Abstract
Objectives
To describe the risk factors for SARS-CoV-2 infection in UK healthcare workers (HCWs).
Methods
We conducted a prospective sero-epidemiological study of HCWs at a major UK teaching hospital using a SARS-CoV-2 immunoassay. Risk factors for seropositivity were analysed using multivariate logistic regression.
Results
410/5,698 (7·2%) staff tested positive for SARS-CoV-2 antibodies. Seroprevalence was higher in those working in designated COVID-19 areas compared with other areas (9·47% versus 6·16%) Healthcare assistants (aOR 2·06 [95%CI 1·14-3·71]; p=0·016) and domestic and portering staff (aOR 3·45 [95% CI 1·07-11·42]; p=0·039) had significantly higher seroprevalence than other staff groups after adjusting for age, sex, ethnicity and COVID-19 working location. Staff working in acute medicine and medical sub-specialities were also at higher risk (aOR 2·07 [95% CI 1·31-3·25]; p<0·002). Staff from Black, Asian and minority ethnic (BAME) backgrounds had an aOR of 1·65 (95% CI 1·32 – 2·07; p<0·001) compared to white staff; this increased risk was independent of COVID-19 area working. The only symptoms significantly associated with seropositivity in a multivariable model were loss of sense of taste or smell, fever, and myalgia; 31% of staff testing positive reported no prior symptoms.
Conclusions
Risk of SARS-CoV-2 infection amongst HCWs is highly heterogeneous and influenced by COVID-19 working location, role, age and ethnicity. Increased risk amongst BAME staff cannot be accounted for solely by occupational factors.
Key words: SARS-CoV-2, COVID-19, sero-epidemiology, healthcare workers, risk factor analysis
Background
With >580 million cases and >6 million deaths reported to date globally, the ongoing COVID-19 pandemic continues to impact daily life (1). A nationwide lockdown in the UK on 23rd March 2020 succeeded in slowing infection rates during the “first wave” (2); however, subsequent waves and the emergence of novel dominant variants have continued to place unprecedented pressure on the NHS (3, 4) and drive infections globally (5, 6, 7). The logistics of managing patients with COVID-19 presented unique challenges to hospitals and NHS trusts across the UK; evidence and practices evolved rapidly as experience was gained. Healthcare workers (HCWs) are at a higher risk of SARS-CoV-2 infection than the general population (8, 9), and subsequent evidence has emerged for risk factors associated with SARS-CoV-2 infection in front-line HCWs (10, 11, 12, 13).
Protecting HCWs by identifying risk factors for SARS-CoV-2 infection will continue to be paramount3 as the UK accepts coronavirus as a common endemic disease. Controlling transmission within a hospital setting, as well as from hospitals back into the community, was a key element in controlling the pandemic (14, 15). However, defining HCW specific risk-factors remains a challenge. Additionally, higher rates of symptomatic SARS-CoV-2 infection, hospitalisation and death have been observed amongst patients from ethnic minority populations in the UK (16) and worldwide (17, 18); the reasons for this disparity are unclear. Reported infections in HCW suggests higher mortality in HCWs from minority backgrounds (19); however, it is not yet clear to what extent workplace exposures influence infection. Here, we present the results of a large sero-epidemiological study of SARS-CoV-2 seropositivity in staff at a teaching hospital in the East of England undertaken during the first wave of the COVID-19 pandemic.
Methods
Setting
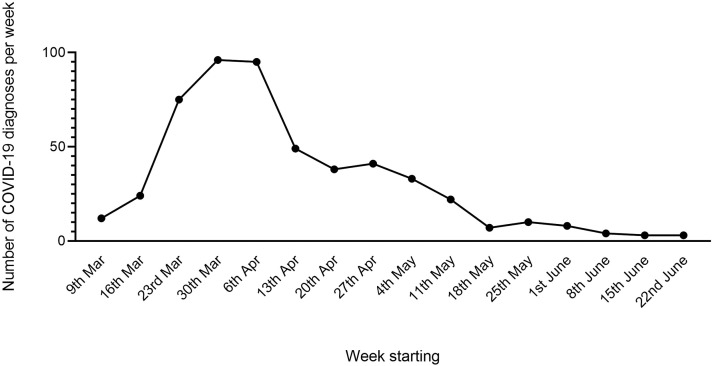
Cambridge University Hospitals NHS Foundation Trust (CUH) is a tertiary referral centre and teaching hospital with 1,000 beds and 11,545 staff serving a population of 580,000 people in the East of England. The facility is equipped with a 20-bed ICU, a 23-bed neurosciences and trauma ICU, and an Emergency Department that receives ∼14,000 attendees a month. Between March and June 2020, CUH treated 525 patients with PCR-confirmed COVID-19 (Figure 1 ). The peak of COVID-19 admissions occurred in late March and early April 2020, with comparatively few COVID-19 admissions from June 2020 onwards. The definition of COVID-19 working for the purpose of risk stratification included clinical areas designated as either “Red” (patients with PCR-confirmed SARS-CoV-2 infection) or “Amber” (patients for whom there is a high clinical suspicion of COVID-19).
Figure 1.
Epidemic curve of COVID-19 admissions at Cambridge University Hospitals NHS Foundation Trust
As of September 2020, the East of England reported 27,516 laboratory-confirmed cases of SARS-CoV-2 infection (20), with a corresponding population rate of 441·2 per 100,000 people as of September 2020. This rate was substantially less than the worst affected regions of North West England (772·9/100,000) and Yorkshire and the Humber (693·2/100,000) (20). According to the 2011 England and Wales census (21) 85·3% of the population of the East of England are White British, 5·5% are White Other, 4·8% are Asian, 2% are Black, and 1·9% are of Mixed ethnicity. The proportion of Black, Asian, and Minority Ethnic (BAME) staff employed at CUH is representative of the overall NHS workforce (22) (21·2% vs 20·7%, respectively).
An asymptomatic staff screening programme using SARS-CoV-2 PCR testing was established in April 2020 (23). A staff screening programme for SARS-CoV-2 serological testing was initiated on the 10th of June 2020. All staff members were invited by email to participate in the serological screening programme and asked to self-refer for a clinic appointment. Written informed consent was obtained from all participants enrolled into this study. As part of this process all participants were invited to join the NIHR BioResource – COVID-19 Research Cohort (IRAS 220277). At enrolment, participants completed a questionnaire asking about demographic characteristics, healthcare role, ethnicity, previous symptoms consistent with COVID-19 and previous results of SARS-CoV-2 PCR testing. A total of 7·8 ml of blood was collected, including one serum sample and one whole blood sample. The serum sample was assayed for total SARS-CoV-2 antibody; both residual serum and whole blood were stored for future analyses.
Laboratory assays
Serological testing for antibodies directed against SARS-CoV-2 was performed using the Centaur XP SARS-Cov-2 Total Antibody assay (Siemens Healthcare Limited, Surrey, UK). This method is a fully automated high throughput enzyme linked chemiluminescent bridging immunoassay which targets the S1RBD antigen of SARS-CoV-2 and can detect all Ig subclasses (IgG, IgM, and IgA). The quantity of SARS-CoV2 antibodies correlates directly with relative light units (RLU), which is converted to Index Values with a measuring interval of 0.05 ->10 index, where values below 1 are reported as nonreactive and those ≥1.0 are reported as reactive, as validated by the manufacturer by clinical correlation. The method was independently validated by Public Health England and has a reported sensitivity and specificity of 98.1% (95% CI 96.6 – 99.1) and 99.9% (95% CI 99.4 – 100) respectively. Samples were processed in the Biochemistry laboratory at CUH following the SOP as stated by the manufacturer in their Instruction for Use (IFU) after a local verification using guidance from The Royal College of Pathologists (24).
As previously described, the RT-PCR assay used at CUH designates a cycle threshold (Ct) of ≤36 to correspond to a positive result (23).
Statistical analysis
Seroprevalence is reported as a percentage ([proportion with antibodies/number tested] x 100). Logistic regression was used for univariable and multivariable analyses of seroprevalence comparisons. The Wilcoxon rank-sum test was used for comparison of median Ct values. Data were analysed using Stata v14.2 (StataCorp, College Station, Texas).
Results
Baseline information
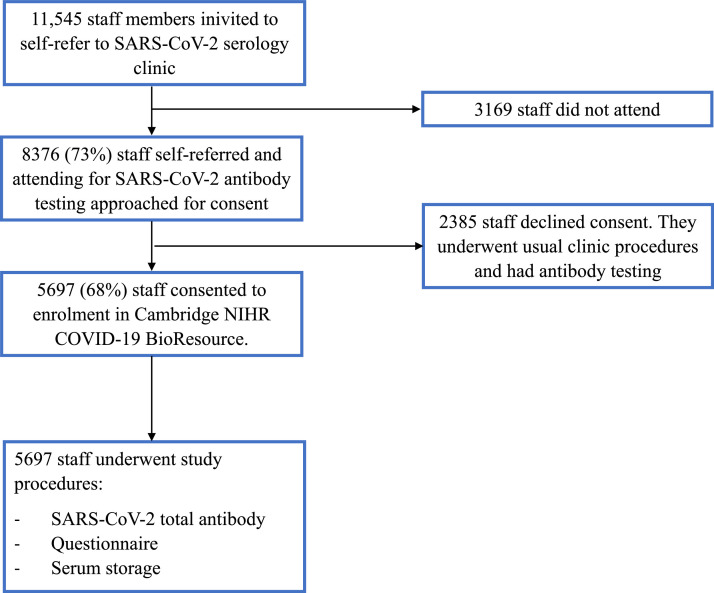
The CUH staff serology screening clinic was operational between 10th June and the 7th of August 2020. A total of 8,376 (73%) staff attended the clinic for SARS-CoV-2 serology; 5,697/8,376 (68%) of these consented to be enrolled in the study (Figure 2 ). 1,700/5,967 (28·5%) of study participants reported that they had worked in a designated COVID-19 area within the CUH structure during the peak of the epidemic between February and June 2020. The median age of participants was 38 years (range 17-83 years) and 22·7% (1,293/5,697) were male (Table 1 ). A total of 22 staff required hospital admission for COVID-19. No CUH staff members died.
Figure 2.
Study flowchart
Table 1.
Baseline demographics
| Baseline variable | Male | Female |
|---|---|---|
| n (%) | 1,293 (22.7) | 4,404 (77.3) |
| Age (median [IQR]) | 38 (30 – 49) | 38 (29 – 49) |
| Age bracket | ||
| 16 – 24 years | 66 | 360 |
| 25 – 34 years | 451 | 1427 |
| 35 – 44 years | 336 | 1035 |
| 45 – 54 years | 250 | 919 |
| 55 – 64 years | 166 | 555 |
| 65 – 74 years | 21 | 74 |
| 75 + years | 3 | 4 |
| COVID working (n, %) | 493 (38.1) | 1,291 (29.3) |
| Ethnicity | ||
| White (n, %) | 887 (68.6) | 3,580 (81.3) |
| BAME (all) (n, %) | 382 (29.5) | 752 (17.1) |
| Asian (n, %) | 276 (21.4) | 495 (11.2) |
| Black (n, %) | 30 (2.3) | 90 (2.0) |
| Chinese (n, %) | 21 (1.6) | 57 (1.3) |
| Mixed (n, %) | 21 (1.6) | 47 (1.1) |
| Other (n, %) | 34 (2.6) | 63 (1.4) |
| Not stated (n, %) | 24 (1.9) | 72 (1.6) |
Seroprevalence
The overall seroprevalence of total SARS-CoV-2 antibodies amongst all staff in this study was 7·2% (410/5,698). Amongst those reporting having worked in a dedicated COVID-19 area between February and June 2020, the seroprevalence increased to 9·5% (169/1,784; Table 2 ). Conversely, the comparable seroprevalence in those reporting they had never worked in COVID-19 area was 6·2% (241/3,913; p<0·0001). The prevalence of seropositivity in male staff (8·0%; 104/1293) was not significantly different (p=0·18) than that observed in female staff (6·95%; 306/4404). The risk of seropositivity decreased with age, with an odds ratio (OR) of 0·83 (95% CI 0·76 – 0·91) for every 10-year increase in age (p<0·0001).
Table 2.
Odds ratio (OR) and adjusted odds ratio (aOR) for variables associated with seropositivity
| Variable | Seropositivity n (%) | Unadjusted OR (96% CI) | p value | Adjusted OR (95% CI) | p value |
|---|---|---|---|---|---|
| No COVID working | 241/3913 (6.16) | 1 | - | 1 | - |
| COVID working | 169/1784 (9.47) | 1.59 (1.30 – 1.96) | <0.001 | 1.50 (1.22 – 1.84) | <0.001 |
| Female sex | 1 | - | 1 | - | |
| Male sex | 104/1293 (8.04) | 1.17 (0.93 – 1.47) | 0.18 | 1.10 (0.87 – 1.39) | 0.43 |
| Age | - | <0.001* | - | <0.001* | |
| Age 16-24 | 47/456 (10.3) | 1 | - | 1 | - |
| Age 25-34 | 152/1878 (8.1) | 0.77 (0.54 – 1.08) | 0.13 | 0.72 (0.51 – 1.02) | 0.069 |
| Age 35-44 | 102/1371 (7.4) | 0.70 (0.49 – 1.0) | 0.054 | 0.69 (0.48 – 0.99) | 0.044 |
| Age 45-54 | 71/1169 (6.1) | 0.56 (0.38 – 0.83) | 0.003 | 0.57 (0.38 – 0.83) | 0.004 |
| Age 55-64 | 32/721 (4.4) | 0.40 (0.25 – 0.64) | <0.001 | 0.44 (0.27 – 0.70) | 0.001 |
| Age 65-74 | 6/95 (6.3) | 0.59 (0.24 – 1.4) | 0.24 | 0.66 (0.27 – 1.59) | 0.34 |
| Age 75+ | 0/7 (0) | - | - | - | - |
| Job role | - | 0.0042* | - | 0.098* | |
| Administrative | 19/412 (4.61) | 1 | - | 1 | - |
| Nursing staff | 261 / 3471 (7.52) | 1.68 (1.04 – 2.71) | 0.033 | 1.52 (0.94 – 2.46) | 0.088 |
| Junior doctor | 10/118 (8.47) | 1.92 (0.87 – 4.24) | 0.11 | 1.43 (0.64 – 3.23) | 0.39 |
| Consultant | 10/174 (5.75) | 1.26 (0.57 – 2.77) | 0.56 | 1.19 (0.53 – 2.68) | 0.42 |
| Healthcare assistant | 36/319 (11.29) | 2.63 (1.48 – 4.68) | 0.001 | 2.06 (1.14 – 3.71) | 0.016 |
| Theatre staff | 3/24 (12.5) | 2.95 (0.81 – 10.78) | 0.10 | 2.40 (0.65 – 8.87) | 0.19 |
| Physiotherapist | 9/84 (10.71) | 2.48 (1.08 – 5.69) | 0.032 | 1.82 (0.78 – 4.24) | 0.16 |
| Domestic and porter | 4/27 (14.81) | 3.60 (1.13 – 11.44) | 0.030 | 3.45 (1.07 – 11.2) | 0.039 |
| Other | 58/1068 (5.43) | 1.19 (0.67 – 2.02) | 0.53 | 1.06 (0.62 – 1.80) | 0.85 |
| Ethnicity | - | - | <0.001* | - | <0.001* |
| White | 275/4467 (6.16) | 1 | - | 1 | - |
| Black | 22/120 (18.33) | 3.42 (2.12 – 5.52) | <0.001 | 3.42 (2.12 – 5.53) | <0.001 |
| Asian | 85/771 (11.02) | 1.89 (1.46 – 2.44) | <0.001 | 1.69 (1.30 – 2.19) | <0.001 |
| Chinese | 5/78 (6.41) | 1.04 (0.42 – 2.60) | 0.93 | 1.04 (0.42 – 2.60) | 0.94 |
| Mixed | 2/68 (2.94) | 0.46 (0.11 – 1.90) | 0.28 | 0.42 (0.10 – 1.71) | 0.23 |
| Other | 9/97 (9.28) | 1.56 (0.78 – 3.12) | 0.21 | 1.36 (0.68 – 2.74) | 0.38 |
| Not stated | 12/96 (12.5) | 2.18 (1.17 – 4.04) | 0.013 | 2.10 (1.13 – 3.90) | 0.019 |
aORs calculated using a multivariable model containing serostatus, age, sex, ethnicity, job role and COVID-working location.
*p value for the likelihood ratio test for the overall effect of variable
Occupation
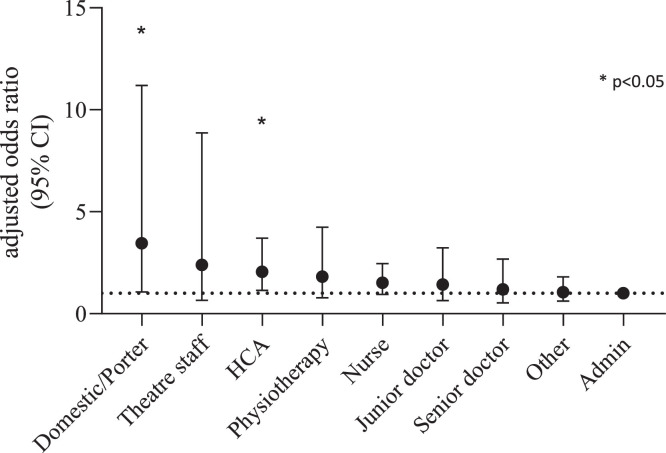
On univariate analysis, a number of HCW roles were associated with greater risk of the detection of SARS-CoV-2 antibodies. Nursing staff (OR 1·68 [95% CI 1·04 – 2·71]; p=0·033), healthcare assistants (HCAs) (OR 2·63 [95% CI 1·48 – 4·86]; p=0·001) physiotherapists (OR 2·48 [95% CI 1·08 – 5·69]; p=0·032) and porters and domestic staff (OR 3·60 [95% CI 1·13 – 11·44]; p=0·03) all displayed a higher risk compared to administrative staff (Table 2), who had the lowest seroprevalence at 4·6% (19/412). Security staff at CUH are employed by a third-party contractor and did not attend the staff serology testing clinic.
Department
Staff working specifically in the ICUs had a seroprevalence of 6·33% (10/158), and staff working specifically in the Emergency Department had a seroprevalence of 9·1% (9/99). However, neither of these staff groups had significantly different seropositivity using univariate analysis (p>0·1 in both groups) compared to non-ICU and non-Emergency Department staff respectively.
Ethnicity
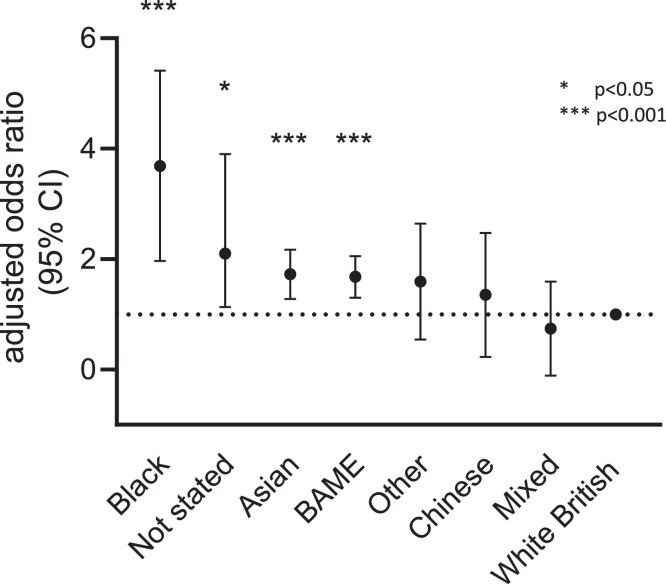
We observed substantial heterogeneity in the proportion of seropositivity between self-reported ethnic groups (Table 2). Staff identifying as White had an overall seropositivity rate of 6·1%. In comparison, Asian staff (Indian/Pakistani/Bangladeshi/Other Asian) and Black staff (Black African/Black Caribbean/Other black) had a seroprevalence of 11·0% (85/771) and 18·3% (22/120), respectively. White staff were more likely to have reported symptoms than Asian or Black staff (29%, 28% and 19% respectively). Despite this, seroconversion following symptoms consistent with COVID-19 was significantly higher in Black staff (p=0·002) and in Asian staff (p<0·001) compared to white staff; 41% (9/22), 26·6% (54/203), and 14·1% (148/1052) of staff had SARS-CoV-2 antibodies after reporting consistent symptoms in Black, Asian and White staff, respectively. The proportion of staff reporting having worked in a COVID-19 area was 38·5%, 60·3% and 32·1% for Black, Asian, and White staff, respectively.
Multivariable analyses
After describing several variables associated with SARS-CoV-2 seropositivity in a univariate analysis we included these variables to assess the risk associated with age, sex, ethnicity, job role and COVID-working status in a multivariable model. Increasing age remained protective for seropositivity on multivariable analysis (aOR 0·85 per 10 years increase in age [95% CI 0·78 – 0·93]; p<0·001). The aOR of having detectable SARS-CoV-2 antibodies in those that reported working in COVID-19 areas was 1·50 (95% CI 1·22 – 1·84; p<0·0001). Nursing staff and physiotherapists were no longer significantly associated with seropositivity on multivariable analysis (Table 2, Figure 3 ). HCAs remained at a significantly higher risk of being seropositive (aOR 2·06 [95% CI 1·14 – 3·71 – 2·4]; p=0·016), as were domestic and portering staff (aOR 3·45 [95% CI 1·07 – 11·2]; p=0·039).
Figure 3.
Adjusted odds ratio for SARS-CoV-2 seropositivity according to job role
Ethnicity remained strongly associated with seropositivity (Table 2, Figure 4 ). The aORs in Asian and black staff in the multivariable model were 1·69 (95% CI 1·30 – 2·19; p<0·0001) and 3·42 (95% CI 2·12 – 5·53; p<0·0001), respectively (Table 2). There was no significant evidence that the effect of ethnicity was modified by COVID working location (p value of interaction 0·96), and we also observed a similar increase in risk associated with ethnicity when data were stratified by CUH COVID-19 working location. For Asian staff, the aOR for seroconversion was 1·59 (95% CI 1·09 – 2·32; p=0·016) for those working in COVID-19 areas compared to 1·76 (95% CI 1·21 – 2·55; p=0·003) for those not working in COVID-19 areas. For black staff the aOR for seroconversion when working in COVID-19 areas was 3·91 (95% CI 1·78 – 8·59; p=0·001), compared to 3·06 (95% CI 1·65 – 5·64; p<0·001) who reported working in a non-COVID-19 area.
Figure 4.
Adjusted odds ratio for SARS-CoV-2 seropositivity according to ethnic group
The aOR for seropositivity in staff self-reporting as BAME (as a binary variable compared to white staff in a separate multivariable model) was 1·65 (95% CI 1·32 – 2·07; p<0·0001) after controlling for age, sex, job role and COVID-19 working location. For staff self-reporting as BAME, the aOR for seroconversion was 1·59 (95% CI 1·13 – 2·23; p=0·007) for those working in COVID-19 areas and 1·68 (95% CI 1·23 – 2·39; p=0·001) for those who reported not working in a COVID-19 area during the epidemic.
Symptoms
Participants were asked about any symptoms consistent with COVID-19 since February 2020. Critically, seroprevalence was significantly higher in the group reporting symptoms (17·2%; 266/1,548) in comparison to those without symptoms (3·1%; 117/3827, p<0·0001). Almost 31% (126/410) of seropositive HCWs reported not having any symptoms consistent with COVID-19 since February 2020. After adjusting for age, sex, and ethnicity, the aOR of seropositivity was 6·97 (95% CI 5·54 – 8·78; p<0·0001) in the group who reported prior symptoms compared to those who did not. The loss of the sense of taste or smell was the strongest predictor of seropositivity on univariate analysis; however, only 44% (154/351) of those reporting the loss of taste or smell were seropositive (Table 3 ). In a multivariable logistic regression model containing all collected symptoms (Table 3), loss of sense of taste or smell (aOR 7·85 [95% CI 5·79 – 10·65], p<0·0001), myalgia (aOR 1·71 [95% CI 1·18 – 2·48], p<0·0005) and fever (aOR 1·44 [95% CI 1·02 – 2·04], p<0·038) were the only symptoms positively associated with seropositivity. Notably, reporting a sore throat at the time of symptoms was negatively associated with seropositivity (aOR 0·7 [95% CI 0·50 – 0·99], p=0·043) in the multivariable model.
Table 3.
Unadjusted odds ratio (OR) and adjusted odds ratio (aOR) of SARS-CoV-2 seropositivity by reported symptoms
| Univariable |
Multivariable |
|||||||
|---|---|---|---|---|---|---|---|---|
| Symptom | Number reporting symptoms n, (%) | Antibody positive | Antibody negative | % positive | OR | p value | OR | p value |
| Loss of taste or smell | 351 (6.2) | 154 | 197 | 43.9 | 15.5 (12.2 – 19.9) | <0.001 | 10.70 (7.80 – 14.70) | <0.001 |
| Myalgia | 807 (14.2) | 166 | 641 | 20.6 | 4.9 (4.0 – 6.1) | <0.001 | 1.71 (1.18 – 2.48) | 0.005 |
| Fever | 740 (13.0) | 147 | 593 | 19.9 | 4.4 (3.60 – 5.51) | <0.001 | 1.44 (1.02 – 2.04) | 0.038 |
| Cough | 874 (15.3) | 154 | 720 | 17.6 | 3.82 (3.10 – 4.73) | <0.001 | 1.33 (0.93 – 1.90) | 0.11 |
| Headache | 847 (14.9) | 157 | 690 | 18.5 | 4.13 (3.34 – 5.12) | <0.001 | 1.32 (0.91 – 1.91) | 0.14 |
| Nausea/vomiting/diarrhoea | 330 (5.8) | 60 | 270 | 18.2 | 3.19 (2.36 – 4.30) | <0.001 | 1.08 (0.73 – 1.58) | 0.71 |
| Nasal Discharge | 453 (8.0) | 72 | 381 | 15.9 | 2.74 (2.08 – 3.61) | <0.001 | 0.82 (0.57 – 1.17) | 0.27 |
| Shortness of breath | 494 (8.7) | 85 | 409 | 17.2 | 3.12 (2.41 – 4.04) | <0.001 | 0.76 (0.51 – 1.13) | 0.18 |
| Hoarse voice | 314 (5.5) | 46 | 268 | 14.7 | 2.37 (1.70 – 3.29) | <0.001 | 0.75 (0.50 – 1.15) | 0.19 |
| Wheeze | 285 (5.0) | 46 | 239 | 16.1 | 2.67 (1.91 – 3.72) | <0.001 | 0.74 (0.47 – 1.17) | 0.20 |
| Sore throat | 806 (14.2) | 117 | 689 | 14.5 | 2.66 (2.12 – 3.35) | <0.001 | 0.70 (0.50 – 0.99) | 0.043 |
Seroconversion after positive SARS-CoV-2 PCR
From 5,991 enrolled participants, 2,825 (47%) reported having had a SARS-CoV-2 PCR test between February 2020 and the time of blood sampling, primarily through the CUH HCW testing programme. Of these, 51 (2·05%) tested PCR positive for SARS-CoV-2 RNA, 47 had detectable SARS-CoV-2 antibodies, and four had no detectable SARS-CoV-2 antibodies. All serological samples in these cases were taken >21 days after positive PCR tests. The median SARS-CoV-2 PCR Ct value in those who seroconverted was 30 (IQR 24 – 34), in comparison to 36 (IQR 35·5 – 37) in those who did not seroconvert (p=0·006). The four staff who had previously tested SARS-CoV-2 PCR positive and were antibody negative all reported having symptoms consistent with COVID-19 infection at the time of PCR testing, although none reported the loss of taste or smell. Nine (18%) of the staff previously testing SARS-CoV-2 PCR positive, and who were antibody positive, were asymptomatic at the time of PCR testing.
Discussion
In this comprehensive assessment of factors associated with seropositivity for SARS-CoV-2 antibodies in HCWs at a large UK tertiary referral centre we were able to identify key at-risk occupational groups. Specifically, staff working in areas where patients with confirmed SARS-CoV-2 infection are cared for, those employed as HCA or domestic and portering staff, those of younger age, and those working in acute medicine or a medical sub-speciality were more likely to have SARS-CoV-2 antibodies. A reduced risk of SARS-CoV-2 seropositivity was associated with White ethnicity, being employed in an administrative role, and belonging to an older age group.
We found that the seroprevalence of SARS-CoV-2 antibodies in staff working in non-COVID facing areas was slightly higher (6·16%) than in the general population in the East of England (5·0%) (25) and comparable to the national prevalence (6·0%) (25). This is in keeping with previous retrospective serological HCW studies reporting relatively low seroprevalences in Germany (1·6%) (26), Wuhan (3·8%) (27) and Belgium (7·6%) (28). Amongst Asian staff working at CUH the seroprevalence was also comparable to East of England data (10·5% vs 10·1%, respectively), as was the seroprevalence amongst Black staff at CUH compared to regional data (18% vs 15%, respectively) (25). Overall, we observed significantly higher seroprevalence in all BAME staff compared to White staff, and to a greater extent in Black and Asian staff specifically. These differences have been observed nationally and are not unique to HCWs. The finding that the increased risk associated with BAME staff was not influenced by COVID-19 area working (and independent of job role), as well as the ethnic differences in symptomatic seroconversion rates, demonstrates that the increased prevalence of antibodies in BAME HCWs cannot be accounted for by purely occupational factors.
In staff who were previously SARS-CoV-2 PCR positive, 92% (47/51) had detectable antibodies. There was a significant difference in Ct values between those who did and did not seroconvert. A potential explanation for this difference is that higher viral loads may be required to generate a sustained antibody response (29). Alternatively, a false positive SARS-CoV-2 PCR result or the detection of viral nucleic acid without infectious virus would also explain a lack of seroconversion.
Consistent with previous studies, we demonstrate that whilst reporting prior symptoms consistent with COVID-19 increased the chances of seropositivity, differentiating previous COVID-19 infection from other common respiratory tract infections based on symptoms alone is unreliable (10). specifically, the only symptoms that significantly predicted seropositivity on a multivariable logistic regression model were the loss of sense of taste or smell, myalgia and fever. Prior reporting of cough or shortness of breath were not good predictors of the presence of SARS-CoV-2 antibodies in a multivariable model. These data also reiterate previous findings that asymptomatic SARS-COV-2 infection amongst healthcare workers is common, with 31% of seropositive staff having never reported consistent symptoms, and 18% of PCR positive staff never having reported consistent symptoms. Our data highlight the importance of the contribution of the asymptomatically infected population to the spread of the disease(30, 31). Consequently, asymptomatic screening of staff in healthcare settings became a major component of routine disease surveillance (23, 32), and is likely to be of benefit in future waves associated with novel variants and in future pandemics.
The development and widespread rollout of mRNA vaccines since this study was conducted has resulted in lower re-infection rates and symptomatic disease in HCWs in the UK, and elsewhere (33, 34). However, the understanding of HCW infection risk remains critical in the protection of HCWs to novel variants (and vaccine escape), as well as those unable to receive vaccination.
We acknowledge several limitations to our study. Variables such as ethnicity, COVID-working location and job role were self-reported; however, we have no reason to think these variables were party to recall bias and it is unlikely to impact on the results to any large degree. The proportion of staff reporting being of Black ethnicity was relatively small, although the proportion of BAME staff is consistent with the wider NHS workforce, and our conclusions are therefore broadly generalisable. The terminology and designation of COVID-facing clinical areas was an evolving factor throughout the course of the epidemic and is likely to be variable between hospital trusts and regions, as will the re-distribution of workforces and workflows through hospitals. Additionally, there will have been heterogeneity in infection rates and admission pressures between different regions and between different hospitals within the same regions that may influence HCW exposure to infection differently. Consequently, this variation between practices may impact the specific risk factors assessed in this study to varying extents between different healthcare trusts. We were also unable to assess the use of PPE and adherence to PPE protocols in this study design. The selected assay may have reduced sensitivity in individuals who generated robust antibody responses to other SARS-COV-2 antigens or those producing low affinity antibodies during early disease. Similar considerations apply to other commercial assays (35), and a subsequent comparison demonstrated assay equivalence with the selected platform having higher accuracy (36), and an independently reported sensitivity and specificity of 98·1% (95% CI 96·6 – 99·1) and 99·1% (95% CI 99·4 – 100) respectively (36). We also note that symptom data were recorded retrospectively and may have been subject to recall bias.
Conclusions
Our study confirms prior findings and provides new information on the risk factors for SARS-COV-2 infection and antibody response in HCWs. We found that the occupational exposure to SARS-COV-2 is heterogenous across job roles, hospital department, and ethnicity. It is clear that HCWs who remain on the frontline of the COVID-19 pandemic require more protection from occupational exposure with accurate stratification of risk factors to develop mitigation strategies despite effective vaccines. The association with ethnic group is concerning and a deeper understanding of the societal and/or genetic factors predisposing the BAME population to SARS-COV-2 infection and seroconversion is needed.
Declarations
Ethical approval
Ethical approval for this study was granted by the East of England – Cambridge Central Research Ethics Committee (IRAS ID: 220277).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding
SGB, IGG and MPW are funded by Wellcome Senior Fellowships (Grant ID 215515/Z/19/Z, 207498/Z/17/Z, 108070/Z/15/Z). DJC and SL received funding for this work from Addenbrooke's Charitable Trust (Grant ID 900254). MET is supported by the Academy of Medical Sciences, the Health Foundation and the NIHR Cambridge Biomedical Research Centre. BW is funded by the National Institute for Health Research Cambridge Biomedical Research Centre at the Cambridge University Hospitals NHS Foundation Trust.
Authors’ Contributions
DJC and SL contributed to study design, data collection, analysis, data interpretation, figures and tables, literature review and wrote the first draft of the manuscript. LW contributed to data collection and analysis and data interpretation. AS and MF contributed to study design, data collection, analysis and data interpretation. RB, KS, MPW, BW, DS, MJ, LR, MR, AC, KD, MM, AL, GG, OO, EW, OS, KT, RT, IH, DH, SH, SP, NK, KS, BG, NW and HS contributed to data collection and analysis and data interpretation. DDA and SS contributed to data analysis and interpretation and writing. JB, MET and GD contributed to data collection, analysis and data interpretation. IGG and SGB contributed to study design, data collection, analysis, data interpretation, figures and tables, literature review and contributed to the first draft of the manuscript. All authors reviewed and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests
Acknowledgements
We thank NIHR BioResource volunteers for their participation, and gratefully acknowledge NIHR BioResource centres, NHS Trusts and staff for their contribution. We thank the National Institute for Health Research, NHS Blood and Transplant, and Health Data Research UK as part of the Digital Innovation Hub Programme. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. The authors also thank the Occupational Health Department at CUH for facilitating sample and data collection during the staff serology testing clinic.
References
- 1.World Health Organisation. Coronavirus Disease (COVID-2019) Situation Reports. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed September 23rd 2020.
- 2.Anderson RM, Hollingsworth TD, Baggaley RF, Maddren R, Vegvari C. COVID-19 spread in the UK: the end of the beginning? Lancet. 2020;396(10251):587–590. doi: 10.1016/S0140-6736(20)31689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UK Health Security Agency. COVID-19 Variants: Genomically Conformed Case Numbers [Available from: https://www.gov.uk/government/publications/covid-19-variants-genomically-confirmed-case-numbers.
- 4.Office for national statistics. COVID-19 Latest Insights. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19latestinsights/overview. Accessed August 2022.
- 5.Lin L, Zhao Y, Chen B, He D. Multiple COVID-19 waves and vaccination effectiveness in the United States. Int J Environ Res Public Health. 2022;19(4) doi: 10.3390/ijerph19042282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viana R, Moyo S, Amoako DG, Tegally H, Scheepers C, Althaus CL, et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature. 2022;603(7902):679–686. doi: 10.1038/s41586-022-04411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volz E, Mishra S, Chand M, Barrett JC, Johnson R, Geidelberg L, et al. Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature. 2021;593(7858):266–269. doi: 10.1038/s41586-021-03470-x. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen LH, Drew DA, Graham MS, Joshi AD, Guo CG, Ma W, et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5(9):e475–ee83. doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez-Ochoa SA, Franco OH, Rojas LZ, Raguindin PF, Roa-Diaz ZM, Wyssmann BM, et al. COVID-19 in healthcare workers: a living systematic review and meta-analysis of prevalence, risk factors, clinical characteristics, and outcomes. Am J Epidemiol. 2020 doi: 10.1093/aje/kwaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eyre DW, Lumley SF, O'Donnell D, Campbell M, Sims E, Lawson E, et al. Differential occupational risks to healthcare workers from SARS-CoV-2 observed during a prospective observational study. Elife. 2020;9 doi: 10.7554/eLife.60675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shields A, Faustini SE, Perez-Toledo M, Jossi S, Aldera E, Allen JD, et al. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax. 2020 doi: 10.1136/thoraxjnl-2020-215414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimcheff DE, Schildhouse RJ, Hausman MS, Vincent BM, Markovitz E, Chensue SW, et al. Seroprevalence of severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2) infection among VA healthcare system employees suggests higher risk of infection when exposed to SARS-CoV-2 outside of the work environment. Infect Control Hosp Epidemiol. 2020:1–25. doi: 10.1017/ice.2020.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bandyopadhyay S, Baticulon RE, Kadhum M, Alser M, Ojuka DK, Badereddin Y, et al. Infection and mortality of healthcare workers worldwide from COVID-19: a systematic review. BMJ Glob Health. 2020;5(12) doi: 10.1136/bmjgh-2020-003097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Lancet COVID-19: protecting health-care workers. Lancet. 2020;395(10228):922. doi: 10.1016/S0140-6736(20)30644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Zhou M, Liu F. Reasons for healthcare workers becoming infected with novel coronavirus disease 2019 (COVID-19) in China. J Hosp Infect. 2020;105(1):100–101. doi: 10.1016/j.jhin.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sapey E, Gallier S, Mainey C, Nightingale P, McNulty D, Crothers H, et al. Ethnicity and risk of death in patients hospitalised for COVID-19 infection in the UK: an observational cohort study in an urban catchment area. BMJ Open Respir Res. 2020;7(1) doi: 10.1136/bmjresp-2020-000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vahidy FS, Nicolas JC, Meeks JR, Khan O, Pan A, Jones SL, et al. Racial and ethnic disparities in SARS-CoV-2 pandemic: analysis of a COVID-19 observational registry for a diverse US metropolitan population. BMJ Open. 2020;10(8) doi: 10.1136/bmjopen-2020-039849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020;382(26):2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kursumovic E, Lennane S, Cook TM. Deaths in healthcare workers due to COVID-19: the need for robust data and analysis. Anaesthesia. 2020;75(8):989–992. doi: 10.1111/anae.15116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gov.uk Coronavirus . September 2020. (COVID-19) in the UK. Cases in United Kingdowm.https://coronavirus.data.gov.uk/cases Available at. Acessed 23rd. [Google Scholar]
- 21.Office for National Statistics . 2011. UK census.https://www.ons.gov.uk/census/2011census Available at: Accessed 23rd September 2020. [Google Scholar]
- 22.Gov.uk. NHS Workforce. Available at: https://www.ethnicity-facts-figures.service.gov.uk/workforce-and-business/workforce-diversity/nhs-workforce/latest. Accessed 23rd September 2020. .
- 23.Rivett L, Sridhar S, Sparkes D, Routledge M, Jones NK, Forrest S, et al. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. Elife. 2020;9 doi: 10.7554/eLife.58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Royal College of Pathologists . 2020. Verification and Validation Methodology and Sample Sets for Evaluation of Assays for SARS-CoV-2 (COVID-19)https://www.rcpath.org/uploads/assets/541a4523-6058-4424-81c119dd2ab0febb/Verification-validation-of-sample-sets-assays-SARS-CoV-2.pdf Available here. Accessed 23rd. [Google Scholar]
- 25.Ward H, Atchison C, Whitaker M, Ainslie KEC, Elliott J, Okell L, et al. SARS-CoV-2 antibody prevalence in England following the first peak of the pandemic. Nat Commun. 2021;12(1):905. doi: 10.1038/s41467-021-21237-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korth J, Wilde B, Dolff S, Anastasiou OE, Krawczyk A, Jahn M, et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128 doi: 10.1016/j.jcv.2020.104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu X, Sun J, Nie S, Li H, Kong Y, Liang M, et al. Seroprevalence of immunoglobulin M and G antibodies against SARS-CoV-2 in China. Nat Med. 2020;26(8):1193–1195. doi: 10.1038/s41591-020-0949-6. [DOI] [PubMed] [Google Scholar]
- 28.Steensels D, Oris E, Coninx L, Nuyens D, Delforge ML, Vermeersch P, et al. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA. 2020;324(2):195–197. doi: 10.1001/jama.2020.11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun J, Tang X, Bai R, Liang C, Zeng L, Lin H, et al. The kinetics of viral load and antibodies to SARS-CoV-2. Clin Microbiol Infect. 2020;26(12):1690 e1-e4. doi: 10.1016/j.cmi.2020.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med. 2020;173(5):362–367. doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu X, Yang R. COVID-19 transmission through asymptomatic carriers is a challenge to containment. Influenza Other Respir Viruses. 2020;14(4):474–475. doi: 10.1111/irv.12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall VJ, Foulkes S, Charlett A, Atti A, Monk EJM, Simmons R, et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN) Lancet. 2021;397(10283):1459–1469. doi: 10.1016/S0140-6736(21)00675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall VJ, Foulkes S, Saei A, Andrews N, Oguti B, Charlett A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397(10286):1725–1735. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malhotra S, Mani K, Lodha R, Bakhshi S, Mathur VP, Gupta P, et al. COVID-19 infection, and reinfection, and vaccine effectiveness against symptomatic infection among health care workers in the setting of omicron variant transmission in New Delhi, India. Lancet Reg Health Southeast Asia. 2022;3 doi: 10.1016/j.lansea.2022.100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Public Health England . 2020. Evaluation of Sensitivity and Specificity of Four Commercially Available SARS-CoV-2 Antibody Immunoassays.https://www.gov.uk/government/publications/covid-19-head-to-head-laboratory-evaluation-of-4-commercial-serological-assays Available at: [Google Scholar]
- 36.National S-C-SAEG. Performance characteristics of five immunoassays for SARS-CoV-2: a head-to-head benchmark comparison. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.