Abstract
As of 2022, the global population has access to several mRNA and traditional inactivated vaccines. However, their effectiveness in preventing infection, hospitalization, and COVID-associated mortality in Jordan has yet to be evaluated. The purpose of this observational study was to evaluate the relative effectiveness of three approved vaccines against COVID-19 in a sample of the Jordanian population. The study was conducted between July 2021 and 2022 in a sample of adult patients presenting to hospitals across Jordan and receiving one of the three vaccines – Pfizer (BNT162b2), Astra Zeneca (ChAdOx1-S), or Sinopharm (BBIBP-CorV). Data were collected to measure the rates of infection without hospitalization, infection with hospitalization, and death. The sample included patients with one of the following chronic conditions: cardiovascular disease, respiratory disease, or diabetes. Primary data were obtained from patients' health records. The sample included 6132 adults from Jordan, with a mean age 52 ± 17 years. The rates of death in patients receiving two doses of any vaccine ranged between 0.175 and 2.77%, compared with 0.69–13.53% in patients receiving only one dose. The rates of hospitalization were 6–7.97% with two doses, compared to 7.98–25.13% with one dose. The rates of infection without hospitalization were significantly higher in the two-dose group (6–25.1%) compared with those who had received only one dose of any COVID-19 vaccine (0.69–10.61%). In conclusion, receiving two doses of a COVID-19 vaccine was associated with lower odds of mortality and hospitalization and higher odds of infection. More research is needed to evaluate the safety and efficacy of vaccines against SARS-CoV-2.
Keywords: COVID-19, Vaccines, Coronavirus, Hospitalization, Infection, Mortality
1. Introduction
On March 11, 2020, the World Health Organization (WHO) declared the beginning of the COVID-19 pandemic [1]. Within months after declaring the epidemic, the world saw the first candidate vaccines, including BNT162b2 from Pfizer-BioNTech and ChaAdOx1 adenoviral vaccine from Oxford-AstraZeneca [1]. The initial goal was to create a vaccine effective enough to prevent infection with SARS-CoV-2 [2]. It was also expected that COVID-19 vaccination would reduce hospitalizations and mortality caused by SARS-CoV-2. Several trials of mRNA vaccines demonstrated that they were 92–95% effective in preventing COVID-19 infection [[2], [3], [4], [5]].
Today, all COVID-19 vaccines are administered to induce immunity against symptomatic SARS-CoV-2 infection and minimize the risks of severe COVID-19. After administration, COVID-19 vaccines launch a chain of immune reactions involving B cells [6]. They cause an antibody response, with antibodies binding to the spike protein to prevent SARS-CoV-2 from entering the cell [6]. The immunological memory created by the COVID-19 vaccines is the primary factor protecting individuals from symptomatic and severe infection, thus lowering the risks of COVID-related death.
Despite the growing number of adults receiving COVID-19 vaccines, the knowledge of their effectiveness in different countries and population groups is scarce [6]. Studies of the comparative effectiveness and safety of mRNA and classic inactivated vaccines against COVID-19 continue, most conducted in Europe or the United States [1,3,[7], [8], [9]]. A few studies were conducted in Egypt, Iran, and Pakistan [[10], [11], [12]]. Until present, only one study of COVID-19 vaccines has been conducted in Jordan, but its goal was to evaluate the scope of vaccine side effects rather than measure their comparative effectiveness [13]. Therefore, the effectiveness of mRNA and traditional inactivated COVID-19 vaccines in Jordan remains an under-researched area.
Added to this is the lack of data and understanding regarding the effects of COVID-19 vaccines on infection, hospitalization, and mortality in at-risk groups. These include individuals with cardiovascular disease, diabetes, and chronic respiratory disease [14]. Knowing this, the purpose of this observational study was to evaluate the comparative effectiveness of three COVID-19 vaccines currently approved for use in Jordan – Pfizer-BioNTech (BNT162b2), Astra Zeneca (ChAdOx1-S), and Sinopharm (BBIBP-CorV) in a large sample of Jordanian adults with chronic cardiovascular and respiratory conditions and diabetes.
2. Materials and methods
This observational study was conducted between July 2021 and January 2022 in several hospitals in different regions within Jordan. The study population included adults with at least one chronic condition–cardiovascular, respiratory, or diabetes–presenting to one of the clinics for COVID-19 vaccination. Patients received one or two doses of one of the following vaccines: Pfizer, Astra Zeneca, or Sinopharm. Patients' health records were used to evaluate the following outcome measures: infection rates (symptomatic), hospitalization rates, and mortality rates. Simple descriptive statistics were used to calculate the percentage of patients with one or two doses of a COVID-19 vaccine with symptomatic infection, requiring hospitalization, or dying as a result of COVID-19. Outcomes were measured for all three vaccines. The relative effectiveness of each of the three vaccines in preventing infection, hospitalization, and death from COVID-19 was also evaluated. All hospitals confirmed that the cause of mortality was the result of COVID-19. Statistical analysis.
IBM SPSS v25.0 was used for the statistical analysis. The data was presented as frequency and percentage (%). Columns were used for data visualization; each column represents a percentage of the population and not absolute numbers to avoid misrepresentation errors (one group is larger than the other). Differences between groups were analyzed using the Chi-Square test of independence and the Z-test for column proportion. A P value of less than 0.05 was considered statistically significant. A Bonferroni correction was applied for all pairwise comparisons using the Z-test.
3. Results
The sample included 6132 adults presenting to hospitals in Jordan. The mean age of study participants was 52 ± 17 years, 61.98% of them being male (N = 3801) and 38.01% being female (N = 2331). Of these, 2017 patients received one dose of a COVID-19 vaccine, and 4115 patients received two doses. In the one-dose group, 1317 patients received Sinopharm, 320 patients received Pfizer, and 380 patients received AstraZeneca. In the two-dose group, 1317 patients received Sinopharm, 1006 received Pfizer, and 1792 received AstraZeneca. All patients had at least one of the three chronic conditions: cardiovascular disease, chronic respiratory disease, and/or diabetes.
3.1. Overall effectiveness of COVID-19 vaccines
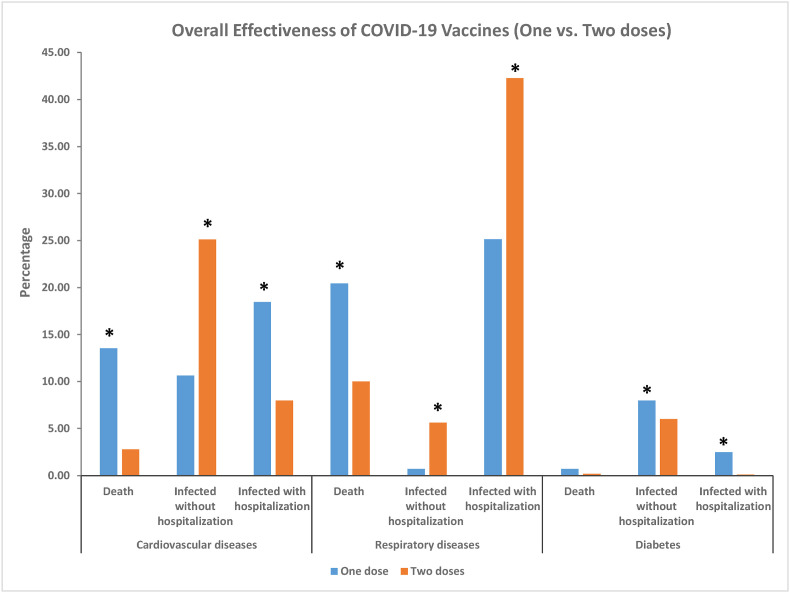
Receiving two doses of any vaccine was associated with lower mortality (X2 = 45.84, p < 0.001). During the study period, 2.77% of patients (N = 114) with cardiovascular disease died of COVID-19 after two doses of a COVID-19 vaccine, compared to 13.53% of patients after one dose (N = 273) (Z-test p < 0.001). The rate of mortality among patients with chronic respiratory disease after two doses was 9.99% (N = 411), compared with 20.42% (N = 412) after receiving the first dose (Z-test p < 0.001). In patients with diabetes, mortality due to COVID-19 was 0.175% (N = 7) after two doses, compared to 0.69% (N = 14) after the first dose. No significant difference was found (Z-test p > 0.05) (Table .1 , Fig. 1 ).
Table 1.
Total infections, hospitalizations, and mortality in patients with one or two doses of a COVID-19 vaccine.
| Disease | Complication | One dose (N = 2017) |
Two doses (N = 4115) |
X2 for “Disease” | X2 for “Complications” | ||
|---|---|---|---|---|---|---|---|
| N | % | N | % | ||||
| Cardiovascular diseases | Death | 273 | 13.53 | 114 | 2.77 | 476.613a | 45.848a |
| Infected without hospitalization | 214 | 10.61 | 1033 | 25.10 | |||
| Infected with hospitalization | 372 | 18.44 | 328 | 7.97 | |||
| Respiratory diseases | Death | 412 | 20.43 | 411 | 9.99 | 291.263a | 131.810a |
| Infected without hospitalization | 14 | 0.69 | 232 | 5.64 | |||
| Infected with hospitalization | 507 | 25.14 | 1739 | 42.26 | |||
| Diabetes | Death | 14 | 0.69 | 7 | 0.17 | 57.660a | 330.981a |
| Infected without hospitalization | 161 | 7.98 | 247 | 6.00 | |||
| Infected with hospitalization | 50 | 2.48 | 4 | 0.10 | |||
Significant at p < 0.001.
Fig. 1.
Complications in patients with different chronic diseases after receiving one or two doses of any vaccine. *Significant difference according to Z-test of proportions at p < 0.001 level. Bonferroni correction was used for all pairwise comparisons. Differences were found between all groups except the mortality rates in patients with diabetes.
Receiving two doses of a COVID-19 vaccine was associated with lower rates of hospitalization for patients with cardiovascular disease and diabetes (X2 = 330.981, p < 0.001). Hospitalization rates for individuals with cardiovascular disease were 7.97% (N = 328) after the second dose, compared with 18.44% (N = 372) after the first dose (Z-test p < 0.001). In patients with diabetes, COVID-19-related hospitalization rates were 0.1% (N = 4) after two doses, compared to 2.48% (N = 50) after the first dose (Z-test p < 0.001). The trend was not sustained among individuals with chronic respiratory disease, where the rates of hospitalization increased after the second dose – 42.46% (N = 1739) against 25.13% (N = 507) after the first dose (Z-test p < 0.001) (Table .1, Fig. 1).
The rates of infection without hospitalization were significantly higher in patients with cardiovascular disease and chronic respiratory disease after the second dose (X2 = 131.810, p < 0.001). 25.1% of patients with cardiovascular disease (N = 1033) were infected with COVID-19 after two doses, compared with 10.61% (N = 214) after the first dose (Z-test p < 0.001). Likewise, 5.64% of individuals with chronic respiratory disease (N = 232) had infections not requiring hospitalization, compared to 0.69% of patients receiving only one dose (N = 14) (Z-test p < 0.001). The trend was not sustained among individuals with diabetes, where the rates of infection slightly decreased after two doses of a COVID-19 vaccine (6%, N = 247) compared with one dose (7.98%; N = 161), however the result was statistically significant (Z-test p < 0.001) (Table .1, Fig. 1).
3.2. Comparative effectiveness of COVID-19 vaccines
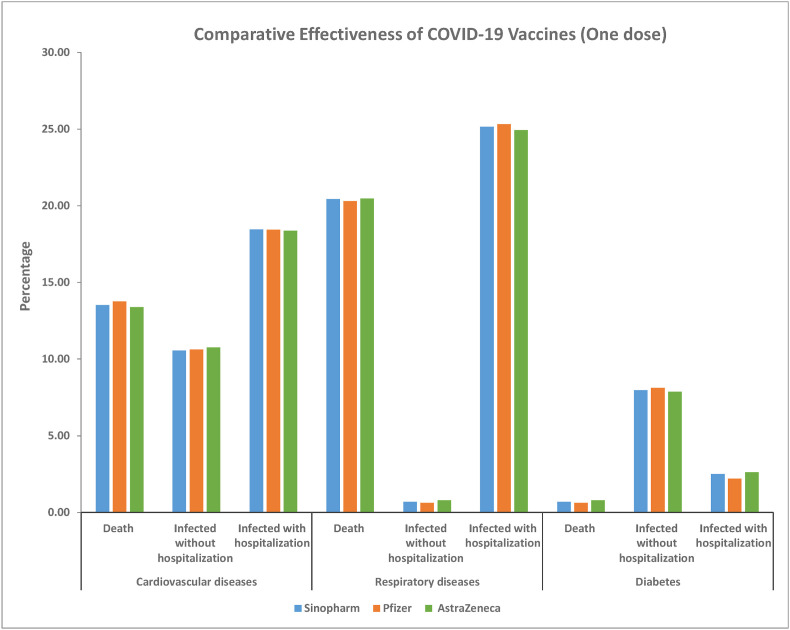
The rates of infection, hospitalization, and death did not differ significantly among patients receiving each of the three vaccines (X2 = 0.155, 0.089, 0.091, p = 0.997, 0.999 and 0.999, respectively). The rates of death after one dose of Sinopharm vaccine were 13.51% for patients with cardiovascular disease (N = 178), 20.425% for patients with respiratory disease (N = 269), and 0.683% for patients with diabetes (N = 9). The rates of death after one dose of Pfizer were 13.75% for patients with cardiovascular disease (N = 44), 20.31% for patients with respiratory disease (N = 65), and 0.625% for patients with diabetes (N = 2). The rates of death after one dose of AstraZeneca were 13.42% among patients with cardiovascular disease (N = 51), 20.5% among patients with respiratory disease (N = 78), and 0.789% among patients with diabetes (N = 3), no significant difference was found between any pairwise comparisons of all 3 vaccines (Z-test p < 0.001) (Table .2 , Fig. 2 ).
Table 2.
Comparative effectiveness of one-dose vaccination regimens.
| Disease | Complication | Sinopharm (N = 1317) |
Pfizer (N = 320) |
AstraZeneca (N = 380) |
X2 for “Disease” | X2 for “Complications” | |||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||||
| Cardiovascular diseases | Death | 178 | 13.53 | 44 | 13.75 | 51 | 13.39 | 0.012 | 0.077 |
| Infected without hospitalization | 139 | 10.56 | 34 | 10.63 | 41 | 10.76 | |||
| Infected with hospitalization | 243 | 18.47 | 59 | 18.44 | 70 | 18.37 | |||
| Respiratory diseases | Death | 269 | 20.44 | 65 | 20.31 | 78 | 20.47 | 0.020 | 0.007 |
| Infected without hospitalization | 9 | 0.68 | 2 | 0.63 | 3 | 0.79 | |||
| Infected with hospitalization | 331 | 25.15 | 81 | 25.31 | 95 | 24.93 | |||
| Diabetes | Death | 9 | 0.68 | 2 | 0.63 | 3 | 0.79 | 0.172 | 0.101 |
| Infected without hospitalization | 105 | 7.98 | 26 | 8.13 | 30 | 7.87 | |||
| Infected with hospitalization | 33 | 2.51 | 7 | 2.19 | 10 | 2.62 | |||
No significant differences were found between all 3 vaccines regarding the mortality or morbidity in patients with chronic diseases.
Fig. 2.
Complications in patients with different chronic diseases after receiving one doses of any of the 3 vaccines. No significant difference was found between all 3 vaccines in any pairwise comparison by Z-test of proportions.
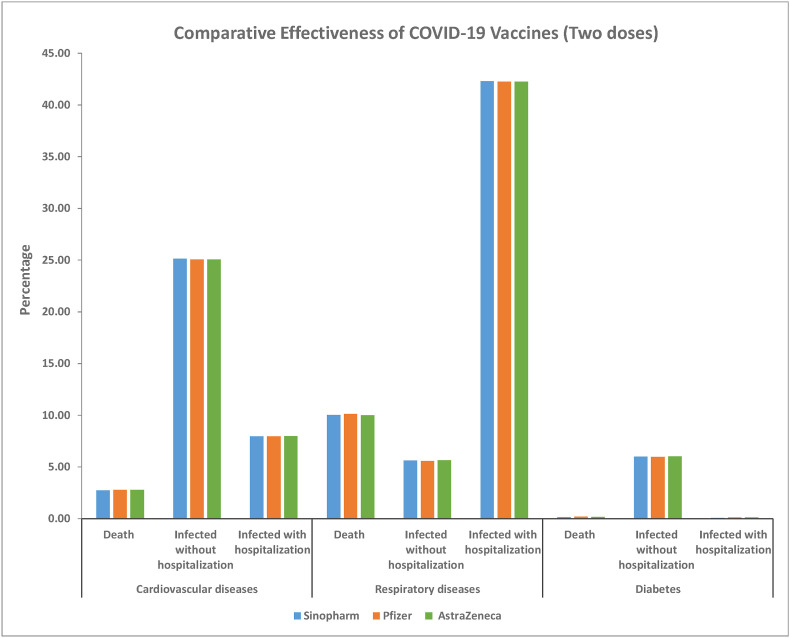
The rates of infection, hospitalization, and death were identical among patients receiving two doses of the COVID-19 vaccine (X2 = 0.101, 0.007, 0.077, p = > 0.999 for all tests). In patients with cardiovascular disease, mortality was 2.7% after two doses of Sinopharm, 2.783% after two doses of Pfizer, and 2.79% after two doses of AstraZeneca. The rates of death in patients with respiratory disease were 10.02% for Sinopharm, 9.94% for Pfizer, and 9.98% for AstraZeneca. Mortality among patients with diabetes was 0.1518% for two doses of Sinopharm, 0.198% for two doses of Pfizer, and 0.167% for two doses of AstraZeneca. No significant difference was found between all 3 groups receiving the different vaccines (Z-test p > 0.05 for all pairwise comparisons) (Table .3 , Fig. 3 ).
Table 3.
Comparative effectiveness of two-dose vaccination regimens.
| Disease | Complication | Sinopharm (N = 1317) |
Pfizer (N = 1006) |
AstraZeneca (N = 1792) |
X2 for “Disease” | X2 for “Complications” | |||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||||
| Cardiovascular diseases | Death | 36 | 2.73 | 28 | 2.78 | 50 | 2.79 | 0.025 | 0.091 |
| Infected without hospitalization | 331 | 25.13 | 252 | 25.05 | 449 | 25.06 | |||
| Infected with hospitalization | 105 | 7.97 | 80 | 7.95 | 143 | 7.98 | |||
| Respiratory diseases | Death | 132 | 10.02 | 102 | 10.14 | 179 | 9.99 | 0.084 | 0.089 |
| Infected without hospitalization | 74 | 5.62 | 56 | 5.57 | 101 | 5.64 | |||
| Infected with hospitalization | 557 | 42.29 | 425 | 42.25 | 757 | 42.24 | |||
| Diabetes | Death | 2 | 0.15 | 2 | 0.20 | 3 | 0.17 | 0.214 | 0.155 |
| Infected without hospitalization | 79 | 6.00 | 60 | 5.96 | 108 | 6.03 | |||
| Infected with hospitalization | 1 | 0.08 | 1 | 0.10 | 2 | 0.11 | |||
No significant differences were found between all 3 vaccines regarding the mortality or morbidity in patients with chronic diseases.
Fig. 3.
Complications in patients with different chronic diseases after receiving two doses of any of the 3 vaccines. No significant difference was found between all 3 vaccines in any pairwise comparison by Z-test of proportions.
The rates of infection were much higher after two doses of each vaccine for patients with cardiovascular disease and respiratory disease; for patients with diabetes, each of the three vaccines reduced slightly the risks of symptomatic infection not requiring hospitalization (Table 3). All three vaccines failed to reduce hospitalization in patients with respiratory disease – the percentage of those requiring hospitalization was 42.49% for two doses of Sinopharm, 42.147% for two doses of Pfizer, and 42.24% after two doses of AstraZeneca, compared to the mean of 25.15% after one dose of COVID-19 vaccine (Table 3).
4. Discussion
4.1. Total effectiveness
Two doses of any COVID-19 vaccine have been proven to reduce the rates of hospitalization and COVID-related mortality compared with one dose. The mean percent of death with one dose was 11.55%, decreasing to a mean of 4.31% after the second dose (Table 2, Table 3). These findings are in line with other published studies [5,15,16]. Investigated the effectiveness and safety of Pfizer's BNT162b2 and found it was 93.4% effective against hospitalization and 91.1% effective against mortality. Bernal et al. (2021) estimated that administering two doses of Pfizer vaccine in older adults reduced the odds of emergency hospital admission by 43% and the risk of death by 51% [15]. Although the numbers vary across studies, they create a strong rationale for a two-dose vaccination regimen in at-risk groups regardless of the type of COVID-19 vaccine used.
The most significant reductions were noted among patients with cardiovascular disease, with COVID-19 mortality decreasing 4.88 times after the second dose (Table 2, Table 3). Death rates were the lowest among patients with diabetes and the highest among patients with respiratory conditions, even after completing the two-dose regimen. Cardiovascular disease remains one of the principal risk factors for severe COVID-19 and death [14,17]. However, the exact mechanism of severe COVID-19 in patients with underlying cardiovascular conditions requires further analysis. Therefore, it is difficult to explain the variability of vaccine effectiveness among different at-risk groups. According to Sabatini et al. (2020), viral infections increase the incidence of cardiac injury; they can also induce an immune response, releasing pro-inflammatory cytokines that result in cardiac complications. Patients with respiratory disease and diabetes should also receive two doses of the COVID-19 vaccine since it can reduce the risks of hospitalization and death [18]. In fact, two doses of Sinopharm, Pfizer, and AstraZeneca vaccines were effective in reducing the rates of hospitalization due to SARS-CoV-2 infection, compared with one dose. Similar findings were previously reported by Botton et al. (2022), with the Pfizer vaccine reducing hospitalization in at-risk groups by 86%. However, while hospitalizations decreased significantly for individuals with cardiovascular disease and diabetes, hospitalizations in patients with respiratory disease increased after the second dose [2]. Overall, the rates of infection with hospitalization were 25.13% with one dose and 42.46% with two doses of any vaccine (Table 1, Table 2, Table 3). These results contradict the findings by Thompson et al. (2021), who found mRNA vaccines to be at least 90% effective against hospitalization in patients with chronic respiratory disorders [19]. They contradict other empirical findings [3,[7], [8], [9],15]. Yan et al. (2021) suggest that, until present, no trials have evaluated the exact mechanism of this relationship and the efficacy of COVID-19 vaccines in this group [14]. Kandi (2021) notes variations in the immune response to COVID-19 vaccines in individuals with chronic and debilitating disorders [20]. These controversies create a room for further experimental research in this field is needed, with an emphasis on the pathophysiology of SARS-CoV-2 in patients with chronic respiratory illness [20].
A notable finding is that the rates of symptomatic infections increased after the second dose, compared with the number of infections after the first dose (Table 1). The mean percent of infections after one dose was 6.43%, and increased to a mean of 12.25% after the second dose. After the second dose, infections increased most prominently in patients with respiratory conditions; the rates of infection declined slightly in patients with diabetes. These findings raise questions about the effectiveness of COVID-19 vaccines against symptomatic infection with SARS-CoV-2. Other studies are almost unanimous in that vaccination with two doses reduces the odds of symptomatic infection or prevents it [1.5,16]. In the study by Rizwan et al. (2021), only 1.55% of respondents developed symptomatic infection after two doses of the Sinopharm vaccine [12]. Unfortunately, studies of COVID-19 vaccine effectiveness are few, and these findings will create the groundwork for future research.
4.2. Comparative effectiveness
In this study, the effectiveness of three COVID-19 vaccines was compared in relation to infection rates, hospitalization, and mortality. Three types of vaccines were tested: mRNA vaccines (Pfizer), non-replicating viral vector-based vaccines (AstraZeneca), and one was an inactivated vaccine (Sinopharm). While the type, nature, and pattern of adaptive immunity vary significantly based on vaccine type, this research has demonstrated that each of the three vaccines had a similar effect on the three outcome measures noted above (Fig. 2, Fig. 3).
The rates of death, symptomatic infection, and hospitalization were similar for each of the three vaccines in all three groups of participants. For example, hospitalization rates in patients with cardiovascular disease after the first dose were 10.55% for Sinopharm, 10.629% for Pfizer, and 10.79% for AstraZeneca. After the second dose, the rates of hospitalization in this group were 25% for Sinopharm, 25.05% for Pfizer, and 25.05% for AstraZeneca. Death among patients with respiratory conditions was highest after two doses of Sinopharm (10.02%) but only slightly higher than mortality after two doses of Pfizer (9.94%) or AstraZeneca (9.98%). In the diabetes group, death rates were the highest after two doses of Pfizer (0.198%), while the rates of hospitalization were the lowest after two doses of Sinopharm (0.076%). The percent of symptomatic infections without hospitalization was the highest in patients with the cardiovascular group, for each of the three vaccines. Meanwhile, patients with respiratory conditions reported the most significant increase in infection rates after two doses, compared with one dose of each vaccine (5.6% against 0.7%, accordingly) (Fig. 2, Fig. 3).
Studies published so far provide a complex picture of vaccine effectiveness against COVID-19. Alqassieh et al. (2021) reported higher IgG titers after two doses of Pfizer compared to two doses of Sinopharm, suggesting that Pfizer could be more effective against symptomatic COVID-19 and poor COVID-19 outcomes [6]. Bernal et al. (2021) found Pfizer and AstraZeneca to be equally effective at preventing symptomatic infection and COVID-19 mortality [15]. Likewise, a large population study by Voko et al. (2022) did not reveal any meaningful differences between Pfizer, Sinopharm, and AstraZeneca in terms of hospitalization and mortality in at-risk groups [21]. Similar results were reported by Ghiasi et al. (2021). Nevertheless, future research should determine an optimal combination of vaccines or vaccination regimens for individuals with different underlying conditions [22].
4.2.1. Limitations
This study is subject to several limitations. First, with the design being observational, we could not account for all confounding variables. Because the analysis of COVID-19 vaccines is currently underway, new and emerging findings may explain the variability of infection, hospitalization, and mortality rates with each of the three vaccines in each of the three at-risk groups included in the study.
Second, this study reveals the effectiveness of three vaccines in reducing poor COVID-19 outcomes in patients with chronic conditions. However, the results do not allow us to evaluate the sustainability of these effects in a long-term perspective. The growing number of published reports suggests that the vaccine immunity for COVID-19 has a tendency to wither over time. For example, Rosenberg et al. (2021) reported that the protection from COVID-19 in older adults after two doses of Pfizer declined from 94.8% to 88.6% over three months [8]. Longitudinal studies are needed to fully assess the effectiveness of COVID-19 vaccines in at-risk adults.
Third, while the effectiveness of COVID-19 vaccines is obvious, their safety profile requires further investigation. Multiple studies reported side effects and adverse events after administering COVID-19 vaccines. Lee et al. (2021) investigated severe hyperglycemia in patients with diabetes induced by a COVID-19 vaccine [23]. Similar findings were reported by Mishra et al. (2021) [24]. According to Elgendy et al. (2022), individuals experienced severe side effects after the first dose of Sinopharm and AstraZeneca, and after the second dose of Pfizer [14]. Specifically, in the Jordanian population, mild side effects (fatigue, body pain, soreness and swelling at the injection site, headache, muscle pain, fever, tiredness, anorexia, sweating, dizziness, dry cough, anxiety, shortness of breath, tachycardia, abdominal pain, sore throat, pain when swallowing, joint pain, and nasal discharge) and no lasting longer than a week were present in 88.9% of respondents receiving any of the three vaccines – Pfizer, AstraZeneca, or Sinopharm. Therefore, while this study demonstrates the efficacy of COVID-19 vaccines, decisions regarding vaccination should be based on a thorough analysis of the vaccines' safety profile. The benefits of vaccination should overweigh any possible risks, suggesting the need for more research in this field.
After the second dose, infections increased most prominently in patients with respiratory conditions; the rates of infection declined slightly in patients with diabetes. These findings raise questions about the effectiveness of COVID-19 vaccines against symptomatic infection with SARS-CoV-2. The most plausible explanation for this result is that we are comparing different groups that were subjected to different conditions, and the symptomatic COVID-19 infection could be referred to various factors other than the number of vaccine doses.
Ultimately, the sample was limited to Jordanian adults with chronic conditions. Thus, the findings reported herein may not be generalized to broader populations within and outside of Jordan. Nevertheless, they can guide vaccination decisions and campaigns to stop the transmission of COVID-19 within and across communities.
Ethics approval
The study protocol conformed to the ethical guidelines of the 1975 Helsinki Declaration. Also, the Ethical Committee approval was obtained from the Ethical Committee of Middle East University-Jordan.
CRediT authorship contribution statement
Mohamed J. Saadh: Writing – review & editing, Writing – original draft, Supervision, Resources, Investigation, Formal analysis, Conceptualization. Saif Aldeen Jaber: Writing – original draft, Validation, Investigation, Formal analysis, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Hall V.J., Foulkes S., Saei A., et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397:1725–1735. doi: 10.1016/S0140-6736(21)00790-X. A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Botton J., Dray-Spira R., Baricault B., Drouin J., Bertrand M., Jabagi M.J., et al. Reduced risk of severe COVID-19 in more than 1.4 million elderly people aged 75 years and older vaccinated with mRNA-based vaccines. Vaccines. 2022;40:414–417. doi: 10.1016/j.vaccine.2021.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bajema K.L., Dahl R.M., Prill M.M., Meites E., Rodriguez-Barradas M.C., Marconi V.C., et al. Effectiveness of COVID-19 mRNA vaccines against COVID-19–associated hospitalization—five veterans affairs medical centers, United States, february 1–august 6, 2021. MMWR (Morb. Mortal. Wkly. Rep.) 2021;70(37):1294. doi: 10.15585/mmwr.mm7037e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chodick G., Tene L., Rotem R.S., Patalon T., Gazit S., Ben-Tov A., et al. The effectiveness of the TWO-DOSE BNT162b2 vaccine: analysis of real-world data. Clin. Infect. Dis. 2022;74:472–478. doi: 10.1093/cid/ciab438. S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saciuk Y., Kertes J., Mandel M., Hemo B., Stein N.S., Zohar A.E. A, Pfizer-BioNTech vaccine effectiveness against Sars-Cov-2 infection: findings from a large observational study in Israel. Prev. Med. 2021;155 doi: 10.1016/j.ypmed.2021.106947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alqassieh R., Suleiman A., Abu-Halaweh S., Santarisi A., Shatnawi O., Shdaifat L., et al. Pfizer-BioNTech and Sinopharm: a comparative study on post-vaccination antibody titers. Vaccines. 2021;9:1223. doi: 10.3390/vaccines9111223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barda N., Dagan N., Balicer R.D. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. Reply. N. Engl. J. Med. 2021;384:1970. doi: 10.1056/NEJMc2104281. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg E.S., Dorabawila V., Easton D., Bauer U.E., Kumar J., Hoen R., et al. COVID-19 vaccine effectiveness in New York state. N. Engl. J. Med. 2022;386:116–127. doi: 10.1056/NEJMoa2116063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ejaz I., Rasheed A., Seher Munir M., Shahid F., Zia A., Mukhtar S. Comparison of efficacy and antibody levels among healthcare providers after second dose of two different COVID vaccines. J. Pharm. Res. Int. 2021;33:292–298. [Google Scholar]
- 11.Elgendy M.O., El-Gendy A.O., Mahmoud S., Mohammed T.Y., Abdelrahim M.E., Sayed A.M. Side effects and efficacy of COVID-19 vaccines among the Egyptian population. Vaccines. 2022;10:109. doi: 10.3390/vaccines10010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizwan W., Qureshi A.U., Rana M.N., Duggal M.N., Sohaib M., Sadiq M.J. Safety profile of Sinopharm COVID-19 vaccine and breakthrough infections in Pakistan. Ann. King Edw. Med. Univ. 2021;27:516–523. [Google Scholar]
- 13.Omeish H., Najadat A., Al-Azzam S., Tarabin N., Abu Hameed A., Al-Gallab N., et al. Immunotherapeutics, Reported COVID-19 vaccines side effects among Jordanian population: a cross sectional study. Hum. Vaccines Immunother. 2021:1–8. doi: 10.1080/21645515.2021.1981086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan Z., Yang M., Lai C.L. COVID-19 vaccinations: a comprehensive review of their safety and efficacy in special populations. Vaccines. 2021;9:1097. doi: 10.3390/vaccines9101097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernal J.L., Andrews N., Gower C., Robertson C., Stowe J., Tessier E., et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. Br. Med. J. 2021:373. doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macchia A., Ferrante D., Angeleri P., Biscayart C., Mariani J., Esteban S., M R., et al. Evaluation of a COVID-19 vaccine campaign and SARS-CoV-2 infection and mortality among adults aged 60 years and older in a middle-income country. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.30800. e2130800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehra M.R., Desai S.S., Kuy S., Henry T.D., Patel A.N. Retraction: cardiovascular disease, drug therapy, and mortality in Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2007621. Mass Medical Soc: N. Engl. J. Med. 2582-2582. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Sabatino J., De Rosa S., Di Salvo G., Indolfi C.J. Correction: impact of cardiovascular risk profile on COVID-19 outcome. A meta-analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0237131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson M.J.P. Wie wirksam schützen Impfstoffe vor schweren COVID-19-Verläufen? N. Engl. J. Med. 2021;75:925–926. [Google Scholar]
- 20.Kandi V. SARS-CoV-2 genetic variations, immunity, and efficacy of vaccines: the current perspectives and future implications. Am. J. Infect. Dis. 2021;9:90–97. [Google Scholar]
- 21.Vokó Z., Kiss Z., Surján G., Surján O., Barcza Z., Pályi B., et al. Infection, Nationwide effectiveness of five SARS-CoV-2 vaccines in Hungary—the HUN-VE study. Clin. Microbiol. Infect. 2021;28:398–404. doi: 10.1016/j.cmi.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghiasi N., Valizadeh R., Arabsorkhi M., Hoseyni T.S., Esfandiari K., Sadighpour T., Jahantigh H.R. Efficacy and side effects of Sputnik V, Sinopharm and AstraZeneca vaccines to stop COVID-19; a review and discussion. Immunopathol. persa. 2021;7:31. [Google Scholar]
- 23.Lee H.J., Sajan A., Tomer Y.J. Hyperglycemic emergencies associated with COVID-19 vaccination: a case series and discussion. J. Endocr. Soc. 2021;5 doi: 10.1210/jendso/bvab141. bvab141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishra A., Ghosh A., Dutta K., Tyagi K., Misra A., Syndrome M. Exacerbation of hyperglycemia in patients with type 2 diabetes after vaccination for COVID19: report of three cases. Diabetes Metabol. Syndr. 2021;15:102151. doi: 10.1016/j.dsx.2021.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]