Abstract
We aimed to verify the frailty status and the factors associated with the change in frailty status during the COVID-19 pandemic. A three-wave cohort study was conducted every six months, from May to July 2020, November 2020 to January 2021, and again from May to July 2021. The frailty status was assessed using the frailty screening index. Multivariate generalized linear mixed-effects models were used to determine whether changes in frailty status were associated with health conditions and lifestyle. The 404 survey forms were analyzed. Decline in chewing function (beta = 0.552) and leg muscle strength weakness (beta = 0.515) were significantly associated with the change in frailty status over six months, and leg muscle strength weakness (beta = 0.512) was significantly associated over 12 months. Risk factors associated with worsening health should be assessed for appropriate support. It is especially important to assess subjective leg muscle weakness in older adults.
Keywords: Frailty, Frailty transition, Community-dwelling, COVID-19
Introduction
Coronavirus disease 2019 (COVID-19) continues to spread worldwide, affecting the health and lifestyle of people everywhere. Many older adults have experienced immense lifestyle changes due to societal countermeasures taken to prevent the spread of COVID-19. Lifestyle changes such as social distancing and refraining from going out may result in increased frailty among older adults. Going forward, this frailty will be referenced as ‘corona-frailty’.1 Because Japan is a hyper-aged society, understanding COVID-19’s impact on frailty among older populations is important. However, the actual conditions and countermeasures for frailty have not been sufficiently clarified because the impact of COVID-19 has not yet diminished, and surveys in the community have been restricted by infection control.
Following the lockdown measures taken due to the COVID-19 pandemic, there was a significant decline in physical activity among cognitively healthy older adults.2 The COVID-19 pandemic has affected the frequency of and opportunities for exercise. Subsequently, activities have decreased in older adults with frailty or disease.3 Physical activity based on an online survey and assessed using an accelerometer sensor declined significantly during the COVID-19 pandemic.4 , 5 In addition to physical activity, life-space mobility substantially decreases, and restrictions on living space are associated with a negative impact on quality of life.6 Moreover, psychological distress increases7 as self-reported mental health declines significantly.8 The COVID-19 pandemic has also caused changes in lifestyle and behavior. Nutritional status and sleep patterns worsened in vulnerable older adults during the COVID-19 lockdown.9 Meal size decreases with increased frailty, and opportunities to talk with others decrease, regardless of frailty status.10 Approximately one-third of older adults cancelled their appointments for medical care during the early stage of the COVID-19 pandemic.11 These findings highlight the secondary impact of the COVID-19 pandemic on older adults. Most of these impacts are related to physical activity, mental health, lifestyle, behavior, and quality of life.
So far, we have indicated the factors associated with frailty transitions based on a two-wave cohort study as an interim report in our three-wave cohort study.12 This finding verified that subjective leg weakness could help identify frailty transitions among non-frail older adults. Our protocol was implemented at the beginning of the COVID-19 pandemic in Japan.13 Freid and colleagues14 suggested the original assessment of frailty before the COVID-19 pandemic. This assessment involves a measurement of grip strength and walking speed. However, due to COVID-19 countermeasures, we could not use this assessment. Hence, the frailty screening index (FSI) developed by Yamada and Arai15 was used. Clarifying the actual conditions and factors associated with changes in frailty status among community-dwelling older adults may indicate which older adults need specialized support. Moreover, social care services may be developed to prevent vulnerable conditions for current and future pandemics. We hypothesised that factors other than leg weakness are associated with changes in frailty status. Based on a three-wave cohort study conducted during the COVID-19 pandemic, this cohort study aimed to verify the actual longitudinal changes in frailty status every six months and its factors among community-dwelling older adults with frailty and leg weakness.
Methods
Participants and procedure
This prospective cohort study was conducted in Takasaki City, Gunma Prefecture, Japan. The participants were 1,953 community dwelling older adults (≥ 65 years) who resided in local housing and received the survey forms distributed by the local volunteers and community general support centres. Moreover, care home residents were excluded from the studies involving community-dwelling older adults living at home without staff support. The baseline survey was conducted from 11 May to 10 July 2020. The six-month follow-up survey was carried out from 11 November 2020 to 10 January 2021 and the 12-month follow-up survey was conducted from 11 May to 10 July 2021. The instructions for this study, as well as a self-reported questionnaire, were distributed via post in compliance with social distancing requirements. If a person agreed to participate, they recorded wrote their names on the survey form and returned them to us by post. Items regarding age, sex, morbidity, and living arrangements were included in the survey. The participants selected their morbidity from the list, including hypertension, osteoporosis, dyslipidemia, diabetes mellitus, osteoarthritis, etc. To indicate the dramatic changes in frailty during the COVID-19 pandemic, more short interval period surveys should be conducted. However, considering the burden on the participant and distribution, the survey was conducted every six months.
This study was approved by the Research Ethics Committee of Takasaki University of Health and Welfare (approval number: 2009) and registered with the University Hospital Medical Information Network (UMIN000040335).
Measurements
The study examined frailty status and progression using the frailty screening index (FSI).15 The predictive validity for disability and concurrent validity for social frailty in the FSI have been confirmed.15 , 16 The frailty status was determined based on the FSI score. Scores of ≥3, 1 to 2, and 0 were defined as frail, pre-frail, and robust, respectively.15
Two types of self-administered questionnaires were used to assess health conditions and lifestyle. The first was the questionnaire for medical checkup of old-old (QMCOO)17 which assesses health conditions and lifestyles comprehensively and has criterion-related validity with frailty.18 To avoid overburdening older adults, it included only 15 items. Because the method for assessing frailty status using the QMCOO has not yet been established, the FSI was applied to determine frailty status.
The QMCOO can assess health conditions in normal periods during non-pandemic phases, and does not require to answer conditions or changes affected by the countermeasures for COVID-19 in the questionnaire. Therefore, it could not assess recent changes due to COVID-19 countermeasures. Therefore, the second was the questionnaire for change for life (QCL), comprising five items to assess the impact of COVID-19 countermeasures on changes related to health conditions and lifestyle.10 The answer options used a 5-point Likert scale to make it easier for older adults to answer. The participants were asked about subjective changes in the last month due to COVID-19 countermeasures. Each item was scored using the following scale: increased or stronger = 1, slightly increased or stronger = 2, unchanged = 3, slightly decreased or weaker = 4, and decreased or weaker = 5. Only items about worry or anxiety had the following scores: decreased = 1, slightly decreased = 2, unchanged = 3, slightly increased = 4, and increased = 5.
Statistical analyses
Demographic variables, QMCOO, and QCL at baseline are presented as frequencies and percentages. Multimorbidity was defined as the presence of greater than one chronic disease,19 and was determined using morbidities selected from the list. The metric used in this study was the change in frailty status from baseline to six- and 12-month follow-up.
Age, sex, multimorbidity, living arrangements, and items of the QMCOO and QCL were verified using univariate generalized linear mixed-effects models for each change in frailty status, improvement, stability, and worsening, followed by pairwise comparisons. Three items of the QMCOO, namely, items 6, 7, and 9, were also included in the FSI. Therefore, they were excluded from the analysis. Multivariate generalized linear mixed-effects models were used to determine whether changes in frailty status were associated with health conditions and lifestyles. The change in frailty status was the dependent variable. Demographic variables and each item score in the QMCOO and the QCL were independent variables if these were associated with a p-value less than 0.1 with the change in frailty status based on univariate models. In univariate and multivariate generalized linear mixed-effects models, a binomial distribution with a logit link was specified for change in frailty status, and random intercepts were allowed for frailty status at baseline. Generalized linear mixed effects models were chosen as they can deal with ordinal scaled changes in frailty status and avoid Type I errors due to by-participant analysis.20 In multivariate generalized linear mixed-effects models, missing data were confirmed to be completely missing at random and imputed using multiple imputation techniques. A chained equations procedure was employed for five imputed datasets, which was similar to the percentage of incomplete cases or five sets, and the results were summarized using the standard Rubin's rule.21 Moreover, standardized regression coefficients (beta) were calculated to evaluate the impact, independent of the unit of variables.
Statistical analyses were performed using R version 4.1.2, with p < 0.05, indicating statistical significance.
Results
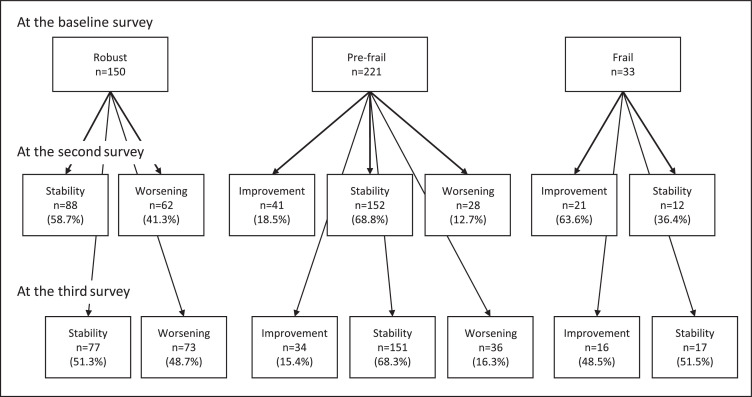
A flowchart of the participants is shown in Figure 1 . In the baseline survey, 1,217 participants completed the survey forms (62.3%). Considering the baseline, six- and 12-month surveys, 434 older adults returned the survey forms. In total, 404 older adults who could assess frailty status using the FSI were analyzed. The follow-up rates were 34.2% among robust, 34.2% among pre-frail, and 25.2% among frail participants. The mean interval between the baseline and the second and third survey was 184.9±17.4 and 364.0±16.0 days, respectively. According to the baseline survey, 151 participants were robust, 221 were pre-frail, and 33 were frail, accounting for 37.3%, 54.6%, and 8.1% of the total survey population, respectively. Among robust participants at baseline, worsening meant transition to pre-frail or frail (n=62 [41.3%] in the second survey, n=73 [48.7%] in the third survey). Among pre-frail at baseline, improvement meant transition to robust (n=41 [18.5%] in the second survey, n=34 [15.4%] in the third survey) and worsening meant transition to frail (n=28 [12.7%] in the second survey, n=36 [16.3%] in the third survey). Among frail at baseline, improvement meant transition to pre-frail or robust (n=21 [63.6%] in the second survey and n=16 [48.5%] in the third survey). These are shown in Figure 2 .
Fig. 1.
Flow diagram of the participants enrolled in the study.
Fig. 2.
The number of the change in frailty status in each survey.
Table 1 presents the baseline characteristics according to the change in frailty status at baseline to the second or third survey. In the baseline and the second survey, the QMCOO scores revealed statistically significant differences in eating difficulties and leg muscle strength (p < 0.001), except for age. Further, the QMCOO helped also us identify significant differences in the occurrences of choking on tea or soup events and leg muscle strength—except for age—(p = 0.027 and p < 0.001, respectively) in the baseline and the third survey.
Table 1.
The baseline characteristics according to change in frailty status at baseline to the second or third survey.
| Total n=404 | 6 months |
12 months |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Improvement n=62 | Stabilty n=252 | Worsening n=90 | p-value | Improvement n=50 | Stabilty n=245 | Worsening n=109 | p-value | ||||||||||
| Age, mean ± SD (years) | 78.8 ± 5.9 | 78.8 ± 6.1 | 78.7 ± 6.0 | 79.4 ± 5.8 | 0.018 | 78.1 ± 5.9 | 79.1 ± 6.1 | 78.6 ± 5.6 | 0.005 | ||||||||
| Female, n (༅) | 312 | (77.2) | 45 | (72.6) | 198 | (78.6) | 69 | (76.7) | 0.253 | 38 | (76.0) | 187 | (76.3) | 87 | (76.7) | 0.767 | |
| Multimorbidity, n (%) | 70 | (17.3) | 12 | (19.4) | 39 | (15.5) | 19 | (21.1) | 0.168 | 10 | (20.0) | 37 | (15.1) | 23 | (21.1) | 0.344 | |
| Living, n (%) | 0.891 | 0.484 | |||||||||||||||
| With Cohabitant | 90 | (22.7) | 12 | (20.0) | 60 | (23.9) | 18 | (20.9) | 12 | (24.0) | 54 | (22.4) | 24 | (20.9) | |||
| Alone | 307 | (77.3) | 48 | (80.0) | 191 | (76.1) | 68 | (79.1) | 38 | (76.0) | 187 | (77.6) | 82 | (79.1) | |||
| the Questionnaire for medical checkup of the old-old | |||||||||||||||||
| 1 | How is your health condition?, n (%) | 0.337 | 0.532 | ||||||||||||||
| 1. Excellent | 81 | (20.4) | 9 | (14.8) | 53 | (21.5) | 19 | (21.1) | 4 | (8.0) | 56 | (23.4) | 21 | (21.1) | |||
| 2. Good | 107 | (27.0) | 14 | (23.0) | 72 | (29.3) | 21 | (23.3) | 15 | (30.0) | 66 | (27.6) | 26 | (23.3) | |||
| 3. Fair | 176 | (44.3) | 29 | (47.5) | 103 | (41.9) | 44 | (48.9) | 26 | (52.0) | 90 | (37.7) | 60 | (48.9) | |||
| 4. Poor | 32 | (8.1) | 8 | (13.1) | 18 | (7.3) | 6 | (6.7) | 5 | (10.0) | 26 | (10.9) | 1 | (6.7) | |||
| 5. Very poor | 1 | (0.3) | 1 | (1.6) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 1 | (0.4) | 0 | (0.0) | |||
| 2 | Are you satisfied with your daily life?, n (%) | 0.725 | 0.328 | ||||||||||||||
| 1. Satisfied | 127 | (31.9) | 17 | (27.4) | 82 | (33.2) | 28 | (31.5) | 10 | (20.0) | 84 | (34.9) | 33 | (31.5) | |||
| 2. Moderately satisfied | 221 | (55.5) | 33 | (53.2) | 136 | (55.1) | 52 | (58.4) | 29 | (58.0) | 128 | (53.1) | 64 | (58.4) | |||
| 3. Moderately dissatisfied | 37 | (9.3) | 7 | (11.3) | 24 | (9.7) | 6 | (6.7) | 7 | (14.0) | 23 | (9.5) | 7 | (6.7) | |||
| 4. Dissatisfied | 13 | (3.3) | 5 | (8.1) | 5 | (2.0) | 3 | (3.4) | 4 | (8.0) | 6 | (2.5) | 3 | (3.4) | |||
| 3 | Do you eat three times a day? (No), n (%) | 19 | (4.7) | 0 | (0.0) | 15 | (6.0) | 4 | (4.4) | 0.999 | 1 | (2.0) | 11 | (4.5) | 7 | (4.4) | 0.270 |
| 4 | Do you have any difficulties eating tough foods compared to 6 months ago? (Yes), n (%) | 114 | (28.3) | 11 | (17.7) | 85 | (33.9) | 18 | (20.0) | <0.001 | 15 | (30.0) | 72 | (29.5) | 27 | (20.0) | 0.314 |
| 5 | Have you choked on your tea or soup recently? (Yes), n (%) | 88 | (21.9) | 12 | (19.4) | 57 | (22.8) | 19 | (21.3) | 0.135 | 8 | (16.0) | 58 | (24.0) | 22 | (21.3) | 0.027 |
| 8 | Have you experienced a fall in the past year? (Yes), n (%) | 91 | (22.6) | 15 | (24.2) | 57 | (22.6) | 19 | (21.3) | 0.128 | 10 | (20.0) | 62 | (25.4) | 19 | (21.3) | 0.050 |
| 10 | Do your family or friends point out your memory loss? (e.g., “You ask the same question over and over again.”) (Yes), n (%) | 29 | (7.2) | 6 | (9.7) | 17 | (6.8) | 6 | (6.8) | 0.544 | 5 | (10.0) | 18 | (7.4) | 6 | (6.8) | 0.717 |
| 11 | Do you find yourself not knowing today's date? (Yes), n (%) | 72 | (18.0) | 11 | (17.7) | 41 | (16.3) | 20 | (22.7) | 0.305 | 9 | (18.0) | 42 | (17.3) | 21 | (22.7) | 0.447 |
| 12 | Do you smoke?, n (%) | (100.0) | 0.763 | 0.271 | |||||||||||||
| 1. No, I do not smoke | 343 | (85.8) | 52 | (83.9) | 214 | (85.9) | 77 | (86.5) | 44 | (89.8) | 209 | (86.0) | 90 | (86.5) | |||
| 2. I quit smoking | 35 | (8.8) | 7 | (11.3) | 21 | (8.4) | 7 | (7.9) | 4 | (8.2) | 22 | (9.1) | 9 | (7.9) | |||
| 3. Yes, I smoke | 22 | (5.5) | 3 | (4.8) | 14 | (5.6) | 5 | (5.6) | 1 | (2.0) | 12 | (4.9) | 9 | (5.6) | |||
| 13 | Do you go out at least once a week? (No), n (%) | 26 | (6.5) | 5 | (8.1) | 17 | (6.8) | 4 | (4.5) | 0.513 | 5 | (10.0) | 15 | (6.1) | 6 | (4.5) | 0.994 |
| 14 | Do you keep regular communication with your family and friends? (No), n (%) | 17 | (4.2) | 5 | (8.1) | 11 | (4.4) | 1 | (1.1) | 0.877 | 3 | (6.0) | 11 | (4.5) | 3 | (1.1) | 0.619 |
| 15 | When you are not feeling well, do you have anyone you can talk with? (No), n (%) | 15 | (3.7) | 4 | (6.5) | 9 | (3.6) | 2 | (2.2) | 0.865 | 4 | (8.0) | 7 | (2.9) | 4 | (2.2) | 0.463 |
| the Questionnaire for Change of Life | |||||||||||||||||
| 1 | Amount of daily movement | 0.152 | 0.895 | ||||||||||||||
| 1. Increased | 3 | (0.7) | 0 | (0.0) | 3 | (1.2) | 0 | (0.0) | 0 | (0.0) | 3 | (1.2) | 0 | (0.0) | |||
| 2. Slightly increased | 12 | (3.0) | 2 | (3.2) | 4 | (1.6) | 6 | (6.7) | 1 | (2.0) | 6 | (2.5) | 5 | (6.7) | |||
| 3. Unchanged | 207 | (51.5) | 30 | (48.4) | 128 | (51.0) | 49 | (55.1) | 21 | (42.0) | 121 | (49.8) | 65 | (55.1) | |||
| 4. Slightly decreased | 112 | (27.9) | 19 | (30.6) | 66 | (26.3) | 27 | (30.3) | 17 | (34.0) | 64 | (26.3) | 31 | (30.3) | |||
| 5. Decreased | 68 | (16.9) | 11 | (17.7) | 50 | (19.9) | 7 | (7.9) | 11 | (22.0) | 49 | (20.2) | 8 | (7.9) | |||
| 2 | Leg muscle strength | <0.001 | <0.001 | ||||||||||||||
| 1. Stronger | 2 | (0.5) | 0 | (0.0) | 2 | (0.8) | 0 | (0.0) | 0 | (0.0) | 2 | (0.8) | 0 | (0.0) | |||
| 2. Slightly stronger | 3 | (0.7) | 1 | (1.6) | 2 | (0.8) | 0 | (0.0) | 0 | (0.0) | 3 | (1.2) | 0 | (0.0) | |||
| 3. Unchanged | 202 | (50.1) | 27 | (43.5) | 127 | (50.4) | 48 | (53.9) | 24 | (48.0) | 115 | (47.1) | 63 | (53.9) | |||
| 4. Slightly weaker | 152 | (37.7) | 24 | (38.7) | 95 | (37.7) | 33 | (37.1) | 20 | (40.0) | 94 | (38.5) | 38 | (37.1) | |||
| 5. Weaker | 44 | (10.9) | 10 | (16.1) | 26 | (10.3) | 8 | (9.0) | 6 | (12.0) | 30 | (12.3) | 8 | (9.0) | |||
| 3 | Meal size | 0.184 | 0.442 | ||||||||||||||
| 1. Increased | 1 | (0.2) | 0 | (0.0) | 1 | (0.4) | 0 | (0.0) | 0 | (0.0) | 1 | (0.4) | 0 | (0.0) | |||
| 2. Slightly increased | 23 | (5.5) | 5 | (8.1) | 13 | (5.2) | 5 | (5.6) | 3 | (6.0) | 13 | (5.3) | 7 | (5.6) | |||
| 3. Unchanged | 313 | (77.8) | 44 | (71.0) | 198 | (78.6) | 71 | (79.8) | 36 | (72.0) | 192 | (78.7) | 85 | (79.8) | |||
| 4. Slightly decreased | 59 | (14.6) | 11 | (17.7) | 35 | (13.9) | 13 | (14.6) | 10 | (20.0) | 34 | (13.9) | 15 | (14.6) | |||
| 5. Decreased | 7 | (1.7) | 2 | (3.2) | 5 | (2.0) | 0 | (0.0) | 1 | (2.0) | 4 | (1.6) | 2 | (0.0) | |||
| 4 | Worry or anxiety | 0.903 | 0.526 | ||||||||||||||
| 1. Decreased | 1 | (0.3) | 0 | (0.0) | 0 | (0.0) | 1 | (1.1) | 0 | (0.0) | 1 | (0.4) | 0 | (1.1) | |||
| 2. Slightly decreased | 8 | (2.0) | 3 | (4.8) | 4 | (1.6) | 1 | (1.1) | 1 | (2.0) | 6 | (2.5) | 1 | (1.1) | |||
| 3. Unchanged | 215 | (53.8) | 27 | (43.5) | 137 | (54.4) | 51 | (57.3) | 23 | (46.0) | 126 | (51.6) | 66 | (57.3) | |||
| 4. Slightly increased | 144 | (36.0) | 28 | (45.2) | 86 | (34.1) | 30 | (33.7) | 20 | (40.0) | 90 | (36.9) | 34 | (33.7) | |||
| 5. Increased | 32 | (8.0) | 4 | (6.5) | 22 | (8.7) | 6 | (6.7) | 5 | (10.0) | 19 | (7.8) | 8 | (6.7) | |||
| 5 | Opportunities to talk to people | 0.142 | 0.199 | ||||||||||||||
| 1. Increased | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | |||
| 2. Slightly increased | 4 | (1.0) | 1 | (1.6) | 2 | (0.8) | 1 | (1.1) | 0 | (0.0) | 2 | (0.8) | 2 | (1.1) | |||
| 3. Unchanged | 151 | (37.6) | 27 | (43.5) | 83 | (33.1) | 41 | (46.1) | 22 | (44.0) | 89 | (36.6) | 40 | (46.1) | |||
| 4. Slightly decreased | 126 | (31.3) | 17 | (27.4) | 81 | (32.3) | 28 | (31.5) | 16 | (32.0) | 70 | (28.8) | 40 | (31.5) | |||
| 5. Decreased | 121 | (30.1) | 17 | (27.4) | 85 | (33.9) | 19 | (21.3) | 12 | (24.0) | 82 | (33.7) | 27 | (21.3) | |||
Multimorbidity was considered when a participant had more than one chronic disease. Three items of the Questionnaire for medical checkup of the old-old; item 6, 7, and 9, were excluded from analysis because these were the same as the items of the Frailty Screening Index.
After some variables which were associated with a p-value less than 0.1 with change based on univariable generalized linear mixed effects models were entered into multivariable generalized linear mixed effects models. The change in frailty status during six months was the dependent variable. Age, difficulty eating hard food in the QMCOO, and leg muscle strength in the QCL were the independent variables. Five imputed datasets were used because there were three incomplete cases (0.7%). Difficulty in eating (beta = 0.552, 95% confidence interval [CI] = 0.187-0.917) and leg muscle strength (beta = 0.515, 95% CI = 0.111-0.918) were significantly associated with changes in frailty status (Table 2 ). Positive coefficients (beta) indicated that poor responses to the independent variables tended to worsen the frailty status. For the change in frailty status over 12 months, age, choking on tea or soup, fall in the past year in the QMCOO, and leg muscle strength in the QCL were independent variables. Five imputed datasets were used because there were four incomplete cases (1.0%). Leg muscle strength alone was significantly associated with changes in frailty status (β = 0.512, 95% CI = 0.099-0.925, Table 2).
Table 2.
Results of multivariable generalized linear mixed models for change in frailty status during 6 and 12 months.
| 6 months |
12 months |
||||
|---|---|---|---|---|---|
| Standardized regression coefficients | (95%CI) | Standardized regression coefficients | (95%CI) | ||
| Age | 0.265 | (-0.079 - 0.610) | 0.331 | (-0.037 - 0.699) | |
| the Questionnaire for medical checkup of the old-old | |||||
| 4 Do you have any difficulties eating tough foods compared to 6 months ago? | 0.552⁎⁎ | (0.187 - 0.917) | - | ||
| 5 Have you choked on your tea or soup recently? | - | 0.319 | (-0.076 - 0.715) | ||
| 8 Have you experienced a fall in the past year? | - | 0.174 | (-0.204 - 0.552) | ||
| the Questionnaire for Change of Life | |||||
| 2 Leg muscle strength | 0.515* | (0.111 - 0.918) | 0.512* | (0.099 - 0.925) | |
CI: confidence interval
p<0.05;
p<0.01
Discussion
To the best of our knowledge, this is the first survey conducted every six months after the initial constraints due to the COVID-19 pandemic. As the survey was conducted specifically to comply with social distancing, follow-up rates were low. Even with the lowered response rate, this study may have the potential to enhance the existing literature on frailty conditions among community-dwelling older adults.
One of our purposes was to attempt to identify factors associated with changes in frailty status. Many previous studies have positively and significantly associated older age with frailty.22 Among frail older adults, the prevalence of multimorbidity is 72%.23 The novelty of this study is that it revealed associations between decline in chewing function, subjective leg muscle weakness, and change in frailty status during six or 12 months; these were not in relation to age or multimorbidity. Oral health is positively associated with frailty.24 Frail older adults had significantly poorer oral function, such as oral bite force and masseter muscle thickness, than pre-frail and robust adults.25 A longitudinal study indicated that oral frailty strongly predicts physical frailty.26 This indicates that poor oral health is associated with a higher risk of worsening, and a lower chance of improvement, in frailty status.27 In the current study, chewing was particularly associated with changes in frailty status in this study. A decline in chewing ability can result in malnutrition, which devolves into sarcopenia and, eventually, frailty.28 Subjective leg muscle weakness alone, was associated with a change in frailty status over six and 12 months. A multimodal approach, including a combined intervention of nutrition and exercise, is recommended to improve muscle mass and strength through resistance training, especially in pre-frailty or frailty.29 , 30 The prevalence of frailty, or worse frailty levels increase with higher levels of sedentary time, and reductions in sedentary levels are needed to prevent frailty.31 Leg stability is crucial for mobility and daily tasks such as walking, which can help prevent inadvertent sedentary behaviour. Sarcopenia may be accompanied by leg muscle weakness and muscle mass loss. Therefore, one of the main contributors to physical frailty is leg muscle weakness. In the current study, the unique feature was that the evaluation of leg muscle strength was based on the participant's report and not on actual measurements. If muscle strength could not be measured, reported subjective feelings about muscle weakness would be an important predictor of vulnerability.
We must use this opportunity to further develop health and social care services to improve care for community-dwelling older adults.32 Since 2020, repeated waves of COVID-19 have consistently affected the survey areas of this study in Japan. With each wave, older adults were required to restrict and modify their lifestyle and engagement in physical activities. Consequently, in Japan, changes in lifestyle and decreased physical activity are the observable side effects of countermeasures to prevent the COVID-19 spread.3 , 5 , 9 The COVID-19 crisis should be utilized as an opportunity to review care models by devoting sufficient time and resources to the needs of frail older adults in a variety of settings.33 Therefore, vulnerable subjects who need support from others can and should be properly supported by experts, supporters, and the administration as soon as possible. Although the factors associated with frailty have been suggested by previous studies, they should be assessed by social care service experts, supporters, and administrations among community-dwelling older adults to prevent vulnerable conditions for current and future infection pandemics.
This study has several limitations. First, the follow-up rate in this study was low. This study may have underestimated or overestimated the impact of the pandemic on frailty. Its number of frail participants was particularly low. Participants might not have returned their survey forms because of difficulties with poor mobility or comprehension. In this case, the results obtained were from participants in good condition and might lack severe conditions for frailty. Thus, these findings should be treated as a preliminary report. Second, the subjects were older adults who could return the survey form. An attempt was made to collect maximum sociodemographic information; however, it may not have been sufficient to define the survey population. Furthermore, cognitive deficits in participants were not adequately considered although the participants who foresaw cognitive decline were 7.2% or 18.0% based on the item 10 or 11 of QMCOO. Third, during the COVID-19 pandemic, the infection situation changed dramatically in a short period of time, and frailty status could have changed in tandem. The 3 wave, every six months, survey in a year would have been lengthy. Finally, we could not quantify the actual lifestyle changes that resulted from societal countermeasures designed to curb the spread of COVID-19. Furthermore, among the analysed participants could be older adults who stayed home because of pre-existing health issues or did not like going out in the first place, leaving the impact of the COVID-19 pandemic to remain unclear.
Conclusion
This cohort study was carried out every six months beginning in May 2020 and terminating in July 2021, as this was the first year of the COVID-19 pandemic in Japan. Approximately 41.1% and 48.3% of robust older adults transitioned to pre-frail or frail in the six- and 12-month follow-up surveys respectively. Among the population that was pre-frail at baseline, 13-18% worsened or improved over the course of the study. Among frail participants, 48.5% and 63.6% improved at the six- and 12-month follow-up, respectively. Decline in chewing function and subjective leg muscle strength weakness were significantly associated with the change in frailty status in the six-month follow-up surveys, and subjective leg muscle strength weakness was still significantly associated in the 12-month follow-up surveys. The current study identified risk factors for identifying vulnerable community-dwelling older adults using questionnaires, which are also useful tools for identifying those in need of help and interventions during a pandemic.
Public health practitioners have a better chance of preventing and improving frailty if they are equipped with knowledge of the factors associated with its prediction. Future studies should determine whether the relationship between chewing function, leg muscle weakness, and changes in frailty status was unique to during the pandemic. As this three-wave cohort study was conducted during the COVID-19 pandemic, the next cohort study is expected to be conducted and validated during a non-pandemic period.
Author contributions
Tomoyuki Shinohara: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript. Kosuke Saida: acquisition of data and interpretation of data. Shigeya Tanaka and Akihiko Murayama: Critical revision of the manuscript for important intellectual content. Daisuke Higuchi: analysis and interpretation of data, critical revision of the manuscript for important intellectual content.
Funding
The parts of this work are supported by the Nippon Life Insurance Foundation (Grant 2020-0203-04) and the Japan Society for the Promotion of Science KAKENHI (Grant 19K19712).
Declaration of competing interest
The authors have no declarations of interest.
Acknowledgements
We would like to express our sincere gratitude to Kenichi Sudo, Munehisa Sudo, Ayako Yamazaki, Junko Ishii, Norie Torizuka, Kumi Aoki, Yumi Ino, Miyuki Ogawa, Seiichi Asanuma, Izumi Tsu-tsumi, Susumu Shimomura, Yuriko Yoshiara, Kazuaki Kuwabara, Nobuko Kaseda, Masaaki Arai, Ryo Koike, Chieko Mesaki, and all district welfare commissioners who cooperated with us in Takasaki. This study was supported by the Nippon Life Insurance Foundation (Grant 2020-0203-04) and the Japan Society for the Promotion of Science KAKENHI (Grant 19K19712).
References
- 1.Shinohara T, Saida K, Tanaka S, Murayama A. Rapid Response: Impact of the COVID-19 pandemic on frailty in the elderly citizen; corona-frailty. BMJ. 2020;369:m1543. doi: 10.1136/bmj.m1543. [DOI] [PubMed] [Google Scholar]
- 2.Salman D, Beaney T, E Robb C, et al. Impact of social restrictions during the COVID-19 pandemic on the physical activity levels of adults aged 50-92 years: a baseline survey of the CHARIOT COVID-19 Rapid Response prospective cohort study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2021-050680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawamura K, Kamiya M, Suzumura S, et al. Impact of the coronavirus disease 2019 outbreak on activity and exercise levels among older patients. J Nutr Health Aging. 2021;25:921–925. doi: 10.1007/s12603-021-1648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernández-García ÁI, Marin-Puyalto J, Gómez-Cabello A, et al. Impact of the Home Confinement Related to COVID-19 on the Device-Assessed Physical Activity and Sedentary Patterns of Spanish Older Adults. Biomed Res Int. 2021 doi: 10.1155/2021/5528866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamada M, Kimura Y, Ishiyama D, et al. The Influence of the COVID-19 pandemic on physical activity and new incidence of frailty among initially non-frail older people in japan: a follow-up online survey. J Nutr Health Aging. 2021;25:751–756. doi: 10.1007/s12603-021-1634-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saraiva MD, Apolinario D, Avelino-Silva TJ, et al. The impact of frailty on the relationship between life-space mobility and quality of life in older adults during the COVID-19 pandemic. J Nutr Health Aging. 2021;25:440–447. doi: 10.1007/s12603-020-1532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Fu P, Li J, et al. Changes in psychological distress before and during the COVID-19 pandemic among older adults: the contribution of frailty transitions and multimorbidity. Age Ageing. 2021;50:1011–1018. doi: 10.1093/ageing/afab061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pérez LM, Castellano-Tejedor C, Cesari M, Inouye SK. Depressive Symptoms, Fatigue and Social Relationships Influenced Physical Activity in Frail Older Community-Dwellers during the Spanish Lockdown due to the COVID-19 Pandemic. Int J Environ Res Public Health. 2021;18:808. doi: 10.3390/ijerph18020808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machón M, Mateo-Abad M, Vrotsou K, et al. Health status and lifestyle habits of vulnerable, community-dwelling older people during the COVID-19 lockdown. J Frailty Aging. 2021;10:286–289. doi: 10.14283/jfa.2021.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shinohara T, Saida K, Tanaka S, Murayama A. Association between frailty and changes in lifestyle and physical or psychological conditions among older adults affected by the coronavirus disease 2019 countermeasures in Japan. Geriatr Gerontol Int. 2021;21:39–42. doi: 10.1111/ggi.14092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuster NA, de Breij S, Schaap LA, et al. Older adults report cancellation or avoidance of medical care during the COVID-19 pandemic: results from the Longitudinal Aging Study Amsterdam. Eur Geriatr Med. 2021;12:1075–1083. doi: 10.1007/s41999-021-00514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shinohara T, Saida K, Tanaka S, Murayama A, Higuchi D. Transition to frailty in older Japanese people during the coronavirus disease 2019 pandemic: a prospective cohort study. Arch Gerontol Geriatr. 2022;98 doi: 10.1016/j.archger.2021.104562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinohara T, Saida K, Tanaka S, Murayama A. Do lifestyle measures to counter COVID-19 affect frailty rates in elderly community dwelling? Protocol for cross-sectional and cohort study. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-040341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 15.Yamada M, Arai H. Predictive value of frailty scores for healthy life expectancy in community-dwelling older Japanese Adults. J Am Med Dir Assoc. 2015;16 doi: 10.1016/j.jamda.2015.08.001. 1002.e7–11. [DOI] [PubMed] [Google Scholar]
- 16.Yamada M, Arai H. Social frailty predicts incident disability and mortality among community-dwelling japanese older adults. J Am Med Dir Assoc. 2018;19:1099–1103. doi: 10.1016/j.jamda.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Satake S, Arai H. Questionnaire for medical checkup of old-old (QMCOO) Geriatr Gerontol Int. 2020;20:991–992. doi: 10.1111/ggi.14004. [DOI] [PubMed] [Google Scholar]
- 18.Shinohara T, Saida K, Tanaka S, Murayama A, Higuchi D. Construct validity of the questionnaire for older senior citizens based on a confirmatory factor analysis: a study during the period of self-restraint to prevent the spread of coronavirus disease 2019. Geriatr Gerontol Int. 2021;21:1018–1025. doi: 10.1111/ggi.14285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. Multimorbidity, technical series on safer primary care. accessed 25 May 2022. https://www.who.int/publications/i/item/multimorbidity.
- 20.Murayama K, Sakaki M, Yan VX, Smith GM. Type I error inflation in the traditional by-participant analysis to metamemory accuracy: a generalized mixed-effects model perspective. J Exp Psychol Learn Mem Cogn. 2014;40:1287–1306. doi: 10.1037/a0036914. [DOI] [PubMed] [Google Scholar]
- 21.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 22.Feng Z, Lugtenberg M, Franse C, et al. Risk factors and protective factors associated with incident or increase of frailty among community-dwelling older adults: A systematic review of longitudinal studies. PLoS One. 2017;12 doi: 10.1371/journal.pone.0178383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vetrano DL, Palmer K, Marengoni A, et al. Frailty and multimorbidity: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci. 2019;74:659–666. doi: 10.1093/gerona/gly110. [DOI] [PubMed] [Google Scholar]
- 24.Tôrres LH, Tellez M, Hilgert JB, Hugo FN, de Sousa MD, Ismail AI. Frailty, frailty components, and oral health: a systematic review. J Am Geriatr Soc. 2015;63:2555–2562. doi: 10.1111/jgs.13826. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe Y, Hirano H, Arai H, et al. Relationship between frailty and oral function in community-dwelling elderly adults. J Am Geriatr Soc. 2017;65:66–76. doi: 10.1111/jgs.14355. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka T, Takahashi K, Hirano H, et al. Oral frailty as a risk factor for physical frailty and mortality in community-dwelling elderly. J Gerontol A Biol Sci Med Sci. 2018;73:1661–1667. doi: 10.1093/gerona/glx225. [DOI] [PubMed] [Google Scholar]
- 27.Slashcheva LD, Karjalahti E, Hassett LC, Smith B, Chamberlain AM. A systematic review and gap analysis of frailty and oral health characteristics in older adults: A call for clinical translation. Gerodontology. 2021;38:338–350. doi: 10.1111/ger.12577. [DOI] [PubMed] [Google Scholar]
- 28.Frailty Morley JE.Oral. J Nutr Health Aging. 2020;24:683–684. doi: 10.1007/s12603-020-1438-9. [DOI] [PubMed] [Google Scholar]
- 29.Jadczak AD, Makwana N, Luscombe-Marsh N, Visvanathan R, Schultz TJ. Effectiveness of exercise interventions on physical function in community-dwelling frail older people: an umbrella review of systematic reviews. JBI Database System Rev Implement Rep. 2018;16:752–775. doi: 10.11124/JBISRIR-2017-003551. [DOI] [PubMed] [Google Scholar]
- 30.Mareschal J, Genton L, Collet TH, Graf C. Nutritional intervention to prevent the functional decline in community-dwelling older adults: a systematic review. Nutrients. 2020;12:2820. doi: 10.3390/nu12092820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kehler DS, Hay JL, Stammers AN, et al. A systematic review of the association between sedentary behaviors with frailty. Exp Gerontol. 2018;114:1–12. doi: 10.1016/j.exger.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 32.O'Hanlon S, Dhesi J, Aronson L, Inouye SK. COVID-19: a call for mobilizing geriatric expertise. Eur Geriatr Med. 2021;12:597–600. doi: 10.1007/s41999-021-00500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crosignani S, Fantinati J, Cesari M. Frailty and geriatric medicine during the pandemic. Front Med (Lausanne) 2021;673814 doi: 10.3389/fmed.2021.673814. [DOI] [PMC free article] [PubMed] [Google Scholar]