Abstract
OBJECTIVE.
Elevated inflammation and psychological distress in patients with breast cancer (BCa) have been related to poorer health outcomes. Regulation of the hypothalamic-pituitary adrenal (HPA) axis and signaling of the receptor for advanced glycation end products (RAGE) are important in the inflammatory response and have been associated with increased stress and poorer health outcomes in patients with cancer. This study examined relationships among circulating cortisol, a measure of HPA axis activity and physiological stress; s100A8/A9, a RAGE ligand and emerging cancer-related biological measure; and self-reported cancer-related distress.
METHODS.
Patients with BCa (N=183, stage 0–IIIb) were recruited 2–10 weeks post-surgery but prior to receiving adjuvant therapies. Participants provided blood samples, from which serum cortisol and s100A8/A9 levels were determined, and completed a psychosocial questionnaire. Regression analyses, adjusting for age, cancer stage, time since surgery, race, and menopausal status, were conducted examining the relationships between cortisol, s100A8/A9, and cancer-related distress (Impact of Event Scale [IES]–Revised).
RESULTS.
Cortisol and s100A8/A9 levels were positively related (β=.218, t(112)=2.332, p=.021), although the overall model was not significant. Cortisol levels were also positively associated with IES-Intrusions (β=.192, t(163)=2.659, p=.009) and IES-Hyperarousal subscale scores (β=.171, t(163)=2.304, p=.022).
CONCLUSIONS.
Patients with higher cortisol levels also reported higher s100A8/A9 levels and more cancer-related distress. The relationship between cortisol and s100A8/A9 supports a link between the stress response and pro-inflammatory physiological processes known to predict greater metastatic risk in BCa. Stress processes implicated in cancer biology are complex and replication and extension of these initial findings is important.
Keywords: breast cancer, stress, s100A8/A9, cortisol, biobehavioral
INTRODUCTION
Cortisol, a marker of physiological stress (1), is a steroid hormone secreted by the adrenal cortex and is considered a reliable measure of hypothalamic-pituitary-adrenal (HPA) axis function (2). Women with BCa have been shown to have higher cortisol levels than healthy controls, and their cortisol levels appear to increase with disease severity (3, 4). Alterations in cortisol levels or regulation may contribute to cancer progression and disease outcomes as cortisol can modulate the immune response and release of proinflammatory cytokines, such that glucocorticoid receptor desensitization can leave inflammatory response cascades relatively unchecked impacting processes involved in cancer (5). Cortisol has also been implicated in chemoresistance in the majority of tumor cells, including breast (6). There is a strong evidence base evaluating how stress processes and distress states relate to general indicators of inflammation and immune function in the context of cancer (7), however, inconsistencies in findings leave debate around how precisely cortisol relates to psychosocial factors in patients with BCa (8).
Also involved in stress-related inflammatory pathways is the receptor for advanced glycation end products (RAGE), a cell surface receptor that is part of the immunoglobulin sub-family of proteins (9). Found on immune and cancer cells, it is activated by ligands such as the heterodimer s100A8/A9 (calprotectin) (10). Evidence suggests elevated leukocyte inflammatory signaling seen in conjunction with elevated stress may be due to effusion of myeloid-derived suppressor cells from the bone marrow with SNS activation (11), which stimulate immune and cancer cells via RAGE (9). Increased RAGE activation, via s100A8/A9 (10), has been linked to greater risk of BCa metastasis because it modifies tumor processes, including increased cell migration and invasion, proliferation, and resistance to apoptosis (9, 12). A growing body of evidence establishes s100A8/A9 as crucial in establishing the “pre-metastatic niche,” a fertile environment for malignant cells from the primary tumor to invade other organs and tissues, facilitating progression from early stage to metastatic breast cancer (9, 13). S100 proteins, and specifically s100A8/A9 in breast cancer, have also been found to mediate chemoresistance, further promoting opportunity for metastasis (14, 15). Identifying and modifying promising prognostic markers such as s100A8/A9 early in the disease course, may impact the cancer trajectory (3, 15, 16).
While several key players have been proposed as relevant to the impact of stress factors on inflammatory processes relevant to BCa progression (7, 17, 18), less is known about how stress-related neuroendocrine regulation relates to RAGE activation in BCa. One identified pathway is that cortisol increases production of s100A8/A9 from polymorphonuclear neutrophils (PMNs) which can lead to reactivation of dormant tumor cells (19). We hypothesized greater cortisol levels would predict greater s100A8/A9 levels in post-surgical patients with BCa as well as higher self-reported cancer-related distress. We also hypothesized a positive association between cancer-related distress and s100A8/A9 levels.
METHODS
Participants and Procedures.
This was a secondary analysis of baseline data from an IRB-approved randomized controlled trial registered as National Institutes of Health Clinical Trial NCT02103387. Additional information on trial methodology and primary outcome results can be found in a previously published report (20). From 2006–2013, recently diagnosed BCa patients (stage 0–IIIb) aged 21+ were recruited from clinics and hospitals in South Florida 2–10 weeks post-surgery but before initiating adjuvant therapies to participate in an IRB-approved randomized controlled trial. They were excluded during screening for severe psychiatric illness, non-fluency in English, prior history of cancer (with the exception of nonmelanoma skin cancer), stage IV BCa, other serious chronic medical conditions, and initiation of neoadjuvant or adjuvant therapy (chemotherapy/radiotherapy). Of 739 women approached, 545 were excluded (318 for not meeting study criteria and 227 for participant refusal or nonavailability). Eleven withdrew from the study prior to baseline data collection. Informed consent was obtained from all individual participants included in the study. Participants (N=183) completed a baseline assessment including a psychosocial questionnaire and a blood sample from which serum levels of cortisol and s100A8/A9 were determined. Participants additionally self-reported demographic and medical information, which was later verified through medical chart review by the study team.
Measures.
A licensed phlebotomist obtained blood samples (35ml) from participants between 4pm and 6:30pm. Cortisol levels in serum were determined with enzyme-linked immunosorbent assay (ELISA, Diagnostic Systems Laboratories, Webster, Texas), inter-assay coefficient of variation (CV) = 6%; intra-assay CV = 5%. Serum s100A8/A9 levels were obtained by an ultra-sensitive ELISA (Calprotectin, Human ELISA; Hycult Biotech Inc, Wayne, PA), inter-assay CV = 4.3%; intra-assay CV = 5.3%. Self-reported cancer-related distress over the past seven days was assessed using the 22-item Impact of Event Scale—Revised (IES-R) (21), which includes three subscales Intrusions (IES-I), Hyperarousal (IES-H), and Avoidance (IES-A). The IES-R has been used in previous studies of stress and stress management in BCa patients (20, 22). Cronbach alpha indicated high reliability (IES-I α=.87; IES-H α=.83; IES-A α=.81).
Analytic Plan.
Analyses were conducted using the Statistical Package for the Social Sciences-version 25. An alpha level of .05 was used and tests were two-tailed. Consistent with prior reports from this trial, for psychosocial data, outliers (>3 SD from the mean) were winsorized. Analyses used raw values for physiological data with extreme values of >3 SD from the mean removed (s100A8/A9 N=1; cortisol N=3). Associations were tested with multiple regression. Covariates were selected based on previous research indicating associations with stress adaptation (23) and inflammatory markers (24, 25), and included age, disease stage, days since surgery, menopausal status, and race. All available data were used and analyses used listwise deletion for missing data (see Table 1 for Ns). The study variable with the largest amount of missing data was s100A8/A9 (N=122/183) as this was a post-hoc assay done with cryopreserved serum samples and could only be conducted for participants who had a sufficient amount of remaining intact sample. There were no significant differences (p>.05) in demographic or medical variables between participants with s100A8/A9 data and the full sample.
Table 1.
Sample Characteristics
| Variable | N | Mean (SD) or Count (%) |
|---|---|---|
| Age (years), range=28–80 | 183 | 54.28(10.06) |
| Surgery to baseline (days) | 183 | 37.42(22.3) |
| BMI (weight/height*703) | 154 | 26.91(5.34) |
| Premenopausal (yes/peri or post) | 178 | 57 (32.0%) |
| Household income (thousands) | 183 | 100.61(67.89) |
| Education (years) | 179 | 15.49(2.99) |
| Married/partnered (yes/no) | 183 | 117(63.9%) |
| Employed (yes/no) | 183 | 129(70.5%) |
| Race/Ethnicity | 183 | |
| Non-Hispanic White | 76(42.2%) | |
| Hispanic | 76(42.2%) | |
| Black | 16(8.9%) | |
| Other | 12(6.7%) | |
| Stage | 182 | |
| 0 | 35(19.2%) | |
| I | 94(51.6%) | |
| II | 44(24.2%) | |
| III | 9(4.9%) | |
| Positive Nodes (yes/no) | 183 | 37(21.6%) |
| Hormonal Status | ||
| Her2 neu (yes/no) | 143 | 17(11.9%) |
| ER Positive (yes/no) | 164 | 141(86.0%) |
| PR Positive (yes/no) | 159 | 122(76.7%) |
| ER or PR Positive (yes/no) | 158 | 137(86.7%) |
| Surgery | 183 | |
| Lumpectomy | 89(48.6%) | |
| Mastectomy | 94(51.4%) | |
| Study Variables | ||
| Serum s100A8/A9 (ng/mL) | 122 | 3936.06 (3079.66) |
| Serum Cortisol (nmol/L) | 177 | 13.39 (7.50) |
| IES-I (0–4 range) | 179 | 1.31(.86) |
| IES-H (0–4 range) | 179 | .8491(.77) |
RESULTS
Participants had a mean age of 54.28 (SD=10.06) and were majority Hispanic (42.2%) or Non-Hispanic White (42.2%). See Table 1 for additional demographic and medical information. There was a significant positive relationship between cortisol and s100A8/A9 (β=.218, t(112)=2.332, p=.021), though the overall model controlling for age, stage, time since surgery, menopause status, and race was not significant (F(6, 112)=1.263, p=.280, R2=.063, R2 change=.045). The overall multiple regression model testing the relationship between cortisol and the full scale IES, controlling for covariates, was significant (F(6, 160)=4.650, p=.<.001, R2=.148, R2 change=.024), and revealed a significant positive association between cortisol levels and IES scores (β=.159, t(160)=2.139, p=.034). Similarly, the overall multiple regression model testing the relationship between cortisol and the IES-I subscale, controlling for covariates, was significant (F(6, 163)=5.824, p<.001, R2=.177, R2 change=.036), and revealed a significant positive association between cortisol and IES-I (β=.192, t(163)=2.659, p=.009), see Figure 1. The overall model testing the relationship between cortisol and IES-H, controlling for covariates, was also significant (F(6, 163)=4.334, p<.001, R2=.138, R2 change =.028), and there was a significant positive association between cortisol and IES-H (β=.171, t(163)=2.304, p=.022). Cortisol levels were not related to IES-A scores (p>.10). Neither full scale IES nor subscales were related to s100A8/A9 levels (p’s>.10).
Figure 1.
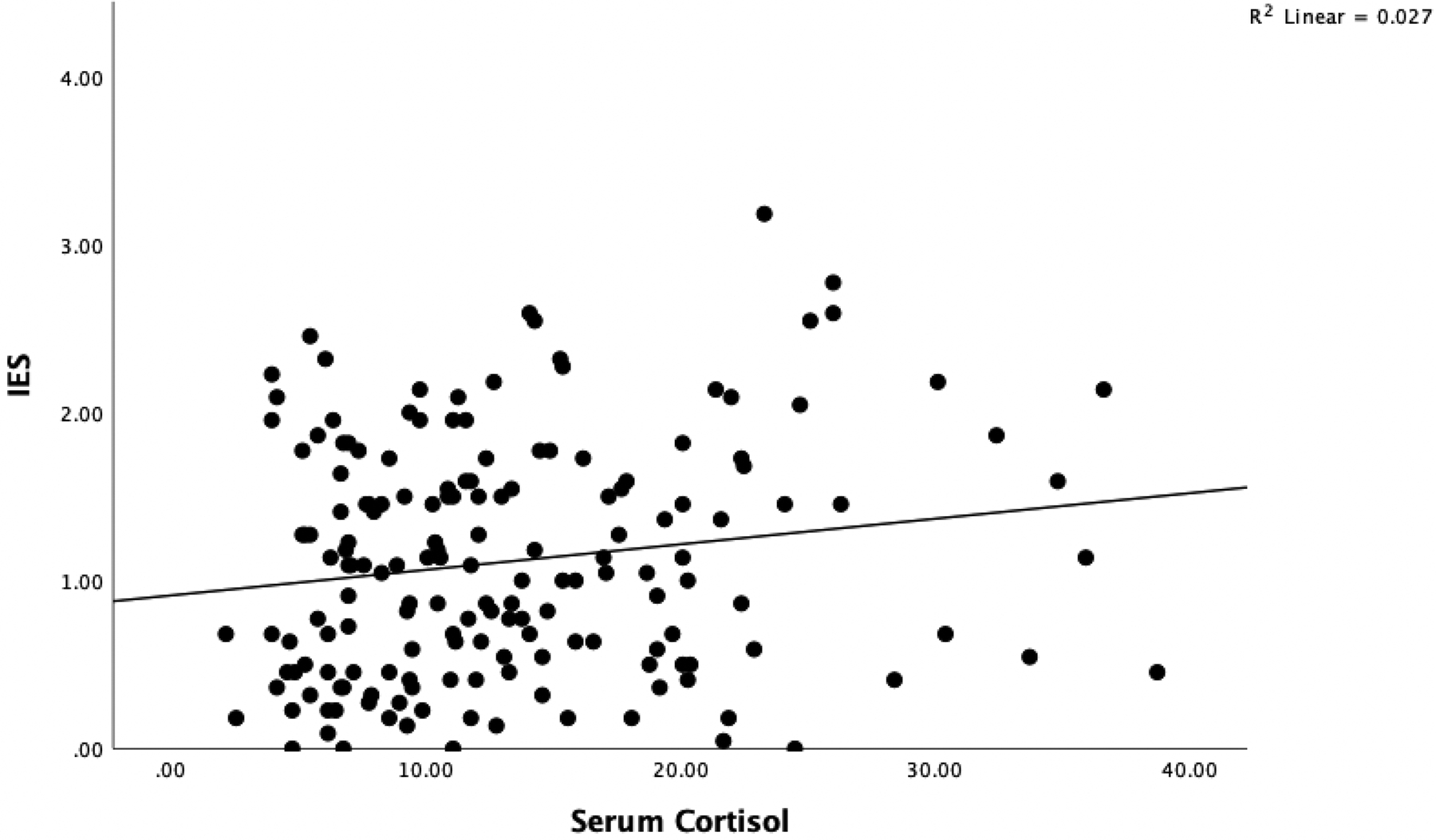
Scatterplot of simple regression for serum cortisol levels and IES-I scores.
DISCUSSION
We documented for the first time that greater circulating levels of cortisol were associated with greater s100A8/A9 levels in BCa patients. The literature suggests that the relationship between cortisol dysregulation and cancer outcomes may be explained at least in part by upregulation of inflammatory processes that facilitate disease progression and metastasis (7, 26–28). The glucocorticoid-resistance model posits that with frequent stress, persistent activation of the HPA axis leads to heightened levels of circulating cortisol, which in turn, causes desensitization of glucocorticoid receptors in inflammatory immune cells like monocytes (29). Since glucocorticoid receptors are responsible for modulation of the immune response including release of pro-inflammatory cytokines, desensitization can leave inflammatory response cascades relatively unchecked (29). The current study examined afternoon levels of serum cortisol, consistent with other prior studies examining stress in BCa (17, 18). Dysregulated cortisol levels – indexed by flatter diurnal pattern – have been linked to poorer disease outcomes in cancer (30–32), and higher levels of afternoon cortisol can contribute in part to a flatter diurnal cortisol slope. To better understand the extent to which elevations of single-assessment serum levels seen here may or may not be a representation of cortisol dysregulation, future work should investigate other measures of cortisol, such as salivary cortisol collection for diurnal rhythm analysis (33) and assays that assess glucocorticoid sensitivity (34). If results of such investigations are consistent, results would support that adequate management of cancer-related distress could be valuable for BCa patients during this period.
Binding of s100A8/A9 to RAGE receptors facilitates regulation of the nuclear factor-κB and mitogen-activated protein kinase (MAPK) signaling pathways involved in production of pro-inflammatory cytokines. SNS-related neurohormones can stimulate bone marrow to release cells that produce s100A8/A9 (35). As such, we hypothesized that cortisol levels would positively relate to s100A8/A9 levels, which our results supported. Consistent with our results, a recent investigation in a murine lung cancer model showed addition of cortisol to PMNs was associated with the greatest increase in s100A8/A9 compared to other stress hormones such as norepinephrine (19). Importantly s100A8/A9 induced these PMNs to accumulate oxidized lipids and release of these lipids up-regulated fibroblast growth factor and appeared to reactivate dormant tumor cells and formation of new tumor lesions (19). To better understand how stress may relate to cancer outcomes via RAGE-mediated processes, future work should also investigate relationships between s100A8/A9 and other neurohormones in BCa.
Additionally, in line with our hypotheses, cortisol levels were positively related to cancer-related distress. This is in agreement with a large body of existing literature that establishes cortisol as a stress biomarker (36) and prior work that highlights a relationship between cortisol and psychological distress in BCa patients (18, 37, 38). Our results suggested women with BCa who self-reported more cancer-related distress in the form of thought intrusions or hyperarousal after surgery also showed a greater HPA-mediated physiological stress response as measured by serum cortisol levels. Cortisol levels were not related to cancer-related distress in the form of avoidance, consistent with prior work from our group showing intrusions and hyperarousal are the IES subscales that tend to demonstrate relationships with stress and inflammatory processes and greater sensitivity to change through stress-management interventions (20, 39).
Interestingly, self-reported cancer-related distress was not directly related to s100A8/A9 levels. In the current study, the IES was selected to represent psychological distress. This scale was chosen as it distinctly captures both the physiological (hyperarousal; IES-H) and the cognitive component of distress (intrusions; IES-I), and has been shown to be influenced by stress management interventions in BCa (20, 22). It is possible, however, that other indicators of psychological adaptation (e.g., depressive symptoms) not assessed here and/or examination of associations between s100A8/A9 and psychological wellbeing in a metastatic sample as opposed to early stage (0-IIIb) would have yielded different relationships with s100A8/A9. In fact, a recent publication reported lower levels of social and family well-being and higher levels of depressive symptoms were related to greater s100A8/A9 in women with metastatic breast cancer (40). The examination of distress in BCa is of great importance with one study categorizing 40% of BCa patients assessed as highly distressed (41). The current study, however, did not specifically select for distressed patients and participants on average endorsed being bothered by symptoms “a little bit” over the past week. Similarly, there was variation in cortisol and s100A8/A9 levels. Mean levels of s100A8/A9 in this study, as expected, were greater than mean levels in prior reports with non-cancer samples (42, 43) and lower than mean levels in non-metastatic breast cancer patients with high distress (39) and patients with metastatic breast cancer (40). It would be valuable in a future study to determine if a significant relationship would emerge between distress and s100A8/A9 in a distressed sample.
Finally, it is possible that the IES measure of cancer-related distress assessed during the recently diagnosed period captured acute distress that better relates temporally to cortisol compared to s100A8/A9, which may be more related to longer-term psychological status, assessed with measures of depression or social and family wellbeing (38). This post-surgical period was chosen for collection of inflammatory measures, as surgery-related inflammation has likely resolved and adjuvant treatments have not begun. This is additionally a time of particular interest in psycho-oncology as women often have elevated distress during this period (44). This may not necessarily be representative of the biopsychosocial processes at play throughout the disease trajectory. Future work should involve longitudinal designs to gain better insight into how these variables relate over time and potential mechanisms at work.
To note, the current sample was comprised of largely middle-class, well-educated, and highly motivated women who were willing to be part of a research study. While a strength of the study was that over 50% of the sample identified as an ethnicity other than non-Hispanic white, only English-speaking patients were able to participate. Also of note, psychosocial data were collected via self-report which is subject to potential participant recall and reporting biases. Furthermore, other demographic or medical variables not included in this study may influence relationships examined here, such as anti-inflammatory treatments, NSAID use, or acute illness. As this study was cross-sectional in nature, no claims can be made concerning causation nor temporality of relationships. Bi-directional relationships exist in stress and inflammatory pathways, and the relationship seen here between cortisol and s100A8/A9 could be independent of stress perceptions via self-reports.
Since this was the first investigation of the associations of cortisol with s100A8/A9 and cancer-related distress, and because the relationship between the two variables but not the overall model was significant, replication is needed before conclusions can be confidently drawn. While the variables examined here are part of the biopsychosocial model of stress in disease (7, 45), inflammatory pathways involved are numerous and complex and more work is needed characterizing the psychological, neuroendocrine, and immunologic variables and networks involved. Despite limitations, this study adds to the literature by demonstrating that among BCa patients in the post-surgical period, greater cortisol levels are associated with greater s100A8/A9 levels, an emerging inflammatory biomarker known to promote processes mediating BCa metastasis.
Conflict of Interest and Source of Funding:
Dr. Antoni is a consultant for Blue Note Therapeutics and Atlantis Healthcare and receives a small royalty from APA and Oxford Press for books he wrote on stress management. Dr. Taub is a consultant for Blue Note Therapeutics. This study was funded by NCI R01CA64710 and Sylvester Cancer Center. Dr. Taub is funded by NCI T32CA193193.
Acronyms:
- BCa
breast cancer
- IES-I
Impact of Event Scale – Intrusions
- IES-H
Impact of Event Scale – Hyperarousal
- HPA
hypothalamic-pituitary adrenal
- RAGE
receptor for advanced glycation end product
- ELISA
enzyme-linked immunosorbent assay
- PMNs
polymorphonuclear neutrophils (PMNs)
REFERENCES
- 1.Kirschbaum C, Hellhammer DH: Salivary cortisol in psychobiological research: an overview. Neuropsychobiology. 1989, 22:150–169. [DOI] [PubMed] [Google Scholar]
- 2.Antoni MH: Psychoneuroendocrinology and psychoneuroimmunology of cancer: Plausible mechanisms worth pursuing? Brain, behavior, and immunity. 2003, 17:S84–91. [DOI] [PubMed] [Google Scholar]
- 3.Abercrombie HC, Giese-Davis J, Sephton S, Epel ES, Turner-Cobb JM, Spiegel D: Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology. 2004, 29:1082–1092. [DOI] [PubMed] [Google Scholar]
- 4.Van Der Pompe G, Antoni MH, Heijnen CJ: Elevated basal cortisol levels and attenuated ACTH and cortisol responses to a behavioral challenge in women with metastatic breast cancer. Psychoneuroendocrinology. 1996, 21:361–374. [DOI] [PubMed] [Google Scholar]
- 5.Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, Stefanek M, Sood AK: The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nature Reviews Cancer. 2006, 6:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang C, Beckermann B, Kallifatidis G, Liu Z, Rittgen W, Edler L, Büchler P, Debatin K-M, Büchler MW, Friess H: Corticosteroids induce chemotherapy resistance in the majority of tumour cells from bone, brain, breast, cervix, melanoma and neuroblastoma. International Journal of Oncology. 2006, 29:1295–1301. [PubMed] [Google Scholar]
- 7.Antoni MH, Dhabhar FS: The impact of psychosocial stress and stress management on immune responses in patients with cancer. Cancer. 2019, 125:1417–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vedhara K, Miles JN, Sanderman R, Ranchor AV: Psychosocial factors associated with indices of cortisol production in women with breast cancer and controls. Psychoneuroendocrinology. 2006, 31:299–311. [DOI] [PubMed] [Google Scholar]
- 9.Yin C, Li H, Zhang B, Liu Y, Lu G, Lu S, Sun L, Qi Y, Li X, Chen W: RAGE-binding S100A8/A9 promotes the migration and invasion of human breast cancer cells through actin polymerization and epithelial–mesenchymal transition. Breast cancer research and treatment. 2013, 142:297–309. [DOI] [PubMed] [Google Scholar]
- 10.Leukert N, Vogl T, Strupat K, Reichelt R, Sorg C, Roth J: Calcium-dependent tetramer formation of S100A8 and S100A9 is essential for biological activity. Journal of molecular biology. 2006, 359:961–972. [DOI] [PubMed] [Google Scholar]
- 11.Talmadge JE, Gabrilovich DI: History of myeloid-derived suppressor cells. Nature Reviews Cancer. 2013, 13:739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drews-Elger K, Iorns E, Dias A, Miller P, Ward TM, Dean S, Clarke J, Campion-Flora A, Rodrigues DN, Reis-Filho JS: Infiltrating S100A8+ myeloid cells promote metastatic spread of human breast cancer and predict poor clinical outcome. Breast cancer research and treatment. 2014, 148:41–59. [DOI] [PubMed] [Google Scholar]
- 13.Rafii S, Lyden D: S100 chemokines mediate bookmarking of premetastatic niches. Nature Cell Biology. 2006, 8:1321–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hua X, Zhang H, Jia J, Chen S, Sun Y, Zhu X: Roles of S100 family members in drug resistance in tumors: Status and prospects. Biomedicine and Pharmacotherapy. 2020, 127:110156. [DOI] [PubMed] [Google Scholar]
- 15.Bresnick AR, Weber DJ, Zimmer DB: S100 proteins in cancer. Nature Reviews Cancer. 2015, 15:96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allgöwer C, Kretz A-L, von Karstedt S, Wittau M, Henne-Bruns D, Lemke J: Friend or foe: S100 proteins in cancer. Cancers. 2020, 12:2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cruess DG, Antoni MH, McGregor BA, Kilbourn KM, Boyers AE, Alferi SM, Carver CS, Kumar M: Cognitive-behavioral stress management reduces serum cortisol by enhancing benefit finding among women being treated for early stage breast cancer. Psychosomatic Medicine. 2000, 62:304–308. [DOI] [PubMed] [Google Scholar]
- 18.Phillips KM, Antoni MH, Carver CS, Lechner SC, Penedo FJ, McCullough ME, Gluck S, Derhagopian RP, Blomberg BB: Stress management skills and reductions in serum cortisol across the year after surgery for non-metastatic breast cancer. Cognitive Therapy and Research. 2011, 35:595–600. [Google Scholar]
- 19.Perego M, Tyurin VA, Tyurina YY, Yellets J, Nacarelli T, Lin C, Nefedova Y, Kossenkov A, Liu Q, Sreedhar S: Reactivation of dormant tumor cells by modified lipids derived from stress-activated neutrophils. Science Translational Medicine. 2020, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gudenkauf LM, Antoni MH, Stagl JM, Lechner SC, Jutagir DR, Bouchard LC, Blomberg BB, Glück S, Derhagopian RP, Giron GL: Brief cognitive–behavioral and relaxation training interventions for breast cancer: A randomized controlled trial. Journal of Consulting and Clinical Psychology. 2015, 83:677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss DS: The impact of event scale: revised. Cross-cultural assessment of psychological trauma and PTSD: Springer, 2007, 219–238. [Google Scholar]
- 22.Antoni MH, Wimberly SR, Lechner SC, Kazi A, Sifre T, Urcuyo KR, Phillips K, Smith RG, Petronis VM, Guellati S and Wells KA: Reduction of cancer-specific thought intrusions and anxiety symptoms with a stress management intervention among women undergoing treatment for breast cancer. American Journal of Psychiatry. 2006, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montazeri A: Health-related quality of life in breast cancer patients: a bibliographic review of the literature from 1974 to 2007. Journal of experimental & clinical cancer research. 2008, 27:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Connor M-F, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK: To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain, behavior, and immunity. 2009, 23:887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouchard LC, Antoni MH, Blomberg BB, Stagl JM, Gudenkauf LM, Jutagir DR, Diaz A, Lechner S, Glück S, Derhagopian RP: Post-Surgical Depressive Symptoms and Pro-Inflammatory Cytokine Elevations in Women Undergoing Primary Treatment for Breast Cancer. Psychosomatic Medicine. 2016, 78:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutgendorf SK, Sood AK, Antoni MH: Host factors and cancer progression: biobehavioral signaling pathways and interventions. Journal of Clinical Oncology. 2010, 28:4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seruga B, Zhang H, Bernstein LJ, Tannock IF: Cytokines and their relationship to the symptoms and outcome of cancer. Nature Reviews Cancer. 2008, 8:887–899. [DOI] [PubMed] [Google Scholar]
- 28.E Goldberg J L Schwertfeger K: Proinflammatory cytokines in breast cancer: mechanisms of action and potential targets for therapeutics. Current drug targets. 2010, 11:1133–1146. [DOI] [PubMed] [Google Scholar]
- 29.Miller GE, Cohen S, Ritchey AK: Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health psychology. 2002, 21:531. [DOI] [PubMed] [Google Scholar]
- 30.Filipski E, King VM, Li X, Granda TG, Mormont M-C, Liu X, Claustrat B, Hastings MH, Lévi F: Host circadian clock as a control point in tumor progression. Journal of the National Cancer Institute. 2002, 94:690–697. [DOI] [PubMed] [Google Scholar]
- 31.Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D: Diurnal cortisol rhythm as a predictor of breast cancer survival. Journal of the National Cancer Institute. 2000, 92:994–1000. [DOI] [PubMed] [Google Scholar]
- 32.Sapolsky RM, Donnelly TM: Vulnerability to stress-induced tumor growth increases with age in rats: role of glucocorticoids. Endocrinology. 1985, 117:662–666. [DOI] [PubMed] [Google Scholar]
- 33.Gatti R, Antonelli G, Prearo M, Spinella P, Cappellin E, Elio F: Cortisol assays and diagnostic laboratory procedures in human biological fluids. Clinical biochemistry. 2009, 42:1205–1217. [DOI] [PubMed] [Google Scholar]
- 34.Bower JE, Ganz PA, Aziz N, Olmstead R, Irwin MR, Cole SW: Inflammatory responses to psychological stress in fatigued breast cancer survivors: relationship to glucocorticoids. Brain, Behavior, and Immunity. 2007, 21:251–258. [DOI] [PubMed] [Google Scholar]
- 35.Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK: Sympathetic nervous system regulation of the tumour microenvironment. Nature Reviews Cancer. 2015, 15:563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chrousos GP, Gold PW: The concepts of stress and stress system disorders: overview of physical and behavioral homeostasis. Jama. 1992, 267:1244–1252. [PubMed] [Google Scholar]
- 37.Bower JE, Ganz PA, Aziz N: Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosomatic Medicine. 2005, 67:277–280. [DOI] [PubMed] [Google Scholar]
- 38.Raghavendra RM, Vadiraja H, Nagarathna R, Nagendra H, Rekha M, Vanitha N, Gopinath K, Srinath B, Vishweshwara M, Madhavi Y: Effects of a yoga program on cortisol rhythm and mood states in early breast cancer patients undergoing adjuvant radiotherapy: a randomized controlled trial. Integrative cancer therapies. 2009, 8:37–46. [DOI] [PubMed] [Google Scholar]
- 39.Diaz A, Taub CJ, Lippman ME, Antoni MH, Blomberg BB: Effects of brief stress management interventions on distress and leukocyte nuclear factor kappa B expression during primary treatment for breast cancer: A randomized trial. Psychoneuroendocrinology. 2021, 126:105163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reis JC, Travado L, Seixas E, Sousa B, Antoni MH: Low social and family well-being is associated with greater RAGE ligand s100A8/A9 and interleukin-1 beta levels in metastatic breast cancer patients. Brain, Behavior, & Immunity-Health. 2022, 21:100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herschbach P, Keller M, Knight L, Brandl T, Huber B, Henrich G, Marten-Mittag B: Psychological problems of cancer patients: a cancer distress screening with a cancer-specific questionnaire. British journal of cancer. 2004, 91:504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jonasson L, Grauen Larsen H, Lundberg A, Gullstrand B, Bengtsson A, Schiopu A: Stress-induced release of the S100A8/A9 alarmin is elevated in coronary artery disease patients with impaired cortisol response. Scientific Reports. 2017, 7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drosatos I-A, Tsoporis JN, Izhar S, Gupta S, Tsirebolos G, Sakadakis E, Triantafyllis AS, Rigopoulos A, Rigopoulos D, Rallidis LS: Differential Regulation of Circulating Soluble Receptor for Advanced Glycation End Products (sRAGEs) and Its Ligands S100A8/A9 Four Weeks Post an Exercise Intervention in a Cohort of Young Army Recruits. Biomolecules. 2021, 11:1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lester J, Crosthwaite K, Stout R, Jones RN, Holloman C, Shapiro C, Andersen BL: Women with breast cancer: self-reported distress in early survivorship. Oncology nursing forum: 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hudson BI, Lippman ME: Targeting RAGE signaling in inflammatory disease. Annual review of medicine. 2018, 69:349–364. [DOI] [PubMed] [Google Scholar]


