunization.
Keywords: adeno-associated virus (AAV), Ebola, vectored immunoprophylaxis, immunotherapy, monoclonal antibody, AAV6.2FF, filovirus
Graphical abstract
Vectorized monoclonal antibody (mAb) expression mediated by adeno-associated virus generates protective and sustained concentrations of therapeutic mAbs in mice that protect against challenge with EBOV, even at low serum concentrations, and does not impede the endogenous antibody response to heterologous viral infection.
Introduction
Highly pathogenic members of the Filoviridae family, including Ebola virus (EBOV), have shown immense epidemic potential and constitute a major public health concern.1 Passive antibody transfer is a method used to treat many infectious diseases, including EBOV disease (EVD).2 Some of the first antibodies raised against EBOV were developed by the Public Health Agency of Canada (PHAC) and included murine monoclonal antibodies (mAbs) 2G4 and 5D2.3 2G4 is a neutralizing antibody that binds to the viral glycoprotein (GP) base at a shallow angle,4, 5, 6 while 5D2 is a non-neutralizing antibody that binds the GP mucin-like domain, which is cleaved prior to fusion in the endosome.7 2G4 was further developed as part of a three-component mAb cocktail, ZMapp, which conferred 100% survival in nonhuman primates (NHPs) as late as 5 days after challenge with EBOV8 and was also used to treat humans during the 2014–16 West Africa Ebola epidemic (NCT02363322).9
While 2G4 and 5D2 were generated by vaccinating mice with a recombinant vesicular stomatitis Indiana virus expressing the EBOV GP,3 newer methods of isolating antibody sequences by high throughput sequencing of B cells from naturally infected survivors yields potent mAbs of human origin.10 For example, human mAbs 100 and 114 were able to confer 100% survival in NHPs administered the cocktail of antibodies as late as 5 days after challenge with EBOV (variant Kikwit),11 and in the case of mAb114, it was well tolerated in humans.12 These antibodies bind to critical structural epitopes to interfere with the ability of EBOV GP to mediate endosomal escape and represent an ideal class of mAbs for clinical development.13 Isolation and characterization of mAbs from human survivors of EVD continues, with a surge occurring after the 2014 West Africa Ebola outbreak.14
Development of pan-ebolavirus mAbs has been possible through immunization of NHPs with either a cocktail of recombinant filovirus GPs or recombinant VSVs displaying GPs from distinct ebolaviruses, such as EBOV, Sudan virus (SUDV), and Bundibugyo virus, resulting in potent, neutralizing, pan-ebolavirus mAbs such as ADI-15878, FVM04, and CA45.15, 16, 17, 18, 19
AAV vectored expression of pathogen-specific mAbs offers a promising alternative to traditional vaccines in addition to a strategy for long-term passive immunization.20,21 The ability of AAV-mediated mAb expression to confer immunity without the need to stimulate the endogenous immune system represents an important application for this prophylactic therapy. Immunocompromised individuals,22 as well as adults of advanced age who respond poorly to conventional vaccines due to age-related decline in immunity, could benefit greatly from this alternative prophylactic approach.23,24
Previous reports utilizing the AAV6.2FF capsid, which consists of F129L, Y445F, and Y731F mutations in the AAV6 capsid backbone, demonstrated rapid and robust transgene expression from both the muscle and lung.25 This capsid elicits long-term systemic expression of mAbs, which conferred protection in multiple infectious disease models, including at mucosal surfaces.26, 27, 28 Here, we further explore the immunity mediated by this AAV6.2FF-mAb approach at reduced vector doses, and we use human IgG1 (hIgG1) filovirus mAbs in immunocompetent mice to expand the utility of our AAV6.2FF-mAb expression platform. In dose reduction experiments, we demonstrate AAV6.2FF vectors expressing murine IgG2a, and later hIgG1 mAbs, have the ability to prevent morbidity and mortality in the mouse-adapted EBOV challenge model, even with low level mAb expression. Furthermore, we investigate the expression and protection of pan-ebolavirus mAbs in the EBOV mouse model to expand the potential arsenal of therapeutics against EVD for future epidemics.
Results
A cocktail of AAV6.2FF-2G4/AAV6.2FF-5D2 prevents morbidity and mortality in mice challenged with mouse-adapted EBOV
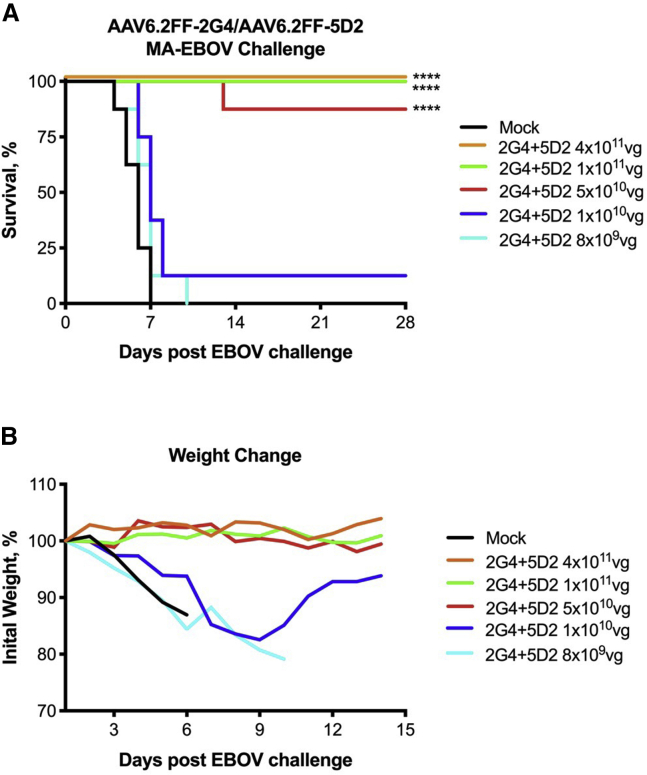
We previously demonstrated that a 4 × 1011 vector genome (vg) dose of AAV6.2FF expressing anti-EBOV GP antibodies 2G4 and 5D2 as murine IgG2a mAbs (2 × 1011 vg each of AAV6.2FF-5D2 and AAV6.2FF-2G4 were administered to mice in separate leg muscles) conferred 100% survival when administered only 7 days prior to challenge.26 In an effort to determine the minimum therapeutic dose of this vector cocktail, we performed a dose reduction experiment in which mice were intramuscularly (IM) administered (in separate leg muscles) AAV doses ranging from a total of 4 × 1011 vg to 8 × 109 vg 28 days prior to mouse-adapted EBOV (MA-EBOV) challenge. At the 4 × 1011 vg, 1 × 1011 vg, and 5 × 1010 vg doses, 100%, 100%, and 88% survival was observed, respectively (Figure 1A). The 1 × 1010 vg dose resulted in a single survivor, and the lowest dose, 8 × 109 vg, did not protect any animals. There was no morbidity associated with the survivors of the 4 × 1011 vg, 1 × 1011 vg, and 5 × 1010 vg doses; however, the single mouse that survived at the 1 × 1010 vg dose did experience significant weight loss prior to recovery (Figure 1B).
Figure 1.
AAV6.2FF-2G4/AAV6.2FF-5D2 dose-reduction experiment
BALB/c mice (n = 8/group) were administered a range of total vector genomes (vg) of AAV6.2FF-2G4 and AAV6.2FF-5D2 intramuscularly, with each vector administered to separate muscle groups. Mock animals were administered a dose of 4 × 1011 vg of AAV6.2FF expressing luciferase. 28 days following AAV administration, mice were challenged with 1000xLD50 MA-EBOV and monitored for (A) survival and (B) weight loss (plotted as group averages). Survival of treated groups was compared with the mock group using the Mantel-Cox log rank test. ∗∗∗∗p < 0.0001.
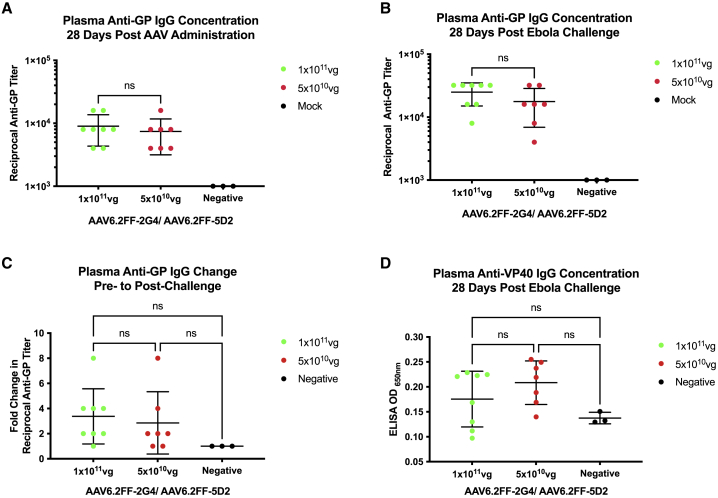
Immediately prior to challenge, serum was collected from the mice in the dose reduction experiment to determine the reciprocal EBOV GP antibody titer (Figure 2A). These titers represent the concentrations of AAV6.2FF-mediated expression of mAbs 2G4 and 5D2 28 days after vector administration in the mice that survived the subsequent challenge. GP antibody titers were also determined 28 days after MA-EBOV challenge to investigate if there was an increase in GP antibody concentrations due to the contribution of the endogenous humoral response against EBOV infection (Figure 2B). Note that for this analysis, only mice that survived challenge with MA-EBOV were included as only they would have had a chance to mount an immune response to MA-EBOV. Additionally, serum from the mice administered a dose of 4 × 1011 vg were not evaluated since the survival was equivalent to mice that received a dose of 1 × 1011 vg. The difference in pre- and post-challenge GP antibody titers ranged from 1X to 8X; however, all the mice included in this analysis survived, and therefore the pre-challenge GP antibody titers were sufficiently protective (Figure 2C). Sera taken from surviving mice 28 days after EBOV challenge were also examined for antibodies against the EBOV matrix protein, VP40, since up to 40% of whole viral protein mass is composed of VP40, and VP40-specific antibodies have been demonstrated in human serum samples following infection (Figure 2D).29,30 VP40 antibodies were not detected significantly above background in any mouse. This result, combined with the minimal increase of GP antibody titers after challenge in the majority of animals and negligible weight loss, indicates that AAV6.2FF-2G4/AAV6.2FF-5D2 treatment may have neutralized EBOV before extensive replication occurred.
Figure 2.
EBOV GP reciprocal antibody titers before and after challenge from mice that survived MA-EBOV challenge
Sera from the surviving mice in the AAV6.2FF-2G4/AAV6.2FF-5D2 dose reduction experiment (1 × 1011 vg, n = 8, and 5 × 1010 vg, n = 7) were analyzed by EBOV GP ELISA (A) 28 days after AAV administration but before challenge and (B) 28 days after challenge. (C) The fold change in pre- to post-challenge reciprocal anti-GP titers observed at each dose. (D) 28 days post-challenge serum samples were analyzed at a 1:100 dilution by EBOV VP40 ELISA; negative controls were serum samples from naive, untreated mice separate from the challenge studies. A one-way ANOVA with Tukey’s multiple comparisons test revealed no significant differences between groups. All error bars represent the standard deviation of the mean.
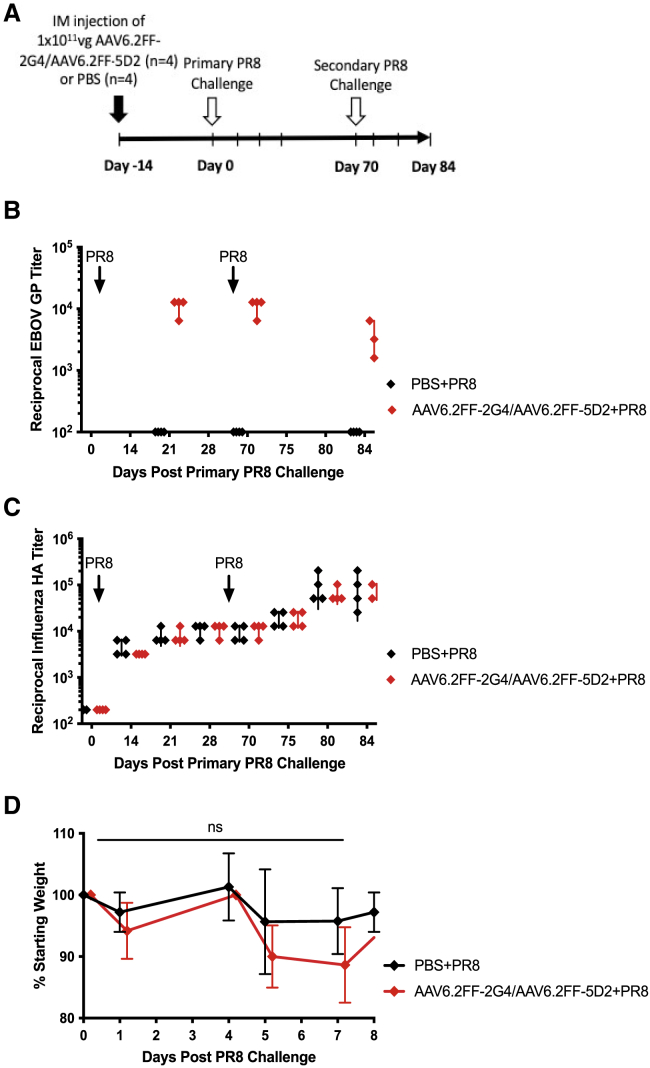
Protective mAb titers mediated by AAV6.2FF-2G4/AAV6.2FF-5D2 do not impair the endogenous antibody response to a heterologous virus infection
To investigate the ability of the endogenous antibody immune response to respond appropriately to a heterologous infection in the context of AAV6.2FF vectorized mAb expression, mice pre-treated with either AAV6.2FF-2G4/AAV6.2FF-5D2 or PBS were exposed to a non-lethal challenge of influenza A virus (strain PR8), and the antibody response to the influenza hemagglutinin (HA) protein was evaluated (Figure 3). Reciprocal antibody titers against EBOV GP were below background in the PBS-treated group but did reach protective concentrations in AAV6.2FF-2G4/AAV6.2FF-5D2-treated mice (Figure 3B). Influenza virus HA antibody titers were indistinguishable between the treatment groups for both primary and secondary influenza virus exposures (Figure 3C), indicating AAV6.2FF-mAb treatment does not hamper the primary or secondary B cell response in mice. Mice were weighed daily for the first week following primary influenza virus infection, and no significant difference between treatment groups was observed (Figure 3D). Moreover, mice remained clinically healthy with no apparent adverse effects.
Figure 3.
Endogenous humoral response to influenza A virus in the context of protective 2G4/5D2 antibody titers
(A) Schematic of experimental design. BALB/c mice (n = 4/group) were administered 1 × 1011 vg of AAV6.2FF-2G4/AAV6.2FF-5D2 IM 14 days prior to primary sub-lethal exposure to 600 HA units of influenza A virus (strain PR8) by IP injection. (B) Reciprocal EBOV GP titers from serum samples from AAV6.2FF-2G4/AAV6.2FF-5D2- or PBS-treated groups. (C) Reciprocal HA titers following primary and secondary exposure to 600 HA units of influenza A virus (strain PR8) in mice treated with AAV6.2FF-2G4/AAV6.2FF-5D2 or PBS. (D) Average weight change in mice following primary influenza A virus. A two-way ANOVA and Šídák’s test for multiple comparisons was conducted to compare weights between the two treatment groups. All error bars represent the standard deviation of the mean.
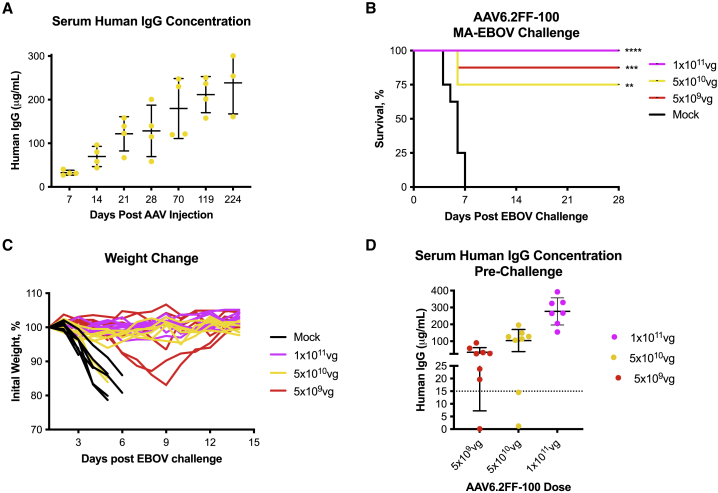
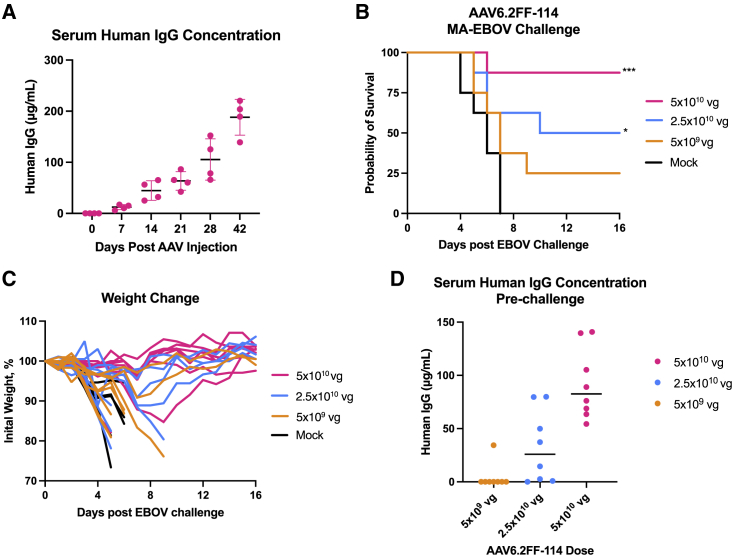
Serum 100 and 114 concentrations as low as 20 μg/mL and 80 μg/mL, respectively, confer survival against EBOV challenge
We previously selected murine IgG2a antibodies to vectorize mAb sequences 5D2 and 2G4 in part to avoid an immune response against human IgG (hIgG). However, B cell mining of human survivors has resulted in exceptionally potent mAbs against filoviruses, and expressing human antibodies in mice would streamline preclinical development. Therefore, two AAV6.2FF vectors were engineered to express mAb100 and mAb11411 as hIgG1 (AAV6.2FF-100 and AAV6.2FF-114). The mAbs contain human IgG1 constant domains, allowing for the precise quantification of transgene expression mediated by AAV6.2FF, which was a technical limitation when using vectors expressing murine IgG2a in a mouse model. The AAV6.2FF-100 and AAV6.2FF-114 vectors were administered via the IM route to mice to monitor the kinetics of hIgG1 expression (Figure 4A and Figure 5A, respectively). Concentrations of mAb100 and mAb114 increased steadily with the time course, demonstrating continuous antibody expression as well as potentially indicating hIgG1 recycling by murine FcRn receptors.31 Furthermore, expression of mAb100 at concentrations of >200 μg/mL at 32 weeks after AAV administration suggests a lack of immune response against either the human Fc domain or the variable regions of this particular hIgG1 antibody. Finally, despite sustained, high-concentration expression of hIgG1 from AAV6.2FF, there was no apparent negative effect on the concentration of endogenous murine IgG levels (Figure S1).
Figure 4.
AAV6.2FF-100 mediates complete protection from EBOV challenge
(A) Expression kinetics of mAb100 as measured by the concentration of human IgG in the serum of BALB/c mice (n = 4) that received 5 × 1010 vg of AAV6.2FF-100 via IM route and were monitored over the course of 224 days (32 weeks). (B–C) BALB/c mice (n = 8/group) were administered various doses of AAV6.2FF-100 by IM injection. Mock animals were administered a dose of 1 × 1011 vg of AAV6.2FF expressing an irrelevant influenza mAb, FluA-20. 28 days after AAV administration, mice were challenged with 1000xLD50 MA-EBOV and monitored for survival (B) and weight change (C) (individual mice plotted). (D) Serum concentrations of human IgG (100) were quantified 1 day prior to challenge. Dotted line denotes minimum threshold concentration of human IgG required to mediate survival in this challenge model. Survival of treated groups was compared with the mock group using the Mantel-Cox log rank test. ∗∗∗∗p < 0.0001, ∗∗p < 0.01, and ∗p < 0.05 for mice treated with 1 × 1011 vg, 5 × 1010 vg, and 5 × 109 vg, respectively, of AAV6.2FF-100 during the 1000xLD50 MA-EBOV challenge. All error bars represent the standard deviation of the mean.
Figure 5.
AAV6.2FF-114 mediates moderate protection from EBOV challenge
(A) Expression kinetics of mAb114 measured as human IgG in the serum of BALB/c mice (n = 4) that received 5 × 1010 vg of AAV6.2FF-114 IM and were monitored over the course of 42 days. BALB/c mice (n = 8/group) were administered various doses of AAV6.2FF-114 via IM administration, while controls were administered 5 × 1010 vg of AAV6.2FF expressing an irrelevant influenza mAb, FluA-20. At 28 days after AAV administration, mice were challenged with 1000xLD50 MA-EBOV and monitored for (B) survival and (C) weight loss (individual mice plotted). (D) Serum concentrations of human IgG were quantified 1 day prior to challenge. Survival of treated groups was compared with the mock group using the Mantel-Cox log rank test. ∗∗∗p < 0.001 and ∗p < 0.05 for mice treated with 5 × 1010 vg and 5 × 109 vg, respectively, of AAV6.2FF-114 during the 1000xLD50 MA-EBOV challenge. All error bars represent the standard deviation of the mean.
To evaluate the efficacy of AAV6.2FF-100, groups of mice were administered three different doses of vector and challenged 28 days later with a lethal dose of MA-EBOV. A 1 × 1011 vg dose of AAV6.2FF-100 conferred 100% survival, whereas doses of 5 × 1010 vg and 5 × 109 vg conferred 75% and 87.5% survival, respectively (Figure 4B). Weight loss was not observed for any mice in the high-dose (1 × 1011 vg) group, suggesting AAV6.2FF-100 was able to induce sterilizing immunity at this dose (Figure 4C). At the medium dose tested (5 × 1010 vg), six of eight mice survived, and similar to the high dose, weight loss was not observed in the survivors. Unfortunately, one of the mice in the medium-dose group had no detectable human IgG at time of challenge, likely due to a technical issue during vector administration, resulting in reduced survival compared with the low-dose group. At the lowest dose (5 × 109 vg), seven of eight mice survived the challenge; however, two surviving mice experienced weight loss, demonstrating that this dose was likely approaching the minimum efficacious threshold for this vector. Interestingly, in the AAV6.2FF-2G4/AAV6.2FF-5D2 dose reduction experiment, the lowest dose of 8 × 109 vg resulted in 0% protection, while a slightly lower dose of 5 × 109 vg of AAV6.2FF-100 yielded 87.5% survival, highlighting the benefit of selecting potent antibodies that are highly expressed from the AAV-mAb platform (See Table S1 for available IC50 values and Figure S3 for neutralizing activity).
Serum samples were collected immediately prior to challenge, allowing quantification of mAb100 at the time of challenge, to further understand the minimum mAb concentration required to confer protection (Figure 4D). The high dose of AAV6.2FF-100 yielded an average human IgG serum concentration of 277 μg/mL, the medium dose generated an average of 104 μg/mL, and the low dose had an average of 40 μg/mL, demonstrating scaling of the vector dose to output antibody concentration is not necessarily linear. Two mice in the 5 × 1010 vg group had much lower serum 100 concentrations compared with the rest of the group, 1.1 μg/mL and 14.5 μg/mL, and died. We believe this may have been due to technical issues during vector administration. Furthermore, the mouse that succumbed to infection in the 5 × 109 vg group also had low human IgG expression at 0.13 μg/mL, while the mice that survived had concentrations that ranged from 19.6 to 91 μg/mL for the low-dose group, 105–196 μg/mL for the medium-dose group, and 155–392 μg/mL for the high-dose group. Therefore, the minimum protective threshold of AAV6.2FF-mediated expression of mAb100 is somewhere between 15 and 20 μg/mL, which represents a reasonable target for clinical translation.
AAV6.2FF-114 was somewhat less efficacious than AAV6.2FF-100 in the murine EBOV challenge model, with 87.5%, 50%, and 25% of mice administered with AAV6.2FF-114 at doses of 5 × 1010 vg, 2.5 × 1010 vg, and 5 × 109 vg, respectively, surviving challenge with MA-EBOV (Figure 5B). Weight loss was observed in two mice from the high-dose (5 × 1010 vg) group, with one displaying rapid weight loss and succumbing to infection at 6 days after challenge, similar to control animals, and the other showing lower and slower weight loss and recovering from infection (Figure 5C). Three mice in the medium-dose (2.5 × 1010 vg) group had rapid weight loss and succumbed to infection, while a fourth mouse experienced a gradual decline in weight over 9 days prior to reaching endpoint. There was considerable weight loss in the low-dose (5 × 109 vg) group, with the only surviving mouse having moderate weight loss preceding recovery (Figure 5C). The fact that the majority of mice in the medium- and low-dose groups experienced weight loss after challenge demonstrates that the minimum efficacious threshold of this vector/mAb combination likely lies somewhere between 5 × 1010 vg (high dose) and 2.5 × 1010 vg (medium dose).
Similar to the AAV6.2FF-100 challenge experiment, serum samples were collected 1 day prior to challenge to determine serum human IgG concentration at the time of challenge and the minimum concentration of mAb114 required for protection (Figure 5D). The high-, medium-, and low-dose groups had pre-challenge human IgG serum concentrations between 54 and 141 μg/mL, 0–80 μg/mL, and 0–34 μg/mL, respectively. Only one mouse in the low-dose group had human IgG concentrations above the level of detection; however, there were two mice that survived. It is possible that the mouse with human IgG concentrations below the level of detection that survived might have inadvertently received a lower challenge dose of MA-EBOV, although this is highly speculative. Interestingly, of the two highest human IgG-expressing mice in the medium-dose group, both at concentrations of 80 μg/mL, one experienced gradual weight loss and reached endpoint, while the other had minimal weight loss and survived (Figure 5C). Similarly, one mouse in the high-dose group expressing 76 μg/mL died, while multiple mice expressing less serum human IgG survived infection. Notwithstanding the variability in human IgG expression and survival in the high-, medium-, and low-dose groups receiving AAV6.2FF-114, these results would suggest that the minimum protective threshold of serum mAb114 is likely less than 50 μg/mL, but further experimentation is required to define a more accurate threshold for protection.
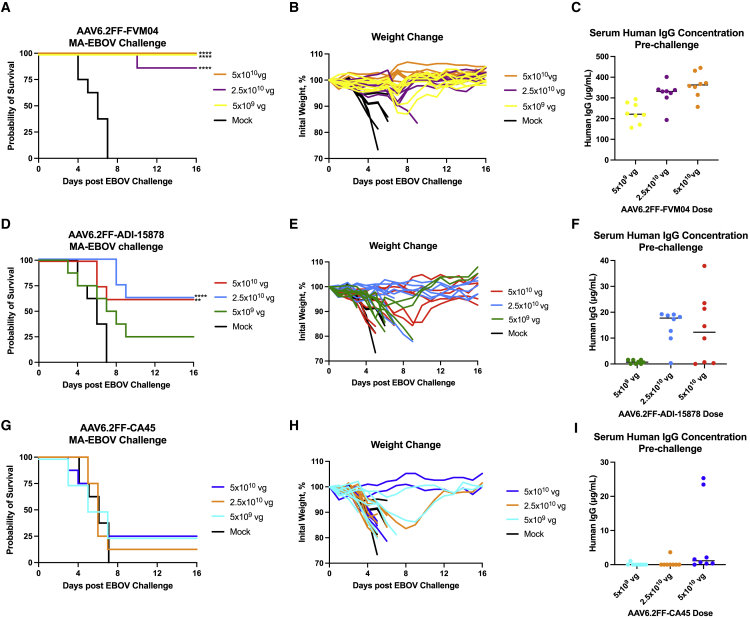
AAV-vectorized pan-ebolavirus mAbs protect mice against MA-EBOV challenge with variable efficacy
In an effort to expand the AAV6.2FF-mAb platform technology to other ebolaviruses, three pan-ebolavirus mAbs were engineered as hIgG1 mAbs and expressed from AAV6.2FF: ADI-15878,32 which was derived from a human survivor of the 2014 West African EBOV outbreak, and CA4515 and FVM04,18,33 which were derived from macaques immunized with GPs from EBOV, SUDV, and Marburg virus (Figure S2). Mice were IM administered AAV6.2FF-FVM04, AAV6.2FF-ADI-15878, or AAV6.2FF-CA45 at low, mid, or high doses of 5 × 109 vg, 2.5 × 1010 vg, or 5 × 1010 vg, respectively, and challenged 28 days later with 1000xLD50 MA-EBOV. AAV6.2FF-FVM04 administered at a dose of 5 × 1010 vg and 5 × 109 vg conferred 100% survival, whereas a dose of 2.5 × 1010 vg resulted in 87.5% survival (Figure 6A). Minimal weight loss was observed in all AAV-treated mice, apart from one mouse in the medium-dose group, which succumbed to infection 10 days after challenge (Figure 6B). No weight loss was observed for any mice in the high-dose group, 5 × 1010 vg, suggesting that AAV6.2FF-FVM04 may have conferred sterilizing immunity at this dose. Serum samples were collected 1 day prior to challenge, and serum human IgG was quantified. A dose reduction in AAV correlated with a reduction in serum human IgG concentration, with serum human IgG concentrations ranging from 257 to 445 μg/mL in the high-dose group (n = 8), 193–401 μg/mL in the medium-dose group (n = 8), and 155–294 μg/mL in the low-dose group (n = 8) (Figure 6C). Although there was one mouse in the medium-dose group with a human IgG serum concentration of 302 μg/mL that reached endpoint following MA-EBOV challenge, other AAV-treated mice expressing lower concentrations of human IgG survived challenge, suggesting that this was not the minimum threshold of protection. Since all but the one mouse with serum human IgG levels in the range of 155–445 μg/mL survived challenge, with little to no weight loss, further AAV6.2FF-FVM04 dose-reduction experiments are needed to determine the minimum serum human IgG concentration needed for protection.
Figure 6.
AAV6.2FF-FVM04 provides excellent protection, whereas AAV6.2FF-ADI-15878 and AAV6.2FF-CA45 provide moderate protection against MA-EBOV challenge
BALB/c mice (n = 8/group) were administered high (5 × 1010 vg), mid (2.5 × 1010 vg), and low (5 × 109 vg) doses of either AAV6.2FF-FVM04 (A–C), AAV6.2FF-ADI-15878 (D–F), or AAV6.2FF-CA45 (G–I) IM, while controls were administered 5 × 1010 vg of an AAV6.2FF vector expressing an irrelevant influenza mAb, FluA-20. 28 days after AAV administration, mice were challenged with 1000xLD50 MA-EBOV IP and monitored for (A, D, G) survival and (B, E, H) weight loss (individual mice plotted). (C, F, I) Serum concentrations of human IgG were quantified immediately prior to challenge. Survival of treated groups was compared with the mock group using the Mantel-Cox log rank test. ∗∗∗∗p < 0.0001 for mice treated will all three doses of AAV6.2FF-FVM04 and 2.5 × 1010 vg of AAV6.2FF-ADI-15878, and ∗∗p < 0.01 for mice treated with 5 × 1010 vg ADI-15878 during the 1000xLD50 MA-EBOV challenge. All error bars represent the standard deviation of the mean.
When administered at the same dose, AAV6.2FF-ADI-15878 provided lower levels of protection than AAV6.2FF-FVM04, with 62.5% protection from the 5 × 1010 vg and 2.5 × 1010 vg doses, and 50% protection from the 5 × 109 vg dose (Figure 6D). Mice that succumbed to infection with MA-EBOV experienced moderate to substantial weight loss across the three treatment groups. Mice in the medium-dose group (2.5 × 1010 vg) that survived challenge lost the least amount of weight; however, there was variable weight loss in the high-dose group, suggesting that higher doses of AAV might be needed to induce sterilizing immunity. There was no correlation between survival and dosage between the treatment groups, which can be explained by variable serum human IgG concentrations (Figure 6F). Although the vector administered to mice in this experiment was from the same virus stock, there did not appear to be a dose response with respect to the concentration of serum human IgG between the treatment groups. Interestingly, while AAV6.2FF-ADI-15878 did not lead to high levels of antibody expression, mice with serum human IgG concentrations as low as 1.6 μg/mL were protected from challenge, suggesting that the minimum threshold of serum human IgG for ADI-15878 is very low.
AAV6.2FF-mediated expression of CA45 yielded the lowest rates of survival among the pan-ebolavirus mAbs, with 25% survival in the high-dose (5 × 1010 vg) and medium-dose (2.5 × 1010 vg) groups and 12.5% survival in the low-dose (5 × 109 vg) group (Figure 6G). There were moderately high levels of weight loss across all three treatment groups. Only two mice in the high-dose group and one mouse in the medium-dose group did not experience weight loss, suggesting that a higher dose of AAV6.2FF-CA45 might be required to confer sterilizing immunity (Figure 6H). While there was a trend toward higher AAV dosage and higher levels of serum human IgG, more than a few mice had serum human IgG concentrations below the level of detection (Figure 6I). In the high-dose AAV group, the two surviving mice had serum human IgG levels of 23 μg/mL and 25 μg/mL, while the other mice had serum human IgG levels from non-detectable to 2.1 μg/mL. There was no correlation between serum human IgG concentrations and survival, as the two surviving mice in the low-dose (5 × 109 vg) group had serum human IgG concentrations of non-detectable and 0.1 μg/mL, while none of the mice with up to 2.1 μg/mL in the high-dose group survived. Due to the variability in protective serum human IgG concentrations across treatment groups, further experiments would be required to accurately determine the minimum protective threshold of AAV6.2FF-mediated expression of CA45.
Post-exposure administration of AAV6.2FF-mAbs results in partial survival
Vectorized mAb expression has clear pharmacokinetic advantages as an alternative to long-term passive recombinant antibody therapy; however, it remained unclear if AAV6.2FF-mAbs would be effective in a therapeutic setting since these AAV vectors take time to generate protective mAb concentrations.
The mouse model of EBOV infection is rapid and stringent, with death usually occurring 5–7 days following challenge with a dose of 1000xLD50. While such high challenge doses offer a robust test for prospective countermeasures, they do not necessarily recapitulate human contact exposure to EBOV. Moreover, in most cases of human infection, there is typically an incubation period of 6–10 days prior to the presentation of initial symptoms.34 In an attempt to more accurately model the course of clinical infection, the challenge dose of MA-EBOV was reduced to 100xLD50 to investigate whether post-exposure use of AAV6.2FF-mAbs could provide a survival benefit. Groups of mice were treated IM with either 1 × 1011 vg of AAV6.2FF-2G4/AAV6.2FF-5D2 (5 × 1010 vg each in separate leg muscles) or 5 × 1010 vg of AAV6.2FF-100 immediately following IP injection of 100xLD50 MA-EBOV. Interestingly, two of eight mice from each group survived despite 100% death in the mock-treated group (Figure 7A). Of the surviving mice, one from each group experienced weight loss, while the other gained weight throughout the monitoring period (Figure 7B). It is important to note that 25% survival was observed for both treatments, despite AAV6.2FF-100 being administered at half the dose of AAV6.2FF-2G4/AAV6.2FF-5D2.
Figure 7.
Post-challenge protection mediated by AAV6.2FF-2G4/AAV6.2FF-5D2 and AAV6.2FF-100
BALB/c mice (n = 8/group) were challenged with a dose of 100xLD50 (A, B) or 30xLD50 (C, D) MA-EBOV IP and were immediately injected IM with 1 × 1011 vg of the AAV6.2FF-2G4/AAV6.2FF-5D2 cocktail or 5 × 1010 vg AAV6.2FF-100 or PBS (mock) and monitored for 28 days for (A, C) survival and (B, D) weight loss (individual mice plotted). Survival of treated groups was compared with the mock group using the Mantel-Cox log rank test. ∗∗p < 0.01 for 1 × 1011 vg and ∗p < 0.05 for 5 × 1010 vg of AAV6.2FF-2G4/AAV6.2FF-5D2 cocktail in the 100xLD50 MA-EBOV. No significant differences were observed in the 30xLD50 MA-EBOV challenge experiment.
In an effort to further reduce the stringency of the model, groups of mice were challenged with 30xLD50 MA-EBOV intraperitoneally and then immediately administered either 1 × 1011 vg of AAV6.2FF-2G4/AAV6.2FF-5D2 (5 × 1010 vg each in separate leg muscles) or 5 × 1010 vg of AAV6.2FF-100 intramuscularly (Figure 7C). At this challenge dose, we observed 50% survival in the AAV6.2FF-100 treatment group, 25% survival in the AAV6.2FF-2G4/AAV6.2FF-5D2 group, and 37.5% survival in the mock group. Weight loss was observed across all groups, with weight gained back in those mice that survived (Figure 7D). Unfortunately, at the 30xLD50 challenge dose of MA-EBOV, there was no statistically significant difference between the treatment groups and the mock group.
AAV6.2FF mediates long-term mAb expression for the natural lifespan of a mouse
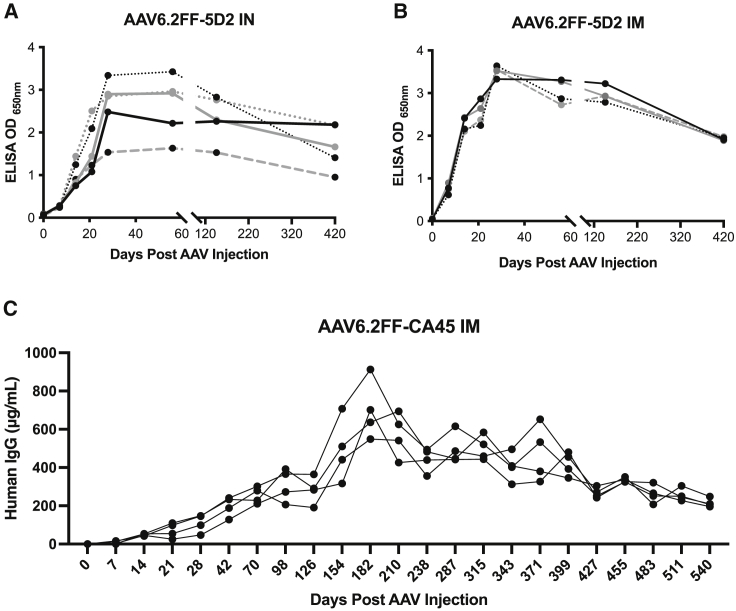
In order for AAV vectored immunoprophylaxis to be useful in the fight against infectious diseases, particularly in immunocompromised individuals, durable production of biologically active mAbs is critical. For this reason, we evaluated the kinetics and duration of mAb expression for first-generation AAV vectors expressing murine IgG2a mAbs as well as for second-generation AAV vectors expressing hIgG mAbs. In the case of AAV6.2FF-2G4 and AAV6.2FF-5D2, we observed peak AAV6.2FF-mAb expression 28 days post IM or intranasal (IN) vector administration, and these high concentrations plateaued until day 146 (Figures 8A and 8B). Between day 146 and 420, mAb concentrations decreased for both methods of vector delivery; however, these terminal concentrations were still greater than 0.8 OD650nm, which was previously demonstrated to be a protective mAb concentration.26 Interestingly, despite IM injection yielding much more consistent serum mAb concentrations than IN administration of the same vector dose, both routes demonstrated very similar patterns of mAb expression kinetics over the 420-day experiment, regardless of the magnitude of peak expression.
Figure 8.
Long-term expression of murine IgG2a 5D2 and human IgG1 CA45
AAV6.2FF-5D2 (1 × 1011 vg) was administered to 6-week-old female BALB/c mice by (A) IM (n = 4) or (B) IN (n = 5) instillation. Serum samples were collected at weekly intervals and analyzed by EBOV GP ELISA at a 1:100 dilution. AAV6.2FF-CA45 (1 × 1011 vg) was administered IM (C) to 6-week-old female BALB/c mice (n = 4). Serum samples were collected for the lifespan of the mice and plasma human IgG levels were quantified using a commercial ELISA. Each animal is plotted individually.
Today, many monoclonal antibodies used to treat disease come from human survivors, as opposed to immunized animals. When expanding our AAV6.2FF-mAb platform, developing vectors expressing human IgG was a top priority, though the concern of anti-drug antibodies and longevity of expressing human monoclonal antibodies in a murine model did arise.26 To determine if long-term expression was possible, or if potential human IgG immune clearance was an issue, mice were IM administered 1 × 1011 vg AAV6.2FF-CA45, and their serum human IgG expression levels were monitored for the lifespan of the animals. Human IgG serum concentrations on day 14 were 42–54 μg/mL and continued to increase to 48–147 μg/mL on day 28 after administration (Figure 8C), showing that the putative level of protection for serum CA45 was achieved within 2 weeks if AAV was administered at a dose of 1 × 1011 vg, and it was well above by day 28, our typical day for EBOV challenge (Figure 6I). We observed peak expression of human IgG in the serum from 548 to 912 μg/mL at day 182, with a steady decline in expression to 195–247 μg/mL on their last bleed at 540 days after AAV administration. The mice died or reached endpoint due to natural causes within a week of each other, shortly after day 540. The levels of human IgG in the serum past day 7 at a dose of 1 × 1011 vg appear to be beyond the minimum protective concentration against 1000xLD50 MA-EBOV challenge in a mouse model, and the mice sustained these protective concentrations for the duration of their lives. Interestingly, BALB/c mice administered 1 × 1011 vg of AAV6.2FF-CA45 IN had no detectable serum human IgG at any point in their lives (data not shown), whereas the AAV6.2FF expressing a murine IgG2a had similar expression when administered both IM and IN.
Discussion
Antibody gene transfer mediated by AAV vectors is a promising alternative vaccination and passive immunotherapy strategy, particularly for individuals with compromised immune systems and for pathogens that lack effective vaccines, such as HIV35,36 and RSV.37 In this study, we demonstrated that AAV-mediated expression of ebolavirus-specific mAbs as either murine IgG2a or human IgG1 protects mice from lethal challenge with MA-EBOV when used prophylactically. With the exception of 5D2, all mAbs evaluated in this study were neutralizing. These same AAV-expressed mAbs also conferred modest protection when used in a post-exposure scenario. Despite serum human IgG1 mAb reaching concentrations greater than 900 μg/mL, there was no evidence that this impaired the host’s endogenous immune response to infection with a heterologous pathogen. We also demonstrate that life-long expression of mAbs from an AAV vector is possible in a murine model and that AAV vectors can be effectively re-administered to mice previously exposed to AAV, demonstrating the potential power of this platform for conferring sustained immunity against multiple pathogens to susceptible individuals.
The lack of significant weight loss in the AAV6.2FF-2G4/AAV6.2FF-5D2 animals combined with minimal increases in pre- to post-challenge GP antibody titers and negative VP40 antibody titers suggests that these AAV6.2FF-mAb vectors effectively blunted virus infection. VP40 forms the highly ordered matrix structure that coats the inside of the viral particle.38 While VP40 is the most abundant protein in EBOV virions, VP40 is not present on the surface of the EBOV particle, and a lack of VP40 antibodies is suggestive of sterilizing immunity.39 However, to be confident this is the case, follow-up studies would need to monitor viral load after challenge.
Although the concentration of AAV-expressed mAbs was sufficient to prevent both morbidity and mortality following MA-EBOV challenge, it was unclear whether high concentrations of AAV-expressed antibody would affect the endogenous antibody response, potentially similar to how colostrum prevents an infant from effectively generating an immune response to a vaccine.40,41 We showed that naive mice or those treated with AAV6.2FF-2G4/AAV6.2FF-5D2 responded immunologically with equivalent influenza HA antibody titers, thus were able to respond normally to an infection while expressing protective 2G4/5D2 mAb concentrations. This finding demonstrates AAV6.2FF-mAb therapeutic vaccines could be regularly used without fear of suppressing the endogenous immune system and could also potentially be used in combination with conventional vaccines. However, it is unclear whether the hyper IgG affected the glomerular flow in some way prior to challenge or during the EBOV challenge via immune complexes. Future investigations are needed to address this possibility.
Hyperglobulinemia is a possible side effect of AAV6.2FF-mAb therapies. It is difficult to find a clinical definition for IgG-related hyperglobulinemia as the condition is generally related to IgD and IgE.42,43 A normal IgG concentration for a mouse is 2–5 mg/mL.44 AAV6.2FF-100/114 administered at 1 × 1010 vg IM expressed an average of 239 μg/mL of human IgG compared with an average of 1,121 μg/mL of murine IgG, resulting in human IgG making up 18% of total IgG in mice (Figure S1). Although there were high concentrations of human IgG mAbs in the serum of mice after AAV vector administration, no apparent side effects related to high-concentration mAb expression were observed in the present investigation. ZMapp was dosed at 50 mg/kg over multi-day treatment courses in humans to maintain a therapeutic threshold9; however, much lower but more consistent serum mAb concentrations mediated by AAV6.2FF should be able to maintain therapeutic efficacy without the peak and trough pharmacokinetics associated with repeat recombinant mAb administration.45
There is strong evidence of the prophylactic efficacy of AAV6.2FF-mAb therapies,26, 27, 28 allowing for post-exposure use to extend the potential applications of this therapy. Potential exposures to EBOV in a lab accident or health-care setting are realistic possibilities. For therapeutic use, AAV6.2FF-mAbs could be combined with an initial bolus of recombinant antibody to extend the therapeutic window while also providing immediate intervention. The MA-EBOV challenge model is rapid with a small therapeutic window, so even partial survival at a 100xLD50 challenge dose suggests that post-exposure and/or therapeutic applications of AAV6.2FF-mAbs for less virulent pathogens might be feasible. The experiment was repeated with a further reduced challenge dose of 30xLD50 MA-EBOV; however, the results were inconclusive as the LD50 dose for MA-EBOV does not scale linearly and was not uniformly lethal.
AAV6.2FF vectors expressing ebolavirus mAbs as human IgG1 antibodies were highly effective at protecting mice from MA-EBOV challenge, even at low mAb concentrations as in the case of 100 (20 μg/mL) and ADI-15878 (2–10 μg/mL), which suggests that scaling AAV doses for human applications should be feasible. For some AAV-mAbs, such as AAV6.2FF-FVM04, antibody expression was so efficient that the dose of AAV needed to express therapeutic concentrations of mAbs is likely quite low. Indeed, AAV6.2FF-FVM04 would be an ideal candidate to include in a pan-ebolavirus or pan-filovirus AAV-mAb cocktail. Expansion of the AAV6.2FF-mAb platform to express pan-reactive antibodies that protect against multiple different ebolaviruses highlights the suitability of this technology for applications beyond EBOV infections. Finally, the fact that AAV-driven expression of human IgG1 Fc domain-containing mAbs persisted for the lifetime of a mouse, without any apparent immune-mediated destruction of AAV-transduced cells, suggests that it may be possible to use the same vector design when translating from small animal models to NHPs and ultimately humans without the need to re-engineer vectors to express species-specific IgG constant domains. Indeed, we have shown that AAV-mediated expression of human IgG1 mAbs is feasible in mice,26 Syrian hamsters,27 and sheep.28 More recently, data from a phase 1, dose-escalation clinical trial (NCT03374202) evaluating AAV8-mediated expression of the broadly neutralizing anti-HIV mAb VRC07 from an expression cassette that we based our EBOV mAb expression platform on in this body of work is encouraging in that all eight individuals produced measurable amounts of serum VRC07, with maximal concentrations >1 μg/mL in three participants.46 In four individuals, VRC07 serum concentrations remained stable near maximal concentration for up to 3 years of follow-up, and unlike the first human clinical trial to evaluate AAV-mediated expression of an HIV bNAb, ADA responses were observed in only three of eight participants, and only in two cases did this lead to decreased serum VRC07, demonstrating that AAV-mAb expression is potentially a viable alternative platform for protection against infectious diseases.
While AAV VIP is particularly well suited for chronic viral infections like HIV47 and hepatitis C virus,48 it also has utility for acute viral infections. For example, ebolavirus infections and outbreaks occur in areas of the world where HIV and malaria are endemic and lead to generalized immunosuppression, which may make infected individuals less responsive to traditional vaccines and/or unable to mount a robust immune response to an infection.49,50 Additionally, there are reports of persistent ebolavirus infections,51,52 in some cases leading to inadvertent transmission via semen53 or breastmilk.54 In some circumstances, vaccination is contraindicated in immunosuppressed individuals, which in the case of SARS-CoV-2 has led to persistent infections and the emergence of variants due to replication for extended period of time without immune pressure.55 Consequently, this proof of concept study could be extended to other infectious diseases that lack approved vaccines or antivirals. Indeed, we have demonstrated that AAV VIP can be used to protect against challenge with C. Difficile toxins,27 thereby extending the application to bacterial infections, particularly those that have acquired antibiotic resistance.
In conclusion, we show that sustained in vivo production of AAV-vectored delivery of ebolavirus neutralizing antibodies leads to long-term, systemic transgene expression that conferred protection from viral challenge in a murine model. These findings, combined with our recent safety study28 showing that AAV6.2FF-mediated mAb expression in murine and ovine models exhibited a lack of toxicological findings by both serum biochemistry and histopathology analysis, provide promise that this antibody gene transfer platform will offer protection against filovirus challenges in larger animal models. These experiments are ongoing.
Materials and methods
AAV vectors
All monoclonal antibody genes were expressed using the bicistronic expression cassette developed by Fang et al.56 and optimized by Balazs et al.,57 including a CASI promoter, heavy and light chains separated by a furin-2A cleavage site, and a WPRE and SV40 poly A signal between the AAV2 inverted terminal repeats (ITR). Murine mAbs were engineered using the murine IgG2a heavy chain and kappa light chain sequences. Murine mAbs were engineered with their original kappa light chains. For consistency, human origin mAbs were cloned as human IgG1 molecules with a lambda light chain constant domain. All vectors were produced using the AAV6.2FF capsid25 as described.58
BSL2 mouse experiments and animal ethics statement
Experiments involving animals were approved by University of Guelph Animal Care Committee according to the guidelines outlined by the Canadian Council on Animal Care. All experiments were completed using 6-week-old female BALB/c mice (Charles River). IM injections were diluted to a 40–50 μL volume in PBS and administered to the gastrocnemius muscle using a 29G needle. AAV cocktails were administered at equimolar quantities by separate injections to two flanks to prevent co-transduction. Intranasal administration of vector with a 50 μL administration volume was performed as previously described.59 Non-lethal influenza virus (strain PR8; A/PR/8/34(H1N1)) challenge was completed by IP injection of 600 HA units diluted in PBS to a 500 μL volume for both primary and secondary exposures.
BSL4 mouse experiments and animal ethics statement
All work with infectious EBOV was performed in the containment level 4 laboratory at the Canadian Science Center for Human and Animal Health (CSCHAH), PHAC, Winnipeg, Manitoba, in accordance with standard operating protocols. Animal experiments were reviewed and approved by the CSCHAH Animal Care Committee in accordance with guidelines from the Canadian Council on Animal Care. All procedures were performed on animals under anesthesia using inhalational isoflurane. Seriously ill animals that reached the humane clinical endpoint, as well as all surviving animals at 28 days dpi, were euthanized by induction of deep anesthesia with isoflurane followed by cervical dislocation. Endpoint criteria included several parameters such as weight, behavior, activity, coat condition, etc. All staff working on animal experiments completed education and training programs according to the standard protocols appropriate for this biosafety.
Evaluation of AAV efficacy in mice
Female BALB/c mice, 5–6 weeks old, were purchased from the Charles River Laboratories. Groups of mice (eight per group) were treated with a high, medium, or low dose (see figure legends for specific vg for high-, medium-, and low-dose groups and Table S2 for doses reported in vg/kg) of AAV6.2FF-mAb vectors. For challenge experiments involving AAVs expressing 114, FVM04, 15,878, and CA45, the control group was injected with 5 × 1010 vg of an AAV vector expressing an irrelevant mAb (FluA20). For all other challenge experiments, control mice were administered PBS. All AAV vectors were diluted in PBS to 50 μL final volume, except for the high-dose group of AAV6.2FF-CA45, for which the final volume was 90 μL. AAV vectors were administered in the quadriceps via a single IM injection, except for the high dose of AAV6.2FF-CA45, which was administered via IM injection of 45 μL into the quadriceps of each hind leg. Serum samples were collected on the day prior to and 27 days after AAV treatment from all mice for human IgG ELISA assay. Mice were challenged with 1,000 median lethal dose (LD50) of MA-EBOV (variant Mayinga) in 200 μL DMEM via the intraperitoneal (IP) route (100 μL/side). Animals were monitored for 28 days after challenge for survival; body weight and clinical signs were recorded daily up to 16 days after challenge. Post-exposure EBOV challenges were completed using an IP dose of 100 and 30 times LD50.
Biodistribution analysis
Genomic DNA was extracted from mouse tissues using the Qiagen DNeasy Blood & Tissue kit (Hilden, Germany). AAV ITR copy number was quantified by Taqman qPCR (IDT, Coralville, Iowa) and normalized by nanodrop DNA concentrations.60
Enzyme-linked immunosorbent assays
Serum samples were collected by saphenous bleed in EDTA collection tubes (BD, Franklin Lakes, New Jersey) and aliquoted for storage at −80°C. Human IgG and murine IgG concentrations were quantified using commercially available kits (Abcam, Cambridge, United Kingdom, ab195215 and ab157719). Reciprocal antibody titers were determined by coating half-area 96-well plates (Corning, New York) with 1 μg/mL recombinant EBOV GP (IBT Bioservices, Rockville, DC, 0501-001), EBOV VP40 (IBT Bioservices, Rockville, DC, 0564-001), or Influenza A virus HA (SinoBiological Beijing, China, 11,684-V08H) protein overnight at 4°C. Plates were washed four times with 0.2% PBS-Tween20 (PBS-T) and blocked with SuperBlock buffer (Fisher, Waltham, Massachusetts). 2-fold serial dilutions were incubated at 37°C for 1 h and then washed four times with PBS-T. Secondary antibody (Pierce P31430) was incubated at 1:2,000 for 1 h at 37°C, washed four times with PBS-T, and incubated with TMB substrate (Pierce PI34021) for 15 min before reading absorbance values at 650 nm. Reciprocal titer was defined as the highest 2-fold serum dilution that gave an OD650 value 2-fold greater than negative control wells.
Statistics
Statistical analysis was performed using GraphPad Prism 7 software. Kaplan-Meyer survival plots were analyzed by Mantel-Cox log rank test for statistical significance compared with mock treated controls. All error bars represent the mean ± standard deviation.
Data availability
Data generated or analyzed during this study can be found within the published article and its supplementary files.
Acknowledgments
Funding for this work was provided by the Canadian Institutes of Health Research (CIHR) project grant #352532, the Public Health Agency of Canada (PHAC), and the Mitacs Accelerate Program (grant #IT18741). Basic science components of the research were supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant awarded to SKW (grant #499834). Stipend funding for L.P.V., A.D.R., and S.P.T. was provided by the Ontario Graduate Scholarship and Ontario Veterinary College Graduate Scholarship programs.
Author contributions
Conceptualization, L.P.V., A.D.R., X.Q., L.B., and S.K.W. Methodology, L.P.V., A.D.R., C.W., S.H., G.S., W.Z., S.P.T., D.S., K.F., and K.T. Writing – original draft preparation, L.P.V. and A.D.R. Writing – review & editing, A.D.R., C.W., B.T., R.R.K., L.B., and S.K.W. Supervision, S.B., D.S., B.W.B., X.Q., L.B., and S.K.W. Project Administration, X.Q., L.B., and S.K.W. Funding acquisition, X.Q. and S.K.W.
Declaration of interests
L.P.L. and S.K.W. are inventors on a US patent for the AAV6.2FF capsid. This patent (US20190216949) is licensed to Avamab Pharma Inc., where B.T., L.P.L., and S.K.W. are co-founders and B.T. serves as an executive. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; nor in the decision to publish the results.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtm.2022.08.003.
Supplemental information
References
- 1.Languon S., Quaye O. Filovirus disease outbreaks: a chronological overview. Virology. 2019;10 doi: 10.1177/1178122X19849927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tshiani Mbaya O., Mukumbayi P., Mulangu S. Review: insights on current FDA-approved monoclonal antibodies against ebola virus infection. Front. Immunol. 2021;12:721328. doi: 10.3389/fimmu.2021.721328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qiu X., Alimonti J.B., Melito P.L., Fernando L., Stroher U., Jones S.M. Characterization of Zaire ebolavirus glycoprotein-specific monoclonal antibodies. Clin. Immunol. 2011;141:218–227. doi: 10.1016/j.clim.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Audet J., Wong G., Wang H., Lu G., Gao G.F., Kobinger G., Qiu X. Molecular characterization of the monoclonal antibodies composing ZMAb: a protective cocktail against Ebola virus. Sci. Rep. 2014;4:6881. doi: 10.1038/srep06881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murin C.D., Fusco M.L., Bornholdt Z.A., Qiu X., Olinger G.G., Zeitlin L., Kobinger G.P., Ward A.B., Saphire E.O. Structures of protective antibodies reveal sites of vulnerability on Ebola virus. Proc. Natl. Acad. Sci. USA. 2014;111:17182–17187. doi: 10.1073/pnas.1414164111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson E., Bryan C., Fong R.H., Barnes T., Pfaff J.M., Mabila M., Rucker J.B., Doranz B.J. Mechanism of binding to ebola virus glycoprotein by the ZMapp, ZMAb, and MB-003 cocktail antibodies. J. Virol. 2015;89:10982–10992. doi: 10.1128/JVI.01490-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiu X., Fernando L., Melito P.L., Audet J., Feldmann H., Kobinger G., Alimonti J.B., Jones S.M. Ebola GP-specific monoclonal antibodies protect mice and Guinea pigs from lethal Ebola virus infection. PLoS Negl. Trop. Dis. 2012;6:e1575. doi: 10.1371/journal.pntd.0001575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiu X., Wong G., Audet J., Bello A., Fernando L., Alimonti J.B., Fausther-Bovendo H., Wei H., Aviles J., Hiatt E., et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514:47–53. doi: 10.1038/nature13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davey R.T., Dodd L., Proschan M.A., Neaton J., Neuhaus Nordwall J., Koopmeiners J.S., Beigel J., Tierney J., Lane H.C., Fauci A.S., et al. A randomized, controlled trial of ZMapp for ebola virus infection. N. Engl. J. Med. 2016;375:1448–1456. doi: 10.1056/NEJMoa1604330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowe J.E. Principles of broad and potent antiviral human antibodies: insights for vaccine design. Cell Host Microbe. 2017;22:193–206. doi: 10.1016/j.chom.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corti D., Misasi J., Mulangu S., Stanley D.A., Kanekiyo M., Wollen S., Ploquin A., Doria-Rose N.A., Staupe R.P., Bailey M., et al. Protective monotherapy against lethal Ebola virus infection by a potently neutralizing antibody. Science. 2016;351:1339–1342. doi: 10.1126/science.aad5224. [DOI] [PubMed] [Google Scholar]
- 12.Gaudinski M.R., Coates E.E., Novik L., Widge A., Houser K.V., Burch E., Holman L.A., Gordon I.J., Chen G.L., Carter C., et al. Safety, tolerability, pharmacokinetics, and immunogenicity of the therapeutic monoclonal antibody mAb114 targeting Ebola virus glycoprotein (VRC 608): an open-label phase 1 study. Lancet. 2019;393:889–898. doi: 10.1016/S0140-6736(19)30036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Misasi J., Gilman M.S., Kanekiyo M., Gui M., Cagigi A., Mulangu S., Corti D., Ledgerwood J.E., Lanzavecchia A., Cunningham J., et al. Structural and molecular basis for Ebola virus neutralization by protective human antibodies. Science. 2016;351:1343–1346. doi: 10.1126/science.aad6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bornholdt Z.A., Turner H.L., Murin C.D., Li W., Sok D., Souders C.A., Piper A.E., Goff A., Shamblin J.D., Wollen S.E., et al. Isolation of potent neutralizing antibodies from a survivor of the 2014 Ebola virus outbreak. Science. 2016;351:1078–1083. doi: 10.1126/science.aad5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao X., Howell K.A., He S., Brannan J.M., Wec A.Z., Davidson E., Turner H.L., Chiang C.I., Lei L., Fels J.M., et al. Immunization-elicited broadly protective antibody reveals ebolavirus fusion loop as a site of vulnerability. Cell. 2017;169:891–904.e5. doi: 10.1016/j.cell.2017.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brannan J.M., He S., Howell K.A., Prugar L.I., Zhu W., Vu H., Shulenin S., Kailasan S., Raina H., Wong G., et al. Post-exposure immunotherapy for two ebolaviruses and Marburg virus in nonhuman primates. Nat. Commun. 2019;10:105. doi: 10.1038/s41467-018-08040-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holtsberg F.W., Shulenin S., Vu H., Howell K.A., Patel S.J., Gunn B., Karim M., Lai J.R., Frei J.C., Nyakatura E.K., et al. Pan-ebolavirus and pan-filovirus mouse monoclonal antibodies: protection against ebola and Sudan viruses. J. Virol. 2016;90:266–278. doi: 10.1128/JVI.02171-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keck Z.Y., Enterlein S.G., Howell K.A., Vu H., Shulenin S., Warfield K.L., Froude J.W., Araghi N., Douglas R., Biggins J., et al. Macaque monoclonal antibodies targeting novel conserved epitopes within filovirus glycoprotein. J. Virol. 2016;90:279–291. doi: 10.1128/JVI.02172-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wec A.Z., Bornholdt Z.A., He S., Herbert A.S., Goodwin E., Wirchnianski A.S., Gunn B.M., Zhang Z., Zhu W., Liu G., et al. Development of a human antibody cocktail that deploys multiple functions to confer pan-ebolavirus protection. Cell Host Microbe. 2019;25:39–48.e5. doi: 10.1016/j.chom.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rghei A.D., van Lieshout L.P., Santry L.A., Guilleman M.M., Thomas S.P., Susta L., Karimi K., Bridle B.W., Wootton S.K. AAV vectored immunoprophylaxis for filovirus infections. Trop Med. Infect. Dis. 2020;5 doi: 10.3390/tropicalmed5040169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deal C.E., Balazs A.B. Engineering humoral immunity as prophylaxis or therapy. Curr. Opin. Immunol. 2015;35:113–122. doi: 10.1016/j.coi.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanders J.W., Ponzio T.A. Vectored immunoprophylaxis: an emerging adjunct to traditional vaccination. Trop. Dis. Travel Med. Vaccines. 2017;3:3. doi: 10.1186/s40794-017-0046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen J.C., Toapanta F.R., Chen W., Tennant S.M. Understanding immunosenescence and its impact on vaccination of older adults. Vaccine. 2020;38:8264–8272. doi: 10.1016/j.vaccine.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demicheli V., Jefferson T., Di Pietrantonj C., Ferroni E., Thorning S., Thomas R.E., Rivetti A. Vaccines for preventing influenza in the elderly. Cochrane Database Syst. Rev. 2018;2:CD004876. doi: 10.1002/14651858.CD004876.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Lieshout L.P., Domm J.M., Rindler T.N., Frost K.L., Sorensen D.L., Medina S.J., Booth S.A., Bridges J.P., Wootton S.K. A novel triple-mutant AAV6 capsid induces rapid and potent transgene expression in the muscle and respiratory tract of mice. Mol. Ther. Methods Clin. Dev. 2018;9:323–329. doi: 10.1016/j.omtm.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Lieshout L.P., Soule G., Sorensen D., Frost K.L., He S., Tierney K., Safronetz D., Booth S.A., Kobinger G.P., Qiu X., Wootton S.K. Intramuscular adeno-associated virus-mediated expression of monoclonal antibodies provides 100% protection against ebola virus infection in mice. J. Infect. Dis. 2018;217:916–925. doi: 10.1093/infdis/jix644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guilleman M.M., Stevens B.A.Y., Van Lieshout L.P., Rghei A.D., Pei Y., Santry L.A., Thompson B., Wootton S.K. AAV-mediated delivery of actoxumab and bezlotoxumab results in serum and mucosal antibody concentrations that provide protection from C. difficile toxin challenge. Gene Ther. 2021 doi: 10.1038/s41434-021-00236-y. [DOI] [PubMed] [Google Scholar]
- 28.Rghei A.D., van Lieshout L.P., McLeod B.M., Yanlong P., Lopes J.A., Zielinska N., Baracuhy E., Stevens B.A.Y., Thomas S.P., Yates J.G.E., et al. Safety and tolerability of the adeno-associated virus vector, AAV6.2FF, expressing a monoclonal antibody in murine and ovine animal models. Biomedicines. 2021;9:9. doi: 10.3390/biomedicines9091186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becquart P., Mahlakõiv T., Nkoghe D., Leroy E.M. Identification of continuous human B-cell epitopes in the VP35, VP40, nucleoprotein and glycoprotein of Ebola virus. PLoS One. 2014;9:e96360. doi: 10.1371/journal.pone.0096360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elliott L.H., Kiley M.P., McCormick J.B. Descriptive analysis of Ebola virus proteins. Virology. 1985;147:169–176. doi: 10.1016/0042-6822(85)90236-3. [DOI] [PubMed] [Google Scholar]
- 31.Andersen J.T., Daba M.B., Berntzen G., Michaelsen T.E., Sandlie I. Cross-species binding analyses of mouse and human neonatal Fc receptor show dramatic differences in immunoglobulin G and albumin binding. J. Biol. Chem. 2010;285:4826–4836. doi: 10.1074/jbc.M109.081828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wec A.Z., Herbert A.S., Murin C.D., Nyakatura E.K., Abelson D.M., Fels J.M., He S., James R.M., de La Vega M.A., Zhu W., et al. Antibodies from a human survivor define sites of vulnerability for broad protection against ebolaviruses. Cell. 2017;169:878–890.e5. doi: 10.1016/j.cell.2017.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howell K.A., Qiu X., Brannan J.M., Bryan C., Davidson E., Holtsberg F.W., Wec A.Z., Shulenin S., Biggins J.E., Douglas R., et al. Antibody treatment of ebola and Sudan virus infection via a uniquely exposed epitope within the glycoprotein receptor-binding site. Cell Rep. 2016;15:1514–1526. doi: 10.1016/j.celrep.2016.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bwaka M.A., Bonnet M.J., Calain P., Colebunders R., De Roo A., Guimard Y., Katwiki K.R., Kibadi K., Kipasa M.A., Kuvula K.J., et al. Ebola hemorrhagic fever in Kikwit, Democratic Republic of the Congo: clinical observations in 103 patients. J. Infect. Dis. 1999;179:S1–S7. doi: 10.1086/514308. [DOI] [PubMed] [Google Scholar]
- 35.Brady J.M., Baltimore D., Balazs A.B. Antibody gene transfer with adeno-associated viral vectors as a method for HIV prevention. Immunol. Rev. 2017;275:324–333. doi: 10.1111/imr.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson P.R., Schnepp B.C., Zhang J., Connell M.J., Greene S.M., Yuste E., Desrosiers R.C., Clark K.R. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat. Med. 2009;15:901–906. doi: 10.1038/nm.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tycko J., Adam V.S., Crosariol M., Ohlstein J., Sanmiguel J., Tretiakova A.P., Roy S., Worgall S., Wilson J.M., Limberis M.P. Adeno-associated virus vector-mediated expression of antirespiratory syncytial virus antibody prevents infection in mouse airways. Hum. Gene Ther. 2021;32:1450–1456. doi: 10.1089/hum.2021.079. [DOI] [PubMed] [Google Scholar]
- 38.Bornholdt Z.A., Noda T., Abelson D.M., Halfmann P., Wood M.R., Kawaoka Y., Saphire E.O. Structural rearrangement of ebola virus VP40 begets multiple functions in the virus life cycle. Cell. 2013;154:763–774. doi: 10.1016/j.cell.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sullivan N.J., Sanchez A., Rollin P.E., Yang Z.Y., Nabel G.J. Development of a preventive vaccine for Ebola virus infection in primates. Nature. 2000;408:605–609. doi: 10.1038/35046108. [DOI] [PubMed] [Google Scholar]
- 40.Edwards K.M. Maternal antibodies and infant immune responses to vaccines. Vaccine. 2015;33:6469–6472. doi: 10.1016/j.vaccine.2015.07.085. [DOI] [PubMed] [Google Scholar]
- 41.Niewiesk S. Maternal antibodies: clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front. Immunol. 2014;5:446. doi: 10.3389/fimmu.2014.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hashemi H., Mohebbi M., Mehravaran S., Mazloumi M., Jahanbani-Ardakani H., Abtahi S.H. Hyperimmunoglobulin E syndrome: genetics, immunopathogenesis, clinical findings, and treatment modalities. J. Res. Med. Sci. 2017;22:53. doi: 10.4103/jrms.JRMS_1050_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kostjukovits S., Kalliokoski L., Antila K., Korppi M. Treatment of hyperimmunoglobulinemia D syndrome with biologics in children: review of the literature and Finnish experience. Eur. J. Pediatr. 2015;174:707–714. doi: 10.1007/s00431-015-2505-9. [DOI] [PubMed] [Google Scholar]
- 44.Klein-Schneegans A.S., Kuntz L., Fonteneau P., Loor F. Serum concentrations of IgM, IgG1, IgG2b, IgG3 and IgA in C57BL/6 mice and their congenics at the lpr (lymphoproliferation) locus. J. Autoimmun. 1989;2:869–875. doi: 10.1016/0896-8411(89)90013-9. [DOI] [PubMed] [Google Scholar]
- 45.Joos B., Trkola A., Kuster H., Aceto L., Fischer M., Stiegler G., Armbruster C., Vcelar B., Katinger H., Günthard H.F. Long-term multiple-dose pharmacokinetics of human monoclonal antibodies (MAbs) against human immunodeficiency virus type 1 envelope gp120 (MAb 2G12) and gp41 (MAbs 4E10 and 2F5) Antimicrob. Agents Chemother. 2006;50:1773–1779. doi: 10.1128/AAC.50.5.1773-1779.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Casazza J.P., Cale E.M., Narpala S., Yamshchikov G.V., Coates E.E., Hendel C.S., Novik L., Holman L.A., Widge A.T., Apte P., et al. Safety and tolerability of AAV8 delivery of a broadly neutralizing antibody in adults living with HIV: a phase 1, dose-escalation trial. Nat. Med. 2022;28:1022–1030. doi: 10.1038/s41591-022-01762-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gardner M.R. Promise and progress of an HIV-1 cure by adeno-associated virus vector delivery of anti-HIV-1 biologics. Front. Cell. Infect. Microbiol. 2020;10:176. doi: 10.3389/fcimb.2020.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Jong Y.P., Dorner M., Mommersteeg M.C., Xiao J.W., Balazs A.B., Robbins J.B., Winer B.Y., Gerges S., Vega K., Labitt R.N., et al. Broadly neutralizing antibodies abrogate established hepatitis C virus infection. Sci. Transl. Med. 2014;6:254ra129. doi: 10.1126/scitranslmed.3009512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwenti T.E. Malaria and HIV coinfection in sub-Saharan Africa: prevalence, impact, and treatment strategies. Res. Rep. Trop. Med. 2018;9:123–136. doi: 10.2147/RRTM.S154501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Calle C.L., Mordmüller B., Singh A. Immunosuppression in malaria: do plasmodium falciparum parasites hijack the host? Pathogens. 2021;10 doi: 10.3390/pathogens10101277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Forrester J.V. Ebola virus and persistent chronic infection: when does replication cease? Ann. Transl. Med. 2018;6:S39. doi: 10.21037/atm.2018.09.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sissoko D., Duraffour S., Kerber R., Kolie J.S., Beavogui A.H., Camara A.M., Colin G., Rieger T., Oestereich L., Pályi B., et al. Persistence and clearance of Ebola virus RNA from seminal fluid of Ebola virus disease survivors: a longitudinal analysis and modelling study. Lancet Glob Health. 2017;5:e80–e88. doi: 10.1016/S2214-109X(16)30243-1. [DOI] [PubMed] [Google Scholar]
- 53.Mate S.E., Kugelman J.R., Nyenswah T.G., Ladner J.T., Wiley M.R., Cordier-Lassalle T., Christie A., Schroth G.P., Gross S.M., Davies-Wayne G.J., et al. Molecular evidence of sexual transmission of ebola virus. N. Engl. J. Med. 2015;373:2448–2454. doi: 10.1056/NEJMoa1509773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sissoko D., Keïta M., Diallo B., Aliabadi N., Fitter D.L., Dahl B.A., Akoi Bore J., Raymond Koundouno F., Singethan K., Meisel S., et al. Ebola virus persistence in breast milk after No reported illness: a likely source of virus transmission from mother to child. Clin. Infect. Dis. 2017;64:513–516. doi: 10.1093/cid/ciw793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Truong T.T., Ryutov A., Pandey U., Yee R., Goldberg L., Bhojwani D., Aguayo-Hiraldo P., Pinsky B.A., Pekosz A., Shen L., et al. Increased viral variants in children and young adults with impaired humoral immunity and persistent SARS-CoV-2 infection: a consecutive case series. EBioMedicine. 2021;67:103355. doi: 10.1016/j.ebiom.2021.103355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fang J., Qian J.J., Yi S., Harding T.C., Tu G.H., VanRoey M., Jooss K. Stable antibody expression at therapeutic levels using the 2A peptide. Nat. Biotechnol. 2005;23:584–590. doi: 10.1038/nbt1087. [DOI] [PubMed] [Google Scholar]
- 57.Balazs A.B., Chen J., Hong C.M., Rao D.S., Yang L., Baltimore D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2012;481:81–84. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Lieshout L.P., Domm J.M., Wootton S.K. AAV-mediated gene delivery to the lung. Methods Mol. Biol. 2019;1950:361–372. doi: 10.1007/978-1-4939-9139-6_21. [DOI] [PubMed] [Google Scholar]
- 59.Santry L.A., Ingrao J.C., Yu D.L., de Jong J.G., van Lieshout L.P., Wood G.A., Wootton S.K. AAV vector distribution in the mouse respiratory tract following four different methods of administration. BMC Biotechnol. 2017;17:43. doi: 10.1186/s12896-017-0365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aurnhammer C., Haase M., Muether N., Hausl M., Rauschhuber C., Huber I., Nitschko H., Busch U., Sing A., Ehrhardt A., Baiker A. Universal real-time PCR for the detection and quantification of adeno-associated virus serotype 2-derived inverted terminal repeat sequences. Hum. Gene Ther. Methods. 2012;23:18–28. doi: 10.1089/hgtb.2011.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data generated or analyzed during this study can be found within the published article and its supplementary files.