Graphical abstract
Highlights
-
•
Multivariate optimization strategy predicted the ultrasound-assisted extraction (UAE) conditions for Nardostachys jatamansi root extracts.
-
•
GC–MS analysis pointed out improvement in the concentration of the bioactive sesquiterpenes and steroidal compounds with the UAE method.
-
•
Optimized extracts using the UAE method demonstrated better anti-oxidant and AChE inhibitory potentials.
-
•
UAE could be assumed as a ‘green approach’ for the extraction of selected medicinal herbs.
Abstract
Extracts from medicinal plants are generally obtained by conventional methods like percolation and maceration. Owing to limitations of traditional methods and to meet the rising demand of extracts, the development of new green approaches is need of hour. In the present research, we have developed an ultrasound-assisted extraction (UAE) method for the Nardostachys jatamansi (NJ) D. Don, DC roots and optimized the extraction parameters for possible improved extract yield. A multivariate optimization strategy using the Centre Composite Design coupled with response surface methodology was applied. A numerical optimization approach accurately predicted the extraction conditions (sonication time ∼ 20 min, ethanol ∼ 70 % and a liquid/solid ratio of about 21:1). Scanning electron microscopy of the plant samples after UAE also indicated the cavitation effect due to sound waves. GC–MS analysis of the optimized ultrasound extract (OUNJ) confirmed improvement in the concentration of various secondary metabolites like jatamansone (91.8 % increase), spirojatamol (42.3 % increase), globulol (130.4 % increase), sitosterol (84.6 % increase) as compared to the soxhlet extract (SXNJ). Different anti-oxidant parameters (DPPH, Glutathione, Catalase SOD and NO) were also significantly altered (p < 0.05) in the optimized extracts. The IC50 to inhibit acetylcholinesterase activity (AChE) in vitro and its concentration in brain homogenates were significantly (p < 0.05) improved by OUNJ extract as compared to the SXNJ ones. To conclude, we can say that established optimized conditions for UAE of N. jatamansi roots not only reduce the extraction time but also improved the pharmacological potential of the extracts.
1. Introduction
There is a considerable demand for extracts of herbs by the different manufacturing or cosmetic units in India and different regions of world. Also, the plant-based formulations are fetching more attention from people all around the world, as these products are considered more effective, cheaper, and with lesser side effects. Globally, the commercial significance of herbal formulations/products is growing steadily, as well. Moreover, it is also expected that worldwide trade of such products could go above 100 billion USD in coming years. Traditional techniques of extraction constitute very time-consuming processes and are not considered to be environment friendly and it would be harsh to rely only on conventional extraction methods to deal with such demands. Hence to meet the rising demand of extracts and to maintain their sustainable supply, the application of unorthodox techniques of extraction is need of hour. Thus, unconventional methods such as ultrasound-assisted extraction (UAE), supercritical fluid extraction (SFE) and pressurized liquid extraction (PLE) should be addressed [1]. These processes are not only fast but could also improve the yields of extracts/secondary metabolites and could prove as an alternative for the industry to get the herbal extracts in a sustainable, economical and greener manner [2].
In the last decade, UAE has been often employed for the extraction of medicinal plants [3], [4]. In this technique, the energy of sound waves above the 20 KHz frequency produced the acoustic cavitation effect in the particles of the crude drug to bring out the solubilized extract. Channels are created in the herb particles due to strong shear forces generated by the bursting of bubbles near the surface of the plant material. These channels help to the diffusion of the solvents more efficiently inside the plant matrix and improve the mass transfer, which leads to enhanced yields of extracts and secondary metabolites [5], [6]. In the past, various researchers have utilized this technique for the enhanced recovery of plant bioactive components/extracts in a fast, cost-effective and environmentally friendly approach [2], [5].
The multivariate optimization strategies could statistically collate the effects of different variables in a complex system to get the desired results [7], [8]. The influencing factors in UAE like sonication power, solvent to solute ratio, time of extraction, choice of solvent, pH etc, which extensively affect the complexity of the extraction process, could be optimized by the application of design approaches for the accuracy and reliability of the data. In the recent years, many optimization approaches have been mingled in the UAE process to get the optimum conditions for extraction of herbs [2]. Moreover, the response surface methodology (RSM) coupled with different optimization design has proven to be precise in determining the optimum region as compared to single factor analysis (SFA) [9].
The selected medicinal herb, Nardostachys jatamansi (NJ) D.Don, DC (Family- Caprifoliaceae), is also a highly traded medicinal plant in India with significant therapeutic potential [10], [11]. Generally, it is collected from wild sources in alpine regions of the Himalayas from Himachal Pradesh to Sikkim and Bhutan at an altitude of 3000–5000 m. The herb, Jatamansi or Indian spikenard, is mentioned in ‘Sushrut Samhita’, ‘Charak Samhita’ and various other ayurvedic literatures, for preventing mental disorders such as epilepsy, anxiety, depression, etc [12], [13]. It is also highly mentioned in the traditional literature for improvement in cognition, alertness and concentration [14]. Various pharmacological reports have also supported the anti-oxidant, learning and memory enhancement, CNS-protective, anti-anxiety, anticonvulsant, antidepressant, anti-inflammatory and anti-Parkinson’s potentials of selected herbs [15], [16], [17], [18]. The potent pharmacological importance of Jatamansi fetches the attention of industries and numerous commercial formulations of this plant such as ‘Mentat’ ‘Mentat DS’ (Himalaya Herbal Healthcare, India), ‘Intellimax’ (Piramal Phytocare, India) are available in the market for improvement of the brain health. Besides the improvement in CNS disorders, this herb is also used commercially for the management of skin disorders and hair fall [19].
Owing to the pharmacological importance and industrial prospects of N. jatamansi, this plant was selected for the present study. It is aimed to extract the herb by an un-conventional method, UAE and to optimize the various extraction parameters for possible improvement in the yields of plant actives/extracts. Scanning Electron Microscopy (SEM) was also performed to confirm the mechanical effects of sound waves on plant samples. On the other hand, the chemical composition of the extracts was analyzed using the gas chromatography coupled mass spectroscopy (GC–MS) technique. As the therapeutic potential, especially the nootropic effect of N. jatamansi is mainly due to its anti-oxidant and anti-cholinesterase properties, the different extracts were also evaluated for their anti-oxidant and acetylcholinesterase (AChE) inhibitory potential.
2. Materials and methods
2.1. Plant samples and reagents
The roots of N. jatamansi were procured from a local market in Hisar, Haryana and authenticated by an eminent botanist. The roots were processed, pulverized and sieved (size 40) to get the coarse powder of the herb. The powdered herb was kept in air-tight containers till use. The solvents employed in the extraction process were of analytical grade. Scopolamine and donepezil were purchased from Sigma Aldrich, USA.
2.2. Extraction of the selected herb
The root powder of the plant was extracted using different extraction methods. Generally, the selected plant, N. jatamansi, is extracted by conventional methods such as the maceration and percolation ones. In the present study, plant samples were extracted by the conventional percolation method (CPM) using soxhlet apparatus. For this, the powder sample (10 g) was filled in the extraction chamber and the extraction was carried out using ethanol as solvent. The process was continued till exhaustion of the extraction process [20], [21]. The powdered herb was also extracted by a non-conventional green technique such as UAE. The probe ultrasonicator (UP-800, Chrome Tech. Co, ltd, Taiwan) was used for the extraction by sound waves. The apparatus was equipped with a sound abating enclosure along with a probe (Ø −5/8″) and a temperature control sensor. The extraction was carried out at different experimental conditions as suggested by the design software at a fixed frequency of 24 KHz, higher intensity and with a pulse of 0.5 sec. During the process, the tip of the ultrasonic probe was dipped to 1cm-height in the solvent [22]. After the extraction process using the two different methods, the solution was centrifuged for 10 min at 5000 rpm and filtered through Whatman filter paper. The filtrate was concentrated to get the solid extract and the percentage yield (w/w) was determined. The extract was kept in an air-tight container till next experiments.
2.3. Experimental design
The various process variables like the sonication time (X1), the solvent concentration (X2) and the volume of the solvent (X3) were optimized for extract yield, EY, (Y1), total phenolic content (TPC, Y2) and total flavonoid content (TFC, Y3) in the sonication-based extraction of the herb. The optimization range of the various independent variables is given in Table 1. The central composite design (CCD) was employed, which suggested the various experimental conditions (20 runs) to carry out the extraction process in UAE (see Table 2). The extract yield, TPC and TFC of the different runs were also determined by specified procedures. The linear (βi), quadratic (βii) and interactive (βij) terms for different independent variables, indicating their effect on various responses (Y) along with the coefficient of interception (β0) and error (ε) were also represented by a standard polynomial equation (eq. 1).
| (1) |
Table 1.
Actual and predicted extraction yields, TPC and TFC at different extraction conditions in UAE.
| Std | Run |
Sonication time (min) (X1) |
Solvent Conc. (%) (X2) |
Volume of solvent (mL) (X3) |
EY (%, w/w) |
TPC mg of GAE/g of extract |
TFC (mg of RUE/g of extract) |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Actual yield (w/w) | Predicted yield (w/w) | Actual yield (w/w) | Predicted yield (w/w) | Actual yield (w/w) | Predicted yield (w/w) | |||||
| 8 | 1 | 30.00 | 80.00 | 25.00 | 3.9 | 4.11 | 39.34 | 39.01 | 17.8 | 17.69 |
| 1 | 2 | 10.00 | 60.00 | 15.00 | 4.10 | 3.68 | 28.9 | 28.68 | 10.8 | 10.37 |
| 2 | 3 | 30.00 | 60.00 | 15.00 | 1.70 | 1.61 | 18.01 | 17.48 | 10.1 | 9.66 |
| 9 | 4 | 3.18 | 70.00 | 20.00 | 2.2 | 2.57 | 30 | 30.27 | 12.1 | 12.08 |
| 18 | 5 | 20.00 | 70.00 | 20.00 | 7.2 | 7.19 | 52.60 | 52.44 | 33.7 | 33.98 |
| 6 | 6 | 30.00 | 60.00 | 25.00 | 3.9 | 3.77 | 35.90 | 35.45 | 18.00 | 17.40 |
| 16 | 7 | 20.00 | 70.00 | 20.00 | 7.00 | 7.19 | 52.9 | 52.44 | 34.00 | 33.98 |
| 4 | 8 | 30.00 | 80.00 | 15.00 | 4.10 | 4.05 | 35.2 | 35.22 | 14.8 | 14.21 |
| 10 | 9 | 36.82 | 70.00 | 20.00 | 2.01 | 1.95 | 27.1 | 27.60 | 12.3 | 13.08 |
| 14 | 10 | 20.00 | 70.00 | 28.41 | 6.2 | 6.22 | 50.8 | 51.35 | 29.2 | 29.39 |
| 3 | 11 | 10.00 | 80.00 | 15.00 | 4.90 | 4.82 | 39.5 | 39.40 | 13.10 | 13.17 |
| 7 | 12 | 10.00 | 80.00 | 25.00 | 2.9 | 2.78 | 31 | 30.98 | 15.9 | 15.80 |
| 17 | 13 | 20.00 | 70.00 | 20.00 | 7.2 | 7.19 | 51.9 | 52.44 | 34.20 | 33.98 |
| 13 | 14 | 20.00 | 70.00 | 11.59 | 5.84 | 6.12 | 43.1 | 43.32 | 20.10 | 20.67 |
| 12 | 15 | 20.00 | 86.82 | 20.00 | 3.6 | 3.52 | 30.9 | 30.89 | 10.2 | 10.38 |
| 19 | 16 | 20.00 | 70.00 | 20.00 | 7.4 | 7.19 | 52.30 | 52.44 | 33.8 | 33.98 |
| 11 | 17 | 20.00 | 53.18 | 20.00 | 1.9 | 2.28 | 18.1 | 18.88 | 7.20 | 7.78 |
| 20 | 18 | 20.00 | 70.00 | 20.00 | 7.4 | 7.19 | 52.5 | 52.44 | 34.5 | 33.98 |
| 5 | 19 | 10.00 | 60.00 | 25.00 | 3.90 | 3.74 | 35.00 | 34.44 | 17.2 | 17.26 |
| 15 | 20 | 20.00 | 70.00 | 20.00 | 7.00 | 7.19 | 52.6 | 52.44 | 33.8 | 33.98 |
Table 2.
ANOVA applied on the results obtained for EY, TPC and TFC in UAE of NJ extracts.
| EY | TPC | TFC | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | Sum of Squares | Df | Mean | F | p-value | Sum of Squares | Df | Mean | F | p-value | Sum of Squares | Df | Mean | F | p-value | |||
| Model | 77.84 | 9 | 8.65 | 104.91 | < 0.0001 | Sig. | 2613.66 | 9 | 290.41 | 1027.34 | < 0.0001 | Sig. | 1922.38 | 9 | 213.60 | 734.64 | < 0.0001 | Sig. |
| X1 | 0.46 | 1 | 0.46 | 5.64 | 0.0390 | 8.58 | 1 | 8.58 | 30.37 | 0.0003 | 1.19 | 1 | 1.19 | 4.10 | 0.0703 | |||
| X2 | 1.87 | 1 | 1.87 | 22.73 | 0.0008 | 174.07 | 1 | 174.07 | 615.78 | < 0.0001 | 8.14 | 1 | 8.14 | 28.01 | 0.0004 | |||
| X3 | 0.012 | 1 | 0.012 | 0.15 | 0.7104 | 77.72 | 1 | 77.72 | 274.95 | < 0.0001 | 91.78 | 1 | 91.78 | 315.67 | < 0.0001 | |||
| X1 X2 | 0.85 | 1 | 0.85 | 10.25 | 0.0095 | 24.61 | 1 | 24.61 | 87.04 | < 0.0001 | 1.53 | 1 | 1.53 | 5.27 | 0.0446 | |||
| X1X3 | 2.21 | 1 | 2.21 | 26.75 | 0.0004 | 74.60 | 1 | 74.60 | 263.91 | < 0.0001 | 0.36 | 1 | 0.36 | 1.24 | 0.2911 | |||
| X2 X3 | 2.20 | 1 | 2.20 | 26.75 | 0.0004 | 100.47 | 1 | 100.47 | 355.40 | < 0.0001 | 9.03 | 1 | 9.03 | 31.06 | 0.0002 | |||
| X12 | 43.88 | 1 | 43.88 | 532.36 | < 0.0001 | 995.56 | 1 | 995.56 | 3521.89 | < 0.0001 | 824.95 | 1 | 824.95 | 2837.30 | < 0.0001 | |||
| X22 | 33.16 | 1 | 33.16 | 402.31 | < 0.0001 | 1368.14 | 1 | 1368.14 | 4839.89 | < 0.0001 | 1116.87 | 1 | 1116.87 | 3841.30 | < 0.0001 | |||
| X32 | 1.88 | 1 | 1.88 | 22.77 | 0.0008 | 47.01 | 1 | 47.01 | 166.32 | < 0.0001 | 144.29 | 1 | 144.29 | 496.26 | < 0.0001 | |||
| Residual | 0.82 | 10 | 0.082 | 2.83 | 10 | 0.28 | 2.91 | 10 | 0.29 | |||||||||
| Lack of Adjustment | 0.66 | 5 | 0.13 | 4.15 | 0.0721 | Not Sig. | 2.25 | 5 | 0.45 | 3.93 | 0.0797 | Not Sig. | 2.45 | 5 | 0.49 | 5.32 | 0.0452 | Not Sig. |
| Pure Error | 0.16 | 5 | 0.032 | 0.57 | 5 | 0.11 | 0.46 | 5 | 0.092 | |||||||||
| C.V. % | 6.09 | 1.37 | 2.61 | |||||||||||||||
| PRESS | 5.34 | 18.01 | 19.52 | |||||||||||||||
| R2 | 0.9895 | 0.9989 | 0.9985 |
|||||||||||||||
| Adjusted R2 | 0.9801 | 0.9979 | 0.9971 |
|||||||||||||||
| Predicted R2 | 0.9322 |
0.9931 |
0.9899 | |||||||||||||||
| Adequate Precision | 27.497 | 92.993 | 68.711 |
|||||||||||||||
The design expert software (7.0.3, Statease Inc, Minneapolis, USA) was used to determine the significance of the obtained results, the applied model and to get the diagnostic curves [23].
2.4. Analysis of TPC and TFC
The TPC and TFC of the various samples were determined by a previously established method [18]. The Folin-Ciocalteu (FC) reagent was used to determine the phenolic content and was expressed as mg of gallic acid equivalent, GAE/g of extracts. Briefly, the various extracts (0.5 g) were dissolved in methanol (10 mL) and the FC reagent (1.5 mL) was added to the solution. The solution was stirred continuously to mix the aqueous Na2CO3 (1.5 mL) for 10 min. Later, this solution was incubated at 45 °C on a water bath for 30 min. Likewise, for the TFC, the extract solution was prepared in methanol (10 mg/mL) and aqueous aluminum chloride was added to this solution. The mixture was incubated at room temperature for 1 h. Finally, the absorbance of the samples was noted at λmax of 760 and 415 nm, for TPC and TFC, respectively, using a UV spectrophotometer (UV-1800, Shimadzu Scientific Instruments Private Limited). The TFC was expressed as mg of rutin equivalent, RUE/g of extract.
2.5. SEM analysis
The powder (untreated) and the marc of the plant samples following the different extraction processes were prepared for scanning electron microscopy. For this, the herb powder was dried for at least two hours under vacuum between 40°-50 °C. The samples were sputtered, coated with gold and scanned at higher vacuum mode for any structural changes under an electron microscope (Zeiss EVO40) [24], [25].
2.6. GC–MS analysis of extracts
Separation and identification of the various constituents of the different extracts were carried out using GC–MS analysis (Shimadzu QP-2010 with Thermal Desorption System TD 20 equipped with MS capillary column). A previously reported method was employed to analyze the NJ extracts with minor modifications [26]. Briefly, the sample (1 µL) was injected in split mode and electronic ionization operating at 70 eV. Helium (He) was used as carrier gas (1.5 mL/min) and injector temperature was kept at 260 °C. Initially, the oven temperature was kept at 60 °C for 5 min and increased to 150 °C at a rate of 3 °C/min and held for 10 min. Finally, the temperature was enhanced up to 250 °C with an increment of 4 °C/min. MS chromatograms of the extracts were recorded and the retention time (RT) and the percentage area (%) of the different compounds were noted.
2.7. Evaluation of the biological activity of the different extracts
Extracts of the selected herb are highly utilized commercially for the development of Ayurvedic formulations in various disorders. Hence, the extracts of the N. jatamansi roots obtained by the conventional percolation method (SXNJ) and the sonication technique, at optimized conditions (OUNJ) were evaluated for their in vitro and in vivo antioxidant potential. The marketed formulations of the NJ extracts are also used for cognition improvement. Therefore, the resulting extracts were tested for their AChE inhibitory potential, as well.
2.7.1. In vitro anti-oxidant assay
The in-vitro anti-oxidant potential of the extracts can be determined by analyzing the inhibitory concentration (IC) needed to scavenge the free radicals of 2,2-Diphenyl Picrylhydrazyl (DPPH) [27]. For the assay, the test solution was prepared by mixing DPPH and the extract/standard (0.2 mL). The solution was incubated at room temperature for 30 min and absorbance was measured in an UV–Visible spectrophotometer (λmax 517 nm). The percentage (%) inhibition of DPPH was calculated by measuring the absorbance of the solution (t0) and after 30 min (t30). The various concentrations of the test samples were prepared to determine the IC50 against fifty percent of free radicals of DPPH.
2.7.2. In vivo anti-oxidant assay
The study protocol was approved by the institutional animal ethical committee (IAEC), MDU, Rohtak, vide reference number 1767/RE/S/14/CPCSEA/CAH/76–85 dated 26–02-2021. The animals (Swiss albino mice) were procured from the Lala Lajpat Rai University of Veterinary and Animal Sciences (LLRU-VAS), Hisar, India. All the mice were provided the dried feed and water ad-libitum and maintained as per the provisions of the committee for the purpose of control and supervision of experiments on animals (CPCSEA). The small animals (25–30 g) were divided into five groups (n = 5) and acclimatized to lab conditions for ten days. The first group received the normal saline and termed as control, the second group was injected with scopolamine at a dose of 0.4 mg/kg through the intra-peritoneally (i.p.) route (negative control), the third group (positive control) received the donepezil (3 mg/kg, ip.) and the fourth and fifth groups were termed as test groups and mice were given the extracts in an oral dose of 200 mg/kg for two weeks. On the 15th day, animals of the test groups were also given scopolamine (0.4 mg/kg i.p.) before evaluating various biochemical parameters. Various in-vivo anti-oxidant parameters such as reduced glutathione, catalase, nitric oxide and superoxide dismutase (SOD) were determined [28]. For this, the whole brain was isolated from the animals of each group and washed with normal saline. The brain samples were homogenized with phosphate buffer (pH 7.4) and centrifuged at 10,000 rpm for 15min. The homogenate was used to determine various biochemical parameters as per the established procedures. The concentration of reduced glutathione was determined by the Ellman’s method. To describe briefly, equal quantities of brain homogenates and trichloroacetic acid (10 %) were mixed and centrifuged for 15 min. The supernatant was separated and mixed with phosphate buffer (pH 8.4), 5,5′-dithiobis (2-nitrobenzoic acid, (DTNB) and distilled water. The absorbance of the mixture was recorded in UV–Visible spectrophotometer at λmax of 412 nm. The catalase activity was evaluated by Aebi’s method [29]. To carry out this, 100 μL of the brain homogenate, phosphate buffer (0.1 mM, pH 7.4) and hydrogen peroxide (30 mM) were mixed and the reaction mixture was analyzed at λmax 240 nm in an UV spectrophotometer. Changes in absorbance were noted and expressed as nmol of H2O2 consumed/min/mg/protein. The nitric oxide (NO) contents in the brain homogenates were evaluated using the Griess reagent and expressed as µmol/mg of tissue [30]. Briefly, the homogenate was mixed with phosphate buffer and sodium nitroprusside and incubated at 25 °C. After 3 h, the Griess reagent (0.5 mL) was mixed with an equal quantity of the solution and again incubated for 30 min at room temperature. Due to the binding of nitrite with the Griess reagent, the colored azo derivatives (chromophores) are formed and can be measured by a spectrophotometer at λmax of 540 nm. The superoxide dismutase (SOD) concentration was also estimated in the different samples of brain homogenates by a spectrophotometric method [31]. To perform this, n-butanol was mixed in brain samples with continuous stirring. The mixture was incubated at room temperature for 15 min and then centrifuged to separate the butanol layer. The absorbance of the solution was measured (λmax 560 nm) to calculate the SOD concentration.
2.7.3. Evaluation of the AChE inhibitory potential
The well-established Ellman’s method was employed to evaluate the AChE inhibitory potential (in vitro and in vivo) of the different extracts [32]. Briefly, the extracts were dissolved at different concentrations in Tris HCl (50 mM). The AChE enzyme (for in vitro assays) and brain homogenates (for in vivo assays) were mixed with phosphate buffer (0.1 M, pH 8) and dithio-bis-nitrobenzoic acid (DTNB). The reaction mixture was incubated for 15 min at room temperature. The substrate, acetylcholine iodide, was added to the mixture before analyzing it with an UV–Visible spectrophotometer at λmax of 412 nm.
2.8. Statistical analysis
All the procedures were conducted in triplicate and results expressed as mean ± S.D. Also, the significance of the data was evaluated by variance analysis (ANOVA) followed by Tukey test using the Graphpad Prism 9.0 software. The probability to get the observed results, p < 0.05 was considered as statistically significant.
3. Results and Discussion
Study of the literature reported that there is a tremendous ambiguity in the taxonomic nomenclature of the selected medicinal plant. N. jatamansi is massively confused with Valeriana jatamansi due to overlapping of trade or vernacular names. Mabberlerie and Notlie [33] have indicated that scientists are still using the wrong citation of this herb in their papers and there is an urgent need to address this problem. Moreover, as per the reports of the National Medicinal Plant Board of India, this plant is mostly collected from wild sources by untrained personnel. Hence, to begin with research on the procured sample of Jatamansi, it was identified and authenticated as Nardostachys jatamansi (D. Don) DC by Dr. Sunita Garg (Principal Scientist) of the CSIR-NISCAIR (NISCAIR/RHMD/Consult/2018/3157–06-2 dated 19/03/2018), based on detailed scrutiny of literature and matching the morphological features with the authentic sample deposited in the Raw Material Herbarium and Museum of Delhi. The selected plant displays great therapeutic potential and its properties are also mentioned in the ancient Indian literature for the treatment of various ailments. It is traditionally used in the prevention of mental illness and improvement of learning and memory. Several reports have indicated that the phenolics and flavonoids present in this herb are responsible for its anti-oxidant potential [34], [35]. It is among the top traded medicinal plants around the Globe. Moreover, about 25 commercial Ayurvedic formulations are available in the market, which signifies the importance of this herb for the prevention or the treatment of various diseases. The herb is generally extracted by conventional extraction methods (percolation or maceration) by manufacturing companies to meet the demand of extracts in India and other countries. Nevertheless, regarding the limitations of traditional methods such as high time and solvent consumption, the paradigm has now shifted toward non-conventional extraction procedures. The present research aimed at establishing an UAE method for the extraction of NJ roots as well as the optimization of various extraction parameters, so that extracts can be prepared in a faster way by adopting the green approach.
Literature indicated that in >60 % of cases, roots of N. jatamansi (D. Don) DC are extracted by the percolation method using alcohol or water as solvents [19]. Therefore, we also prepared extracts of this plant by the conventional percolation method using hydroalcoholic solvent (20:80) in the soxhlet apparatus. Moreover, the extraction of plant actives, including total phenolics is generally carried out with polar solvents (ethanol or water) [36]. Hence, we employed the hydroalcoholic solvent for extraction by the soxhlet method and the yield of extracts, TPC and TFC were calculated to be 6.20 ± 0.81 w/w, 38.56 ± 1.04 mg GAE/g, 28.33 ± 0.94 mg RUE/g, respectively.
Nowadays, the UAE is extensively used for the improved and efficient extraction of crude plant samples compared to traditional methods. It is considered not only to be a cost-effective method, but it also expedites the extraction process in an environmentally-friendly manner. Vibrations caused by the sound waves produce small jets inside the solvent and create microbubbles, which grow in size and burst near the surface of the particles to bring out the cavitation effect. This process leads to enhanced migration of the solvents inside the plant samples, improved solubilization of extractable matter and efficient migration of extracts into the surrounding medium. Extraction using UAE is generally affected by numerous variables such as sonication frequency, process temperature, solvent nature and its viscosity, process duration, solvent concentration and pulse of sound waves generation, etc. Therefore, some preliminary experiments and an extensive literature survey were conducted to select the process variables and their range for optimization. As a result, a sonication frequency of 24 KHz, a processing temperature of 70 °C and ethanol as solvent were selected for the UAE of NJ roots. Also, the maximum exposure to heating in solution mixtures can be prevented by interval pulse modulation to enhance the experimental results. Therefore, a pulse of 0.5 sec at low intensity was selected on basis of the results of preliminary experiments. The optimization of the process can confirm the reliability, reproducibility and effectiveness of the extraction process in UAE. Hence, the central composite design (CCD) coupled with response surface methodology (RSM) was applied for the optimization of various process variables like duration of sonication (X1, 10–30 min). The viscosity and the volume of the solvent with respect to plant particles, significantly affected the movement of sound waves in the medium. Therefore, the volume of added water needs to be increased to reduce the viscosity of ethanol (concentration of solvent, X2, 60–80 %). The ratio of the solvent to the plant sample volume of solvent, X3, (15–25 mL/g) was also optimized for better extraction efficiency. A total of twenty experiments were conducted which represents six axial points, eight factorial points and six central points.
3.1. Adjustment of the developed model
The different experimental conditions followed, and the actual and predicted values of the different dependent variables (EY, TPC and TFC) are presented in Table 1. Data were analyzed and the statistical significance of the obtained results was predicted by applying variance analysis (Table 2).
Polynomial regression equations were also generated (Eq. 2,3,4) to indicate the magnitude of the effect of the different process variables on the selected responses (Y1, Y2, Y3).
| (2) |
| (2) |
| (3) |
Statistical analysis of the data confirmed the adequate functionality of the results by the developed model (p < 0.001). Results of the experiments also indicated that the linear and interactive effects of independent variables (X1, X2 and X3) on dependent variables were also significant (p < 0.05) except for TFC (X1 and X1X3 > 0.05). The values of the correlation coefficient R2 for all the responses were found to be close to one, which suggests that most of the variations can be explained by the developed model. Also, the closeness of R2 and the predicted R2 to each other confirmed that the variables (dependent and independent) were well correlated. The diagnostic curves also confirmed the suitability of the developed model and the obtained results. The graphs between the predicted results and the actual results, the residuals against the actual runs and the predicted runs, indicated that all the results obtained were within the confidence limit of the predicted ones Fig. 1 (Fig. 1.a, 2.a, 3.a). The box-cox plots indicated that the λ values were close to those predicted and no power transform was suggested by the software (Fig. 1.b., 2.b, 3.b).Fig. 2.Fig. 3..
Fig. 1.
Diagnostic plot for EY a. Actual results versus predicted results and residuals against predicted and actual runs; b. Box-cox plot for power transform.
Fig. 2.
Diagnostic plot for TPC a. Actual results versus predicted results and residuals against predicted and actual runs; b. Box-cox plot for power transform.
Fig. 3.
Diagnostic plot for TFC, a. Actual results versus predicted results and residuals against predicted and actual runs; b. Box-cox plot for power transform.
Fig. 4.
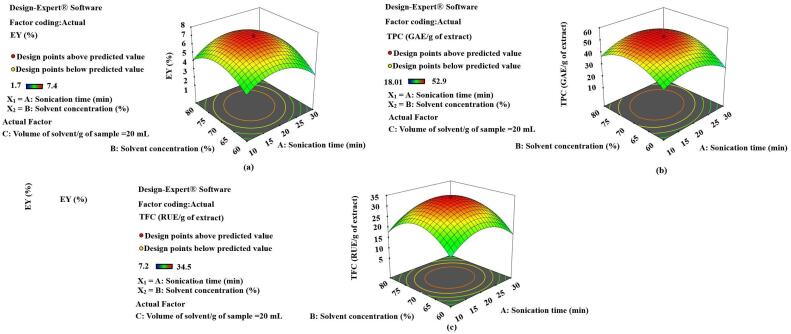
3-D diagrams indicating the effect of different variables (X1,X2) on EY, TPC and TFC.
The model developed for the different variables and responses also predicted the low coefficient of variance and a high adequate precision (see Table 2), which illustrates the accuracy and the consistency in the obtained results. Moreover, the non-significance of the pure error in the developed model was exhibited by absence of the adjustment value (p > 0.05).
3.2. Response surface analysis in UAE
The effect of selected variables on different responses has been figured in different 3-D diagrams (Fig. 4, 5, 6). Various factors influence the yield of extract and plant active contents in UAE. Some process parameters like temperature during extraction (70 °C), frequency of sound waves (24 KHz) and impulse (0.5 sec) have been fixed, based on some preliminary experiments. The sonication time (X1), the concentration of the solvent (X2) and the volume of the solvent (X3) with respect to the plant samples have also been optimized for EY, TPC and TFC in SXNJ and OUNJ extracts. From Fig. 4, one can analyze the effect of different sonication times and solvent concentrations at a fixed ratio of solvent to samples. It has been observed that when increasing the time of sonication, 10 to 20 min and the solvent concentration from 60 to 70 %, there was a significant rise in the EY, TPC and TFC (p < 0.01). Enhancement in the yield as well as in the phenolic and the flavonoid contents could be attributed to an increased polarity of the solvent mixture, the target compounds being more soluble in polar solvents. Moreover, it has also been reported that sound waves travel better in polar solvents to produce the acoustic cavitation effect [37], [38]. However, after that point, no more improvement in the response was observed, instead it declined sharply. Reduction in the yields of TPC and TFC could be due to an oxidation of plant metabolites by free ions generated by the dissociation of a higher amount of water molecules present as solvent [39].
Fig. 5.
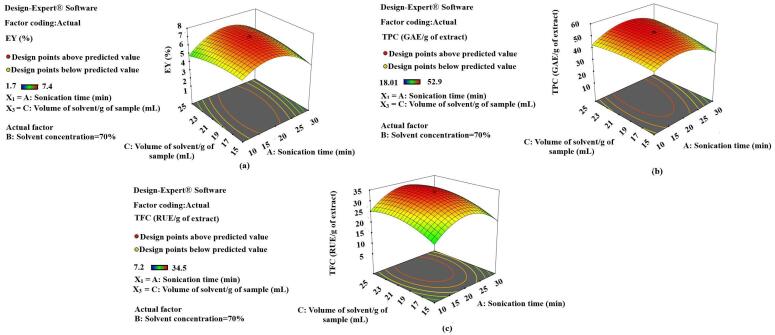
3-D diagrams indicating the effect of different variables (X1, X3) on EY, TPC and TFC.
Also, the duration of the exposure to sound waves affects the yields of the constituents. The response surface 3-D diagrams (Fig. 5) also indicated the effect of sonication time along with solvent concentration and volume of solvent/g of samples. A review of the literature suggests that optimization of the sonication time is highly required for better yields of phytoconstituents. Sonication times of 5–60 min are generally applied depending on the cellular structure of the plant sample and the resistance to heat mass transfer [2]. For example, hard nuts of Areca catechu were extracted for 50 min whereas a sonication time of only 5.9 min was sufficient for Thymus leaf extraction by UAE [40], [41]. In the present study, a range of 10–30 min for optimization of the sonication time was chosen by conducting some preliminary experiments. The exact optimum was selected by analyzing the results of various experiments conducted under different sets of conditions. These indicated that EY, TPC and TFC enhanced significantly with a rise in the sonication time but after certain periods, they decreased abruptly. These results are in agreement with previous ones regarding the extraction of phenolic compounds from olive leaves, which was found to be optimum at 20 min of UAE and thereafter decreasing with additional sonication of the samples [42]. This suggests that sonication times exceeding an optimum could lead to a reduction in yields of plant actives due to the shift of mass transfers inside the cells of the plant sample. Also, exposure to sound waves for longer than essential is not cost-effective and could lead to secondary metabolite deterioration due to the heat produced by the ultrasonic probe [5], [43].
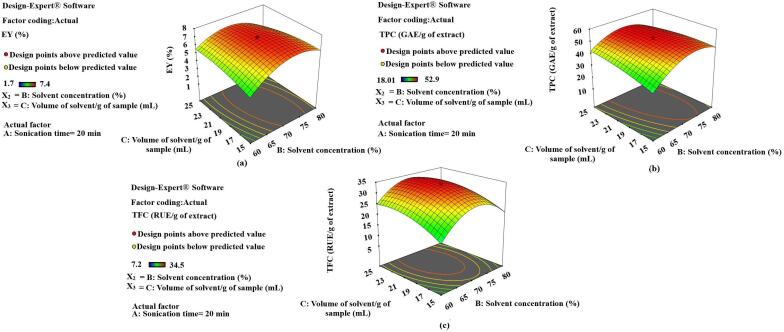
The volume of the solvent required per g of plant sample is also an important variable in the optimization of UAE of herbs. The 3-D diagram presented in Fig. 6 indicates the effect of the volume of the solvent along with sonication time and solvent concentration on various dependent variables (EY, TPC, TFC). The optimum volume of the solvent, near to 20 mL/g of sample, allowed the recovering of the highest yields. These results also matched with past researchers’ observations reporting optimized solvent to solid ratios of 15:1 and 25:1 for the UAE of Hypericum perforatum and Hemerocallis citrinabaroni, respectively [44], [45]. A suitable amount of solvent is always required as it helps in the solubilization of non-polar metabolites and also acts as a medium for the traveling of the sound waves. Nevertheless, after exceeding a certain ratio, this will hamper the sonication process by causing a disturbance in the movement of radiations. Moreover, higher concentrations of solute particles could also increase the operation cost as more power is required for producing the cavitation effect [46].
Fig. 6.
3-D diagrams indicating the effect of different variables (X2, X3) on EY, TPC and TFC.
3.3. Validation of the developed model
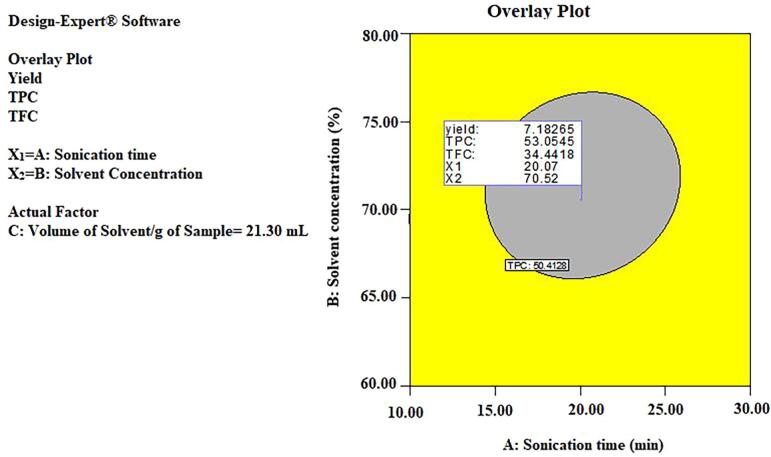
The developed model was also used to maximize the experimental conditions by a numerical optimization approach and prediction of the sonication time (20.10 min), the solvent concentration (70.46 %) and the volume of the solvent (21.43 mL) for the extraction of the selected plant by the sonication method. An overlay plot was also generated for the point prediction of extraction conditions and responses (Fig. 7). The experiments were also conducted at the predicted optimized conditions and the EY, TPC and TFC were calculated to be 7.85 ± 0.62 % (w/w), 52.94 ± 0.12 GAE/g, and 33.78 ± 0.15 RUE/g, respectively. All the results were within the 95 % confidence limit and confirmed the accuracy and predictability of the developed model for the selected variables.
Fig. 7.
Overlay plot for the point prediction of optimized extraction conditions.
It has been found that the volume of solvent/g of solid was optimized to 20:1 in UAE extracts as compared to 30:1 in SXNJ extracts, which did not only efficiently improve the yield of extract, TPC and TFC (p < 0.05) but also reduced the extraction time from 13 h in SXNJ to approximately 20 min in OUNJ. Similar results were also reported in the past, where the UAE was found to enhance the yields of TPC and TFC from the flowers of Jatropha integerrima as compared to conventional methods at optimized conditions [47]. Also, the seeds of Eurylae ferox were efficiently extracted within 21 min (as suggested by the CCD) of the sonication process [48]. From these results, we can suggest that the CCD coupled RSM could be successfully employed to optimize the extraction conditions in UAE for the selected herb.
3.4. Scanning electron microscopy (SEM)
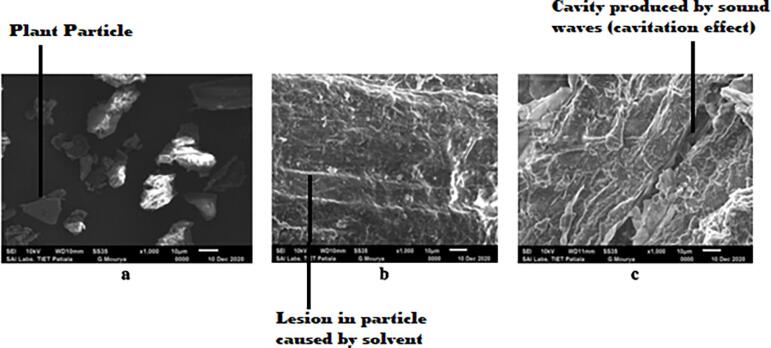
Microscopical alterations in the morphology of the plant samples which affect the different extraction processes, can be analyzed by using scanning electron microscopy (SEM). The magnified image (1000 × ) of untreated and processed (after extraction) plant samples can be visualized in Fig. 8. Before extraction, the powder of the herb revealed different particles without any striations on the surface (Fig. 8 a). After extraction by the conventional percolation method, the particles became flat and lesions were formed on their surface due to softening by the solvent (Fig. 8b). Herb processing with UAE brought out significant structural changes in the plant samples (Fig. 8c). Due to the mechanical effect of the microjets produced by sound waves in the solution, cavities formed on the surface of the solid particles. Moreover, the intensity of the cavitation enhanced for the solvents with low vapor pressure and surface tension [49]. Hence, binary mixtures of polar solvents, ethanol and water, with vapor pressures of 0.08 and 0.03 atm, respectively, further helped in inducing the cavitation effect. Also, heating of the solution due to the sonication process caused the crumbling of the cell surface [50], [51]. Based on SEM studies of the different plant samples, it can be deduced that sound waves significantly alter the cellular structure of the particles and thus could be helpful in improved and fast movement of the solvent to bring out a better extraction of plant actives. These results are also concomitant with previous studies showing that SEM analysis of Soybean flakes after the UAE confirmed the microfractures formed in the samples [39].
Fig. 8.
Microscopical pictures (1000x) of the plant samples (a) Untreated (b) after extraction by CPM (c) after UAE.
3.5. GC–MS analysis
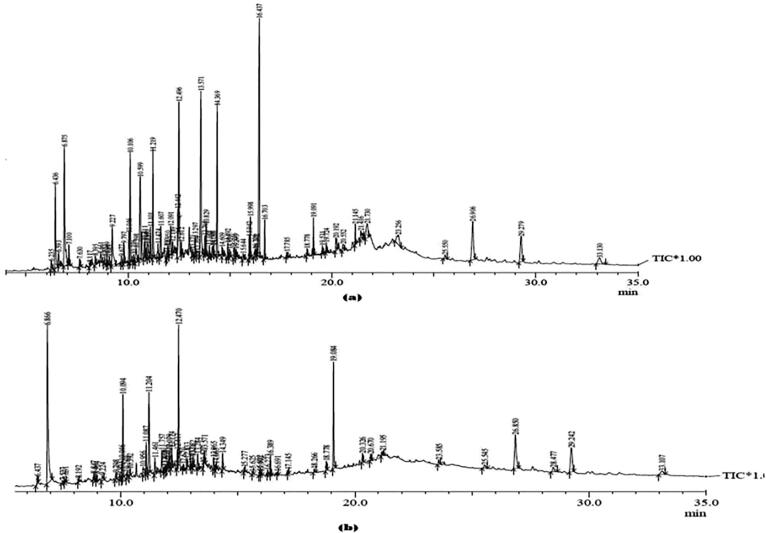
Separation and identification of the various constituents present in the different extracts of the roots of NJ were carried out using GC–MS analysis. The chromatogram indicating the peaks of the different plant actives is shown in Fig. 9. Moreover, the retention times (RT) of the various phytoconstituents along with their relative % area, are presented in Table 3. Analysis of the SXNJ and OUNJ extracts provided an idea about the presence of various sesquiterpenes, steroids and ester compounds in both extracts. The yields of therapeutically important sesquiterpenes such as jatamansone (↑91.8 %), spirojatamaol (↑42.1 %), globulol (↑130.4 %) and the steroid-like sitosterol (↑84.6 %) were significantly enhanced in the OUNJ extracts as compared to the SXNJ ones. Also, some compounds like the nootkatone (RT 12.67), nardostachnol (RT 11.08), stigma-3,5-diene-7-one (RT 28.47) and pentafluoro-propionic acid dodecyl ester (RT 10.04) were only recovered in OUNJ. GC–MS analysis depicted that the concentration of all plant actives was significantly improved, these results being in agreement with previous studies showing that UAE of Enicostema littorate and Momordica charantia improves the yield of secondary metabolites like swertiamarin (↑2.8-fold) and charantin (↑2.74-fold) as compared to conventional extraction methods [52], [53]. Heating of the plant samples and the cavitation effect produced by the sonication process at the optimized conditions of UAE could be responsible for the improved yields of plant actives [2].
Fig. 9.
GC–MS chromatograms of different extracts (a) SXNJ (b) OUNJ. This figure should be read concomitantly with Table 3.
Table 3.
Different phytoconstituents identified in GC–MS analysis of SXNJ and OUNJ extracts of the selected plant along with their retention times (RT) and % area.
| SXNJ | OUNJ | |||
|---|---|---|---|---|
| Chemical Constituents | RT (min) | Area (%) | RT (min) | Area (%) |
| Spirojatamol | 10.106 | 4.13 | 10.094 | 5.87 |
| Nardostachnol | – | – | 11.087 | 2.38 |
| Jatamansone | 11.219 | 3.97 | 11.204 | 7.57 |
| Valerenal | 11.607 | 1.45 | 11.937 | 0.36 |
| Globulol | 12.496 | 3.85 | 12.470 | 8.87 |
| Nootkatone | – | – | 12.670 | 0.44 |
| Ethyl oleate | 15.998 | 1.12 | 15.992 | 0.22 |
| Octadecanoic acid, ethyl ester | 16.228 | 0.40 | 15.992 | 0.20 |
| Stigmasta-5,22-dien-3-ol,acetate, −3-beta | – | – | 23.585 | 0.57 |
| Gamma-sitosterol | 26.906 | 3.83 | 26.850 | 7.07 |
| Gamma-sitostenone | 29.279 | 2.56 | 29.242 | 5.59 |
| Stigmastane-3,6-dione,5-alpha | 33.130 | 1.12 | 33.107 | 1.18 |
| Cyclopentane, 1-(3-methylbutyl) | 9.227 | 2.99 | 9.224 | 0.78 |
| Aromadendrene, dehydro | 11.101 | 1.21 | 11.757 | 2.13 |
| Naphthalene,1,2,3,5,6,7,8,8A-octahydro-1,8A-dimethyl-7-(1-methylethenyl) | 8.969 | 0.34 | 8.964 | 0.42 |
| 2,11-dioxatetracyclo(4,3,1,1(3,10,)0,(6,9))undec-4-ene,3,7,7,10-tetramethyl | 11.474 | 0.71 | 11.461 | 1.55 |
| Isovalencenal | 12.189 | 0.51 | 12.174 | 1.58 |
| Sinularene | 12.612 | 0.39 | – | – |
| Stigmasta-3,5-diene-7-one | – | – | 28.477 | 2.26 |
| Cyclopentaneacetaldehyde,2-formyl-3-methyl-alpha-methylene | 6.436 | 3.68 | 6.437 | 0.66 |
| 2-(1-Methylcyclopropyl)aniline | 6.875 | 6.36 | 6.866 | 20.82 |
| 6-isopropenyl-4,8A-dimethyl-3,5,6,8,8A-hexahydro-1H-naphthalene-2-one | 12.091 | 1.72 | 12.079 | 1.22 |
| Coumarin-6-ol,3,4-dihydro-4,4,5,7-tetramethyl-methylsulfate(ester) | – | – | 12.417 | 0.92 |
| Ergost-5-en-3-ol, 3-beta | 25.550 | 0.32 | 25.545 | 0.70 |
| Stigmasta-3,5-diene | – | – | 28.477 | 0.97 |
| Pentafluropropionic acid, dodecyl ester | – | – | 10.046 | 1.29 |
3.6. anti-oxidant potential of the extracts
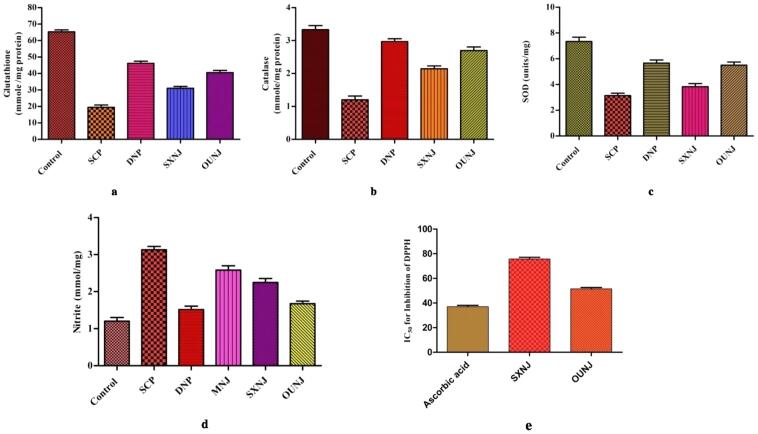
Oxidative stress and reactive oxygen species (ROS) are held responsible for numerous diseases in humans including neurodegeneration, Alzheimer disease and inflammation, etc. [54], [55], [56], [57], [58]. Extracts of N. jatamansi are also included in a number of marketed formulations for the prevention and treatment of such diseases [14], [17]. Thus, the anti-oxidant potential of the different extracts of N. jatamansi (D. Don) DC, SXNJ and OUNJ, were evaluated by in-vitro and in-vivo assays. The DPPH assay was used to evaluate the anti-oxidant potential of the extracts under in vitro conditions. Scavenging of free radicals of DPPH by antioxidants present in the extracts faded the violet color of the solution. Also, alteration in color intensity was proportional to antioxidants concentration in the test solution [59]. In the present case, we observed that the IC50 to curb the free radicals was significantly reduced (p < 0.05) in the OUNJ extracts as compared to the SXNJ ones (Fig. 10e). Moreover, the extracts were also evaluated for their in vivo anti-oxidant potential. Scopolamine was used to increase the oxidative stress level in experimental animals and it was found that enhanced stress significantly altered various biochemical parameters such as glutathione, catalase, SOD and nitrite in the negative control group (p < 0.01) (Fig. 10a,10b,10c,10d). It has been observed that changes in the concentration of glutathione, catalase and nitrite in the OUNJ group were significantly different as compared to the negative control group (p < 0.01) as well as the SXNJ group animals (p < 0.05). Data indicated that SXNJ extracts altered the selected biochemical parameters, as well (p < 0.05). Thus, interpretation of the results regarding the anti-oxidant assays confirmed the protective effect of OUNJ extracts as well as SXNJ extracts against scopolamine-induced oxidative stress. It has also been observed that the antioxidant protective effect was significantly improved in the optimized extracts as compared to those obtained by conventional percolation (SXNJ). The enhanced protective effect of the OUNJ extracts could be attributed to increased phenolic and flavonoid contents in those extracts. Phenolic or flavonoid compounds are proven to possess anti-oxidant properties by donating hydrogen atoms to free radicals [60], [61]. It was also reported that the quenching of free radicals was improved with an increased concentration of such metabolites [62]. Literature suggests that along with phenolic or flavonoid compounds, sesquiterpenes also possess anti-oxidant properties [63]. The globulol and nootkatone sesquiterpenes found in the NJ extracts were also found to scavenge free radicals in various past studies [64], [65]. In the present work, GC–MS analyses of the extracts proved that the concentrations of various sesquiterpenes like jatamansone, spirojatamol, globulol, nootkatone were significantly increased in the OUNJ extracts as compared to the SXNJ ones. The observed improved protection against the oxidative stress by OUNJ extracts could thus be attributed to the enhanced concentration of these various sesquiterpenes along with TPC and TFC.
Fig. 10.
In vivo biochemical estimation of different anti-oxidant stress parameters (a) reduced glutathione (b) catalase (c) SOD (d) nitrite content in various groups (Control; SCP- Scopolamine induced group (negative control); DNP- Donepezil treated (positive control); SXNJ- test group (soxhlet extracts of N. jatamansi); OUNJ- test group (optimized extract formed by UAE of N. jatamansi) and (e) IC50 for scavenging of DPPH radicals (in vitro).
3.7. AChE inhibitory potential of the different extracts
N. jatamansi root extracts possess a remarkable pharmacological potential useful in various neurodegenerative ailments including Alzheimer disease [17], [66]. In the past, the NJ extracts were also reported to inhibit the AChE enzyme, which plays a fundamental role in Alzheimer disease [67]. The importance of AChE inhibitors has been well established in attenuating Alzheimer disease symptoms [14], [68], [69], [70]. Thus, the effect of the optimized extracts along with conventional extracts on the AChE (in vitro and in vivo) has been studied in the present research. In vitro assays indicated that the IC50 for AChE inhibition was significantly reduced from 69.11 ± 2.38 µg/mL in the SXNJ extracts to 51.15 ± 1.28 µg/mL in the OUNJ ones (p < 0.05) (Fig. 11b). Moreover, analysis of AChE in brain homogenates of experimental animals confirmed that scopolamine significantly enhanced its concentration (Fig. 11a). Treatment with the optimized extract (OUNJ) significantly reduced AChE concentrations as compared to the negative control (p < 0.01) and the SXNJ extract (p < 0.05). As well, the extract obtained by the conventional method (SXNJ) significantly inhibited AChE at the test doses. The present results could be attributed to an improved concentration of secondary metabolites especially that of sesquiterpenes, like jatamansone, spirojatamaol, globulol, etc in the OUNJ extracts. Past studies proved that cholinergic transmission can be ameliorated by various sesquiterpenes through AChE inhibition and thus could be employed for the management of Alzheimer disease [14]. Moreover, the concentration of nootkatone increased in the OUNJ extracts and it has been reported that this plant active could play a significant role in reduction of the neuro-inflammation associated with Alzheimer disease by altering cholinergic transmission, oxidative stress and expression of the transcription factor NF- κB-P65 [65]. Various researchers have also shown that the phenolic content from herbs could be linked to free radical scavenging in brain tissues and can improve the cognitive function of experimental animals [71], [72]. Thus, we can say that improvement in the concentration of various plant actives reported in this work, is responsible for the significant alteration in the anti-oxidant as well as the AChE inhibition potential of the optimized extracts.
Fig. 11.
(a) AChE concentrations in brain tissues (in vivo) (Control; SCP- Scopolamine induced group (negative control); DNP- Donepezil treated (positive control); SXNJ- test group (soxhlet extracts of N. jatamansi); OUNJ- test group (optimized extract formed by UAE of N. jatamansi) (b) IC50 for AChE inhibition in µg/mL (in vitro).
4. Conclusion
Nardostachys jatamansi (D. Don) DC is a potential medicinal herb, utilized in substantial ayurvedic formulations for the prevention and treatment of various ailments. In past years, the commercial significance of this medicinal plant had also increased potentially and thus the demand for herb extracts is enhanced in India and abroad. Conventional methods of extraction alone cannot meet the demand of the industry. Moreover, traditional extraction methods carry the limitations of not being environmentally friendly as well as being time-consuming. Hence, the paradigm has to be shifted toward non-conventional techniques such as UAE for improving extraction in less time and also accounting for a green approach. In the present research, the UAE method has been developed and optimized for the extraction of NJ roots by using a response surface methodology. It has been found that extracts obtained at optimized conditions of UAE not only possess similar metabolites (compared to a conventional extract) but the quality of the process was also improved in terms of concentration of the plant actives. Moreover, the time of extraction as well as the pharmacological potential (anti-oxidant and AChE inhibition activities), were also improved in the optimized extracts. To conclude with, we can suggest that after suitable improvement of the developed method, application at an industrial scale can be considered to meet the rising demand of extracts of that plant.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Authors expressed their sincere gratitude to Taif University Researchers Supporting Project number (TURSP-2020/38), Taif University, Taif, Saudi Arabia for kind support.
Contributor Information
Vineet Mittal, Email: drvineet.pharma@mdurohtak.ac.in.
Philippe Jeandet, Email: philippe.jeandet@univ-reims.fr.
Data availability
Data will be made available on request.
References
- 1.Lee M.H., Lin C.C. Comparison techniques for extraction of isoflavones from the root of Radix Puerariae: Ultrasonic and pressurized solvent extractions. Food Chem. 2007;105:223–228. doi: 10.1016/j.foodchem.2006.11.009. [DOI] [Google Scholar]
- 2.Arya A., Chahal R., Nanda A., Kaushik D., Bin-Jumah M., Rahman M.H., Abdel-Daim M.M., Mittal V. Statistically Designed Extraction of Herbs Using Ultrasound Waves: A Review. Curr. Pharm. Des. 2021;27:1–18. doi: 10.2174/1381612827666210222114441. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Z.-S., Wang L.-J., Li D., Jiao S.-S., Chen X.D., Mao Z.-H. Ultrasound assisted extraction of oil from flaxseed. Sep. Purif. Technol. 2008;62(1):192–198. [Google Scholar]
- 4.Oroian M., Ursachi F., Dranca F. Influence of ultrasonic amplitude temperature time and solvent concentration on bioactive compounds extraction from propolis. Ultrason. Sonochem. 2020;164 doi: 10.1016/j.ultsonch.2020.105021. [DOI] [PubMed] [Google Scholar]
- 5.A. Nanda, V. Mittal, Design Based Ultrasound-Assisted Extraction of Marrubium vulgare Linn and Comparative Evaluation of Extracts for Furan Labdane Diterpene (Marrubiin) Concentration and Antihypertensive Potential, Curr. Bioact. Comp. 16 (2020) 924-936, DOI: 102174/1573407215666190524102431.
- 6.S.Q. Sun, Y.J. Wang, W. Xu, C.J. Zhu, X.X. Liu, Optimizing ultrasound-assisted extraction of prodigiosin by response surface methodology, Prep. Biochem. Biotechnol. 45 (2015) 101-108, doi: 101080/108260682013877029. [DOI] [PubMed]
- 7.Bezerra M.A., Santelli R.E., Oliveira E.P., Villar L.S., Escaleira L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta. 2008;76:965–977. doi: 10.1016/j.talanta.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Konar N., Poyrazoğlu E.S., Demir K., Artik N. Determination of conjugated and free isoflavones in some legumes by LC–MS/MS. J. Food Compos. Anal. 2012;25:173–178. doi: 10.1016/j.jfca.2011.11.004. [DOI] [Google Scholar]
- 9.Tye H. Application of statistical ‘design of experiments’ methods in drug discovery. Drug Discov. Today. 2004;9:485–491. doi: 10.1016/S1359-6446(04)03086-7. [DOI] [PubMed] [Google Scholar]
- 10.Mulliken T., Petra C. Federal Agency for Natural Conservation, Bonn, Germany; BfN-Skripten: 2008. Review of the status, harvest, trade andmanagement of seven Asian CITES-listed medicinal and aromatic plant species; pp. 11–138. [Google Scholar]
- 11.Jhunjhunwala A. Local market report: Natural essential oils of Indian origin. Indian Perfum. 2010;54:13. [Google Scholar]
- 12.Subashini R., Gnanapragasam A., Senthilkumar S., Yogeeta S., Devaki T. Protective effect of Nardostachys jatamansi (Rhizomes) on mitochondrial respiration and lysosomal hydrolases during doxorubicin induced myocardial injury in rats. J. Health Sci. 2007;53:67–72. doi: 10.1248/jhs.53.67. [DOI] [Google Scholar]
- 13.Wang M., Yang T.T., Rao Y., Wang Z.M., Dong X., Zhang L.H., Han L., Zhang Y., Wang T., Zhu Y., Gao X.M. A review on traditional uses, phytochemistry, pharmacology, toxicology and the analytical methods of the genus Nardostachys. J. Ethnopharmacol. 2021;280 doi: 10.1016/j.jep.2021.114446. [DOI] [PubMed] [Google Scholar]
- 14.A. Arya, R. Chahal, R. Rao, M. Rahman, D. Kaushik, M.F. Akhtar, A. Saleem, S. Khalifa, H.R. El-Seedi, M. Kamel, G.M. Albadrani, M.M. Abdel-Daim, V. Mittal, Acetylcholinesterase Inhibitory Potential of Various Sesquiterpene Analogues for Alzheimer’s Disease Therapy, Biomolecules. 11 (2021) 350, 103390/biom11030350. [DOI] [PMC free article] [PubMed]
- 15.Joshi R.K., Satyal P., Setzer W.N. Himalayan aromatic medicinal plants: a review of their ethnopharmacology, volatile phytochemistry, and biological activities. Medicines (Basel). 2016;3:6. doi: 10.3390/medicines3010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saroya A.S., Singh J. In: Pharmacotherapeutic Potential of Natural Products in Neurological Disorders. Saroya A.S., Singh J., editors. Springer Singapore; Singapore: 2018. Neuropharmacology of Nardostachys jatamansi DC; pp. 167–174. [Google Scholar]
- 17.H. Joshi, M. Parle, Nardostachys jatamansi improves learning and memory in mice, J. Med. Food. 9 (2006) 113–118 doi: 101089/jmf20069113. [DOI] [PubMed]
- 18.S.K. Sharma, A.P. Singh, In vitro antioxidant and free radical scavenging activity of Nardostachys jatamansi DC, J. Acupunct. Meridian. Stud. 5 (2012) 112-118, doi: 101016/jjams201203002. [DOI] [PubMed]
- 19.Dhiman N., Bhattacharya A., Nardostachysjatamansi (D. Don), DC.-Challenges and opportunities of harnessing the untapped medicinal plant from the Himalayas. J. of ethnopharmacol. 2020;246 doi: 10.1016/j.jep.2019.112211. [DOI] [PubMed] [Google Scholar]
- 20.Devgun M., Nanda A., Ansari S.H. Comparative evaluation of the anti-diabetic activity of Pterocarpus marsupium Roxb Heartwood in the alloxan induced diabetic rats using extracts obtained by optimized conventional and non- conventional methods. Pak. J. Pharm. Sci. 2013;26:973–976. [PubMed] [Google Scholar]
- 21.Mittal V., Nanda A. Intensification of marrubiin concentration by optimization of microwave-assisted (low CO2 yielding) extraction process for Marrubium vulgare using central composite design and antioxidant evaluation. Pharm. Biol. 2017;55(1):1337–1347. doi: 10.1080/13880209.2017.1297837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ismail B.B., Guo M., Pu Y., Wang W., Ye X., Liu D. Valorisation of baobab (Adansonia digitata) seeds by ultrasound assisted extraction of polyphenolics. Optimisation and comparison with conventional methods. Ultrasonics Sonochemistry. 2019;52:257–267. doi: 10.1016/j.ultsonch.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 23.Yolmeh M., Habibi Najafi M.B., Farhoosh R. Optimisation of ultrasound-assisted extraction of natural pigment from annatto seeds by response surface methodology (RSM) Food Chemistry. 2014;155:319–324. doi: 10.1016/j.foodchem.2014.01.059. [DOI] [PubMed] [Google Scholar]
- 24.Abugabr Elhag H.E.E., Naila A., Nour A.H., Ajit A., Sulaiman A.Z., Aziz B.A. Optimization of protein yields by ultrasound assisted extraction from Eurycoma longifolia roots and effect of agitation speed. Journal of King Saud University - Science. 2019;31(4):913–930. [Google Scholar]
- 25.Lou Z., Wang H., Zhu S., Zhang M., Gao Y., Ma C., Wang Z. Improved extraction and identification by ultra-performance liquid chromatography tandem mass spectrometry of phenolic compounds in burdock leaves. J. Chromatogr. A. 2010;1217(16):2441–2446. doi: 10.1016/j.chroma.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Razack S., Kumar K., Nallamuthu I., Naika M., Khanum F. I, Nallamuthu. M, Naika, F, Khanum, Antioxidant Biomolecule Oxidation Protective Activities of Nardostachys jatamansi DC and Its Phytochemical Analysis by RP-HPLC and GC-MS, Antioxidants. 2015;4(1):185–203. doi: 10.3390/antiox4010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bersuder P., Hole M., Smith G. Antioxidants from a heated histidine glucose model system 1: investigations of the antioxidant role of histidine and isolation of antioxidant by high performance liquid chromatography. J. Am. Oil. Chemists. Soc. 1998;75(1998):181–187. [Google Scholar]
- 28.Ellman G.L. Tissue sulfhydryl groups. Tissue sulfhydryl groups. Arch. Biochem. Biophy. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 29.H. Aebi, Catalase In Methods of enzymatic analysis, Academic press. (1974) 673-684, 101016/B978-0-12-091302-250032-3.
- 30.Lieben C.K.J., Blokland A., Şık A., Sung E., van Nieuwenhuizen P., Schreiber R. The selective 5-HT6 receptor antagonist Ro4368554 restores memory performance in cholinergic and serotonergic models of memory deficiency in the rat. Neuropsycho pharmacology. 2005;30(12):2169–2179. doi: 10.1038/sj.npp.1300777. [DOI] [PubMed] [Google Scholar]
- 31.Kakkar P., Das B., Viswanathan P.N. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984;21:130–132. http://nopr.niscair.res.in/handle/123456789/19932 [PubMed] [Google Scholar]
- 32.Ellman G.L., Courtney K.D., Andres V., Featherstone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology. 1961;7(2):88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 33.Mabberley D.J., Noltie H.J. A note on Valeriana jatamansi Jones (Caprifoliaceae s.l.) Blumea. 2014;59:37–41. doi: 10.3767/000651914X683476. [DOI] [Google Scholar]
- 34.Lyle N., Bhattacharyya D., Sur T.K., Munshi S., Paul S., Chatterjee S., Gomes A. Stress modulating antioxidant effect of Nardostachys jatamansi. Indian J. Biochem. Biophys. 2009;46:93–98. http://nopr.niscair.res.in/handle/123456789/3323 [PubMed] [Google Scholar]
- 35.N. Lyle, A. Gomes, T. Sur, S. Munshi, S. Paul, S. Chatterjee, D. Bhattacharyya, The role of antioxidant properties of Nardostachys jatamansi in alleviation of the symptoms of the chronic fatigue syndrome, Behav. Brain Res. 14202 (2009) 285-290, doi:101016/jbbr200904005. [DOI] [PubMed]
- 36.Alara O.R., Abdurahman N.H., Ukaegbu C.L. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021;4:200–214. doi: 10.1016/j.crfs.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao S., Kwok K.C., Liang H. Investigation on ultrasound assisted extraction of saikosaponins from Radix Bupleuri. Separ. Purif. Tech. 2007;55:307–312. doi: 10.1016/j.seppur.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boonkird S., Phisalaphong C., Phisalaphong M. Ultrasound-assisted extraction of capsaicinoids from Capsicum frutescens on a laband pilot-plant scale. Ultrason. Sonochem. 2008;15:1075–1079. doi: 10.1016/j.ultsonch.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Li H., Pordesimo L., Weiss J. High intensity ultrasound-assisted extraction of oil from soybeans. Food Res. Int. 2004;37:731–738. doi: 10.1016/j.foodres.2004.02.016. [DOI] [Google Scholar]
- 40.Chavan Y., Singhal R.S. Ultrasound-assisted extraction (UAE) of bioactives from arecanut (Areca catechu L.) and optimization study using response surface methodology. Innov. Food Sci. Emerg. Technol. 2013;17:106–113. doi: 10.1016/j.ifset.2012.10.001. [DOI] [Google Scholar]
- 41.Favre L.C., Dos Santos C., López-Fernández M.P., Mazzobre M.F., Buera M.D.P. Optimization of β-cyclodextrin-based extraction of antioxidant and anti-browning activities from thyme leaves by response surface methodology. Food Chem. 2018;265:86–95. doi: 10.1016/j.foodchem.2018.05.078. [DOI] [PubMed] [Google Scholar]
- 42.Şahin S., Şamlı R. Optimization of olive leaf extract obtained by ultrasound- assisted extraction with response surface methodology. Ultrason. Sonochem. 2013;20:595–602. doi: 10.1016/j.ultsonch.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 43.Yingngam B., Monschein M., Brantner A. Ultrasound-assisted extraction of phenolic compounds from Cratoxylum formosum ssp. formosum leaves using central composite design and evaluation of its protective ability against H2O2-induced cell death, Asian Pac. J Trop. Med. 2014;7:S497–S505. doi: 10.1016/S1995-7645(14)60281-9. [DOI] [PubMed] [Google Scholar]
- 44.Tahmasebi-Boldaji R., Hatamipour M.S., Khanahmadi M., Sadeh P., Najafipour L. Ultrasound-assisted packed-bed extraction of hypericin from Hypericum perforatum L. and optimization by response surface methodology. Ultrason. Sonochem. 2019;57:89–97. doi: 10.1016/j.ultsonch.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 45.Yang R.F., Geng L.L., Lu H.Q., Fan X.D. Ultrasound-synergized electrostatic field extraction of total flavonoids from Hemerocallis citrine baroni. Ultrason. Sonochem. 2017;34:571–579. doi: 10.1016/j.ultsonch.2016.06.037. [DOI] [PubMed] [Google Scholar]
- 46.Vinatoru M. An Overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001;8:303–313. doi: 10.1016/S1350-4177(01)00071-2. [DOI] [PubMed] [Google Scholar]
- 47.Xu D.P., Zhou Y., Zheng J., Li S., Li A.N., Li H.B. Optimization of Ultrasound- Assisted Extraction of Natural Antioxidants from the Flower of Jatropha integerrima by Response Surface Methodology. Molecules. 2015;21:E18. doi: 10.3390/molecules21010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y., Wei S., Liao M. Optimization of ultrasonic extraction of phenolic compounds from Euryale ferox seed shells using response surface methodology. Ind. Crops Prod. 2013;49:837–843. doi: 10.1016/j.indcrop.2013.07.023. [DOI] [Google Scholar]
- 49.Chivate M., Pandit A. Quantification of cavitation intensity in fluid bulk. Ultrason. Sonochem. 1995;2:S19–S25. doi: 10.1016/1350-4177(94)00007-F. [DOI] [Google Scholar]
- 50.Hara A., Radin N. Lipid extraction of tissues with a lowtoxicity solvent. Analytical Biochemistry. 1978;90:420–426. doi: 10.1016/0003-2697(78)90046-5. [DOI] [PubMed] [Google Scholar]
- 51.Schäfer K. Accelerated solvent extraction of lipids for determining the fatty acid composition of biological material. Analytica chimica acta. 1998;358:69–77. doi: 10.1016/S0003-2670(97)00587-4. [DOI] [Google Scholar]
- 52.Ahamad J., Amin S., Mir S.R. Optimization of ultrasound-assisted extraction of charantin from Momordica charantia fruits using response surface methodology. J. Pharm. Bioallied Sci. 2015;7:304–307. doi: 10.4103/0975-7406.168032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ahamad J., Amin S., Ahmad J.R., Mir S. Response surface methodology for optimization of ultrasound assisted extraction of swertiamarin from Enicostema littorale Blume. Curr. Bioact. Compd. 2016;12:87–92. doi: 10.2174/157340721202160504222203. [DOI] [Google Scholar]
- 54.Celik S., Ozkaya A. Effects of Intraperitoneally Administered Lipoic Acid, Vitamin E, and Linalool on the Level of Total Lipid and Fatty Acids in Guinea Pig Brain with Oxidative Stress Induced by H 2 O 2. BMB Reports. 2002;35(6):547–552. doi: 10.5483/bmbrep.2002.35.6.547. [DOI] [PubMed] [Google Scholar]
- 55.Roy A., Jana A., Yatish K., Freidt M.B., Fung Y.K., Martinson J.A., Pahan K. Reactive oxygen species up-regulate CD11b in microglia via nitric oxide: Implications for neurodegenerative diseases. Free Radical Biology and Medicine. 2008;45(5):686–699. doi: 10.1016/j.freeradbiomed.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yadav R.S., Sankhwar M.L., Shukla R.K., Chandra R., Pant A.B., Islam F., Khanna V.K. Attenuation of arsenic neurotoxicity by curcumin in rats. Toxicol. Appl. Pharmacol. 2009;240(3):367–376. doi: 10.1016/j.taap.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 57.Chen S.-G., Gu R.-X., Dai H., Wei D.-Q. Virtual screening for alpha7 nicotinic acetylcholine receptor for treatment of Alzheimer's disease. Journal of Molecular Graphics and Modelling. 2013;39:98–107. doi: 10.1016/j.jmgm.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 58.A.C. Souza, C.A. Bruning, C.I. Acker, J.S. Neto, C.W. Nogueira, 2-Phenylethynyl-butyltellurium enhances learning and memory impaired by scopolamine in mice, Behav. Pharmacol. 24 (2013) 249-254, doi: 101097/FBP0b013e32836353a5. [DOI] [PubMed]
- 59.Ardestani A., Yazdanparast R. Antioxidant and free radical scavenging potential of Achillea santolina extracts. Food Chem. 2007;104:121–219. doi: 10.1016/j.foodchem.2006.10.066. [DOI] [Google Scholar]
- 60.Rice-Evans C.A., Miller N.J., Bramley P.M., Pridham J.B. The relative antioxidant activity of plant derived polyphenolic flavonoids. Free Radic. Res. 1995;22:375–383. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- 61.Aiyegoro O.A., Okoh A.I. Prelimnary phytochemical screening and invitro antioxidant activities of the aqueous extract of Helichrysum longifolium DC. BMC Complement. Altern. Med. 2010;10:21. doi: 10.1186/1472-6882-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saeed N., Khan M.R., Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilisleptophylla L. BMC Complement Altern. Med. 2012;12:221–232. doi: 10.1186/1472-6882-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neganova M.E., Afanas’eva S.V., Klochkov S.G., Shevtsova E.F. Mechanisms of antioxidant effect of natural sesquiterpene lactone and alkaloid derivatives. Bull. Exp. Biol. Med. 2012;152(6):720–722. doi: 10.1007/s10517-012-1615-x. [DOI] [PubMed] [Google Scholar]
- 64.S. Rodriguez, R.A. Sueiro, A.P. Murray, J.M. Leiro, Bioactive sesquiterpene obtained from Schinusareira L. (Anacardiaceae) essential oil, In Multidisciplinary Digital Publishing Institute Proceedings, 41 (2019) 85, 10.3390/ecsoc-23-06649.
- 65.Wang Y., Wang M., Xu M., Li T., Fan K., Yan T., Xiao F., Bi K., Jia Y. Nootkatone, a neuroprotective agent from Alpiniae Oxyphyllae Fructus, improves cognitive impairment in lipopolysaccharide-induced mouse model of Alzheimer's disease. Int. immunopharmacol. 2018;62:77–85. doi: 10.1016/j.intimp.2018.06.042. [DOI] [PubMed] [Google Scholar]
- 66.Rokaya M.B., Münzbergová Z., Timsina B. Ethnobotanical study of medicinal plants from the Humla district of western Nepal. J. Ethnopharmacol. 2010;130:485–504. doi: 10.1016/j.jep.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 67.Mathew M., Subramanian S. In vitro screening for anti-cholinesterase and antioxidant activity of methanolic extracts of ayurvedic medicinal plants used for cognitive disorders. PloS one.9. 2014:e86804. doi: 10.1371/journal.pone.0086804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.A. Nordberg, C. Ballard, R. Bullock, T. Darreh-Shori, M. Somogyi, A review of butyrylcholinesterase as a therapeutic target in the treatment of Alzheimer's disease, Prim. Care Companion CNS Disord. 15 (2013) 26731, doi: 104088/PCC12r01412. [DOI] [PMC free article] [PubMed]
- 69.Davidsson P., Blennow K., Andreasen N., Eriksson B.o., Minthon L., Hesse C. Differential increase in cerebrospinal fluid-acetylcholinesterase after treatment with acetylcholinesterase inhibitors in patients with Alzheimer's disease. Neuroscience Letters. 2001;300(3):157–160. doi: 10.1016/s0304-3940(01)01586-5. [DOI] [PubMed] [Google Scholar]
- 70.K. Rockwood, Biomarkers to measure treatment effects in Alzheimer's disease: What should we look for? Int. J. Alzheimers Dis. 598175 (2011) 1-14, 104061/2011/598175. [DOI] [PMC free article] [PubMed]
- 71.Arya A., Kaushik D., Almeer R., Bungau S.G., Sayed A.A., Abdel-Daim M.M., Bhatia S., Mittal V. Application of Green Technologies in Design-Based Extraction of Celastrus paniculatus (Jyotishmati) Seeds, SEM, GC-MS Analysis, and Evaluation for Memory Enhancing Potential. Front. Nutr. 2022;9:1–19. doi: 10.3389/fnut.2022.871183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharififar F., Moshafi M.H., Shafazand E., Koohpayeh A. Acetyl cholinesterase inhibitory, antioxidant and cytotoxic activity of three dietary medicinal plants. Food Chemistry. 2012;130(1):20–23. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.