Abstract
Lantana camara L. is widely used in folk medicine for alleviation of inflammatory disorders, but studies that proved this folk use and that revealed the molecular mechanism of action in inflammation mitigation are not enough. Therefore, this study aimed to identify L. camara phytoconstituents using UPLC-MS/MS and explain their multi-level mechanism of action in inflammation alleviation using network pharmacology analysis together with molecular docking and in vitro testing. Fifty-seven phytoconstituents were identified in L. camara extract, from which the top hit compounds related to inflammation were ferulic acid, catechin gallate, myricetin and iso-ferulic acid. Whereas the most enriched inflammation related genes were PRKCA, RELA, IL2, MAPK 14 and FOS. Furthermore, the most enriched inflammation-related pathways were PI3K-Akt and MAPK signaling pathways. Molecular docking revealed that catechin gallate possessed the lowest binding energy against PRKCA, RELA and IL2, while myricetin had the most stabilized interaction against MAPK14 and FOS. In vitro cytotoxicity and anti-inflammatory testing indicated that L. camara extract is safer than piroxicam and has a strong anti-inflammatory activity comparable to it. This study is a first step in proving the folk uses of L. camara in palliating inflammatory ailments and institutes the groundwork for future clinical studies.
Subject terms: Computational biology and bioinformatics, Plant sciences, Diseases
Introduction
Inflammation is a complex process, triggered by injury, infection, or genetic alterations, leading to signaling cascades stimulation, transcription factors activation, gene expression, elevated levels of inflammatory enzymes, and liberation of diverse oxidants and pro-inflammatory mediators in inflammatory cells. Immoderate levels of oxidants and inflammatory molecules are harmful to normal tissue. They can cause toxicity, lack of barrier function, anomalous cell proliferation, impeding normal function of tissues and organs and eventually resulting in systemic disorders1. The ordinary inflammation treatment mostly includes steroidal and non-steroidal anti-inflammatory drugs and opiates. These agents have many adverse effects such as gastric ulcers, tolerance, and dependence2. Therefore, the attention is now paid to natural products with the aim to gain more effective anti-inflammatory agents with less or no side effects. Recently, many medicinal plants have been successfully used for alleviation of inflammation as they exhibited significant anti-inflammatory activities, such as macrophage differentiation, lymphocyte activation, and propagation of apoptosis3. A remarkable example of these medicinal plants is Lantana camara L. (Verbanaceae)2,4.
Lantana camara L. is a flowering plant belongs to family Verbanaceae, also called wild or red sage. It is indigenous to Africa and tropical America, and it is the most prevalent species of genus Lantana. It is considered as a medicinal aromatic plant, a notorious weed and as a common ornamental garden plant4. It is used in folk medicine in alleviation of several inflammatory ailments, such as rheumatism, swellings, bronchitis, and asthma5. In addition, it is used as an adjuvant therapy in mitigation of cancers, chicken pox, measles, eczema, tumors, high blood pressure, bilious fevers, and catarrhal infections6. Furthermore, it is utilized to palliate malaria, tuberculosis, lymphadenitis, mumps, stomachache, and bone pain7.
Former in vivo and in vitro studies of L. camara showed that it exerts its anti-inflammatory activity through suppressing several inflammatory mediators such as COX-2, LOX, NO, ROS, NF-kB, or inhibition of inflammatory signals transmission4. Moreover, L. camara extract showed an inhibition to edema induced by carrageenan2,8, and it was found to suppress iNOS which has an important role in inflammation9.
The plant extracts have a complicated nature which makes it difficult to explain their molecular mechanisms of action. This may be because the plant extracts can act on several targets at the same time, or due to the synergism between their chemical constituents10. Recently, the molecular targets and the related disease pathways of plant constituents have been successfully predicted via the application of network pharmacology-based analysis. This technique allows to envisage compound-target-gene-disease network hence, facilitating the projection of the multi-target mechanism of plant extracts11,12. Network pharmacological analysis has been successfully used to explain the mechanism of action of many medicinal plants in alleviating different diseases13–17.
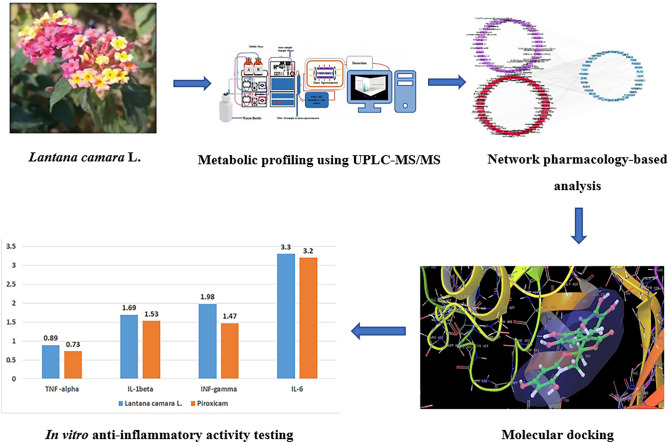
Although there are some studies in literature that explain the mechanisms of action of L. camara in mitigating inflammatory disorders2,4,8,9,18–20, this work was carried out to allow for more in-depth understanding of these molecular mechanisms of action using network pharmacology-based and molecular docking analyses, in addition to in vitro studies, thus proving the folk uses of L. camara as anti-inflammatory natural product. The workflow of this study is illustrated in Fig. 1.
Figure 1.
Study workflow diagram.
Results
UPLC-MS/MS analysis of L. camara extract
The base peak chromatogram of L. camara extract showed the presence of 57 metabolites (Supplementary Fig. S1) which were recognized through comparing their retention times to external standards, CRC, Wiley, reference literature in addition to our in-house database. The UPLC-MS/MS metabolic profile was presented in Table 1.
Table 1.
Metabolites identified in the extract of L. camara using UPLC-MS/MS in negative ionization mode.
| Peak number | Retention time (min) | Identified compounds | M−Ha | Molecular weight | Chemical class | Element composition | MSn fragmentsb |
|---|---|---|---|---|---|---|---|
| 1 | 1.14 | 1-cinnamoyl rhamnoside | 293 | 294 | Aromatic acid glycoside | C15H18O6 | 148 |
| 2 | 1.26 | Gallic acid | 169 | 170 | Phenolic acid | C7H6O5 | 125, 107 |
| 3 | 1.46 | Chlorogenic acid | 353 | 354 | Phenolic acid | C16H18O9 | 191, 179 |
| 4 | 1.67 | Ferulic acid | 193 | 194 | Phenolic acid | C10H10O4 | 149, 175 |
| 5 | 2.16 | Isoferulic acid | 193 | 194 | Phenolic acid | C10H10O4 | 149, 175 |
| 6 | 4.72 | Cinnamic acid | 147 | 148 | Aromatic monocarboxylic acid | C9H8O2 | 103, 129 |
| 7 | 5.55 | 2-Oxoisocaproate | 129 | 130 | Short-chain keto acids | C6H10O3 | 115, 100, 70 |
| 8 | 7.26 | Catechin gallate | 441 | 442 | Flavonoid gallic acid ester | C22H18O10 | 109, 121, 271, 289 |
| 9 | 8.35 | Isorhamnetin-3-O-rutinoside | 623 | 624 | Flavonoid | C28H32O16 | 315, 300, 271, 255 |
| 10 | 8.85 | Pectolinarin | 621 | 622 | Flavonoid | C29H34O15 | 314, 299, 284, 234 |
| 11 | 9.70 | verbascoside | 623 | 624 | Phenylethanoid glycoside | C29H36O15 | 179, 161, 461, 315, 135 |
| 12 | 10.95 | isoverbascoside | 623 | 624 | Phenylethanoid glycoside | C29H36O15 | 179, 161, 461, 315, 135 |
| 13 | 11.34 | Theveside | 389 | 390 | Iridoid glycosides | C16H22O11 | 227, 345, 371 |
| 14 | 12.07 | Geniposide | 387 | 388 | Iridoid glycosides | C17H24O10 | 225, 207, 123, 101 |
| 15 | 12.44 | 8-epiloganin | 389 | 390 | Iridoid glycosides | C17H26O10 | 359, 227, 329, 311 |
| 16 | 12.72 | Chrysoeriol-O-hexoside | 461 | 462 | Flavonoid | C22H22O11 | 299, 284 |
| 17 | 13.58 | Rhamnocitrin-O-glucoside | 461 | 462 | Flavonoid | C22H22O11 | 299, 446 |
| 18 | 13.84 | Linaroside | 475 | 476 | Flavonoid | C23H24O11 | 313, 460, 445 |
| 19 | 14.23 | Durantoside I | 551 | 552 | Iridoid glycosides | C26H32O13 | 389, 521, 491 |
| 20 | 15.88 | Scoparin(Chrysoeriol 8-C-glucoside) | 461 | 462 | Flavonoid | C22H22O11 | 371, 341, 298 |
| 21 | 16.05 | 6- Methoxy-5-hydroxynaphtho[2,3-b]furan-4,9-dione | 243 | 244 | Furanonaphthoquinone | C13H8O5 | 228, 215, 187 |
| 22 | 16.13 | Afzelechin | 273 | 274 | Flavonoid | C15H14O5 | 257, 137 |
| 23 | 20.90 | Myricetin | 317 | 318 | Flavonoid | C15H10O8 | 151,179 |
| 24 | 23.06 | Kaempferol | 285 | 286 | Flavonoid | C15H10O6 | 239, 187,143 |
| 25 | 24.21 | Chrysoeriol | 299 | 300 | Flavonoid | C16H12O6 | 284, 255 |
| 26 | 24.48 | Cirsiliol | 329 | 330 | Flavonoid | C17H14O7 | 314, 299, 285, 271 |
| 27 | 25.15 | pectolinarigenin | 313 | 314 | Flavonoid | C17H14O6 | 299, 284, 234 |
| 28 | 26.32 | Penduletin | 343 | 344 | Flavonoid | C18H16O7 | 328, 313, 298 |
| 29 | 26.89 | 3',4'-Dimethoxy-7-hydroxyflavanone | 297 | 298 | Flavonoid | C17H14O5 | 284, 254, 135 |
| 30 | 26.98 | Lamiide | 421 | 422 | Terpene glycoside | C17H26O12 | 391, 259, 361, 343 |
| 31 | 27.97 | 3,22,24-Trihydroxy-12-oleanen-28-oic acid; (3β,22β)-form, 3-Ketone | 485 | 486 | Oleane-type triterpene | C30H46O5 | 467, 441 |
| 32 | 28.35 | 3,12,13-Trihydroxy-28-oleananoic acid; (3β,12β,13β)-form, 3-ketone | 487 | 488 | Oleane-type triterpene | C30H48O5 | 443, 469 |
| 33 | 28.68 | 3,24-Dioxo-12-oleanen-28-oic acid | 467 | 468 | Oleane-type triterpene | C30H44O4 | 423 |
| 34 | 28.72 | 24-Hydroxy-3-oxo-12-oleanen-28-oic acid | 469 | 470 | Oleane-type triterpene | C30H46O4 | 451, 425 |
| 35 | 28.80 | Lantanolic acid | 469 | 470 | Oleane-type triterpene | C30H46O4 | 421, 391, 420, 377 |
| 36 | 28.83 | Icterogenin | 567 | 568 | Oleane-type triterpene | C35H52O6 | 451, 407, 98 |
| 37 | 28.87 | Lantanilic acid | 567 | 568 | Oleane-type triterpene | C35H52O6 | 451, 407, 98 |
| 38 | 28.94 | Camaric acid | 567 | 568 | Oleane-type triterpene | C35H52O6 | 451, 407, 98 |
| 39 | 28.97 | 22-Tigloyloxylantanolic acid | 567 | 568 | Oleane-type triterpene | C35H52O6 | 549, 523 |
| 40 | 29.00 | Lantadene A | 551 | 552 | Oleane-type triterpene | C35H52O5 | 98, 507 |
| 41 | 29.19 | Lantadene B | 551 | 552 | Oleane-type triterpene | C35H52O5 | 98, 435, 391 |
| 42 | 29.32 | Dihydrorehmannic acid | 553 | 554 | Oleane-type triterpene | C35H54O5 | 535, 509 |
| 43 | 30.28 | Lantoic acid | 485 | 486 | Ursane-type triterpene | C30H46O5 | 437, 421, 407 |
| 44 | 30.78 | 3,24-Dioxo-12-ursen-28-oic acid | 467 | 468 | Ursane-type triterpene | C30H44O4 | 423 |
| 45 | 31.20 | 24-Hydroxy-3-oxo-12-ursen-28-oic acid | 469 | 470 | Ursane-type triterpene | C30H46O4 | 451, 425 |
| 46 | 31.37 | 3,25-Epoxy-3-hydroxy-12-ursen-28-oic acid | 469 | 470 | Ursane-type triterpene | C30H46O4 | 451, 425 |
| 47 | 31.43 | Pomonic acid | 469 | 470 | Ursane-type triterpene | C30H46O4 | 451, 407 |
| 48 | 31.55 | Lantic acid | 469 | 470 | Ursane-type triterpene | C30H46O4 | 421, 391, 420, 377 |
| 49 | 31.64 | Pomolic acid | 471 | 472 | Ursane-type triterpene | C30H48O4 | 453, 411 |
| 50 | 31.73 | Camarinic acid | 527 | 528 | Ursane-type triterpene | C32H48O6 | 58, 451, 407 |
| 51 | 31.82 | Ursoxy acid | 483 | 484 | Ursane-type triterpene | C31H48O4 | 453, 439 |
| 52 | 31.90 | Lantacin | 569 | 570 | Ursane-type triterpene | C35H54O6 | 98, 453, 409 |
| 53 | 34.54 | Myristoleic acid | 225 | 226 | Unsaturated fatty acid | C14H26O2 | 54, 181, 207 |
| 54 | 36.57 | Linolenic acid | 277 | 278 | Unsaturated fatty acid | C18H30O2 | 261, 235, 54 |
| 55 | 37.28 | Linoleic acid methyl ester | 293 | 294 | Unsaturated fatty acid ester | C19H34O2 | 66, 278 |
| 56 | 38.28 | Arachidic acid | 311 | 312 | Saturated fatty acid | C20H40O2 | 293, 267, 59 |
| 57 | 38.32 | Behenic acid | 339 | 340 | Saturated fatty acid | C22H44O2 | 321, 295, 59 |
aM−H is the quasi-molecular ion that results from ionization of metabolites using Electrospray Ionization technique (ESI).
bMSn fragments are the fragments obtained from MS2 fragmentation of ionized metabolites in collision cell of the triple quadrapole mass analyzer.
Fishing of inflammatory target proteins for L. camara metabolites and networks construction
Interactions between L. camara endogenous metabolites with the proteins involved in inflammation were unveiled via constructing a constituent-target (C-T) network (Supplementary Fig. S2). Out of the identified 57 compounds from UPLC-MS/MS analysis, only 39 compounds were potential candidates for inflammation-related protein targets, and 35 inflammation-related target genes were eventually fished out based on screening results from STITCH public database. Regarding STITCH 5.0 database, “combined score” is the parameter utilized to evaluate the strength of interactions between the input compound and the genes. Compounds possessing high combined scores have accurate and strong interactions with their corresponding genes13. In this study, only compounds having interaction scores higher than 0.4 were retained13 (Table 2).
Table 2.
Potential protein targets of L. camara constituents.
| Target protein short name | Full name of protein | Interacting compound (s) (combined interaction score) |
|---|---|---|
| APP | Amyloid-beta precursor protein | Myricetin (1), ferulic acid (0.79), isoferulic acid (0.67), chlorogenic acid (0.83), pectolinarigenin (0.53) |
| BCL2 | Apoptosis regulator Bcl-2 | Catechin gallate (1) |
| BRAF | Serine/threonine-protein kinase B-raf | Myricetin (1) |
| CD81 | CD81 antigen | Lantoic acid (0.49), pomolic acid (0.57), 3β,22β)-form, 3-O-(3-methyl-2-butenoyl) (0.64), dihydrorehmannic acid (0.65), lantadene C (0.47), 3,24-dihydroxy-12-ursen-28-oic acid; 3 β form, 3-ketone (0.42), lantanolic acid (0.43), pomonic acid (0.4), icterogenin (0.45), lantanillic acid (0.42), camaric acid (0.41), lantacin (0.51), 3,24-dihydroxy-12-oleanen-28-oic acid; 3α form, 3-ketone, 24-aldehyde (0.49), 3,22-dihydroxy-12-oleanen-28-oic acid; (3 β,22 β)-form, 3-ketone, 22-angeloyl (0.47), 3,22-dihydroxy-12-oleanen-28-oic acid; (3 β, 22 β)-form, 3-ketone, 22-(3-methyl-2-butenoyl) (0.49) |
| CREB1 | Cyclic AMP-responsive element-binding protein 1 | Pectolinarin (0.42), linaroside (0.47), pectolinarigenin (0.79), penduletin (0.4) |
| CXCL12 | Stromal cell-derived factor 1 | Ferulic acid (0.69), isoferulic acid (0.53) |
| FLT3 | Receptor-type tyrosine-protein kinase FLT3 | Myricetin (1) |
| FOS | Proto-oncogene c-Fos | Ferulic acid (0.74), isoferulic acid (0.56) |
| IL2 | Interleukin-2 | Scoparin (0.5), pectolinarin (0.47), narcissin (0.4), 8-epiloganin (0.42), chrysoeriol-7-O-GLUCOSIDE (0.81), linaroside (0.6), rhamnocitrin-O-GLUCOSIDE (0.57) |
| INSR | Insulin receptor | Myricetin (1) |
| JUN | Transcription factor AP-1 | Ferulic acid (0.7) |
| KIT | Mast/stem cell growth factor receptor Kit | Pectolinarigenin (1) |
| LPAR1 | Lysophosphatidic acid receptor 1 | Linoleic acid methyl ester (0.48), Linolenic acid (0.43), myristoleic acid (0.47) |
| LPAR2 | Lysophosphatidic acid receptor 2 | Arachidic acid (0.4), behenic acid (0.4), linoleic acid methyl ester (0.48), Linolenic acid (0.43), myristoleic acid (0.47) |
| LPAR3 | Lysophosphatidic acid receptor 3 | Arachidic acid (0.4), behenic acid (0.4), linoleic acid methyl ester (0.48), Linolenic acid (0.43), myristoleic acid (0.47) |
| LPAR4 | Lysophosphatidic acid receptor 4 | Linoleic acid methyl ester (0.48), myristoleic acid (0.41) |
| MAPK14 | Mitogen-activated protein kinase 14 | Catechin gallate (1) |
| MAPT | Microtubule-associated protein tau | Ferulic acid (0.69), isoferulic acid (0.67), myricetin (1) |
| MET | Hepatocyte growth factor receptor | Catechin gallate (1) |
| MMP9 | Matrix metalloproteinase-9 | Ferulic acid (0.74), isoferulic acid (0.64) |
| PGF | Placenta growth factor | Afzelechin (0.59) |
| PIK3CG | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform | Myricetin (1) |
| PLA2G2C | Putative inactive group IIC secretory phospholipase A2 | Arachidic acid (0.52), behenic acid (0.52), myristoleic acid (0.43) |
| PLA2G4B | Cytosolic phospholipase A2 beta | Arachidic acid (0.46), behenic acid (0.46) |
| PLA2G5 | Calcium-dependent phospholipase A2 | Arachidic acid (0.43), behenic acid (0.43) |
| PPARA | Peroxisome proliferator-activated receptor alpha | Arachidic acid (1), behenic acid (1) |
| PRKCA | Protein kinase c alpha | Linoleic acid methyl ester (0.51), verbascoside (1) |
| PTGER2 | Prostaglandin E2 receptor EP2 subtype | Arachidic acid (0.45), behenic acid (0.45), ferulic acid (0.44), isoferulic acid (0.44) |
| PTPN1 | Tyrosine-protein phosphatase non-receptor type 1 |
3,22-Dihydroxy-12-oleanen-28-oic acid; 3 β,22 β form, 3-ketone, 22-(3-methyl-2-butenoyl) (0.61), 3,22-dihydroxy-12-oleanen-28-oic acid; (3 β,22 β)-form, 3-ketone, 22-angeloyl (0.61), 3,24-dihydroxy-12-oleanen-28-oic acid; 3α;-form, 3-ketone, 24-aldehyde (0.79), 3,24-dihydroxy-12-ursen-28-oic acid; 3 β-form, 3-ketone (0.64), camaric acid (0.45), camarinic acid (0.42), dihydrorehmannic acid (0.67), icterogenin (0.54), lantadene C (0.59), lantanillic acid (0.44), lantanolic acid (0.6), lantic acid (0.61), lantoic acid (0.49), pomolic acid (1), pomonic acid (0.82), Ursoxy acid (0.58) |
| PTPN6 | Tyrosine-protein phosphatase non-receptor type 6 | 3,22-Dihydroxy-12-oleanen-28-oic acid; (3β,22β)-form, 3-O-(3-methyl-2-butenoyl) (0.51), 3,24-dihydroxy-12-oleanen-28-oic acid; 3α;-form, 3-Ketone, 24-aldehyde (0.53), 3,24-dihydroxy-12-ursen-28-oic acid; 3β-form, 3-ketone (0.45), dihydrorehmannic acid (0.54), lantadene C (0.42), lantanolic acid (0.54), pomolic acid (0.54) |
| RELA | Transcription factor p65 | Ferulic acid (0.5), isoferulic acid (0.54) |
| RPS6KA3 | Ribosomal protein S6 kinase alpha-3 | Narcissin (0.62), rhamnocitrin-O-glucoside (0.72) |
| SYK | Tyrosine-protein kinase SYK | Myricetin (1) |
| TLR2 | Toll-like receptor 2 | Arachidic acid (1), behenic acid (1), linoleic acid methyl ester (0.46), linolenic acid (0.48) |
| VEGFA | Vascular endothelial growth factor a | Afzelechin (1) |
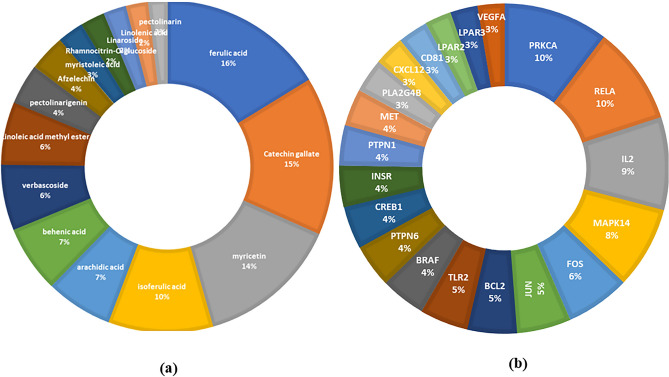
The constructed C-T network (Supplementary Fig. S2) comprised 74 nodes (39 constituents and 35 target genes) and 479 edges with an average of 3.043 targets for each constituent, indicating the multi-target properties of the L. camara phytoconstituents. As deduced from Fig. 2a, the highest percentages of interactions were demonstrated by ferulic acid, followed by catechin gallate, then myricetin and iso-ferulic acid. Inspection of the targeted genes (Fig. 2b, Table 2) indicated that the genes PRKCA, RELA, IL2, MAPK14 and FOS were the most enriched ones possessing the highest combined scores and interaction percentages with the constituents in the C-T network, proposing their possible key role in suppressing inflammation. In addition, protein–protein interactions were examined using STRING database then visualized through P-P network analysis. From this network, strong correlations between the identified potential anti-inflammatory target proteins were spotted suggesting that they probably regulate the functions of each other (Supplementary Fig. S3).
Figure 2.
Doughnut charts showing the distributions % of the compound–target gene (C–T) interactions on L. camara constituents (a) and the identified inflammation-related proteins (b).
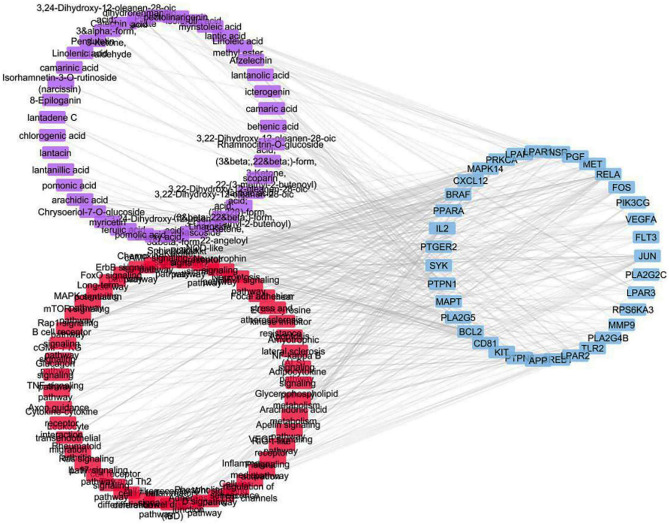
Potential metabolic pathways of inflammation were explored by forwarding the target genes to the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis21–23 where annotation was restricted to Homo sapiens. As shown in Supplementary Fig. S4 and Table 3, the target genes were involved in 47 inflammation-related pathways (having P-values < 0.05). The most enriched pathways were observed to be PI3K-Akt signaling pathway exhibiting the largest number of gene count followed by MAPK signaling pathway, Rap1 signaling pathway, Ras signaling pathway and Phospholipase D signaling pathway. The constructed networks were merged to generate the compound–target–pathway network (Fig. 3) which implied strong co-relations between the studied compounds and inflammation-related targets and pathways.
Table 3.
KEGG pathway analysis of potential target genes functions.
| Pathway ID | Pathway name | Gene count | False discovery rate (P-value) | Matching proteins in the network |
|---|---|---|---|---|
| hsa04151 | PI3K-Akt signaling pathway | 17 | 6.54E−11 | IL2, FLT3, KIT, INSR, MET, PIK3CG, LPAR1, SYK, BCL2, RELA, CREB1, LPAR3, LPAR4, PRKCA, LPAR2, PGF, VEGFA |
| hsa04010 | MAPK signaling pathway | 16 | 1.59E−11 | MAPK14, FLT3, FGF2, KIT, BRAF, INSR, FOS, MET, MAPT, JUN, RPS6KA3, RELA, PLA2G4B, PRKCA, PGF, VEGFA |
| hsa04015 | Rap1 signaling pathway | 12 | 9.69E−12 | MAPK14, KIT, BRAF, INSR, MET, LPAR1, LPAR3, LPAR4, PRKCA, LPAR2, PGF, VEGFA |
| hsa04014 | Ras signaling pathway | 11 | 1.63E−09 | FLT3, PLA2G2C, KIT, INSR, MET, PLA2G5, RELA, PLA2G4B, PRKCA, PGF, VEGFA |
| hsa04072 | Phospholipase D signaling pathway | 10 | 6.41E−08 | KIT, INSR, PIK3CG, LPAR1, SYK, LPAR3, PLA2G4B, LPAR4, PRKCA, LPAR2 |
| hsa05418 | Fluid shear stress and atherosclerosis | 7 | 4.56E−10 | MAPK14, FOS, JUN, MMP9, BCL2, RELA, VEGFA |
| hsa04024 | cAMP signaling pathway | 7 | 2.50E−05 | PTGER2, BRAF, FOS, JUN, RELA, PPARA, CREB1 |
| hsa04510 | Focal adhesion | 7 | 0.00011 | BRAF, MET, JUN, BCL2, PRKCA, PGF, VEGFA |
| hsa04662 | B cell receptor signaling pathway | 6 | 3.60E−08 | CD81, FOS, JUN, SYK, RELA, PTPN6 |
| hsa04668 | TNF signaling pathway | 6 | 0.00000111 | MAPK14, FOS, JUN, MMP9, RELA, CREB1 |
| hsa04660 | T cell receptor signaling pathway | 6 | 3.63E−06 | IL2, MAPK14, FOS, JUN, RELA, PTPN6 |
| hsa04722 | Neurotrophin signaling pathway | 6 | 6.06E−05 | MAPK14, BRAF, JUN, RPS6KA3, BCL2, RELA |
| hsa04060 | Cytokine-cytokine receptor interaction | 6 | 0.0085 | IL2, FLT3, KIT, MET, CXCL12, VEGFA |
| hsa04064 | NF-kappa B signaling pathway | 5 | 3.35E−07 | SYK, CXCL12, BCL2, RELA |
| hsa04066 | HIF-1 signaling pathway | 5 | 3.44E−06 | INSR, BCL2, RELA, PRKCA, VEGFA |
| hsa04620 | Toll-like receptor signaling pathway | 5 | 4.45E−06 | MAPK14, TLR2, FOS, JUN, RELA |
| hsa01521 | EGFR tyrosine kinase inhibitor resistance | 5 | 4.51E−06 | BRAF, MET, BCL2, PRKCA, VEGFA |
| hsa04657 | IL-17 signaling pathway | 5 | 1.40E−05 | MAPK14, FOS, JUN, MMP9, RELA |
| hsa05323 | Rheumatoid arthritis | 5 | 4.85E−05 | TLR2, FOS, JUN, CXCL12, VEGFA |
| hsa04659 | Th17 cell differentiation | 5 | 0.00013 | IL2, MAPK14, FOS, JUN, RELA |
| hsa04658 | Th1 and Th2 cell differentiation | 5 | 0.00032 | IL2, MAPK14, FOS, JUN, RELA |
| hsa04071 | Sphingolipid signaling pathway | 4 | 1.33E−13 | MAPK14, BCL2, RELA, PRKCA |
| hsa04670 | Leukocyte transendothelial migration | 4 | 1.46E−06 | MAPK14, MMP9, CXCL12, PRKCA |
| hsa04611 | Platelet activation | 4 | 0.00000329 | MAPK14, PIK3CG, SYK, PLA2G4B |
| hsa04210 | Apoptosis | 4 | 6.21E−06 | FOS, JUN, BCL2, RELA |
| hsa04750 | Inflammatory mediator regulation of TRP channels | 4 | 0.000014 | MAPK14, PTGER2, PLA2G4B, PRKCA |
| hsa04370 | VEGF signaling pathway | 4 | 0.0000473 | MAPK14, PLA2G4B, PRKCA, VEGFA |
| hsa05321 | Inflammatory bowel disease (IBD) | 4 | 6.06E−05 | IL2, TLR2, JUN, RELA |
| hsa04621 | NOD-like receptor signaling pathway | 4 | 0.00013 | MAPK14, JUN, BCL2, RELA |
| hsa04150 | mTOR signaling pathway | 4 | 0.00026 | BRAF, INSR, RPS6KA3, PRKCA |
| hsa04520 | Adherens junction | 4 | 0.0037 | INSR, MET, PTPN1, PTPN6 |
| hsa00590 | Arachidonic acid metabolism | 3 | 8.61E−11 | PLA2G2C, CBR1, PLA2G5, PLA2G4B |
| hsa04062 | Chemokine signaling pathway | 3 | 6.06E−05 | BRAF, CXCL12, RELA |
| hsa04022 | cGMP-PKG signaling pathway | 3 | 0.00042 | INSR, PIK3CG, CREB1 |
| hsa04720 | Long-term potentiation | 3 | 0.00042 | BRAF, RPS6KA3, PRKCA |
| hsa04012 | ErbB signaling pathway | 3 | 0.0014 | BRAF, JUN, PRKCA |
| hsa04360 | Axon guidance | 3 | 0.0027 | MET, CXCL12, PRKCA |
| hsa00564 | Glycerophospholipid metabolism | 3 | 0.0117 | PLA2G2C, PLA2G5, PLA2G4B |
| hsa04068 | FoxO signaling pathway | 3 | 0.032 | MAPK14, BRAF, INSR |
| hsa04920 | Adipocytokine signaling pathway | 2 | 0.0033 | RELA, PPARA |
| hsa04218 | Cellular senescence | 2 | 0.0056 | MAPK14, RELA |
| hsa04622 | RIG-I-like receptor signaling pathway | 2 | 0.0185 | MAPK14, RELA |
| hsa04217 | Necroptosis | 2 | 0.0193 | BCL2, PLA2G4B |
| hsa05014 | Amyotrophic lateral sclerosis (ALS) | 2 | 0.036 | MAPK14, BCL2 |
| hsa04310 | Wnt signaling pathway | 2 | 0.0442 | JUN, PRKCA |
| hsa04922 | Glucagon signaling pathway | 2 | 0.0489 | PPARA, CREB1 |
| hsa04371 | Apelin signaling pathway | 1 | 0.0027 | PIK3CG |
Figure 3.
Compound–target–pathway network (compounds are represented in violet color, targets are presented in blue color and pathways are presented in red color).
Gene ontology (GO) enrichment analysis for targets
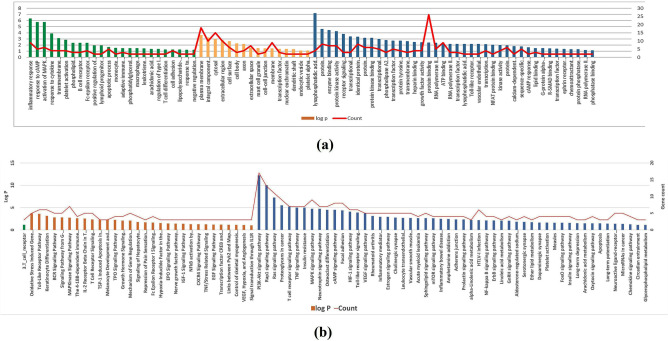
Gene ontology (GO) enrichment analysis was carried out to the identified targets via importing to DAVID bioinformatics resources with limiting annotations to Homo sapiens, thus revealing the most enriched pathways and GO terms which have the highest log P value and gene counts. As depicted in Fig. 4a, the identified targets are associated with numerous biological processes, the most enriched ones are inflammatory response, response to cAMP, activation of MAPK activity and response to cytokine. The most significant molecular cellular components were plasma membrane, integral component of plasma membrane, cytosol and extracellular region. It was also concluded that the most enriched molecular functions were lysophosphatidic acid receptor activity, protein heterodimerization activity, enzyme binding and protein kinase activity. Nevertheless, functional annotations using DAVID bioinformatics resources revealed 1 BBID pathway named 3.T-cell receptor, and 30 BIOCARTA pathways such as: oxidative stress induced gene expression via Nrf2, Toll like receptor pathway, keratinocyte differentiation and BCR signaling pathway. Additionally, 52 KEGG pathways involving PI3K-Akt signaling pathway, Rap1 signaling pathway, Ras signaling pathway and proteoglycans in cancer were identified (Fig. 4b). All these recognized pathways possessed P-value less than or equal to 0.05, implying their striking association with inflammation.
Figure 4.
(a) Gene Ontology analysis of inflammation targets determined by DAVID database. Biological processes, molecular functions and cellular components are represented by green, orange and blue bars, respectively. (b) Major BBID (green), BIOCARTA (orange) and KEGG (blue) pathways clusters generated from DAVID database. The significance of enrichment is indicated by log P-value with bar charts. Red lines represent the number of genes enriched by each term.
Molecular docking studies of L. camara hit compounds in the active sites of the most enriched inflammation-related target genes
The calculation of the docking XP G scores of L. camara top hit compounds; ferulic acid, catechin gallate, myricetin and iso-ferulic acid, against the active sites of the most enriched inflammation-related target genes; PRKCA, RELA, IL2, MAPK14 and FOS, was carried out using Glide module embedded in Schrodinger suite software. From Supplementary Table S1, it can be concluded that catechin gallate had the lowest binding energy against PRKCA, RELA and IL2, while myricetin possessed the most stable interaction against MAPK14 and FOS.
Validation of molecular docking protocol
Validation procedures for each docking software were attained using two methods. First is the redocking procedure which evaluates the accuracy of the docking poses and during which, the co-crystallized ligands were docked back into the receptor binding cavity. The re-docked complex was superimposed on to the reference co-crystallized complex and the RMSD value between the initial conformation and the re-docked one is calculated. A cut off value of 2 Å was set; therefore, complexes encompassing above this value were considered incorrect24. For each of the studied proteins: 4RA4, 1M49 and 6HWU, the re-docked complex was superimposed on to the reference co-crystallized complex to a great extent (Supplementary Fig. S5). Moreover, the RMSD value between the initial conformation and the re-docked one was calculated and all the three crystallographic structures displayed good values of 1.172, 0.386, 0.558 respectively (supplementary Table S2) indicating the efficiency of the docking protocol.
Second is the utilization of enrichment calculations that are crucial for evaluating the quality of scoring and eliminating random or by chance selection of actives25. A validation set comprising active compounds for each of the investigated proteins was seeded into 1000 built-in Schrodinger® decoys. Decoys are compounds that are similar in physical properties with respect to the reference ligand that might not bind effectively to a protein26. Validation parameters such as receiver operating characteristic (ROC), AUC-ROC, BEDROC and enrichment factor (EF at 2%, 5% and 10%) were then estimated. From the ROC plots, the area under the curve (AUC) computed the probability of how highly a randomly selected active is ranked compared to a randomly chosen decoy. The ideal range of AUC is 0–1, a value near ≤ 0.5 indicates that the software randomly selects true actives and false actives, where a value close to 1 highlights greater possibility to identify true actives before false ones25. As depicted in (supplementary Table S2), it was observed that all proteins scored promising AUC-ROC values. Comparing EF values revealed that the investigated proteins were able to extract actives from a seeded random set, when the top 2, 5 and 10% of the total set were considered, noting that the maximum attainable enrichment factors are 50, 20, and 10 for EF(2%), EF(5%), and EF(10%), respectively27. Using BEDROC as a criterion to assess early recognition of actives from decoys at different tuning parameter value α28, all the proteins recorded the high scores at all α values. To conclude, all the enrichment values obtained for each docking procedure suggested that GLIDE software was able to filter the enriched database efficiently.
ADME filtration of L. camara top hit compounds
QikProp module was utilized to calculate the ADME characteristics of the L. camara hit compounds, in order to assess their drug-likeness. L. camara hit compounds were regarded as drug candidates as they conformed to Lipinski's rule of 529, and Jorgensen’s rule of 330 (Supplementary Table S3).
In vitro cytotoxicity and anti-inflammatory activity of L. camara extract
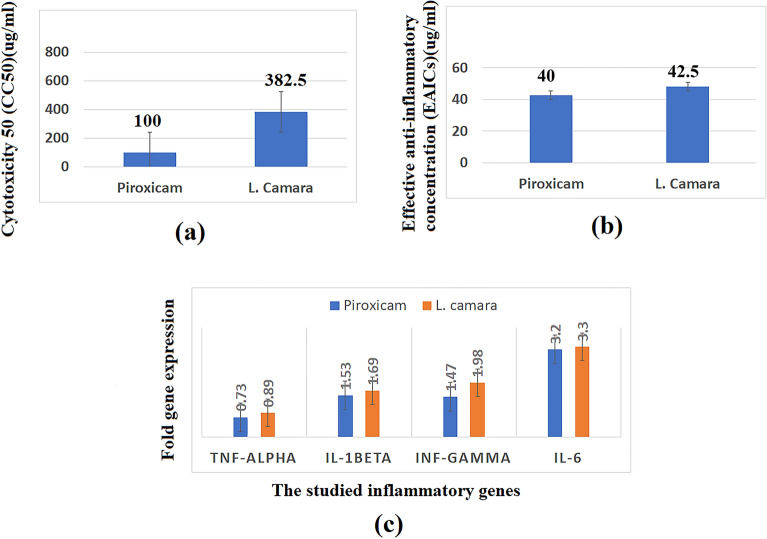
In order to assess the safety of the tested extract, the cell cytotoxicity 50 (CC50), which is the drug concentration required for reducing the cell viability by 50%, was determined for the extract and the standard anti-inflammatory drug (piroxicam) using MTT test. The tested extract showed higher CC50 value (382.5 µg/mL) than that of piroxicam (100 µg/mL) indicating that the extract is safer than piroxicam (Fig. 5a). Afterwards, anti-inflammatory activities of the extract compared to piroxicam were estimated using lipopolysaccharides (LPS)-stimulated WBC cells (Fig. 5b). Both extract and piroxicam showed comparable effective anti-inflammatory concentrations (EAICs) (48.08 µg/mL and 42.50 µg/mL, respectively), suggesting the promising activity of the extract as anti-inflammatory candidate. To determine the mechanism of anti-inflammatory activity at the genetic level, the gene expression of four pro-inflammatory markers (TNF-α, IL-1β, INF-γ, IL-6) was measured by real time polymerase chain reaction (PCR) in normal WBCs and lipopolysaccharide (LPS)-treated WBCs (Fig. 5c). Regarding TNF-α, lipopolysaccharide (LPS) upregulated the expression of this gene by 2.1-folds. Upon treatment of the WBCs with the tested extract this upregulation was abolished to 0.89-fold which was comparable to that exerted by piroxicam (0.73-fold). Meanwhile, LPS upregulated the expression of IL-1β by 5.23-folds which was attenuated by the extract to 1.69-folds. This value was in close agreement to that obtained by piroxicam (1.53-folds). Interestingly, the upregulation of the gene expression of INF-γ and IL-6 was significantly decreased by the tested extract and piroxicam to a similar level (error bars were shown in Fig. 5 and p values for all experiments were less than 0.05). It can be concluded that L. camara extract can serve as potential anti-inflammatory natural product assigning to its noticeable inhibition of the upregulated TNF-α, IL-1β, INF-γ, IL-6 expression levels. These results were compatible with that obtained from network pharmacology and molecular docking analyses that revealed the multi-target and multi-pathways nature of the tested extract regarding anti-inflammatory activity.
Figure 5.
Bar chart showing (a) cytotoxicity (CC50 µg/mL), (b) effective anti-inflammatory concentrations (EAICS) (µg/mL) of L. camara extract and piroxicam, (c) TNF-α, IL-1β, INF-γ, IL-6 (fold change in gene expression) by L. camara extract and piroxicam (standard anti-inflammatory drug).
Discussion
LC–MS/MS analysis results in 57 identified metabolites which belong to different chemical classes such as, flavonoids, phenolic acids, iridoids, phenyl ethanoid glycosides, triterpenes, and fatty acids.
Flavonoids
This class is represented by 15 peaks (8, 9, 10, 16, 17, 18, 20, 22–29), peaks 9, 10, 16, 17, 20 and 22 represented flavonoid glycosides. Compound 10 showed daughter MS2 fragment of its aglycone (M-H-2CH3) at 299 Da31 thus it was identified as a di-methoxylated flavone. Based on the mass data with that reported in literature, compound 10 was identified as pectolinarin32. Compound 8 is a glycoside with rutin sugar part as it showed a characteristic peak at 315 Da (M-H-308) along with its characteristic daughter fragments at 300, 271 and 255 Da. By referring to literature it was tentatively identified as isorhamnetin-O-rutinoside33. Compounds 16 and 17 showed (M-H-162) peak that indicated the loss of one hexose unit. Compound 20 was C-glycoside that deduced from its characteristic fragments at 371, 341 and 298 Da as scoparin34. Compounds (23–29) represented flavone aglycones and by referring to literature they were tentatively identified as afzelechin, myrcetin, kaempferol, chrysoeriol, cirsiliol, pectolinarigenin, Penduletin and 3′,4′-Dimethoxy-7-hydroxyflavanone35–40. Moreover, compound 8 was identified as catechin gallate41.
Phenolic acids
This class was represented by four peaks (2, 3, 4 and 5). Mass fragmentation of phenolic acids is generally characterized by loss of water and CO2 and loss of methyl groups in case of methylated phenolic acids42. By referring to literature, 2, 3, 4 and 5 were identified as gallic acid, chlorogenic acid, ferulic and iso-ferulic acid42–45.
Phenylethanoid glycosides
This class was represented by two peaks (11 and 12). Phenyletanoids are β-glucopyranose directly attached to a hydroxyphenyl ethyl moiety. Moreover, at the positions C-4 and C-6 the substitution by hydroxyl derivatives of cinnamic acid (such as caffeoyl and feruloyl) usually occurs. At the C-2 or C-3 position of β-glucopyranose, another sugar moiety is usually located46. Two highly abundant mass fragments at 161 and 179 Da indicated the presence of caffeoyl moiety attached to the glucose unit47. Meanwhile, three sequential losses of caffeoyl moiety, deoxyhexose (rhamnose) moiety (M-H-162-146)47 and glucose unit from the parent ion followed by dehydration to yield another daughter fragment (M-H-162-146-162-18) at 135 Da assigned for anhydrophenolethanol moiety48. Compounds 11 and 12 were tentatively identified as verbascoside48 and isoverbascoside49, respectively.
Iridoid glycosides
Five peaks represented this class (13, 14, 15, 19 and 30). Generally, they showed the characteristic peak (M+HCOO-H)− and they showed their major MS2 fragment (M−H)−. Formate anion (M+HCOO-H)− is commonly resulted from iridoid glycosides bearing an ester group or a carboxyl group at C-450. Compound 13 showed characteristic daughter peaks at 371 Da, 345 Da and 209 Da owing to loss of water, CO2 and glucose moieties, respectively51. By referring to literature it was tentatively identified as theveside. Moreover, compound 15 showed characteristic peaks as a result of losing methoxy group (M−H-30) at 359 Da. Furthermore, there were fragments due to loss of glucose unit (M−H-162) from the precursor ion at 259 Da along with a methyl ester loss represented by the mass fragment (M−H-60) at 329 Da with subsequent dehydration to afford the major product ion (M−H-78) at 311 Da, respectively. Based on the mentioned information and by referring to literature, compounds 14, 15 and 19 were tentatively identified as geniposide52, 8-epiloganin53,54 and durantoside I, respectively55. Moreover, peak 30 was tentatively identified as lamiide56.
Triterpenes
Oleanane-type triterpenes were represented by 12 peaks (peaks from 31 to 42) and ursane-type triterpenes were represented by 10 peaks (peaks from 43 to 52). Oleanane and ursane-type triterpenes were characterized by the presence of the most important mass fragments due to loss of angeloyl or methyl butenoyl or hydroxyl moiety followed by loss of CO2. Based on this information and by referring to literature, compounds from 31 to 42 were tentatively identified as 3,12,13-trihydroxy-28-oleananoic acid; 3-ketone, 3,24-dioxo-12-oleanen-28-oic acid, 24-hydroxy-3-oxo-12-oleanen-28-oic acid, lantanolic acid, icterogenin, lantanilic acid, camaric acid, 22-tigloyloxylantanolic acid, lantadene A, lantadene B and dihydrorehmannic acid, respectively57–60. On the other hand, compounds from 43 to 52 were tentatively identified as, lantoic acid, 3,24-dioxo-12-ursen-28-oic acid, 24-hydroxy-3-oxo-12-ursen-28-oic acid, 3,25-epoxy-3-hydroxy-12-ursen-28-oic acid, pomonic acid, lantic acid, pomolic acid, camarinic acid, ursoxy acid and lantacin, respectively57–60.
Fatty acids
Three peaks (53, 54 and 55) represented unsaturated fatty acids. The mass fragmentation of unsaturated fatty acids is represented by two characteristic fragments due to loss of water and CO261 along with their characteristic fragment at 54 m/z that result from double-bond transfer and α-cleavage62,63. Compounds 53, 54 and 55 were identified as myristoleic acid, linolenic acid and linoleic acid methyl ester, respectively64. Meanwhile, two peaks (56 and 57) represented saturated fatty acids, the mass fragmentation of saturated fatty acids is represented by two characteristic fragments result from loss of water and CO265 along with the fragment of Mclafferty rearrangement that was detected at 59 Da66. Compounds 56 and 57 were tentatively identified as arachidic acid and behenic acid, respectively64.
PubMed literature review was implemented to validate the role of the hit compounds identified from network pharmacology analysis in alleviation of inflammation. As can be observed in Supplementary Table S4, ferulic acid precluded methotrexate-induced hepatotoxicity via inducing Nrf2/HO-1 signaling and PPARγ, as well as abolishing oxidative stress and inflammation67. Catechin gallate diminished the levels of cyclo-oxygenase and lipoxygenase inflammatory mediators thus alleviated UV radiation-induced erythema68. Another previous work confirmed the protective effect of myricetin against liver fibrosis in a diet-induced non-alcoholic steatohepatitis rat model through inhibiting the TREM-1-TLR2/4-MyD88 signaling molecules in macrophages69. Meanwhile, isoferulic acid attenuated the production of PI3K/Akt-dependent NF-κB activity, thus, could serve as a potential drug for treating neuritis and other neuronal ailments70.
In addition, several studies have documented the relation between the recognized most enriched genes and inflammation. For example, controlling the expression of PRKCA levels relieved Barrett's esophagus, esophagitis71, multiple sclerosis72 and inhibited LPS-induced acute lung injury and inflammatory response73. Moreover, regulation of nuclear-cytoplasmic shuttling of RELA aids in attenuation of inflammation74. It was also proved that loss of epithelial RELA results in deregulated intestinal proliferative/apoptotic homeostasis and susceptibility to inflammation75. Furthermore, expression and induction of a pancreatitis-associated protein (PAP1) depended on RelA/p65 levels, suggesting its multidimensional roles in treating cerulein pancreatitis76. Also, allergic inflammation was claimed to be influenced by nuclear factor κB1/RelA expression in human lung epithelial cells77. Meanwhile, interleukin-2 (IL-2) is the canonical T-cell growth factor that stimulates clonal expansion of T cells following antigen stimulation, hence plays a critical role in orchestrating optimal immune and inflammatory responses78. Therefore, targeting such protein contributes to alleviate inflammatory bowel diseases as well as suppressing inflammation synergized by respiratory viral infections79. Additionally, P38α/MAPK14 is intracellular signaling regulator involved in biosynthesis of inflammatory mediator cytokines as TNF-α, IL-1, IL-6, and IL-1β, which induced the production of inflammatory proteins such as iNOS, NF-kB, and COX-280. Also, regulation of MAPK14 expression prevented aggravation of myocarditis81, multiple sclerosis82 and inflammatory bowel diseases83. Other recent work confirmed the vital role of MAPK14 in relieving the inhibitory control by autophagy on inflammation in response to a stress signal84.
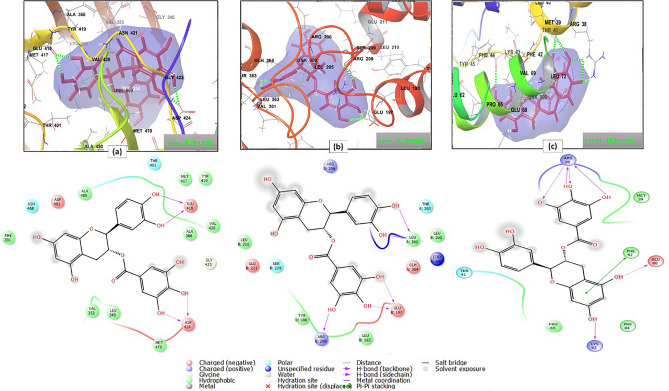
Molecular docking analysis revealed the strong binding of the top hit compounds on the active sites of the most enriched genes. For example; the 2D and 3D interaction diagrams of catechin gallate in the active site of protein kinase C alpha type (PBD ID 4RA4) (Fig. 6a) showed that the strong binding—as expressed by its XP G score- was attributed to the formation of two hydrogen bonds between 3 and 4′ hydroxyl groups and Glu418, two hydrogen bonds between 3 and 4″ hydroxyl groups and Asp424, In addition to hydrophobic interactions with Phe350, Ala480, Met417, Tyr419, Ala366, Val420, Met470, Leu345 and Val353. Moreover, polar interactions with Asn468 and Thr401 and charged negative interactions with Asp481, Glu418 and Asp424 were observed85 (Supplementary Table S5).
Figure 6.
2D and 3D interaction diagrams of (a) catechin gallate in the active site of protein kinase C alpha type (PDB ID 4RA4) (b) catechin gallate in the active site of transcription factor p65 (PDB ID 3QXY) (c) catechin gallate in the active site of interleukin-2 (PDB ID 1M49).
Meanwhile, catechin gallate occupied the active site of transcription factor p65 (PBD ID 3QXY) with two hydrogen bonds between 3 and 4′ hydroxyl groups and LeuA 362, two hydrogen bonds between 3″, 4″ hydroxyl groups and GluB 197 and another one between 5″ hydroxyl group and ArgB 208. Additionally, hydrophobic interactions with LeuB 205, LeuA 362, LeuB 193, TyrB 188 and LeuB 210, and polar interactions with ThrA 363 and SerB 209 were denoted. Moreover, charged positive interactions with ArgB 206 and ArgB 208, and charged negative interactions with GlhA 364, GluB 197 and GluB 211 were involved in binding82 (Fig. 6b, Supplementary Table S5).
However, binding of catechin gallate with interleukin-2 (PBD ID 1M49) showed two hydrogen bonds between the hydroxyl groups at C-5 and C-7 and Glu68 and Lys43, respectively, together with four hydrogen bonds between 3″, 4″ and 5″ hydroxyl groups and Arg38. Also, a pi–pi stacking interaction between the aromatic ring A of the flavone moiety and Phe42 and hydrophobic interactions with Pro65, Phe44, Phe42 and Met39 were unveiled. Two charged positive interactions with Lys43 and Arg38, one charged negative interaction with Glu 68, beside one polar interaction with Thr41 were also deduced86 (Fig. 6c, Supplementary Table S5).
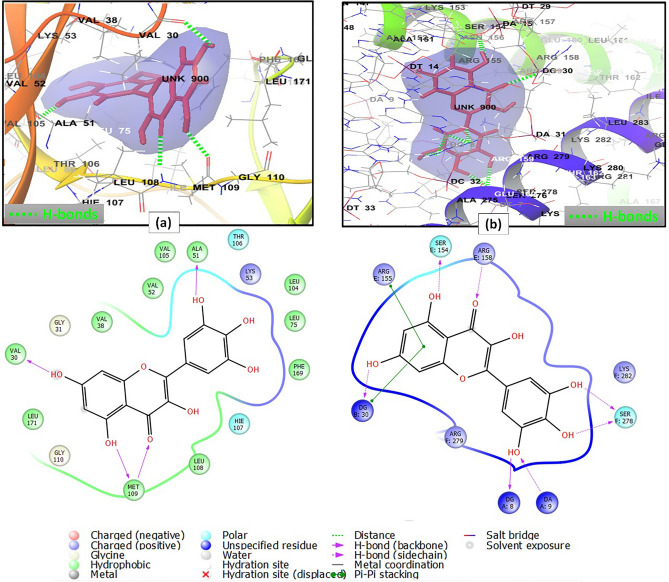
On the other hand, the interaction pattern of myricetin with mitogen-activated protein kinase 14 (PBD ID 6HWU) included the formation of four hydrogen bonds between the following pairs: 3′ hydroxyl group and Ala51; 4 carbonyl group, 5 hydroxyl group and Met109; and 7 hydroxyl group and Val30; in addition to hydrophobic interactions with Val30, Leu171, Met109, Leu108, Val105, Ala51, Val52, Leu104, Leu75, Phe169 and Val38. There were also polar interactions with Thr106 and Hie107 and a charged positive interaction with Lys5387 (Fig. 7a, Supplementary Table S5). Furthermore, the binding mode of myricetin with proto-oncogene c-Fos (PBD ID 1FOS) revealed the presence of four hydrogen bonds between 4 carbonyl group and ArgE 158, C-5 hydroxyl group and SerE 154, C-3′, C-4′ hydroxyl groups and SerF 278. Myricetin also engaged in a pi–pi stacking interaction through its flavone aromatic ring A moiety and ArgE 155. Charged positive interactions with the backbone amino acid residues ArgE 155, ArgE 158, LysF 282, ArgF 279, polar interactions with SerE 154 and SerF 278, and a hydrophobic interaction with AlaE 151 were also considered88 (Fig. 7b, Supplementary Table S5).
Figure 7.
2D and 3D interaction diagrams of (a) myricetin in the active site of mitogen-activated protein kinase 14 (PDB ID 6HWU) (b) myricetin in the active site of proto-oncogene c-Fos (PDB ID 1FOS).
The drug-likeness of compounds can be predicted by applying Lipinski's rule of 5. As claimed by Lipinski's rule of 5, a compound of known pharmacological activity is regarded active (having good absorption and/or permeation) if it possesses less than 10 hydrogen-bond acceptors (acptHB), less than 5 hydrogen-bond donors (donorHB), a molecular weight (mol_MW) lower than 500 Da and a calculated QPlogPo/wvalue less than five29. Only compounds conformed to minimally three of the above characteristics were considered active.
In addition, the oral bioavailability (OB) of the top hit phytoconstituents was evaluated using the descriptor Jorgensen’s rule of 330. Only compounds demonstrating OB ≥ 30% were considered active. The hit L. camara constituents obey the above criteria and hence, were considered as drug candidates (Supplementary Table S3).
Methods
Chemicals and plant material
All chemicals utilized in this study were procured from (St. Louis, Mo., USA). Dimethyl sulfoxide (DMSO), piroxicam, lipopolysaccharides (LPS), SYBR green master mix, trypan blue and MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) dyes, RNA and cDNA extraction kits, nuclease free water, RNase inhibitor and reverse transcriptase were bought from Sigma (St. Louis, Mo., USA). Fetal Bovine serum, Roswell Park Memorial Institute (RPMI) 1640 Medium, l-glutamine were obtained from Lonza (Belgium). dT primer, dNTPs (deoxynucleotide triphosphate) were purchased from Thermo Fisher Scientific.
The aerial parts of L. camara were collected from Antoniades Garden, Alexandria, Egypt with permission from the Agriculture Research Center, Giza, Egypt at "9 Cairo University Road, Giza District, Giza Governorate". The plant collection was accomplished in accordance with the national guidelines. The identity of the plant was confirmed by Dr. Therese Labib, specialist of plant identification in El Orman Garden, Cairo, Egypt. A voucher specimen (No. LC-250) was deposited at the herbarium of the Department of Pharmacognosy, Faculty of Pharmacy, Alexandria University.
Preparation of L. camara extract
Air-dried powdered leaves of L. camara (500 g) were extracted by sonication in 1 L of 95% ethanol in an ultrasonic bath apparatus 28 kHz/1100 W (3 L Alpha Plus, Japan) for 30 min at 35 °C. The obtained extract was filtered, and the procedure was repeated twice. The obtained extracts were combined and evaporated to dryness under reduced pressure using rotary evaporator at 45 °C to obtain 200 g dry residue. A portion of the dry residue of L. camara extract was dissolved in HPLC-grade methanol to obtain a sample solution of concentration 1 mg/mL. This sample solution was filtered using a membrane disc filter (0.2 μm), then degassed by sonication. After that, a full loop injection volume (10 μL) of this solution was injected into the chromatographic column.
Analysis of L. camara extract using UPLC-MS/MS technique
The chromatographic analysis was accomplished using an UPLC XEVO TQD triple quadruple instrument Waters Corporation, Milford, MA01757 USA equipped with a Waters Acquity QSM pump, a LC-2040 autosampler, degasser in addition to Waters Acquity CM detector. The dimensions of Waters Acquity UPLC BEH C18 column was 50 mm (length), 2.1 mm (internal diameter) and 1.7 μm (particle size). The operation of the column was at a flow rate of 0.2 mL/ min and the system was thermostated at 30 °C. The mobile phase that used for analyses consisted of two phases; phase A: ultrapure water + 0.1% formic acid, and phase B: methanol + 0.1% formic acid. Elution was gradient one and its program was as following: 0.0–2.0 min, 10% B; 2.0–5.0 min, 30% B; 5.0–15.0 min, 70% B; 22.0 min, 90% B; 22.0–25.0 min, 90% B; 26.0 min, 100% B; 26.0–29.0 min, 100% B; 30.0 min, 10% B. Then 4 min were set at the initial conditions to re-equilibrate the column.
The mass spectrometric analysis and metabolites annotation were carried out according to the method described by Darwish et al.89 as shown in the Supplementary data.
Network pharmacology-based analysis
The 2D structures of the identified compounds yielded from UPLC-MS/MS analysis were converted to SMILES format using Schrodinger software (LLC, New York, NY, 2015), then furtherly subjected to network pharmacology- based analysis. The identification of the target genes linked to the selected constituents was performed using STITCH database (http://stitch.embl.de/, ver.5.0) with the ‘Homo sapiens’ species settings. UniProt (http://www.uniprot.org/)90,91 was utilized for retrieving gene information including name, gene ID and accession number. To retrieve information about functional annotation and the signaling pathways, bioprocesses, cellular components and molecular functions that were highly associated with inflammation target proteins, DAVID ver. 6.8 (https://david.ncifcrf.gov/) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways (http://www.genome.jp/kegg/pathway.html) were employed. An adjusted p-value < 0.05 was set as a cut-off value for enriching the significance of contributing pathways to inflammation.
Three types of networks: constituent-target gene, gene-pathway, and constituent-gene-pathway networks were constructed and visualized by Cytoscape 3.8.2 (http://www.cytoscape.org/) in order to visualize the interactions between compounds, target proteins and inflammation-related pathways. In the graphical network, each constituent, gene protein and pathway were described by node, and the interactions were encoded by edges. The network parameters were calculated using the network analyzer plug-in in Cytoscape where the weight of nodes in each constructed network was evaluated using Cytoscape combined score of interactions. Protein–protein interaction network (PPI network) was constructed using STRING database (https://string-db.org/).
Molecular docking studies
Molecular docking studies were performed using Glide module integrated in Schrodinger® software. The Protein Data Bank (PDB) was utilized to retrieve the crystal structures of the most enriched target proteins recognized from network pharmacology analysis, named; protein kinase C alpha type (4RA4), transcription factor p65 (3QXY), interleukin-2 (1M49), mitogen-activated protein kinase 14 (6HWU) and proto-oncogene c-Fos (1FOS). These crystal structures were saved as pdb files for further preparation using the PrepWiz module. Location of the binding site for the docking experiments was determined using the receptor grid generation module. Some protein models have no co-crystallized ligands (ex: 3QXY and 1FOS), so the ligand was set as the centroid of specified selected residues retrieved from literature. Hence, the size of the receptor grid predetermined as (20 × 20 × 20 Å3) was adjusted to accommodate ligands with size ≤ 20 Å to exclude large molecules with overestimated docking scores. For other models with co-crystallized ligands (ex: 4RA4, 1M49 and 6HWU) the boxes enclosing the centroids of co-crystallized ligands were set as the grids. 3D-structures of the top hit compounds recognized from network pharmacology analysis (ferulic acid, catechin gallate, myricetin and isoferulic acid) were imported as SDF files to be prepared using Ligprep module generating molecules with correct chiralities, ionization states, tautomers, stereochemistries and ring conformations. The generated compounds from the LigPrep file were flexibily docked using extra precision (XP) docking, and 2D and 3D ligand-target interactions were visualized in maestro interface.
The docking protocol was validated using two methods: (i) redocking of the co-crystallized ligands into the binding sites of their corresponding proteins then the resulting complexes were superimposed on to the reference co-crystallized complexes and the root mean square deviation (RMSD) was calculated. This was done to ensure exact binding of the inhibitor to the active site where less deviation compared to the actual co-crystallized complex is more favorable. This method was exclusively performed for proteins bearing co-crystallized ligands (Protein kinase C alpha type, 4RA4; Interleukin-2, 1M49; and Mitogen-activated protein kinase 14, 6HWU). (ii) Enrichment calculations: for each of the investigated proteins, a validation set composed of known active ligands compiled from literature was constructed (Supplementary Table S6). The validation set compounds were seeded in 1000 Schrodinger® built-in decoys then docked against the active site of target protein using XP mode. Protein–ligand complexes were validated using GLIDE enrichment calculator using numerous validation parameters such as receiver operating characteristic (ROC), AUC- ROC, BEDROC and enrichment factor (EF at 2%, 5% and 10%). These calculations aimed to enrich the docking procedure and to discriminate active compounds from non-active ones thus, avoiding false positive hits production.
ADME and drug-likeness of top hit compounds
The top hit constituents related to inflammation were assessed for drug-likeness by calculating in-silico absorption, distribution, metabolism, and excretion (ADME) criteria and adopting Lipinski's rule of five29, by the aid of Qikprop module (Schrodinger suite 2017A). Only compounds with predicted oral bioavailability ≥ 30 and satisfying at least three criteria from Lipinski's rule of five were considered active.
In vitro cytotoxicity and anti-inflammatory activity testing
It was carried out according to the method described by Darwish et al.92 as shown in the Supplementary data.
Conclusion
In this study, the phytoconstituents of L. camara extract were identified using UPLC-MS/MS analysis, then they were subjected to network pharmacology analysis that declared ferulic acid, catechin gallate, myricetin and isoferulic acid as the endogenous metabolites mostly associated to inflammation, and PRKCA, RELA, IL2, MAPK 14 and FOS as the main inflammation-related genes. The identified target genes were involved in 47 inflammation-related pathways, where the most enriched ones were PI3K-Akt signaling and MAPK signaling pathways. Molecular docking of top hit compounds on the active sites of the most enriched genes revealed that catechin gallate possessed the lowest binding energy against PRKCA, RELA and IL2, while myricetin exhibited the most stable interaction against MAPK14 and FOS. The extract was then forwarded to in vitro cytotoxicity and anti-inflammatory testing indicating comparable results to those of piroxicam. This study provides a profound explanation of the mechanism of the proposed anti-inflammatory activity of L. camara and recommends this plant as a source of potential anti-inflammatory agents. Further in vivo and clinical studies are recommended to affirm our outcomes.
Supplementary Information
Author contributions
A.A.E., R.S.D. and H.M.D. planned the overall study protocol, analyzed and visualized the data, wrote and revised the manuscript. D.A.G., A.M.Y. and S.A.A. conducted the in vitro cytotoxicity and anti-inflammatory testing. All authors have read and approved the final manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
All data generated or analyzed during this study are included in this article (and its supplementary information files).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-19137-0.
References
- 1.Gopi S, Amalraj A, Kunnumakkara A, Thomas S. Inflammation and Natural Products. Academic Press; 2021. [Google Scholar]
- 2.Patil SM, Saini R. Anti-inflammatory and analgesic activities of methanol extract of roots of Lantana camara Linn. J. Pharm. Res. 2012;5:1034–1036. [Google Scholar]
- 3.Tasneem S, Liu B, Li B, Choudhary MI, Wang W. Molecular pharmacology of inflammation: Medicinal plants as anti-inflammatory agents. Pharmacol. Res. 2019;139:126–140. doi: 10.1016/j.phrs.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Ashal TF, Ifora I, Oktavia S. Potential anti-inflammatory effects of Lantana camara L.: A review. Int. Res. J. Pharm. Med. Sci. 2020;3:1–4. [Google Scholar]
- 5.Kohli RK, Batish DR, Singh HP, Dogra KS. Status, invasiveness and environmental threats of three tropical American invasive weeds (Parthenium hysterophorus L., Ageratum conyzoides L., Lantana camara L.) in India. Biol. Invasions. 2006;8:1501–1510. doi: 10.1007/s10530-005-5842-1. [DOI] [Google Scholar]
- 6.Naz R, Bano A. Phytochemical screening, antioxidants and antimicrobial potential of Lantana camara in different solvents. Asian Pac. J. Trop. Dis. 2013;3:480–486. doi: 10.1016/S2222-1808(13)60104-8. [DOI] [Google Scholar]
- 7.Wu P, et al. Bioactive triterpenoids from Lantana camara showing anti-inflammatory activities in vitro and in vivo. Bioorg. Chem. 2020;101:104004. doi: 10.1016/j.bioorg.2020.104004. [DOI] [PubMed] [Google Scholar]
- 8.Bairagi SM, Pathan IB, Nema N. Analgesic and anti-inflammatory activity of crude leaf and bark extract of Lantana camara. Marmara Pharm. J. 2017;21:810–817. doi: 10.12991/mpj.2017.7. [DOI] [Google Scholar]
- 9.Ghosh S, DasSarma M, Patra A, Hazra B. Anti-inflammatory and anticancer compounds isolated from Ventilago madraspatana Gaertn., Rubia cordifolia Linn. and Lantana camara Linn. J. Pharm. Pharmacol. 2010;62:1158–1166. doi: 10.1111/j.2042-7158.2010.01151.x. [DOI] [PubMed] [Google Scholar]
- 10.Caesar LK, Cech NB. Synergy and antagonism in natural product extracts: when 1 + 1 does not equal 2. Nat. Prod. Rep. 2019;36:869–888. doi: 10.1039/C9NP00011A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hopkins AL. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008;4:682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Fan TP, Jia W, Lu A, Zhang W. Network pharmacology in traditional chinese medicine. Evid.-Based Complement. Altern. Med. 2014 doi: 10.1155/2014/138460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibrahim RS, El-Banna AA. Network pharmacology-based analysis for unraveling potential cancer-related molecular targets of Egyptian propolis phytoconstituents accompanied with molecular docking and in vitro studies. RSC Adv. 2021;11:11610–11626. doi: 10.1039/D1RA01390D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao M, et al. An integrated approach to uncover quality marker underlying the effects of Alisma orientale on lipid metabolism, using chemical analysis and network pharmacology. Phytomedicine. 2018;45:93–104. doi: 10.1016/j.phymed.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Ma J, et al. The ethnopharmacology, phytochemistry and pharmacology of Angelica biserrate—A review. J. Ethnopharmacol. 2019;231:152–169. doi: 10.1016/j.jep.2018.10.040. [DOI] [PubMed] [Google Scholar]
- 16.Shawky E, Nada AA, Ibrahim RS. Potential role of medicinal plants and their constituents in the mitigation of SARS-CoV-2: Identifying related therapeutic targets using network pharmacology and molecular docking analyses. RSC Adv. 2020;10:27961–27983. doi: 10.1039/D0RA05126H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taha KF, Khalil M, Abubakr MS, Shawky E. Identifying cancer-related molecular targets of Nandina domestica Thunb. by network pharmacology-based analysis in combination with chemical profiling and molecular docking studies. J. Ethnopharmacol. 2020;249:1–11. doi: 10.1016/j.jep.2019.112413. [DOI] [PubMed] [Google Scholar]
- 18.Sore MA, Mwonjoria JK, Juma KK, Ngugi PM, Mwaniki NEN. Evaluation of analgesic, anti-inflammatory and toxic effects of Lantana camara L. Int. J. Phytopharmacol. 2017;8:89–97. [Google Scholar]
- 19.Silva TSC, et al. Antinociceptive and anti-inflammatory effects of Lantana camara L. extract in mice. Revista Brasileira de Plantas Medicinais. 2015;17:224–229. doi: 10.1590/1983-084X/11_109. [DOI] [Google Scholar]
- 20.Gamal El-Din MI, et al. Comparative LC–LTQ–MS–MS analysis of the leaf extracts of Lantana camara and Lantana montevidensis growing in Egypt with insights into their antioxidant, anti-inflammatory, and cytotoxic activities. Plants. 2022;11:1699. doi: 10.3390/plants11131699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanehisa M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019;28:1947–1951. doi: 10.1002/pro.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanehisa M, Furumichi M, Sato Y, Ishiguro-Watanabe M, Tanabe M. KEGG: integrating viruses and cellular organisms. Nucleic Acids Res. 2021;49:D545–D551. doi: 10.1093/nar/gkaa970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mateev, E., Georgieva, M. & Zlatkov, A. Improved Molecular Docking of MAO-B Inhibitors with Glide. (2022).
- 25.Lokhande, K. B., Ghosh, P., Nagar, S. & Venkateswara Swamy, K. Novel B, C-ring truncated deguelin derivatives reveals as potential inhibitors of cyclin D1 and cyclin E using molecular docking and molecular dynamic simulation. Mol. Divers. 1–15 (2021). [DOI] [PubMed]
- 26.Hevener KE, et al. Validation of molecular docking programs for virtual screening against dihydropteroate synthase. J. Chem. Inf. Model. 2009;49:444–460. doi: 10.1021/ci800293n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halgren TA, et al. Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 2004;47:1750–1759. doi: 10.1021/jm030644s. [DOI] [PubMed] [Google Scholar]
- 28.Zhao W, Hevener KE, White SW, Lee RE, Boyett JM. A statistical framework to evaluate virtual screening. BMC Bioinform. 2009;10:1–13. doi: 10.1186/1471-2105-10-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lipinski CA. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004;1:337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Zhang K, Yan G, Zhang A, Sun H, Wang X. Recent advances in pharmacokinetics approach for herbal medicine. RSC Adv. 2017;7:28876–28888. doi: 10.1039/C7RA02369C. [DOI] [Google Scholar]
- 31.Zhao X, Zhang S, Liu D, Yang M, Wei J. Analysis of flavonoids in Dalbergia odorifera by ultra-performance liquid chromatography with tandem mass spectrometry. Molecules. 2020;25:1–16. doi: 10.3390/molecules25020389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao M, et al. Qualitative and quantitative analyses of active constituents in Trollius ledebourii. J. Chromatogr. Sci. 2018;56:619–635. doi: 10.1093/chromsci/bmy035. [DOI] [PubMed] [Google Scholar]
- 33.Rini Vijayan KP, Raghu AV. Tentative characterization of phenolic compounds in three species of the genus Embelia by liquid chromatography coupled with mass spectrometry analysis. Spectrosc. Lett. 2019;52:653–670. doi: 10.1080/00387010.2019.1682013. [DOI] [Google Scholar]
- 34.Raslan MA, Abdel-Rahman RF, Fayed HM, Ogaly HA, Tahere RF. Metabolomic profiling of Sansevieria trifasciata hort ex. Prain leaves and roots by HPLC-PAD-ESI/MS and its hepatoprotective effect via activation of the NRF2/ARE signaling pathway in an experimentally induced liver fibrosis rat model. Egypt. J. Chem. 2021;64:6647–6671. [Google Scholar]
- 35.Velamuri R, Sharma Y, Fagan J, Schaefer J. Application of UHPLC-ESI-QTOF-MS in phytochemical profiling of sage (Salvia officinalis) and rosemary (Rosmarinus officinalis) Planta Medica Int. Open. 2020;07:e133–e144. doi: 10.1055/a-1272-2903. [DOI] [Google Scholar]
- 36.BelhadjSlimen I. LC-MS analysis of phenolic acids, flavonoids and betanin from spineless Opuntia ficus-indica fruits. Cell Biol. 2017;5:17. doi: 10.11648/j.cb.20170502.12. [DOI] [Google Scholar]
- 37.Bystrom LM, Lewis BA, Brown DL, Rodriguez E, Obendorf RL. Characterization of phenolics by LC-UV/vis, LC-MS/MS and sugars by GC in Melicoccus bijugatus Jacq. “Montgomery” fruits. Food Chem. 2008;111:1017–1024. doi: 10.1016/j.foodchem.2008.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Enomoto H, Takahashi S, Takeda S, Hatta H. Distribution of flavan-3-ol species in ripe strawberry fruit revealed by matrix-assisted laser desorption/ionization-mass spectrometry imaging. Molecules. 2020;25:1–14. doi: 10.3390/molecules25010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin Y, et al. Metabolite identification of myricetin in rats using HPLC coupled with ESI-MS. Chromatographia. 2012;75:655–660. doi: 10.1007/s10337-012-2239-z. [DOI] [Google Scholar]
- 40.March RE, Miao X-S. fragmentation study of kaempferol using electrospray quadrupole time-of-flight mass spectrometry at high mass resolution. Int. J. Mass Spectrom. 2004;231:157–167. doi: 10.1016/j.ijms.2003.10.008. [DOI] [Google Scholar]
- 41.Yuzuak S, Ballington J, Xie D-Y. HPLC-qTOF-MS/MS-based profiling of flavan-3-ols and dimeric proanthocyanidins in berries of two muscadine grape hybrids FLH 13–11 and FLH 17–66. Metabolites. 2018;8:1–24. doi: 10.3390/metabo8040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinosaki NBM, et al. Structural study of phenolic acids by triple quadrupole mass spectrometry with electrospray ionization in negative mode and H/D isotopic exchange. J. Braz. Chem. Soc. 2020;31:402–408. [Google Scholar]
- 43.Fang N, Yu S, Prior RL. LC/MS/MS characterization of phenolic constituents in dried plums. J. Agric. Food Chem. 2002;50:3579–3585. doi: 10.1021/jf0201327. [DOI] [PubMed] [Google Scholar]
- 44.Li C, Seeram NP. Ultra-fast liquid chromatography coupled with electrospray ionization time-of-flight mass spectrometry for the rapid phenolic profiling of red maple (Acer rubrum) leaves. J. Sep. Sci. 2018;41:2331–2346. doi: 10.1002/jssc.201800037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang X, et al. On the origin of the methyl radical loss from deprotonated ferulic and isoferulic acids: Electronic excitation of a transient structure. J. Am. Soc. Mass Spectrom. 2013;24:941–948. doi: 10.1007/s13361-013-0604-2. [DOI] [PubMed] [Google Scholar]
- 46.Xue Z, Yang B. Phenylethanoid glycosides: Research advances in their phytochemistry, pharmacological activity and pharmacokinetics. Molecules. 2016;21:1–25. doi: 10.3390/molecules21080991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li C, Liu Y, Abdulla R, Aisab HA, Suo Y. Characterization and identification of chemical components in Neopicrorhiza scrphulariiflora roots by liquid chromatography-electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Anal. Methods. 2014;6:3634–3643. doi: 10.1039/C4AY00157E. [DOI] [Google Scholar]
- 48.Attia YM, El-Kersh DM, Wagdy HA, Elmazar MM. Verbascoside: Identification, quantification, and potential sensitization of colorectal cancer cells to 5-FU by targeting PI3K/AKT pathway. Sci. Rep. 2018;8:1–12. doi: 10.1038/s41598-018-35083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ashour MA. Isolation, HPLC/UV characterization and antioxidant activity of phenylethanoids from Blepharis edulis (Forssk.) Pers. growing in Egypt. Bull. Faculty Pharm. Cairo Univ. 2021;50:67–72. doi: 10.1016/j.bfopcu.2012.03.003. [DOI] [Google Scholar]
- 50.Ren L, et al. Studies of iridoid glycosides using liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007;21:3039–3050. doi: 10.1002/rcm.3189. [DOI] [PubMed] [Google Scholar]
- 51.Li H, et al. Chemical profiling of Re-Du-Ning injection by ultra-performance liquid chromatography coupled with electrospray ionization tandem quadrupole time-of-flight mass spectrometry through the screening of diagnostic ions in MSe mode. PLoS ONE. 2015;10:1–19. doi: 10.1371/journal.pone.0121031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang S-C, Liao H-J, Lee W-C, Huang C-M, Tsai T-H. Using orthogonal array to obtain gradient liquid chromatography conditions of enhanced peak intensity to determine geniposide and genipin with electrospray tandem mass spectrometry. J. Chromatogr. A. 2008;1212:68–75. doi: 10.1016/j.chroma.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 53.Calixto NO, et al. Chemical constituents of Psychotria nemorosa gardner and antinociceptive activity. J. Braz. Chem. Soc. 2017;28:707–723. [Google Scholar]
- 54.Czerwińska ME, Kalinowska E, Popowski D, Bazylko A. Lamalbid, chlorogenic acid, and verbascoside as tools for standardization of Lamium album flowers—development and validation of HPLC–DAD method. Molecules. 2020;25:1721. doi: 10.3390/molecules25071721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Apisornopas J, et al. Synthetic analogues of durantoside I from Citharexylum spinosum L. and their cytotoxic activity. Bioorganic Med. Chem. Lett. 2018;28:1558–1561. doi: 10.1016/j.bmcl.2018.03.068. [DOI] [PubMed] [Google Scholar]
- 56.Kostova MB, Konaklieva MI, Alipieva KI, Popov SS, Handjieva NV. ESI-MS study of some C10 iridoid glycosides. Instrum Sci. Technol. 2005;33:691–702. doi: 10.1080/10739140500311288. [DOI] [Google Scholar]
- 57.Ayatollahi AM, et al. Pentacyclic triterpenes in Euphorbia microsciadia with their T-cell proliferation activity. Iran. J. Pharm. Res. 2011;10:287–294. [PMC free article] [PubMed] [Google Scholar]
- 58.Begum S, Zehra SQ, Siddiqui BS, Fayyaz S, Ramzan M. Pentacyclic triterpenoids from the aerial parts of Lantana camara and their nematicidal activity. Chem. Biodivers. 2008;5:1856–1866. doi: 10.1002/cbdv.200890173. [DOI] [PubMed] [Google Scholar]
- 59.Begum S, et al. Leishmanicidal triterpenes from Lantana camara. Chem. Biodivers. 2014;11:709–718. doi: 10.1002/cbdv.201300151. [DOI] [PubMed] [Google Scholar]
- 60.Chen Q, Zhang Y, Zhang W, Chen Z. Identification and quantification of oleanolic acid and ursolic acid in Chinese herbs by liquid chromatography–ion trap mass spectrometry. Biomed. Chromatogr. 2010;25:1381–1388. doi: 10.1002/bmc.1614. [DOI] [PubMed] [Google Scholar]
- 61.Ghallab DS, Mohyeldin MM, Shawky E, Metwally AM, Ibrahim RS. Chemical profiling of Egyptian propolis and determination of its xanthine oxidase inhibitory properties using UPLC–MS/MS and chemometrics. LWT Food Sci. Technol. 2020;136:1–16. [Google Scholar]
- 62.Gross, J. H. Fragmentation of organic ions and interpretation of EI mass spectra. In Mass Spectrometry 325–437. (Springer, 2017).
- 63.Hui-Qin W, et al. Gas chromatographic retention time rule and mass spectrometric fragmentation rule of fatty acids and its application in food. Chin. J. Anal. Chem. 2007;35:998–1003. doi: 10.1016/S1872-2040(07)60065-6. [DOI] [Google Scholar]
- 64.Darwish RS, et al. Differential anti-inflammatory biomarkers of the desert truffles Terfezia claveryi and Tirmania nivea revealed via UPLC-QqQ-MS-based metabolomics combined to chemometrics. Lwt. 2021;150:111965. doi: 10.1016/j.lwt.2021.111965. [DOI] [Google Scholar]
- 65.Ghallab DS, Mohyeldin MM, Shawky E, Metwally AM, Ibrahim RS. Chemical profiling of Egyptian propolis and determination of its xanthine oxidase inhibitory properties using UPLC–MS/MS and chemometrics. LWT. 2020;136:110298–110313. doi: 10.1016/j.lwt.2020.110298. [DOI] [Google Scholar]
- 66.Gross JH. Mass Spectrometry. Springer International Publishing; 2017. [Google Scholar]
- 67.Mahmoud AM, Hussein OE, Hozayen WG, Bin-Jumah M, Abd El-Twab SM. Ferulic acid prevents oxidative stress, inflammation, and liver injury via upregulation of Nrf2/HO-1 signaling in methotrexate-induced rats. Environ. Sci. Pollut. Res. 2020;27:7910–7921. doi: 10.1007/s11356-019-07532-6. [DOI] [PubMed] [Google Scholar]
- 68.Rhodes LE, et al. Universities of Leeds, Sheffield and York Chapter 4. Br. J. Nutr. 2013;110:891–900. doi: 10.1017/S0007114512006071. [DOI] [PubMed] [Google Scholar]
- 69.Yao Q, Li S, Li X, Wang F, Tu C. Myricetin modulates macrophage polarization and mitigates liver inflammation and fibrosis in a murine model of nonalcoholic steatohepatitis. Front. Med. 2020;7:1–16. doi: 10.3389/fmed.2020.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dilshara MG, et al. Downregulation of NO and PGE2 in LPS-stimulated BV2 microglial cells by trans-isoferulic acid via suppression of PI3K/Akt-dependent NF-κB and activation of Nrf2-mediated HO-1. Int. Immunopharmacol. 2014;18:203–211. doi: 10.1016/j.intimp.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 71.Guo Y, et al. Clinical significance of the correlation between PLCE 1 and PRKCA in esophageal inflammation and esophageal carcinoma. Oncotarget. 2017;8:33285–33299. doi: 10.18632/oncotarget.16635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barton A, et al. Association of protein kinase C alpha (PRKCA) gene with multiple sclerosis in a UK population. Brain. 2004;127:1717–1722. doi: 10.1093/brain/awh193. [DOI] [PubMed] [Google Scholar]
- 73.Wang M, et al. EGCG promotes PRKCA expression to alleviate LPS-induced acute lung injury and inflammatory response. Sci. Rep. 2021;11:1–13. doi: 10.1038/s41598-020-79139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kelly D, et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shutting of PPAR-γ and ReIA. Nat. Immunol. 2004;5:104–112. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- 75.Steinbrecher K, Harmel-Laws E, Sitcheran R, Baldwin A. Loss of epithelial RelA results in deregulated intestinal proliferative/apoptotic homeostasis and susceptibility to inflammation1. J. Immunol. 2008;180:2588–2599. doi: 10.4049/jimmunol.180.4.2588. [DOI] [PubMed] [Google Scholar]
- 76.Algül H, et al. Pancreas-specific RelA/p65 truncation increases susceptibility of acini to inflammation-associated cell death following cerulein pancreatitis. J. Clin. Investig. 2007;117:1490–1501. doi: 10.1172/JCI29882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jagannathan L, et al. Nuclear factor κB1/RelA mediates inflammation in human lung epithelial cells at atmospheric oxygen levels. J Cell Physiol. 2016;231:1611–1620. doi: 10.1002/jcp.25262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hoyer KK, Dooms H, Barron L, Abul Abbas K. Interleukin-2 in the development and control of inflammatory disease. Immunol. Rev. 2008;226:19–28. doi: 10.1111/j.1600-065X.2008.00697.x. [DOI] [PubMed] [Google Scholar]
- 79.McKinstry KK, et al. Memory CD4 T cell-derived IL-2 synergizes with viral infection to exacerbate lung inflammation. PLoS Pathog. 2019;15:1–24. doi: 10.1371/journal.ppat.1007989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ali EMH, et al. Design, synthesis and anti-inflammatory activity of imidazol-5-yl pyridine derivatives as p38α/MAPK14 inhibitor. Bioorg. Med. Chem. 2021;31:115969. doi: 10.1016/j.bmc.2020.115969. [DOI] [PubMed] [Google Scholar]
- 81.Zhang L, et al. Circular RNA circACSL1 aggravated myocardial inflammation and myocardial injury by sponging miR-8055 and regulating MAPK14 expression. Cell Death Dis. 2021;12:1–18. doi: 10.1038/s41419-020-03229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fazia T, et al. Investigating the causal effect of brain expression of CCL2, NFKB1, MAPK14, TNFRSF1A, CXCL10 genes on multiple sclerosis: A two-sample mendelian randomization approach. Front. Bioeng. Biotechnol. 2020;8:1–16. doi: 10.3389/fbioe.2020.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grossi V, Hyams JS, Glidden NC, Knight BE, Young EE. Characterizing clinical features and creating a gene expression profile associated with pain burden in children with inflammatory bowel disease. Inflamm. Bowel Dis. 2020;26:1283–1290. doi: 10.1093/ibd/izz240. [DOI] [PubMed] [Google Scholar]
- 84.She H, He Y, Zhao Y, Mao Z. Release the autophage brake on inflammation: The MAPK14/p38α-ULK1 pedal. Autophagy. 2018;14:1097–1098. doi: 10.1080/15548627.2018.1446626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakano S, et al. Computational molecular docking and X-ray crystallographic studies of catechins in new drug design strategies. Molecules. 2018;23:2020. doi: 10.3390/molecules23082020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ganeshpurkar A, Saluja A. In silico interaction of catechin with some immunomodulatory targets: A docking analysis. Indian J. Biotechnol. 2018;17:626–631. [Google Scholar]
- 87.Kang KA, et al. Myricetin protects cells against oxidative stress-induced apoptosis via regulation of PI3K/Akt and MAPK signaling pathways. Int. J. Mol. Sci. 2010;11:4348–4360. doi: 10.3390/ijms11114348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Park S. Polyphenol compound as a transcription factor inhibitor. Nutrients. 2015;7:8987–9004. doi: 10.3390/nu7115445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Darwish RS, et al. Chemical profiling and unraveling of anti-COVID-19 biomarkers of red sage (Lantana camara L.) cultivars using UPLC-MS/MS coupled to chemometric analysis, in vitro study and molecular docking. J. Ethnopharmacol. 2022;291:115038. doi: 10.1016/j.jep.2022.115038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chandran U, Patwardhan B. Network ethnopharmacological evaluation of the immunomodulatory activity of Withania somnifera. J. Ethnopharmacol. 2017;197:250–256. doi: 10.1016/j.jep.2016.07.080. [DOI] [PubMed] [Google Scholar]
- 91.Shi X-Q, et al. A network pharmacology approach to investigate the blood enriching mechanism of Danggui buxue Decoction. J. Ethnopharmacol. 2019;235:227–242. doi: 10.1016/j.jep.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 92.Darwish RS, et al. Comparative metabolomics reveals the cytotoxic and anti-inflammatory discriminatory chemical markers of raw and roasted colocynth fruit (Citrullus colocynthis L.) RSC Adv. 2021;11:37049–37062. doi: 10.1039/D1RA07751A. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article (and its supplementary information files).