Abstract
Women with pathogenic germline BRCA1 or BRCA2 variants have a higher risk of breast cancer than in the general population. International guidelines recommend specific clinical and radiological breast follow-up. This specific breast screening program has already been shown to be of clinical benefit, but no information is available concerning the use of prognostic factors or specific survival to guide follow-up decisions. We evaluated “high-risk” screening in a retrospective single-center study of 520 women carrying pathogenic germline variants of the BRCA1 or BRCA2 gene treated for breast cancer between January 2000 and December 2016. We compared two groups of women: the incidental breast cancer group (IBCG) were followed before breast cancer diagnosis (N = 103), whereas the prevalent breast cancer group (PBCG) (N = 417) had no specific follow-up for high risk before breast cancer diagnosis. Breast cancers were diagnosed at an earlier stage in the IBCG than in the PBCG: T0 in 64% versus 19% of tumors, (p < 0.00001), and N0 in 90% vs. 75% (p < 0.00001), respectively. Treatment differed significantly between the 2 groups: less neoadjuvant chemotherapy (7.1% vs. 28.5%, p < 0.00001), adjuvant chemotherapy (47.7% vs. 61.9%, p = 0.004) and more mastectomies (60% vs. 42% p < 0.0001) in the IBCG vs PBCG groups respectively. Overall and breast cancer-specific mortality were similar between the two groups. However, the patients in the IBCG had a significantly longer metastasis-free survival than those in the PBCG, at three years (96.9% [95% CI 93.5–100] vs. 92.30% [95% CI 89.8–94.9]; p = 0.02), suggesting a possible long-term survival advantage.
Subject terms: Cancer genetics, Breast cancer, Cancer genetics
Introduction
Women with pathogenic germline BRCA1 or BRCA2 variants have a high risk of breast and ovarian cancer. Their cumulative risk of breast cancer by the age of 80 years has been estimated at 72% (95% CI: 65–79%) for women with BRCA1 variants and 69% (95% CI: 61–77%) for those BRCA2 variants. Annual breast cancer incidence increases steadily with age, reaching a maximum between the ages of 30 and 50 years for BRCA1 carriers, and between the ages of 40 and 60 years for BRCA2 carriers. Thereafter, breast cancer incidence remains constant for both types of BRCA variant (20–30 per 1000 person-years) [1]. A number of national guidelines recommend specific breast screening programs, with annual mammograms and breast magnetic resonance imaging (MRI) beginning at the age of 25 or 30 years. Ultrasound scans may also be considered [2–4]. Since 2009, French national guidelines have recommended a similar radiological approach [5, 6]. Bilateral prophylactic mastectomy is an alternative to radiological surveillance [7, 8]. However, this surgery is not without complications and has a major impact on body image, anxiety and sexuality [9–11]. According to French national guidelines, it is reasonable to perform such surgery from the age of 30 years onwards [5]. Many studies have evaluated the impact of specific high-risk breast screening on the clinical characteristics of tumors in cohorts of patients with pathogenic BRCA1 or BRCA2 variants or patients considered at risk of breast cancer [12–24]. However, only a few studies have assessed the impact of specific screening on overall survival or relapse-free interval in susceptible women [14, 25–27]. The purpose of this observational and retrospective study was to evaluate the benefits of intensive clinical and radiological surveillance in terms of breast cancer characteristics in a French single-center cohort of women carrying pathogenic germline BRCA1 or BRCA2 variants. Women with genetic alterations of BRCA1 or BRCA2 identified at Institut Curie and treated for breast cancer were assigned to two groups. The first was a group of women receiving radiological follow-up for a high risk of breast cancer linked to genetic knowledge obtained before the diagnosis of breast cancer: the incident breast cancer group (IBCG). These women were cancer-free at the time of genetic testing, the variant having been detected in a relative. The women in the other group, the prevalent breast cancer group (PBCG), underwent classic breast monitoring before cancer diagnosis (annual breast examination with or without a mammogram every year, depending on family history), but their genetic status was unknown at the time of screening, they had the follow-up of women “in real life”: a regular or not, follow-up with their gynecologists, and according to the family history, they could already have started the breast radiological monitoring, on an annual basis or every 18 months/2 years. The primary outcome was impact on histological and clinical tumor features. The secondary outcomes concerned prognosis: specific or overall survival and metastasis-free survival. We also investigated whether treatment decisions were modified by the knowledge of genetic status.
Materials and methods
Study population
Between January 2000 and December 2016, 585 women carrying a pathogenic germline BRCA1/2 variant and treated for at least one breast cancer, were seen at Institut Curie for genetic testing or for breast cancer treatment. The study was approved by the Breast Cancer Study Group of Institut Curie and was conducted according to institutional and ethical rules concerning research on tissue specimens and patients. We excluded two women carrying double pathogenic variants (BRCA1/BRCA2 and BRCA1/CDH1), and 63 women for whom no precise data concerning the date of breast cancer diagnosis were available. The final analysis concerned 625 breast cancers in 520 women (unilateral tumors and some contralateral recurrences). Ipsilateral recurrences were not taken into account.
Study design
Two groups were defined on the basis of the type of clinical and radiological surveillance at the time of breast cancer diagnosis. The women in the IBCG were monitored according to national guidelines for high-risk group: clinical breast examination every six months, breast MRI and mammography annually, from the age of 30 years, and, ultrasound examinations if deemed necessary by the radiologist. These three imaging examinations were performed in this order, within two months. MRI examinations began before the age of 30 years in women with a familial history of breast cancer in a young relative. After the age of 65 years, women were followed annually by mammography and ultrasound (if requested by the radiologist). Women not screened in this way before breast cancer diagnosis were included in the PBCG. It was possible for a woman to switch groups between the first breast cancer and a subsequent cancer, on the basis of genetic testing. Women in the IBCG who became pregnant discontinued screening until four to six months after delivery. We included women whose breast cancers were diagnosed as a result of genetic analysis, during the first radiological screening, in the PBCG. It was therefore possible to assess the possible benefits of enhanced surveillance only from the second clinical and radiological examination onwards, not at the first round, and we took into account all breast cancers occurring during follow-up or between rounds of screening.
Outcomes
The primary outcome of this study was the oncological, clinical, and pathological characteristics of the breast cancers diagnosed in the two groups (PBCG and IBCG). The secondary outcomes were metastasis-free interval, overall and breast cancer-specific mortality, calculated by screening group.
Examinations
The imaging examinations performed in this study were mammograms (oblique and craniocaudal views for women with a history of breast cancer, and oblique views only for women with no history of breast cancer), gadolinium-chelate contrast-enhanced breast MRI, and ultrasound scans if requested by the radiologist. All of these examinations were assessed according to the Breast Imaging Reporting and Data System (BI-RADS). A screening test was considered positive if the BI-RADS assessment category was 4 or more. BI-RADS category assessment was not mandatory before 2004. For tumors diagnosed before 2004, we considered screening tests to be positive if the examination was followed by a histological examination. Multicentric tumors in a single breast were considered as single cancer, and only the size of the largest invasive component was taken into account.
Specific features of the tumors
For each cancer, clinical stage at diagnosis was reported according to the clinical or histological classification of the American Joint Committee on Cancer (AJCC). Histological type (invasive ductal carcinoma (IDC), invasive lobular carcinoma (ILC), mixed, or ductal carcinoma in situ (DCIS)) and Elston and Ellis grade were then analyzed. Hormone receptor status (considered positive if 10% or more of cells expressed hormonal receptors) and HER2/neu receptor overexpression were also noted.
Treatments
All treatments administered were recorded for each breast cancer. The type of surgery was analyzed: mastectomy or breast-conserving surgery, axillary lymphadenectomy, or sentinel node biopsy. Adjuvant or neo-adjuvant chemotherapy, endocrine therapy, and radiotherapy were recorded, together with any bilateral or contralateral prophylactic breast surgery and prophylactic salpingo-oophorectomy.
Data analysis
Statistical analysis was performed with R Software (R Core Team, 2016). The differences in tumor characteristics between the two groups were compared by χ² tests, with Yates correction or Fisher’s exact tests if required. A p value of less than 0.05 in a two-tailed test was considered statistically significant. Furthermore, after the diagnosis of breast cancer during the first round of monitoring, 26 women were placed in the PBCG. For the analysis of prognosis, patients assigned to the PBCG for their first cancer, and then to the IBCG for a subsequent contralateral cancer, were included in the PBCG; 41 women were in this situation. Indeed, prognosis is linked principally to the characteristics of the first breast cancer. Prognosis was analyzed in both univariate (by log rank test) and multivariate analyses, taking into account age at diagnosis, mutational status (BRCA1 or BRCA2) and the occurrence of a contralateral breast cancer during follow-up.
Results
The IBCG contained 155 breast tumors (24.8%) with a median follow-up of 5.4 years, and the PBCG contained 470 breast tumors (75.2%) with a median follow-up of 9.3 years (Table 1).
Clinical and pathological data
Table 1.
Characteristics by group.
| Number of patients: N (%) | IBCG (103) (%) | PBCG (417) (%) |
|---|---|---|
| BRCA1m | 65 (64.5) | 221 (53) |
| BRCA2m | 38 (35.5) | 196 (47) |
| BC Unilaterality | 92 (89.3) | 323 (77.4) |
| Bilaterality | 11 (10.7) | 94 (22.6) |
| Follow-up (median) | 65 months (5.4 years) | 111 months (9.3 years) |
| Age at first breast cancer | Range: 24–81, median: 42 mean: 44.3 | Range: 21–80, median: 40 mean: 41.8 |
| <30 | 6 (5.8) | 36 (8.6) |
| (30–40) | 29 (28.2) | 165 (39.6) |
| (40–50) | 40 (38.8) | 123 (29.5) |
| (50–60) | 19 (18.5) | 65 (15.6) |
| ≥60 | 9 (8.7) | 28 (6.7) |
| BMI | N = 102: median:22.6 Mean: 24 | N = 406: median: 22.5 mean: 23.5 |
| Death during follow-up | 6 | 64 |
| Breast cancer | 4 | 50 |
| Ovarian cancer | 0 | 8 |
| Pancreatic cancer | 1 | 1 |
| Other cancer | 0 | 4* |
| Other cause | 1 | 1 |
| Number of prophylactic salpingo-oophorectomies | 83 | 298 |
| Median age at surgery: | 45.4 (35–71) | 48.9 (31–73) |
Abbreviations: IBCG: incident breast cancer group, PBCG: prevalent breast cancer group.
*: 1 cholangiocarcinoma, 2 lung cancers, and 1 colorectal cancer.
The tumor could be detected by palpation for 30% of the IBCG tumors and 76% of the PBCG tumors (p < 0.00001) (Table 2). Overall, 64% of the tumors in the IBCG and 19% in the PBCG were detected at the subclinical stage (T0) (p < 0.00001). There was also a significant difference in clinical node status between the two groups (90% vs. 75% N0, p < 0.00001). No metastatic disease was reported at diagnosis in the IBCG, whereas 10 tumors were already metastatic at diagnosis in the PBCG, but this difference was not statistically significant (NS) (p = 0.12) (eFigure 1).
Table 2.
Clinical and pathological status, according to screening program.
| IBCG N = 155 (%) | PBCG N = 470 (%) | P value | |
|---|---|---|---|
| Diagnosis by clinical examination of the breast | 149 | 426 | <0.00001 |
| Yes | 45 (30.0) | 325 (76.0) | |
| No | 104 (70.0) | 101 (24.0) | |
| Clinical tumor size (TNM stage) | 149 | 422 | <0.00001 |
| 0 (tumor not palpable) | 96 (64.0) | 79 (19.0) | |
| 1 (<20 mm) | 39 (26.0) | 142 (33.0) | |
| 2 (20-50 mm) | 14 (10.0) | 141 (33.0) | |
| 3 (>50 mm) | 0 (0) | 47 (12.0) | |
| 4 (extension to skin/chest wall or inflammatory) | 0 (0) | 13 (0) | |
| Clinical node involvement (TNM stage) | 149 | 421 | <0.00001 |
| 0 | 134 (90.0) | 275 (75.0) | |
| 1 (1 to 3 lymph nodes involved) | 13 (9.0) | 134 (32.0) | |
| 2–3 (>4 lymph nodes involved) | 2 (1.0) | 12 (2.0) | |
| Clinical M (TNM stage) | 149 | 422 | 0.12 |
| M0 | 149 (100.0) | 412 (98.0) | |
| M1 | 0 (0) | 10 (2.0) | |
| Histological type | 152 | 463 | <0.0001 |
| DCIS | 36 (23.7) | 35 (7.6) | |
| Low grade | 4 (11.1) | 3 (9) | NS |
| Intermediate grade | 8 (22.2) | 13 (37) | |
| High grade | 24 (66.7) | 19 (54) | |
| IDC | 108 (71.0) | 405 (87.5) | |
| ILC+/−IDC | 5 (3.3) | 17 (3.6) | |
| Other | 3 (2.0) | 6 (1.3) | |
| Unknown | 3 | 7 | |
| Molecular phenotype (invasive tumors) | 115 | 422 | NS |
| HER2+++ | 2 (1.7) | 14 (3.3) | |
| HR−/HER2− | 61 (53.0) | 201 (47.6) | |
| HR−/HER2? | 1 (0.9) | 13 (3.0) | |
| HR+/HER2? | 1 (0.9) | 27 (6.4) | |
| HR+/HER2− | 50 (43.6) | 167 (39.7) | |
| Unknown | 4 | 13 | |
| Nodal status (invasive tumors) | 119 | 435 | <0.0001 |
| Negative | 84 (81.5) | 185 (63.1) | |
| Positive | 19 (18.5) | 108 (36.9) | |
| Unknown | 16 | 142 | |
| EE Grade | 116 | 428 | NS |
| I–II | 41 (36.9) | 139 (33.2) | |
| III | 70 (63.1) | 280 (66.8) | |
| Unknown | 5 | 9 |
Abbreviations: IBCG: incident breast cancer group, PBCG: prevalent breast cancer group.
DCIS: ductal carcinoma in situ, IDC: invasive ductal carcinoma, ILC: invasive lobular carcinoma.
HER2+++: human epidermal growth factor receptor-2 amplified, HR: hormone receptor status.
Positive or negative. EE grade: Elston and Ellis grade.
The frequency of DCIS without invasive component was significantly higher in the IBCG than in the PBCG (23.7% vs. 8% p < 0.0001) and in the IBCG, 24 (66.7%) were High-grade DCIS. The frequency of negative lymph nodes on pathology was significantly higher in the IBCG (81.5% vs. 63.1%, p < 0.0001). Histological prognosis grade for invasive breast cancer did not differ significantly between screening groups. The proportion of the triple-negative phenotype was similar in the two groups: 52.2% vs. 47% (NS).
-
Sensitivity of examinations
In the IBCG, 76% of tumors were imaged by breast MRI and 60.5% by mammography (Table 3). Complementary breast ultrasound scans were performed at diagnosis for 147 tumors (90.7%), and all three examinations were performed at diagnosis for 106 tumors. Moreover, 42.4% of these tumors were identified by all three examinations: breast MRI, mammography, and breast ultrasound. For 29 tumors (27.3%), only one of the three examinations yielded a positive result (for example, positive breast MRI but no abnormality detected on the other two examinations). The sensitivity of the examinations was 76% for breast MRI (including all types of cancer: invasive and DCIS), 60.5% for mammography, and 76% for ultrasound scans. In total, 18 of the 32 tumors not visible on MRI (56%) were DCIS.
-
Treatments
Radical mastectomy was performed for 60% of IBCG tumors vs. 42% of PBCG tumors, and breast-conserving surgery was performed for 37% (vs. 56%, p < 0.0001) (eFigure 2). In addition, among these 94 women who received a therapeutic mastectomy, 75 (80%) subsequently received a contralateral preventive mastectomy during the follow-up. Treatment data were missing for three women. Axillary lymphadenectomy was performed for 23.9% of the IBCG tumors (vs. 67.2% of the PBCG tumors) and sentinel node biopsy was performed for 61.3% of the IBCG tumors (vs. 26.6%); no axillary surgery was performed for 12.2% of IBCG tumors (vs 4.9%, p < 0.0001). Radiotherapy was performed in addition to surgery in 64.5% of cases in the IBCG (vs. 87.6% in the PBCG, p < 0.0001). Neoadjuvant chemotherapy was administered for 7.1% of IBCG tumors (vs. 28.5% of PBCG tumors, p < 0.00001) and adjuvant chemotherapy was administered for 47.7% of IBCG tumors (vs. 61.9% of PBCG tumors, p < 0.004) (eFigure 3). Endocrine therapy was provided for 30.3% of IBCG tumors (vs. 40.6% of PBCG tumors, p = 0.06), (Table 4). Significantly less neoadjuvant chemotherapy was prescribed for triple-negative breast cancer in the IBCG than in the PBCG (11% versus 40%, p < 0.05).
Breast cancer-specific survival, overall survival, and metastasis-free survival
Table 3.
Sensitivity of the examinations.
| IBCG | |
|---|---|
| N = 155 (%) | |
| All 3 examinations performed | 106 |
| All 3 examinations positive for cancer diagnosis | 45 (42.4) |
| Tumors with only one positive examination | 29 (27.3) |
| Only MRI positive | 14 (13.2) |
| Only mammogram positive | 8 (7.5) |
| Only ultrasound positive | 7 (6.6) |
| Breast MRI sensitivity | 97/128 (76.0) |
| Mammogram sensitivity | 80/133 (60.5) |
| Ultrasound sensitivity | 102/136 (75.0) |
Abbreviations: IBCG: incident breast cancer group: women were followed according to the French guidelines, with a clinical examination every six months and breast MRI, mammogram+/− ultrasound annually.
Table 4.
Breast cancer treatment according to the type of follow-up.
| Population n = 625 (%) | IBCG n = 155 (%) | PBCG n = 470 (%) | p- value | |
|---|---|---|---|---|
| Breast surgery | <0.0001 | |||
| No | 3 (0.2) | 0 (0) | 3 (0.6) | |
| Mastectomy | 293 (47) | 94 (60.6) | 199 (42.3) | |
| Breast-conserving surgery | 324 (52) | 58 (37.5) | 266 (56.7) | |
| Unknown | 5 (0.8) | 3 (1.9) | 2 (0.4) | |
| Axillary surgery | <0.0001 | |||
| No | 42 (6.7) | 19 (12.2) | 23 (4.9) | |
| Lymphadenectomy | 353 (56.5) | 37 (23.9) | 316 (67.2) | |
| Sentinel node biopsy | 220 (35.2) | 95 (61.3) | 125 (26.6) | |
| Unknown | 10 (1.6) | 4 (2.6) | 6 (1.3) | |
| Neoadjuvant chemotherapy | <0.00001 | |||
| No | 474 (75.9) | 140 (90.3) | 334 (71.0) | |
| Yes | 145 (23.2) | 11 (7.1) | 134 (28.5) | |
| Unknown | 6 (0.9) | 4 (2.6) | 2 (0.5) | |
| Adjuvant chemotherapy | =0.004 | |||
| No | 248 (39.7) | 76 (49.0) | 172 (36.6) | |
| Yes | 365 (58.4) | 74 (47.7) | 291 (61.9) | |
| Unknown | 12 (1.9) | 5 (2.6) | 7 (1.5) | |
| Radiotherapy | <0.0001 | |||
| No | 102 (16.3) | 51 (32.9) | 51 (10.8) | |
| Yes | 512 (81.9) | 100 (64.5) | 412 (87.7) | |
| Unknown | 11 (1.8) | 4 (2.6) | 7 (1.5) | |
| Hormone treatment | ||||
| No | 367 (58.7) | 98 (63.2) | 269 (57.2) | =0.06 |
| Yes | 238 (38.1) | 47 (30.3) | 191 (40.6) | |
| Unknown | 20 (3.2) | 10 (6.5) | 10 (2.2) |
***p < 0.0001, ****p < 0.00001.
NSS group: non specific screening group, ISP group: Intensive screening group.
Abbreviations: IBCG: incident breast cancer group.
PBCG: prevalent breast cancer group.
The two groups did not differ for overall or breast cancer-specific survival, with a follow-up of 101 months (range: 1–229 months). During follow-up, 70 deaths occurred in the two groups (Table 1). These deaths included four deaths from breast cancer in the IBCG, 50 in the PBCG, and eight deaths from ovarian cancer, all in the PBCG. For this particular risk, which is specific to pathogenic germline BRCA1/2 variants, 83 women in the IBCG and 298 women in the PBCG underwent prophylactic salpingo-oophorectomy at a median age of 45.4 years and 48.9 years, respectively (Table 1). Other BRCA-related tumors were also observed, with two women (one in each group) dying from pancreatic cancer. There were four deaths from other cancers (one cholangiocarcinoma, two lung cancers, and one colorectal cancer) and two deaths from other causes.
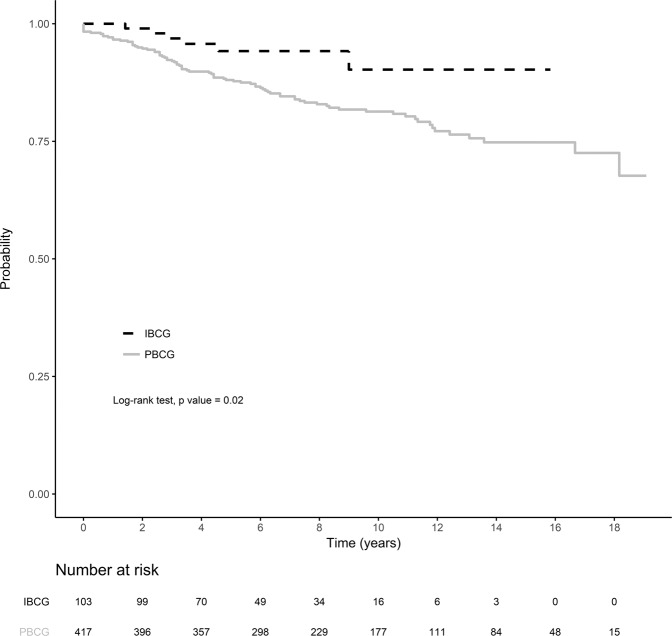
Four women from the IBCG and 68 from the PBCG are being treated for metastatic breast cancer. This corresponds to a statistically significant difference in metastasis-free survival rates at three years between the two cohorts: 96.9% [95% CI: 93.5–100] for the IBCG vs. 92.3% [95% CI: 89.8–94.9] for the PBCG; p = 0.02 (Fig. 1). This difference remained significant in multivariate analysis including mutational status (BRCA1 versus BRCA2) and age at first diagnosis into account (Cox model) (p-value = 0.03).
Fig. 1. Metastasis-free survival by screening group.
Metastasis-free survival was compared in the two groups: IBPG (incident breast cancer group) and the PBCG (prevalent breast cancer group). Metastasis-free survival was defined as time from histological diagnosis to breast cancer-specific metastasis or death, whichever occurred first. Differences in breast cancer-specific metastasis-free survival were compared in log-rank tests. Red: IBCG group (103 women, 6 with metastasis or death). Blue: PBCG group (417 women, 82 with metastasis or death). ***p < 0.0001, ****p < 0.00001.
Discussion
The goal of this study was to evaluate the benefits of national “high-risk” breast cancer screening guidelines in terms of the characteristics of tumors and prognosis. Enhanced radiological monitoring including breast MRI is known to be beneficial for women at high risk of breast cancer. In our study, this close monitoring significantly improves the clinical detection of smaller breast cancers and lowers axillary lymph node involvement, which translates into a significantly longer metastasis-free interval.
However, these potentially interesting results must be seen in the context of the biases inherent to retrospective studies. Breast cancer diagnosis (improvement of imaging) and treatment have changed considerably over the last 20 years, both in terms of the identification of pathological elements required for its management (HER2 status) and in terms of surgical (sentinel lymph node biopsy rather than lymphadenectomy) and oncological (modification of adjuvant chemotherapy) indications. All these changes can also modify the prognosis of treated breast cancers. Otherwise, the absence of precise data on breast monitoring of women in the PBCG, before cancer diagnosis does not provide a homogenous view of these patients.
Moreover, we choose not to take into account in the metastasis-free survival the lead-time bias; this statistical bias needs an adjustment in particular in breast organized screening in postmenopausal women who often present breast tumors with low grade. Koscielny and Tubiana in 1991 were able to deduce the tumor doubling time as a function of grade and axillary involvement [28]. We have no data on the natural history of those cancers, often high grade; in women carrying a germline pathogenic predisposition. Under these conditions, the lied time bias must be short and certainly not modify the data.
Furthermore, high-risk follow-up significantly increases the rate of DCIS detection. This type of breast cancer has a better prognosis, and can be managed with complementary treatments with lower levels of associated morbidity [16, 20]. Given the risk of breast cancer in this population, we do not believe that the higher rates of DCIS detection reported here are due to overdiagnosis. Another study showed that the early changes to mammary myoepithelial cell differentiation observed in sporadic DCIS were also found in healthy breast tissue from women with a predisposition to breast cancer [29].
We also report lower levels of associated treatments (neoadjuvant or adjuvant chemotherapy and complementary radiotherapy) likely to generate immediate or delayed adverse effects potentially decreasing quality of life (infertility or lymphoedema) [30, 31] that may be more frequent in women with mutations [32–35]. Thus, the monitoring of carriers of pathogenic germline BRCA1/2 variants can reduce the morbidity associated with surgical, oncological, and radiotherapy treatments, however, this follow-up could generate harmful effects (false positives, anxiety…). Women with a germline BRCA1/2 pathogenic variant have, in literature, the rate of ACR3 in MRI above 6 to 10% [36–39]. In Edmonds et al., during the first cycle the rate is evaluated at 8.5% then passes to 2% during the follow cycles [36]. In addition, in Vreemann et al., radiological false positives, as well as those leading to a negative biopsy in BRCA mutated women, were compared with women without a genetic predisposition [40]. In view of the higher risk of breast cancer in the group of women carrying a BRCA1/2 pathogenic variant, there were significantly fewer false positives in this group. Taking into account the risk of cancer in these women (annual risk of breast cancer that varies between 1 and 2%), Furthermore, this false positive rate remains acceptable and the benefits of intensive screening outweigh the risks of harm.
Remarkably, when women know their genetic status, they often request therapeutic radical mastectomy, even though they could potentially benefit from breast-conserving surgery. Of note, breast-cancer specific mortality is identical between mastectomy and breast-conserving surgery in the general population [41]. However, in the meta-analysis performed by Valachis, the risk of ipsilateral breast cancer appears to be higher in these women seven years after the end of radiotherapy [42]. Furthermore, knowledge of their genetic predisposition provides these women with a better understanding of the risk of contralateral breast cancer risk relative to that in non-carriers (1.5–3% increase in risk annually vs. 0.5% for non-carriers) [1, 42, 43], which can be managed by contralateral radical mastectomy. When in possession of this information, given the possibility of prophylactic surgery on the contralateral breast, which can be performed sometime after breast cancer treatment, women more frequently request a mastectomy, even when breast-conserving surgery is possible. Thus, we can identify in our IBCG population, 94 women who performed a therapeutic mastectomy and among them, 75 women (80%) had a contralateral preventive mastectomy during follow-up.
Despite significant differences in breast cancer stage and axillary involvement between the groups, there was no significant difference in breast cancer-specific mortality or overall mortality. This lack of difference can be explained by the favorable prognosis for breast cancer at the five-year time point. Moreover, the presence of a pathogenic BRCA1/2 variant is not associated with a negative prognosis. A comparison of the breast cancer-specific survival of women with breast cancer as a function of the presence or absence of such alterations, with matching according to the molecular characteristics of cancer, showed this survival to be similar in the two groups [44]. Indeed, breast cancer-specific survival may even be slightly better in the first two years after triple-negative breast cancer in women with BRCA1/2 mutations [44]. Most of the breast cancers in our cohort were diagnosed at an early stage (less than 15% T3 or 4 in the PBCG and 0 T3 or 4 in the IBCG) [45]. It was not possible to demonstrate a benefit in terms of breast cancer-specific mortality in this study. Such a demonstration would require a very long follow-up, which was not possible for the IBCG since breast MRI was not incorporated into the French guidelines for the management of BRCA1/2 carriers until 2009. However, metastasis-free survival seemed to be better in the IBCG than in the PBCG which could potentially result in a specific survival benefit for women in the IBCG. This finding thus indicates that close follow-up significantly decreases the risk of having metastatic breast disease, and this information is of the utmost importance for our patients. It is interesting to compare our study with that of Hadar et al., which is the most similar in design to our study [27]. Hadar et al. showed that enhanced radiological surveillance may improve patient survival, through the identification of a larger number of DCIS (although patients were older at diagnosis in his cohort and the frequency of DCIS may be higher than published rates) [15, 16, 18, 44, 45]. Our data highlight a similar pattern, and confirm the benefit of this follow-up in terms of the histological prognostic characteristics of invasive breast cancers.
The starting point for follow-up adapted to breast and ovarian cancer risk remains the targeted test or genetic analysis. However, Alegre et al., show that knowledge about genetic predisposition is poorly transmitted within families [46]. It is very important, in the context of breast surveillance to improve prognosis, to enhance communication within families, and to offer genetic analysis to any woman with personal or familial criteria for such analysis [47]. In addition, a knowledge of genetic status makes it possible to provide care in specialized units aware of the follow-up recommendations and used to these specific situations [48, 49].
Conclusion
This is the largest published study to date on the benefits of enhanced follow-up in women with a pathogenic germline BRCA1 or BRCA2 variant treated for breast cancer. It provides a number of interesting findings: close clinical and radiological surveillance improves the clinical prognosis criteria of identified breast cancers (based on tumor size and lymph node involvement), but also the histological prognosis criteria (higher proportion of DCIS). Together, these two factors lead to a significant decrease in associated treatments (neoadjuvant and adjuvant chemotherapy and radiotherapy), reducing the morbidity linked to the management of breast cancer. However, this approach has not yet displayed any proven benefits in terms of breast cancer-specific survival, although the findings for earlier signs are encouraging (significant increase in metastasis-free survival). In light of the benefits identified here, studies should be performed following women in two groups managed in different ways: enhanced clinical and radiological monitoring or prophylactic breast surgical management, because each of these options is beneficial, but has its own specific constraints. Finally, these data argue for the earliest possible identification of women with a genetic predisposition: it is imperative that women with personal or family indications for genetic analysis are correctly referred to genetic services.
Supplementary information
Author contributions
Study design: CS, SMH, EMF, DSL. Data collection: CS, SMH, EMF. Data analysis: EMF, MC. Data interpretation: CS, SMH, EMF. Writing: CS, SMH, EMF. Extensive revision of the manuscript: CS, SMH, MC, CM, PC, FR, ML, EG, AD, NC, SF, FC, DSL, EMF.
Funding
This project has not received specific funding.
Data availability
The clinical data analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Ethical approval
The study was approved by the Breast Cancer Study Group of Institut Curie and was conducted according to institutional and ethical rules concerning research on tissue specimens and patients.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Claire Saule, Solveig Menu-Hespel.
Supplementary information
The online version contains supplementary material available at 10.1038/s41431-022-01049-2.
References
- 1.Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips K-A, Mooij TM, Roos-Blom M-J, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317:2402–16. doi: 10.1001/jama.2017.7112. [DOI] [PubMed] [Google Scholar]
- 2.National Collaborating Centre for Cancer (UK). Familial Breast Cancer: Classification and Care of People at Risk of Familial Breast Cancer and Management of Breast Cancer and Related Risks in People with a Family History of Breast Cancer [Internet]. Cardiff (UK): National Collaborating Centre for Cancer (UK); 2013 [cited 2020 Feb 18]. (National Institute for Health and Clinical Excellence: Guidance). Available from: http://www.ncbi.nlm.nih.gov/books/NBK247567/. [PubMed]
- 3.Paluch-Shimon S, Cardoso F, Sessa C, Balmana J, Cardoso MJ, Gilbert F, et al. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann Oncol. 2016;27:v103–10. doi: 10.1093/annonc/mdw327. [DOI] [PubMed] [Google Scholar]
- 4.Daly MB, Pilarski R, Yurgelun MB, Berry MP, Buys SS, Dickson P, et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 1.2020 [Internet]. J Natl Compr Cancer Netw.: JNCCN. 2020 [cited 2020 Dec 8]. Available from: https://pubmed.ncbi.nlm.nih.gov/32259785/.
- 5.Synthèse - Femmes porteuses d’une mutation de BRCA1 ou BRCA2/Détection précoce du cancer du sein et des annexes et stratégies de réduction du risque - Ref: RECOBRCASYNTH17 | Institut National Du Cancer [Internet]. [cited 2017 Aug 15]. Available from: http://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publications/Synthese-Femmes-porteuses-d-une-mutation-de-BRCA1-ou-BRCA2-Detection-precoce-du-cancer-du-sein-et-des-annexes-et-strategies-de-reduction-du-risque.
- 6.Chirurgie prophylactique des cancers avec prédisposition génétique - Ref: RECOCHIRPRO09 [Internet]. [cited 2020 Feb 25]. Available from: https://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publications/Chirurgie-prophylactique-des-cancers-avec-predisposition-genetique.
- 7.Jakub JW, Peled AW, Gray RJ, Greenup RA, Kiluk JV, Sacchini V, et al. Oncologic safety of prophylactic nipple-sparing mastectomy in a population with BRCA mutations: a multi-institutional study. JAMA Surg. 2018;153:123–9. doi: 10.1001/jamasurg.2017.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartmann LC, Lindor NM. The role of risk-reducing surgery in hereditary breast and ovarian cancer. N. Engl J Med. 2016;374(Feb):454–68. doi: 10.1056/NEJMra1503523. [DOI] [PubMed] [Google Scholar]
- 9.Heemskerk-Gerritsen BAM, Jager A, Koppert LB, Obdeijn AI-M, Collée M, Meijers-Heijboer HEJ, et al. Survival after bilateral risk-reducing mastectomy in healthy BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat. 2019;177(Oct):723–33. doi: 10.1007/s10549-019-05345-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurian AW, Sigal BM, Plevritis SK. Survival analysis of cancer risk reduction strategies for BRCA1/2 mutation carriers. J Clin Oncol. 2010;28(Jan):222–31. doi: 10.1200/JCO.2009.22.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rojas KE, Butler E, Gutierrez J, Kwait R, Laprise J, Wilbur JS, et al. Choosing high-risk screening vs. surgery and the effect of treatment modality on anxiety and breast-specific sensuality in BRCA mutation carriers. Gland Surg. 2019;8(Jun):249–57. doi: 10.21037/gs.2019.04.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krammer J, Pinker-Domenig K, Robson ME, Gönen M, Bernard-Davila B, Morris EA, et al. Breast cancer detection and tumor characteristics in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat. 2017;163(Jun):565–71. doi: 10.1007/s10549-017-4198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phi X-A, Saadatmand S, De Bock GH, Warner E, Sardanelli F, Leach MO, et al. Contribution of mammography to MRI screening in BRCA mutation carriers by BRCA status and age: individual patient data meta-analysis. Br J Cancer. 2016;114(Mar):631–7. doi: 10.1038/bjc.2016.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans DG, Gareth ED, Kesavan N, Nisha K, Lim Y, Yit L, et al. MRI breast screening in high-risk women: cancer detection and survival analysis. Breast Cancer Res Treat. 2014;145(Jun):663–72. doi: 10.1007/s10549-014-2931-9. [DOI] [PubMed] [Google Scholar]
- 15.Passaperuma K, Warner E, Causer PA, Hill KA, Messner S, Wong JW, et al. Long-term results of screening with magnetic resonance imaging in women with BRCA mutations. Br J Cancer. 2012;107(Jun):24–30. doi: 10.1038/bjc.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warner E, Hill K, Causer P, Plewes D, Jong R, Yaffe M, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29(May):1664–9. doi: 10.1200/JCO.2009.27.0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leach MO, Boggis CRM, Dixon AK, Easton DF, Eeles RA, Evans DGR, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS) Lancet. 2005;365(May):1769–78. doi: 10.1016/S0140-6736(05)66481-1. [DOI] [PubMed] [Google Scholar]
- 18.Kriege M, Brekelmans CTM, Boetes C, Besnard PE, Zonderland HM, Obdeijn IM, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N. Engl J Med. 2004;351(Jul):427–37. doi: 10.1056/NEJMoa031759. [DOI] [PubMed] [Google Scholar]
- 19.Murakami W, Tozaki M, Nakamura S, Ide Y, Inuzuka M, Hirota Y, et al. The clinical impact of MRI screening for BRCA mutation carriers: the first report in Japan. Breast Cancer. 2019;26(Sep):552–61. doi: 10.1007/s12282-019-00955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chéreau E, Uzan C, Balleyguier C, Chevalier J, de Paillerets BB, Caron O, et al. Characteristics, treatment, and outcome of breast cancers diagnosed in BRCA1 and BRCA2 gene mutation carriers in intensive screening programs including magnetic resonance imaging. Clin Breast Cancer. 2010;10(Apr):113–8. doi: 10.3816/CBC.2010.n.022. [DOI] [PubMed] [Google Scholar]
- 21.Saadatmand S, Geuzinge HA, Rutgers EJT, Mann RM, de Roy van Zuidewijn DBW, Zonderland HM, et al. MRI versus mammography for breast cancer screening in women with familial risk (FaMRIsc): a multicentre, randomised, controlled trial. Lancet Oncol. 2019;20(Aug):1136–47. doi: 10.1016/S1470-2045(19)30275-X. [DOI] [PubMed] [Google Scholar]
- 22.Riedl CC, Luft N, Bernhart C, Weber M, Bernathova M, Tea M-KM, et al. Triple-modality screening trial for familial breast cancer underlines the importance of magnetic resonance imaging and questions the role of mammography and ultrasound regardless of patient mutation status, age, and breast density. J Clin Oncol. 2015;33(Apr):1128–35. doi: 10.1200/JCO.2014.56.8626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bick U, Engel C, Krug B, Heindel W, Fallenberg EM, Rhiem K, et al. High-risk breast cancer surveillance with MRI: 10-year experience from the German consortium for hereditary breast and ovarian cancer. Breast Cancer Res Treat. 2019;175(May):217–28. doi: 10.1007/s10549-019-05152-9. [DOI] [PubMed] [Google Scholar]
- 24.Evans DG, Howell SJ, Gandhi A, van Veen EM, Woodward ER, Harvey J, et al. Breast cancer incidence and early diagnosis in a family history risk and prevention clinic: 33-year experience in 14,311 women. Breast Cancer Res Treat. 2021;189(Oct):677–87. doi: 10.1007/s10549-021-06333-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Møller P, Stormorken A, Jonsrud C, Holmen MM, Hagen AI, Clark N, et al. Survival of patients with BRCA1-associated breast cancer diagnosed in an MRI-based surveillance program. Breast Cancer Res Treat. 2013;139(May):155–61. doi: 10.1007/s10549-013-2540-z. [DOI] [PubMed] [Google Scholar]
- 26.Saadatmand S, Obdeijn I-M, Rutgers EJ, Oosterwijk JC, Tollenaar RA, Woldringh GH, et al. Survival benefit in women with BRCA1 mutation or familial risk in the MRI screening study (MRISC) Int J Cancer. 2015;137(Oct):1729–38. doi: 10.1002/ijc.29534. [DOI] [PubMed] [Google Scholar]
- 27.Hadar T, Mor P, Amit G, Lieberman S, Gekhtman D, Rabinovitch R, et al. Presymptomatic awareness of germline pathogenic BRCA variants and associated outcomes in women with breast cancer. JAMA Oncol. 2020;6:1460–3. [DOI] [PMC free article] [PubMed]
- 28.Tubiana M, Koscielny S. Natural history of human breast cancer: recent data and clinical implications. Breast Cancer Res Treat. 1991;18(Aug):125–40. doi: 10.1007/BF01990028. [DOI] [PubMed] [Google Scholar]
- 29.Ding L, Su Y, Fassl A, Hinohara K, Qiu X, Harper NW, et al. Perturbed myoepithelial cell differentiation in BRCA mutation carriers and in ductal carcinoma in situ. Nat Commun. 2019;10:4182. doi: 10.1038/s41467-019-12125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ter Welle-Butalid MEE, Vriens IJHI, Derhaag JGJ, Leter EME, de Die-Smulders CEC, Smidt MM, et al. Counseling young women with early breast cancer on fertility preservation. J Assist Reprod Genet. 2019;36(Dec):2593–604. doi: 10.1007/s10815-019-01615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terada M, Yoshimura A, Sawaki M, Hattori M, Naomi G, Kotani H, et al. Patient-reported outcomes and objective assessments with arm measurement and bioimpedance analysis for lymphedema among breast cancer survivors. Breast Cancer Res Treat. 2020;179(Jan):91–100. doi: 10.1007/s10549-019-05443-1. [DOI] [PubMed] [Google Scholar]
- 32.Friedlaender A, Vuilleumier A, Viassolo V, Ayme A, De Talhouet S, Combes J-D, et al. BRCA1/BRCA2 germline mutations and chemotherapy-related hematological toxicity in breast cancer patients. Breast Cancer Res Treat. 2019;174(Apr):775–83. doi: 10.1007/s10549-018-05127-2. [DOI] [PubMed] [Google Scholar]
- 33.Iqbal J, Nussenzweig A, Lubinski J, Byrski T, Eisen A, Bordeleau L, et al. The incidence of leukaemia in women with BRCA1 and BRCA2 mutations: an International Prospective Cohort Study. Br J Cancer. 2016;114:1160–4. doi: 10.1038/bjc.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Churpek JE, Marquez R, Neistadt B, Claussen K, Lee MK, Churpek MM, et al. Inherited mutations in cancer susceptibility genes are common among survivors of breast cancer who develop therapy-related leukemia. Cancer. 2016;122(Jan):304–11. doi: 10.1002/cncr.29615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomao F, Musacchio L, Di Mauro F, Boccia SM, Di Donato V, Giancotti A, et al. Is BRCA mutational status a predictor of platinum-based chemotherapy related hematologic toxicity in high-grade serous ovarian cancer patients? Gynecol Oncol. 2019;154:138–43. doi: 10.1016/j.ygyno.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Edmonds CE, Lamb LR, Mercaldo SF, Sippo DA, Burk KS, Lehman CD. Frequency and cancer yield of BI-RADS Category 3 lesions detected at high-risk screening breast MRI. AJR Am J Roentgenol. 2020;214(Feb):240–8. doi: 10.2214/AJR.19.21778. [DOI] [PubMed] [Google Scholar]
- 37.Chikarmane SA, Tai R, Meyer JE, Giess CS. Prevalence and predictive value of BI-RADS 3, 4, and 5 lesions detected on breast MRI: correlation with study indication. Acad Radio. 2017;24(Apr):435–41. doi: 10.1016/j.acra.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Lourenco AP, Donegan L, Khalil H, Mainiero MB. Improving outcomes of screening breast MRI with practice evolution: initial clinical experience with 3T compared to 1.5T. J Magn Reson Imaging. 2014;39(Mar):535–9. doi: 10.1002/jmri.24198. [DOI] [PubMed] [Google Scholar]
- 39.Panigrahi B, Harvey SC, Mullen LA, Falomo E, Di Carlo P, Lee B, et al. Characteristics and outcomes of BI-RADS 3 lesions on breast MRI. Clin Breast Cancer. 2019;19(Feb):e152–9. doi: 10.1016/j.clbc.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 40.Vreemann S, van Zelst JCM, Schlooz-Vries M, Bult P, Hoogerbrugge N, Karssemeijer N, et al. The added value of mammography in different age-groups of women with and without BRCA mutation screened with breast MRI. Breast Cancer Res. 2018;20(Aug):84. doi: 10.1186/s13058-018-1019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N. Engl J Med. 2002;347(Oct):1227–32. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 42.Valachis A, Nearchou AD, Lind P. Surgical management of breast cancer in BRCA-mutation carriers: a systematic review and meta-analysis. Breast Cancer Res Treat. 2014;144(Apr):443–55. doi: 10.1007/s10549-014-2890-1. [DOI] [PubMed] [Google Scholar]
- 43.van den Broek AJ, van’t Veer LJ, Hooning MJ, Cornelissen S, Broeks A, Rutgers EJ, et al. Impact of age at primary breast cancer on contralateral breast cancer risk in BRCA1/2 mutation carriers. J Clin Oncol. 2016;34(Feb):409–18. doi: 10.1200/JCO.2015.62.3942. [DOI] [PubMed] [Google Scholar]
- 44.Copson ER, Maishman TC, Tapper WJ, Cutress RI, Greville-Heygate S, Altman DG, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 2018;19(Feb):169–80. doi: 10.1016/S1470-2045(17)30891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(Jan):288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 46.Alegre N, Perre PV, Bignon YJ, Michel A, Galibert V, Mophawe O, et al. Psychosocial and clinical factors of probands impacting intrafamilial disclosure and uptake of genetic testing among families with BRCA1/2 or MMR gene mutations. Psychooncology. 2019;28:1679–86. doi: 10.1002/pon.5142. [DOI] [PubMed] [Google Scholar]
- 47.Pujol P, Barberis M, Beer P, Friedman E, Piulats JM, Capoluongo ED, et al. Clinical practice guidelines for BRCA1 and BRCA2 genetic testing. Eur J Cancer. 2021;146(Mar):30–47. doi: 10.1016/j.ejca.2020.12.023. [DOI] [PubMed] [Google Scholar]
- 48.Dhar SU, Cooper HP, Wang T, Parks B, Staggs SA, Hilsenbeck S, et al. Significant differences among physician specialties in management recommendations of BRCA1 mutation carriers. Breast Cancer Res Treat. 2011;129(Aug):221–7. doi: 10.1007/s10549-011-1449-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eccles BK, Copson E, Maishman T, Abraham JE, Eccles DM. Understanding of BRCA VUS genetic results by breast cancer specialists. BMC Cancer. 2015;15(Nov):936. doi: 10.1186/s12885-015-1934-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The clinical data analyzed during the current study are available from the corresponding author on reasonable request.