Abstract
Purpose
HER2-positive colorectal cancer was drawn increasing attention in recent years. Accumulating evidence showed HER2-positive metastatic colorectal cancer could benefit from HER2-targeted therapy. While HER2 expression and the relationship between HER2 status and clinicopathological characteristics of overall colorectal cancer remains largely unknown. The aim of this study was to evaluate HER2 expression in colorectal cancer and compare the clinicopathological features between HER2-positive and HER2-negative colorectal cancer.
Methods
We retrospectively analyzed 3910 primary colorectal cancer patients treated in our institution from January 2016 to December 2019. Medical records and pathology reports after surgery were collected to provide information about HER2 status and other clinicopathological characteristics.
Results
We identified 3347 HER2-negative and 79 HER2-positive colorectal cancer patients in our cohort. The chi-square test showed that vessel invasion was significantly more common in HER2-positive colorectal cancer patients. Crude analysis showed HER2 positive was associated with vessel invasion in colorectal cancer [OR and 95% CI 0.534 (0.341, 0.835), p = 0.006]. After adjusting for N stage, a significant association was still observed between HER2 status and vessel invasion in colorectal cancer [OR and 95% CI 0.550 (0.322, 0.941), p = 0.029]. Survival analysis showed that there was no significant difference in 3-year overall survival rate between HER2 positive and HER2 negative group (p = 0.603).
Conclusion
Our findings indicate that the rate of HER2 positivity in colorectal cancer was relatively low, and HER2 status was strongly associated with vessel invasion while having no significant influence on the 3-year overall survival rate in colorectal cancer patients.
Keywords: Colorectal cancer, HER2, Immunohistochemistry, Vessel invasion
Introduction
Human epidermal growth factor receptor 2 (HER2) is one of the members of the EGFR family of receptor tyrosine kinases [1]. Former studies had proved that HER2 overexpression is closely related to tumorigenesis and progression [2–4]. In addition, HER2 is overexpressed in about 30% of breast cancer and 20% of advanced gastric cancer [5–8]. Based on the evidence from previous clinical trials, HER2 had been well-established as a therapeutic target in breast cancer and gastric cancer [9–13].
In recent years, many studies revealed that HER2 is a promising target in HER2-amplified metastatic colorectal cancer (CRC) [14–16]. Moreover, HER2 could be a negative biomarker for EGFR-targeted treatments in CRC [17]. However, the most current research was focused on HER2 positive metastatic CRC (mCRC), and the clinical and pathological features of overall HER2-positive CRC remain largely unknown.
In this retrospective study, we aimed to compare the clinical and pathological features between HER2 negative and HER2 positive CRC and provide meaningful information about HER2-positive CRC.
Methods
Patients
This study was approved by the ethics committee of Fudan University Shanghai Cancer Center (No.2206255-Exp1). Patients newly diagnosed with CRC between January 2016 and December 2019 were retrospectively analyzed in this study. The inclusion criteria were as follows: (1) primary CRC with radical resection. (2) primary CRC with complete records of clinical and pathological data, including sex, age, T stage, N stage, distant metastasis, vessel invasion, perineural invasion, tumor differentiation, and HER2 status. (3) primary CRC without neoadjuvant treatment. (4) primary CRC without other severe diseases, especially cancer.
Clinical and pathological data were collected from the department of information of our center, including age, sex, TNM stage, vessel invasion, perineural invasion, tumor differentiation, and HER2 status. These patients were followed up by telephone or by visiting the hospital. The follow-up interval was 3 months for the first 2 years after surgery and 6 months for the third year after surgery. The overall survival (OS) was defined as the time from surgery to death or the last follow-up.
HER2 testing
HER2 status was measured by immunohistochemistry, which was evaluated according to the HERACLES criteria (IHC score 0: no staining or staining in < 10% of cells; IHC score 1 + : faint staining or moderate staining < 50% of cells or intense staining ≤ 10% cells; IHC score 2 + : moderate staining in ≥ 50% of cells; IHC score 3 + : intense staining in ≥ 50% of cells. In situ hybridization (ISH) was mandatory when IHC score 2 + . HER2 positivity, defined as IHC score 3 + and ISH HER2: CEP17 ratio higher than two in more than 50% of cells).
Only IHC score 0–1 + was considered HER2 negative, and IHC score 3 + was considered HER2 positive in this study due to ISH of CRC was not routinely performed in our institute.
Statistical analysis
SPSS version 23.0 was recruited to conduct all statistical analyses. Student’s t-test was used to compare differences in continuous variables between HER2 negative and HER2 positive groups. The chi-square test or Fisher’s exact test was used to compare categorical variables between HER2 negative and HER2 positive groups. Univariate and multivariate logistic regression models were applied to assess the influence of HER2 status on clinical and pathological characteristics by calculating the odd ratios and their corresponding 95% confidence intervals (CIs). The cumulative survival probabilities of patients were estimated using the Kaplan–Meier method and compared with the log-rank test. P < 0.05 was considered to be statistically significant.
Results
Relationship between HER2 status and clinicopathologicl factors
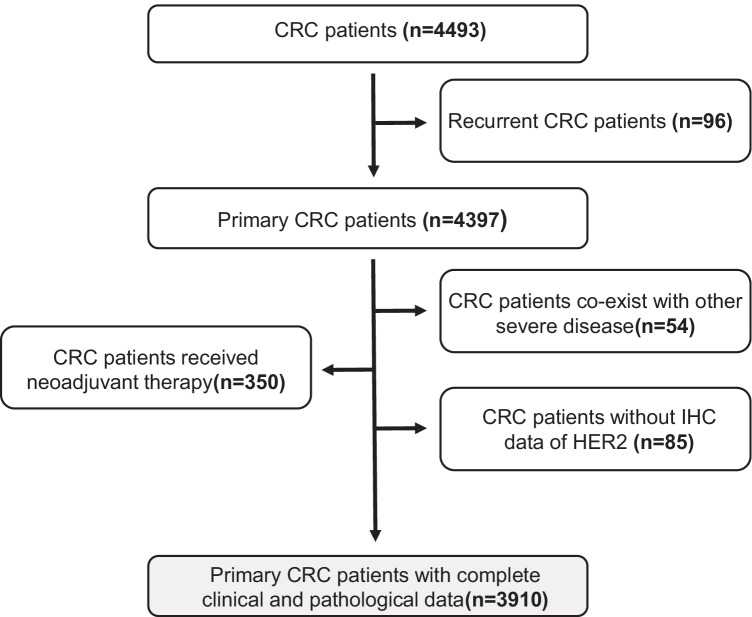
We collected 4493 CRC patients treated in our department from January 2016 to December 2019. Among them, 96 were recurrent CRC cases, 404 patients co-exist with other severe diseases or received neoadjuvant treatment (12 with a lung cancer history, 25 with breast cancer history, 17 with a renal cancer history, and 350 received neoadjuvant chemotherapy or radiotherapy), and 85 cases without IHC results of HER2 for unknown reasons. A total of 3910 primary CRC cases with complete clinical and pathological data were enrolled in this study (Fig. 1).
Fig. 1.
Flow diagram of the patient selection process-01
The overall HER2 status in this cohort tested by IHC was shown in Table 1. According to the HER2 testing criteria described above, 3347 cases were regarded as HER2 negative CRC and 79 as HER2 positive in total.
Table 1.
Demographics and clinical characteristics of patients in this study
| Characteristics |
IHC ( −) N (%)/mean ± sd |
IHC (1 +) N (%)/mean ± sd |
IHC (2 +) N (%)/mean ± sd |
IHC (3 +) N (%)/mean ± sd |
|---|---|---|---|---|
| Age (year) | 60.35 ± 11.89 | 60.51 ± 11.61 | 60.24 ± 11.72 | 58.29 ± 10.39 |
| Sex | ||||
|
Male Female |
1480 (60) 945 (40) |
599 (65) 323 (35) |
318 (65.7) 166 (34.3) |
43 (54.4) 36 (45.6) |
| T stage | ||||
|
T1 T2 T3 T4 |
133 (5.5) 347 (14.3) 1566 (64.6) 379 (15.6) |
34 (3.7) 128 (13.9) 631 (68.4) 129 (14) |
22 (4.5) 67 (13.8) 335 (69.2) 60 (12.4) |
7 (8.9) 9 (11.4) 52 (65.8) 11 (13.9) |
| N stage | ||||
|
N0 N1 N2 |
1318 (54.4) 670 (27.6) 437 (18) |
524 (56.8) 254 (27.6) 144 (15.6) |
267 (55.2) 139 (28.7) 78 (16.1) |
36 (45.6) 27 (34.2) 16 (20.3) |
| Distant metastasis | ||||
|
Yes No |
2084 (85.9) 341 (14.1) |
765 (83) 157 (17) |
390 (80.6) 94 (19.4) |
69 (87.3) 10 (12.7) |
| TNM stage | ||||
|
I II III IV |
360 (14.9) 856 (35.3) 869 (35.8) 340 (14) |
127 (13.8) 351 (38.1) 289 (31.3) 155 (16.8) |
61 (12.6) 181 (37.4) 148 (30.6) 94 (19.4) |
10 (12.7) 23 (29.1) 36 (45.6) 10 (12.7) |
| Vessel invasion | ||||
|
Yes No |
1489 (61.4) 936 (38.6) |
595 (64.5) 327 (35.5) |
310 (64) 174 (36) |
37 (46.8) 42 (53.2) |
| Perineural invasion | ||||
|
Yes No |
1616 (66.6) 809 (33.4) |
615 (66.7) 307 (33.3) |
305 (63) 179 (37) |
47 (59.5) 32 (40.5) |
| Tumor differentiation | ||||
|
High Poor-moderate |
2293 (94.6) 132 (5.4) |
877 (95.1) 45 (4.9) |
455 (94) 29 (6) |
76 (96.2) 3 (3.8) |
| Total | 2425 (62) | 922 (23.6) | 484 (12.4) | 79 (2) |
Then we divided these patients into two groups by HER2 status and analyzed the association between clinicopathological characteristics and HER2 status (Table 2). The average age was 58.29 and 60.43 years old in the HER2 positive and HER2 negative groups, respectively. There was no significant difference in age between these two groups (p = 0.113). Moreover, chi-square analysis showed that there were no significant differences between sex, T stage, N stage, distant metastasis, TNM stage perineural invasion, and tumor differentiation with HER2 status. However, significant differences were found between vessel invasion and HER2 positive and HER2 negative groups (p = 0.005).
Table 2.
Number of patients in HER2 positive and HER2 negative groups, respectively
|
HER2 positive N (%)/mean ± sd |
HER2 negative N (%)/mean ± sd |
Statistic | P | |
|---|---|---|---|---|
| Age (year) | 58.29 ± 10.391 | 60.43 ± 11.89 | − 1.587 | 0.113 |
| Sex | ||||
|
Male Female |
43 (54.4) 36 (45.6) |
2079 (62.1) 1268 (37.9) |
1.933 | 0.164 |
| T stage | ||||
|
T1 T2 T3 T4 |
7 (8.9) 9 (11.4) 52 (65.8) 11 (13.9) |
167 (5) 475 (14.2) 2197 (65.6) 508 (15.2) |
2.717 | 0.43 |
| N stage | ||||
|
N0 N1 N2 |
36 (45.6) 27 (34.2) 16 (20.3) |
1842 (55) 924 (27.6) 581 (17.4) |
2.833 | 0.243 |
| Distant metastasis | ||||
|
Yes No |
10 (12.7) 69 (87.3) |
498 (14.9) 2849 (85.1) |
0.301 | 0.583 |
| TNM stage | ||||
|
I II III IV |
10 (12.7) 23 (29.1) 36 (45.6) 10 (12.7) |
487 (14.6) 1207 (36) 1158 (34.6) 495 (14.8) |
4.132 | 0.248 |
| Vessel invasion | ||||
|
Yes No |
42 (53.2) 37 (46.8) |
1263 (37.7) 2084 (62.3) |
7.791 | 0.005 |
| Perineural invasion | ||||
|
Yes No |
32 (40.5) 47 (59.5) |
1116 (33.3) 2230 (66.7) |
1.772 | 0.183 |
| Tumor differentiation | ||||
|
High Poor–moderate |
3 (3.8) 76 (96.2) |
177 (5.3) 3170 (94.7) |
0.798 | 0.396 |
Logistic regression univariate and multivariate analyses were used to analyze the associations between HER2 status and other clinical and pathological characteristics. On crude analysis, HER2-positive CRC patients were more likely to with vessel invasion, OR and 95% CI 0.534 (0.341, 0.835), p = 0.006. After adjusting for N stage, a similar association was seen, OR and 95% CI 0.550 (0.322, 0.941), p = 0.029. While sex, T stage, N stage, distant metastasis, TNM stage, perineural invasion, and tumor differentiation were not showed significant association with HER2 status (Table 3).
Table 3.
Odds ratios (OR) (with 95% CI) of HER2 positive relative to HER2 negative by different characteristics of CRC patients
| Crude | Adjusted | ||
|---|---|---|---|
| OR (95% CI) P | OR (95% CI) P | ||
| Sex |
Male Female |
Ref. 0.729 (0.465, 1.141) 0.166 |
\ |
| T stage |
T1–T2 T3–T4 |
Ref. 1.072 (0.615, 1.868) 0.806 |
\ |
| N stage |
N0 N1–N2 |
Ref. 0.684 (0.437, 1.071) 0.097 |
Ref. 0.946 (0.553, 1.619) 0.839 |
| Distant metastasis |
Yes No |
Ref. 1.206 (0.617, 2.357) 0.584 | \ |
| TNM stage |
I-II III-IV |
Ref. 0.700 (0.445, 1.100) 0.122 | \ |
| Vessel invasion |
Yes No |
Ref. 0.534 (0.341, 0.835) 0.006 |
Ref. 0.550 (0.322, 0.941) 0.029 |
| Perineural invasion |
Yes No |
Ref. 0.735 (0.466, 1.158) 0.184 | \ |
| Tumor differentiation |
High Poor–moderate |
Ref. 1.415 (0.442, 4.528) 0.559 |
\ |
Prognostic value of HER2 status in CRC patients
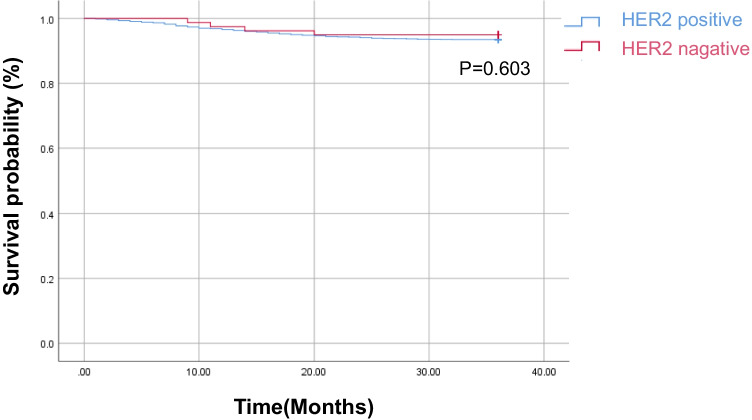
Furthermore, we analyzed the relationship between HER2 status and 3-year overall survival (OS) in CRC patients. A total of 3257 patients in the HER2 negative group and 79 in the HER2 positive group were followed up, and the follow-up rate was 97.7%. The results showed that there was no significant difference in OS rate between the HER2 positive and HER2 negative groups (p = 0.603, Fig. 2).
Fig. 2.
OS curve of colorectal cancer patients based on HER2 status
Discussion
The diagnostic criterion for HER2 positivity in CRC has not reached an agreement worldwide. The rate of HER2 positivity in CRC varied widely in different researches due to inconsistent diagnostic criteria [18].
In this study, we adopted the HERACLES criteria, which were proposed by Valtorta and his colleagues [19]. They established an archival test cohort which was conducted on formalin-fixed paraffin-embedded archival samples by three pathologists and concluded an agreement by collegial review and discussion, thus formulating a diagnostic algorithm for HER2 positivity in CRC, referred to as HERACLES Diagnostic Criteria. Then, the clinical validation cohort was established on KRAS 12/13 wild-type mCRC patients to validate the accuracy of this criteria.
HER2 expression in CRC can also be detected by molecular techniques except for IHC and ISH. Shimada Y et al. proved that comprehensive genomic sequencing (CGS) has the same utility as IHC and ISH for detecting HER2-positive CRC patients [20]. Nakamura Y et al. demonstrated that circulating tumor DNA (ctDNA) is an efficient marker for predicting HER2-amplified mCRC in clinical practice [21].
Some clinical trials had acquired promising results regarding HER2-targeted therapy in mCRC. The most well-known studies were HERACLES and MyPathway [22, 23]. HERACLES study preliminary proved that trastuzumab combined with lapatinib was effective in treating KRAS exon 2 wild type, HER2 positive mCRC (response rate was 30%, 4% for complete response, and 26% for partial response). MyPathway study showed that pertuzumab plus trastuzumab was well tolerated and effective in treatment-refractory HER2-amplified mCRC (response rate was 32%, 2% for complete response, and 30% for partial response). Grelly et al. summarized 9 completed or ongoing clinical trials concerning HER2-targeted therapy in mCRC [24]. Though HER2-targeted therapy showed promising results in mCRC patients, the prognostic role of HER2 in CRC remains unclear, further large cohort studies with stratified and long-term follow-up dates were required to elucidate this point.
Several previous studies also analyzed the association between HER2 status and pathological characteristics in CRC patients. Zhang et al. reported that HER2 status was significantly associated with T stage and TNM stage in CRC, but not associated with lymphovascular invasion [25]. Seo et al. reported that HER2 amplification was not associated with any pathological variables except tumor location in the rectum [26].
In conclusion, our study demonstrated that the rate of HER2 positive in CRC was relatively low. Moreover, HER2-positive CRC patients were more likely with vessel invasion than HER2-negative CRC patients. However, while had no significant influence on 3-year overall survival in CRC patients.
Author contribution
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Mingdian Wang, Xiang Wang, and Yiwei Li. The first draft of the manuscript was written by Maoguang Ma, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of FUSCC (Shanghai, China).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflict of interests
The authors declare no competing interests.
Footnotes
Mingdian Wang and Xiang Wang have contributed equally to this work.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xinxiang Li, Email: xinxiangli@fudan.edu.cn.
Maoguang Ma, Email: mamaoguang@shca.org.cn.
References
- 1.Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW, Jr, Leahy DJ. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756–760. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 2.Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469–6487. doi: 10.1038/sj.onc.1210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vranić S, Bešlija S, Gatalica Z. Targeting HER2 expression in cancer: new drugs and new indications. Bosn J Basic Med Sci. 2021;21:1–4. doi: 10.17305/bjbms.2020.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neve RM, Lane HA, Hynes NE. The role of overexpressed HER2 in transformation . Ann Oncol : Official J Euro Soc Med Oncol. 2001;12(Suppl 1):S9–13. doi: 10.1093/annonc/12.suppl_1.S9. [DOI] [PubMed] [Google Scholar]
- 5.Harbeck N, Gnant M. Breast cancer. Lancet (London, England) 2017;389:1134–1150. doi: 10.1016/S0140-6736(16)31891-8. [DOI] [PubMed] [Google Scholar]
- 6.Incorvati JA, Shah S, Mu Y, Lu J. Targeted therapy for HER2 positive breast cancer. J Hematol Oncol. 2013;6:38. doi: 10.1186/1756-8722-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boku N. HER2-positive gastric cancer. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2014;17:1–12. doi: 10.1007/s10120-013-0252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target, Annals of oncology : official journal of the European Society for. Med Oncol. 2008;19:1523–1529. doi: 10.1093/annonc/mdn169. [DOI] [PubMed] [Google Scholar]
- 9.Meric-Bernstam F, Johnson AM, Dumbrava EEI, Raghav K, Balaji K, Bhatt M, Murthy RK, Rodon J, Piha-Paul SA. Advances in HER2-targeted therapy: novel agents and opportunities beyond breast and gastric cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2019;25:2033–2041. doi: 10.1158/1078-0432.CCR-18-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunte S, Abraham J, Montero AJ. Novel HER2-targeted therapies for HER2-positive metastatic breast cancer. Cancer. 2020;126:4278–4288. doi: 10.1002/cncr.33102. [DOI] [PubMed] [Google Scholar]
- 11.Shitara K, Iwata H, Takahashi S, Tamura K, Park H, Modi S, Tsurutani J, Kadowaki S, Yamaguchi K, Iwasa S, Saito K, Fujisaki Y, Sugihara M, Shahidi J, Doi T. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive gastric cancer: a dose-expansion, phase 1 study. Lancet Oncol. 2019;20:827–836. doi: 10.1016/S1470-2045(19)30088-9. [DOI] [PubMed] [Google Scholar]
- 12.Tabernero J, Hoff PM, Shen L, Ohtsu A, Shah MA, Cheng K, Song C, Wu H, Eng-Wong J, Kim K, Kang YK. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018;19:1372–1384. doi: 10.1016/S1470-2045(18)30481-9. [DOI] [PubMed] [Google Scholar]
- 13.Doi T, Shitara K, Naito Y, Shimomura A, Fujiwara Y, Yonemori K, Shimizu C, Shimoi T, Kuboki Y, Matsubara N, Kitano A, Jikoh T, Lee C, Fujisaki Y, Ogitani Y, Yver A, Tamura K. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol. 2017;18:1512–1522. doi: 10.1016/S1470-2045(17)30604-6. [DOI] [PubMed] [Google Scholar]
- 14.Ross JS, Fakih M, Ali SM, Elvin JA, Schrock AB, Suh J, Vergilio JA, Ramkissoon S, Severson E, Daniel S, Fabrizio D, Frampton G, Sun J, Miller VA, Stephens PJ, Gay LM. Targeting HER2 in colorectal cancer: the landscape of amplification and short variant mutations in ERBB2 and ERBB3. Cancer. 2018;124:1358–1373. doi: 10.1002/cncr.31125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siena S, Sartore-Bianchi A, Marsoni S, Hurwitz HI, McCall SJ, Penault-Llorca F, Srock S, Bardelli A, Trusolino L. Targeting the human epidermal growth factor receptor 2 (HER2) oncogene in colorectal cancer. Annals of oncology : official journal of the European Society for Medical Oncology. 2018;29:1108–1119. doi: 10.1093/annonc/mdy100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sartore-Bianchi A, Lonardi S, Martino C, Fenocchio E, Tosi F, Ghezzi S, Leone F, Bergamo F, Zagonel V, Ciardiello F, Ardizzoni A, Amatu A, Bencardino K, Valtorta E, Grassi E, Torri V, Bonoldi E, Sapino A, Vanzulli A, Regge D, Cappello G, Bardelli A, Trusolino L, Marsoni S, Siena S. Pertuzumab and trastuzumab emtansine in patients with HER2-amplified metastatic colorectal cancer: the phase II HERACLES-B trial. ESMO open. 2020;5:e000911. doi: 10.1136/esmoopen-2020-000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawada K, Nakamura Y, Yamanaka T, Kuboki Y, Yamaguchi D, Yuki S, Yoshino T, Komatsu Y, Sakamoto N, Okamoto W, Fujii S. Prognostic and predictive value of HER2 amplification in patients with metastatic colorectal cancer. Clin Colorectal Cancer. 2018;17:198–205. doi: 10.1016/j.clcc.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 18.De Cuyper A, Van Den Eynde M, Machiels JP. HER2 as a predictive biomarker and treatment target in colorectal cancer. Clin Colorectal Cancer. 2020;19:65–72. doi: 10.1016/j.clcc.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Valtorta E, Martino C, Sartore-Bianchi A, Penaullt-Llorca F, Viale G, Risio M, Rugge M, Grigioni W, Bencardino K, Lonardi S, Zagonel V, Leone F, Noe J, Ciardiello F, Pinto C, Labianca R, Mosconi S, Graiff C, Aprile G, Frau B, Garufi C, Loupakis F, Racca P, Tonini G, Lauricella C, Veronese S, Truini M, Siena S, Marsoni S, Gambacorta M. Assessment of a HER2 scoring system for colorectal cancer: results from a validation study, Modern pathology : an official journal of the United States and Canadian Academy of Pathology. Inc. 2015;28:1481–1491. doi: 10.1038/modpathol.2015.98. [DOI] [PubMed] [Google Scholar]
- 20.Shimada Y, Yagi R, Kameyama H, Nagahashi M, Ichikawa H, Tajima Y, Okamura T, Nakano M, Nakano M, Sato Y, Matsuzawa T, Sakata J, Kobayashi T, Nogami H, Maruyama S, Takii Y, Kawasaki T, Homma KI, Izutsu H, Kodama K, Ring JE, Protopopov A, Lyle S, Okuda S, Akazawa K, Wakai T. Utility of comprehensive genomic sequencing for detecting HER2-positive colorectal cancer. Hum Pathol. 2017;66:1–9. doi: 10.1016/j.humpath.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura Y, Okamoto W, Kato T, Esaki T, Kato K, Komatsu Y, Yuki S, Masuishi T, Nishina T, Ebi H, Sawada K, Taniguchi H, Fuse N, Nomura S, Fukui M, Matsuda S, Sakamoto Y, Uchigata H, Kitajima K, Kuramoto N, Asakawa T, Olsen S, Odegaard JI, Sato A, Fujii S, Ohtsu A, Yoshino T. Circulating tumor DNA-guided treatment with pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer: a phase 2 trial. Nat Med. 2021;27:1899–1903. doi: 10.1038/s41591-021-01553-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, Zagonel V, Leone F, Depetris I, Martinelli E, Troiani T, Ciardiello F, Racca P, Bertotti A, Siravegna G, Torri V, Amatu A, Ghezzi S, Marrapese G, Palmeri L, Valtorta E, Cassingena A, Lauricella C, Vanzulli A, Regge D, Veronese S, Comoglio PM, Bardelli A, Marsoni S, Siena S. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17:738–746. doi: 10.1016/S1470-2045(16)00150-9. [DOI] [PubMed] [Google Scholar]
- 23.Meric-Bernstam F, Hurwitz H, Raghav KPS, McWilliams RR, Fakih M, VanderWalde A, Swanton C, Kurzrock R, Burris H, Sweeney C, Bose R, Spigel DR, Beattie MS, Blotner S, Stone A, Schulze K, Cuchelkar V, Hainsworth J. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019;20:518–530. doi: 10.1016/S1470-2045(18)30904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greally M, Kelly CM, Cercek A. HER2: an emerging target in colorectal cancer. Curr Probl Cancer. 2018;42:560–571. doi: 10.1016/j.currproblcancer.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Wu J, Wang L, Zhao H, Li H, Duan Y, Li Y, Xu P, Ran W, Xing X. HER2 and BRAF mutation in colorectal cancer patients: a retrospective study in Eastern China. PeerJ. 2020;8:e8602. doi: 10.7717/peerj.8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seo AN, Kwak Y, Kim DW, Kang SB, Choe G, Kim WH, Lee HS. HER2 status in colorectal cancer: its clinical significance and the relationship between HER2 gene amplification and expression. PLoS ONE. 2014;9:e98528. doi: 10.1371/journal.pone.0098528. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.