Abstract
The use of low-dose aspirin in older adults is increasing as is the prevalence of osteoporosis. Aspirin has been shown in numerous studies to affect bone metabolism. However, there is no clear link between low-dose aspirin use and bone mineral density (BMD). This study examined differences in bone mineral density between low-dose aspirin users and non-aspirin users in adults aged 50–80 years. We conducted a cross-sectional study of 15,560 participants who participated in the National Health and Nutrition Examination Survey (NHANES) 2017-March 2020. We used a multivariate logistic regression model to evaluate the relationship between low-dose aspirin and femoral neck BMD, femoral total BMD, intertrochanteric BMD, and the first lumbar vertebra BMD (L1 BMD) in patients aged 50 to 80 years. A total of 1208 (Group 1: femoral neck BMD, total femur BMD, and intertrochanter BMD) and 1228 (Group 2: L1 BMD) adults were included in this study. In both group 1 and group 2, BMD was higher in the low-dose aspirin group than in the non-aspirin group (Total femur BMD β = 0.019, 95% CI 0.004–0.034; Femoral neck BMD β = 0.017, 95% CI 0.002–0.032; Intertrochanter BMD β = 0.025, 95% CI 0.007–0.043; L1 BMD β = 0.026, 95% CI 0.006–0.046). In subgroup analyses stratified by gender, this positive association existed in both gender after adjusting for confounders. On subgroup analyses stratified by age, this positive association existed in three different age groups after adjusting for confounders. To test whether the effect of low-dose aspirin on BMD was affected by gender and age, the interaction P value was greater than 0.05. These findings from a human study looking into the relationship between low-dose aspirin use and BMD suggest that regular low-dose aspirin may be associated with a higher BMD. The association between low-dose aspirin and BMD did not differ by age group or gender.
Subject terms: Health care, Medical research
Introduction
Osteoporosis (OP) is a systemic bone metabolic disease in which bone loss exceeds bone formation, leading to reduced bone mass, bone microarchitecture deterioration, bone fragility, and fracture susceptibility1. Many endogenous and exogenous factors influence bone metabolism. Prostaglandin E2 (PGE2) is a precursor of inflammatory factors synthesized by arachidonic acid in the enzyme Cyclooxygenase (COX), which affects bone metabolism2. It is critical for osteoblast and bone tissue formation and may influence osteoclast formation by altering the RANKL-OPG axis2–6. Aspirin belonging to NSAIDs inhibits the synthesis of PGE2 by inhibiting COX, thus affecting bone metabolism7. Aspirin could either result in decreased or increased BMD, depending on the balance of prostaglandins in bone tissue8. Aspirin inhibited osteoclast formation by inhibiting the activation of NF-κB and MAPKS in RANKL-induced RAW264.7 cells, thereby increasing bone mineral density9. A recent meta-analysis by Barker et al. found that aspirin was associated with higher total hip and lumbar spine BMD in women and men10. However, aspirin, on the other hand, increases physiological nitric oxide (NO) production11,12, which may decrease osteoblasts and increase osteoclast activity, resulting in a reduction in BMD.
Osteoporosis and cardiovascular disease often coexist and our of major public health concern13. Aspirin is a cyclooxygenase inhibitor used mainly by the elderly to prevent cardiovascular disease14. Low-dose aspirin (75–100 mg/day orally) may be considered to prevent Atherosclerotic Cardiovascular Disease (ASCVD) in people aged 40 to 70 with no risk of bleeding15. Data from the 2010 U.S. National Health Interview Survey found that 19% of 27,157 subjects aged 18 years and older took aspirin16. Among current preventive aspirin users, 70% took low-dose aspirin (75–100 mg/day orally) daily17. There are about 2 million osteoporosis-related fractures annually, and the net cost to the U.S. Medicare system is $19 billion18,19. Clinical significance of osteoporosis is highlighted by the increasing prevalence of fragility fractures. Around 536,000 new brittle fractures occur annually in the U.K., including 79,000 hip fractures and 66,000 clinically diagnosed spinal fractures20. As measured by BMD, osteoporosis was defined at the bone mass level 21. Measurements at the hip and spine are typical areas for assessing and monitoring BMD are commonly used as an outcome measure in trials for about 30 years22. Given the widespread use of aspirin in older adults, it may be essential to ascertain whether it affects bone mineral density. This study aimed to investigate the association between low-dose aspirin use and bone mineral density among adults 50–80 years of age using the National Health and Nutrition Examination Survey (NHANES) data.
Materials and methods
Study population
Data for this study came from NHANES conducted between 2017 and March 2020, using a complex, multi-stage, hierarchical, clustered probability sample design to select a representative sample of civilians rather than a simple random sample based on the U.S. population23. The Ethics Review Committee of the National Center for Health Statistics approved all NHANES protocols and obtained written informed consent from all participants. The relevant guidelines and regulations performed all methods.
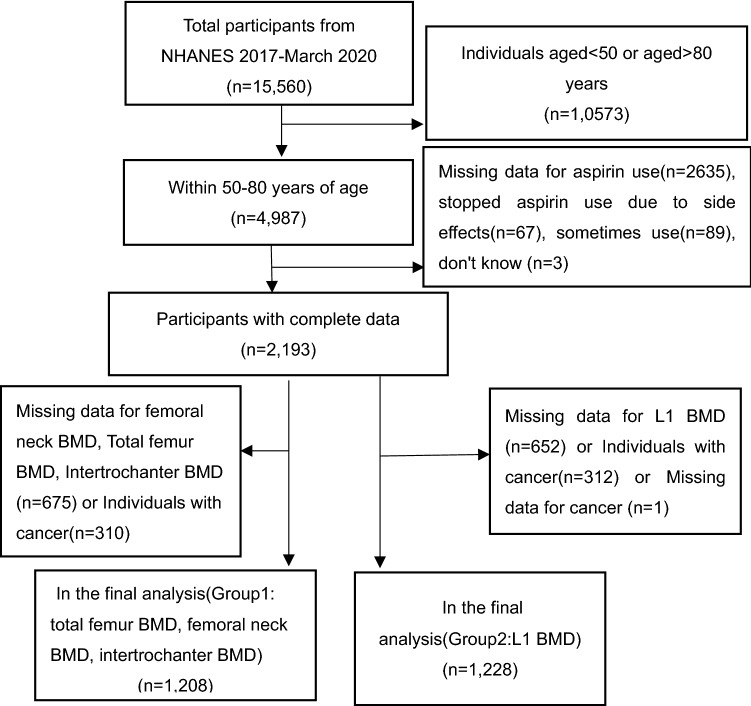
A total of 15,560 participants were analyzed from 2017 to 2020 (Fig. 1). Excluding patients younger than 50 or older than 80 (n = 10,857) there were 4987 participants. 2794 people who were not eligible were excluded for the following reasons: Missing data for aspirin use (n = 2635), stopped aspirin use due to side effects (n = 67), sometimes using aspirin (n = 89), do not know (n = 3). Additionally, after excluding individuals with cancer and missing data for BMD there were 1208 patients with femoral BMD (group 1) and 1128 with L1 BMD (group 2). The National Center for Health Statistics’ Institutional Review Board reviewed and approved the survey protocols, and each participant signed a consent form voluntarily24.
Figure 1.
Flow chart of NHANES sample selection from March 2017 to March 2020.
Evaluation of exposures
The exposure variables in this study were based on in-person home interviews with participants who reported aspirin use prophylaxis and followed recommendations for low-dose aspirin use. The questions to prevent aspirin use were asked at home by trained interviewers using a computer-assisted Personal Interview (CAPI) system. Questions ascertaining aspirin use, “Have you taken a daily low-dose aspirin as recommended by your doctor and other health care provider to prevent heart disease, stroke, or cancer?”. Categorical answers included “don’t know (n = 3), sometimes take aspirin (n = 89), stopped taking medication due to side effects (n = 67)” were excluded from the statistical analysis. The American college of cardiology foundation and the American heart association (ACC/AHA) defined the use of low-dose aspirin for preventive reasons as taking 75–100 mg orally daily15.
Outcome
Association of aspirin use with femoral neck BMD, total femur BMD, intertrochanter BMD and L1 BMD, a measurement that has been used in the assessment and treatment of osteoporosis as a result of a clinical trial. All subjects in this study received a dual-energy X-ray absorptiometry (DXA) whole-body scan except for pregnant women, individuals who had used a radiographic contrast agent (such as dye or barium) within the past seven days; and individuals who weighed more than 208 kg (DXA table limit). A routine scan of the left hip was performed unless the participants reported a left hip fracture and had surgery on the left hip. In 2017–2018, the femur and spine scans were obtained using Apex 3.2 software on Hologic Discovery Type A Densimeter (Hologic, Inc., Bedford, Massachusetts). The femur and spine scans were taken on the Hologic Horizon Model A densimeter (Hologic, Inc., Bedford, Massachusetts) from March 2019 to March 2020. All scans obtained during March 2017–2020 were analyzed using APEX V4.0 (Hologic) software. Experts checked the scans for accuracy and consistency.
Covariates
The categorical variables listed below were used in this study as covariates: gender, race, heart attack, vigorous work activity, education level, fractures, anti-osteoporotic, prednisone or cortisone, smoking status, and alcohol consumption. Standardized questionnaires were used to obtain the above variables25. Our analysis included successive covariables: age, BMI, Hba1c, the ratio of family income to poverty, C Reactive Protein (CRP), serum creatinine, and serum uric acid.
Race or ethnicity was categorized as non-Hispanic whites, non-Hispanic blacks, Mexican–American, Other Hispanic, and Other Race—Including Multi-Racial. Education level was categorized as Less than high school, high school, and more than high school. Less than high school was defined as Less than 9th grade and 9-11th grade (Includes 12th grade with no diploma). High school was defined as high school graduate/GED or equivalent. More than high school was defined as some college or AA (Associate of Arts) degree and college graduate or above. The ratio of family income to poverty was calculated by dividing total annual family (or individual) income by the poverty guidelines specific to the survey year26. Smoking status was categorized as Every day, Some days, not at all. Vigorous work activity was assessed via a history of participating in one of the following activities during the past week. This is based on responses to the question, “Does your job involve a high-intensity activity, such as lifting or lifting heavy objects, digging or construction work, for at least ten consecutive minutes, that causes a significant increase in breathing or heart rate?” Vigorous work activity was categorized as “yes” or “no” according to the participants’ answered. Alcohol consumption was categorized as never in the last year, nearly every day, 2 to 4 times a week, less than 2 times a week. This is based on responses to the question, “During the past 12 months, how often did you drink any alcoholic beverage?” Fracture history was categorized based on the question “Did the doctor tell you about any other fractures?” Categorize fractures as “yes” or “no”.
Consistency checking is built into CAPI systems to reduce data entry errors. CAPI also uses online help filters to help interviewers define critical terms in the questionnaire. After collection, interview data are reviewed by NHANES field office staff to ensure the accuracy and completeness of selected items. Interviewers were asked to conduct taped interviews, which were reviewed by National Center for Health Statistics (NCHS) staff and interviewers.
Statistical analysis
Non-response and unequal selection probability samples were interpreted with weights. Data analysis in this study was conducted by the analysis guidelines prepared by the U.S. National Bureau of Statistics, using R version 3.4.3 (http://www.R-project.org) and filling in statistical software (http://www.empowerstats.com). 0.05 (P value) was defined as a significant level. A multivariate logistic regression model was used to evaluate the relationship between low-dose aspirin use status and femoral neck BMD, total femur BMD, intertrochanter BMD and L1 BMD. Following the Strengthening, the Reporting of Observational Studies in Epidemiology (STROBE) statement guidelines27, subgroup analyses stratified by age and gender were chosen, and interaction tests were run to make the data more useful. The relevant guidelines and regulations performed all methods. Three models were constructed: Model 1: no covariables are adjusted; Model 2: age and race or age and sex are adjusted; Model 3: all covariables are adjusted.
Ethical statement
The Ethics Review Committee of the National Center for Health Statistics approved all NHANES protocols and obtained written informed consent from all participants. All methods were performed by the relevant guidelines and regulations.
Results
According to the characteristics of the weighted sample shown in Table 1, the following are the characteristics of non-aspirin and low-dose aspirin users (group1: total femur BMD, femoral neck BMD, intertrochanter BMD group): compared with the non-aspirin group (n = 353), the low-dose aspirin group (n = 855) was older (65.7 ± 8.5 VS 63.0 ± 8.3 years P < 0.05). And total femur BMD was higher (0.925 ± 0.149 0.946 ± 0.167 P = 0.037). In the non-aspirin group, the proportion was 40.3% in females, 37.4% in the 50–60 years old group, 40.5% in the 60–70 years old group, and 22.0% in 70–80 years old group. Women accounted for 42.5% of the low-dose aspirin group. 27.7% of participants were 50–60 years old, 36.3% were 60–70, and 36.0% were 70–80 years old. Total femur BMD, intertrochanter BMD, BMI, Hba1c, heart attack, smoking status, fractures, and education level were significantly different between the two groups (P < 0.05).
Table 1.
Weighted characteristics of study sample non-aspirin users and low-dose aspirin users (Group1: Total femur BMD, Femoral neck BMD, Intertrochanter BMD).
| Non-aspirin use (n = 353) | Low-dose aspirin use (n = 855) | P value | |
|---|---|---|---|
| Total femur BMD (gm/cm2) | 0.925 ± 0.149 | 0.946 ± 0.167 | 0.037 |
| Femoral neck BMD (gm/cm2) | 0.756 ± 0.132 | 0.774 ± 0.151 | 0.053 |
| Intertrochanter BMD (gm/cm2) | 1.099 ± 0.174 | 1.126 ± 0.198 | 0.030 |
| BMI (kg/m2) | 29.13 ± 5.15 | 30.03 ± 5.86 | 0.012 |
| Hba1c (%) | 6.01 ± 1.26 | 6.24 ± 1.08 | 0.002 |
| Serum creatinine(mmol/L) | 83.29 ± 23.64 | 83.62 ± 30.23 | 0.852 |
| Serum uric acid (umol/L) | 328.27 ± 77.46 | 329.13 ± 80.70 | 0.864 |
| Gender (%) | 0.464 | ||
| Male | 59.73 | 57.45 | |
| Female | 40.26 | 42.54 | |
| Age (years) | 63.07 ± 8.39 | 65.78 ± 8.51 | < 0.001 |
| Age groups (%) | < 0.001 | ||
| 50–60 years | 37.44 | 27.67 | |
| 60–70 years | 40.50 | 36.25 | |
| 70–80 years | 22.04 | 36.07 | |
| Race (%) | 0.096 | ||
| Mexican American | 5.55 | 4.29 | |
| Other Hispanic | 8.79 | 5.23 | |
| Non-Hispanic White | 69.69 | 71.43 | |
| Non-Hispanic Black | 9.70 | 10.44 | |
| Other race-including multi-racial | 6.25 | 8.59 | |
| Heart attack (%) | < 0.001 | ||
| Yes | 5.73 | 16.57 | |
| No | 94.26 | 83.38 | |
| Recorded | 0.04 | ||
| Education level (%) | 0.011 | ||
| Less than high school | 10.11 | 11.77 | |
| High school | 27.41 | 35.96 | |
| More than high school | 62.47 | 52.21 | |
| Recorded | 0.03 | ||
| Fractures (%) | 0.001 | ||
| Yes | 35.73 | 25.40 | |
| No | 64.19 | 74.47 | |
| Recorded | 0.07 | 0.12 | |
| Smoking status (%) | 0.00 | ||
| Every day | 16.04 | 10.18 | |
| Some days | 4.03 | 1.25 | |
| Not at all | 33.55 | 35.24 | |
| Recorded | 46.36 | 53.31 | |
Mean ± SD for continuous variables: P value was calculated by the weighted linear regression model.
Per cent for categorical variables: P value was calculated by weighted chi-square test. Weights are created in NHANES to account for the complex survey design (including oversampling), survey non-response, and post-stratification adjustment to match total population counts from the Census Bureau.
In Table 2, the following are the characteristics of non-aspirin and low-dose aspirin users (group2: L1BMD): compared with the non-aspirin group (n = 350), the low-dose aspirin group (n = 878) was older (65.8 ± 8.5 and 63.0 ± 8.4 years P < 0.05). In the non-aspirin group, the proportion was 41.8% in females, 58.2% in males, 38.6% in the 50–60 years old group, and 38.5% in the 60–70 years old group, and 22.9% in 70–80 years old group. Women accounted for 44.7% of the low-dose aspirin group, while men accounted for 55.3%. A total of 28.3% in the 50–60 years old group, 34.9% in the 60–70 years old group, and 36.8% in the 70–80 years old group. L1 BMD, BMI, Hba1c, Heart attack, smoking status, and education level were also significantly different between the two groups (P < 0.05).
Table 2.
Weighted characteristics of study sample non-aspirin users and low-dose aspirin users (Group2: L1BMD).
| Non-aspirin use (n = 350) | Low-dose aspirin use (n = 878) | P value | |
|---|---|---|---|
| L1 BMD (gm/cm2) | 0.963 ± 0.159 | 1.006 ± 0.189 | < 0.001 |
| Age(years) | 63.03 ± 8.46 | 65.72 ± 8.60 | < 0.001 |
| Age groups (%) | < 0.001 | ||
| 50–60 years | 38.64 | 28.31 | |
| 60–70 years | 38.48 | 34.88 | |
| 70–80 years | 22.87 | 36.80 | |
| BMI (kg/m2) | 29.55 ± 5.55 | 30.87 ± 6.53 | < 0.001 |
| Hba1c (%) | 6.07 ± 1.27 | 6.27 ± 1.11 | 0.008 |
| Serum creatinine (mmol/L) | 83.06 ± 22.27 | 84.18 ± 34.46 | 0.574 |
| Serum uric acid (umol/L) | 327.82 ± 76.09 | 331.71 ± 81.46 | 0.443 |
| Gender (%) | 0.353 | ||
| Male | 58.21 | 55.29 | |
| Female | 41.78 | 44.70 | |
| Race (%) | 0.198 | ||
| Mexican American | 5.48 | 4.70 | |
| Other Hispanic | 8.48 | 5.18 | |
| Non-Hispanic White | 68.64 | 70.71 | |
| Non-Hispanic Black | 10.39 | 10.43 | |
| Other race-including multi-racial | 6.99 | 8.96 | |
| Education level (%) | 0.002 | ||
| Less than high school | 10.31 | 12.22 | |
| High school | 26.81 | 35.59 | |
| More than high school | 62.87 | 52.18 | |
| Heart attack (%) | < 0.001 | ||
| Yes | 5.46 | 15.98 | |
| No | 94.38 | 83.97 | |
| Recorded | 0.15 | 0.04 | |
| Fractures (%) | 0.085 | ||
| Yes | 34.20 | 27.81 | |
| No | 65.72 | 72.03 | |
| Recorded | 0.07 | 0.15 | |
| Smoking status (%) | 0.002 | ||
| Every day | 14.91 | 10.68 | |
| Some days | 4.11 | 1.30 | |
| Not at all | 32.93 | 35.38 | |
| Recorded | 48.03 | 52.63 | |
Mean ± SD for continuous variables: P value was calculated by the weighted linear regression model.
Per cent for categorical variables: P value was calculated by weighted chi-square test. Weights are created in NHANES to account for the complex survey design (including oversampling), survey non-response, and post-stratification adjustment to match total population counts from the Census Bureau.
Table 3 shows the gender-based stratified analysis and interaction tests. Table 3 shows the gender-based stratified analysis and interaction tests. Low-dose aspirin was positively associated with bone mineral density in both males (Total femur BMD β = 0.025, 95% CI 0.004–0.046; Femoral neck BMD β = 0.014, 95% CI − 0.006 to 0.035; Intertrochanter BMD β = 0.032, 95% CI 0.007–0.057; L1 BMD β = 0.041, 95% CI 0.013–0.069) and females (Total femur BMD β = 0.017, 95% CI − 0.004 to 0.038; Femoral neck BMD β = 0.021, 95% CI − 0.001 to 0.043; Intertrochanter BMD β = 0.021, 95% CI − 0.006 to 0.049; L1 BMD β = 0.005, 95% CI − 0.023 to 0.034), P interaction > 0.05, indicating that the effect of low-dose aspirin on BMD was not significantly different between genders.
Table 3.
Subgroup analysis was stratified by gender.
| Total femur BMD (gm/cm2) | Femoral neck BMD (gm/cm2) | Intertrochanter BMD (gm/cm2) | L1 BMD (gm/cm2) | |
|---|---|---|---|---|
| Male | ||||
| Non-aspirin users | Reference | Reference | Reference | Reference |
| Low-dose aspirin users | 0.025 (0.004, 0.046) P = 0.0179 | 0.014 (− 0.006, 0.035) P = 0.1775 | 0.032 (0.007, 0.057) P = 0.0113 | 0.041 (0.013, 0.069) P = 0.0042 |
| Female | ||||
| Non-aspirin users | Reference | Reference | Reference | Reference |
| Low-dose aspirin users | 0.017 (− 0.004, 0.038) P = 0.1218 | 0.021 (− 0.001, 0.043) P = 0.0572 | 0.021 (− 0.006, 0.049) P = 0.1235 | 0.005 (− 0.023, 0.034) P = 0.7127 |
| P interaction | P = 0.7746 | P = 0.4587 | P = 0.7612 | P = 0.0882 |
Age, Race, Heart attack, Vigorous work activity, BMI, Hba1c, Education level, Ratio of family income to poverty, Fractures, Anti-osteoporotic, Prednisone or Cortisone, HS C-Reactive Protein, Smoking status, Serum Creatinine, Serum Uric acid, Alcohol consumption were adjusted.
Table 4 shows the stratified analysis and interaction tests by age. Stratified analysis by age showed that the positive association between low-dose aspirin use and bone mineral density remained in three different age groups: 50–60 years (Total femur BMD β = 0.020, 95% CI − 0.010 to 0.049; Femoral neck BMD β = 0.026, 95% CI − 0.004 to 0.056; Intertrochanter BMD β = 0.028, 95% CI − 0.008 to 0.064; L1 BMD β = 0.026, 95% CI − 0.007 to 0.059), 60–70 years (Total femur BMD β = 0.037, 95% CI 0.013–0.061; Femoral neck BMD β = 0.014, 95% CI − 0.011 to 0.040; Intertrochanter BMD β = 0.047, 95% CI 0.018–0.077; L1 BMD β = 0.029, 95% CI − 0.013 to 0.062), 70–80 years (Total femur BMD β = 0.010, 95% CI − 0.009 to 0.039; Femoral neck BMD β = 0.013, 95% CI − 0.013 to 0.039; Intertrochanter BMD β = 0.008, 95% CI − 0.028 to 0.044; L1 BMD β = 0.011, 95% CI − 0.030 to 0.052). The interaction p value of low-dose aspirin on bone mineral density was greater than 0.05 in 50–60 years old, 60–70 years old and 70–80 years old, indicating that there was no significant difference in the effect of low-dose aspirin on bone mineral density in different age groups.
Table 4.
Subgroup analyses stratified by age.
| Total femur BMD (gm/cm2) | Femoral neck BMD (gm/cm2) | Intertrochanter BMD (gm/cm2) | L1 BMD (gm/cm2) | |
|---|---|---|---|---|
| 50–60 years | ||||
| Non-aspirin users | Reference | Reference | Reference | Reference |
| Low-dose aspirin users | 0.020 (− 0.010, 0.049) P = 0.1907 | 0.026 (− 0.004, 0.056) P = 0.0931 | 0.028 (− 0.008, 0.064) P = 0.1289 | 0.026 (− 0.007, 0.059) P = 0.1190 |
| 60–70 years | ||||
| Non-aspirin users | Reference | Reference | Reference | Reference |
| Low-dose aspirin users | 0.037 (0.013, 0.061) P = 0.0024 | 0.014 (− 0.011, 0.040) P = 0.2614 | 0.047 (0.018, 0.077) P = 0.0015 | 0.029 (− 0.004, 0.062) P = 0.0887 |
| 70–80 years | ||||
| Non-aspirin users | Reference | Reference | Reference | Reference |
| Low-dose aspirin users | 0.010 (− 0.019, 0.039) P = 0.4979 | 0.013 (− 0.013, 0.039) P = 0.3352 | 0.008 (− 0.028, 0.044) P = 0.6663 | 0.011 (− 0.030, 0.052) P = 0.5989 |
| P interaction | P = 0.3347 | P = 0.7506 | P = 0.2308 | P = 0.7528 |
Gender, Race, Heart attack, Vigorous work activity, BMI, Hba1c, Education level, Ratio of family income to poverty, Fractures, Anti-osteoporotic, Prednisone or Cortisone, HS C-Reactive Protein, Smoking status, Serum Creatinine, Serum Uric acid, Alcohol consumption were adjusted.
Total femur BMD β = 0.019, 95% CI 0.004–0.034; Femoral neck BMD β = 0.017, 95% CI 0.002–0.032; Intertrochanter BMD β = 0.025, 95% CI 0.007–0.043; L1 BMD β = 0.026, 95% CI 0.006–0.046.
Table 5 shows the results of multiple regression analysis. In the unadjusted model 1, low-dose aspirin use was positively associated with bone mineral density (Total femur BMD β = 0.021, 95% CI 0.001–0.041; Femoral neck BMD β = 0.018, 95% CI − 0.000 to 0.036; Intertrochanter BMD β = 0.026, 95% CI 0.003–0.050; L1 BMD β = 0.043, 95% CI 0.021–0.066). After adjusting for confounders, this positive association persisted in model 2 (adjusted for age, gender, and race) (Total femur BMD β = 0.041, 95% CI 0.024–0.057; Femoral neck BMD β = 0.034, 95% CI 0.019 to 0.050; Intertrochanter BMD β = 0.048, 95% CI 0.029–0.067; L1 BMD β = 0.051, 95% CI 0.030–0.072) and model 3 (adjusted for all covariates) (Total femur BMD β = 0.019, 95% CI 0.004–0.034; Femoral neck BMD β = 0.017, 95% CI 0.002–0.032; Intertrochanter BMD β = 0.025, 95% CI 0.007–0.043; L1 BMD β = 0.026, 95% CI 0.006–0.046 P < 0.05). On average, low-dose aspirin users had 0.019 g/cm2 higher total femoral BMD, 0.017 g/cm2 higher femoral neck BMD, 0.025 g/cm2 higher intertrochanteric BMD, and 0.026 g/cm2 higher L1 BMD than non-aspirin users.
Table 5.
Associations between low-dose aspirin use and bone mineral density.
| Model1, β (95% CI, P) | Model2, β (95% CI, P) | Model3, β (95% CI, P) | |
|---|---|---|---|
| Total femur BMD (gm/cm2) | |||
| Non-aspirin use | Reference | Reference | Reference |
| Low-dose aspirin use | 0.021 (0.001, 0.041) P = 0.03786 | 0.041 (0.024, 0.057) P < 0.00001 | 0.019 (0.004, 0.034) P = 0.01137 |
| Femoral neck BMD (gm/cm2) | |||
| Non-aspirin use | Reference | Reference | Reference |
| Low-dose aspirin use | 0.018 (− 0.000, 0.036) P = 0.05388 | 0.034 (0.019, 0.050) P = 0.00001 | 0.017 (0.002, 0.032) P = 0.02779 |
| Intertrochanter BMD (gm/cm2) | |||
| Non-aspirin use | Reference | Reference | Reference |
| Low-dose aspirin use | 0.026 (0.003, 0.050) P = 0.03016 | 0.048 (0.029, 0.067) P < 0.00001 | 0.025 (0.007, 0.043) P = 0.00743 |
| L1 BMD (gm/cm2) | |||
| Non-aspirin use | Reference | Reference | Reference |
| Low-dose aspirin use | 0.043 (0.021, 0.066) P = 0.00017 | 0.051 (0.030, 0.072) P < 0.00001 | 0.026 (0.006, 0.046) P = 0.00941 |
Model 1 no covariates were adjusted.
Model 2 age, gender, and race were adjusted.
Model 3 Gender, Age, Race, Heart attack, Vigorous work activity, BMI, Hba1c, Education level, Ratio of family income to poverty, Fractures, Anti-osteoporotic, Prednisone or Cortisone, HS C-Reactive Protein, Smoking status, Serum Creatinine, Serum Uric acid, Alcohol consumption were adjusted.
Discussion
This cross-sectional study investigated the relationships of low-dose aspirin use with total femur BMD, femoral neck BMD, intertrochanter BMD, and L1BMD. In summary, our results mainly show significantly higher total femur BMD, intertrochanter BMD, and L1 BMD in low-dose aspirin users compared to non-aspirin users in the general US population and significantly greater femoral neck BMD but only after multivariate adjustment. Stratified analysis and interaction test showed that the effect of low-dose aspirin on bone mineral density had no significant difference among different age groups and genders.
In Tables 1 and 2, aspirin users have higher BMI and higher Hba1c, which may be because that obese patients or diabetic patients are more likely to suffer from cardiovascular diseases and need aspirin. The number of fractures in patients who took aspirin was significantly lower than in those who did not. This could mean that people with broken bones are more likely to have cardiovascular disease or that aspirin use might reduce the risk of fractures. This requires further study, the lower prevalence of daily smoking among aspirin users does not mean that smokers are less likely to develop the cardiovascular disease but rather that daily smokers are likely to be younger and have a lower risk of cardiovascular disease. In Table 3, in stratified analysis by gender, total femur BMD, intertrochanter BMD, and L1BMD were significantly higher in male aspirin users than in non-aspirin users. However, the P values of the interaction were all greater than 0.05, which may indicate no difference in the effect of aspirin on BMD between the genders. In Table 4, in age stratification, although the total femur BMD and intertrochanter BMD of patients aged 60 to 70 who used aspirin were higher than that of those who did not use aspirin, all P values of interaction were more significant than 0.05, which may indicate that there was no difference in the effect of aspirin on BMD among age groups. In Table 5, in the multiple regression model, after adjusting variables in model 2 and Model 3, total femur BMD, femoral neck BMD, intertrochanter BMD, and L1BMD of aspirin users was significantly higher than that of patients of non-aspirin users (P < 0.05), and femoral neck BMD of aspirin users in model 1 was higher than that of non-aspirin users not significant (P = 0.053). Possibly due to the influence of sample size, it is expected that increasing sample size will significantly increase bone mineral density.
The majority of low-dose aspirin users are elderly, who have higher rates of cardiovascular disease morbidity and mortality28,29. In addition to its antiplatelet activity, aspirin may also affect oxidative stress, vascular inflammation, and insulin sensitivity30–35. Recent studies have shown that low-dose aspirin can affect bone metabolism10,14,36–41. But these research results are contradictory and controversial.
Early results suggest that aspirin can improve BMD and bone structure in young rat models of osteoporosis42–44. These results come from animal studies, and there is no definitive evidence in humans. Another study observed that, after adjusting for confounders, women who took aspirin five to seven times a week had higher bone mineral density in the hips and spine than women who did not take aspirin40. The findings are difficult to generalize to other populations because the subjects were all white women40. Carbone et al. found significant increases in systemic BMD in subjects using aspirin alone and aspirin plus selective nonsteroidal anti-inflammatory drugs relative to COX239. Only two ethnic groups were represented in the study: 43.1% African American and 56.9% Caucasian. The average age of the study participants was 73.6 years (19–70 years). One study showed that long-term use of low-dose aspirin was not associated with decreased bone mineral density in the general population45. Individuals over 65 were excluded from the study, even though it was a large population study with multiple confounders. However, due to the large elderly population of low-dose aspirin users, the study’s sample size was limited to 47 low-dose aspirin users, so the results are not representative of the general population. Despite a growing number of observational studies, there have been no randomized controlled trials of the effect of aspirin treatment on fracture risk in humans, and the relationship between aspirin use and bone mineral density is contradictory37,39,40,46.
This research has several limitations. As the study is cross-sectional, it cannot describe changes in BMD with aspirin over time and causality in the relationship cannot be inferred. Secondly, we had no information on the duration of aspirin therapy or the exact dose. We were unable to a relationship of aspirin dose to BMD because this study lacked information on the exact dose of aspirin. Thirdly we only measured BMD, but other measures that capture bone quality and microarchitecture, including Computerized tomography (CT), were unavailable, or bone turnover markers were47,48. Fourthly, while we adjusted for the presence of cardiovascular disease, we were not able to specifically take account of medications like thiazide diuretics and statins which are associated with higher BMD.
On the other hand, to better summarize the U.S. population, this study included a representative multiracial population sample with a large sample size that could be further analyzed by subgroup. In addition, we adjusted for multiple potential confounding factors, including heart attack, vigorous work activity, education level, fractures, anti-osteoporotic, prednisone or Cortisone, HS C-Reactive Protein, smoking status, serum creatinine, serum uric acid, and alcohol consumption.
Overall, our research suggests that low dose aspirin may improve bone mineral density irrespective of age or gender. However, as studies are cross-sectional further research to assess longitudinal changes in BMD in low-dose aspirin users should be conducted. Additionally, randomized controlled trials would be required to prove causality.
Acknowledgements
The authors thank the staff and the participants of the NHANES study for their valuable contributions.
Author contributions
All authors have contributed significantly to the work of the report in terms of concept, study design, execution, data collection, analysis and interpretation; Participate in the drafting, revision or critical review of clauses; The main manuscript was written by H.L. and Q.S., and the figure and tables were written by X.T. and Y.T. X.X. is mainly responsible for the design guidance of the project. All the authors have reviewed the manuscript.
Data availability
All data generated or analyzed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med.94(6), 646–650 (1993). [DOI] [PubMed]
- 2.Raisz LG. Potential impact of selective cyclooxygenase-2 inhibitors on bone metabolism in health and disease. Am. J. Med. 2001;110(Suppl 3A):43s–45s. doi: 10.1016/S0002-9343(00)00684-7. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Y, Boudreau DM, Freedman AN. Trends in the use of aspirin and nonsteroidal anti-inflammatory drugs in the general U.S. population. Pharmacoepidemiol. Drug Saf. 2014;23(1):43–50. doi: 10.1002/pds.3463. [DOI] [PubMed] [Google Scholar]
- 4.Suda K, Udagawa N, Sato N, Takami M, Itoh K, Woo JT, Takahashi N, Nagai K. Suppression of osteoprotegerin expression by prostaglandin E2 is crucially involved in lipopolysaccharide-induced osteoclast formation. J. Immunol. 2004;172(4):2504–2510. doi: 10.4049/jimmunol.172.4.2504. [DOI] [PubMed] [Google Scholar]
- 5.Liu H, Li W, Liu Y, Zhang X, Zhou Y. Co-administration of aspirin and allogeneic adipose-derived stromal cells attenuates bone loss in ovariectomized rats through the anti-inflammatory and chemotactic abilities of aspirin. Stem Cell Res. Ther. 2015;6:200. doi: 10.1186/s13287-015-0195-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agas D, Marchetti L, Capitani M, Sabbieti MG. The dual face of parathyroid hormone and prostaglandins in the osteoimmune system. Am. J. Physiol. Endocrinol. Metab. 2013;305(10):E1185–E1194. doi: 10.1152/ajpendo.00290.2013. [DOI] [PubMed] [Google Scholar]
- 7.Boutaud O, Sosa IR, Amin T, Oram D, Adler D, Hwang HS, Crews BC, Milne G, Harris BK, Hoeksema M, et al. Inhibition of the biosynthesis of prostaglandin E2 by low-dose aspirin: Implications for adenocarcinoma metastasis. Cancer Prev. Res. 2016;9(11):855–865. doi: 10.1158/1940-6207.CAPR-16-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blackwell KA, Raisz LG, Pilbeam CC. Prostaglandins in bone: Bad cop, good cop? Trends Endocrinol. Metab. 2010;21(5):294–301. doi: 10.1016/j.tem.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng YP, Yang C, Li Y, Fan Y, Yang HJ, Liu B, Sang HX. Aspirin inhibits osteoclastogenesis by suppressing the activation of NF-κB and MAPKs in RANKL-induced RAW264.7 cells. Mol. Med. Rep. 2016;14(3):1957–1962. doi: 10.3892/mmr.2016.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker AL, Soh SE, Sanders KM, Pasco J, Khosla S, Ebeling PR, Ward SA, Peeters G, Talevski J, Cumming RG, et al. Aspirin and fracture risk: A systematic review and exploratory meta-analysis of observational studies. BMJ Open. 2020;10(2):e026876. doi: 10.1136/bmjopen-2018-026876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grosser N, Schröder H. Aspirin protects endothelial cells from oxidant damage via the nitric oxide-cGMP pathway. Arterioscler. Thromb. Vasc. Biol. 2003;23(8):1345–1351. doi: 10.1161/01.ATV.0000083296.57581.AE. [DOI] [PubMed] [Google Scholar]
- 12.Taubert D, Berkels R, Grosser N, Schröder H, Gründemann D, Schömig E. Aspirin induces nitric oxide release from vascular endothelium: A novel mechanism of action. Br. J. Pharmacol. 2004;143(1):159–165. doi: 10.1038/sj.bjp.0705907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad K, Budoff MJ, Delaney JA, Mao S, Gao Y, Nasir K, Tracy R, Li D. Association of aspirin and other nonsteroidal anti-inflammatory drugs with vertebral trabecular bone: Data from multiethnic study of atherosclerosis, a population-based multicenter cohort study. J. Comput. Assist. Tomogr. 2020;44(4):562–568. doi: 10.1097/RCT.0000000000001022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chin KY. A review on the relationship between aspirin and bone health. J. Osteoporos. 2017;2017:3710959. doi: 10.1155/2017/3710959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e563–e595. doi: 10.1161/CIR.0000000000000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis JS, Lee HY, Kim J, Advani SM, Peng HL, Banfield E, Hawk ET, Chang S, Frazier-Wood AC. Use of non-steroidal anti-inflammatory drugs in US adults: Changes over time and by demographic. Open Heart. 2017;4(1):e000550. doi: 10.1136/openhrt-2016-000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu Q, Dillon CF, Eberhardt MS, Wright JD, Burt VL. Preventive aspirin and other antiplatelet medication use among U.S. adults aged ≥ 40 years: Data from the National Health and Nutrition Examination Survey, 2011–2012. Public Health Rep. 2015;130(6):643–654. doi: 10.1177/003335491513000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J. Bone Miner. Res. 2007;22(3):465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 19.Lewiecki EM. Managing osteoporosis: Challenges and strategies. Clevel. Clin. J. Med. 2009;76(8):457–466. doi: 10.3949/ccjm.76a.09019. [DOI] [PubMed] [Google Scholar]
- 20.Svedbom A, Hernlund E, Ivergård M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B, Kanis JA. Osteoporosis in the European Union: A compendium of country-specific reports. Arch. Osteoporos. 2013;8(1):137. doi: 10.1007/s11657-013-0137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregson CL, Armstrong DJ, Bowden J, Cooper C, Edwards J, Gittoes NJL, Harvey N, Kanis J, Leyland S, Low R, et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch. Osteoporos. 2022;17(1):58. doi: 10.1007/s11657-022-01061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hart DJ, Cronin C, Daniels M, Worthy T, Doyle DV, Spector TD. The relationship of bone density and fracture to incident and progressive radiographic osteoarthritis of the knee: The Chingford Study. Arthritis Rheum. 2002;46(1):92–99. doi: 10.1002/1529-0131(200201)46:1<92::AID-ART10057>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 23.Dillon CF, Weisman MH. US National Health and Nutrition examination survey arthritis initiatives, methodologies and data. Rheum. Dis. Clin. N. Am. 2018;44(2):215–265. doi: 10.1016/j.rdc.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: Plan and operations, 1999–2010. Vital Health Stat. 2013;56:1–37. [PubMed] [Google Scholar]
- 25.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–1994. Series 1: Programs and collection procedures. Vital Health Stat. 32, 1–407 (1994). [PubMed]
- 26.Patel CJ, Pho N, McDuffie M, Easton-Marks J, Kothari C, Kohane IS, Avillach P. A database of human exposomes and phenomes from the US National Health and Nutrition Examination Survey. Sci. Data. 2016;3:160096. doi: 10.1038/sdata.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, et al. Heart disease and stroke statistics-2018 update: A report from the American Heart Association. Circulation. 2018;137(12):e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 29.Patrono C, Bachmann F, Baigent C, Bode C, De Caterina R, Charbonnier B, Fitzgerald D, Hirsh J, Husted S, Kvasnicka J, et al. Expert consensus document on the use of antiplatelet agents. The task force on the use of antiplatelet agents in patients with atherosclerotic cardiovascular disease of the European society of cardiology. Eur. Heart J. 2004;25(2):166–181. doi: 10.1016/j.ehj.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE, Shulman GI. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J. Clin. Investig. 2002;109(10):1321–1326. doi: 10.1172/JCI0214955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costanzo A, Moretti F, Burgio VL, Bravi C, Guido F, Levrero M, Puri PL. Endothelial activation by angiotensin II through NFkappaB and p38 pathways: Involvement of NFkappaB-inducible kinase (NIK), free oxygen radicals, and selective inhibition by aspirin. J. Cell. Physiol. 2003;195(3):402–410. doi: 10.1002/jcp.10191. [DOI] [PubMed] [Google Scholar]
- 32.Ristimäe T, Zilmer M, Zilmer K, Kairane C, Kullisaar T, Teesalu R. Effect of low-dose aspirin on the markers of oxidative stress. Cardiovasc. Drugs Ther. 1999;13(6):485–490. doi: 10.1023/A:1007867402152. [DOI] [PubMed] [Google Scholar]
- 33.Reilly M, Delanty N, Lawson JA, FitzGerald GA. Modulation of oxidant stress in vivo in chronic cigarette smokers. Circulation. 1996;94(1):19–25. doi: 10.1161/01.CIR.94.1.19. [DOI] [PubMed] [Google Scholar]
- 34.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 2002;196(8):1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raghavan RP, Laight DW, Cummings MH. Aspirin in type 2 diabetes, a randomised controlled study: Effect of different doses on inflammation, oxidative stress, insulin resistance and endothelial function. Int. J. Clin. Pract. 2014;68(2):271–277. doi: 10.1111/ijcp.12310. [DOI] [PubMed] [Google Scholar]
- 36.Minn YK, Suk SH, Do SY. Osteoporosis as an independent risk factor for silent brain infarction and white matter changes in men and women: The PRESENT project. Osteoporos. Int. 2014;25(10):2465–2469. doi: 10.1007/s00198-014-2785-3. [DOI] [PubMed] [Google Scholar]
- 37.Bleicher K, Cumming RG, Naganathan V, Seibel MJ, Sambrook PN, Blyth FM, Le Couteur DG, Handelsman DJ, Creasey HM, Waite LM. Lifestyle factors, medications, and disease influence bone mineral density in older men: Findings from the CHAMP study. Osteoporos. Int. 2011;22(9):2421–2437. doi: 10.1007/s00198-010-1478-9. [DOI] [PubMed] [Google Scholar]
- 38.Chen CH, Lin CL, Kao CH. Association between chronic hepatitis B virus infection and risk of osteoporosis: A Nationwide Population-Based Study. Medicine. 2015;94(50):e2276. doi: 10.1097/MD.0000000000002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carbone LD, Tylavsky FA, Cauley JA, Harris TB, Lang TF, Bauer DC, Barrow KD, Kritchevsky SB. Association between bone mineral density and the use of nonsteroidal anti-inflammatory drugs and aspirin: Impact of cyclooxygenase selectivity. J. Bone Miner. Res. 2003;18(10):1795–1802. doi: 10.1359/jbmr.2003.18.10.1795. [DOI] [PubMed] [Google Scholar]
- 40.Bauer DC, Orwoll ES, Fox KM, Vogt TM, Lane NE, Hochberg MC, Stone K, Nevitt MC. Aspirin and NSAID use in older women: Effect on bone mineral density and fracture risk. Study of Osteoporotic Fractures Research Group. J. Bone Miner. Res. 1996;11(1):29–35. doi: 10.1002/jbmr.5650110106. [DOI] [PubMed] [Google Scholar]
- 41.Chai SC, Foley EM, Arjmandi BH. Anti-atherogenic properties of vitamin E, aspirin, and their combination. PLoS ONE. 2018;13(10):e0206315. doi: 10.1371/journal.pone.0206315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamaza T, Miura Y, Bi Y, Liu Y, Akiyama K, Sonoyama W, Patel V, Gutkind S, Young M, Gronthos S, et al. Pharmacologic stem cell based intervention as a new approach to osteoporosis treatment in rodents. PLoS ONE. 2008;3(7):e2615. doi: 10.1371/journal.pone.0002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei J, Wang J, Gong Y, Zeng R. Effectiveness of combined salmon calcitonin and aspirin therapy for osteoporosis in ovariectomized rats. Mol. Med. Rep. 2015;12(2):1717–1726. doi: 10.3892/mmr.2015.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen ZW, Wu ZX, Sang HX, Qin GL, Wang LS, Feng J, Wang J, Li XJ, Wang JC, Zhang D. Effect of aspirin administration for the treatment of osteoporosis in ovariectomized rat model. Zhonghua Yi Xue Za Zhi. 2011;91(13):925–929. [PubMed] [Google Scholar]
- 45.Bonten TN, de Mutsert R, Rosendaal FR, Jukema JW, van der Bom JG, de Jongh RT, den Heijer M. Chronic use of low-dose aspirin is not associated with lower bone mineral density in the general population. Int. J. Cardiol. 2017;244:298–302. doi: 10.1016/j.ijcard.2017.06.089. [DOI] [PubMed] [Google Scholar]
- 46.Hill DD, Cauley JA, Bunker CH, Baker CE, Patrick AL, Beckles GLA, Wheeler VW, Zmuda JM. Correlates of bone mineral density among postmenopausal women of African Caribbean ancestry: Tobago women's health study. Bone. 2008;43(1):156–161. doi: 10.1016/j.bone.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hollaender R, Hartl F, Krieg MA, Tyndall A, Geuckel C, Buitrago-Tellez C, Manghani M, Kraenzlin M, Theiler R, Hans D. Prospective evaluation of risk of vertebral fractures using quantitative ultrasound measurements and bone mineral density in a population-based sample of postmenopausal women: Results of the Basel Osteoporosis Study. Ann. Rheum. Dis. 2009;68(3):391–396. doi: 10.1136/ard.2007.083618. [DOI] [PubMed] [Google Scholar]
- 48.Naylor K, Eastell R. Bone turnover markers: Use in osteoporosis. Nat. Rev. Rheumatol. 2012;8(7):379–389. doi: 10.1038/nrrheum.2012.86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.