TLR7 mutations predispose individuals to SLE
Systemic lupus erythematosus (SLE), a complicated autoimmune disease with multiple organ involvement, is characterized by excessive autoantibody production and immune complex deposition. According to the European League Against Rheumatism and the American College of Rheumatology classifications of SLE, clinical manifestations and immunological indicators are two major criteria for lupus classification. A 7-year-old patient presented by Brown et al. exhibited thrombocytopenia, hypocomplementemia, and elevated autoantibodies, which are critical serological features of lupus.1 Moreover, damage to diverse tissues including joints, kidneys, and neurological systems was observed. Altogether, this patient was diagnosed with pediatric lupus. Additionally, this individual responded well to current SLE therapies including prednisone, azathioprine, mycophenolate, and rituximab. Various etiologies, including genetic predisposition and environmental factors, result in the high heterogeneity of SLE. Notably, genetic factors play a crucial role in childhood-onset refractory SLE. Although genome-wide association studies have presented circumstantial evidence that Toll like receptor 7 (TLR7) signaling might be a central hub in lupus pathogenesis, direct evidence is lacking.2 Noteworthily, whole-exome sequencing was performed on the patient and a de novo nucleotide substitution on TLR7 was identified, which resulted in a missense mutation from tyrosine to histamine on the 264th amino acid within the highly conserved ligand-binding domain (Y264H). Further functional validation of this de novo mutation has potential for the development of prognostic tools and effective targeted therapies in patients with SLE.
TLR7Y264H drives lupus development
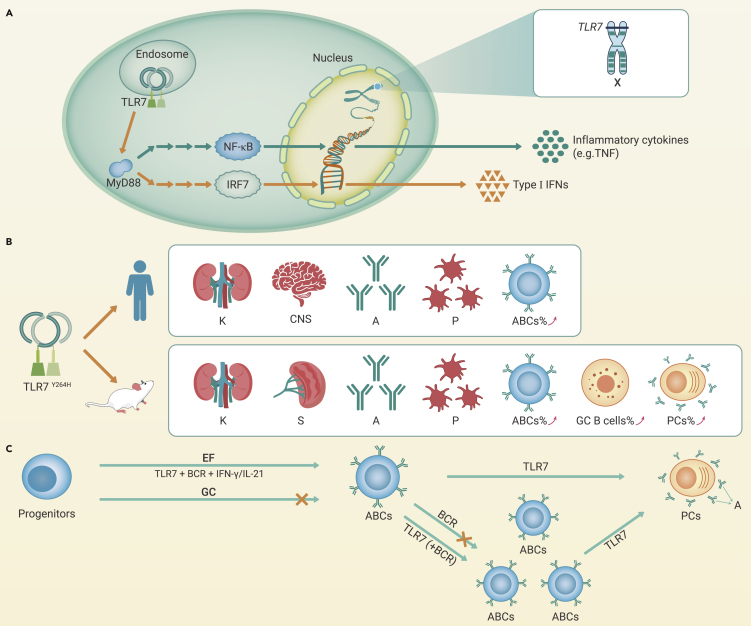
TLR7 recognizes intracellular single-stranded RNA, and aberrant TLR7 activation promotes autoimmune responses. Brown et al. hypothesized that TLR7Y264H might be a gain-of-function (GOF) mutation, and further investigation confirmed that TLR7Y264H exhibited a higher binding affinity for guanosine, which amplified the downstream nuclear factor-κB signaling and promoted the inflammatory cytokine production (Figure 1A). Furthermore, TLR7Y264H was demonstrated to be a sustained self-activation variant that underwent spontaneous cleavage and activation in the absence of exogenous ligands. These findings may explain the pathogenicity of TLR7Y264H. Notably, TLR7 might also be aberrantly activated by abnormal microbiota, and the translocation of pathobionts, such as Lactobacillus reuteri and Enterococcus gallinarum, reportedly engages type I interferon pathways via TLR7 activation.3 Consistent with the observation from the patient, TLR7Y264H mice displayed spontaneous symptoms of lupus, (Figure 1B). Notably, TLR7 is located on the X chromosome, which might explain the female predominance of SLE patients. Collectively, these results validate the hypothesis that TLR7 is one of the causative genes of lupus.
Figure 1.
The role of TLR7 in SLE pathogenesis
(A) The pathways of cytokine production driven by TLR7. TLR7 recruits MyD88 and initiates a signal cascade by activation of interferon regulatory factor 7 (IRF7) and nuclear factor kappa-B (NF-κB), which drives the production of type I interferons (IFNs) and inflammatory cytokines, respectively.
(B) TLR7Y264H variant reproduced SLE manifestations in transgenic mice. Both patient and mutant mice showed elevated production of autoantibodies, decreased proportion of platelets, and damaged multiple organs. The expansion of ABCs was observed in the patient, and ABCs, GC B cells, and plasma cells (PCs) were increased likewise in TLR7Y264H mutant mice.
(C) The role of TLR7 in ABCs differentiation, proliferation, and activation. Progenitors differentiate into ABCs mainly in an EF-dependent response under the signal of B-cell receptor (BCR) and TLR7/9 synergistic with IFN-γ or IL-21. The proliferation of ABC is more sensitive to TLR7 than BCR signal, but there is a synergistic effect with TLR7 and BCR co-stimulation. Activated ABCs can differentiate into PCs under TLR7 signaling. K, kidney; CNS, central nervous system; A, autoantibodies; P, platelets; S, spleen.
TLR7 variant activates autoreactive B cells
Production of autoantibodies against cellular and nuclear components and deposition of immune complexes in various organs lead to tissue inflammation and injury in SLE. Thus, antibody-producing B cells have long been considered central players in lupus pathogenesis. Traditionally, high-affinity immunoglobulin G autoantibodies are thought to arise through the germinal center (GC) response in a T cell-dependent manner. Recently, mechanisms such as class switching and somatic hypermutation of B cells in extrafollicular (EF) locations have attracted attention.4 Although both GC and EF responses have been implicated in SLE, given the heterogeneity of lupus, novel signal molecules and specific activation pathways of various B cell populations in different contexts require further exploration.
The noteworthy work presented by Brown et al. confirmed that pathogenic antibodies could be generated in a GC-independent manner in a proportion of patients with SLE. Age-associated B cells (ABCs), a B cell subpopulation derived mainly from the EF response, are highly suspected of participating in lupus pathogenesis.5 Evidence suggests that ABC numbers increase in aged mice but are prematurely expanded in different autoimmune disorders. Signals that activate the B cell receptor and TLR7/9 synergistically with interferon-gamma or interleukin-21 from T cells act on progenitors and initiate their differentiation towards ABCs through the EF pathway. Unlike follicular B cells, ABCs cannot proliferate with B cell receptor engagement alone but are susceptible to TLR7 stimulation. Thus, the activation and proliferation of ABCs are tightly controlled by TLR7 signaling (Figure 1C).
Intrinsic TLR7 signaling in ABCs is crucial for differentiation and subsequent antibody production. Both B cell-specific TLR7 deletion and B cell-specific ablation of myeloid differentiation primary response protein 88 (MyD88) could reduce the autoantibody titers in lupus-like mice.5 These results collectively raised the question of whether pathogenic antibody production in TLR7Y264H transgenic mice is due to ABCs expansion. This was addressed by observing the aberrant expansion of ABCs and plasma cells in both the patient and TLR7Y264H mice plus the fact that TLR7 or MyD88 deficiency ameliorated the disease by reducing ABCs. Despite the rarity of patients with SLE harboring TLR7 GOF variants, the TLR7-driven EF response is significant in SLE progression. Therefore, the TLR7-driven mechanisms described by Brown et al. have critical implications for the development of effective targeted therapies in patients with SLE.
However, further studies are required to explore the role of ABCs in lupus pathogenesis. Moreover, considering the significant contribution of innate immunity to the pathogenesis of SLE, the influence of TLR7 GOF mutation on innate immune cells remains to be elucidated. Future studies investigating these questions may increase understanding of the contribution of TLR7 to SLE pathogenesis.
Potential applications targeted TLR7/MyD88 axis
Since conventional therapies for lupus are not yet optimal, numerous drugs candidate with various targets have been developed or are under development, and B cell-targeting approaches are some of the most promising. Although different B-cell targeted agents, including belimumab, telitacicept, and rituximab, have been tested or even approved in the clinic, they are currently used off label as a first-line treatment supplement because they do not display superior efficacy to standard immunosuppression. Thus, researchers continue to explore new targets with better therapeutic efficacy.
With the recognition of its role in the pathogenesis of lupus, targeting TLR7 for SLE treatment brings new hope to patients. Brown et al. provided support for the benefits of blocking the TLR7-MyD88 axis in SLE. Notably, hydroxychloroquine, recommended for most individuals with lupus, can gently reduce TLR7 activation by inhibiting endosome acidification. Enpantoran (M5049), a TLR7/8 inhibitor, is currently used in a phase II randomized controlled trial for SLE. Additionally, the insensitivity of pathogenic ABCs to B lymphocyte stimulator might explain the non-response to belimumab, and combining TLR7 inhibitors with belimumab might provide superior efficacy.
In summary, the highly heterogeneous pathogenesis and diverse clinical manifestations of SLE present considerable challenges to both scientists and clinicians. However, the study presented by Brown et al. has the potential to contribute to novel and efficacious therapies for SLE. From a translational point of view, the next step in establishing the TLR7-MyD88 axis as a therapeutic target for SLE will be to demonstrate its clinical benefits in proof-of-concept trials with patients.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81788101, 32141004, and 82171798) and the CAMS Innovation Fund for Medical Sciences (CIFMS) (2021-1-I2M-040, 2021-1-I2M-017, 2021-1-I2M-047, 2021-1-I2M-016, and 2021-1-I2M-026).
Declaration of interests
The authors declare no competing interests.
Published Online: August 6, 2022
Contributor Information
Hao Li, Email: hli13@bidmc.harvard.edu.
Xuan Zhang, Email: zxpumch2003@sina.com.
References
- 1.Brown G.J., Cañete P.F., Wang H., et al. TLR7 gain-of-function genetic variation causes human lupus. Nature. 2022;605:349–356. doi: 10.1038/s41586-022-04642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fillatreau S., Manfroi B., Dörner T. Toll-like receptor signalling in B cells during systemic lupus erythematosus. Nat. Rev. Rheumatol. 2021;17:98–108. doi: 10.1038/s41584-020-00544-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang M., Liu Y., Zhao L., Zhang X. Modulating gut microbiota in autoimmune diseases: a cutting-edge strategy from prophylaxis to therapeutics. Sci. Bull. 2022;67:771–773. doi: 10.1016/j.scib.2021.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Jenks S.A., Cashman K.S., Woodruff M.C., et al. Extrafollicular responses in humans and SLE. Immunol. Rev. 2019;288:136–148. doi: 10.1111/imr.12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancro M.P. Age-associated B cells. Annu. Rev. Immunol. 2020;38:315–340. doi: 10.1146/annurev-immunol-092419-031130. [DOI] [PubMed] [Google Scholar]