Abstract
Background
Adrenaline is routinely administered during cardiac arrest resuscitation. Using a novel murine model of cardiac arrest, this study evaluates the effects of adrenaline use on survival and end-organ injury.
Methods
A total of 58 mice, including cardiac arrest (CA) and sham (SHAM) groups received intravenous potassium chloride either as a bolus (CA) or slow infusion (SHAM), inducing ECG-confirmed asystole (in CA only) for 4-minutes prior to intravenous adrenaline (+ADR;250 ul,32 ug/ml) or saline (-ADR;250 ul) and manual chest compressions (300 BPM) for 4-minutes. Mice with return of spontaneous circulation (ROSC) were assessed at 24- or 72-h timepoints.
Results
Among animals that underwent CA, rates of ROSC (n = 21 (95 %) vs n = 14 (82 %), P = 0.18) and survival to the planned endpoint (n = 11 (50 %) vs n = 12 (71 %), P = 0.19) were similar when comparing those treated with (CA+ADR) and without (CA-ADR) adrenaline. However, in CA animals that initially achieved ROSC, subsequent mortality was approximately 3-fold greater with adrenaline treatment (48 % vs 14 %, P = 0.042). Among SHAM animals, adrenaline use had no impact on survival rates or other endpoints. Greater myocardial injury occurred in CA+ADR vs CA-ADR, with increased Hs-Troponin levels measured at 24- (26.0 ± 0.9 vs 9.4 ± 5.3 ng/mL, P = 0.015) and 72-h (20.9 ± 8.3 vs 5.0 ± 2.4 ng/mL, P = 0.012), associated with increased expression of pro-inflammatory and fibrotic genes within cardiac and renal tissue.
Conclusion
Adrenaline did not improve ROSC or overall survival but following successful ROSC, its use resulted in 3-fold greater mortality rates. Adrenaline was also associated with increased myocardial injury, end-organ inflammation, and fibrosis. These findings underscore the need for further preclinical evaluation of alternate pharmacologic adjuncts for cardiopulmonary resuscitation that improve survival and limit end-organ injury.
Keywords: Cardiac arrest, Adrenaline, Epinephrine, Resuscitation, Post-cardiac arrest syndrome
Abbreviations: CA, cardiac arrest; ADR, adrenaline (epinephrine); OHCA, out of hospital cardiac arrest; ROSC, return of spontaneous circulation
Introduction
Out of hospital cardiac arrest (OHCA) is a major public health challenge. Despite improvement in the resuscitative care for OHCA, survival continues to remain poor with approximately 8 % of patients discharged from hospital alive.1 In patients who achieve return of spontaneous circulation [ROSC], morbidity and mortality are driven by whole body ischemia and the resulting post-cardiac arrest syndrome that is characterized by myocardial dysfunction, hypoxic brain injury and systemic inflammation.1
Current resuscitation guidelines support the administration of intra-arrest bolus adrenaline in cardiac arrest.2 Its use in this context is underpinned by animal and human studies which have demonstrated that adrenaline, mediated through α1 adrenoceptor activation, increases vasomotor tone, improving coronary perfusion pressure and therefore the likelihood of ROSC.3 However, several studies have shown that adrenaline use during cardiac arrest resulted in impaired blood flow to vital organs and worse neurological outcomes compared to placebo or alternate vasopressors.4., 5., 6. Beyond the neurological sequelae, the impact of adrenaline use during cardiac arrest on end-organ injury remains poorly defined. Using a novel murine model of asystolic cardiac arrest, due to its moderate ischemic insult and high rates of survival to endpoint assessment, we sought to characterize the impact of intra-arrest adrenaline exposure on end-organ injury, inflammation, and fibrosis.
Methods
Animal Experiments
All animal research was conducted in accordance with Australian National Health and Medical Research (NHMRC) guidelines, and Alfred Research Alliance (ARA) Animal Ethics approvals (Approval P6581). 58 male C57Bl6 mice, sourced from AMREP Animal Services, were randomized and coded with investigators blinded from treatment allocation. Mice were anaesthetised with a ketamine/xylazine/atropine anaesthetic cocktail (K:100 mg/kg, X:20 mg/kg, A:1.2 mg/kg), electrocardiogram probes were placed in both forearms and the left hind limb, and endotracheal intubation performed. An intravenous catheter was inserted into the right jugular vein to administer IV potassium chloride (40 μl,0.5 M) either as bolus to induce arrest (CA) or slowly infused over 1 minute without causing arrest (SHAM with humoral equivalence). At the time of this publication, this approach to SHAM is a novel introduction to cardiac arrest studies in preclinical models.7 There were therefore four treatment groups used in the study; SHAM-ADR, SHAM+ADR, CA-ADR and CA+ADR. The current study was intended to validate a novel model of cardiac arrest and the impacts of intra-arrest adrenaline use. The primary outcome was survival to the planned endpoint at 24- or 72-h.
All CA mice immediately entered asystole with ventilation removed for 4 minutes. Re-ventilation and manual chest compressions (300BPM) were then delivered until sinus rhythm resumed by surface ECG indicating ROSC (Supplemental Fig. 1). 30-seconds after resuscitation commenced, mice received an IV infusion of either saline (−ADR;250 μl) or a single standard-dose of adrenaline (+ADR;250 μl,32 μg/ml [pH-balanced]).7 All animals that achieved ROSC and independence from ventilation survived until their planned endpoint (“recovery/survival”), at either 24- or 72-h. Immediately following euthanasia by cervical dislocation, a sternotomy was performed and the heart rapidly excised. Tissues and plasma were collected, snap-frozen in liquid nitrogen and stored at −80 °C for further analyses.
Tissue gene expression
To examine mRNA expression in cardiac and renal tissue, real-time polymerase chain reaction was performed (RT-PCR).
Biomarker assessment
Plasma cytokines (Bio-Rad, Bio-Plex Pro Kit) and troponin (Life Diagnostics CTNI-1-HSP) were measured from plasma collected at either 24- or 72-h timepoints. Plasma catecholamines (noradrenaline, adrenaline, and dihydroxyphenylalanine) were extracted from alumina absorption and concentration determined by high-performance liquid chromatography with coulometric detection as previously described.8
Histological analysis
Histological analysis was performed on 4-µm paraffin sections stained with Masson’s trichrome to analyze the collagen present in heart samples.
Statistical analyses
GraphPad Prism (v.9) package was used for statistical analyses. Two-tail independent sample t-tests (with Welch correction in the case of different variance), ANOVA (with Tukey adjustment for multiple comparisons), and Chi Square tests were used as appropriate. Values are presented as mean ± SEM, and P < 0.05 was considered significant.
Results
Survival outcomes
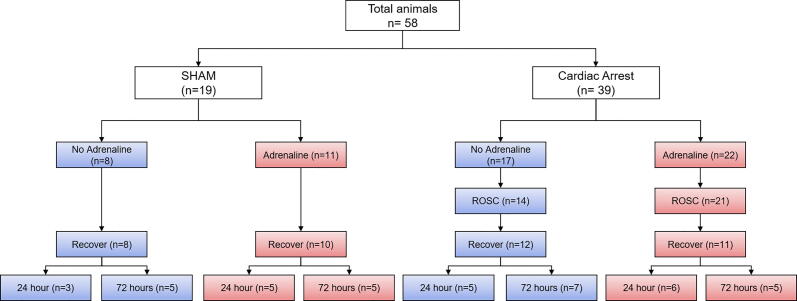
A total of 58 animals were included in the study (Fig. 1). At baseline, there were no differences between groups in anthropometric measurements (Supplemental Table 1). There were 19 animals assigned to SHAM (SHAM+ADR, n = 11; SHAM-ADR, n = 8) and 39 to CA (CA+ADR, n = 22; CA-ADR, n = 17). Among animals that underwent CA, rates of ROSC (n = 21 (95 %) vs n = 14 (82 %), P = 0.18) and survival to the planned endpoint (n = 11 (50 %) vs n = 12 (71 %), P = 0.19) were similar between CA+ADR and CA-ADR groups, respectively. Notably, in mice that achieved ROSC, adrenaline was associated with a 3-fold greater rate of mortality (n = 10 of 21 (48 %) in CA+ADR vs n = 2 of 14 (14 %) in CA-ADR, P = 0.041) (Fig. 2). In animals that underwent the SHAM procedure, adrenaline administration was not associated with any significant difference in survival to the assigned endpoint (SHAM+ADR, n = 10 (91 %) vs SHAM-ADR, n = 8 (100 %), P = 0.38), or any other endpoint assessed.
Fig. 1.
Consort diagram demonstrating the cohort derivation.
Fig. 2.
Survival outcomes in cardiac arrest with (CA + ADR) and without (CA-ADR) adrenaline for ROSC and survival to planned endpoint. Presented within the survival to planned endpoint columns are the percentage of animals that achieved initial ROSC but did not survive to their planned endpoint.
Myocardial injury
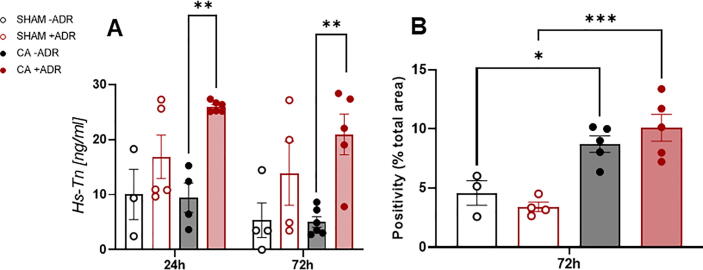
In surviving CA animals, adrenaline resulted in greater myocardial injury. Plasma troponin levels were significantly higher with CA+ADR when measured at both 24-h (25.98 ± 0.86 ng/ml vs 9.45 ± 4.6 ng/ml, P = 0.005) and 72-h (20.94 ± 7.39 ng/ml vs 5.02 ± 2.21 ng/ml, P = 0.003), compared with CA-ADR (Fig. 3A). Furthermore, CA, independent of adrenaline status, resulted in a greater degree of relative myocardial fibrosis determined through Masson trichrome staining (Fig. 3B).
Fig. 3.
Cardiac biomarkers and myocardial fibrosis. A) Plasma high-sensitivity troponin measured in 24 and 72-h cohorts. B) Relative Myocardial area fibrosis assessed through Masson’s trichrome staining of the 72-h cohort. *P < 0.05, **P < 0.01, ***P < 0.001.
Serum cytokines and catecholamines
Assessment of plasma cytokines (Supplemental Fig. 2A) revealed no significant differences in any of IL6, IL17 or RANTES between groups. However, CA+ADR resulted in significantly increased levels of the circulating chemokine CXCL1, compared with CA-ADR. Catecholamine levels were measured at 24-h (Supplemental Fig. 2B). There were no differences between groups in noradrenaline and dihydroxyphenylalanine levels. However, plasma adrenaline levels at 24-h were significantly higher in the CA+ADR group, compared with CA-ADR and SHAM+ADR.
Inflammatory and pro-fibrotic gene expression in cardiac and renal tissue
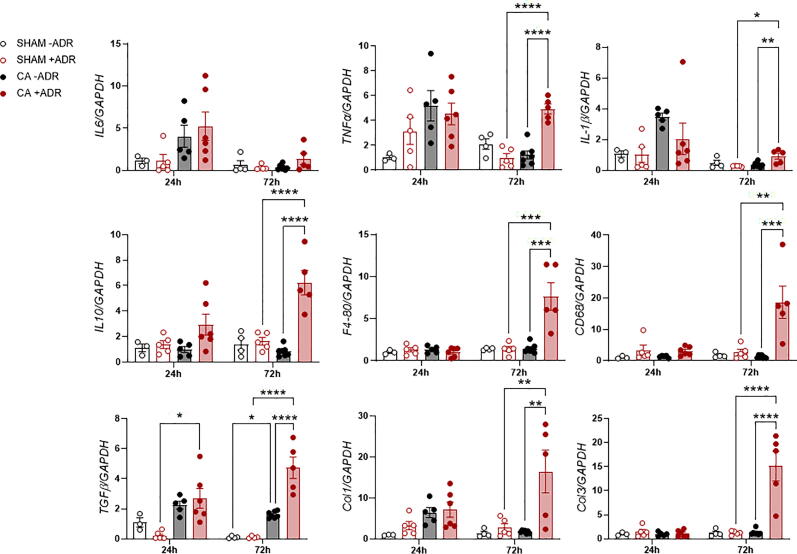
RT-PCR was performed on both cardiac and renal tissue. Within cardiac tissue at 72-h, CA+ADR, compared to CA-ADR, was associated with significantly increased expression of inflammatory (TNF-α, IL1β, IL10, F4-80 and CD68; all P < 0.01) and fibrotic (TGFβ, Col1 and Col3; all P < 0.01) genes (Fig. 4). There were no differences between SHAM-ADR and SHAM+ADR in cardiac tissue at 72-h. There was a similar pattern of increased relative expression of inflammatory and fibrotic genes within renal tissue (Supplemental Fig. 3), with the addition of early upregulation of IL6, TNF-α, CD68, and TGF-β observed at 24 h (all P < 0.01). These data suggest that this model of 4-min cardiac arrest results in significant end-organ injury and that adrenaline exposure appears to exacerbate CA-mediated injury.
Fig. 4.
Real time quantitative polymerase chain reaction (RT-PCR) of inflammatory and fibrotic genes of interest in cardiac tissue. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Discussion and conclusion
In the current study we investigated the effects of adrenaline on ROSC, survival and associated end-organ injury in a novel murine model of cardiac arrest, and report the following key findings: first, the use of adrenaline did not improve overall rates of survival and was associated with significantly increased rates of mortality in animals that initially achieve ROSC; second, adrenaline appears to potentiate the deleterious effects of the post-cardiac arrest syndrome with increased cardiac injury, systemic inflammation, and the expression of pro-inflammatory and fibrotic genes in cardiac and renal tissue. These findings are of particular relevance given the widespread support of adrenaline in contemporary advanced life-support guidelines and treatment algorithms for the resuscitation of cardiac arrest .
Survival from OHCA remains poor despite concerted public health initiatives and incremental improvements in resuscitative care.9., 10., 11. The use of adrenaline has been well described for its ability to improve rates of ROSC.3., 12., 13. Despite improving the likelihood of achieving ROSC, adrenaline has not been shown to improve functional outcomes and in some studies may result in worse rates of mortality in the subgroup of patients that initially achieve ROSC.12., 13., 14. The current study’s findings are consistent with these data, with significantly higher rates of mortality demonstrated in adrenaline-treated animals that initially achieved ROSC. While outside the scope of the current study, these findings are hypothesis-generating and warrant further investigation.
Cardiac arrest is characterized by whole body ischemia and in those who achieve ROSC, reperfusion can result in secondary injury.15 The use of adrenaline in this context is underpinned by animal and human studies which have demonstrated that adrenaline, mediated through increased vascular vasomotor tone, improves coronary perfusion pressure and the likelihood of ROSC.16., 17., 18. While adrenaline improves macrocirculatory surrogates of perfusion such as mean arterial pressure, its impact on oxygen delivery and exchange, which occurs at the level of the microcirculation in capillary beds, is less well defined.19 In swine models of resuscitated cardiac arrest, intra-arrest adrenaline has been shown to result in reduced microcirculatory blood flow, assessed by orthogonal polarization spectral imaging of sublingual mucosa, following ROSC.20., 21. The impact of adrenaline at the level of the microcirculation, may exacerbate tissue hypoxia and injury and therefore provide a mechanistic understanding for the increased cardiac injury, pro-inflammatory and fibrotic state observed in adrenaline-treated mice in the current study. In this context, our mechanistic data add to an emerging and broader safety concern with respect to the use of adrenaline in other cardiac emergencies recently observed in human studies.22., 23., 24.
While the current study reports an experimental platform for the investigation of current and potential pharmacologic interventions in cardiac arrest we acknowledge several limitation. The study was a pilot aimed at developing and validating the cardiac arrest model and investigating the influence of adrenaline on cardiac arrest outcomes. As such, no formal sample size estimates were conducted in the preparatory phase. Furthermore, the dose of adrenaline used were higher on a weight basis than that used in humans, although they were broadly similar to that reported recently in animal models. Furthermore, in the current study we used potassium chloride to induce cardiac arrest and it is acknowledged that the majority of cardiac arrest is the result of ventricular fibrillation or ventricular tachycardia in the setting of ischemia or underlying ventricular dysfunction. Finally, we acknowledge that the murine inflammatory response to cardiac arrest might differ to that in humans.
In conclusion, this focused investigation challenges the suitability of adrenaline use in resuscitation and underscores the need for further pre-clinical evaluation of alternate pharmacologic adjuncts for cardiopulmonary resuscitation that improve rates of survival and limit end-organ injury.
Sources of Funding
This work was funded by the National Health and Medical Research Council (NHMRC) (D.M. Kaye) and philanthropic funding form the Nossbaum Foundation. Dr Bloom is the recipient of postgraduate scholarships from the NHMRC and National Heart Foundation (NHF). Dr Stub is supported through a NHF fellowship.
CRediT authorship contribution statement
Daniel G. Donner: Conceptualization, Methodology, Formal analysis, Writing – original draft. Jason E. Bloom: Conceptualization, Methodology, Formal analysis, Writing – original draft. Waled A. Shihata: Conceptualization, Methodology, Writing – review & editing, Supervision. Aascha A. Brown: Conceptualization, Methodology, Data curation. Rosalind Cook: Conceptualization, Methodology. Tsin Yee Tai: Methodology, Formal analysis. Gavin W. Lambert: Formal analysis, Writing – review & editing. Po-Yin Chu: Conceptualization, Methodology. William Chan: Writing – review & editing, Supervision. Dion Stub: Conceptualization, Methodology, Writing – review & editing, Supervision. Bing H. Wang: Conceptualization, Methodology, Writing – review & editing, Supervision. David M. Kaye: Conceptualization, Methodology, Formal analysis, Writing – original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We acknowledge the assistance of Camilla Cohen at the Monash Histology Platform.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resplu.2022.100292.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Stub D., Bernard S., Duffy S.J., Kaye D.M. Post Cardiac Arrest Syndrome: A Review of Therapeutic Strategies. Circulation. 2011;123:1428–1435. doi: 10.1161/CIRCULATIONAHA.110.988725. [DOI] [PubMed] [Google Scholar]
- 2.Soar J., Böttiger B.W., Carli P., Couper K., Deakin C.D., Djärv T., et al. European Resuscitation Council Guidelines 2021: Adult advanced life support. Resuscitation. 2021;161:115–151. doi: 10.1016/j.resuscitation.2021.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Perkins G.D., Ji C., Deakin C.D., Quinn T., Nolan J.P., Scomparin C., et al. A Randomized Trial of Epinephrine in Out-of-Hospital Cardiac Arrest. N Engl J Med. 2018;379:711–721. doi: 10.1056/NEJMoa1806842. [DOI] [PubMed] [Google Scholar]
- 4.Gueugniaud P.Y., David J.S., Chanzy E., Hubert H., Dubien P.Y., Mauriaucourt P., et al. Vasopressin and Epinephrine vs. Epinephrine Alone in Cardiopulmonary Resuscitation. N Engl J Med. 2008;359:21–30. doi: 10.1056/NEJMoa0706873. [DOI] [PubMed] [Google Scholar]
- 5.Lindner K.H., Prengel A.W., Pfenninger E.G., Lindner I.M., Strohmenger H.U., Georgieff M., et al. Vasopressin Improves Vital Organ Blood Flow During Closed-Chest Cardiopulmonary Resuscitation in Pigs. Circulation. 1995;91:215–221. doi: 10.1161/01.cir.91.1.215. [DOI] [PubMed] [Google Scholar]
- 6.Prengel A.W., Lindner K.H., Keller A. Cerebral Oxygenation During Cardiopulmonary Resuscitation With Epinephrine and Vasopressin in Pigs. Stroke. 1996;27:1241–1248. doi: 10.1161/01.str.27.7.1241. [DOI] [PubMed] [Google Scholar]
- 7.Vognsen M., Fabian-Jessing B.K., Secher N., Løfgren B., Dezfulian C., Andersen L.W., et al. Contemporary animal models of cardiac arrest: A systematic review. Resuscitation. 2017;113:115–123. doi: 10.1016/j.resuscitation.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 8.Lambert G.W., Jonsdottir I.H. Influence of voluntary exercise on hypothalamic norepinephrine. J Appl Physiol. 1998;85:962–966. doi: 10.1152/jappl.1998.85.3.962. [DOI] [PubMed] [Google Scholar]
- 9.Buick J.E., Drennan I.R., Scales D.C., Brooks S.C., Byers A., Cheskes S., et al. Improving Temporal Trends in Survival and Neurological Outcomes After Out-of-Hospital Cardiac Arrest. Circ: Cardiovascular Quality Outcomes. 2018;11 doi: 10.1161/CIRCOUTCOMES.117.003561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks S.C., Clegg G.R., Bray J., Deakin C.D., Perkins G.D., Ringh M., et al. Optimizing Outcomes After Out-of-Hospital Cardiac Arrest With Innovative Approaches to Public-Access Defibrillation: A Scientific Statement From the International Liaison Committee on Resuscitation. Resuscitation. 2022 doi: 10.1016/j.resuscitation.2021.11.032. S0300957221004883. [DOI] [PubMed] [Google Scholar]
- 11.Stub D., Bernard S., Pellegrino V., Smith K., Walker T., Sheldrake J., et al. Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial) Resuscitation. 2015;86:88–94. doi: 10.1016/j.resuscitation.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs I.G., Finn J.C., Jelinek G.A., Oxer H.F., Thompson P.L. Effect of adrenaline on survival in out-of-hospital cardiac arrest: A randomised double-blind placebo-controlled trial. Resuscitation. 2011;82:1138–1143. doi: 10.1016/j.resuscitation.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 13.Hagihara A., Hasegawa M., Abe T., Nagata T., Wakata Y., Miyazaki S. Prehospital Epinephrine Use and Survival Among Patients With Out-of-Hospital Cardiac Arrest. JAMA. 2012;307:1161. doi: 10.1001/jama.2012.294. [DOI] [PubMed] [Google Scholar]
- 14.Dumas F., Bougouin W., Geri G., Lamhaut L., Bougle A., Daviaud F., et al. Is Epinephrine During Cardiac Arrest Associated With Worse Outcomes in Resuscitated Patients? J Am Coll Cardiol. 2014;64:2360–2367. doi: 10.1016/j.jacc.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 15.Patil K.D., Halperin H.R., Becker L.B. Cardiac Arrest: Resuscitation and Reperfusion. Circ Res. 2015;116:2041–2049. doi: 10.1161/CIRCRESAHA.116.304495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paradis N.A., Martin G.B., Rosenberg J., Rivers E.P., Goetting M.G., Appleton T.J., et al. The effect of standard- and high-dose epinephrine on coronary perfusion pressure during prolonged cardiopulmonary resuscitation. JAMA. 1991;265:1139–1144. [PubMed] [Google Scholar]
- 17.Callaway C.W., Donnino M.W. Testing Epinephrine for Out-of-Hospital Cardiac Arrest. N Engl J Med. 2018;379:787–788. doi: 10.1056/NEJMe1808255. [DOI] [PubMed] [Google Scholar]
- 18.Hardig B.M., Götberg M., Rundgren M., Götberg M., Zughaft D., Kopotic R., et al. Physiologic effect of repeated adrenaline (epinephrine) doses during cardiopulmonary resuscitation in the cath lab setting: A randomised porcine study. Resuscitation. 2016;101:77–83. doi: 10.1016/j.resuscitation.2016.01.032. [DOI] [PubMed] [Google Scholar]
- 19.Totaro R.J., Raper R.F. Epinephrine-induced lactic acidosis following cardiopulmonary bypass. Crit Care Med. 1997;25:1693–1699. doi: 10.1097/00003246-199710000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Fries M., Weil M.H., Chang Y.T., Castillo C., Tang W. Microcirculation during cardiac arrest and resuscitation. Crit Care Med. 2006;34:S454–S457. doi: 10.1097/01.CCM.0000247717.81480.B2. [DOI] [PubMed] [Google Scholar]
- 21.Ristagno G., Sun S., Tang W., Castillo C., Weil M.H. Effects of epinephrine and vasopressin on cerebral microcirculatory flows during and after cardiopulmonary resuscitation. Crit Care Med. 2007;35:2145–2149. doi: 10.1097/01.ccm.0000280427.76175.d2. [DOI] [PubMed] [Google Scholar]
- 22.Levy B., Clere-Jehl R., Legras A., Morichau-Beauchant T., Leone M., Frederique G., et al. Epinephrine Versus Norepinephrine for Cardiogenic Shock After Acute Myocardial Infarction. J Am Coll Cardiol. 2018;72:173–182. doi: 10.1016/j.jacc.2018.04.051. [DOI] [PubMed] [Google Scholar]
- 23.Levy B., Buzon J., Kimmoun A. Inotropes and vasopressors use in cardiogenic shock: when, which and how much? Curr Opin Crit Care. 2019;25:384–390. doi: 10.1097/MCC.0000000000000632. [DOI] [PubMed] [Google Scholar]
- 24.Ichikawa Y., Sawada Y., Nakajima J., Isshiki Y., Fukushima K., Aramaki Y., et al. Relationship between the Plasma Levels of Catecholamines and Return of Spontaneous Circulation in Patients with Out-of-Hospital Cardiac Arrest. Ng KC, editor. Emergency Med Int. 2021;29:1–6. doi: 10.1155/2021/5324038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.