Abstract
Introduction
Acute respiratory distress syndrome (ARDS) is characterized by hypoxemia and increased lung permeability and would result in acute respiratory failure and with high mortality. In patients who survive from acute lung injury (ALI)/ARDS, it is an active process of the transition from injury to resolution depending on the coordinated immune system. The roles of regulatory CD4+T cells (Tregs) are now gradually being clarified during inflammation and resolution of ARDS. However, clear conclusions about roles of Tregs in ALI/ARDS are only a few.
Objective
This review provides an overview of phenotype, differentiation, and suppressive mechanisms of Tregs and focuses on keys of biology of Tregs in alveolar space during the inflammatory response and resolution of ALI/ARDS.
Data Source
Literature search of Web of Science, PubMed, and EMBASE was made to find relative articles about Tregs in ALI/ARDS. We used the following search terms: Tregs, ALI, ARDS, inflammation, and resolution.
Conclusion
More and more studies have indicated Tregs involved in the processes of inflammation and resolution of ALI/ARDS. A deep understanding of the roles of Tregs may indicate new treatments for patients of ARDS. Therapies aimed at expansion or adaptive transfer of Tregs could be an effective therapy to ARDS patients.
Keywords: ALI, ARDS, inflammation, regulatory T cells, resolution
The roles of regulatory CD4+T lymphocytes (Tregs) are now increasingly being recognized during resolution of ARDS. A dissection of the roles of Tregs may indicate the design of new treatments for ARDS patients. Therapies aimed at expansion or adaptive transfer of Tregs could be an effective therapy to ARDS patients.
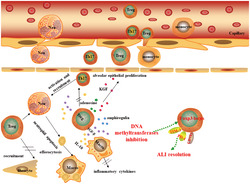
1. INTRODUCTION
The acute onset of ALI and ARDS is mainly manifested by respiratory distress and refractory hypoxemia with high mortality and poor prognosis. Now, the main clinical treatment methods are supportive treatment. Except for small tidal volume mechanical ventilation and prone position ventilation, 1 which can reduce the mortality rate of ALI/ARDS, there are no other effective drug treatment measures. The existing schemes cannot achieve satisfactory results, and the mortality is still up to 40%. 2 In addition to the above treatments, research on regulating the immune response of ALI/ARDS is also receiving increasing attention. Studies indicated that the recovery of lung injury depends on highly coordinated immune system. 3 , 4 , 5 , 6 , 7 , 8 In one our retrospective study, we found NLR (neutrophil and lymphocyte ratio) was significantly correlated with the prognosis of the ARDS. The prognosis was poor when the ratio was greater than 14, indicating that lymphocytes may be play an important role in the onset and development of ARDS. 9
At present, the most widely studied lymphocyte subsets in ALI/ARDS are regulatory T cells (Tregs). In alveolar space, the processes of inflammation and resolution are closely related to the subpopulations and functions of Tregs. Researchers have identified the phenotype, mechanisms, and signal pathways of Tregs involved in acute inflammation and resolution in ALI/ARDS. Now, we will focus on keys of Tregs biology (subpopulations, differentiation, and function) of alveolar space during the inflammatory response and resolution of ALI/ARDS and explore new areas of therapeutic potential of ARDS.
2. PHENOTYPE AND DEVELOPMENT OF Tregs
Tregs play an important role during the suppression of immune response through different mechanisms. 10 , 11 Tregs mainly include two subgroups: nTregs (thymus‐derived Tregs) and iTregs (induced Tregs), which peripherally antigen‐induced Tregs generated in the periphery from naive CD4+T cells under certain antigenic stimuli or suppressor cytokines. iTregs are usually more plastic than nTregs. 12 Although two Treg subpopulations exhibit different developmental mechanisms, they have a synergetic effect to maintain immune homeostasis and share similar phenotypes and suppressive function.
The precursors that express TCRs with high affinity for self‐antigens can develop into nTregs. 13 nTreg differentiation in thymus consists of two steps. The combination of a strong TCR signal with costimulatory molecules result in the upregulation of CD25 of naïve CD4+ T cells. Then, signal transducer and activator of transcription 5 (STAT‐5) are the downstream of CD25, can bind a regulatory sequence in the Foxp3 gene, and then promote Foxp3 expression, which is a necessary marker during the development and function of Tregs in the thymus and peripheral lymphoid organs, 14 , 15 , 16 and Foxp3 locus conserved noncoding regions (CNS) of the regions also involved in Treg induction and stability (Figure 1A). 17 After generation in thymus, nTregs migrate to the periphery to perform their suppressive function. 18
FIGURE 1.
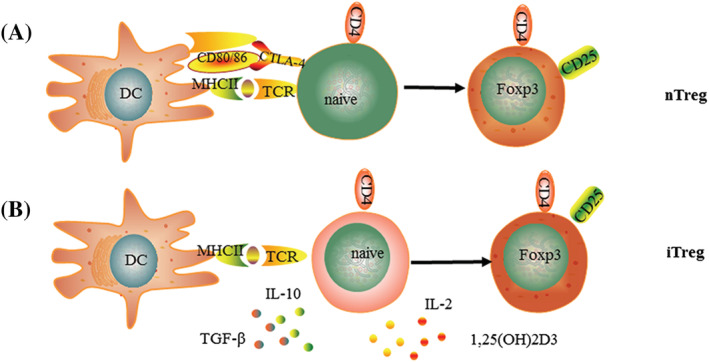
(A) nTreg develops in thymus. A strong TCR signal associated with CTLA‐4/CD80/CD86 upregulates CD25 in naïve CD4+T cells. Then, signals through CD25 lead to the expression of Foxp3. (B) The regulatory phenotype of iTreg induced in peripheral lymphoid organs stimulated by antigens and suppressive cytokines
nTregs express constitutively CD4 and CD25, but CD25 is also upregulated in effector T cells (Teffs) when activated. nTregs also constitutively express CTLA‐4 (cytotoxic T Lymphocyte antigen 4), CD62L, CD103, and GITR (glucocorticoid‐induced tumor necrosis factor receptor related protein), but expression of these markers is also affected by T cell activation and do not provide more specificity than CD25. 10 , 19 , 20 , 21 CD127 have been thought to be characteristic of nTregs, 22 but its expression is downregulated after Teff activation. 23 Thus, CD127 is only useful to identify Tregs in non‐inflammatory conditions. nTregs also express some non‐specific makers such as LAG‐3 (protein lymphocyte activation gene 3) and TLRs 4, 5, 7, and 8. 24
iTregs express different levels of CD25. Most of iTregs highly express CD25, and a small group expresses small amounts of CD25, but both populations express Foxp3 (Figure 1B). iTregs are generated in the periphery induced from naive CD4+T cells with anti‐inflammatory cytokines and dendritic cells (DCs). A high concentration of TGF‐β is a critical cytokine for the generation and phenotype in T cells. 25 , 26 , 27 TGF‐β affected the peripheral pool of Tregs, 28 and the role of TGF‐β in development of iTregs was initially refuted. However, recent studies have showed that TGF‐β is necessary requirement for the generation of iTregs, indicating that TGF‐β also can induce Foxp3 expression of thymic Treg precursors in the context of TCR. 29 Tolerogenic CD103+DCs can generated RA (retinoic acid) and 1,25(OH)2D3 (1,25‐dihydroxyvitamin D3) to provide a favorable environment for iTregs differentiation. 30 , 31 , 32 1,25(OH)2D3 combined with IL‐2 can induce both CTLA‐4 and Foxp3 expression of iTregs. 33 The PD‐1/PD‐L1 signaling also plays critical roles in the generation, homeostasis, and plasticity of Foxp3+iTreg. 34 , 35 , 36 , 37 , 38 The most common subgroups of Tregs include Tr1 cells (Type 1 regulatory T) and Th3 cells. Tr1 cells express low and transient levels of Foxp3 and secretion of high amounts of IL‐10, which induce anergy and low cell proliferation. 39 They also produce IFN‐γ, TGF‐β, and IL‐15 and low levels of IL‐2 and IL‐4 and induce anergy and low cell proliferation by secretion of IL‐10. 39 , 40 There is no specific marker for Th1 cells, although some research has shown that GATA is a potential candidate. 41 Th3 cells originate from CD4+T cells stimulated by TGF‐β and play critical role in oral tolerance by secretion of TGF‐β and IL‐10. 42
Both nTregs and iTregs express CD25 and Foxp3. Thus, it remains challenging to distinguish these two subsets. Helios, Nrp‐1 (neuropilin‐1), and FR4 (folate receptor 4) are candidates for this discrimination. But these are no specific markers to distinguish nTregs from iTregs. Although nTregs can distinguish from iTregs by higher expression of Helios, a small group of human nTregs does not express Helios. 43 , 44 Furthermore, recent studies have reported that iTregs could also express Helios. 45 Although nTregs exclusively express Nrp‐1, Nrp‐1 can also be induced in activated Teffs in humans. 46 , 47 High amounts of FR4 are found to express constitutively on nTregs, but it also expressed by iTregs. 48 Thus, the contribution of nTregs and iTregs to the disease would be limited by the lack of definite markers, particularly in humans.
3. SUPPRESSIVE MECHANISMS OF Tregs
TCR repertoires of nTregs and iTregs are different leads to their function are distinct. iTregs are mainly involved in the tolerance to non‐self‐antigens while nTregs are preferentially responsible for control of auto‐specific responses. 49 Tregs can regulate both innate and adaptive immune cells in various pathophysiological microenvironment though different suppressive mechanism.
Two main types of suppressive mechanisms of Tregs are contact‐dependent and contact‐independent. Tregs can regulate maturation and function of APC (antigen presenting cell) through the interaction of CTLA‐4, Nrp‐1, and LAG‐3 expressed on Tregs with the CD80/86 costimulatory molecules, MHC class II, and Sema4a expressed on APC. 50 , 51 , 52 Tregs can also induce direct killing of Teffs through interaction of Gal‐9 expressed by Tregs and Tim‐3 expressed by Teffs. Tregs can express CD39/CD73 ectoenzymes to cleavage of ATP into adenosine. 53 , 54 Interaction of adenosine with the A2A receptor increases cAMP levels of target cells, thus inhibit cell proliferation of these target cells. 55 , 56 , 57 Adenosine combined with A2A receptor expressed on Tregs can improve expression of Foxp3 and Tregs function.
Tregs can also secret anti‐inflammatory cytokines, such as TGF‐β, IL‐10, and IL‐35. These immunosuppressive cytokines in turn induce the development of iTregs. TGF‐β can inhibit the proliferation and differentiation of Th1 and Th2 by downregulating of the transcription of T‐bet and GATA‐3. 58 , 59 High level of TGF‐β also affects Th17 cells function. 27 IL‐10 can suppress T cells responses by downregulating of IFN‐γ, IL‐2, and GM‐CSF. 60 Furthermore, IL‐10 can induce IgG4 and suppress IgE of B cells to induce immune tolerance. 61 IL‐35 is a novel anti‐inflammatory cytokine specifically secreted by Tregs, and necessary for maximal suppressive function of Tregs, can induce the development of iTregs, suppress the proliferation of Th1 and Th17 cells by inhibiting the G1 phase of cell division of early T cell rest, 62 and inhibit development and proliferation of Th2 by repressing GATA3 transcription and IL‐4 secretion. 63 IL‐35 can also regulate the plasticity of Th2, mediate differentiation of Th2 cells to Tregs (Figure 2). 64
FIGURE 2.
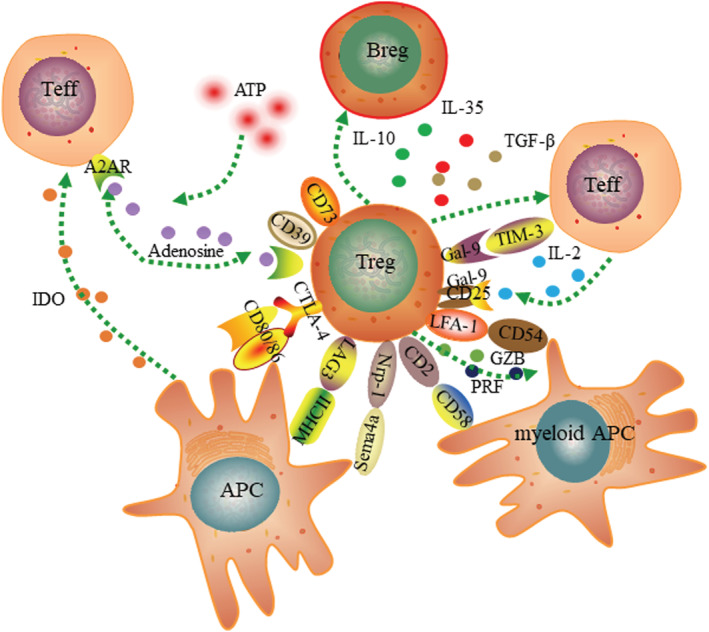
Tregs regulate immune responses through multiple suppressive mechanisms. Tregs can inhibit Teffs through the suppressive cytokines IL‐10, IL‐35, and TGF‐β. Tregs can disrupt metabolic functions of Teff through co‐expressing CD39/CD73 generating adenosine or IL‐2 deprivation. Interaction of CTLA‐4, LAG‐3, and Nrp1 expressed by Tregs with CD80/86 costimulatory molecules and Sema4a and MHCII expressed by APC can suppress maturation and function of Tregs, lead to production of IDO, and finally prevent maturation of APC maturation and activation of Teffs. Tregs also can induce direct killing of Teffs through the production of GZB or the interaction between Gal‐9 expressed by Tregs with Tim‐3 expressed by Teffs. GZB also can lyse myeloid APC through the interaction of CD2 and LFA‐1 expressed by Tregs and CD58 and CD54 expressed by myeloid APC.
IL‐2 is required to expand Tregs and to induce their suppressive function in vitro. However, in vivo, Tregs are strong competitors for IL‐2 compared with their target cells via constitutively expressing CD25. Lack of IL‐2 causes apoptosis of target cells through the Bcl‐2/Bim and independently of PRF/Fas signaling pathway (Figure 2). 65 Mice deficient in IL‐2 develop an unstable population of Tregs and subsequently acquire lymphoproliferative disease. 66 From this side, it is difficult to reconcile with the idea that IL‐2 absorption plays a relevant role as an effector mechanism in vivo. Recent studies have proposed that Tregs can induce direct killing of Teffs through the GZB (granzyme B) production or by the interaction of Gal‐9 (galectin 9) expressed by Tregs with Tim‐3 (T‐cell immunoglobulin and mucin domain‐containing protein 3) expressed by Teff. GZB also can lyse myeloid APC through the interaction of CD2 and LFA‐1 (lymphocyte function‐associated antigen‐1) expressed by Tregs with CD58 and CD54 expressed by myeloid APC (Figure 2). 67
4. TREGS IN ACUTE LUNG INJURY
ARDS is a fatal inflammatory lung disease with high mortality without effective therapies. More and more studies have shown that Tregs resolve inflammation of lung, but mechanisms of Tregs to promote the resolution of lung injury remain unknown. Investigators are ongoing into whether the known mechanisms of suppressive function of Treg are effective in ALI/ARDS. Presumably, if we could understand how Tregs play roles and change in ALI/ARDS, then effective therapies could be designed to treat ALI/ARDS by regulating the number and function of Tregs either endogenously or exogenously.
In Tregs, CD4+CD25+Foxp3+Tregs are the most studied in ALI/ARDS. D'Alessio et al. first show that CD4+CD25+Foxp3+Tregs could mediated the active resolution of ALI mouse model. 8 Many studies have indicated that CD4+CD25+Foxp3+Tregs are associated with severity of ALI/ARDS. Adamzik group found CD4+CD25+Foxp3+Tregs could be activated in ARDS patients and increased in alveola and even could predict poor outcome of ARDS. 68 Sebastien et al. also found that the quantity and function of Tregs changed in ARDS patients. 69 A prospective, observational study performed by Yu group indicated ratio of Th17/Treg ratio >0.79 was the independent predictor for 28‐day mortality in ARDS patients. 70 Our recent studies also found there was imbalance of Tregs and Th17 cells and Tregs/Th17 ratio downregulated in LPS‐induced ALI model. 71
The main mechanisms of CD4+CD25+Foxp3+Tregs in alveolar to improve ALI resolution are mediated by inducing neutrophil apoptosis and suppression of macrophage anti‐inflammatory cytokine secretion 8 , 72 ; control fibrocyte recruitment to lung to inhibit the fibroproliferation though CXCL12‐CXCR4 axis 73 ; CD73‐dependent adenosine generation. 74 Singer group also found that the number and Foxp3 expression, activation state, suppressive phenotype, and proliferative capacity of CD4+CD25+Foxp3+Tregs in lung enhanced in mice treated by DNA methyltransferase inhibitor indicated that epigenetic pathways are very likely to be novel targets for the treatment of ARDS. 75 Epithelial repair also plays important role in resolution of ALI. Data of Dial et al. indicated that Foxp3+Tregs can secret keratinocyte growth factor (KGF) to enhance alveolar epithelial proliferation. 76 Moreover, Tregs also can directly exert tissue repair function, at least in part, through production of amphiregulin in influenza‐induced ALI model (Figure 3). 77
FIGURE 3.
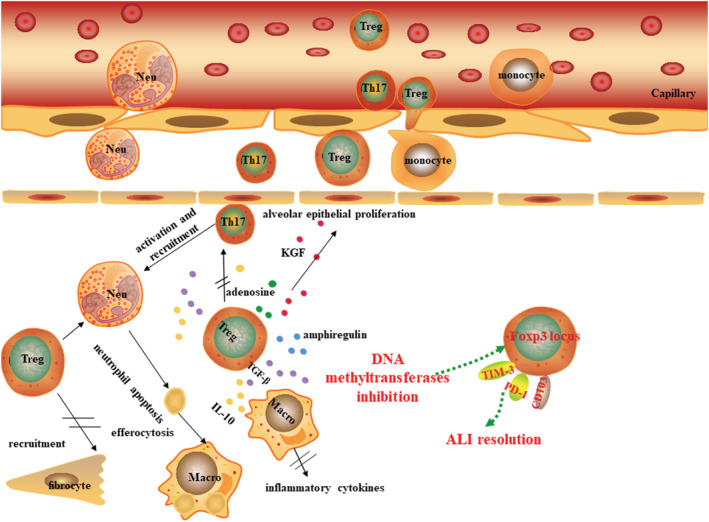
The potential mechanism of Treg suppress the inflammation and promote of resolution of ALI. Neutrophils, macrophages, and Th17 cells are recruited into alveola, and release inflammatory mediators lead to damage of endothelium, thus pulmonary edema during ALI. IL‐10 and TGF‐β secretion of Tregs can inhibit the proliferation and function of Th17 cells and macrophages. TGF‐β also can mediate apoptosis of neutrophil apoptosis and cytokines secretion of macrophage, promote the barrier repair. Tregs also can control of fibrocyte recruitment to the lung to inhibit the fibroproliferation. Tregs also can promote resolution of ALI through CD73‐dependent adenosine generation. Treg number and function can be partly enhanced by DNA methyltransferase inhibition to accelerate repair of lung injury. Moreover, Tregs exert tissue repair function by expression KGF and amphiregulin.
5. FUTURE DIRECTIONS AND PERSPECTIVES
Clarifying the roles of Tregs in ALI resolution may lead to the design of new treatments for patients with ARDS. And therapies aimed at expansion or adaptive transfer of Tregs to ARDS patients could be an effective approach.
Studies have found leukotriene B4 Receptor (BLT1) and alanyl‐glutamine (Ala‐Gln) could recruit CD4+CD25+Foxp3+Tregs of alveoli. The blockade of LTB4‐BLT1 pathway significantly decreased Tregs numbers in BALF and impaired ALI resolution. 78 And intragastric gavage Ala‐Gln could regulate the polarization of Tregs and Th17 cells to promote the resolution of lung inflammation. 79 Moreover, our previous studies also indicated that lung‐resident mesenchymal stem cell can maintain balance of Tregs and Th17 cells and upregulate Treg/Th17 ratio. 80 These data above tell us that finding ways to increase the number or function of Tregs or upregulate the Treg/Th17 cell ratio may be an effective way to alleviate lung injury and promote lung repair.
The adoptive transfer of regulatory lymphocytes to patients with ALI is a good idea. D'Alessio et al. found the transfer of Tregs significantly improved survival rate and resolution of ALI. 8 But there are concerns that Tregs tend to exhibit remarkable plasticity. It remains unknown whether Tregs differentiate into effector T cells after adaptive transfer to ARDS patients. Moreover, therapeutic effect of Tregs depend on its relative contributions and the timing during the course of ALI initiation, pathogenesis and resolution. Up to now, there is only evidence of Tregs transfer to promote the resolution of ALI, but no studied pay attention to the timing of Tregs.
6. CONCLUSIONS
ARDS is a hard clinical problem with high mortality in critically ill patients. No specific therapies are available. Studies of ALI indicate that Tregs attempt to promote the resolution of ALI by regulating actively innate and adaptive immune responses. Identifying how to best isolate and augment Tregs in vivo or ex vivo and avoid Tregs depletion are critical aims that are the potential treatment of ALI/ARDS.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
ETHICS STATEMENT
The Ethics statement is not applicable in this review.
AUTHOR CONTRIBUTIONS
Linlin Wang, Weipeng Jiang, and Xiaocen Wang involved in writing manuscript draft, searched for literatures, and contributed to final draft. Yuanlin Song and Lin Tong revised the manuscript. All authors have read and approved the final draft of the manuscript for publication.
ACKNOWLEDGMENTS
This study was supported by National Natural Science Foundation of China (8200087 and 82130001), National Key R&D Plan (2020YFC2003700), Science and Technology Commission of Shanghai Municipality (20Z11901000, 20DZ2261200, and 20XD1401200), Shanghai Municipal Science and Technology Major Project, Clinical Research Plan of SHDC (SHDC2020CR5010‐002), and Shanghai Municipal Key Clinical Specialty (shslczdzk02201).
Wang L, Jiang W, Wang X, Tong L, Song Y. Regulatory T cells in inflammation and resolution of acute lung injury. Clin Respir J. 2022;16(9):587‐595. doi: 10.1111/crj.13527
Linlin Wang, Weipeng Jiang, and Xiaocen Wang contributed equally.
Funding information Shanghai Municipal Key Clinical Specialty, Grant/Award Number: shslczdzk02201; Clinical Research Plan of SHDC, Grant/Award Number: SHDC2020CR5010‐002; Shanghai Municipal Science and Technology Major Project; Science and Technology Commission of Shanghai Municipality, Grant/Award Numbers: 20Z11901000, 20DZ2261200, 20XD1401200; National Key R&D Plan, Grant/Award Number: 2020YFC2003700; National Natural Science Foundation of China, Grant/Award Numbers: 8200087, 82130001
Contributor Information
Lin Tong, Email: tong.lin@zs-hospital.sh.cn.
Yuanlin Song, Email: song.yuanlin@zs-hospital.sh.cn.
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. Guérin C, Beuret P, Constantin JM, et al. A prospective international observational prevalence study on prone positioning of ARDS patients: the APRONET (ARDS Prone Position Network) study. Intensive Care Med. 2018;44(1):22‐37. doi: 10.1007/s00134-017-4996-5 [DOI] [PubMed] [Google Scholar]
- 2. Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122(8):2731‐2740. doi: 10.1172/JCI60331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6(12):1191‐1197. doi: 10.1038/ni1276 [DOI] [PubMed] [Google Scholar]
- 4. Savill J. Apoptosis in resolution of inflammation. J Leukoc Biol. 1997;61(4):375‐380. doi: 10.1002/jlb.61.4.375 [DOI] [PubMed] [Google Scholar]
- 5. Henson PM. Dampening inflammation. Nat Immunol. 2005;6(12):1179‐1181. doi: 10.1038/ni1205-1179 [DOI] [PubMed] [Google Scholar]
- 6. Henson PM, Hume DA. Apoptotic cell removal in development and tissue homeostasis. Trends Immunol. 2006;27(5):244‐250. doi: 10.1016/j.it.2006.03.005 [DOI] [PubMed] [Google Scholar]
- 7. Ross R. The role of T lymphocytes in inflammation. Proc Natl Acad Sci U S A. 1994;91(8):2879. doi: 10.1073/pnas.91.8.2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. D'Alessio FR, Tsushima K, Aggarwal NR, et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. 2009;119(10):2898‐2913. doi: 10.1172/JCI36498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang Y, Mohan J, Chen C, et al. Neutrophil‐to‐lymphocyte ratio as a prognostic marker in acute respiratory distress syndrome patients: a retrospective study. J Thorac Dis. 2018;10(1):273‐282. doi: 10.21037/jtd.2017.12.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Billerbeck E, Bottler T, Thimme R. Regulatory T cells in viral hepatitis. World J Gastroenterol. 2007;13(36):4858‐4864. doi: 10.3748/wjg.v13.i36.4858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3(3):253‐257. doi: 10.1038/nri1032 [DOI] [PubMed] [Google Scholar]
- 12. Murai M, Krause P, Cheroutre H, Kronenberg M. Regulatory T‐cell stability and plasticity in mucosal and systemic immune systems. Mucosal Immunol. 2010;3(5):443‐449. doi: 10.1038/mi.2010.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21(2):267‐277. doi: 10.1016/j.immuni.2004.07.009 [DOI] [PubMed] [Google Scholar]
- 14. Burchill MA, Yang J, Vang KB, et al. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 2008;28(1):112‐121. doi: 10.1016/j.immuni.2007.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lio CW, Hsieh CS. A two‐step process for thymic regulatory T cell development. Immunity. 2008;28(1):100‐111. doi: 10.1016/j.immuni.2007.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor FOXP3. Science. 2003;299(5609):1057‐1061. doi: 10.1126/science.1079490 [DOI] [PubMed] [Google Scholar]
- 17. Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non‐coding DNA elements in the Foxp3 gene in regulatory T‐cell fate. Nature. 2010;463:808‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salomon B, Lenschow DJ, Rhee L, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12(4):431‐440. doi: 10.1016/S1074-7613(00)80195-8 [DOI] [PubMed] [Google Scholar]
- 19. Ngoma AM, Ikeda K, Hashimoto Y, et al. Impaired regulatory T cell reconstitution in patients with acute graft‐versus‐host disease and cytomegalovirus infection after allogeneic bone marrow transplantation. Int J Hematol. 2012;95(1):86‐89. doi: 10.1007/s12185-011-0976-7 [DOI] [PubMed] [Google Scholar]
- 20. Smyth LJ, Eustace A, Kolsum U, Blaikely J, Singh D. Increased airway T regulatory cells in asthmatic subjects. Chest. 2010;138(4):905‐912. doi: 10.1378/chest.09-3079 [DOI] [PubMed] [Google Scholar]
- 21. Moradi B, Schnatzer P, Hagmann S, et al. CD4+CD25+/highCD127low/− regulatory T cells are enriched in rheumatoid arthritis and osteoarthritis joints‐analysis of frequency and phenotype in synovial membrane, synovial fluid and peripheral blood. Arthritis Res Ther. 2014;16(2):R97. doi: 10.1186/ar4545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seddiki N, Santner‐Nanan B, Martinson J, et al. Expression of interleukin (IL)‐2 and IL‐7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203(7):1693‐1700. doi: 10.1084/jem.20060468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aerts NE, Dombrecht EJ, Ebo DG, Bridts CH, Stevens WJ, De Clerck LS. Activated T cells complicate the identification of regulatory T cells in rheumatoid arthritis. Cell Immunol. 2008;251(2):109‐115. doi: 10.1016/j.cellimm.2008.04.008 [DOI] [PubMed] [Google Scholar]
- 24. Caramalho I, Lopes‐Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express toll‐like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197(4):403‐411. doi: 10.1084/jem.20021633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Amarnath S, Dong L, Li J, Wu Y, Chen W. Endogenous TGF‐beta activation by reactive oxygen species is key to Foxp3 induction in TCR‐stimulated and HIV‐1‐infected human CD4+CD25‐T cells. Retrovirology. 2007;4(1):57. doi: 10.1186/1742-4690-4-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koli K, Myllärniemi M, Keski‐Oja J, Kinnula VL. Transforming growth factor‐beta activation in the lung: focus on fibrosis and reactive oxygen species. Antioxid Redox Signal. 2008;10(2):333‐342. doi: 10.1089/ars.2007.1914 [DOI] [PubMed] [Google Scholar]
- 27. Li MO, Flavell RA. TGF‐β: a master of all T cell trades. Cell. 2008;134(3):392‐404. doi: 10.1016/j.cell.2008.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF‐beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+regulatory T cells. J Exp Med. 2005;201(7):1061‐1067. doi: 10.1084/jem.20042276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen WJ, Konkel JE. Development of thymic Foxp3(+) regulatory T cells: TGF‐β matters. Eur J Immunol. 2015;45(4):958‐965. doi: 10.1002/eji.201444999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun CM, Hall JA, Blank RB, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 Treg cells via retinoic acid. J Exp Med. 2007;204(8):1775‐1785. doi: 10.1084/jem.20070602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Coombes JL, Siddiqui KR, Arancibia‐Cárcamo CV, et al. A functionally specialized population of mucosal CD103+DCs induces Foxp3+ regulatory T cells via a TGF‐beta and retinoic acid‐dependent mechanism. J Exp Med. 2007;204(8):1757‐1764. doi: 10.1084/jem.20070590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Benson MJ, Pino‐Lagos K, Rosemblatt M, Noelle RJ. All‐trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co‐stimulation. J Exp Med. 2007;204(8):1765‐1774. doi: 10.1084/jem.20070719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jeffery LE, Burke F, Mura M, et al. 1,25‐Dihydroxyvitamin D3 and IL‐2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA‐4 and FoxP3. J Immunol. 2009;183(9):5458‐5467. doi: 10.4049/jimmunol.0803217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Francisco LM, Sage PT, Sharpe AH. The PD‐1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236(1):219‐242. doi: 10.1111/j.1600-065X.2010.00923.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Amarnath S, Mangus CW, Wang JC, et al. The PDL1‐PD1 axis converts human TH1 cells into regulatory T cells. Sci Transl Med. 2011;3(111):111ra120. doi: 10.1126/scitranslmed.3003130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yogev N, Frommer F, Lukas D, et al. Dendritic cells ameliorate autoimmunity in the CNS by controlling the homeostasis of PD‐1 receptor (+) regulatory T cells. Immunity. 2012;37(2):264‐275. doi: 10.1016/j.immuni.2012.05.025 [DOI] [PubMed] [Google Scholar]
- 37. Totsuka T, Kanai T, Makita S, et al. Regulation of murine chronic colitis by CD4+CD25‐ programmed death‐1+ T cells. Eur J Immunol. 2005;35(6):1773‐1785. doi: 10.1002/eji.200425109 [DOI] [PubMed] [Google Scholar]
- 38. Totsuka T, Kanai T, Nemoto Y, et al. Immunosenescent colitogenic CD4(+) T cells convert to regulatory cells and suppress colitis. Eur J Immunol. 2008;38(5):1275‐1286. doi: 10.1002/eji.200737914 [DOI] [PubMed] [Google Scholar]
- 39. Strober S, Cheng L, Zeng D, et al. Double negative (CD4‐CD8‐alphabeta+) T cells which promote tolerance induction and regulate autoimmunity. Immunol Rev. 1996;149(1):217‐230. doi: 10.1111/j.1600-065X.1996.tb00906.x [DOI] [PubMed] [Google Scholar]
- 40. Levings MK, Sangregorio R, Galbiati F, Squadrone S, de Waal Malefyt R, Roncarolo MG. IFN‐α and IL‐10 induce the differentiation of human type 1 T regulatory cells. J Immunol. 2001;166(9):5530‐5539. doi: 10.4049/jimmunol.166.9.5530 [DOI] [PubMed] [Google Scholar]
- 41. Fujio K, Okamura T, Yamamoto K. The family of IL‐10‐secreting CD4 + T cells. Adv Immunol. 2010;105:99‐130. doi: 10.1016/S0065-2776(10)05004-2 [DOI] [PubMed] [Google Scholar]
- 42. Wan YY, Flavell RA. ‘Yin‐Yang’ functions of transforming growth factor‐β and T regulatory cells in immune regulation. Immunol Rev. 2007;220(1):199‐213. doi: 10.1111/j.1600-065X.2007.00565.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thornton AM, Korty PE, Tran DQ, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic‐derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184(7):3433‐3441. doi: 10.4049/jimmunol.0904028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Himmel ME, MacDonald KG, Garcia RV, Steiner TS, Levings MK. Helios+ and Helios‐ cells coexist within the natural FOXP3+T regulatory cell subset in humans. J Immunol. 2013;190(5):2001‐2008. doi: 10.4049/jimmunol.1201379 [DOI] [PubMed] [Google Scholar]
- 45. Gottschalk RA, Corse E, Allison JP. Expression of Helios in peripherally induced Foxp3+ regulatory T cells. J Immunol. 2012;188(3):976‐980. doi: 10.4049/jimmunol.1102964 [DOI] [PubMed] [Google Scholar]
- 46. Yadav M, Louvet C, Davini D, et al. Neuropilin‐1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. 2012;209(1713–1722):S1‐S19. doi: 10.1084/jem.20120822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Milpied P, Renand A, Bruneau J, et al. Neuropilin‐1 is not a marker of human Foxp3+ Treg. Eur J Immunol. 2009;39(6):1466‐1471. doi: 10.1002/eji.200839040 [DOI] [PubMed] [Google Scholar]
- 48. Yamaguchi T, Hirota K, Nagahama K, et al. Control of immune responses by antigen‐specific regulatory T cells expressing the folate receptor. Immunity. 2007;27(1):145‐159. doi: 10.1016/j.immuni.2007.04.017 [DOI] [PubMed] [Google Scholar]
- 49. Wyss L, Stadinski BD, King CG, et al. Affinity for self‐antigen selects Treg cells with distinct functional properties. Nat Immunol. 2016;17(9):1093‐1101. doi: 10.1038/ni.3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self‐tolerance maintained by CD25+CD4+regulatory T cells constitutively expressing cytotoxic T lymphocyte‐associated antigen 4. J Exp Med. 2000;192(2):303‐310. doi: 10.1084/jem.192.2.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sarris M, Andersen KG, Randow F, Mayr L, Betz AG. Neuropilin‐1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity. 2008;28(3):402‐413. doi: 10.1016/j.immuni.2008.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liang B, Workman C, Lee J, et al. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene‐3 engagement of MHC class II. J Immunol. 2008;180(9):5916‐5926. doi: 10.4049/jimmunol.180.9.5916 [DOI] [PubMed] [Google Scholar]
- 53. Bopp T, Becker C, Klein M, et al. Cyclic adenosine monophosphateis a key component of regulatory T cell‐mediated suppression. J Exp Med. 2007;204(6):1303‐1310. doi: 10.1084/jem.20062129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204(6):1257‐1265. doi: 10.1084/jem.20062512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sauer AV, Brigida I, Carriglio N, et al. Alterations in the adenosine metabolism and CD39/CD73 adenosinergic machinery cause loss of Treg cell function and autoimmunity in ADA‐deficient SCID. Blood. 2012;119(6):1428‐1439. doi: 10.1182/blood-2011-07-366781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Raker VK, Becker C, Steinbrink K. The cAMP pathway as therapeutic target in autoimmune and inflammatory diseases. Front Immunol. 2016;7:123. doi: 10.3389/fimmu.2016.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zarek PE, Huang CT, Lutz ER, et al. A2A receptor signaling promotes peripheral tolerance by inducing T‐cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111(1):251‐259. doi: 10.1182/blood-2007-03-081646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gorelik L, Flavell RA. Abrogation of TGFβ signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12(2):171‐181. doi: 10.1016/S1074-7613(00)80170-3 [DOI] [PubMed] [Google Scholar]
- 59. Kuwahara M, Yamashita M, Shinoda K, et al. The transcription factor Sox4 is a downstream target of signaling by the cytokine TGF‐β and suppresses T(H)2 differentiation. Nat Immunol. 2012;13(8):778‐786. doi: 10.1038/ni.2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gregori S, Goudy KS, Roncarolo MG. The cellular and molecular mechanisms of immuno‐suppression by human type 1 regulatory T cells. Front Immunol. 2012;3:30. doi: 10.3389/fimmu.2012.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Meiler F, Zumkehr J, Klunker S, Rückert B, Akdis CA, Akdis M̈. In Vivoswitch to IL‐10‐secreting T regulatory cells in high dose allergen exposure. J Exp Med. 2008;205(12):2887‐2898. doi: 10.1084/jem.20080193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wirtz S, Billmeier U, Mchedlidze T, Blumberg RS, Neurath MF. Interleukin‐35 mediates mucosal immune responses that protect against T‐cell‐dependent colitis. Gastroenterology. 2011;141(5):1875‐1886. doi: 10.1053/j.gastro.2011.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Huang A, Cheng L, He M, Nie J, Wang J, Jiang K. Interleukin‐35 on B cell and T cell induction and regulation. J Inflamm (Lond). 2017;14(1):16. doi: 10.1186/s12950-017-0164-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vignali DA, Kuchroo VK. IL‐12 family cytokines: immunological playmakers. Nat Immunol. 2012;13(8):722‐728. doi: 10.1038/ni.2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+FOXP3+regulatory T cells induce cytokine deprivation‐mediated apoptosis of effector CD4+T cells. Nat Immunol. 2007;8(12):1353‐1362. doi: 10.1038/ni1536 [DOI] [PubMed] [Google Scholar]
- 66. Sneller MC, Lane HC. CD4+CD25+ T regulatory cells, immunotherapy of cancer, and interleukin‐2. J Immunother. 2005;28(2):120‐128. doi: 10.1097/01.cji.0000155049.26787.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Magnani CF, Alberigo G, Bacchetta R, et al. Killing of myeloid APCs via HLA class I, CD2 and CD226 defines a novel mechanism of suppression by human Tr1 cells. Eur J Immunol. 2011;41(6):1652‐1562. doi: 10.1002/eji.201041120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Adamzik M, Broll J, Steinmann J, et al. An increased alveolar CD4 + CD25 + Foxp3 + T‐regulatory cell ratio in acute respiratory distress syndrome is associated with increased 30‐day mortality. Intensive Care Med. 2013;39(10):1743‐1751. doi: 10.1007/s00134-013-3036-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Halter S, Aimade L, Barbié M, et al. T regulatory cells activation and distribution are modified in critically ill patients with acute respiratory distress syndrome: a prospective single‐Centre observational study. Anaesth Crit Care Pain Med. 2020;39(1):35‐44. doi: 10.1016/j.accpm.2019.07.014 [DOI] [PubMed] [Google Scholar]
- 70. Yu ZX, Ji MS, Yan J, et al. The ratio of Th17/Treg cells as a risk indicator in early acute respiratory distress syndrome. Crit Care. 2015;19(1):82. doi: 10.1186/s13054-015-0811-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang L, Wang X, Tong L, et al. Recovery from acute lung injury can be regulated via modulation of regulatory T cells and Th17 cells. Scand J Immunol. 2018;88(5):e12715. doi: 10.1111/sji.12715 [DOI] [PubMed] [Google Scholar]
- 72. Huynh ML, Fadok VA, Henson PM. Phosphatidylserine‐dependent ingestion of apoptotic cells promotes TGF‐β1 secretion and the resolution of inflammation. J Clin Invest. 2002;109(1):41‐50. doi: 10.1172/JCI0211638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Garibaldi BT, D'Alessio FR, Mock JR, et al. Regulatory T cells reduce acute lung injury fibroproliferation by decreasing fibrocyte recruitment. Am J Respir Cell Mol Biol. 2013;48(1):35‐43. doi: 10.1165/rcmb.2012-0198OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ehrentraut H, Clambey ET, McNamee EN, et al. CD73+ regulatory T cells contribute to adenosine‐mediated resolution of acute lung injury. FASEB J. 2013;27(6):2207‐2219. doi: 10.1096/fj.12-225201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Singer BD, Mock JR, Aggarwal NR, et al. Regulatory T cell DNA methyltransferase inhibition accelerates resolution of lung inflammation. Am J Respir Cell Mol Biol. 2015;52(5):641‐652. doi: 10.1165/rcmb.2014-0327OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dial CF, Tune MK, Doerschuk CM, Mock JR. Foxp3+ regulatory T cell expression of keratinocyte growth factor enhances lung epithelial proliferation. Am J Respir Cell Mol Biol. 2017;57(2):162‐173. doi: 10.1165/rcmb.2017-0019OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Arpaia N, Green JA, Moltedo B, et al. A distinct function of regulatory T cells in tissue protection. Cell. 2015;162(5):1078‐1089. doi: 10.1016/j.cell.2015.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang L, Zhao L, Lv J, et al. BLT1‐dependent alveolar recruitment of CD4(+) CD25(+) Foxp3(+) regulatory T cells is important for resolution of acute lung injury. Am J Respir Crit Care Med. 2012;186(10):989‐998. doi: 10.1164/rccm.201202-0261OC [DOI] [PubMed] [Google Scholar]
- 79. Hou YC, Pai MH, Liu JJ, Yeh SL. Alanyl‐glutamine resolves lipopolysaccharide‐induced lung injury in mice by modulating the polarization of regulatory T cells and T helper 17 cells. J Nutr Biochem. 2013;24(9):1555‐1563. doi: 10.1016/j.jnutbio.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 80. Wang L, Shi M, Tong L, et al. Lung‐resident mesenchymal stem cells promote repair of LPS‐induced acute lung injury via regulating the balance of regulatory T cells and Th17 cells. Inflammation. 2019;42(1):199‐210. doi: 10.1007/s10753-018-0884-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared.


