Introduction
Immune checkpoint inhibitors encompass a class of agents designed to target tumor- or lymphocyte-expressed programmed cell death-1 protein, programmed cell death-1 ligand, or cytotoxic T-lymphocyte-associated antigen 4 proteins.1,2 These agents are thought to block proteins involved in the downregulation of the immune response and as such have been implicated in the development of self-intolerance, leading to the development of autoimmune-like reactions.3 Pembrolizumab therapy specifically has been associated with cutaneous immunotherapy-related adverse events in up to 42% of patients.4 In one large prospective cohort study investigating cutaneous reactions of COVID-19 messenger ribonucleic acid (RNA) vaccines, 1.9% of the patients developed a cutaneous reaction after their first dose, and 2.3% of the patients did so after the second dose of COVID-19 vaccine. These reactions most commonly involved mild local injection-site reactions, urticarial eruptions, and morbilliform eruptions.5,6 It has been hypothesized that administration of vaccinations on a background of immunotherapy may drive the development of cutaneous eruptions by further unmasking the immune response.7
Here, we present a case of widespread cutaneous eruption after COVID-19 booster administration in the setting of pembrolizumab immunotherapy for the treatment of non-small cell lung cancer.
Case report
A 74-year-old woman with stage III non-small cell lung cancer diagnosed in 2015 was treated with lobectomy and cisplatin/pemetrexed and found to have disease recurrence 3 years after initial diagnosis. She was started on durvalumab therapy in 2018 but found to have disease progression. Tumor immunohistochemical analysis of programmed cell death-1 ligand expression was performed using a commercially available kit, revealing a positivity rate of 2%. In 2020, the patient was begun on a combination of a fixed dose of pembrolizumab 200 mg and pemetrexed 755 mg every 21 days, leading to maintenance of stable disease.
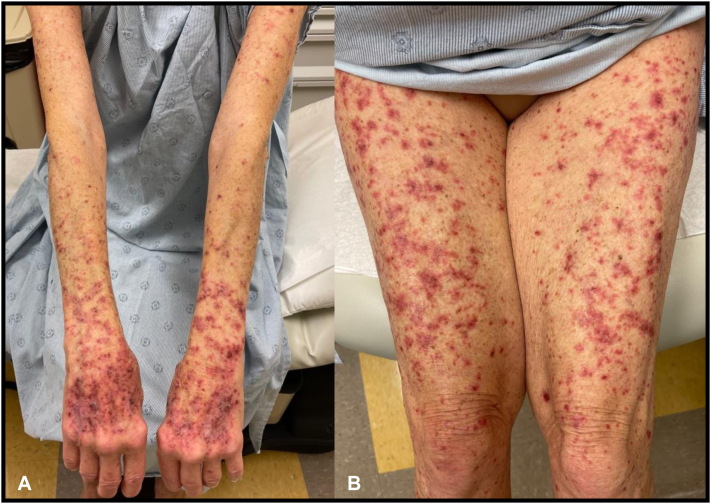
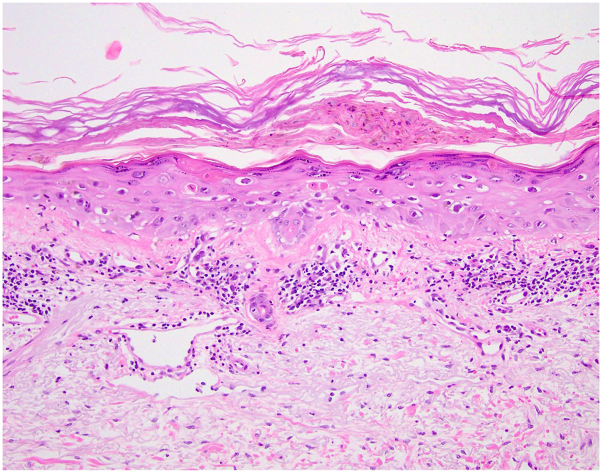
The patient received her first set of COVID vaccines (BioNTech-Pfizer COVID-19 RNA vaccines) in early 2021 (February and March of 2021) and 6 months later received her booster dose of the COVID vaccine. Three days after receiving the COVID-19 booster dose (BioNTech-Pfizer COVID-19 RNA vaccine), she presented with a mild pruritic rash on both forearms. Seven days after booster dose administration, the patient received her cycle 13 infusion of pembrolizumab/pemetrexed, leading to acute worsening and spreading of the cutaneous eruption. She had received the first and second doses of COVID-19 vaccinations 6-7 months previously, each 6.5 weeks apart and with no cutaneous or other adverse reactions. In the previous 10 months of immunotherapy treatment alone, the patient had not developed any cutaneous reactions to the pembrolizumab regimen. On examination, there were numerous 2-5–mm pink-purple papules on both forearms, the dorsal aspects of the hands, chest, legs, buttock, and lower back without any mucosal or palmoplantar involvement and with many of the lesions showing overlying hemorrhagic crust (Fig 1). The patient unsuccessfully trialed hydrocortisone 1% cream before seeing oncodermatology and was started on triamcinolone 0.1% cream applied to affected areas twice daily for 2 weeks. Pembrolizumab cycle 14 treatment was withheld in the interim. She noted mild improvement in those areas to which steroid cream was applied, but over the following 3 weeks, she noticed a pattern of mild resolution of old lesions alongside eruption of newly pruritic pink papules. Lesion development was characterized by a deep-red darkening of color with cessation of pruritus and sensation of warmth but with increased abundance of hemorrhagic crust at outer edges of lesions. Due to the lack of resolution, triamcinolone cream was discontinued, and the more potent clobetasol 0.05% cream was initiated for twice-daily application. A 4-mm punch biopsy was taken to delineate diagnosis and was consistent with parakeratosis with interface dermatitis and basal vacuolar degeneration, keratinocyte apoptosis, and epidermal necrosis with lichenoid reaction consistent with a lichenoid-like eruption (Fig 2). After 2 weeks of clobetasol cream treatment, significant improvement was noted. The rash improved significantly on the upper arms, forearms, dorsal aspects of the thighs, posterior aspects of the thighs, lower legs, and dorsal aspects of the feet and was particularly more confluent and much flatter with more violaceous hyperpigmentation noted. Over the next several weeks, the eruption completely cleared, and the patient’s combination regimen of pembrolizumab/pemetrexed was reinitiated with no further outbreaks of the cutaneous eruption.
Fig 1.
Clinical photographs of upper and lower extremities on initial examination. On exam, there were numerous 2-5mm pink-purple papules on the A) bilateral forearms, dorsal hands, and B) legs, many withoverlying hemorrhagic crust.
Fig 2.
A 4-mm punch biopsy was taken to delineate diagnosis and was consistent with epidermal necrosis with lichenoid reaction. There is a layer of epidermal necrosis sandwiched in between a basket-weave orthokeratosis and reactive epidermis beneath with scattered necrotic cells. There is also an interface mononuclear cell infiltrate with some pigment laden macrophages.
Discussion
Immunotherapy-related cutaneous adverse events have been widely reported in response to programmed cell death-1 protein–blocking agents. However, the incidence of immunotherapy-related adverse events on the background of COVID-19 vaccination has been much less characterized. To our knowledge, this is the first reported case in response to COVID-19 booster dose administration in the setting of pembrolizumab immunotherapy. Only one reported case highlighted worsening of a drug-induced delayed-type hypersensitivity reaction in the setting of COVID-19 vaccine and checkpoint inhibitor therapy (ipilimumab/nivolumab therapy).7 In the majority of cases, the Pfizer COVID-19 vaccine has been associated with only mild cutaneous eruptions; however, a handful of reports have recently showcased the development of more widespread findings like pityriasis lichenoides et varioliformis acuta.6,8 Furthermore, while pembrolizumab therapy alone has been associated with lichenoid-like eruptions, it does not typically present this late (cycle 13). Our case highlights the possibility of an interaction occurring between COVID-19 vaccines and checkpoint inhibitors specifically given the unique timeframe and onset of symptoms seen in this scenario. In our specific case, it is interesting that our patient did not develop any cutaneous reaction to either of the initial 2 vaccines alone, highlighting the possibility that a longer-term exposure to immunotherapy specifically may be required to unmask such a cutaneous response to vaccination. Although the mechanism driving this process has not been fully elucidated, it is believed that vaccine administration likely acts as an initial stimulant, with programmed cell death-1 protein blockade allowing for a more robust T-cell response to foreign antigens. Mechanistically, messenger RNA-based vaccines allow for expression of viral envelope particles in antigen-presenting cells, facilitating antigen-presenting cell activation of innate and adaptive immune system arms. The activation of epidermal-dermal resident T cells via activated antigen-presenting cells presenting viral envelope antigens in the setting of a non-attenuated immune response via checkpoint blockade may drive the severity of cutaneous immunotherapy-related adverse events in such cases.9,10 Overall, this case highlights an underreported phenomenon and emphasizes the importance of consideration by clinicians of timing of vaccine administration in the setting of checkpoint inhibitor therapy and the need to be on high alert for possible severe cutaneous reactions in the setting of concomitant vaccine administration and immunotherapy.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Shrimali R.K., Janik J.E., Abu-Eid R., Mkrtichyan M., Khleif S.N. Programmed death-1 & its ligands: promising targets for cancer immunotherapy. Immunotherapy. 2015;7(7):777–792. doi: 10.2217/imt.15.49. [DOI] [PubMed] [Google Scholar]
- 2.Collins L.K., Chapman M.S., Carter J.B., Samie F.H. Cutaneous adverse effects of the immune checkpoint inhibitors. Curr Probl Cancer. 2017;41(2):125–128. doi: 10.1016/j.currproblcancer.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Yoest J.M. Clinical features, predictive correlates, and pathophysiology of immune-related adverse events in immune checkpoint inhibitor treatments in cancer: a short review. Immunotargets Ther. 2017;6:73–82. doi: 10.2147/ITT.S126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanlorenzo M., Vujic I., Daud A., et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151(11):1206–1212. doi: 10.1001/jamadermatol.2015.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson L.B., Fu X., Hashimoto D., et al. Incidence of cutaneous reactions after messenger RNA COVID-19 vaccines. JAMA Dermatol. 2021;157(8):1000–1002. doi: 10.1001/jamadermatol.2021.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMahon D.E., Amerson E., Rosenbach M., et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J Am Acad Dermatol. 2021;85(1):46–55. doi: 10.1016/j.jaad.2021.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hussain K., Kawsar A., Weir J., et al. Severe cutaneous adverse reaction following COVID-19 vaccination and immunotherapy: a second hit? Clin Exp Dermatol. 2022;47(1):149–151. doi: 10.1111/ced.14852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dawoud N.M., Aslam H., Ali I.M., Dawoud M.M. The first case report of Pityriasis lichenoides chronica following COVID-19 mRNA vaccination. Dermatol Ther. 2022;35(6) doi: 10.1111/dth.15445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miao L., Zhang Y., Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer. 2021;20(1):41. doi: 10.1186/s12943-021-01335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniello L., Elshiaty M., Bozorgmehr F., et al. Therapeutic and prognostic implications of immune-related adverse events in advanced non-small-cell lung cancer. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.703893. [DOI] [PMC free article] [PubMed] [Google Scholar]