Abstract
Objective:
To summarize literature examining the prevalence, impact, and trajectories of sleep disturbance in cardiac rehabilitation (CR) patients and discuss how CR programs may incorporate targeted evaluation and interventions to promote sleep health.
Review Methods:
Narrative review of literature examining the prevalence of sleep disturbance in CR patients, the effects of sleep disturbance on CR outcomes, and trajectories of sleep disturbance in CR.
Summary:
Sleep disturbance is prevalent in CR patient populations and is related to clinical and functional outcomes. Sleep may be an important biobehavioral process to target in CR to improve important patient outcomes and achieve secondary prevention goals.
Keywords: Sleep, Insomnia, Sleep Apnea, Cardiac Rehabilitation
CONDENSED ABSTRACT
Sleep disturbance is common in those attending cardiac rehabilitation and is related to clinical and functional outcomes. Cardiac rehabilitation programs have the potential to improve sleep health with targeted evaluation and intervention.
The weeks and months following a cardiac event are a critical window of opportunity to improve clinical and functional outcomes. Cardiac rehabilitation (CR) is a multifaceted and multidisciplinary form of secondary prevention that effectively contributes to cardiovascular disease (CVD) risk reduction in people living with coronary artery disease and heart failure.1–4 Since its inception, CR has evolved from a program that primarily focuses on supporting acute recovery through early post-event ambulation and physical activity, to a multicomponent program that includes patient assessment, CVD risk factor management, psychosocial management, nutritional counseling, physical activity counseling, and exercise training.5 These components support the broad aims of promoting engagement in healthy behaviors and reducing cardiovascular risk and disability.
Sleep is a universal human need, an important biological process, and a modifiable health behavior that can further improve the functional impact of CR. Sleep disturbance is common in people living with CVD, possibly more so following a cardiac event.6–8 Sleep disturbance may predate and be exacerbated by a cardiac event or may be newly precipitated by a cardiac event; the etiology is likely multifactorial.6 During a cardiac hospitalization, and extending into the recovery period, numerous factors may contribute to sleep disruptions that then persist beyond the period of acute recovery.8,9 These factors could include the disease process itself, exacerbation of comorbid conditions, the hospital environment (e.g., ambient light and noise; monitoring of vital signs), disruptions to patterns of rest-activity, medications (e.g., beta blockers, diuretics), and psychological and physical symptoms (e.g., anxiety, pain, dyspnea, nocturia). Although the current model of CR care provides an excellent infrastructure for promoting sleep health, such content is often absent, potentially missing an important opportunity to improve this vital aspect of health and well-being.
In this review, we first provide an overview of sleep and its links to clinically relevant outcomes. We then summarize research examining the prevalence of different types of sleep disturbance in CR, the effects of sleep disturbance on CR outcomes, and trajectories of change in sleep disturbance during CR. We conclude by discussing how the existing model of CR care may lead to improvements in sleep disturbance and how CR programs may address sleep disturbance and promote sleep health.
REVIEW OF RELEVANT LITERATURE
CLARIFYING SLEEP-RELATED TERMINOLOGY AND LINKS TO CLINICAL AND FUNCTIONAL OUTCOMES
Sleep is a neurobehavioral state of reduced awareness of and engagement with the environment, and is further characterized by its periodic occurrence and rapid reversibility. Sleep is not adequately measured by any one metric, but rather, is characterized by multiple dimensions. Recently, there have been movements within sleep medicine to approach sleep from a health-promotion framework; this framework has value in other models of care, including CR.10 Sleep health is defined as a “multidimensional pattern of sleep-wakefulness…that promotes physical and mental well-being.”10 Measurable and empirically-supported dimensions of sleep health include: regularity in sleep-wake times, satisfaction with sleep (also referred to as sleep quality), alertness during waking, timing of sleep, sleep efficiency, and sleep duration. Each dimension is a continuum, with potential for behavioral modification and improvement. Sleep duration and sleep quality are among the dimensions of sleep studied most commonly in association with cardiovascular outcomes. Sleep duration is the total amount of sleep obtained during a 24 hr period. Current guidelines recommend adults 26–64 yr obtain 7–9 hr of sleep per night and adults ≥ 65 yr obtain 7–8 hr/night.11 Shorter or longer sleep duration has been linked to adverse outcomes including greater risk of CVD mortality,12,13 coronary heart disease,13 stroke,13 and dementia.14 Sleep quality is defined variably, but can be conceptualized as “one’s satisfaction of the sleep experience, integrating aspects of sleep initiation, sleep maintenance, sleep quantity, and refreshment upon awakening.”15 In a recent meta-analysis of prospective cohort studies, poor sleep quality was associated with coronary heart disease but not mortality or other adverse CVD outcomes.16
The above-described conceptualization of multidimensional sleep health reflects an approach wherein the goal is to improve sleep health for individuals who have room for improvement on any dimension (e.g., poor sleep quality; excessive daytime sleepiness and napping). Within the field of sleep medicine, and other specialty areas including cardiology, sleep has more traditionally been approached from a disease-based framework, with focus on diagnosis and management sleep disorders and understanding how sleep disturbance adversely impacts health outcomes. Sleep disorders are generally classified into 6–7 major categories: insomnia, sleep-related breathing disorders, central disorders of hypersomnolence, circadian rhythm sleep-wake disorders, parasomnias, sleep-related movement disorders, and other sleep disorders.17 Examples of sleep disorders, characteristic symptoms, diagnostic considerations, and treatment approaches are outlined in Table 1. Sleep disturbance is a broader term that can be used to refer to difficulties with one of more sleep dimensions that may or may not meet diagnostic criteria for a sleep disorder.
Table 1.
Overview of common sleep disorders, characteristic symptoms, and screening/diagnostic tools
| Categories of sleep disorders | Example disorders | Characteristic symptoms | Screening/Diagnostic tools | Treatment approaches |
|---|---|---|---|---|
| Insomnia | Chronic Insomnia Disorder, Short-Term Insomnia Disorder | Difficulties falling asleep or staying asleep (despite adequate opportunity) with associated daytime impairment | Clinical interview; rating scales (e.g., Insomnia Severity Index, Pittsburgh Sleep Quality Index); sleep diaries72 | Psychological and behavioral interventions (e.g., Cognitive Behavioral Therapy for Insomnia, Brief Behavioral Treatment for Insomnia), pharmacological management72 |
| Sleep-related breathing disorders | OSA, Central Sleep Apnea | Excessive daytime sleepiness, snoring, pauses in breathing or gasping during sleep, awakening with dry mouth or headache | Rating scales (e.g., STOP-BANG, Epworth Sleepiness Scale) for screening with PSG or HSAT for diagnosis73 | PAP therapy, psychological and behavioral interventions (i.e., weight loss, exercise, positional therapy, avoidance of alcohol and sedative hypnotics, motivational interviewing), oral appliances, surgical interventions73, UAS therapy |
| Central disorders of hypersomnolence | Narcolepsy, Idiopathic Hypersomnia, Hypersomnia due to a Medical Disorder | Daytime sleepiness not attributable to a different sleep disorder | Clinical interview; objective assessment (e.g., PSG, rating scales; MSLT, maintenance of wakefulness test, immunoreactivity assay; actigraphy); sleep diaries74 | Pharmacological management,74 psychological and behavioral interventions75 |
| Circadian rhythm sleep-wake disorders | Delayed Sleep-Wake Phase Disorder, Advanced Sleep-Wake Phase Disorder, Shift Work Disorder | Sleep disruption (often insomnia or daytime sleepiness) primarily stemming from misalignment between endogenous circadian rhythm and sleep-wake patterns due to external/ environmental demands (e.g., occupational or social schedules) | Clinical interview; sleep diaries; actigraphy76 | Strategically timed melatonin, light therapy, psychological and behavioral interventions77 |
| Parasomnias | Confusional Arousals, Sleepwalking, REM Sleep Behavior Disorder, Nightmare Disorder | Behaviors such as sleep walking, sleep talking/yelling, distressing dreams | Clinical interview; rating scales; sleep diaries; PSG with video recording and additional EEG derivations78,79 | Pharmacological management, behavioral psychological and behavioral interventions (e.g., Imagery Rehearsal Therapy for nightmares)78,79 |
| Sleep-related movement disorders | Restless Legs Syndrome, Periodic Limb Movement Disorder | Movements occurring during sleep | Clinical interview; rating scales; possible PSG80 | Pharmacological management80 |
Abbreviations: EEG, electroencephalography; HSAT, home sleep apnea testing; MSLT, multiple sleep latency test; OSA, obstructive sleep apnea; PAP, positive airway pressure; PSG, polysomnography; REM, rapid eye movement; UAS, upper airway stimulation
In people with CVD, insomnia and sleep-related breathing disorders, such as sleep apnea (obstructive sleep apnea [OSA] or central sleep apnea), are common. Observational studies indicate that OSA is associated with coronary and cerebrovascular morbidity and mortality.18 Pathophysiological process affecting cardiovascular function (e.g., increased sympathetic nervous system activity, inflammation, endothelial dysfunction) and altered metabolism (e.g., decreased insulin sensitivity, increased leptin resistance, increased lipolysis, and impaired lipoprotein clearance dysfunction) are thought to be primary pathways linking OSA to outcomes like increased blood pressure, pulmonary hypertension, arrhythmias, coronary artery disease, and CVD mortality.18 Clinical practice guidelines recommend positive airway pressure (PAP) therapy for people with OSA who experience sleepiness, reduced quality of life, and/or hypertension.19 Clinical trials of PAP therapy for OSA have been challenging because of heterogenous diagnostic phenotypes and low adherence to treatment. A recent meta-analysis identified that PAP-treated OSA is not associated with lowered risk of major cardiovascular events or CVD mortality.20 However, PAP therapy is associated with other important outcomes including reduced daytime sleepiness21 and improved sleep- and health-related quality of life,19,21 and social functioning.22
Insomnia symptoms refer to difficulties with sleep initiation, maintenance, and/or early morning awakenings. When these symptoms occur despite adequate opportunity for sleep and are accompanied by dissatisfaction with sleep and related daytime impairments or distress, they may reach the threshold for insomnia disorder. An earlier meta-analysis of prospective studies identified a 45% increased risk of CVD morbidity and/or mortality for those reporting insomnia symptoms at baseline.23 A more recent meta-analysis of prospective cohort studies identified that experiences of non-restorative sleep and difficulty falling asleep were associated with increased risk of all-cause mortality and CVD mortality; the formal diagnosis of insomnia disorder and symptoms of difficulty staying asleep and early morning awakenings were not associated with CVD mortality.24 These variable findings may partly be due to variable definitions and diagnostic approaches. Increased sympathetic activity and/or systemic inflammation, and hypothalamic-pituitary-adrenal axis dysregulation are proposed mechanistic pathways linking insomnia to CVD.25 Insomnia symptoms are negatively associated with outcomes such as health-related quality of life,26 cognitive performance,27 and mental health.28 Non-pharmacological insomnia interventions yield at least mild improvements in outcomes such as quality of life, social functioning, and symptoms of depression, anxiety, and fatigue.29
EVIDENCE THAT CR IS A LOGICAL VENUE FOR IMPROVING SLEEP HEALTH
Considering health behaviors across the 24 hr of the day, including waking and sleep, is increasingly recognized as integral to physical and psychological health and well-being. The 24 hr perspective is highly relevant in CR, where adoption and maintenance of multiple healthy lifestyle behaviors is a primary goal. Like other health behaviors targeted in CR (e.g., physical activity, healthy eating, stress management), sleep is a foundational biobehavioral process that underlies good health, well-being, and functioning. We propose that CR outcomes may be enhanced by incorporating routine assessment of sleep disturbance and interventions to improve sleep health (see Figure 1). We propose this based on existing literature suggesting that sleep disturbance: 1) is common in individuals attending CR, 2) is related to outcomes that may impact the CR process of care, and 3) does not resolve without targeted intervention for many people attending CR. In the following sections we evaluate the literature in each of these areas; we focus on insomnia symptoms, sleep quality, and sleep apnea as these are the primary sleep parameters that have been empirically evaluated in the context of CR. We then discuss how CR programs may incorporate processes to evaluate and intervene upon sleep
Figure 1.

Figure 1 is adapted from Thomas et al.71 and represents an expanded model of CR in which sleep health is included as a potential target health behavior.
High prevalence of sleep disturbance in CR
Insomnia Symptoms & Sleep Quality.
At enrollment in CR, 50–85% of patients experience poor sleep quality30–32 and during CR up to 49% experience poor sleep quality.33 Anywhere from 39–45% of patients attending CR experience at least mild symptoms of insomnia, 8–14% moderate symptoms, and up to 4% severe symptoms.34–37 As alluded to earlier, the etiology of poor sleep quality and insomnia in CR patients is likely multifactorial; plausible contributors may include medical comorbidities (e.g., benign prostatic hyperplasia, diabetes, chronic pain), mental health symptoms (e.g., depression, anxiety), physical symptoms (e.g., nocturia, pain related to wound healing), medications, and altered daily routines. Because it is unusual for any one contributor to be completely responsible for sleep problems, optimizing an individual’s medical and psychiatric management is as important for sleep problems as it is for CR generally.
Sleep Apnea.
Estimates from screening questionnaires suggest somewhere between 37–80% of individuals in CR are at high risk for OSA.38–40 By comparison, studies using objective assessment methods (e.g., polysomnography, home sleep apnea testing, ECG Holter) estimate that 40–100% of CR patients have at least mild sleep apnea and 33–85% have at least moderate sleep apnea.30,41–44 Participants in CR also experience higher than normal or excessive daytime sleepiness, a common symptom of sleep apnea.41,45
Effects of sleep disturbance on the CR process of care
Insomnia Symptoms & Sleep Quality.
Among CR participants, poor sleep quality has been cross-sectionally associated with higher levels of depression symptoms and lower quality of life;31 insomnia symptoms have been associated with lower pleasant mood, positive affect, and tranquility, and higher levels of fatigue. Interestingly, more severe insomnia symptoms have been associated with greater improvements in mood and tranquility during a CR exercise session.35 Few studies have adopted experimental or longitudinal approaches to examine how sleep quality and insomnia symptoms, and improvements in these domains, impact CR outcomes. Rouleau et al. found that greater improvements in insomnia symptoms were linked to greater posttreatment improvements in symptoms of depression and anxiety, and total cholesterol.36 The specific pathways through which improvements in insomnia symptoms were related to improvements in total cholesterol were not studied, but based on work in the area of sleep restriction and metabolic functioning, the authors speculate that improvements in sleep duration may have occurred with improvements in insomnia symptoms, contributing to improvements in lipid profiles via improvements in glucose metabolism, calorie consumption, or changes in appetite-regulating hormones.36 The potential for reverse causality (i.e., improvements in depression and anxiety leading to improvements in sleep) and confounding roles of statin medications were also acknowledged.36
Sleep Apnea.
CR participants who are at high versus low risk for sleep apnea have differences in physiological and functional performance measures. Cheung and colleagues compared performance on the 6 min walk test in CR participants considered high versus low risk for sleep apnea based on different screening questionnaires.40 Differences in distance walked only emerged in those identified as high versus low risk by the Berlin Questionnaire. Adjusting for sociodemographic and clinical characteristics, individuals at high risk for sleep apnea walked a significantly shorter distance than those at low risk for sleep apnea.40 Loo et al. compared performance on the 6 min walk test in individuals with and without sleep disordered breathing, enrolled in CR.46 Individuals without sleep disordered breathing outperformed those with objectively-diagnosed sleep disordered breathing on the 6 min walk test; however adjusting for age, sex, and waist circumference, these differences were not statistically significant. Hargens et al. compared a number of physiological and functional outcomes among individuals with and without OSA who were beginning CR. Sleep apnea was associated with diminished left ventricular systolic function, including indices of lower cardiac output, stroke volume and cardiac work index; there were no group differences in heart rate, diastolic blood pressure, or performance on the six-min walk test.45 Finally, using a longitudinal design, Yamamoto and colleagues examined the effects of CR on sleep disordered breathing in patients with stable heart failure. A positive, but not statistically significant, correlation was observed between reductions in apnea-hypopnea index (AHI), an index commonly used to indicate apnea severity, and reduction in the minute ventilation to oxygen uptake slope.48
Trajectories of sleep disturbance during CR
Insomnia Symptoms & Sleep Quality.
Rouleau and colleagues37 identified that individuals experienced a 23% reduction in insomnia symptom severity from baseline to completion of CR. Risom and colleagues33 compared changes in sleep quality in patients with atrial fibrillation who underwent ablation and were randomized to CR versus usual care; improvements in sleep quality were evidenced in both groups. In the CR group the proportion of people reporting poor sleep quality decreased from 87% at mo 1 to 58% at mo 6; in the usual care group this proportion decreased from 82% to 52%.33 There were no significant group differences in sleep quality scores at 6 mo. Collectively, these results suggest that insomnia symptoms and sleep quality improve over time for those in CR and for those receiving usual care. Mediational pathways were not assessed in the above-described studies; however, improvements in cardiac, depressive, and anxiety symptoms have been identified as potential pathways through which sleep quality may improve over the course of CR.
Sleep Apnea.
Physical activity and exercise can contribute to improvements in OSA, with proposed mechanisms including decreased rostral fluid redistribution, stabilization of chemoreceptor sensitivity, improvements in nasal resistance and strength of pharyngeal dilator muscles, and weight loss.49 Select studies have examined whether CR affects sleep apnea, primarily defined by AHI, and other sleep outcomes in patients with sleep apnea. Hupin and colleagues found that AHI decreased pre-post CR, but only for those with severe sleep apnea.50 In patients with coronary artery disease and sleep apnea, AHI was significantly improved after 1 and 6 mo for those who completed CR compared to controls;51,52 group differences in other sleep parameters, however, were not observed.51,52 Studies in people living with heart failure have produced somewhat mixed findings. In one sample, CR was associated with increases in the amount of stage 3 and 4 sleep (considered to be restorative stages of sleep) as well as decreases in number of arousals in patients with OSA; in those with central sleep apnea, neither improvements in central sleep apnea severity nor improvements in sleep architecture were observed.53 In another study, those with heart failure and sleep disordered breathing who completed CR demonstrated significant decreases in AHI and central sleep apneas at 6 mo, whereas the control group did not experience such changes.48 Though limited evidence indicates sleep apnea severity improves over the course of CR, findings in this area are inconsistent and not sufficient to conclude that sleep apnea severity improves in response to CR alone. Such findings further support the need to integrate strategies to address sleep disturbance in CR.
DISCUSSION
Sleep disturbance is common and potentially consequential for individuals in CR. Findings from existing literature highlight that CR programs may contribute to improvements in sleep disturbance; however, they also suggest that CR does not consistently result in better sleep outcomes compared to usual care and that up to half of individuals attending CR continue to experience poor sleep quality. Targeted efforts to evaluate and intervene on sleep may augment improvements in sleep disturbance, and improve sleep health, for a greater number of CR participants. We conclude by summarizing areas for future research and offering future directions for evaluating and intervening to promote sleep health in CR.
GAPS IN THE LITERATURE & AREAS FOR FURTHER RESEARCH
The high prevalence of sleep disturbance in people undergoing CR is established. Nascent research suggests improvements in sleep disturbance are associated with more positive CR outcomes, such as greater improvements in symptoms of depression and anxiety. In broader populations sleep disturbance is associated with lower levels of physical activity,54 sleep restriction is associated with increased food intake, and short sleep duration is associated with poorer dietary quality.55 Sleep disturbance can also exacerbate physical symptoms such as pain and fatigue which, for some, may result in reduced adherence to therapeutic regimens.32,56
CR programs target behaviors and experiences (e.g., physical activity, mood and stress, diet/nutrition) that are bidirectionally related to sleep, and it is plausible that gains in these areas may contribute to improvements in sleep; however, the pathways through which CR improves sleep health are not currently known. Future research is needed to clearly define the pathways through which CR improves sleep health. Importantly, research also suggests that not everyone’s sleep health improves over the course of CR and that CR does not consistently improve sleep outcomes to a greater degree than usual care. Longitudinal studies and randomized controlled trials will provide useful insights into the extent to which sleep health improves over the course CR (with and without targeted intervention), for whom, and through what mechanisms.
FUTURE DIRECTIONS: IMPROVING SLEEP HEALTH IN CR THROUGH EVALUATION AND INTERVENTION
Sleep is increasingly recognized as essential to health and well-being. For instance, a goal of the Healthy People 2030 initiative is to “improve health, productivity, well-being, quality of life, and safety by helping people get enough sleep.”57 Relatedly, a position statement by the American Academy of Sleep Medicine (AASM) highlights the need for greater focus on sleep health in education, clinical practice, hospitals and long-term care, public health promotion, and the workplace.58 Ideally, the growing recognition of the importance of sleep to health and calls for increased focus on sleep health across various socioecological contexts will lead to greater public awareness of sleep health and detection of sleep disorders by providers across disciplines and care settings. Research is needed to determine optimal methods for integrating sleep assessment and implementing sleep therapeutics into the CR model of care. Given the considerable variability in structure and resources available to different CR programs, successful methods of implementation are likely to vary by CR program.
In general, a stepped care approach similar to those adopted for physical and mental health conditions (e.g., pain, depression) may be useful for integrating sleep therapeutics into CR (for example refer to Figure 2). Stepped care models align with population-based care approaches and prioritize screening and assessment to connect individuals with the treatment that is most appropriate and effective yet least resource intensive.
Figure 2.
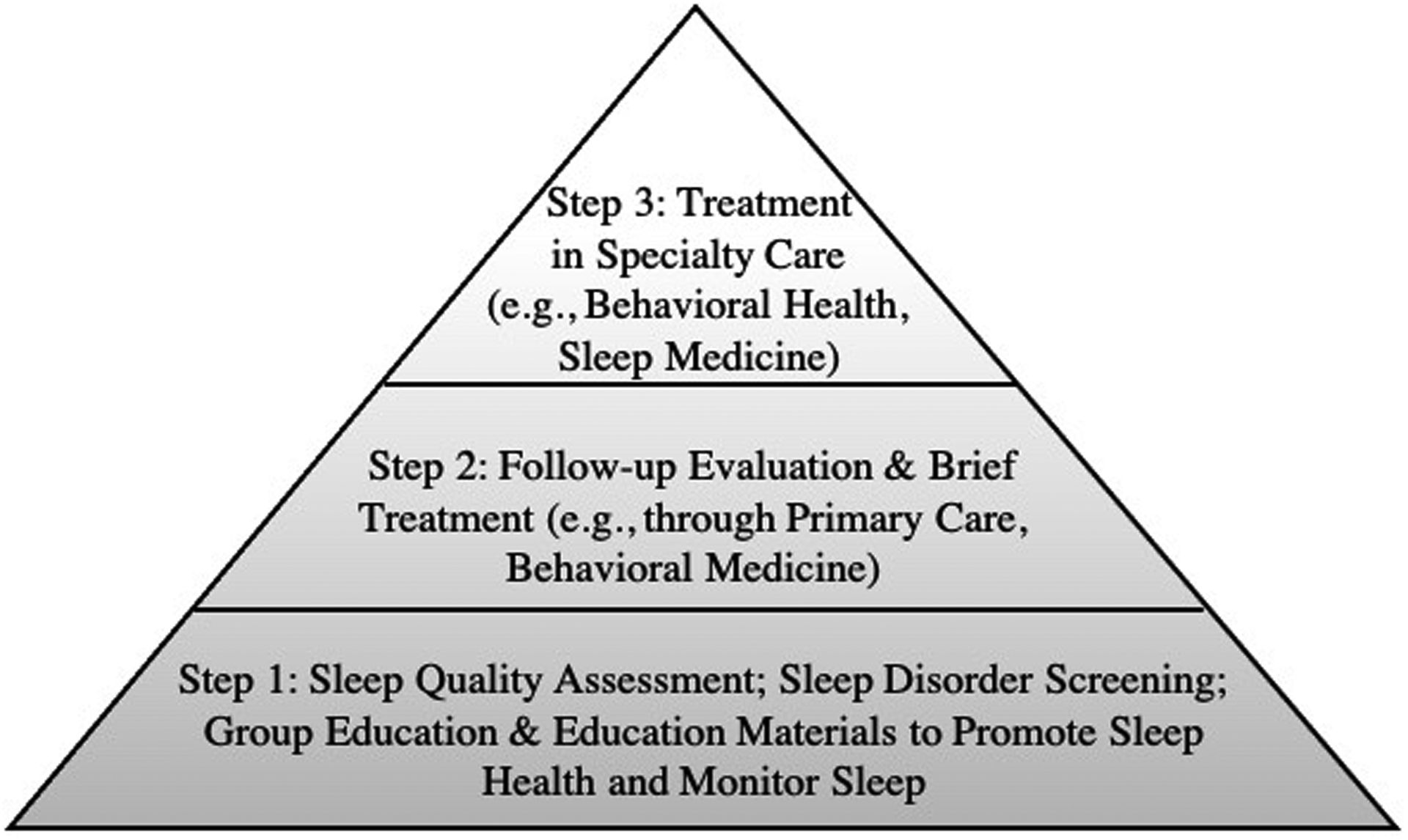
Figure 2 presents an example stepped care model for addressing sleep in CR.
In CR, the first step, applicable to all CR participants, could involve routine brief assessment of sleep quality, screening for sleep disorders that are highly prevalent in CR patients, and provision of educational and monitoring materials to promote sleep health. Based on recommendations from a recent systematic review and meta-analysis,47 the Pittsburgh Sleep Quality Index could be administered upon CR enrollment to assess sleep quality (https://www.sleep.pitt.edu/instruments/), the Insomnia Severity Index could be used to screen for insomnia (https://eprovide.mapi-trust.org/instruments/insomnia-severity-index), and the STOP-BANG could be administered to screen for sleep apnea (http://www.stopbang.ca/osa/screening.php). Each of these self-report measures can be relatively quickly and easily completed by patients and scored by providers; measures may also be bypassed if diagnostic information is already known (e.g., patient with sleep apnea is using PAP therapy). When time and resources are limited, an abbreviated version of the Insomnia Severity Index (59,60) or question about sleep quality (e.g., Sleep Quality Scale61) could be administered to inform the need for follow-up. Routine screening for sleep disorders in CR aligns with guidelines and position statements developed for cardiac patients by international professional organizations (e.g.,62–64). Given the relevance of sleep to CR foci (e.g., physical activity, stress management, eating behaviors), CR providers may see value in incorporating information from sleep assessments/screenings into their CR treatment planning and recommendations/feedback for specialty care coordination.
Within CR, group education sessions and educational or self-help materials that address cross-cutting strategies for improving sleep health (e.g., maintaining a consistent and appropriately timed sleep-wake schedule, limiting time awake in bed and daytime napping, avoiding consumption of caffeine in the afternoon/evenings and alcoholic beverages before bed) may be appropriate for delivery to all CR participants. This approach would be consistent with current recommended interventions for psychosocial core components of CR and because behavioral sleep interventions follow similar principles to other health behavior change interventions already enacted as part of CR, or proposed for inclusion in CR, they may synergize with those other interventions.5,65 It may also be useful for CR participants to complete sleep diaries as a form of self-monitoring. Sleep diaries are logs used to record sleep-related information such as sleep-wake times, time to fall asleep, number of awakenings and time awake in the middle of the night, and sleep quality; diaries can also be used to record information on sleep-related experiences such as use of PAP therapy, experience of nightmares, use of sleep aids, or other symptom experiences (e.g., pain, nocturia). Sleep diaries are similar to other daily logs (e.g., exercise logs, food logs) maintained by patients in CR; they can be easily integrated into CR materials and curricula, are useful for informing treatment planning, and have the potential to result in improvements in sleep.
The second step in the stepped care model would be applicable for those who report poor sleep quality or screen positive for sleep disorders assessed at intake. For these individuals, follow-up evaluation and potential brief treatment for sleep disorders would be considered; CR staff could recommend to the patient and their referring provider and/or primary care provider a referral to sleep medicine specialty care for assessment of sleep apnea or other sleep disorders. Follow-up evaluation and brief treatment could also occur in primary care or through behavioral medicine services (such as those embedded within cardiac rehabilitation). For those with insomnia, brief treatment may involve behavioral interventions such as Cognitive Behavioral Therapy for Insomnia (CBT-I), the recommended first line treatment for insomnia that is efficacious for people living with cardiovascular conditions (e.g., heart failure, hypertension) or Brief Behavioral Treatment for Insomnia (BBTI). For those diagnosed with sleep apnea and prescribed PAP therapy, brief treatment may involve motivational enhancement to promote adherence to PAP therapy. Non-sleep specialists can be trained to deliver select behavioral sleep interventions (e.g., CBT-I).66 Digital therapeutics (e.g., web-based programs, phone applications) and bibliotherapy (e.g., self-help books) are also likely to be useful particularly for programs with limited access to sleep medicine providers. Digital therapeutics and bibliotherapy are both efficacious for improving sleep disturbances such as insomnia.67 Among these, Somryst® is an asynchronous mobile CBT-I application and is the first prescription digital therapeutic authorized by the Food and Drug Administration to treat adults with chronic insomnia.
The third step in the stepped care model would be applicable for those who do not respond to brief treatment or otherwise have more complicated presentations requiring treatment in specialty care, such as care a sleep medicine clinic or through behavioral health. Treatment in this level of care may include more specialized or intensive behavioral interventions and/or pharmacotherapy. Responding to the increasing demand for and shortage of sleep medicine specialists, the AASM is investing substantially in expanding access to sleep medicine services such as through the growth and evolution of sleep training and fellowship programs, promotion of multidisciplinary collaboration, use of consumer technologies, and expansion of sleep medicine via telemedicine.68,69 Other organizations, such as the Veterans Health Administration, are similarly endeavoring to improve access and expand sleep telemedicine.70 Also important to note is that stepped care and related integrated care models have the potential to improve efficiency of service delivery and decrease burden on overtaxed tertiary care providers by reducing referrals to specialty sleep medicine providers that can be appropriately addressed through lower levels of care.
SUMMARY
Sleep disturbance is pervasive in people undergoing CR.32,37 The empirical and clinical consideration of sleep health in CR has been relatively limited despite its relevance to cardiovascular health and biopsychosocial functioning.32,37 By evaluating and intervening on sleep health in CR we might enhance the CR therapeutic model and expand the notion of what vulnerabilities are key to address following a cardiac event.
ACKNOWLEDGMENTS
The views expressed in this article are those of the authors and do not necessarily represent the position or policy of the Department of Veterans Affairs or the United States Government. This work is not subject to US copyright as several of the authors of this manuscript are employees of the U.S. Government.
Sources of Support:
This material is the result of work supported with resources and the use of facilities at the Veterans Integrated Service Network 4 Mental Illness Research, Education, and Clinical Center at the VA Pittsburgh Healthcare System, Pittsburgh, Pennsylvania. Dr. Tighe is supported by Career Development/Capacity Building Award Number IK2 RX003393 from the United States (U.S.) Department of Veterans Affairs Rehabilitation R&D (Rehab RD) Service. Dr. Forman receives funding support through National Institute of Aging grants R01AG060499, R01AG058883, and P30AG024827. Dr. Buysse receives grant support from NIH grants (P50 DA046346, AG047319), PCORI (CER-2018C2-13262), AHRQ, and the VA.
Footnotes
Conflicts of Interest: Drs. Tighe, Beehler, Weiner, and Forman have no conflicts to report. Over the past 3 years, Dr. Buysse has served as a paid consultant to National Cancer Institute, Pear Therapeutics (which markets the Somryst® sleep app), Sleep Number, Idorsia, and Weight Watchers International. Dr. Buysse is an author of the Pittsburgh Sleep Quality Index, Pittsburgh Sleep Quality Index Addendum for PTSD (PSQI-A), Brief Pittsburgh Sleep Quality Index (B-PSQI), Daytime Insomnia Symptoms Scale, Pittsburgh Sleep Diary, Insomnia Symptom Questionnaire, and RU_SATED (copyrights held by University of Pittsburgh). These instruments have been licensed to commercial entities for fees. He is also co-author of the Consensus Sleep Diary (copyright held by Ryerson University), which is licensed to commercial entities for a fee.
REFERENCES
- 1.Antman EM et al. ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction—Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). J. Am. Coll. Cardiol 2004;44:671–719. [DOI] [PubMed] [Google Scholar]
- 2.Braunwald E et al. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction--summary article: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the Management of Patients With Unstable Angina). J. Am. Coll. Cardiol 2002;40:1366–1374. [DOI] [PubMed] [Google Scholar]
- 3.Committee Members et al. ACC/AHA 2002 Guideline Update for the Management of Patients With Chronic Stable Angina—Summary Article: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Chronic Stable Angina). Circulation 2003;107:149–158 (2003). [DOI] [PubMed] [Google Scholar]
- 4.WRITING COMMITTEE MEMBERS et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult—Summary Article: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): Developed in Collaboration With the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: Endorsed by the Heart Rhythm Society. Circulation 2005;112:1825–1852. [DOI] [PubMed] [Google Scholar]
- 5.Balady GJ et al. Core Components of Cardiac Rehabilitation/Secondary Prevention Programs: 2007 Update: A Scientific Statement From the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. J. Cardiopulm. Rehabil. Prev 2007;27:121–129. [DOI] [PubMed] [Google Scholar]
- 6.Redeker NS, Ruggiero J & Hedges C Patterns and predictors of sleep pattern disturbance after cardiac surgery. Res. Nurs. Health 2004;27:217–224 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Alexander M et al. The National Veteran Sleep Disorder Study: Descriptive Epidemiology and Secular Trends, 2000–2010. Sleep 2016;39:1399–1410 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong J-Y, Zhang Y-H & Qin L-Q Obstructive sleep apnea and cardiovascular risk: Meta-analysis of prospective cohort studies. Atherosclerosis 2013;229:489–495. [DOI] [PubMed] [Google Scholar]
- 9.Redeker NS Sleep in Acute Care Settings: An Integrative Review. J. Nurs. Scholarsh 2000;32:31–38. [DOI] [PubMed] [Google Scholar]
- 10.Liao W-C, Huang C-Y, Huang T-Y & Hwang S-L A Systematic Review of Sleep Patterns and Factors That Disturb Sleep After Heart Surgery. J. Nurs. Res 19, 275–288 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Buysse DJ Sleep health: can we define it? Does it matter? Sleep 37, 9–17 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirshkowitz M et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health 1, 40–43 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Krittanawong C et al. Association between short and long sleep durations and cardiovascular outcomes: a systematic review and meta-analysis. Eur. Heart J. Acute Cardiovasc. Care 8, 762–770 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Jiawei Yin et al. Relationship of Sleep Duration With All-Cause Mortality and Cardiovascular Events: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. J. Am. Heart Assoc 6, e005947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan L, Xu W, Cai Y, Hu Y & Wu C Sleep Duration and the Risk of Dementia: A Systematic Review and Meta-analysis of Prospective Cohort Studies. J. Am. Med. Dir. Assoc 20, 1480–1487.e5 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Kline C Sleep Quality. in Encyclopedia of Behavioral Medicine (eds. Gellman MD & Turner JR) 1811–1813 (Springer, 2013). [Google Scholar]
- 17.Kwok Chun Shing et al. Self-Reported Sleep Duration and Quality and Cardiovascular Disease and Mortality: A Dose-Response Meta-Analysis. J. Am. Heart Assoc 7, e008552 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.International Classification of Sleep Disorders. (American Academy of Sleep Medicine, 2014). [Google Scholar]
- 19.Drager Luciano F, McEvoy R. Doug, Barbe Ferran, Lorenzi-Filho Geraldo, & Redline Susan. Sleep Apnea and Cardiovascular Disease. Circulation 136, 1840–1850 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patil SP et al. Treatment of Adult Obstructive Sleep Apnea with Positive Airway Pressure: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med 15, 335–343 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu J et al. Association of Positive Airway Pressure With Cardiovascular Events and Death in Adults With Sleep Apnea: A Systematic Review and Meta-analysis. JAMA 318, 156–166 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao YY et al. Effect of Continuous Positive Airway Pressure Treatment on Health-Related Quality of Life and Sleepiness in High Cardiovascular Risk Individuals With Sleep Apnea: Best Apnea Interventions for Research (BestAIR) Trial. Sleep 40, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis EF et al. Impact of continuous positive airway pressure and oxygen on health status in patients with coronary heart disease, cardiovascular risk factors, and obstructive sleep apnea: A Heart Biomarker Evaluation in Apnea Treatment (HEARTBEAT) analysis. Am. Heart J 189, 59–67 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sofi F et al. Insomnia and risk of cardiovascular disease: a meta-analysis. Eur. J. Prev. Cardiol 21, 57–64 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Ge L et al. Insomnia and risk of mortality from all-cause, cardiovascular disease, and cancer: Systematic review and meta-analysis of prospective cohort studies. Sleep Med. Rev 48, 101215 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Javaheri S & Redline S Insomnia and Risk of Cardiovascular Disease. Chest 152, 435–444 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kyle SD, Morgan K & Espie CA Insomnia and health-related quality of life. Sleep Med. Rev 14, 69–82 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Wardle-Pinkston S, Slavish DC & Taylor DJ Insomnia and cognitive performance: A systematic review and meta-analysis. Sleep Med. Rev 48, 101205 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Hertenstein E et al. Insomnia as a predictor of mental disorders: A systematic review and meta-analysis. Sleep Med. Rev 43, 96–105 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Benz F et al. The efficacy of cognitive and behavior therapies for insomnia on daytime symptoms: A systematic review and network meta-analysis. Clin. Psychol. Rev 80, 101873 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Juskiene A, Podlipskyte A, Bunevicius A & Varoneckas G Type D Personality and Sleep Quality in Coronary Artery Disease Patients With and Without Obstructive Sleep Apnea: Mediating Effects of Anxiety and Depression. Int. J. Behav. Med 25, 171–182 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Banack HR et al. The association between sleep disturbance, depressive symptoms, and health-related quality of life among cardiac rehabilitation participants. J. Cardiopulm. Rehabil. Prev 34, 188–194 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Risom SS, Fevejle Cromhout P, Overgaard D, Hastrup Svendsen J & Kikkenborg Berg S Effect of Rehabilitation on Sleep Quality After Ablation for Atrial Fibrillation: Data From a Randomized Trial. J. Cardiovasc. Nurs 33, 261–268 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurose S et al. Association of locomotive activity with sleep latency and cognitive function of elderly patients with cardiovascular disease in the maintenance phase of cardiac rehabilitation. J. Cardiol 73, 530–535 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Horsley KJ et al. Insomnia symptoms and heart rate recovery among patients in cardiac rehabilitation. J. Behav. Med 39, 642–651 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Rouleau CR et al. The Association Between Insomnia Symptoms and Mood Changes During Exercise Among Patients Enrolled in Cardiac Rehabilitation. J. Cardiopulm. Rehabil. Prev 35, 409–416 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Rouleau CR, Toivonen K, Aggarwal S, Arena R & Campbell TS The association between insomnia symptoms and cardiovascular risk factors in patients who complete outpatient cardiac rehabilitation. Sleep Med. 32, 201–207 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Da Costa D et al. Prevalence and Determinants of Insomnia After a Myocardial Infarction. Psychosomatics 58, 132–140 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Sharma S & Parker AT Prevalence of obstructive sleep apnea in a patient population undergoing cardiac rehabilitation. J. Cardiopulm. Rehabil. Prev 31, 188–192 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Sert-Kuniyoshi FH et al. Screening for obstructive sleep apnea in early outpatient cardiac rehabilitation: feasibility and results. Sleep Med. 12, 924–927 (2011). [DOI] [PubMed] [Google Scholar]
- 41.Cheung Y-Y, Loo G, Gay GMW, Tay H-Y & Lee C-H Screening questionnaires for sleep-disordered breathing and six-minute walk test in patients attending cardiac rehabilitation. Int. J. Cardiol 207, 20–22 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Skobel E et al. Risk factors for, and prevalence of, sleep apnoea in cardiac rehabilitation facilities in Germany: The Reha-Sleep registry. Eur. J. Prev. Cardiol 22, 820–830 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Hupin D et al. Obstructive Sleep Apnea in Cardiac Rehabilitation Patients. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med 14, 1119–1126 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fox H et al. Prevalence of Sleep-Disordered Breathing and Patient Characteristics in a Coronary Artery Disease Cohort Undergoing Cardiovascular Rehabilitation: J. Cardiopulm. Rehabil. Prev 36, 421–429 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Hargens TA, Aron A, Newsome LJ, Austin JL & Shafer BM Effects of obstructive sleep apnea on hemodynamic parameters in patients entering cardiac rehabilitation. J. Cardiopulm. Rehabil. Prev 35, 181–185 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Marzolini S, Sarin M, Reitav J, Mendelson M & Oh P Utility of Screening for Obstructive Sleep Apnea in Cardiac Rehabilitation. J. Cardiopulm. Rehabil. Prev 36, 413–420 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Loo G et al. Sleep-disordered Breathing in Cardiac Rehabilitation: Prevalence, Predictors, and Influence on the Six-Minute Walk Test. Heart Lung Circ. 25, 584–591 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto U et al. Six-Month Aerobic Exercise Training Ameliorates Central Sleep Apnea in Patients With Chronic Heart Failure. J. Card. Fail 13, 825–829 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Javaheri S et al. Sleep Apnea. J. Am. Coll. Cardiol 69, 841–858 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hupin D et al. Obstructive Sleep Apnea in Cardiac Rehabilitation Patients. J. Clin. Sleep Med 14, 1119–1126 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mendelson M et al. Long-term effects of cardiac rehabilitation on sleep apnea severity in patients with coronary artery disease. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med 16, 65–71 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mendelson M et al. Effects of exercise training on sleep apnoea in patients with coronary artery disease: a randomised trial. Eur. Respir. J 48, 142–150 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Ueno LM et al. Effects of exercise training in patients with chronic heart failure and sleep apnea. Sleep 32, 637–647 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kline CE The Bidirectional Relationship Between Exercise and Sleep: Implications for Exercise Adherence and Sleep Improvement. Am. J. Lifestyle Med 8, 375–379 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.St-Onge M-P et al. Sleep Duration and Quality: Impact on Lifestyle Behaviors and Cardiometabolic Health: A Scientific Statement From the American Heart Association. Circulation 134, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith MT & Haythornthwaite JA How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev 8, 119–32 (2004). [DOI] [PubMed] [Google Scholar]
- 57.Healthy People 2030. | health.gov. https://health.gov/healthypeople.
- 58.Ramar K et al. Sleep is essential to health: an American Academy of Sleep Medicine position statement. J. Clin. Sleep Med 17, 2115–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thakral M, Von Korff M, McCurry SM, Morin CM & Vitiello MV ISI-3: evaluation of a brief screening tool for insomnia. Sleep Med. 82, 104–109 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kraepelien M et al. A very brief self-report scale for measuring insomnia severity using two items from the Insomnia Severity Index - development and validation in a clinical population. Sleep Med. 81, 365–374 (2021). [DOI] [PubMed] [Google Scholar]
- 61.Snyder E, Cai B, DeMuro C, Morrison MF & Ball W A New Single-Item Sleep Quality Scale: Results of Psychometric Evaluation in Patients With Chronic Primary Insomnia and Depression. J. Clin. Sleep Med 14, 1849–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Le Grande MR et al. Screening for obstructive sleep apnoea in cardiac rehabilitation: A position statement from the Australian Centre for Heart Health and the Australian Cardiovascular Health and Rehabilitation Association. Eur. J. Prev. Cardiol 23, 1466–1475 (2016). [DOI] [PubMed] [Google Scholar]
- 63.EHRA Scientific Committee Task Force & ESC Scientific Document Group. European Heart Rhythm Association (EHRA)/European Association of Cardiovascular Prevention and Rehabilitation (EACPR) position paper on how to prevent atrial fibrillation endorsed by the Heart Rhythm Society (HRS) and Asia Pacific Heart Rhythm Society (APHRS). EP Eur. 19, 190–225 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yeghiazarians Y et al. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation (2021). [DOI] [PubMed] [Google Scholar]
- 65.Ades PA & Savage PD The Treatment of Obesity in Cardiac Rehabilitation: A Review And Practical Recommendations. J. Cardiopulm. Rehabil. Prev 41, 295–301 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alessi C et al. Cognitive Behavioral Therapy for Insomnia in Older Veterans Using Nonclinician Sleep Coaches: Randomized Controlled Trial. J. Am. Geriatr. Soc 64, 1830–1838 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luik AI, Kyle SD & Espie CA Digital Cognitive Behavioral Therapy (dCBT) for Insomnia: a State-of-the-Science Review. Curr. Sleep Med. Rep 3, 48–56 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watson NF, Rosen IM & Chervin RD The Past Is Prologue: The Future of Sleep Medicine. J. Clin. Sleep Med 13, 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carden KA Sleep is essential: A new strategic plan for the American Academy of Sleep Medicine. J. Clin. Sleep Med 16, 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sarmiento KF et al. National Expansion of Sleep Telemedicine for Veterans: The TeleSleep Program. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med 15, 1355–1364 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thomas RJ et al. Home-Based Cardiac Rehabilitation: A Scientific Statement From The American Association Of Cardiovascular And Pulmonary Rehabilitation, The American Heart Association, And The American College Of Cardiology. J. Cardiopulm. Rehabil. Prev 39, 208–225 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schutte-Rodin S, Broch L, Buysse D, Dorsey C & Sateia M Clinical Guideline for the Evaluation and Management of Chronic Insomnia in Adults. J. Clin. Sleep Med 04, 487–504 (2008). [PMC free article] [PubMed] [Google Scholar]
- 73.Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical Guideline for the Evaluation, Management and Long-term Care of Obstructive Sleep Apnea in Adults. J. Clin. Sleep Med 05, 263–276 (2009). [PMC free article] [PubMed] [Google Scholar]
- 74.Morgenthaler TI et al. Practice Parameters for the Treatment of Narcolepsy and other Hypersomnias of Central Origin. 30, 16 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ong JC, Dawson SC, Mundt JM & Moore C Developing a cognitive behavioral therapy for hypersomnia using telehealth: a feasibility study. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med 16, 2047–2062 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morgenthaler TI et al. Practice Parameters for the Clinical Evaluation and Treatment of Circadian Rhythm Sleep Disorders. Sleep 30, 1445–1459 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Auger RR et al. Clinical Practice Guideline for the Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders: Advanced Sleep-Wake Phase Disorder (ASWPD), Delayed Sleep-Wake Phase Disorder (DSWPD), Non-24-Hour Sleep-Wake Rhythm Disorder (N24SWD), and Irregular Sleep-Wake Rhythm Disorder (ISWRD). An Update for 2015: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med 11, 1199–1236 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morgenthaler TI et al. Position Paper for the Treatment of Nightmare Disorder in Adults: An American Academy of Sleep Medicine Position Paper. J. Clin. Sleep Med 14, 1041–1055 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Standards of Practice Committee et al. Best Practice Guide for the Treatment of REM Sleep Behavior Disorder (RBD). J. Clin. Sleep Med 06, 85–95 (2010). [PMC free article] [PubMed] [Google Scholar]
- 80.Aurora RN et al. The Treatment of Restless Legs Syndrome and Periodic Limb Movement Disorder in Adults—An Update for 2012: Practice Parameters with an Evidence-Based Systematic Review and Meta-Analyses. SLEEP (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]


