Abstract
Background
Striga orobanchioides Benth is a traditionally used Ayurvedic medicinal plant for the treatment of diabetes. Scientific validation of the claim is studied in this research. The significant bioactivity of the plant components is obligatory for its use in medicine.
Objective
The present work is to extract bioactive fractions and chemicals, biological activity of the chemicals and to identify potentially bioactive compound(s) from ethanolic extract of the plant.
Materials and methods
Ethanolic extract of the authenticated plant was fractionated and subjected to in vitro and in vivo antidiabetic and antihyperlipidemic activity. In vitro α-amylase and α-glucosidase enzyme activity was carried out on digestive enzyme. Streptozotocin (STZ) induced diabetes mellitus in rats model was preferred for in vivo activity where antidiabetic parameters body weight, urine volume, blood glucose level, glycosylated hemoglobin, serum insulin, liver glycogen and lipid profile as an antihypertensive parameters were assessed. Isolation of bioactive compounds was carried out by chromatographic techniques and identification of the compound was done by FTIR, Mass spectrometry and NMR spectroscopy. The molecular docking study with α-amylase, α-glucosidase, dipeptidyl peptidase-IV (DPP-IV), glucokinase (GK) as diabetic markers and on 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) and Niemann-Pick C1-Like 1 (NPC1L1) was carried out.
Results
Ethyl acetate-methanol fraction of the ethanol extract showed presence of pentacyclic triterpenoids (81.5% w/w) in GC-HRMS study. Spectroscopic analysis of the isolated compound revealed presence betulin. In vitro antidiabetic activity pointed out robust inhibition of the digestive enzymes by the fractions known as bioactive fraction and betulin. Betulin at a dose of 40 mg/kg treated group showed significant improvement of diabetic conditions. The gene expression studies revealed that betulin in 40 mg/kg dose has positive effects for carbohydrate metabolism in liver, lowers the hepatic inflammation and increases insulin secretion. The plant compound demonstrated significant inhibitory potential on α-amylase, α-glucosidase, DPP-IV and GK enzymes in silico.
Conclusion
The biological study reveals that betulin could dominate the succession of diabetes in dose dependent manner. The plant and specifically Betulin exerts a significant antidiabetic and antihyperlipidemic effects that are more possibly through stimulation of insulin secretion, increase in PPAR-α level with an increase in GRIA2 mRNA expression.
Keywords: α-amylase, α-glucosidase, Betulin, Striga, Ethanolic extract, Molecular docking
1. Introduction
Numerous plant bioactive compounds have been demonstrated to possess functional capabilities used as a complementary treatment for a number of ailments, that implies its role in the prevention of a wide variety of chronic diseases and most of them are described in Ayurveda, Siddha, and Unani system of medicines [1].The potential constituents are found in approximately 70,000 distinct species of plants in which almost 3000 plant species have been found in India [2]. Diabetes mellitus is a metabolic condition that results in an elevation of the body's blood glucose levels due to insufficient insulin or the insulin that is produced is ineffectively utilized [3,4]. Multiple cells are damaged by hyperglycemia, resulting in impairment and pathological impairment to organs such as the pancreas, heart, liver, and kidney. Numerous medicinal herbs have been used in the Ayush method of therapy for the regulation of a variety of health problems in conjunction with DM.
Striga orobanchioides Benth belonging to family Scrophulariaceae, is a scrounger herb, abide on the roots of several herbs such as Euphoria antiguorum, Lepidagathis and Dysophylla [5,6]. In Ayurveda, the plant is narrated as an antidiabetic herb [[7], [8], [9], [10]]. It shows presence of alkaloid, tannins, glycosides, carbohydrates, proteins and flavonoids in the ethanolic extract [11]. The extract is reported to have antiandrogenic [12], antibacterial [13] antihistaminic, and mast cell stabilizing activities [14], also two known flavonoids, apigenin and luteolin, have an antifertility properties [15]. The ethanolic extract of plant was also screened for in vitro and in vivo antioxidant properties which reported to stop the generation of the advanced glycation end products, responsible for the pathogenesis of diabetes [16]. Recently, the investigations indicate that entanglement of antioxidants in food or as a medicament is a substitute for the cure of diabetic issues. The plant domain provides dormant provenance of antioxidant drugs which needs rigorous enslavement [17].
There is range of oral antidiabetic drugs available now a days. Additionally, parenteral Insulin showed remarkable controlling blood sugar level. Despite of availability of such synthetic drugs for diabetes and hyperlipidemia, number of side effects and not receiving complete recovery from the diseases remain a big question [33]. Alternative therapies are in demand in recent years including Ayurveda, homeopathy, unani, and others [31,32]. Some of them showed better result in some patients. These therapy uses plant as a major source of their treatment, the line of treatment may be different. The overall reported investigation suggests that the plant has potential to treat diabetes as reported in ayurveda, traditional medicine and few of the scientific investigation [32]. The mechanism of action of the plant for the activity is not yet clear as well as responsible compound for the activity need to investigate to bring this plant in use as a medicine. Therefore present study aimed to investigate antidiabetic and antihyperlipidemic activity of plant and separation of bioactive compound through bioassay guided fractionation strategy. In vitro enzyme essay, in vivo antidiabetic and antihyperlipidemic activity of the plant fractions, separation and identification of bioactive compound and in silico study has been preferred to investigate the plant.
2. Materials and methods
The experimental protocol was approved by institutional animal ethical committee; Proposal number: ARCMR/PIMS/IAEC/1801.
2.1. Drugs and chemicals
Streptozotocin (Biogenuix Medsystems Pvt. Ltd., New Delhi), metformin (Merck, Germany), α-amylase, α-glucosidase enzyme, starch, Dinitrosalisilic acid, p-nitrophenyl-β-D-glucopyranoside, and acarbose were purchased from Sigma Aldrich (Merck KGaA Life science, India). The other chemicals like petroleum ether, chloroform, ethanol, methanol, ethyl acetate, sodium citrate dihydrate, benzene, citric acid, hydrochloric acid, sodium chloride, potassium chloride, and calcium chloride of analytical grades were purchased from PCL, India. Glucose-oxidase peroxidase kit and Accu check glucometer were purchased from Roche diabetes care Inc.
2.2. Plant material and animals
The whole plant of Striga orobanchioides was obtained from Pimparne Mountain, Sangamner, Maharashtra and was authenticated by Dr. Wabale Anil Sopanrao, Head of Department of Botany, PVP College of Arts, Science and Commerce, Pravaranagar, Maharashtra, India, vide letter number PVPC/2018-19-HD-45 dated 28/08/2018, Specimen no. SRV 123. The animals and food was purchased from Lacsmi Biofarms Pvt. Ltd., Pune.
2.3. Extraction and fractionation of plant materials
The 1000 gm of dried and coarse powder of the whole plant was extracted with ethanol in a Soxhlet extractor for complete extraction [18]. The extract was filtered, concentrated under reduced pressure and dried in desiccator. The ethanolic extract designated as SEE (20 gm) was subjected to fractionation by gradient elution method using mobile phase benzene, benzene:ethyl acetate (5:5), ethyl acetate, ethyl acetate:methanol (5:5)and methanol. All the fractions were subjected for TLC using various mobile phases as benzene, chloroform:ethyl acetate (9:1), toluene:ethylacetate:methanol (7:2:1) [19]. Benzene:ethyl acetate (5:5) fraction described as SEBEF and Ethyl acetate:methanol fraction of the ethanolic extract (SEEMF) was yielded higher quantity. SEEMF (5 gm) was subjected for column chromatography by gradient elution method using mobile phases chloroform:ethyl acetate (8:2), chloroform:ethylacetate:methanol (7:2:1), chloroform:ethylacetate:methanol (6:2:2), chloroform:methanol (7:3). All the fractions were subjected for TLC. One compound was separated from the chloroform:ethyl acetate (8:2) sub-fraction (CEF) of SEEMF and purified.
2.4. Identification of bioactive compound
To identify the bioactive compounds present in CEF, gas chromatograph with high resolution mass spectrophotometer was carried out using gas chromatography apparatus (Agilant,7890) with FID detector, head space injector and combipal auto sampler. The compound was further analyzed by FTIR (JASCO, Japan) and NMR (02 h discovery, Bruker) [35]. Bioactive compound was identified and analyzed by GCHRMS, FTIR and NMR methods.
2.5. Antidiabetic and antihyperlipidemic activity of plant extract, fractions and isolated compound
Ethanol extract and major fractions of the extract was studied for antidiabetic and antihyperlipidemic activity by in vitro and in vivo study. The isolated compound from the sub-fraction was identified and designated as BET. In vitro study on α-amylase and α-glucosidase enzyme and in vivo study in STZ-induced diabetic rats was carried out. Different parameters were assessed in vivo.
2.5.1. In vitro antidiabetic activity on α-amylase enzyme
SEE, SEBEF, SEEMF, and isolated bioactive compound were subjected for α-amylase and β-amylase enzyme assay [20]. The experiment was carried out in triplicate. Acarbose was used as a standard (50–300 μg/mL) to validate the results. The samples of SEE, SEBEF, SEEMF, and BET (Betulin) (50–300 μg/mL) were treated with 50 μL phosphate buffer (pH 6.8), 10 μL α-amylase (2 U/mL in 20 mM PBS buffer) and were incubated for 30 min at 25 °C. Further, 20 μL of 1% soluble starch as a substrate was added (dissolved in 20 mM phosphate buffer, pH 6.8) and further incubated at 37 °C for 30 min. DNS color reagent 100 μL was added and the reaction mixture was allowed to react at 95 °C for 10 min. The absorbance of resulting mixture was measured at 540 nm using UV-spectrophotometer. The control was same as above mentioned reaction, without SEE, SEBEF, SEEMF, and BET [21]. The percent inhibition was calculated by the formula as [(Abs control – Abs sample) x 100]/(Abs control).
2.5.2. In vitro antidiabetic activity on α-glucosidase enzyme
Different concentrations (50–300 μg/mL) of SEE, SEBEF, SEEMF, and BET were added to tube containing 50 μL of 20 mM phosphate buffer (pH 6.8), 10 μL α-glucosidase, 1 U/mL of 20 mM PBS and were incubated at 37 °C for 15 min. The catalytic reaction was begin by adding 20 μL of 5 mM p-NPG and incubated at 37 °C for 20 min. The reaction was ended by adding 50 μL of 0.1 M sodium carbonate (Na2CO3) and the absorbance was recorded at 405 nm using UV-spectrophotometer. The % of inhibition was calculated [21].
2.5.3. In vivo antidiabetic and antihyperlipidemic activity in STZ-induced diabetic rats
Male wistar rats, weighing 250–300 gm were used for screening of antidiabetic and antihyperlipidemic activity. The specific laboratory conditions were maintained for animals and were nourished with specific rodent nourishment and water ad libitum. The animals were maintained at constant temperature (22 ± 2 °C), humidity (55%) and light-dark cycle (12/12 h). After an overnight fasting, diabetes was induced in rats by single intra-peritoneal injection of STZ dissolved in 0.1 M sodium citrate buffer (pH 4.5) at a dose of 55 mg/kg body weight (b.w.). The animals were allowed for free access of food and water, after injection of STZ. The animals were allowed to drink 5% glucose solution overnight to overcome the hypoglycemic shock for 18 h. The development of diabetes was confirmed by measuring blood glucose level on fifth day of STZ injection. The animals having fasting blood glucose levels more than 250 mg/dl were considered as diabetic and used for experimentation. The experimental rats were indiscriminately divided into eleven groups of six animals each and treated for 20 days.
As per literature survey, it was found that acute toxicity study of ethanolic extract of striga plant is studied [14] and also it was found that ethanolic extract of striga plant is studied for anti-oxidant activity [16] and effective dose for anti-oxidant activity was found to be 100 mg/kg. So, two dose levels 50 mg/kg and 100 mg/kg b. w. were selected for the study according to standard method. Betulin was given at dose of 20 mg/kg and 40 mg/kg [34].
NC (Normal control) group in which rats maintained on regular food and drinking water ad libitum, DC (Diabetic control) grouping which rats maintained on regular food and drinking water ad libitum, PC (positive control) group in which rats treated with standard drug metformin (150 mg/kg b.w.), SEE50 and SEE100 groups in which diabetic rats were treated with SEE at a dose of 50 and 100 mg/kg b.w. respectively, SEBEF50 and SEBEF100 in which rats were treated with SEBEF at a dose of 50 and 100 mg/kg b.w. respectively, SEEMF50 and SEEMF100 groups in which diabetic rats were treated with SEEMF at a dose of 50 and 100 mg/kg b.w. respectively, BET20 and BET40 in which diabetic rats were treated with BET at a dose of 20 and 40 mg/kg b.w. respectively. The above mentioned groups were subjected to study the different biochemical and enzymatic parameters to monitor antidiabetic and antihyperlipidemic activities of the samples.
The parameters such as BW (body weight), Vu (urine volume), BGL (blood glucose level), HbA1C (glycosylated hemoglobin), INs (serum insulin), and LG (liver glycogen) were estimated to study the anti-diabetic potential of the extract. All the parameters were determined on days 1, 5, 10, 15 and 20, during the experiment. The BWs were calculated using digital balance in all the groups. Vu was determined by collecting urine at 5 h interval. BGL was estimated by tail flick method using glucometer. Following ether anesthesia, blood was collected from the animals' retro-orbital sinus, and the HbA1C concentration was determined. On day one and day twenty of the experiment, the HbA1C was determined using a mechanized biochemistry analyzer. Plasma insulin level was determined using insulin radioimmunoassay with ELISA kit (Linco Research, Inc., St. Charles, MO). Additionally, blood was taken in dried test tubes and allowed to coagulate at ambient temperature for 30 min at 2000 rpm for 10 min and the resultant translucent liquid was utilized to investigate TC (total cholesterol), TG (triglycerides), HDL (high density lipoprotein), LDL (low density lipoprotein), and VLDL (very low density lipoprotein), ALP (alkaline phosphatase), SGOT (serum glutamic oxaloacetic transaminase), SGPT (serum glutamic pyruvic transaminase) by following the manufacturer's instructions (Sigma Aldrich Merck KGaA Life science, India).
On day 20 of the study, pancreas were isolated and homogenized in Tris HCI buffer (pH 7.4) and centrifuged at 12000 rpm for 30 min at 4 °C. The antioxidant enzymes activities were performed by the standard procedures with little bit alterations, as per the previous methods [22,23]. The supernatants from plasma and pancreas were collected and utilized to perform assays and to determine the actions of various antioxidant enzymes as, CAT (catalase), SOD (superoxide dismutase), GPx (glutathione peroxidase), and GST (glutathione-S-transferase).
2.6. Histopathological studies
The pancreas and the liver of all the groups were isolated, eradicated and washed using ice-cold saline solution after sacrificing the animals by cervical dislocation. Pieces of both were stored in a 10% formalin solution. Paraffin sections of both the tissues were pigmented using hematoxylin and eosin for histopathological studies using a light microscope [36].
2.7. RT-PCR to study the mRNA expression of IRS2, PPARα, and GRIA2
RT-PCR (Real-time polymerase chain reaction) was used to study the mRNA expressions of IRS2 (insulin receptor substrate-2), PPARα (peroxisome proliferator activated receptor-α), and GRIA2 (glutamate ionotropic receptor AMPA-2). Total RNA from liver tissues of all groups was separated using kit (Qiagen RNA isolation kit, Germany) as the procedure described by the manufacturer. 1 μg of RNA was used for cDNA synthesis along with oligodTs to a final volume of 20 μL (QiagencDNA synthesis kit, Germany) as per guidelines by the manufacturer. Primers of IRS2, PPARα and GRIA2 were commercially obtained from Qiagen, Germany. qPCR (Roche, USA) was used to perform gene expression study by diluting 1 μl of cDNA (10 μL reaction with 1X SYBR green, 0.5 μL of each primer) on an Mx3000 P instrument (Stratagene, Cedar Creek, TX, USA). Central control used was Glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Following parameters were used to carry out single step RT-PCR as; primary denaturation at 95 °C for 3 min, followed by 50 cycles of denaturation at 95 °C for 5s, 60 °C for 40s, and 72 °C for 40s. To quantify the results of qPCR, the ΔΔCt method was used [37,38].
2.8. Statistical analysis
The final findings were reported as mean ± standard error mean (SEM) or standard deviation (SD). Graph Pad Prism 8.02; one-way analysis of variance (ANOVA) followed by student t-test was used to investigate the statistical analysis of all the data. Statistical significance between drug-treated groups and negative control group was calculated by Dunnett's comparison test (p < 0.05 was considered as significant).
2.9. Molecular docking (MD)
The Autodockvina 1.1.2 in PyRx-Virtual Screening Tool 0.8 and the Biovia Discovery studio was used to perform MD [24,25]. The Structure of betulin (SDF File) was downloaded from the official website of the US National Library of Medicine PubChem [https://pubchem.ncbi.nlm.nih.gov/]. The energy minimization was performed by Universal Force Field (UFF). The crystal structures of the enzymes were obtained from the RCSB Protein Data Bank [https://www.rcsb.org/]. 3D structures with PDB IDs of the enzymes are illustrated in Fig. 10. For the MD simulation, the three-dimensional grid box of known size (Alpha Amylase, size_x = 54.3200Ao, size_y = 65.5343Ao, size_z = 56.4905Ao; Alpha Glucosidase, size_x = 49.5962 A0, size_y = 52.8182Ao, size_z = 79.2698Ao; DPP-IV, size_x = 58.8950 A0, size_y = 67.5143Ao, size_z = 66.6780Ao; GK, size_x = 57.4468 A0, size_y = 65.5343Ao, size_z = 66.6780Ao; HMG CoA, size_x = 78.5046 A0, size_y = 61.6466Ao, size_z = 79.2698Ao; NPC1L1, size_x = 41.72374 A0, size_y = 57.6876Ao, size_z = 47.7393Ao) was adjusted (to define area for interactions) with exhaustiveness value of 8.
3. Results
3.1. Extraction and fractionation of plant materials
The column chromatography of SEBEF and SEEMF were higher in yield of about 4.9% and 5.3%, respectively.
3.2. Characterization of extract, fractions and bioactive compound of the plant
See Table 1.
Table 1.
The derivatives or compounds detected in GCHRMS analysis of SEEMF and CEF.
| Sr. No. | RT | Area | Mass | Base peak | Derivatives/Compounds |
|---|---|---|---|---|---|
| SEEMF | |||||
| 1 | 48.97 | 192,352,951.85 | 468 | 218 | Pentacyclictriterpenoid derivative |
| 2 | 9.64 | 22,145,254.82 | 126 | 97 | Furancarboxaldehyde |
| 3 | 7.55 | 58,166,095.52 | 144 | 43 | Pyrone derivatives |
| 4 | 19.57 | 66,830,899.52 | 180 | 73 | Mannose |
| 5 | 22.11 | 66,493,460.68 | 504 | 73 | Estriol derivative |
| 6 | 17.65 | 34,717,333.15 | 168 | 125 | Octadecanoic acid |
| CEF of SEEMF | |||||
| 1 | 19.24 | 56,557,932.75 | 442 | 189 | Betulin |
| 2 | 19.61 | 16,198,862.79 | 284 | 88 | Hexadecanoic acid ethyl ester |
| 3 | 22.62 | 20,488,206 | 284 | 43 | Octadecanoic acid C18H36O2 |
| 4 | 22.93 | 22,582,792 | 312 | 88 | Octadecanoic acid ethyl ester |
| 5 | 27.43 | 18,186,842 | 312 | 57 | Hexadecanoic acid -2-methyl propyl ester |
| 6 | 25.91 | 17,459,295 | 354 | 57 | n-tetracosanol |
| 7 | 28.0 | 17,350,197 | 390 | 149 | Diisioctyl ester of benzene carboxylic acid diamer |
| 8 | 28.6 | 29,400,401 | 410 | 57 | octacosanol |
3.3. Antidiabetic and antihyperlipidemic activity of plant extract, fractions and isolated compound
See fig. 1.
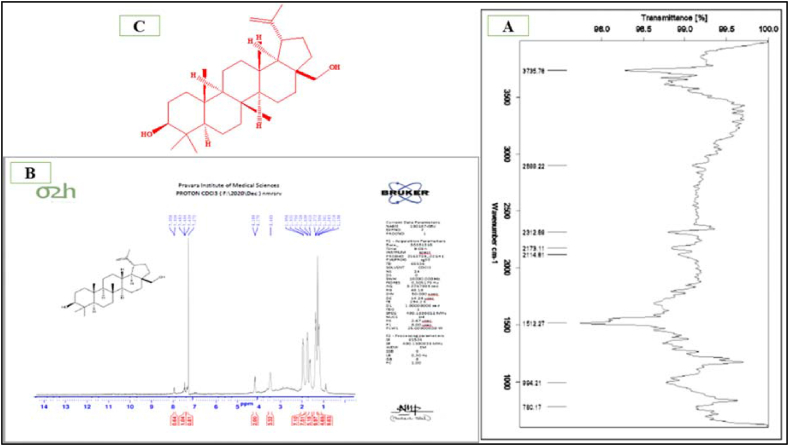
Fig. 1.
FTIR spectra (A), NMR spectra (B) and structure (C) of isolated compound from the plant.
3.3.1. In vitro antidiabetic activity on α-amylase and α-glucosidase enzymes
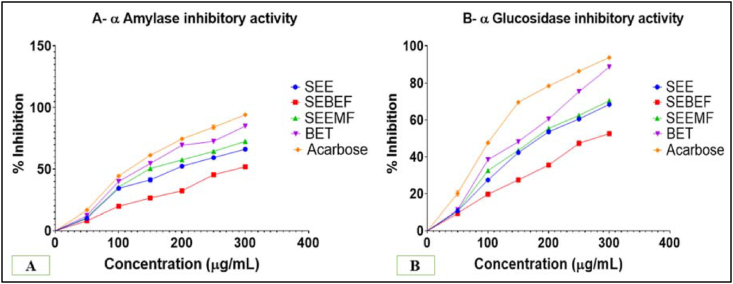
See fig. 2.
Fig. 2.
In vitro antidiabetic activity on α-amylase and α-glucosidase enzymes.
3.3.2. In vivo antidiabetic and antihyperlipidemic activity in STZ-induced diabetic rats
Table 2.
Effect of plant samples on BGL, BW, Vu and HbA1C as an antidiabetic parameters.
| Parameter | Day | NC | DC | PC | SEE50 | SEE100 | SEBEF50 | SEBEF100 | SEEMF50 | SEEMF100 | BET20 | BET40 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BW (gm) | 1 | 276.36 ± 5.54 | 263.89 ± 3.87 | 267.83 ± 2.5 | 267.44 ± 3.05 | 265.13 ± 4.58 | 269.37 ± 3.19 | 264.04 ± 3.64 | 264.13 ± 2.31 | 265.88 ± 4.84 | 269.08 ± 3.95 | 266.22 ± 4.48 |
| 5 | 279.62 ± 5.3 | 232.79 ± 5.7 | 265.59 ± 4.31∗∗∗ | 246.72 ± 4.8 | 257.71 ± 5.07∗∗ | 247.29 ± 5.09 | 252.76 ± 7.34 | 243.3 ± 3.81 | 256.49 ± 5.25∗ | 254.02 ± 2.87∗ | 259.18 ± 5.2∗∗ | |
| 10 | 284.43 ± 5.63 | 220.67 ± 5.85 | 280.91 ± 3.11∗∗∗ | 243.25 ± 3.87 | 254.36 ± 10.74∗∗ | 243.03 ± 3.78 | 255.57 ± 8.86∗∗∗ | 255.57 ± 8.86∗∗ | 256.06 ± 6.95∗∗∗ | 256.06 ± 6.95∗∗∗ | 264.56 ± 4.01∗∗∗ | |
| 15 | 287.78 ± 5.95 | 210.42 ± 4.78 | 292.92 ± 7.75∗∗∗ | 247.57 ± 2.78∗∗ | 251.25 ± 10.63∗∗ | 229.01 ± 6.54 | 249.41 ± 6.16∗∗ | 250.37 ± 2.87∗∗ | 256.69 ± 11.97∗∗∗ | 268.54 ± 5.37∗∗∗ | 283.87 ± 6.08∗∗∗ | |
| 20 | 295.33 ± 5.01 | 200.09 ± 4.23 | 311.89 ± 8.06∗∗∗ | 233.02 ± 7.61∗ | 247.43 ± 14.34∗∗∗ | 222.46 ± 5.32 | 229.07 ± 8.18 | 238.43 ± 4.69∗∗ | 251.67 ± 10.1∗∗∗ | 279.15 ± 2.78∗∗∗ | 295.62 ± 6.71∗∗∗ | |
| BGL (mg/dl) | 1 | 82.62 ± 2.87 | 277.23 ± 2.89 | 279.6 ± 2.57 | 278.25 ± 3.71 | 279.68 ± 3.03 | 281.11 ± 4.14 | 281.86 ± 3.15 | 277.88 ± 3.09 | 286.41 ± 3.14 | 277.55 ± 5.59 | 275.79 ± 3.86 |
| 5 | 81.6 ± 3.45 | 285.07 ± 0.77 | 274.22 ± 2.75∗∗∗ | 280.45 ± 0.84 | 278.08 ± 1.09∗ | 280.3 ± 2.02 | 278.65 ± 2.11 | 280.85 ± 1.42 | 277.98 ± 1.25∗ | 277.24 ± 0.89∗ | 277.54 ± 2.14∗ | |
| 10 | 80.36 ± 3.72 | 291.86 ± 1.91 | 227.63 ± 3.19∗∗∗ | 287.83 ± 2.25 | 280.7 ± 2.58∗ | 282.96 ± 3.05 | 281.77 ± 3.36∗ | 283.99 ± 1.75 | 280.39 ± 2.09∗ | 277.54 ± 1.37∗∗ | 272.91 ± 2.12∗∗∗ | |
| 15 | 79.96 ± 3.91 | 302.72 ± 2.98 | 152.86 ± 5.45∗∗∗ | 285.45 ± 1.94∗ | 281.62 ± 3.25∗∗ | 283.65 ± 1.12∗ | 280.38 ± 2.54∗∗ | 284.38 ± 1.13∗ | 279.53 ± 2.79∗∗ | 263.36 ± 2.94∗∗∗ | 159.53 ± 5.64∗∗∗ | |
| 20 | 81.35 ± 3.44 | 331.93 ± 5.02 | 110.21 ± 3.96∗∗∗ | 289.1 ± 3.19∗ | 287.71 ± 8.05∗ | 309.55 ± 4.02 | 315.87 ± 3.21 | 288.26 ± 6.4∗ | 277.38 ± 18.07∗∗ | 207.04 ± 17.13∗∗∗ | 142.21 ± 7.88∗∗∗ | |
| Vu(ml/5 h) | 1 | 1.38 ± 0.14 | 7.57 ± 0.24 | 6.65 ± 0.39 | 7.42 ± 0.2 | 7.02 ± 0.42 | 6.67 ± 0.47 | 7.55 ± 0.41 | 7.47 ± 0.34 | 7.18 ± 0.49 | 7.1 ± 0.45 | 7.4 ± 0.55 |
| 5 | 1.45 ± 0.11 | 8.35 ± 0.44 | 4.57 ± 0.44∗∗∗ | 7.15 ± 0.36 | 6.28 ± 0.64∗ | 7.22 ± 0.35 | 6.45 ± 0.56 | 7.22 ± 0.28 | 8.13 ± 0.76 | 6.8 ± 0.18 | 5.12 ± 0.56∗∗∗ | |
| 10 | 1.5 ± 0.13 | 9.2 ± 0.69 | 5.35 ± 0.63∗∗∗ | 7.35 ± 0.34 | 6.72 ± 0.65∗ | 7.2 ± 0.27 | 7.1 ± 0.44∗ | 6.08 ± 0.2∗∗ | 6.23 ± 0.71∗∗ | 6.63 ± 0.49∗∗ | 5.83 ± 0.61∗∗∗ | |
| 15 | 1.57 ± 0.2 | 9.43 ± 0.39 | 6.5 ± 0.15∗∗∗ | 7.35 ± 0.36∗ | 7.08 ± 0.31∗∗ | 7.77 ± 0.44 | 7.53 ± 0.6∗ | 7.93 ± 0.43 | 6.68 ± 0.64∗∗ | 7.05 ± 0.63∗∗ | 6.97 ± 0.51∗∗ | |
| 20 | 1.48 ± 0.13 | 10.27 ± 0.06 | 5.28 ± 0.22∗∗∗ | 7.97 ± 0.44∗∗ | 8.08 ± 0.63∗∗ | 7.75 ± 0.42∗∗ | 7.97 ± 0.67∗∗ | 8 ± 0.36∗∗ | 5.47 ± 0.4∗∗∗ | 6.95 ± 0.46∗∗∗ | 6.77 ± 0.36∗∗∗ | |
| HbA1C (%) | 1 | 2.55 ± 0.11 | 8.91 ± 0.2 | 9.01 ± 0.28 | 8.94 ± 0.11 | 8.93 ± 0.15 | 8.9 ± 0.18 | 9.06 ± 0.3 | 9.04 ± 0.16 | 9.05 ± 0.24 | 9.04 ± 0.31 | 8.94 ± 0.27 |
| 20 | 2.85 ± 0.03 | 8.9 ± 0.2 | 3.44 ± 0.14∗∗∗ | 7.72 ± 0.39 | 7.46 ± 0.33∗ | 7.82 ± 0.28 | 7.51 ± 0.32 | 7.68 ± 0.26 | 6.97 ± 0.46∗∗ | 6.47 ± 0.5∗∗∗ | 4.87 ± 0.51∗∗∗ |
Table 3.
Effect of plant samples on different parameters (TG and TC, LDL, VLDL and HDL, Liver damage markers and Insulin level).
| Parameters | Day | NC | DC | PC | SEE50 | SEE100 | SEBEF50 | SEBEF100 | SEEMF50 | SEEMF100 | BET20 | BET40 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Insulin (μU/ml) | 1 | 18.02 ± 0.29 | 7.07 ± 0.2 | 7.16 ± 0.22 | 7.06 ± 0.31 | 7.22 ± 0.27 | 7.19 ± 0.35 | 7.07 ± 0.32 | 7.27 ± 0.36 | 7.06 ± 0.4 | 7.25 ± 0.43 | 7.22 ± 0.25 |
| 20 | 18.19 ± 0.35 | 7.15 ± 0.16 | 17.15 ± 0.37∗∗∗ | 8.39 ± 0.37∗ | 8.42 ± 0.28∗ | 7.86 ± 0.1 | 8.46 ± 0.34∗ | 8.47 ± 0.36∗ | 8.56 ± 0.31∗ | 8.58 ± 0.29∗ | 14.97 ± 0.32∗∗∗ | |
| TC (mg/dl) | 20 | 63.65 ± 0.83 | 175.9 ± 1.27 | 75.28 ± 1.06∗∗ | 166.91 ± 2.11 | 159.13 ± 3.37 | 170.05 ± 1.69 | 164.64 ± 6.59 | 169.19 ± 1.96 | 157.74 ± 2.71∗∗ | 157.47 ± 4.01∗∗ | 135.3 ± 3.55∗∗∗ |
| TG (mg/dl) | 20 | 65.38 ± 0.34 | 190.17 ± 5.13 | 96.74 ± 1.23∗∗∗ | 188.86 ± 2.86 | 171.83 ± 4.92∗ | 176.28 ± 2.27 | 170.63 ± 4.77∗ | 169.19 ± 5.09∗ | 169.05 ± 4.55∗ | 167.46 ± 6.56∗∗ | 121.64 ± 5.43∗∗∗ |
| HDL (mg/dl) | 20 | 14.98 ± 0.89 | 10.66 ± 0.44 | 14.09 ± 0.61∗∗∗ | 12.15 ± 0.37 | 12.52 ± 0.19∗∗ | 10.94 ± 0.44 | 11.47 ± 0.6 | 11.66 ± 0.26 | 12.74 ± 0.14∗∗ | 12.87 ± 0.19∗∗ | 13.17 ± 0.24∗∗∗ |
| LDL (mg/dl) | 20 | 25.23 ± 1.18 | 94.79 ± 0.8 | 31.46 ± 0.33∗∗∗ | 78.28 ± 3.34∗∗ | 78.06 ± 3.92∗∗ | 82.05 ± 3.49 | 81.89 ± 3.54 | 81.48 ± 3.76∗ | 56.3 ± 3.81∗∗∗ | 78.57 ± 4.36∗∗ | 42.06 ± 3.71∗∗∗ |
| VLDL (mg/dl) | 20 | 14.94 ± 0.18 | 28.96 ± 0.67 | 15.74 ± 0.3∗∗∗ | 24.29 ± 1.26∗ | 25.53 ± 0.99 | 26.71 ± 1.46 | 26.4 ± 0.68 | 24.99 ± 1.04∗ | 24.72 ± 0.96∗ | 23.39 ± 0.84∗∗ | 20.44 ± 1.11∗∗∗ |
Table 4.
Effect of extract, fractions and isolated compound on hepatic glucose metabolic enzymes and LG content.
| Parameters | NC | DC | PC | SEE50 | SEE100 | SEBEF50 | SEBEF100 | SEEMF50 | SEEMF100 | BET20 | BET40 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Glycolytic enzymes | |||||||||||
| Glucokinase (u/h/mg protein) | 0.65 ± 0.04 | 0.19 ± 0.02 | 0.58 ± 0.03∗∗∗ | 0.25 ± 0.01 | 0.26 ± 0.01∗ | 0.23 ± 0.01 | 0.22 ± 0.01 | 0.25 ± 0.01 | 0.27 ± 0.02∗ | 0.28 ± 0.01∗∗ | 0.56 ± 0.03∗∗∗ |
| Glucose-6-phosphatedehydrogenase (lU/L) | 4.82 ± 0.15 | 1.89 ± 0.19 | 4.26 ± 0.08∗∗∗ | 2.08 ± 0.16 | 2.61 ± 0.1∗ | 1.91 ± 0.13 | 1.98 ± 0.1 | 2.13 ± 0.28 | 2.58 ± 0.13∗ | 2.63 ± 0.09∗∗ | 4.25 ± 0.16∗∗∗ |
| 2. Gluconeogenic enzymes | |||||||||||
| Glucose-6-phosphatase (μmoles of Pi liberated/min/mg of protein) | 0.179 ± 0.01 | 0.309 ± 0.01 | 0.205 ± 0.01∗∗∗ | 0.275 ± 0.02 | 0.232 ± 0.02∗∗ | 0.293 ± 0.02 | 0.291 ± 0.01 | 0.288 ± 0.02 | 0.247 ± 0.02∗ | 0.229 ± 0.02∗∗ | 0.212 ± 0.01∗∗∗ |
| Fructose 1,6 bis phosphatase (μmoles of Pi liberated/h/mg of protein) | 0.354 ± 0.01 | 0.696 ± 0.02 | 0.398 ± 0.01∗∗∗ | 0.693 ± 0.02 | 0.617 ± 0.01∗ | 0.638 ± 0.02 | 0.689 ± 0.02 | 0.66 ± 0.03 | 0.614 ± 0.02∗ | 0.613 ± 0.02∗ | 0.402 ± 0.01∗∗∗ |
| 3. Hepatic glycogen | |||||||||||
| Glycogen (mg/g liver) | 21.56 ± 0.74 | 10.47 ± 0.29 | 19.14 ± 0.19∗∗∗ | 10.51 ± 0.34 | 11.81 ± 0.27∗∗ | 10.55 ± 0.32 | 10.61 ± 0.18 | 10.75 ± 0.23 | 11.84 ± 0.23∗∗ | 11.89 ± 0.27∗∗ | 18.91 ± 0.17∗∗∗ |
The values are expressed as mean ± SEM, n = 6. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 indicates the significant difference between DC and treatment groups.
Table 5.
Effect of plant samples on different plasma and pancreatic enzymes.
| NC | DC | PC | SEE50 | SEE100 | SEBEF50 | SEBEF100 | SEEMF50 | SEEMF100 | BET20 | BET40 |
|---|---|---|---|---|---|---|---|---|---|---|
| CATALASE [PANCREAS] micromoles of H2O2utilized/mg of protein | ||||||||||
| 6.86 ± 0.24 | 2.48 ± 0.17 | 5.89 ± 0.22∗∗∗ | 2.47 ± 0.23 | 4.54 ± 0.8∗ | 2.25 ± 0.25 | 2.87 ± 0.3 | 2.94 ± 0.09 | 4.58 ± 0.81∗ | 4.67 ± 0.79∗ | 5.19 ± 0.43∗∗ |
| CATALASE [PLASMA] micromoles of H2O2utilized/mg of protein | ||||||||||
| 71.83 ± 1.24 | 41.9 ± 1.6 | 60.24 ± 1.22∗∗∗ | 41.73 ± 1.22 | 53.23 ± 2.59∗ | 41.63 ± 1.44 | 50.77 ± 4.48 | 42.06 ± 1.28 | 53.75 ± 2.89∗ | 52.87 ± 4.19∗ | 59.85 ± 1.52∗∗∗ |
| GPx [PANCREAS] micromoles of glutathione oxidized/mg of protein | ||||||||||
| 0.49 ± 0.01 | 0.18 ± 0.01 | 0.4 ± 0.01∗∗∗ | 0.26 ± 0.02∗ | 0.28 ± 0.03∗∗ | 0.22 ± 0.01 | 0.23 ± 0.01 | 0.24 ± 0.02 | 0.29 ± 0.04∗∗ | 0.3 ± 0.03∗∗ | 0.38 ± 0.01∗∗∗ |
| GPx [PLASMA] micromoles of glutathione oxidized/mg of protein | ||||||||||
| 46.69 ± 0.84 | 22.22 ± 0.85 | 37.83 ± 0.59∗∗∗ | 22.9 ± 0.83 | 28.06 ± 1.57∗ | 22.57 ± 0.93 | 28.31 ± 1.29∗ | 27.17 ± 1.47 | 27.95 ± 2.16∗ | 29.57 ± 2.5∗∗ | 36.98 ± 0.7∗∗∗ |
| GST [PANCREAS] U/mg of Protein | ||||||||||
| 3.96 ± 0.34 | 1.84 ± 0.04 | 2.99 ± 0.28∗∗∗ | 2.01 ± 0.18 | 2.67 ± 0.12∗ | 2.03 ± 0.13 | 2.59 ± 0.23∗ | 2.38 ± 0.16 | 2.69 ± 0.17∗ | 2.73 ± 0.11∗∗ | 2.91 ± 0.22∗∗∗ |
| GST [PLASMA] U/mg of Protein | ||||||||||
| 35.37 ± 0.53 | 15.84 ± 0.31 | 29.25 ± 0.55∗∗∗ | 16.72 ± 0.82 | 22.84 ± 2.17∗∗ | 17.37 ± 0.48 | 21.68 ± 1.4∗ | 17.2 ± 0.29 | 22.98 ± 2.1∗∗ | 23.46 ± 2.19∗∗ | 28.67 ± 0.69∗∗∗ |
| SOD [PANCREAS] amount of enzyme required to inhibit 50% NBT reduction/min | ||||||||||
| 5.34 ± 0.15 | 2.42 ± 0.24 | 4.43 ± 0.2∗∗ | 2.47 ± 0.23 | 3.69 ± 0.46 | 2.07 ± 0.23 | 3.75 ± 0.51 | 2.87 ± 0.09 | 3.72 ± 0.42 | 4.01 ± 0.52∗ | 4.38 ± 0.34∗∗ |
| SOD [PLASMA] amount of enzyme required to inhibit 50% NBT reduction/min | ||||||||||
| 9.32 ± 0.21 | 4.23 ± 0.17 | 8.06 ± 0.14∗∗∗ | 4.29 ± 0.15 | 5.81 ± 0.23∗∗ | 4.14 ± 0.23 | 4.32 ± 0.25 | 4.34 ± 0.22 | 5.83 ± 0.24∗∗ | 5.87 ± 0.76∗∗ | 7.97 ± 0.23∗∗∗ |
3.4. Histopathological studies
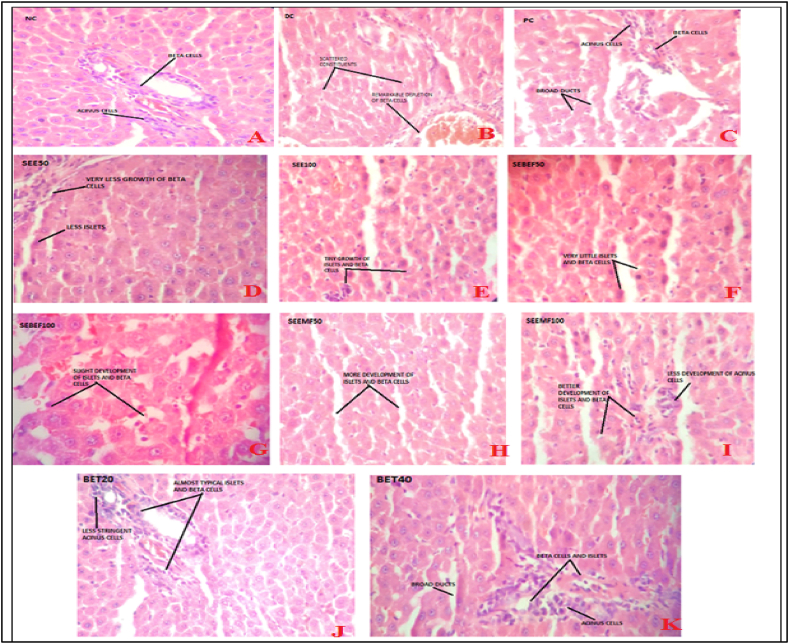
Fig. 3.
The histopathological observations of pancreas tissue samples.
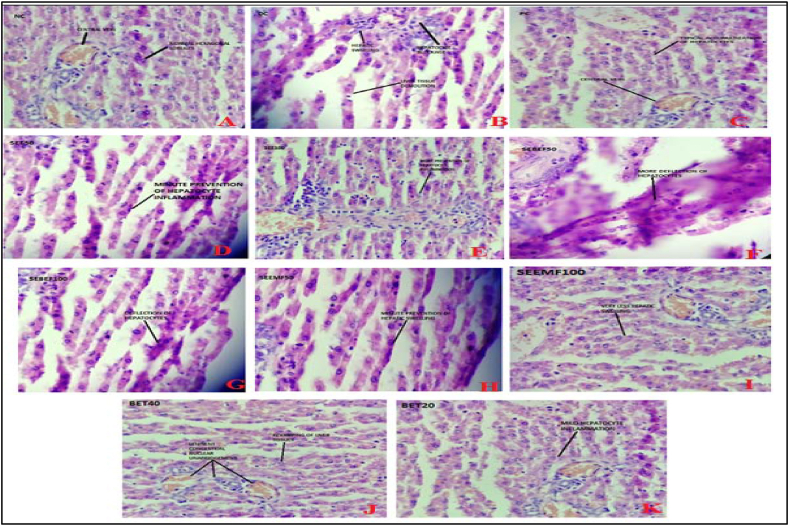
Fig. 4.
The histopathological observations of liver tissue samples.
3.5. RT-PCR to study the mRNA expression of IRS2, PPARα, and GRIA2
See Table 6.
Table 6.
Effects of the samples on mRNA expression of IRS2 gene, PPARα gene, and GRIA2.
| NC | DC | PC | SEE50 | SEE100 | SEBEF50 | SEBEF100 | SEEMF50 | SEEMF100 | BET20 | BET40 |
|---|---|---|---|---|---|---|---|---|---|---|
| IRS2 (Insulin Receptor Substrate-2) gene | ||||||||||
| 1.28 ± 0.04 | 0.53 ± 0.03 | 1.01 ± 0.09∗∗∗ | 0.67 ± 0.02 | 0.77 ± 0.02 | 0.57 ± 0.06 | 0.64 ± 0.02 | 0.57 ± 0.06 | 0.81 ± 0.09∗ | 0.84 ± 0.08∗∗ | 0.99 ± 0.08∗∗∗ |
| PPARα (Peroxisome Proliferator Activated Receptor-α) gene | ||||||||||
| 1.55 ± 0.03 | 0.39 ± 0.02 | 1.48 ± 0.05∗∗∗ | 0.98 ± 0.21∗∗ | 1.05 ± 0.15∗∗ | 0.55 ± 0.07 | 0.65 ± 0.11 | 0.59 ± 0.05 | 1.07 ± 0.16∗∗ | 1.09 ± 0.16∗∗ | 1.44 ± 0.06∗∗∗ |
| GRIA2(Glutamate Ionotropic Receptor AMPA-2) gene | ||||||||||
| 1.92 ± 0.06 | 0.75 ± 0.07 | 1.86 ± 0.08∗∗∗ | 0.79 ± 0.06 | 1.24 ± 0.11∗ | 0.93 ± 0.13 | 1.22 ± 0.11∗ | 1.22 ± 0.09∗ | 1.27 ± 0.12∗∗ | 1.32 ± 0.09∗∗ | 1.73 ± 0.14∗∗∗ |
3.6. Molecular docking (MD)
Table 7.
The docking score of betulin with different enzymes.
| Enzyme Name | Ligand Energy (kcal/mol) | Binding Affinity (kcal/mol) | rmsd/ub | rmsd/lb |
|---|---|---|---|---|
| Alpha Amylase | 1121.26 | −9.6 | 0 | 0 |
| Alpha Glucosidase | −8.8 | 0 | 0 | |
| DPP-IV | −9.6 | 0 | 0 | |
| Glucokinase | −7.9 | 0 | 0 | |
| HMG CoA | −8.5 | 0 | 0 | |
| NPC1L1 | −7.4 | 0 | 0 |
Table 8.
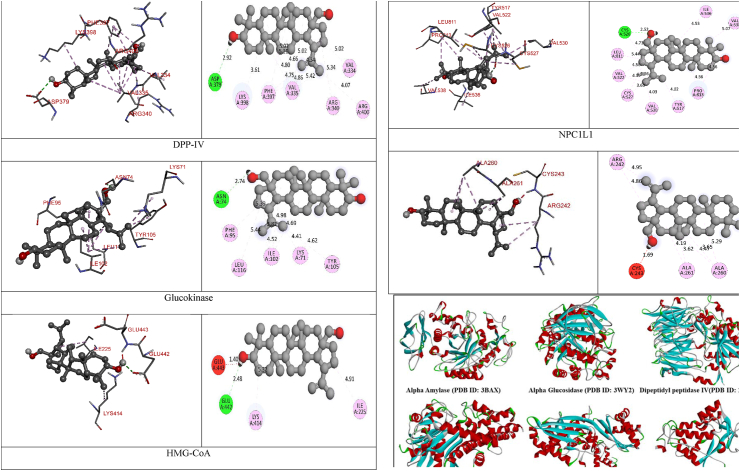
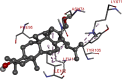
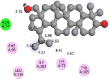
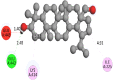
The 2D- and 3D-docking poses of betulin with all the enzymes
| 3D-docking Poses | 2D-docking Poses |
|---|---|
| Alpha Amylase | |
 |
 |
| Alpha Glucosidase | |
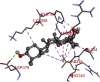 |
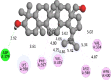 |
| DPP-IV | |
 |
 |
| Glucokinase | |
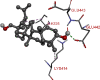 |
 |
| HMG-CoA | |
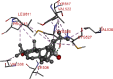 |
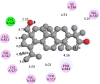 |
| NPC1L1 | |
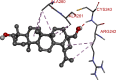 |
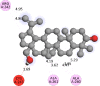 |
Fig. 5.
3D images of enzyme for ligand interactions.
4. Discussion
Authenticated and powdered whole plant extracted with ethanol and produced 22% yield. 20 gm of SEE was further fractionated and obtained two fractions SEBEF and SEEMF in desirable amount which were subjected for animal activity to obtained bioactive fraction. The column chromatography of SEE found that amongst all the fractions, SEBEF and SEEMF were higher in yield. In TLC of SEBEF and SEEMF, it showed two and three spots respectively. SEEMF column chromatography revealed that CEF showed only one spot on TLC with the highest percentage yield of 1.3% amongst all the fractions. The SEEMF showed the presence of the highest percentage of pentacyclic triterpenoid derivatives at retention time (RT) of 48.97 with 81.5% in GCHRMS study and lower percentage of pyrone derivatives, estriol derivatives, octadecanoic acid, furancarboxaldehyde and mannose were observed (Table 1). The GCHRMS chromatogram of CEF showed that at RT 19.24 possesses compound base peak value at m/z 189, and major peaks at m/z 95, 135, 205, 234 present in 25.8% w/w. FTIR analysis (Fig. 1A) and NMR analysis (Fig. 1B) confirmed the structure (Fig. 1C) of isolated compound as betulin. Fig 1 confirmed the structure of isolated, compound Betulin. The peak at m/e 429 reflects the molecular ion peak. The mass peak at m/e 430 represents C C cleavage and m/e at 431 represents the CH3 bending. These peaks gave tentative idea of the compound with its fragments. FTIR spectral analysis provided data for functional group characterization of the compound. The important characteristics of the alkane group are shown with stretching frequency 2888 cm−1. The presence of CH3 bending in the molecule is interpreted by 1512 cm−1. C C stretching are interpreted at frequency 2114 cm−1. Another diagnostic frequency of IR regions are C-O at 994 cm−1, -OH at 2312 cm−1, C-H bending at 780 cm−1. The proton NMR study was final interpretation of the compound which was carried out in CDCl3 solvent. There are about eleven types of peak observed in the molecule. The down field peaks at singlet shifted to 4.75 ppm, singlet shifted to 4.68 ppm and doublet shifted to 4.6 ppm gave idea about aromatic protons. Peaks observed were doublet at 3 ppm, multiplet at 2.13–2.46 ppm, multiplet at 2.14–2.3 ppm, multiplet at 1.8–2.1 ppm, multiplet at 1.5–1.7 ppm, duplet at 1.4 ppm, multiplet at 1.11–1.3 ppm and triplet at 1–1.1 ppm. Based on spectroscopic study of the compound, Betulin was identified as isolated compound from the fraction.
The enzyme inhibition activity of samples were compare with acarbose as shown in Fig. 2. BET exhibited the highest % inhibition and showed 146.10 μg and 151.60 μg IC50 value in α-amylase (Fig. 2A) and α-glucosidase (Fig. 2B) enzyme inhibition activity assay respectively. The IC50 value of SEE, SEBEF and SEEMF was found to be 191.50 μg, 296.00 μg, and 149.90 μg, respectively in α-amylase assay whereas 193.50 μg, 280.40 μg, and 185.10 μg, respectively in α-glucosidase enzyme assay. The IC50 values for standard acarbose was found to be 118.50 μg and 107.50 μg in α-amylase and α-glucosidase enzyme inhibition activity assay (Figure 2A and B) respectively.
The animals in groups received respective samples were studied for 20 days on day 1, 5, 10, 15, 20, and different parameters were assessed. Changes in BGL, BW and Vu were studied for antidiabetic activity and TC, TG, HDL, LDL, VLDL for anti-hyperlipidemic parameters. During study period DC animals showed significant (p < 0.001) change in these parameters. BET20 and BET40 treated groups showed significant decrease (p < 0.001) in BGL, Vu, and significant increase in BW of animals compared to metformin treated groups (Table 2). Our study divulged that in DC groups, TC, TG, LDL and VLDL levels was increased whereas HDL level was decreased (Table 3). The results obtained by treatment with SEBEF were not significant, whereas significant results were obtained by treatment with BET20 and BET40. Hepatic function test was carried out to check the levels of liver damage markers (Table 3). In DC group, the level of liver enzymes ALP, SGOT, SGPT was increased. This level was significantly decreased by treatment with BET40 when compared with DC animals. On day 20 of the treatment with BET40 and metformin treated group showed significant dose dependent increase in INs level than other treatment groups (Table 3). The disclosure of hepatic glucose metabolic enzymes and LG contents are outlined in Table 2. The DC group animals showed decreased levels of hepatic glucose metabolic enzymes and LG. Treatment with BET showed dose dependent significant increased levels of LG, activities of glucokinase, glucose-6-phosphate dehydrogenase, glucose-6-phosphatase, and fructose-1, 6-biphosphatase. Treatment with metformin and BET40 showed highly significant results than that of other treatment groups. HbA1C content was checked for all the groups on day 1 and day 20 of the study (Table 2). It was observed that on day 1 of study, all treatment group animals were having high percentage of HbA1C. After the treatment, the HbA1C percentage was significantly reduced in BET40 treated group compared with metformin treated group, rather SEE100 gave less significant results whereas, SEBEF100 and BET20 gave moderate significant results. BET recovered HbA1C percentage level in more amount in dose dependent manner when compared with diabetic control rats.
The effects of plant samples on Catalase, SOD, GPx, and GST (Table 5) in plasma and pancreas were recorded. DC group showed significant lower levels of activities of enzymatic antioxidants in circulation and pancreas, whereas after treatment with BET40, activities of enzymatic antioxidants were significantly increased in dose dependent manner Table 4.
The histopathological observations of pancreas and liver tissue samples are given in Fig. 3 and Fig. 4. In pancreatic tissue (Fig. 3C) PC showed deformation of common aspects of islets of Langerhans. The majority of acinus cavities disclosed acinar injury characterized by cytoplasmic vacuolization and cell withering. Broad intra- and interlobular channels of ducts were observed. BET20 (Fig. 3J) showed almost typical islets and beta cells but less stringent acinus cells. BET40 (Fig. 3K) showed it divulged rejuvenation of islets of Langerhans. The little empty space in the basal region of acinus cells was also much compact. The broad intra- and interlobular channels of ducts were observed. SEEMF50 (Fig. 3H) showed more development of islets and beta cells. SEEMF100 (Fig. 3I) showed an almost typical structure of islets of Langerhans. The acinus cell modification was less stringent. The boundary line between exocrine and endocrine segments became more definite.
The liver tissue samples (Fig. 4) disclose that the NC (Fig. 4A) group showed the normal microscopic scrutiny of the liver is composed of normal histological features. BET20 (Fig. 4J) showed the prevention of histological changes. Hepatic digression was not seen, but mild inflammation in hepatocytes was observed. BET40 (Fig. 4K) showed a liver with revamping of liver tissue, hepatocytes and showed lenient congestion with nuclear abridgment. The recovery of hepatic lobules and clear hepatic mitigation were observed.
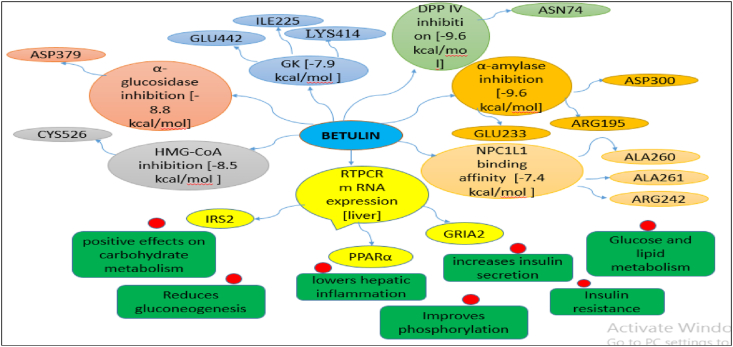
Effects of the samples on mRNA expression of IRS2 gene, PPARα gene, and GRIA2 (Table 6) gene in rat liver were studied. The gene expression of IRS2, PPARα, and GRIA2 gene mRNA was decreased in the DC group, which after treatment was increased and found significantly high in the BET40 treated group than that of the other groups. Hence, BET has positive effects on carbohydrate metabolism in the liver (IRS2 gene), lowers hepatic inflammation (PPARα gene) and increases insulin secretion (GRIA2 gene).
The docking score of betulin with different enzymes are described in Table 7. The best binding mode with zero rmsd/ub and rmsd/lb have been selected as best model of inhibition. The 2D- and 3D-docking poses of betulin with all the enzymes are represented in Table 8. The enzymes are shown in Fig. 5.
In the investigation of the striga plant, betulin, a pentacyclic triterpenoid derivative isolated from bioactive fraction exhibits more efficacious antidiabetic and antihyperlipidemic activity at 40 mg/kg in the STZ-induced diabetic rat model. A bioactive compound identified from CEF GCHRMS spectra was betulin with probable percentage composition of 25.8% w/w. There was no scientific evidence of the pharmacological study on the plant hence antidiabetic study on ethanolic extract was performed followed by its fractions and isolated compound. In the present investigation, SEE, SEBEEF, SEEMF and BET showed stalwart inhibition of digestive enzymes α-amylase and α-glucosidase. It is divulged that intra-peritoneal injection of 55 mg/kg doses of STZ in adult Wistar rats influences investigational diabetes mellitus within five days [22]. In the current investigation, after administration of STZ to rats, they had accounted in expanded BGL on the fourth day, probably because of the wrecking of pancreatic islets and the death of β-cells. It has been proven that STZ creates diabetes by damaging glucose oxidation, decreasing biosynthesis and emanation of insulin [23]. STZ releases a pestilential volume of NO, which disfigurements DNA by impeding aconitase activity, resulting in necrosis by wrecking of β-cells [26].
DM is chiefly kindred with various metabolic disorders, out of which lipid metabolism is remarkably contrived by elevated TC, TG, and LDL concentration and declined concentration of HDL [27]. In the current investigation, the altered lipid profile of diabetic rats was found to manifest elevated TC, TG, LDL, VLDL and declined HDL which were improved in BET treatment. These results may be analogous with the probable defensive effect of the polysaccharides to pancreatic β-cells, which elevates the release of insulin to invigorate the synthesis of fatty acid and chartering of fatty acids into TG from the liver and adipose tissues Fig. 6.
Fig. 6.
The possible molecular pharmacological changes by betulin identified from study.
In the current investigation, DC animals showed declined insulin level, which was significantly elevated by the treatment with BET40. It was maybe due to the role of insulin in managing glucose-stimulated insulin secretion through glucose-sensing or growth of β-cells. It is revealed that certain drugs induce hypoglycemic activity by heightening the insulin effect, either by expanding the pancreatic emanation of insulin from the β-cells or its reveal from bound insulin. However, others perform by the extra-pancreatic mechanism by retardation of hepatic glucose production or alteration of insulin confrontation [28].The level of glycolytic enzymes, glucokinase and glucose-6-phosphate dehydrogenase was decreased in DC animals, maybe due to increased oxidative stress, which indicates suppressed phosphor-gluconate pathway [29].
Molecular docking is a computational tool which enables us to screen the molecules virtually, by which we can get preliminary activity potential of ligand against biological targets. Betulin exhibited very good inhibitory potential against many targets selected for the study. It has been reported that α-amylase contains three significant residues i.e., ASP300, GLU233 and ASP197 are present at the active site cleft which are mainly involved in the hydrolysis of glycosidic bonds present in carbohydrates [30]. In present study, Betulin inhibited the α-amylase with −9.6 kcal/mol binding affinity and formed 3 hydrogen bonds (two conventional and one carbon hydrogen) with GLU233 (2.34673 A0), ARG195 (2.41462 A0), and ASP300 (3.67748 A0). It has developed many hydrophobic (alkyl and Pi-alkyl) with α-amylase. Betulin showed −8.8 kcal/mol binding affinity with α-glucosidase and formed only one conventional hydrogen bond with ASP379 (2.91937 A0). It demonstrated good number of hydrophobic interactions with α-glucosidase. Betulin has inhibited DPP-IV with −9.6 kca/mol binding affinity and formed one conventional hydrogen bond with ASN74 (2.74401 A0). It has developed many alkyl and Pi-alkyl interactions with allosteric site. Betulin exhibited −7.9 kcal/mol binding free energy with GK and formed one conventional hydrogen bond with GLU442 (2.47646 A0). It displayed two hydrophobic interactions with ILE225 (4.90987 A0) and LYS414 (5.3197 A0).
Betulin exhibited −8.5 kcal/mol inhibitory potential against HMG-CoA. It has formed one conventional hydrogen bond with CYS526 (2.52079 A0). It has displayed −7.4 kcal/mol binding affinity with NPC1L1 but unfortunately did not formed any type of hydrogen bond with it. It has developed hydrophobic interactions with ALA260 (3.65013 A0, 4.46702 A0, 5.28745 A0), LA261 (3.62331 A0, 4.19424 A0), and ARG242 (4.94552 A0, 4.86026 A0).
5. Conclusion
The Ayurvedic, traditionally used Striga orobanchioides for diabetes are not evaluated for biological potential. In this project the plant was scientifically investigated for antidiabetic and antihyperlipidemic activity. Ethanol extract, Ethyl acetate-methanol fraction and chloroform-ethyl acetate sub-fraction showed potency of reducing blood sugar level and bad cholesterol in vivo. Betulin isolated from later sub-fraction also demonstrated significant activities compare with other fractions and metformin. The in vivo antidiabetic and antihyperlipidemic parameters are effectively controlled by the samples. The activity could be initiated by stimulating insulin secretion, increasing the PPAR-α level with an increase in GRIA2 mRNA expression. Betulin has demonstrated very good inhibitory potential on α-amylase, α-glucosidase, DPP-IV and GK enzymes. It also demonstrate significant in silico binding with recently studied HMG-CoA and NPC1L1 to develop novel anti-hyperlipidemic drugs. It is concluded that the plant and isolated compound Betulin obtained from bioassay guided fractionation could be effectively used to develop antidiabetic and antihyperlipidemic medicine. However further studies are essential to know other chemicals of the plant which individually or synergistically showed the activities and also important to investigate clinical study of these chemicals.
Authors contribution
Sunayana Vikhe: Conceptualization, Study design, Software, Validation, Investigation, Resources, Writing – original draft. Rahul Kunkulol: Formal analysis, Data curation, Visualization, Supervision, Project administration. Dipak Raut: Writing – review and editing, Visualization, Supervision.
Source of funding
None.
Declaration of competing interest
The authors declared no any conflict of interest.
Acknowledgement
The authors wish to express their gratitude to the Management of Pravara Institute of Medical Sciences, Loni and Management of Pravara Rural College of Pharmacy, Loni for providing facilities and necessary support. Authors are also acknowledging SAIF, IIT Mumbai for providing spectral analysis of the samples.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Saranya R., Thirumalai T., Hemalatha M., Balaji R., David E. Pharmacognosy of enicostemmalittorale: a review. Asian Pac J Trop Biomed. 2013;3(1):79–84. doi: 10.1016/S2221-1691(13)60028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Indumathi C., Durgadevi G., Nithyavani S., Gayathri P.K. Estimation of terpenoid content and its antimicrobial property in Enicostemmalitorrale. Int J ChemTech Res. 2014;6(9):4264–4267. [Google Scholar]
- 3.Pal M. Medicinal chemistry approaches for glucokinase activation to treat type 2 diabetes. Curr Med Chem. 2009;16(29):3858–3874. doi: 10.2174/092986709789177993. [DOI] [PubMed] [Google Scholar]
- 4.Singh R., Lather V., Pandita D., Judge V., N Arumugam K., Singh Grewal A. Synthesis, docking and antidiabetic activity of some newer benzamide derivatives as potential glucokinase activators. Lett Drug Des Discov. 2017;14(5):540–553. [Google Scholar]
- 5.Prasathkumar M., Anisha S., Dhrisya C., Becky R., Sadhasivam S. Therapeutic and pharmacological efficacy of selective Indian medicinal plants–A review. Phytomedicine. 2021 doi: 10.1016/j.phyplu.2021.100029. [DOI] [Google Scholar]
- 6.Asolkar L.V., Kakkar K.K., Chakre O.J., Chopra R.N., Nayar S.L., Chopra I.C. Publications & Information Directorate; 1992. Glossary of Indian medicinal plants. Edition 2002. [Google Scholar]
- 7.Chopra R.N., Nayar S.L., Chopra I.C. Council of Scientific and Industrial Research; New Delhi: 1956. Glossary of Indian medicinal plants; p. 235. [Google Scholar]
- 8.Kirtikar K.R., Basu B.D. 2nd ed. III. International Book Distributor; Dehradun: 2006. Indian medicinal plants; p. 1830. [Google Scholar]
- 9.Pullaiah T., Naidu Chandrashekhar K. 2003. Antidiabetic plants in India; p. 287. [Google Scholar]
- 10.Nadkarni K.M. I. Bombay Popular Prakashan; 1986. Indian materia medica; p. 1171. [Google Scholar]
- 11.Vikhe S., Kunkulol R. Microscopic investigations and pharmacognosy of Striga orobanchioides Benth. Pharm J. 2020;12(6):1325–1331. doi: 10.5530/pj.2020.12.182. [DOI] [Google Scholar]
- 12.Hiremath S.P., Badami S., Swamy H.K.S., Patil S.B., Londonkar R.L. Antiandrogenic activity of Striga orobanchioides. J Ethnopharmacol. 1997;56:55–60. doi: 10.1016/s0378-8741(96)01505-x. [DOI] [PubMed] [Google Scholar]
- 13.Hiremath S.P., Swamy H.K.S., Badami S., Meena S. Antibacterial and antifungal activity of Striga orobanchioides. Indian J Pharmaceut Sci. 1996;58:174–178. [Google Scholar]
- 14.Harish M.S., Nagur M., Badami S. Antihistaminic and mast cell stabilizing activity of Striga orobanchioides. J Ethnopharmacol. 2001;76:197–200. doi: 10.1016/S0378-8741(01)00236-7. [DOI] [PubMed] [Google Scholar]
- 15.Hiremath S.P., Badami S., Swamy H.K.S., Patil S.B. Antifertility and hormonal properties of flavones of Striga orobanchioides. Eur J Pharmacol. 2001;391:193–197. doi: 10.1016/S0014-2999(99)00723-2. [DOI] [PubMed] [Google Scholar]
- 16.Badami S., Gupta M.K., Suresh B. Antioxidant activity of the ethanolic extract of Striga orobanchioides. J Ethnopharmacol. 2003;85(2):227–230. doi: 10.1016/S0378-8741(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 17.Bhattacharya S. Medicinal plants and natural products in amelioration of arsenic toxicity: a short review. Pharm Biol. 2017;55:349–354. doi: 10.1080/13880209.2016.1235207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sunayana Vikhe, Sunil Nirmal. Antiallergic and antihistaminic actions of Ceasalpinia bonducella seeds: possible role in treatment of asthma. J Ethnopharmacol. 2018;216:251–258. doi: 10.1016/j.jep.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Borowitz I.J. Thin-layer chromatography. A laboratory handbook. J Colloid Interface Sci. 1966;21(1):124–125. doi: 10.1016/0095-8522(66)90095-X. [DOI] [Google Scholar]
- 20.Gayathri Nambirajana, Kaleshkumar Karunanidhib, Arun Ganesanb, Rajaram Rajendranb, Ruckmani Kandasamyc, Abbirami Elangovana, et al. Evaluation of antidiabetic activity of bud and flower of Avaram Senna (Cassia auriculata L.) in high fat diet and streptozotocin induced diabetic rats. Biomed Pharmacother. 2018;108:1495–1506. doi: 10.1016/j.biopha.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Hemalatha P., Bomzan D.P., Rao B.S., Sreerama Y.N. Distribution of phenolic antioxidants in whole and milled fractions of quinoa and their inhibitory effects on α- amylase and α-glucosidase activities. Food Chem. 2016;199:330–338. doi: 10.1016/j.foodchem.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 22.Ali Sangi S.M., Jalaud N.A. Prevention and treatment of brain damage in streptozotocin induced diabetic rats with Metformin, Nigella sativa, Zingiber officinale, and Punica granatum. Biomed Res Ther. 2019;7(6):3274–3285. [Google Scholar]
- 23.Bolaffi J.L., Nagamastu S., Harris J., Grodsky G.M. Protection by thymidine, an inhibitor of polyadenosine diphosphate ribosylation of streptozotocin inhibition of insulin secretion. Endocrinology. 1987;20:2117e22. doi: 10.1210/endo-120-5-2117. [DOI] [PubMed] [Google Scholar]
- 24.Dallakyan S., Olson A.J. Humana Press; New York, NY: 2015. Small-molecule library screening by docking with PyRx; pp. 243–250. In Chemical biology. [DOI] [PubMed] [Google Scholar]
- 25.Khan S.L., Siddiqui F.A. Beta-sitosterol: as immunostimulant, antioxidant and inhibitor of SARS-CoV-2 spike glycoprotein. Arch Pharmacol Therapeut. 2020;2(1) [Google Scholar]
- 26.Choudhari V.P., Gore K.P., Pawar A.T. Antidiabetic, antihyperlipidemic activities and herb-drug interaction of a Polyherbal formulation in streptozotocin. J Ayurveda Integr Med. 2017;8(4):218–225. doi: 10.1016/j.jaim.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elberry Ahmed A., Fathalla Harraz M., Ghareib Salah A., Gabr Salah A., Nagy Ayman A., Abdel-Sattar Essam Methanolic extract of Marrubium vulgare ameliorates hyperglycemia and dyslipidemia in streptozotocin-induced diabetic rats. Inter J Diabetes Mellitus. 2011;11:1877–1878. doi: 10.1016/j.ijdm.2011.01.004. [DOI] [Google Scholar]
- 28.Pari L., Satheesh M.A. Antidiabetic activity of Boerhaavia diffusa L on hepatic key enzymes in experimental diabetes. J Ethnopharmacol. 2004;91:109e13. doi: 10.1016/j.jep.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 29.Choukem S.P., Sobngwi E., Garnier J.P., Letellier S., Mauvais-Jarvis F., Calvo F., et al. Hyperglycaemia per se does not affect erythrocyte glucose-6-phosphate dehydrogenase activity in ketosis-prone diabetes. Diabetes Metab. 2015;41:326–330. doi: 10.1016/j.diabet.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Naeem F., Nadeem H., Muhammad A., Zahid M.A., Saeed A. Synthesis. α-Amylase inhibitory activity and molecular docking studies of 2,4-thiazolidinedione derivatives. Open Chem J. 2018;5(1):134–144. doi: 10.2174/1874842201805010134. [DOI] [Google Scholar]
- 31.Rao M.U., Sreenivasulu M., Chengaiah B., Reddy K.J., Chetty C.M. Herbal medicines for diabetes mellitus: a review. Int J PharmTech Res. 2010 Jul;2(3):1883–1892. [Google Scholar]
- 32.Salehi B., Ata A., V Anil Kumar N., Sharopov F., Ramírez-Alarcón K., Ruiz-Ortega A., et al. Antidiabetic potential of medicinal plants and their active components. Biomolecules. 2019 Oct;9(10):551. doi: 10.3390/biom9100551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dey L., Attele A.S., Yuan C.S. Alternative therapies for type 2 diabetes. Alternative Med Rev. 2002 Feb 1;7(1):45–58. [PubMed] [Google Scholar]
- 34.Ma Chunhua. Long Hongyan Protective effect of betulin on cognitive decline in streptozotocin (STZ)-induced diabetic rats. Neurotoxicology. 2016;57:104–111. doi: 10.1016/j.neuro.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Narasimhamurthy Konappa, Arakere C.Udayashankar, Soumya Krishnamurthy, Chamanalli Kyathegowda Pradeep, Srinivas Chowdappa, Sudisha Jogaiah. GC–MS analysis of phytoconstituents from Amomum nilgiricum and molecular docking interactions of bioactive serverogenin acetate with target proteins. Sci Rep. 2020;10:16438. doi: 10.1038/s41598-020-73442-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Histology Laboratory Manual. Vagelos College of physicians & surgeons Columbia university patrice F spitalnik MD histology director. P.N. 85-86.
- 37.Kubota T., Kubota N., Kadowaki T. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia. 2012;55(10):2565–2582. doi: 10.1007/s00125-012-2644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eckstein S.S., Weigert C., Lehmann R. Divergent roles of IRS (insulin receptor substrate) 1 and 2 in liver and skeletal muscle. Curr Med Chem. 2017;24(17):1827–1852. doi: 10.2174/0929867324666170426142826. [DOI] [PubMed] [Google Scholar]