Abstract
TANGO2 disorder is a rare genetic disease with multi-system effects that causes episodic crises. Quality of life and psychosocial effects of this rare disease have not previously been studied. To examine health-related quality of life (HRQoL), illness perceptions, and lived experience, we surveyed 16 children and 31 parents of children with TANGO2 disorder identified via a disease-specific social media group and research foundation email distribution list. We assessed HRQoL by parent proxy-report and child self-report using the Pediatric Quality of Life Inventory (PedsQL™). Parental perceptions of their child’s condition were assessed using the revised illness perceptions questionnaire adapted for TANGO2 disorder (IPQ-R-TANGO2). To collect qualitative data on parents’ lived experience, we used novel open-ended survey questions. Parent proxy-reported (n = 29) physical (78.4 (21)) and psychosocial health (73.4 (12.8)) were highest among toddlers with TANGO2 disorder. Parent proxy-reported physical health was lowest in young adults (34.4 (35.4)), and psychosocial health was lowest in teens (40.8 (10.8)). When compared to previously published PedsQL™ scores in healthy children, parent-proxy reported summary and scale scores for TANGO2 patients were significantly lower (all p < 0.001). Parents’ IPQ-R-TANGO2 responses (n = 26) suggested that parents perceived significant negative consequences of the disease. Parents’ open-ended survey responses (n = 21) highlighted that they derived support from the TANGO2 community. This study characterizes HRQoL in patients with TANGO2 disorder across a range of ages, identifies potential targets for HRQoL improvement, and provides valuable insight into the psychosocial effects of TANGO2 disorder on patients and their families.
Subject terms: Quality of life, Outcomes research
Introduction
TANGO2-related metabolic encephalopathy and arrhythmias, also known as TANGO2 disorder, is a rare, autosomal recessive genetic condition with broad-ranging effects on multiple organ systems. It is characterized by recurrent metabolic crises, developmental delay and/or regression, speech problems, seizures, ataxia, muscle weakness, rhabdomyolysis, and life-threatening arrhythmias. The causative gene was identified recently, in 2016, and little is currently known about the natural history of this disorder [1]. Due to the rarity, recent discovery, and ongoing characterization of this disorder, there have been no investigations into patients’ quality of life and the psychosocial effects on families.
Health-related quality of life (HRQoL) is a multi-dimensional construct that reflects individuals’ perceptions of their health’s effects on physical, psychological, and social functioning. Variations in HRQoL among people with rare diseases may reflect several factors such as disease severity, age of onset, access to adequate healthcare and other resources, and length of diagnostic odyssey, thus it is difficult to generalize about HRQoL among patients with rare diseases [2]. While patients with 22q11 microdeletion syndrome, achondroplasia, and Gaucher disease report lower HRQoL than healthy peers [2, 3], other studies have shown higher HRQoL in patients with congenital adrenal hyperplasia than healthy individuals and mixed findings for school-related functioning in children with intoxication-type inborn errors of metabolism [2, 4]. A recent single-center study of HRQoL among children with various inborn errors of metabolism revealed that those with higher disease burden had lower HRQoL compared to those with less severe disease [5]. Moreover, in multiple studies of rare disease, discrepancies have been noted between child self-report and parent proxy-report of HRQoL, with parents often rating their affected child’s HRQoL lower than children rate their own [2]. Despite these challenges, data on patient experiences of rare diseases collected through patient-reported outcome measures are vital to guide the development of clinical interventions and assess the effectiveness of interventions at improving patient-centered outcomes.
Illness perceptions are the beliefs or perceptions that people have about their illness. The five components are identity (symptoms of illness and illness-related experiences), causality (causes of illness), consequences (implications of illness on quality of life), timeline (illness duration or evolution), and control (ability of personal choices or available treatments to treat or prevent the illness) [6]. Illness perceptions have been associated with several clinical outcomes including treatment adherence, functional recovery, frequency of healthcare use, and levels of patient satisfaction [7].
Among people with genetic diseases, illness perceptions have been associated with variations in quality of life, psychological distress, and adherence to treatment and surveillance. In people with porphyria cutanea tarda, highly negative illness perceptions were more closely associated with psychological distress than higher symptom burden [8]. Children with β-thalassemia intermedia who had positive illness perceptions reported higher quality of life (QoL) [9]. In children with Maroteaux-Lamy syndrome, perceptions of disease burden, treatment burden, and treatment control affected adherence to recommended therapies [10]. Among adults with cystic fibrosis, negative illness perceptions, and not clinical characteristics or symptom burden, were associated with lower psychosocial QoL. Furthermore, higher illness coherence (understanding of the disease) and perceived personal control over illness were associated with higher psychosocial QoL [11].
Illness perceptions have also been studied among family members of people with health conditions. In spouses of individuals with Huntington’s disease, illness perceptions were found to significantly influence both physical and mental well-being [12]. Among people with head and neck cancer, greater discrepancy between patient and caregiver illness perceptions predicted lower HRQoL in patients [13]. In this same population, caregivers’ illness perceptions were more negative than those of patients, underscoring the importance of direct patient input [14], and caregivers’ negative perceptions of treatment efficacy were predictive of post-traumatic stress disorder [15]. Among caregivers of youth with anorexia nervosa, negative illness perceptions were associated with caregiver burden, independent of clinical characteristics [16].
Here we describe self- and parent proxy-reported HRQoL of TANGO2 patients, parents’ perceptions of their child’s illness, and parental lived experiences. We highlight parental reports of positive and challenging aspects of parenting a child with TANGO2 disorder, hopes for their children’s clinical outcomes, and research priorities. By understanding illness perceptions in parents of children with TANGO2 disorder, we hope to identify areas for intervention to improve quality of life for children with this rare disease. Our approach represents an emerging model of patient-centered research in the rare disease realm.
Methods
This study was approved by the Institutional Review Board for Human Subjects Research at Baylor College of Medicine.
Recruitment
All patients at least 5 years of age with TANGO2 disorder and all parents of individuals (any age) with TANGO2 disorder were eligible for participation in this study. Parents of deceased individuals with TANGO2 disorder were also eligible for parts of the study. There were no exclusions based on length of time since patient’s death. We recruited participants through advertisement on the TANGO2 support group on Facebook, emails distributed on the TANGO2 Research Foundation email distribution list, and from attendees of an in-person TANGO2 family conference held in Houston, TX, in June 2019. The conference was supported by the Patient-Centered Outcomes Research Institute (PCORI), and families from the United States, Canada, France, Germany, the Netherlands, and Saudi Arabia attended the conference.
Data collection
Electronic survey data were collected prior to the conference using REDCap (Research Electronic Data Capture), a secure, web-based application designed to support data capture for research studies, hosted at Baylor College of Medicine [17]. Paper survey responses were collected during the conference and transferred to REDCap. Data collection closed after the conference.
Only one parent (determined by participants) of each affected child completed surveys. For families with multiple affected children, one response per affected child was collected for the HRQoL and illness perceptions surveys, and one response per family was collected for the custom open-ended survey. For the open-ended survey, parents were asked to reflect on the overall experience of parenting a child or children with TANGO2 disorder, and thus there was no index child. During the family conference, we shared preliminary survey data with participants and allowed for exchange of ideas and experiences among participants and researchers. Notes taken during the workshop facilitated interpretation of study findings.
Instruments
Outcomes measured, survey instruments, and respondent categories are listed in Table 1. To measure HRQoL, we used the Pediatric Quality of Life Inventory (PedsQL™) Generic Core Scales. The PedsQL™ is a modular instrument that can be used to assess HRQoL in children, adolescents, and young adults 2 to 25 years of age [18]. Versions of the PedsQL™ are available for child self-report (ages 5 and up) and parent proxy-report (ages 2 and up). Parents of all living TANGO2 patients two years of age and older completed the parent proxy-report appropriate for the patient’s age (toddler: 2–4; young child: 5–7; child: 8–12; teen: 13–18; young adult: 19–25). For all TANGO2 patients five years of age and older, parents administered the Young Child self-report to the child. A recent literature review found intellectual disability in 78% of individuals with TANGO2 disorder and developmental delay in 86% [19]. These findings were previously noted in her clinical experience by one member of the study team (SRL). Therefore, in order to be appropriate for the cognitive functioning in this patient population, the Young Child survey was administered to all ages.
Table 1.
Instruments utilized in this study, outcomes measured by each instrument, and respondents for each instrument.
| Instrument | Measured Outcomes | Respondents |
|---|---|---|
| PedsQL™ | Health-related quality of life | All parents - one per living affected child age 2 and up |
| Affected children age 5 and up | ||
| IPQ-R-TANGO2 | Illness perceptions | All parents - one per living or deceased affected child |
| Custom open-ended survey | Lived experiences | All parents - one per family |
| Research goals | ||
| Healthcare goals |
The Generic Core Scales assess HRQoL in four dimensions: Physical Functioning, Emotional Functioning, Social Functioning, and School Functioning. Scores range from 0 to 100 on all dimension scales, Summary Scales, and the Total Scale, with higher scores indicating higher quality of life. The score on the Physical Functioning dimension is also the Physical Health Summary Score. Scores on the Emotional, Social, and School Functioning dimensions are averaged to create the Psychosocial Health Summary Score. The Total Scale Score is the average score across all answered items.
The Revised Illness Perception Questionnaire (IPQ-R) has been widely used to study illness perceptions among people with genetic diseases and their caregivers [6]. The creators of the IPQ-R encourage researchers to adapt the Cause and Identity subscales to particular illnesses. The IPQ-R has been adapted to autism spectrum disorder, hemophilia, HIV, and hypertension, and has been administered to parent or caregiver proxies [20, 21].
Except for Identity, each subscale is composed of several items with five-point Likert-Scale answers. The Identity subscale assesses symptom presence and perceived attribution to the illness. Except for Cause and Identity, each subscale is scored by converting Likert responses to numeric scores and summing numeric scores for the items. The Identity subscale is scored by converting “yes” responses to 1, and “no” responses to 0, and summing the scores for all the items. Each item on the Cause subscale represents a specific causal belief, and thus each is considered individually and there is no associated numeric score [6, 20].
Maximum scores vary across subscales. For those with Likert scale items, maximum scores range from 20-30 (derived by multiplying the number of items in the subscale by five). For the Identity subscale, maximum score is 14. Minimum scores range from 0 to 6. Higher scores indicate stronger belief. For example, higher score on Timeline-Acute/Chronic indicates stronger belief that the symptoms are chronic, higher score on Illness Coherence indicates stronger belief in personal understanding of the illness, and so on, indicated in Supplementary Table 1. There are no established cutoff scores for the IPQ-R subscales, thus based on the maximum scores, we selected >20 to indicate strong belief, 16–20 to indicate moderate belief, and <16 to indicate weak belief for all subscales except for Timeline Cyclical, with a maximum score of 20, where >15 was selected to indicate strong belief. The study team adapted the Identity subscale to TANGO2 disorder utilizing 14 common symptoms. The Cause subscale was adapted from a version of the IPQ-R adapted to autism spectrum disorder [21]. The IPQ-R-TANGO2 is available as Appendix 1. Parents of all living and deceased TANGO2 patients answered the IPQ-R-TANGO2 survey, one per affected child. This study did not employ a self-report of the IPQ-R-TANGO2.
In order to understand the lived experience of parenting a child with TANGO2 disorder and identify research and healthcare goals of these parents, the study team created a novel, custom open-ended survey (Appendix 2). This survey included seven open-ended items inquiring about positive and negative aspects of parenting an affected child; worries and hopes of the parent; desired clinical outcomes for their child; and research questions of interest to parents. An open-ended format was chosen in order to assess the lived experience of this community in an unbiased, exploratory fashion. Parents of all living and deceased TANGO2 patients answered this custom survey, one per family, with no index child.
Data analysis
PedsQL™ responses were scored according to guidelines provided by the measure developer [22], and descriptive statistics were calculated for each dimension scale score as well as the Physical Health Summary Score, Psychosocial Health Summary Score, and Total Scale Score. For analysis of child self-report data, we pooled responses from children of all ages since all responses were collected on the Young Child version. For analysis of parent proxy-report data, we calculated descriptive statistics on pooled responses across all age groups. We also described parent proxy-report responses for each PedsQL™ version administered according to the child’s age group, but small sample sizes prevented statistical comparison of scores between age groups. To evaluate inter-rater reliability between self- and proxy-report scores, we calculated intraclass correlation coefficients (ICCs) using a two-way mixed-effects model, reporting absolute agreement on individual scores. ICCs close to 1 indicate high agreement between scores for the same child with values of 0.61 to 0.80 indicating good agreement and 0.81 to 1.00 indicating excellent agreement [23]. To evaluate mean differences between self- and proxy-report scores, we calculated paired t tests. Additionally, parent proxy-report responses were compared to those previously reported in healthy children [18] using one-sample t tests. Data analyses were performed using Stata IC 15 (College Station, Texas).
IPQ-R-TANGO2 surveys were scored according to guidelines provided by the measure developer [20]. Descriptive statistics were generated using SAS software, Version 9.4© 2013 SAS Institute Inc. Parents’ responses for children of all age groups were analyzed together. Responses representing deceased and living children were analyzed separately as well as together. We used a thematic analysis approach to analyze open-ended responses on the custom survey. One coder (CNM) applied inductive codes facilitated by NVivo 12 Plus. We recorded the prevalence of identified themes.
Results
We received survey responses from 27 families, five of which had two or more affected children. PedsQL™ responses were received from 29 parents and from 16 affected individuals. IPQ-R-TANGO2 responses were received from 26 parents, 24 parents of living children and two parents of deceased children. Responses from 21 parents were received on the custom open-ended survey, representing parenting experience with 24 living children and 3 deceased children. Respondent characteristics are presented in Table 2. Child ages ranged from toddler to young adult, with most children in the 2–4 year age category (13 out of 29 PedsQL™ responses, 12 out of 26 IPQ-R-TANGO2 responses).
Table 2.
Respondent characteristics for each survey, including number of parent and child respondents, number of respondents representing living or deceased affected individuals, and age categories of living individuals represented.
| PedsQL™ | IPQ-R-TANGO2 | Open-ended survey | |
|---|---|---|---|
| Total respondents | 45 | 26 | 21 |
| Parent | 29 | 26 | 21 |
| Child | 16 | - | - |
| Living individuals represented | 29 | 24 | 24 |
| Deceased individuals represented | N/A | 2 | 3 |
| Age of living individuals represented | |||
| Under 2 | N/A | 2 | |
| 2–4 | 13 | 12 | |
| 5–7 | 4 | 3 | |
| 8–12 | 4 | 1 | |
| 13–18 | 6 | 4 | |
| 19–25 | 2 | 2 | |
Health-related quality of life
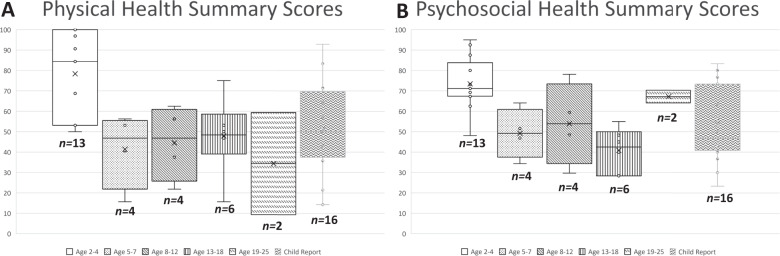
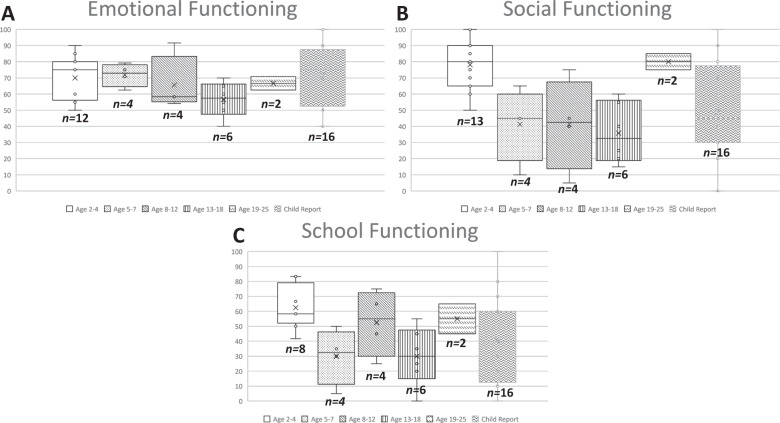
PedsQL™ mean scores and standard deviations (SD) for children with both self-report and parent proxy-report (n = 16) are shown in Table 3 and for all children by age group (n = 29) in Supplementary Table 2. Physical and Psychosocial Health Summary Scores are shown in Fig. 1. Parent proxy responses represented 13 toddlers (age 2–4), four young children (age 5–7), four children (age 8–12), six teens (age 13–18), and two young adults (age 19–25). Mean (SD) parent proxy-reported Physical Health Summary Scores were highest in the Toddler age group (78.4 (21.0)) and lowest in the Young Adult age group (34.4 (35.4)). Psychosocial Summary Scores were highest in the Toddler age group (73.4 (12.8)) and lowest in the Teen age group (40.8 (10.8)). Emotional, Social, and School Functioning scale scores are shown in Fig. 2. Mean (SD) parent proxy-reported Emotional Functioning was highest in Young Children (71.9 (7.1)) and lowest in Teens (56.7 (10.8)). Social Functioning was highest in the Young Adult age group (80.0 (7.1)) and lowest in Teens (35.8 (18.8)). School Functioning was highest in Toddlers (62.5 (14.8)) and lowest in Young Children (30 (18.7)) and Teens (30 (19.5)). Due to the wide range in scores, these variations by age range should be interpreted with caution.
Table 3.
PedsQL™ child-report and parent proxy-report, all children ≥5 years.
| All children ≥5 years, n = 16 | Parent proxy-report, mean (SD) | Child self-report, mean (SD) | Paired t test p value | Child–Parent Agreement, ICC (95% CI) |
|---|---|---|---|---|
| Total scale score | 48.03 (13.46) | 52.85 (16.82) | 0.11 | 0.70 (0.34, 0.88) |
| Physical health summary score | 43.75 (18.99) | 51.19 (22.84) | 0.08 | 0.68 (0.31, 0.88) |
| Psychosocial health summary score | 49.49 (15.16) | 53.75 (18.93) | 0.17 | 0.75 (0.44, 0.91) |
| Emotional functioning scale score | 63.96 (12.33) | 71.88 (21.05) | 0.05 | 0.60 (0.17, 0.84) |
| Social functioning scale score | 44.06 (24.37) | 48.75 (30.30) | 0.38 | 0.72 (0.38, 0.89) |
| School functioning scale score | 38.75 (21.10) | 40.62 (28.86) | 0.71 | 0.70 (0.32, 0.88) |
SD standard deviation, ICC intraclass correlation coefficient, CI confidence interval.
Fig. 1. Parent proxy-reported PedsQLTM Summary scores.
A Box plots of parent proxy-report Physical Health summary scores for toddlers (age 2–4), young children (age 5–7), children (age 8–12), teens (age 13–18), and young adults (age 19–25). Boxes represent 25–7% interquartile range, whiskers indicate maximum and minimum scores, median indicated by horizontal line, and individual instances represented by open circles. B Box plots of parent proxy-report Psychosocial Health summary scores for toddlers (age 2–4), young children (age 5–7), children (age 8–12), teens (age 13–18), and young adults (age 19–25). Boxes represent 25–7% interquartile range, whiskers indicate maximum and minimum scores, median indicated by horizontal line, and individual instances represented by open circles.
Fig. 2. Parent proxy-reported PedsQLTM Scale scores.
A Box plots of parent proxy-report Emotional Functioning scores for toddlers (age 2–4), young children (age 5–7), children (age 8–12), teens (age 13–18), and young adults (age 19–25). Boxes represent 25–7% interquartile range, whiskers indicate maximum and minimum scores, median indicated by horizontal line, and individual instances represented by open circles. B Box plots of parent proxy-report Social Functioning scores for toddlers (age 2–4), young children (age 5–7), children (age 8–12), teens (age 13–18), and young adults (age 19–25). Boxes represent 25–7% interquartile range, whiskers indicate maximum and minimum scores, median indicated by horizontal line, and individual instances represented by open circles. C Box plots of parent proxy-report School Functioning scores for toddlers (age 2–4), young children (age 5–7), children (age 8–12), teens (age 13–18), and young adults (age 19–25). Boxes represent 25–7% interquartile range, whiskers indicate maximum and minimum scores, median indicated by horizontal line, and individual instances represented by open circles.
Among the child self-reported Scale scores, Emotional Functioning (71.9 (21.1)) was highest and School Functioning (40.6 (28.9)) was lowest. Child self-report scores were higher than parent proxy-report scores for Total, Summary, and Scale scores, yet no differences reached conventional levels of statistical significance (Table 3). Agreement between self-report and proxy-report scores ranged from 0.60 (Emotional Functioning Scale Score) to 0.75 (Psychosocial Health Summary Score), indicating good agreement. When compared to previously published scores in healthy children, parent-proxy report Total, Summary, and Scale scores for TANGO2 patients were significantly lower (all p < 0.001), as shown in Supplementary Table 3.
Illness perceptions
Scores on IPQ-R-TANGO2 subscales are shown in Table 4. In general, scores were high (>20) on Timeline Acute/Chronic, Timeline Cyclical, Consequences, and Emotional Representations, representing strong belief in the chronicity, cyclical nature, negative consequences, and negative emotional effects of TANGO2 disorder. Scores were lower (<16) on Treatment Control and Illness Coherence, representing low confidence in treatment efficacy and low personal understanding of the illness. Scores on the Personal Control subscale were moderate for parents of living children (19.3 (5.0)), indicating moderate confidence in personal ability to alter disease course.
Table 4.
Mean scores and standard deviations on the Identity, Timeline Acute/Chronic, Timeline Cyclical, Consequences, Personal Control, Treatment Control, Illness Coherence, and Emotional Representations subscales of IPQ-R-TANGO2.
| IPQ-R-TANGO2 subscale scores (Mean (SD)) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Identity | Timeline Acute/Chronic | Timeline Cyclical | Consequences | Personal Control | Treatment Control | Illness Coherence | Emotional Representations | |
| Respondent group | ||||||||
| Living (n = 24) | 8.3 (3.8) | 25.6 (5.0) | 15.0 (3.1) | 24.2 (5.0) | 19.3 (5.0) | 15.5 (2.8) | 15.8 (5.5) | 23.3 (3.9) |
| Deceased (n = 2) | 11 (1.4) | 29.5 (0.7) | 15.5 (0.7) | 26.5 (2.1) | 26.5 (2.1) | 16.5 (7.8) | 14.0 (1.4) | 26.0 (1.4) |
| All (n = 26) | 8.5 (3.8) | 25.9 (4.9) | 15.1 (3.0) | 24.3 (4.9) | 24.3 (4.9) | 15.5 (3.1) | 15.7 (5.3) | 23.5 (3.8) |
| Maximum score | 14 | 30 | 20 | 30 | 30 | 25 | 25 | 30 |
Mean scores are shown representing living affected children, deceased affected children, and all affected children.
On the Cause subscale, the most commonly endorsed cause for TANGO2 disorder was Genetics. Four parents mentioned crises following vaccinations. Several respondents listed dehydration, fasting, and virus, which may represent perceived causes of metabolic crises, not causes of TANGO2 disorder itself.
Experience of parenting a child with TANGO2
We received 21 responses to the custom survey, representing 20 families and 27 children total. The most commonly cited positive aspect of parenting a child with TANGO2 disorder was improvement in parents themselves (n = 8), such as knowledge gained, increased patience, and advocating for the child. Other positive experiences included participation in the TANGO2 community (n = 4), the happiness and joy of the affected child (n = 4), and resilience and strength of the affected child (n = 4). The most commonly cited challenge was fear of the affected child’s death (n = 21). Other concerns were fear or anxiety surrounding metabolic crises (n = 13), financial and insurance difficulties (n = 11), and burden of healthcare utilization (n = 8).
The most common desired clinical outcome was preventing loss of function and developmental regression (n = 17). Parents also desired as much adult independence as possible (n = 15), as well as effective treatments (n = 14), prevention of metabolic crises (n = 13), and a cure for the disease (n = 13). Parents expressed a strong interest in research aimed at identifying diets or supplements that may prevent crises (n = 8). Another common goal was creation of a set treatment protocol (n = 8). Gene therapy was also desired (n = 5).
Discussion
Due to the recent discovery and rarity of TANGO2 disorder, the psychosocial effects of this disease remain largely unstudied [1]. We sought to understand these effects by researching HRQoL in affected individuals; examining illness perceptions in parents of affected individuals; and asking parents to expound on the lived experience of caregiving for an individual with TANGO2 disorder. Our findings reveal that individuals with TANGO2 disorder experience decreased HRQoL compared to healthy individuals, and that their parents perceive strong negative consequences of the disease. Nevertheless, parents of individuals with TANGO2 disorder describe some positive effects, including parental self-improvement; and hopes for the future, including research questions of interest. Taken together, these data present a rich view into the lives of people affected by this recently characterized rare disease.
Our results suggest that HRQoL is lower for children with TANGO2 disorder than healthy children. Moreover, in our population with TANGO2 disorder, PedsQL™ scores are similar to parent proxy-reported HRQoL in children with methylmalonic acidemia (MMA), which are also decreased compared to healthy children [24]. MMA is characterized by recurrent episodic metabolic crises as well as developmental delay and regression, thus sharing clinical similarities with TANGO2 disorder. The similar clinical profiles in both conditions appear to have similar effects on HRQoL, as measured by the PedsQL™ scales.
Though our participant numbers were too small to make statistical comparisons, we noted higher HRQoL in the toddler (2–4 years) age group compared to older age groups. Given that infants or toddlers with TANGO2 disorder may present with developmental delay, seizures, coma, ataxia, and metabolic crisis, this finding may signify that HRQoL is mediated by factors beyond symptom burden [1, 25]. We observed lowest HRQoL in teens (13–18 years) in our study sample, across Social, Emotional, and School Functioning dimensions, yet a medical rationale to explain this finding is not yet known. Few studies examine the influence of age on HRQoL among people living with genetic disease, and thus the effects of age, if any, are unclear [26–28]. It is notable that young adults with TANGO2 disorder score higher than teens across all psychosocial health-related scales, which may reflect adaptation to the diagnosis. Indeed, Grootenhuis et al. have proposed that adaptation to the diagnosis and adjustment of expectations may improve perceived HRQoL among people with genetic conditions [29]. This theory should undergo further empirical study, specifically with regards to the influence of age, among the TANGO2 population and those affected by other genetic diagnoses.
The parents in our study sample endorsed negative illness perceptions in terms of practical consequences, emotions, and disease chronicity. Since this study did not correlate illness perceptions with clinical characteristics, it is unclear whether these negative perceptions reflected disease severity or other factors. Caregiver illness perceptions have been found to predict perceived caregiver burden, caregiver psychological adjustment, and patient HRQoL, and our findings suggest that parents of children with TANGO2 disorder may be at risk for adverse mental health outcomes [13, 15, 16]. Parents’ responses to the IPQ-R-TANGO2 further demonstrated poor understanding of the illness and weak or moderate belief in treatment efficacy and personal ability to control the disease course. These findings reflect the limited current medical knowledge of this rare disease, suggest that this population may be at risk for depression and anxiety, [30–32] and that caregivers’ negative illness perceptions may affect their children’s HRQoL [14]. Our results imply that while research for treatments is ongoing, it may be possible to ameliorate the negative psychological effects of rare disease through interventions focused on improving families’ understanding of the disorder. Responses on the Cause subscale of IPQ-R-TANGO2 revealed a strong understanding of the genetic underpinnings, and in future work, this subscale could be utilized to investigate perceived causes of metabolic crises.
The open-ended responses on the custom survey provided rich information. When asked about the best aspects of parenting a child with TANGO2 disorder, parents most often cited improvement within themselves, including improved patience, knowledge gained, advocacy skills, and self-efficacy. Identifying these improvements represents the coping strategy positive reappraisal, in which individuals affected by rare disease view negative experiences in a positive light and find strength in difficulties [33, 34]. Respondents expressed that the TANGO2 community was a source of strength and represented a positive effect of the diagnosis. In contrast, parents of children with some other rare diseases may struggle with a lack of community and social support [35, 36].
Our study team’s discussion with parents during the conference adds important context to survey results. Workshop participants pinpointed aspects of the TANGO2 disorder lived experience that they deemed important for further study. Particulars about educational setting, such as accommodations for learning, mainstream versus special education classes, and specific learning disabilities, were of keen interest. Families were eager to leverage the TANGO2 community to identify triggers for metabolic crises and “intermittent random things,” a phrase describing episodic events involving drooling, abnormal posturing, gait abnormality, extreme fatigue, unprovoked emotion, and other behaviors.
Related to the HRQoL findings, multiple family members described triggers for physical symptoms in their children, including altitude changes, travel, and weather changes. From a psychosocial perspective, families expressed that the uncertainty of TANGO2 disorder was one of its most challenging aspects, and proposed that identification of a laboratory test or other indicator of oncoming crisis would improve their quality of life. Their feedback highlighted that identification of an evidence-based diet and supplement regimen to prevent crises and disease progression would increase their sense of personal control over the disease. Similar to parents of other children with rare diseases, parents in our study emphasized the burden of educating healthcare providers about their child’s rare disease [35]. By combining patient-reported outcome measures with interactive community engagement, our approach yielded valuable insights into the daily life and well-being of families impacted by TANGO2 disorder.
Our study had several limitations. Although the sample size is large for this newly identified rare disease, it is nevertheless small and prevented meaningful statistical comparisons of HRQoL between patient age groups. As a result, observed age-related differences in HRQoL and statistical comparisons of self- and proxy-report scores should be interpreted with caution. Moreover, since parents administered the Young Child version of the PedsQL™ to their child, they were not blinded to their child’s responses. The custom open-ended survey included example answers for the “desired clinical outcomes” question (“For instance, improved quality of life, preventing loss of function, and so on”), which likely influenced responses. Another limitation is the focus on parent-report for illness perceptions and lived experiences. With evidence that illness perceptions vary between caregivers and affected individuals, the illness perceptions of TANGO2-affected individuals themselves should be studied in future work [14]. Last, while our study informally incorporated elements of direct caregiver engagement, future work should approach this type of engagement in a more structured manner. Caregivers should be involved in study design and survey selection or creation in order to maximize the effectiveness of future work to inform patient-centered interventions.
Supplementary information
Acknowledgements
The authors would like to thank Denese Neu, the Engagement Officer at PCORI for assisting with the family conference workshops. We are also grateful to the members of the TANGO2 community, who engaged enthusiastically with this research from the start and enabled the authors to complete this project.
Author contributions
CNM was responsible for study design, survey selection, participant recruitment, data collection, co-leading the workshop, data analysis, result interpretation, and writing the manuscript. SRL contributed to participant recruitment, capacity-building, and provided feedback on the manuscript. MSA contributed to participant recruitment and capacity-building. CYM provided substantial feedback on the manuscript. HSS was responsible for study design, survey selection, co-leading the workshop, data analysis, and provided feedback on the manuscript.
Funding
While engaged in this work, CNM was supported by the Medical Genetics Research Fellowship Program (T32 GM07526-42), HSS was supported by a grant from the National Human Genome Research Institute (K99HG011491) and CYM was supported by National Institutes of Health, K23-HL136932. Research reported in this publication was funded through a Patient-Centered Outcomes Research Institute® (PCORI®) Award (EAIN-11402) to SRL and by the TANGO2 Research Foundation.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the Institutional Review Board for Human Subjects Research at Baylor College of Medicine.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41431-022-01127-5.
References
- 1.Lalani SR, Liu P, Rosenfeld JA, Watkin LB, Chiang T, Leduc MS, et al. Recurrent muscle weakness with rhabdomyolysis, metabolic crises, and cardiac arrhythmia due to bi-allelic TANGO2 mutations. Am J Hum Genet. 2016;98:347–57. doi: 10.1016/j.ajhg.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen JS, Biesecker BB. Quality of life in rare genetic conditions: a systematic review of the literature. Am J Med Genet A. 2010;152A:1136–56. doi: 10.1002/ajmg.a.33380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joyce P, O’Rourke C, McDermott B, Heussler H. Health-related quality of life in 22q11.2 deletion syndrome: The child’s perspective. J Paediatr Child Health. 2018;54:311–5. doi: 10.1111/jpc.13746. [DOI] [PubMed] [Google Scholar]
- 4.Zeltner NA, Huener M, Baumgartner MR, Landolt MA. Quality of life, psychological adjustment, and adaptive functioning of patients with intoxication-type inborn errors of metabolism – a systematic review. Orphanet J Rare Dis. 2014;9:159–76. doi: 10.1186/s13023-014-0159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimitrova, N, Glaus, J, Urben, S, Wuthrich, V, Harari, MM, Ballhausen, D. The impact of disease severity on the psychological well-being of youth affected by an inborn error of metabolism and their families: a one-year longitudinal study. Mol Genet Metab Rep. 2021. 10.1016/j.ymgmr.2021.100795. [DOI] [PMC free article] [PubMed]
- 6.Moss-Morris R, Weinman J, Petrie KJ, Horne R, Cameron LD, Buick D. The revised illness perception questionnaire (IPQ-R) Psychol Health. 2002;17:1–16. doi: 10.1080/08870440290001494. [DOI] [Google Scholar]
- 7.Petrie KJ, Jago LA, Devcich DA. The role of illness perceptions in patients with medical conditions. Curr Opin Psychiatry. 2007;20:163–7. doi: 10.1097/YCO.0b013e328014a871. [DOI] [PubMed] [Google Scholar]
- 8.Andersen J, Nordin K, Sandberg S. Illness perception and psychological distress in persons with porphyria cutanea tarda. Acta Derm Venereol. 2016;96:674–8. doi: 10.2340/00015555-2339. [DOI] [PubMed] [Google Scholar]
- 9.Th Atwa Z, Abdel Wahed WY. The impact of illness perception and socio-clinico-demographic factors on perceived quality of life in children and adolescents with thalassemia intermedia. Pediatr Blood Cancer. 2019;66:e27745. doi: 10.1002/pbc.27745. [DOI] [PubMed] [Google Scholar]
- 10.Dilger H, Leissner L, Bosanska L, Lampe C, Plockinger U. Illness perception and clinical treatment experiences in patients with M. Maroteaux-Lamy (mucopolysaccharidosis type IV) and a Turkish migration background in Germany. PLoS ONE. 2013;8:e66804. doi: 10.1371/journal.pone.0066804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawicki GS, Sellers DE, Robinson WM. Associations between illness perceptions and health-related quality of life in adults with cystic fibrosis. J Psychosom Res. 2011;70:161–7. doi: 10.1016/j.jpsychores.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helder DI, Kaptein AA, Van Kempen GMJ, Weinman J, Van Houwelingen JC, Roos RAC. Living with Huntington’s disease: illness perceptions, coping mechanisms, and spouses’ quality of life. Int J Behav Med. 2002;9:37–52. doi: 10.1207/S15327558IJBM0901_03. [DOI] [PubMed] [Google Scholar]
- 13.Richardson AE, Morton RP, Broadbent EA. Changes over time in head and neck cancer patients’ and caregivers’ illness perceptions and relationships with quality of life. Psychol Health. 2016;31:1203–19. doi: 10.1080/08870446.2016.1203686. [DOI] [PubMed] [Google Scholar]
- 14.Richardson AE, Morton R, Broadbent E. Caregivers’ illness perceptions contribute to quality of life in head and neck cancer patients at diagnosis. J Psychosoc Oncol. 2015;33:414–32. doi: 10.1080/07347332.2015.1046011. [DOI] [PubMed] [Google Scholar]
- 15.Richardson AE, Morton RP, Broadbent EA. Illness perceptions and coping predict post-traumatic stress in caregivers of patients with head and neck cancer. Support Care Cancer. 2016;24:4443–50. doi: 10.1007/s00520-016-3285-0. [DOI] [PubMed] [Google Scholar]
- 16.Matthews A, Lenz KR, Peugh J, Copps EC, Peterson CM. Caregiver burden and illness perceptions in caregivers of medically hospitalized youth with anorexia nervosa. Eat Behav. 2018;29:14–8. doi: 10.1016/j.eatbeh.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varni JW, Seid M, Kurtin PS. PedsQL™ 4.0: reliability and validity of the pediatric quality of life inventory™ version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–12. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Schymick J, Leahy P, Cowan T, Ruzhnikov MRZ, Gates R, Fernandez L, et al. Variable clinical severity in TANGO2 deficiency: case series and literature review. Am J Med Genet A. 2021. 10.1002/ajmg.a.62543. [DOI] [PubMed]
- 20.The Illness Perception Questionnaire Website. 2020. http://ipq.h.uib.no//index.html.
- 21.Mire SS, Tolar TD, Brewton CM, Raff NS, Mckee SL. Validating the Revised Illness Perception Questionnaire as a measure of parent perceptions of autism spectrum disorder. J Autism Dev Disord. 2018;48:1761–79. doi: 10.1007/s10803-017-3442-4. [DOI] [PubMed] [Google Scholar]
- 22.Varni JW, MAPI Research Trust. Pediatric quality of life inventory™ PedsQL scoring manual.
- 23.Streiner DL, Norman, GR, Cairney, J. Health measurement scales: a practical guide to their development and use. 5th ed. Oxford University Press: Oxford; 2015.
- 24.Splinter K, Niemi AK, Cox R, Platt J, Shah M, Enns GM, et al. Impaired health-related quality of life in children and families affected by methylmalonic acidemia. J Genet Couns. 2016;25:936–44. doi: 10.1007/s10897-015-9921-x. [DOI] [PubMed] [Google Scholar]
- 25.Dines JN, Golden-Grant K, Lacroix A, Muir AM, Laboy Cintron D, McWalter K, et al. TANGO2: expanding the clinical phenotype and spectrum of pathogenic variants. Genet Med. 2019;21:601–7. doi: 10.1038/s41436-018-0137-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alishbayli G, Kilinc AA, Cokugras H. Evaluation of the health-related quality of life in Turkish cystic fibrosis patients. Pediatr Int. 2021;68:965–70. [DOI] [PubMed]
- 27.Ballard LM, Jenkinson E, Byrne CD, Child JC, Inskip H, Lokulo-Sodipe O, et al. Experience of adolescents living with Silver-Russell syndrome. Arch Dis Child. 2021;106:1195–201. doi: 10.1136/archdischild-2020-321376. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez-Lopez CR, Perestelo-Perez L, Escobar A, Lopez-Bastida J, Serrano-Aguilar P. Health-related quality of life in patients with spinocerebellar ataxia. Neurologia. 2017;32:143–51. doi: 10.1016/j.nrl.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Grootenhuis M, de Boone J, van der Kooi A. Living with muscular dystrophy: health related quality of life consequences for children and adults. Health Qual Life Outcomes. 2007;5:31–8. doi: 10.1186/1477-7525-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dempster M, Howell D, McCorry NK. Illness perceptions and coping in physical health conditions: a meta-analysis. J Psychosom Res. 2015;79:506–13. doi: 10.1016/j.jpsychores.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Solmaz N, Ilhan N, Bulut H. The effect of illness perception on life quality in psoriasis patients. Psychol Health Med. 2021;26:955–67. doi: 10.1080/13548506.2020.1847300. [DOI] [PubMed] [Google Scholar]
- 32.Unal O, Akyol Y, Tander B, Ulus Y, Terzi Y, Kuru O. The relationship of illness perceptions with demographic features, pain, severity, functional capacity, disability, depression, and quality of life in patients with chronic low back pain. Turk J Phys Med Rehabil. 2019;65:301–8. doi: 10.5606/tftrd.2019.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dellenmark-Blom M, Chaplin J, Jonsson L, Gatzinsky V, Quitmann JH, Abrahamsson K. Coping strategies used by children and adolescents born with esophageal atresia – a focus group study obtaining the child and parent perspective. Child Care Health Dev. 2016;42:759–67. doi: 10.1111/cch.12372. [DOI] [PubMed] [Google Scholar]
- 34.Feragen KB, Stock NM, Myhre A, Due-tonnessen BJ. Medical stress reactions and personal growth in parents of children with a rare craniofacial condition. Cleft Palate Craniofac J. 2020;57:228–37. doi: 10.1177/1055665619869146. [DOI] [PubMed] [Google Scholar]
- 35.Currie G, Szabo J. “It is like a jungle, gym, and everything is under construction”: The parent’s perspective of caring for a child with a rare disease. Child Care Health Dev. 2019;45:96–103. doi: 10.1111/cch.12628. [DOI] [PubMed] [Google Scholar]
- 36.Germeni E, Vallini I, Bianchetti MG, Schulz PJ. Reconstructing normality following the diagnosis of a childhood chronic disease: Does “rare” make a difference? Eur J Pediatr. 2018;177:489–95. doi: 10.1007/s00431-017-3085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.