Abstract
The activity of the toluene-responsive ς54 Pu promoter of the pWW0 TOL plasmid of Pseudomonas putida is down-regulated in vivo during exponential growth in rich medium and also by the presence of glucose in the culture. Although the Pu promoter already performs poorly during log growth in minimal medium when amended with casamino acids, the addition of glucose further decreased by two- to threefold the accumulation of β-galactosidase in a Pu-lacZ reporter P. putida strain. Since Pu was still down-regulated during exponential growth regardless of glucose addition, it appeared that the carbohydrate separately influenced promoter activity. This notion was supported by the growth-dependent induction pattern of Pu in a ptsN mutant of P. putida, the loss of which makes Pu no longer responsive to repression by glucose. On the other hand, overexpression of the sigma factor ς54, known to partially alleviate the exponential silencing of the promoter, did not affect glucose inhibition of Pu. These data indicated that exponential silencing and carbon source-dependent repression are two overlapping but genetically distinguishable mechanisms that adapt Pu to the physiological status of the cells and nutrient availability.
The ς54-dependent promoter Pu of Pseudomonas putida drives transcription of an operon borne by the TOL plasmid pWW0 which determines the bioconversion of toluene, m-xylene, and p-xylene into the corresponding carboxylic acids, i.e., benzoate, m-toluate, and p-toluate, respectively (1). This promoter is activated at a distance by the enhancer-binding and toluene-responsive protein XylR (15) with the structural assistance of the integration host factor (IHF). While Pu is functional in vitro by just mixing purified and preactivated XylR with ς54-containing RNAP and IHF (14), promoter activity in vivo is subject to the metabolic status of the cells. This suggests that additional elements adjust transcription to a given physiological state. Various reports have shown that Pu activity is down-regulated in response to exponential growth in rich media, a phenomenon referred to as catabolic repression (7, 12), stationary-phase dependency (4, 10), or exponential silencing (2). However, Pu becomes quickly activated at the onset of stationary phase. At least in part, this effect can be traced to modulation of the activity of the sigma factor (2). On the other hand, the presence of certain carbon compounds, such as glucose or gluconate, also inhibits Pu activity (9). The effects of the growth phase and carbon sources have, however, different extents. β-Galactosidase accumulation experiments (2) and quantitative S1 nuclease assays of transcript production (7, 12) have shown that exponential silencing caused by rapid growth in rich medium completely abolishes Pu activity. In contrast, none of the carbon sources assayed decreased promoter output by more than two-thirds of the maximal activity (3, 9). The gene ptsN, encoding a new member of the phosphoenolpyruvate:sugar phosphotransferase system (PTS), has been recently related to this modulation, since its loss relieves glucose inhibition of Pu in P. putida cells (3). Glucose assimilation is not affected in this mutant, suggesting that this gene participates in sensing the carbohydrate (3) and not in its metabolism.
Taken together, these results indicate the existence of additional control devices that connect Pu activity to the metabolic status of P. putida cells, and they also raise the question of what specific mechanisms and environmental signals are involved. In other words, are growth phase control and nutrient control different aspects of the same regulatory mechanism, or are there distinct physiological controls? Similar physiological control phenomena have been described for Po, a different, yet related, ς54 promoter which drives the expression of an operon for degradation of phenol and m-cresol in Pseudomonas sp. strain CF600. In this instance, however, the degree of repression observed in various culture conditions can be related to the corresponding growth rates, regardless of the carbon source added (17). In reality, the entire physiological control of Po can be traced to its strict requirement of ppGpp for promoter activity (18). Yet, this is clearly not the case for Pu. Holtel et al. (9) could not find a correlation between Pu activity and growth rates in various carbon sources. Moreover, Pu activity in P. putida cells growing at identical rates in a medium with casamino acids was inhibited by glucose but not by fructose (3). Finally, Pu is not dependent on ppGpp for full activity in vivo (our unpublished observations).
In this work, we employed a genetic approach to examine the relationship between the phenomenon of exponential silencing and the C source inhibition of Pu. To this end, we investigated whether the lack of ptsN, a genetic background that abolishes C-dependent inhibition (3), could also suppress exponential silencing. Conversely, we also tested whether overexpression of the sigma factor ς54, which alleviates the exponential-phase regulation of a Pu-lacZ fusion when cells are grown in rich medium (2), could also mitigate carbon repression. In order to answer these questions, we have used a specialized P. putida strain bearing a reporter system integrated into its chromosome which reproduces faithfully all regulatory elements involved in the physiological control of Pu. The results below show that the C source and the growth phase enter the system as distinct physiological signals, the responses to which can be separated genetically.
MATERIALS AND METHODS
Strains, plasmids, media, and general methods.
P. putida MAD2 (8) and its ptsN::Km derivative (3) were used in all cases to examine Pu activity. This strain is a tellurite-resistant derivative of P. putida KT2442 harboring a chromosomal Pu-lacZ fusion along with an xylR allele named xylRΔA. The loss of the N-terminal A domain of the protein makes XylR constitutively active in the absence of an inducer (m-xylene) (8). The ptsN::Km derivative and the details on the construction of plasmid pJM154 can be found in reference 3. P. putida rpoN ΩKm has been described before (11). The tetracycline-resistant broad-host-range, Ptac/lacIq-dependent expression vector pVLT31 and its derivative bearing the P. putida rpoN gene have been reported elsewhere (2, 5). Promoter activity was monitored by assaying the accumulation of β-galactosidase in cells of P. putida MAD2 or its ptsN::Km derivative permeabilized with chloroform and sodium dodecyl sulfate as described by Miller (13). Each enzymatic measurement was repeated at least twice in duplicate samples, with deviations being less than 20%. Bacteria were grown in either complete Luria broth (LB) medium or in synthetic mineral M9 medium (13) supplemented with 0.2% casamino acids (M9-caa). In the latter case, glucose was added, where indicated, at a final concentration of 10 mM. Culture media were supplemented, where needed, with kanamycin (Km; 50 μg/ml), streptomycin (Sm; 50 μg/ml), potassium tellurite (Tel; 80 μg/ml), or tetracycline (Tc; 15 μg/ml). Mobilization into a P. putida recipient through triparental matings with helper strain Escherichia coli HB101 (RK600) was described before (6).
Protein techniques.
Western blot assays were made as described by Cases et al. (2). To this end, equal amounts of whole Pseudomonas cells were lysed in a sample buffer with 2% sodium dodecyl sulfate and 5% β-mercapthoethanol and run in denaturing 15% polyacrylamide gels. These were subsequently blotted and probed with a 1:1,000 dilution of a preadsorbed rabbit antiserum against the purified ς54 protein of E. coli kindly provided by A. Ishihama. The band corresponding to this protein was developed with the Amersham Biotech ECL+ Developing system as indicated by the producer.
RESULTS AND DISCUSSION
Monitoring metabolic coregulation of Pu in a Pseudomonas reporter system.
In order to have a reliable in vivo assay to examine the growth conditions that down-regulate Pu, we employed strain P. putida MAD2 (8). This strain bears all regulatory elements that control expression of Pu assembled in a mini-Tn5 transposon inserted into the chromosome of P. putida. This includes a transcriptional Pu-lacZ fusion that results from placing a 312-bp DNA fragment from the TOL plasmid spanning positions −205 to +93 of Pu in front of a promoterless lacZ gene (see Materials and Methods). xylR is entered in the reporter element in the form of a truncated gene encoding a variant named XylRΔA, in which the N-terminal domain has been deleted. Such a deletion results in the constitutive activity of the protein independently of effector addition (8). Therefore, this reporter system reflects the physiological regulation of Pu as a phenomenon different from its activation by m-xylene, a feature endowed to the protein by the A domain which is absent in XylRΔA (2, 3, 8).
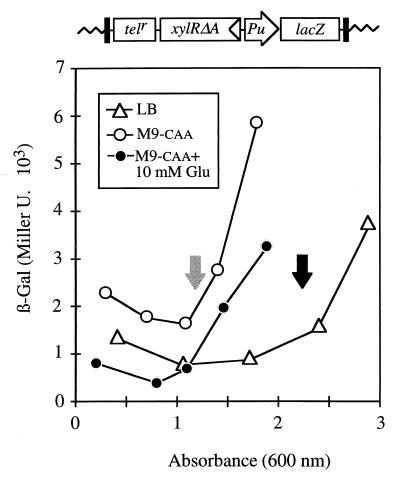
In order to monitor simultaneously the effects of glucose and growth phase in our reporter system, we employed the medium M9-caa (3) alone or supplemented with 10 mM glucose, a known repressive carbohydrate. The presence of an excess (0.2%) of casamino acids in the medium (i) affords equal growth rates regardless of the addition of an additional carbon source (3), (ii) causes exponential silencing (12), and (iii) prevents the production of ppGpp and the stringent response (18), but it does not inhibit the consumption of glucose (3). P. putida MAD2 cells were grown in this semisynthetic medium with or without the additional carbon source, and the induction pattern of the Pu-lacZ fusion was monitored along growth, as shown in Fig. 1. As predicted, β-galactosidase accumulation was significantly lower when cells were grown in the medium amended with glucose than when they were grown in the medium without an extra carbon source. The variation, however, did not exceed two- to threefold. Despite these differences, Pu activity was systematically triggered when the growth rate slowed in any of the media tested, a result consistent with the previous observations made with richer media, such as LB. These results fully validated the experimental assay system and suggested that the C source did not entirely account by itself for the induction levels of P. putida MAD2 in the various media. Furthermore, they suggested that the exponential silencing of Pu and the control of this promoter by glucose, shown in Fig. 1, may obey different signals, albeit overlapping somewhat during batch growth.
FIG. 1.
Evolution of Pu activity during growth in different media. P. putida MAD2 cells bearing all elements required for Pu regulation assembled a mini-Tn5 (top) and were grown overnight at 30°C in complete LB medium or in M9-caa medium with or without 10 mM glucose as an extra C source, diluted to an A600 of ∼0.05, and regrown in the same conditions. β-Galactosidase levels were followed along the growth curve as shown. Note that the promoter remained partially or fully inhibited (as reflected by β-galactosidase output) until cultures entered stationary phase (indicated by arrows). Note also that addition of glucose reduced Pu activity along the entire growth curve as well as the rate of accumulation of β-galactosidase following the onset of the stationary phase.
The loss of ptsN relieves C source inhibition, but not exponential silencing of Pu.
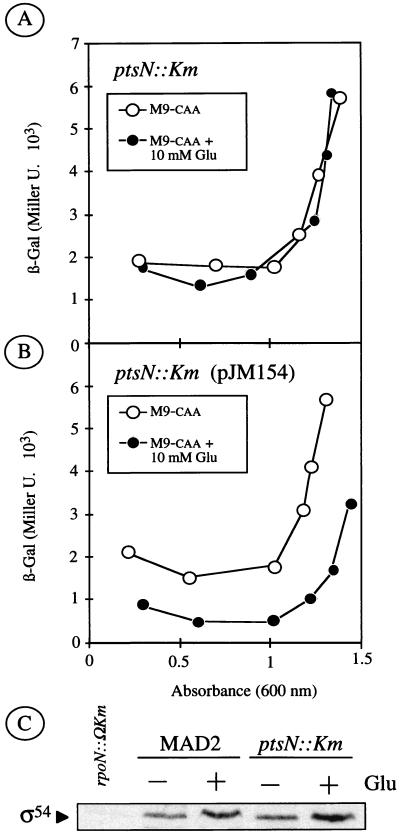
We have previously reported that the product of the ptsN gene is involved in the transduction pathway that leads to carbon-dependent inhibition of the Pu promoter through a mechanism that seems to involve phosphorylation of the encoded IIANtr protein (3). To ascertain whether this mutation also affected the exponential silencing of Pu activity, we compared the output of the promoter in a ptsN::Km derivative of P. putida MAD2 during growth in M9-caa with or without glucose. As shown in Fig. 2A, the ptsN variant of P. putida MAD2 displayed a virtually identical inhibition of Pu during rapid growth. This indicated unequivocally that ptsN was not involved in exponential silencing and argued against a shared mechanism for sensing both exponential growth and repressive carbon sources. As expected from the known phenotype of strains lacking ptsN (3), the mutant showed β-galactosidase levels in the medium with glucose that were significantly higher than those produced by the wild type and virtually the same as those of the parental strain without any carbohydrate added (Fig. 1). But, regardless of the amendments to the M9-caa medium, promoter activity in the ptsN mutant was still clearly subjected to growth-phase control. These results indicated that while the loss of the IIANtr product abolishes the repression exerted by glucose on Pu, it does not affect exponential silencing. Consistent with this notion, the induction rate of Pu in glucose appeared to be somewhat slower in the presence of the carbohydrate, but it still depended on the growth stage.
FIG. 2.
Growth-phase-dependent induction of Pu in a P. putida MAD2 derivative lacking ptsN. (A) P. putida MAD2 ptsN::Km cells from a stationary-phase culture were diluted to an A600 of ∼0.05 and regrown at 30°C in M9-caa medium with or without glucose as an extra C source as indicated. β-Galactosidase levels were followed soon after growth resumed in the fresh medium. (B) Same as above, but instead with P. putida MAD2 ptsN::Km cells transformed with plasmid pJM154, which harbors a copy of the ptsN gene under the control of a Plac promoter. Note that although Pu lacks inhibition by glucose in the ptsN mutant, it still retains its exponential silencing to the same degree as the wild-type strain. (C) Comparison of the intracellular levels of ς54 in P. putida MAD2 and P. putida MAD2 ptsN::Km cells in the different culture conditions described above. The loss of the ptsN gene did not grossly affect ς54 amounts as detected by Western blot with anti-ς54 polyclonal serum.
To verify that the effects observed could unequivocally be traced to ptsN, the mutant strain was transformed with a broad-host-range plasmid carrying a PstI fragment containing only the ptsN sequence placed under the control of a Plac promoter. That transformation of this plasmid (pJM154) in P. putida MAD2 ptsN::Km restored expression of the corresponding gene product was confirmed through Western blot analysis of the resulting strain with a polyclonal anti-IIANtr serum (data not shown). The induction pattern of Pu in such a complemented strain was also virtually identical to that of nonmutated P. putida MAD2 regardless of the extra carbon source employed in the experiment (Fig. 2B).
To rule out that the apparent exponential silencing of the ptsN mutant could be only an artifact caused by a variation of the levels of the ς54 factor in the mutant, we compared the intracellular contents of ς54 in isogenic ptsN+ and mutant ptsN strains of P. putida. To this end, we blotted cell extracts from the two strains grown in M9-caa with or without glucose and we probed them in a Western assay with a specific anti-ς54 polyclonal antibody. The results of Fig. 2C show that the ptsN mutant has ς54 levels comparable to those of the wild type and, therefore, that the distinctive silencing of the mutant is a genuine phenomenon.
Overexpression of ς54 does not relieve C source inhibition of Pu.
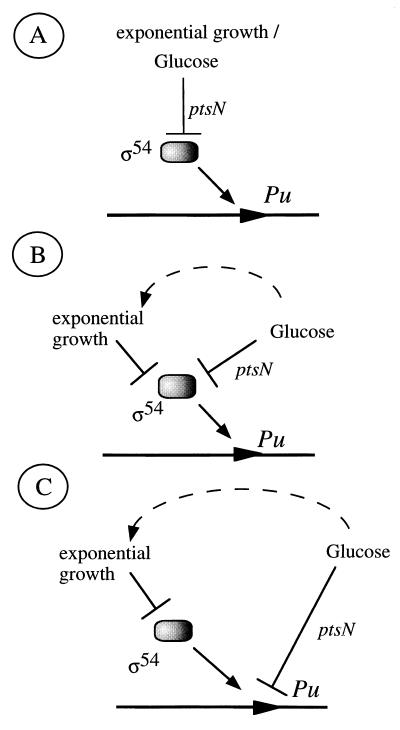
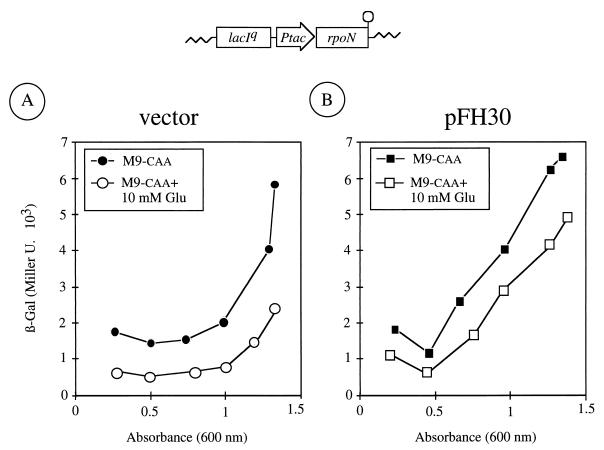
The experiments above rule out a model in which both exponential silencing and C source inhibition are channeled through the IIANtr protein (Fig. 3A). Moreover, they suggest that the exponential silencing of Pu and its down-regulation by glucose are dissimilar phenomena and the mechanisms involved are at least partially independent. However, they do not say whether they work ultimately on the same molecular target. It has been suggested before that the inhibition of Pu activity during rapid growth reflects the physiological control of the activity of the ς54 factor itself. This is based on the observation that overexpression of ς54 relieves in part the inhibition of Pu caused by rapid growth in LB medium (2). On this basis, it became plausible that the signals of fast growth and C source are ultimately channeled towards inhibiting the activity of the factor in vivo (Fig. 3B). If so, overexpression of ς54 would overcome glucose repression as it does with the exponential silencing (2). To examine this possibility we set up an experiment in which ς54 was overproduced in P. putida MAD2 in M9-caa medium with or without glucose. If the scheme of Fig. 3B (ς54 channeling the inhibitory effects of both rapid growth and C source) were true, then increasing the level of the ς54 factor should lessen the negative effects of the two conditions. The results in Fig. 4 show, however, that this was not the case. For this experiment, we employed plasmid pFH30, which bears the rpoN gene of P. putida (encoding ς54) under the control of an inducible Ptac promoter, and the corresponding insertless vector pVLT31 (5). These plasmids were introduced separately into the reporter strain P. putida MAD2, and the activity of Pu was monitored during growth in M9-caa medium with or without glucose amended with isopropyl-β-d-thiogalactopyranoside (IPTG). These results revealed that Pu activity in cells transformed with the insertless vector pVTL31 evolved identically to P. putida MAD2 cells devoid of plasmids (compare Fig. 1 and 4A). However, cells bearing pFH30 and thus overexpressing ς54 (Fig. 4B) did change its induction pattern. Similarly to the situation reported for P. putida MAD2 grown in LB (2), an excess of the sigma factor allowed the accumulation of β-galactosidase to occur much earlier during exponential growth in the M9-caa medium with or without glucose. However, the presence of glucose decreased both the rate and the levels of β-galactosidase accumulation (Fig. 4B), to an extent comparable to that showed by the control strain (Fig. 4A) in M9-caa medium at the onset of stationary phase. Since the growth rates of the strains were identical in all conditions, these results suggested that overexpression of ς54 relieved the exponential silencing but not the down-regulation effect of glucose. This supported the notion that growth phase control and C source inhibition of the Pu promoter might operate independently (Fig. 3C).
FIG. 3.
Alternative models for the physiological regulation of Pu. Three different hypotheses may account for the effects of rapid growth and glucose on the activity of the promoter. In these schemes, the dotted lines symbolize the indirect effect caused by the metabolization of a given carbon source on growth rate. (A) Both signals (exponential growth and C source) could be channeled through ptsN to regulate ς54 activity directly or indirectly. (B) Exponential growth and glucose could be sensed independently, the latter through ptsN. However, both pathways may converge to check ς54 activity or performance. (C) Glucose and growth might act independently on the Pu promoter. As explained in the text, this is the most likely possibility to account for the effects observed.
FIG. 4.
Effect of ς54 overexpression on Pu activity during growth. (A) Cells from a stationary phase culture of P. putida MAD2 transformed with the insertless vector pVLT31 were diluted to an A600 of ∼0.05 and regrown at 30°C in M9-caa medium with 0.5 mM IPTG and 10 mM glucose as indicated. The β-galactosidase levels during subsequent growth are shown. (B) Same as above, but with cells of P. putida MAD2 bearing the ς54-overexpressing plasmid pFH30. A sketch of the relevant portion of this plasmid is shown on top. Note the partial relief of the exponential silencing of Pu in P. putida MAD2 (pFH30) as well as the maintenance of the down-regulation by glucose even when ς54 is in excess.
Two or more independent mechanisms mediate the physiological control of Pu activity.
Two observations reported in this work show that down-regulation of Pu in response to the physiological status involves two signaling pathways which can be separated genetically (Fig. 3C). On the other hand, exponential growth in rich medium strongly represses Pu through a mechanism likely to involve the modulation of ς54 activity and which can be relieved by overexpressing the factor (2). On the other hand, several carbon sources, including glucose (3, 9), down-regulate Pu through a mechanism which requires the ptsN gene (3). Pu activity in the ptsN::Km strain is not inhibited by glucose, but is still subject to exponential silencing. Although this observation ruled out a shared mechanism for sensing both signals (Fig. 3A), it did not rule out that ς54 was the primary target for Pu regulation (Fig. 3B). However, overproduction of ς54 alleviated exponential silencing, but not the inhibition by glucose. These data support the model of Fig. 3C, which proposes two separate channels that bring physiological signals to Pu. However, a connection between both signals is plausible. It is obvious that the presence of a carbon source, such as glucose, could have an effect on the growth rate and the general energy status of the cell (Fig. 3). This indirect outcome could account for the observation made in continuous culture on the general repressive effect of an excess of carbon in the media (7). It is possible that in those conditions the effect of excess C could be channeled through ς54, thereby paralleling the exponential silencing observed in batch culture. In contrast, the inhibition exerted by glucose, which is mediated by ptsN, appears to be specific for some carbohydrates. This is supported by the observation that some carbon sources that typically trigger catabolic repression in Pseudomonas, such as fructose or succinate, do not directly affect Pu activity (3, 9). This is especially significant since the metabolisms of glucose and fructose have been shown to be biochemically close in this genus (16).
ACKNOWLEDGMENTS
We are indebted to A. Ishihama for kindly providing anti-sigma 54 serum, and to J. Pérez-Martín for advice on the work and comments on the manuscript.
This research was supported by contracts BIO4-CT97-2040 and QLRT-99-00041 of the EU and by grant BIO98-0808 of the Comisión Interministerial de Ciencia y Tecnología. I.C. was a predoctoral Fellow of the Spanish Ministry of Education and Culture.
REFERENCES
- 1.Assinder S J, Williams P A. The TOL plasmids: determinants of the catabolism of toluene and xylenes. Adv Microb Physiol. 1990;31:1–69. doi: 10.1016/s0065-2911(08)60119-8. [DOI] [PubMed] [Google Scholar]
- 2.Cases I, Pérez-Martín J, de Lorenzo V. Involvement of ς54 in exponential silencing of the Pseudomonas putida TOL plasmid Pu promoter. Mol Microbiol. 1996;19:7–17. doi: 10.1046/j.1365-2958.1996.345873.x. [DOI] [PubMed] [Google Scholar]
- 3.Cases I, Pérez-Martín J, de Lorenzo V. The IIANtr (PtsN) protein of Pseudomonas putida mediates C source inhibition of the ς54-dependent Pu promoter of the TOL plasmid. J Biol Chem. 1999;274:15562–15568. doi: 10.1074/jbc.274.22.15562. [DOI] [PubMed] [Google Scholar]
- 4.de Lorenzo V, Cases I, Herrero M, Timmis K N. Early and late response of TOL promoters to pathway inducers: identification of postexponential promoters in Pseudomonas putida with lacZ-tet bicistronic reporters. J Bacteriol. 1993;175:6902–6907. doi: 10.1128/jb.175.21.6902-6907.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Lorenzo V, Eltis L, Kessler B, Timmis K N. Analysis of Pseudomonas gene products using lacIq/Ptrp-lac plasmids and transposons that confer conditional phenotypes. Gene. 1993;123:17–24. doi: 10.1016/0378-1119(93)90533-9. [DOI] [PubMed] [Google Scholar]
- 6.de Lorenzo V, Timmis K N. Analysis and construction of stable phenotypes in Gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol. 1994;235:386–405. doi: 10.1016/0076-6879(94)35157-0. [DOI] [PubMed] [Google Scholar]
- 7.Duetz W A, Marqués S, Wind B, Ramos J L, van Andel J G. Catabolite repression of the toluene degradation pathway in Pseudomonas putida harboring pWW0 under various conditions of nutrient limitation in chemostat culture. Appl Environ Microbiol. 1996;62:601–606. doi: 10.1128/aem.62.2.601-606.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernández S, de Lorenzo V, Pérez-Martín J. Activation of the transcriptional regulatory XylR of Pseudomonas putida by release of repression between functional domains. Mol Microbiol. 1995;16:205–213. doi: 10.1111/j.1365-2958.1995.tb02293.x. [DOI] [PubMed] [Google Scholar]
- 9.Holtel A, Marqués S, Möhler I, Jakubzik U, Timmis K N. Carbon source-dependent inhibition of xyl operon during expression of the Pseudomonas putida TOL plasmid. J Bacteriol. 1994;176:1773–1776. doi: 10.1128/jb.176.6.1773-1776.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hugouvieux-Cotte-Pattat N, Köhler T, Rekik M, Harayama S. Growth-phase-dependent expression of the Pseudomonas putida TOL plasmid pWW0 catabolic genes. J Bacteriol. 1990;172:6651–6660. doi: 10.1128/jb.172.12.6651-6660.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Köhler T, Harayama S, Ramos J-L, Timmis K N. Involvement of Pseudomonas putida RpoN ς factor in regulation of various metabolic functions. J Bacteriol. 1989;171:4326–4333. doi: 10.1128/jb.171.8.4326-4333.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marqués S, Holtel A, Timmis K N, Ramos J L. Transcriptional induction kinetics from the promoters of the catabolic pathways of TOL plasmid pWW0 of Pseudomonas putida for metabolism of aromatics. J Bacteriol. 1994;176:2517–2524. doi: 10.1128/jb.176.9.2517-2524.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 14.Pérez-Martín J, de Lorenzo V. In vitro activities of an N-terminal truncated form of XylR, a ς54-dependent transcriptional activator of Pseudomonas putida. J Mol Biol. 1996;258:575–587. doi: 10.1006/jmbi.1996.0270. [DOI] [PubMed] [Google Scholar]
- 15.Ramos J L, Marqués S, Timmis K N. Transcriptional control of the Pseudomonas TOL plasmid catabolic operons is achieved through an interplay of host factors and plasmid encoded regulators. Annu Rev Microbiol. 1997;51:341–373. doi: 10.1146/annurev.micro.51.1.341. [DOI] [PubMed] [Google Scholar]
- 16.Sawyer M H, Baumann P, Berman S M, Canovas J L. Pathways of D-fructose catabolism in Pseudomonas. Arch Microbiol. 1977;112:49–55. doi: 10.1007/BF00446653. [DOI] [PubMed] [Google Scholar]
- 17.Sze C C, Moore T, Shingler V. Growth-phase dependent transcription of the ς54-dependent Po promoter controlling the Pseudomonas-derived (methyl)phenol dmp operon of pVI150. J Bacteriol. 1996;178:3727–3735. doi: 10.1128/jb.178.13.3727-3735.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sze C C, Shingler V. The alarmone (p)ppGpp mediates physiological-responsive control at the ς54-dependent Po promoter. Mol Microbiol. 1999;31:1217–1228. doi: 10.1046/j.1365-2958.1999.01264.x. [DOI] [PubMed] [Google Scholar]