Abstract
BACKGROUND/ AIMS:
Hepatitis E Virus (HEV) infection rarely causes icteric hepatitis yet 10 to 40% of adult Americans have serological evidence of prior infection. The aim of this study was to investigate the incidence, presentation, and outcome of acute and prior HEV infection in a large cohort of patients with suspected drug-induced liver injury (DILI).
APPROACH/ RESULTS:
Serum samples from 2012 patients enrolled in the Drug-Induced Liver Injury Network were tested for anti-HEV IgG. Those with detectable anti-HEV IgG underwent testing for anti-HEV IgM; those with detectable anti-HEV IgM were tested for HEV RNA. Anti-HEV IgG was detected in 407 (20%) patients and associated with increasing subject age and earlier year of enrollment. The median age of seropositive subjects was more than a decade higher than seronegative subjects (59.8 vs 48.7 years). The overall prevalence of anti-HEV declined from 22% (2004 −2011) to 18% (2012 – 2019), suggestive of a cohort effect. The frequency of acute hepatitis E (median ALT= 1231 IU/l) also decreased from 3% (2004-2008) to 1.2% (2009-2013) to 0.6% (2014-2019). These results suggest that acute HEV infection is usually subclinical and was much more frequent in this cohort before 2004.
CONCLUSIONS:
Acute HEV infection accounts for less than 1% of suspected American DILI cases and is more frequent in older men. Prior HEV infection is also most commonly seen in older individuals. Clinicians should consider testing for unsuspected acute HEV infection in older adult patients with acute hepatocellular DILI and jaundice.
Keywords: Causality, drug hepatotoxicity, Hepatitis E, viral hepatitis
Introduction
Establishing a diagnosis of idiosyncratic drug-induced liver injury (DILI) is difficult due to the diverse clinical presentations that mimic other, more common, causes of liver injury (1-3). In addition to looking for a compatible drug latency, dechallenge, and clinical presentation, testing for common alternative causes of liver injury such as acute hepatitis A virus (HAV), hepatitis B virus (HBV), and hepatitis C virus (HCV) infection is recommended (1,2). In contrast, testing for acute hepatitis E virus (HEV) infection is controversial and not routinely undertaken, in part, due to the low incidence of acute HEV in many Western countries but also because of the lack of widely available standardized serological assays (4-6). As a result, the frequency with which acute HEV is the cause of liver injury in patients with suspected DILI is not well established. Nonetheless, recent studies from the United Kingdom and Spain have suggested that 8 to 12% cases of acute liver injury cases initially attributed to medications are actually due to unsuspected acute HEV infection (7,8). In addition, HEV infection has emerged as the most common cause of acute viral hepatitis in Scotland and can be mistaken for acute rejection in liver transplant recipients (9, 10). However, large prospective studies of North American patients with severe acute liver injury requiring hospitalization or with acute liver failure (ALF) have identified unsuspected acute HEV infection in only 1-2% (11,12). In addition, a study from Iceland failed to demonstrate any cases of acute HEV infection amongst 85 consecutive patients with DILI (13).
The Drug-induced Liver Injury Network (DILIN) is conducting an ongoing, multicenter, prospective observational study in children and adults with suspected DILI enrolled at geographically diverse sites in the United States (14). In an analysis of cases enrolled between 2004 and 2008, 9 of 318 (2.8%) consecutive patients were found on testing of stored serum samples to be anti-HEV IgM positive of which 4 were HEV-RNA positive (15). The aim of the current study was to update information on the incidence, clinical presentation, and outcome of acute and prior HEV infections using data from the DILIN prospective study that now contains over 2,000 tested patients enrolled over a 16-year period. In addition, a careful description of available liver biopsy samples from the acute HEV cases was undertaken to better define the histological findings of acute HEV infection (16-19).
METHODS
DILIN prospective study:
All patients presenting with newly diagnosed liver injury suspected to be due to a medication, herbal product or dietary supplement at participating medical centers were enrolled in the DILIN Prospective Study, the full design and details of which have been published (13). Liver injury onset was defined as the first date that a subject met one of the predefined laboratory criteria, which included: (1) serum aspartate aminotransferase (AST) or alanine aminotransferase (ALT) level that exceeded 5 times the upper limit of normal (ULN) (or 5 times pretreatment baseline if baseline values were abnormal); (2) a serum alkaline phosphatase (Alk P) that exceeded 2 times the ULN (or 2 times pretreatment baseline if baseline values were abnormal); any ALT, AST or ALP elevation accompanied by a total bilirubin of 2.5 mg/dL or above, or an international normalized ratio (INR) greater than 1.5 on two consecutive blood draws (14). All study participants provided written informed consent and were required to be enrolled within 6 months of onset. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as approved by the local IRB.
A detailed medical history was obtained at the baseline study visit and additional laboratory and radiological testing were performed to fully characterize the DILI event and exclude competing etiologies via testing for infection with hepatitis A, B, and C viruses, human immunodeficiency virus (HIV), anti-nuclear antibody (ANA) and smooth muscle antibody (SMA) titers, and CMV and EBV infection. Enrolled patients were seen for a follow-up study visit 6 months after initial enrollment and those with evidence of chronic liver injury were asked to return for further study visits for up to 48 months (14). Subjects self-reported race and ethnicity were used in reported analyses.
The severity of the DILI episode was categorized on a 5-point scale from 1+ (mild: bilirubin <2.5 mg/dL and INR <1.5), 2+ (moderate: bilirubin ≥ 2.5 mg/dL but INR <1.5 and not hospitalized), 3+ (moderate-hospitalized: bilirubin ≥2.5 mg and INR <1.5 and hospitalized for the liver injury), 4+ (severe: bilirubin ≥2.5 mg/dL and at least one other sign of liver failure, e.g., INR ≥1.5), and 5+ (fatal: death or liver transplantation due to DILI within six months of onset).
Liver histopathology:
Available liver biopsies were reviewed by an expert hepatic pathologist (DEK) and scored for histological features as well as an overall pattern of liver injury (20).
HEV testing:
Serum samples were obtained from all consenting patients at baseline and at follow up visits, aliquoted and shipped to the NIDDK Central Repository where they were stored at −80° C. An aliquot of serum from all patients at baseline and relevant specimens from follow visits were selected and sent for testing for anti-HEV IgM, anti-HEV IgG, and HEV-RNA at the Hepatitis Viruses Section and later the Hepatic Pathogenesis Section of the Laboratory of Infectious Diseases, National Institute of Allergy and Infectious Diseases (NIAID). Anti-HEV IgM was detected by a class-capture ELISA assay using 10 μl of serum (20, 21). A modification of the ELISA assay was used to test all samples for anti-HEV IgG using 10 μl of serum, and positive controls were run on each plate (21, 22). An independent serum sample from all anti-HEV IgM positive patients was also tested for HEV RNA, using consensus primers, from 140 μl of serum by real-time polymerase chain reaction (PCR) with a lower limit of detection of 50 genome equivalents (GE)/reaction or 3.3 log10 genome equivalents (GE)/mL (23, 24). In addition, serum samples from all patients with HIV infection regardless of anti-HEV test results were also tested for HEV RNA. All testing was done in duplicate and under code. Samples that yielded borderline or discrepant results were retested using a separate serum aliquot shipped from the DILIN Repository.
Characterization of HEV genotype-
HEV genotype was detected by reverse-transcriptase polymerase chain reaction (RT-PCR) using primers derived from a segment of the conserved region of the RNA-dependent RNA polymerase within the ORF-1 of HEV genotype 3. RNA was extracted with TRIZOL LS (Life Technologies) according to the manufacturer’s instructions and reverse transcribed using SuperScript III RT (Life Technologies) using a reverse primer 1 (5’ GCGAAGGGGTTGGTTGGATG). The PCR product was purified with MinElute Gel Extraction Kit (Qiagen, Germantown, MD) and sequenced by ACGT, Inc. sequencing service. The sequence analysis was done using the Sequencher DNA sequence analysis software (Gene Codes Corporation, Ann Arbor, MI) and MacVector (MacVector, Apex, NC).
Definitions:
Acute HEV infection was defined as a patient who was anti-HEV IgM positive on repeated testing with or without detectable HEV RNA. The diagnosis of HEV infection was scored for 3 degrees of likelihood: as definite (1) if HEV RNA was detected at the same time as anti-HEV IgG and IgM; as highly likely (2) if anti-HEV IgG and IgM were present without HEV RNA initially, and a follow up specimen documented the decrease of anti-HEV IgM to borderline or undetectable while anti-HEV IgG remained present; or as probable (3) if anti-HEV IgG and IgM were present without HEV RNA initially and no follow up specimen was available. The presence of anti-HEV IgG alone without anti-HEV IgM was considered evidence of prior and resolved infection with the virus.
Causality assessment:
The causal relationship between the liver injury episode and the implicated drug was evaluated in a standardized fashion by the DILIN Causality Committee (14). A DILIN expert opinion causality score ranging from 1 (Definite > 95% likelihood), 2 (Highly Likely 75%-95% likelihood), 3 (Probable 50%-74% likelihood), 4 (Possible 25%-49% likelihood) to 5 (unlikely < 25% likelihood) was assigned by a consensus agreement of the committee members for all DILIN cases. In subjects with 2 or more implicated drugs, an overall causality score was assigned to the case in addition to an individual causality score for each drug. In this study, all cases regardless of causality score were tested for anti-HEV.
Cases positive for anti-HEV IgM were subjected to repeat causality adjudication by 3 independent reviewers using the results of HEV serological and PCR testing. Cases were re-adjudicated for the probability of DILI on the scale of 1 (definite) to 5 (unlikely). Cases were also judged using the same scale of 1 to 5 as to the likelihood that the acute liver injury was caused by acute hepatitis E based on the clinical, biochemical, and histologic findings.
Data analyses:
Descriptive statistics were computed for demographic and patient characteristics by frequency (percentage) for categorical variables and mean, standard deviation (SD), median, and the range for continuous variables. Differences between the anti-HEV IgG positive and negative test result groups were tested using the non-parametric test, Wilcoxon or Kruskal-Wallis test for continuous variables, and the chi-square test or trend test for categorical variables. A logistic regression model was developed to identify independent pre-specified prognostic factors that were associated with presence of anti-HEV IgG and absence of anti-HEV IgM. All tests were assessed at the 0.05 significance level. Statistical analyses were performed using SAS Version 9.4 (SAS Institute, Cary, NC) at the data-coordinating center at Duke Clinical Research Institute, Durham, North Carolina.
RESULTS
Patient Population.
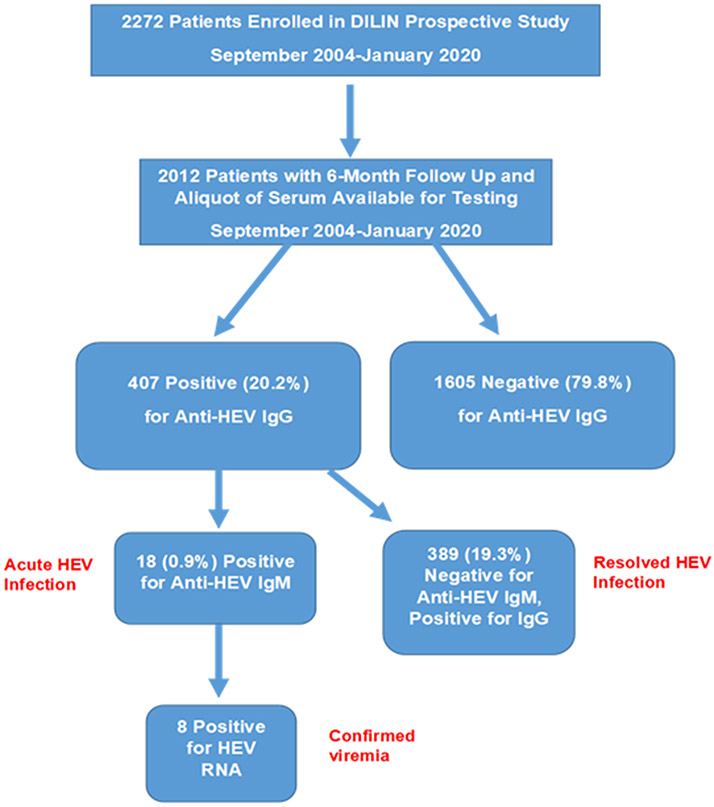
Between September 2004 and January 2020, 2,272 patients were enrolled in the DILIN Prospective study of whom 2,012 completed 6 months of follow up and had an aliquot of serum from the baseline study visit available for anti-HEV IgM and anti-HEV IgG testing (Figure 1). Of the total, 407 (20%) were anti-HEV IgG positive of whom 18 were also positive for anti-HEV IgM. Thus, 18 patients (0.9%) were considered to have acute HEV and 389 (19.3%) resolved HEV infection. The remaining 1,605 (79.8%) patients were negative for anti-HEV and were considered without evidence of HEV infection.
Figure 1-. Overview of study population-.
During the study period, there were 2272 patients enrolled into DILIN with 2012 who had HEV testing completed for analysis with 6 months of follow-up. HEV testing of baseline samples demonstrated that 18 (0.9%) were anti-HEV IgM (+) consistent with acute HEV infection, 389 (19.3%) were anti-HEV IgG + but anti-HEV IgM (−) consistent with prior HEV infection, and 1605 (79.8%) without prior HEV infection.
A comparison of patients with and without anti-HEV IgG is shown in Table 1. Those with anti-HEV were significantly older and more likely to be male and have diabetes compared to those without antibody. There were no differences by race, ethnicity, body mass index, latency to onset, and rates of HIV positivity. There were minor differences in median levels of several laboratory results such as serum AST and ALP. During follow-up, the all-cause and liver-related mortality were higher in the anti-HEV positive group. Many of the differences observed between the two groups appeared to be due to the older age of the antibody positive cohort. Using multivariate analyses, the only baseline clinical features associated with the presence of anti-HEV were patient age and enrollment era (Supplemental Table 1).
Table 1.
Clinical characteristics of the patients with and without anti-HEV
| Anti-HEV Positive N= 407 |
Anti-HEV Negative N=1605 |
P value | |
|---|---|---|---|
| Age (years) | 59.8 (8.4-88) | 48.7 (1.7-93.3) | < 0.001 |
| Male | 192 (47%) | 666 (41%) | 0.04 |
| Race | |||
| White | 330 (81%) | 1241 (78%) | 0.30 |
| Black | 44 (11%) | 212 (13%) | |
| Asian | 16 (4%) | 55 (3%) | |
| Other/Mixed | 17 (4%) | 91 (6%) | |
| Hispanic/Latino | 33 (8%) | 182 (11%) | 0.06 |
| BMI (kg/m2) | 26.8 (12-57) | 26.5 (8-66) | 0.28 |
| Diabetes | 122 (30%) | 366 (23%) | <0.01 |
| HIV positive | 9 (2%) | 46 (3%) | 0.47 |
| Latency to injury onset (days) | 45 (1-4663) | 44 (1-7046) | 0.86 |
| Labs at onset | |||
| AST (U/L) | 355 (33-10,920) | 309 (12-22,370) | 0.02 |
| ALT (U/L) | 471 (23-10,001) | 454 (3-15065) | 0.30 |
| Alk P (U/L) | 215 (41-1952) | 198 (31-4148) | <0.01 |
| Total bilirubin (mg/dL) | 4.6 (0.2-39.5) | 4.4 (0.2-48.4) | 0.32 |
| INR | 1.2(0.8, 6.6) | 1.2(0.8, 13.9) | 0.06 |
| Hepatocellular/ Mixed/ cholestatic | 55/ 21/ 24 % | 55/ 21/ 24 % | 0.95 |
| Hospitalized | 270 (66%) | 993 (62%) | 0.09 |
| DILIN severity score | |||
| 1 (Mild) | 100 (25%) | 400 (25%) | |
| 2 (Moderate) | 67 (16%) | 290 (18%) | 0.51 |
| 3 (Mod-Hospitalized) | 130 (31%) | 502 (31%) | |
| 4 (Severe) | 74 (18%) | 300 (19%) | |
| 5 (Death or Transplant) | 36 (9%) | 103 (6%) | |
| Death, all-cause | 46 (11%) | 118 (7%) | <0.01 |
| Death, liver-related | 26 (6%) | 49 (3%) | <0.01 |
| Chronic injury | 70/355 (20%) | 246/1384 (18%) | 0.40 |
Data reported as median (range) or number (%)
The increase in anti-HEV positivity with age is shown graphically in Figure 2a. The increase with age was highly significant overall (p<.001) and was similar in the 3 major racial groups (Figure 2b), rising from less than 10% below the age of 30 years to more than 30% in the oldest age groups. The apparent higher rates in Asians above the age of 60 was not significant, mainly because of small numbers in those age groups.
Figure 2a-. Seroprevalence of anti-HEV IgG in DILIN patients enrolled in the first 8 years of the study compared to more recently enrolled patients.
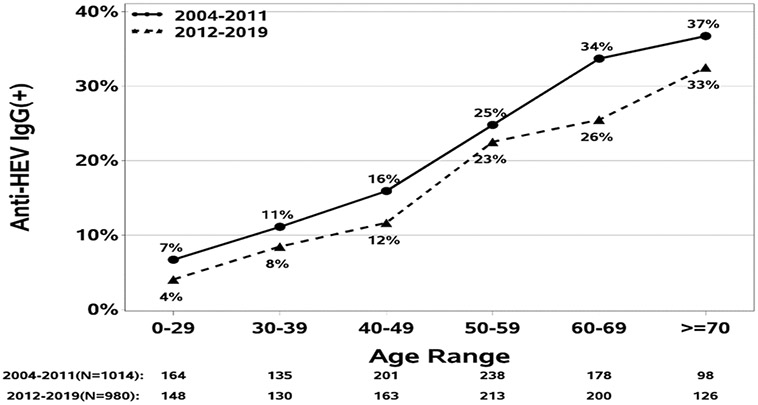
The seroprevalence of anti-HEV IgG increased in both cohorts with subject age but overall likelihood of having prior HEV infection was consistently lower in the later compared to the earlier era.
Figure 2b-. Seroprevalence of anti-HEV IgG by subject age and race.
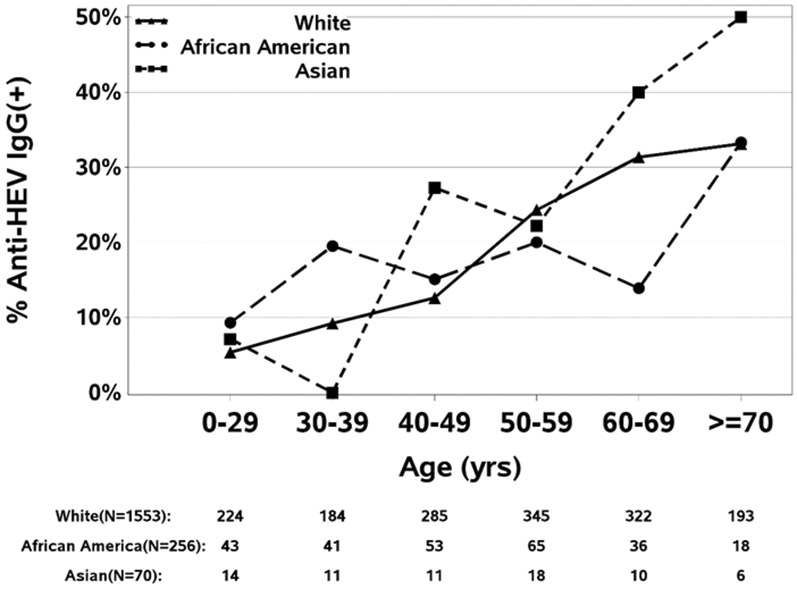
The seroprevalence increased in all racial subgroups amongst older patients.
In further analyses, the prevalence of anti-HEV was analyzed by year of enrollment. There was a steady decline in the prevalence of anti-HEV over time, from 22% between 2004 and 2011 to 18% between 2012 and 2019. Comparing the subjects enrolled during the first half of the study (2004-2011) to those enrolled in the second half (2012-2019) (Figure 2a) demonstrated a similar increase in rates of anti-HEV positivity with age, separated by a 4 to 8% difference at each age cohort, suggestive of a cohort effect in the age association.
A total of 8 major medical centers participated in the DILIN Prospective study over the 16 years of its enrollment. The overall rates of anti-HEV varied among the 8 centers (Supplementary Table 2) and were significantly higher at Mayo, UTSW and Einstein in Philadelphia. The three centers that enrolled patients during all 4 periods demonstrated a decrease in rates of anti-HEV positivity over time (Supplementary Figure 1), indicating that the association of age-related changes in anti-HEV positivity were not due to changes in sites of enrollment.
Acute HEV cases.
There were 18 cases of confirmed acute HEV infection with detectable anti-HEV IgM, 9 of which were previously reported based upon results from 2004 to 2008 (9). Selected factors for each of the 18 cases are summarized in Table 2. Seven patients had detectable HEV RNA in serum at the time of enrollment and an eighth patient had been found to have HEV RNA in a concurrent stool sample tested at the Centers for Disease Control and Prevention. Four of the 7 with HEV RNA detectable in serum could be genotyped, all were genotype 3. Follow up serum samples taken 6 to 8 months after onset were available from 11 patients, all of which were still reactive for anti-HEV IgG and negative or only borderline positive for anti-HEV IgM. All were also negative for HEV-RNA. Using a priori established definitions, HEV infection was scored as definite in 6, highly likely in 9, and probable in 3.
Table 2-.
Clinical features of 18 cases of acute HEV infection
| Case | Drug | Year | Age (yr.) |
Sex | HEV Infection Score |
HEV Causality Score |
Drug Causality Score |
Severity | Initial R-ratio |
Clinical Phenotype |
Liver biopsy Reading |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Azithromycin | 2004 | 74 | M | 3 | 2 | 4 | Moderate | 28.9 | Ac Hep | |
| 2* | Nevirapine | 2005 | 42 | M | 1 | 2 | 4 | Mild | 5.9 | Chr Hep | Chronic Hep |
| 3 | Isoniazid | 2005 | 64 | M | 1 | 3 | 4 | Severe | 12.1 | Ac on Chr | |
| 4 | Ezetimibe | 2005 | 64 | M | 3 | 3 | 4 | Moderate | 6.9 | Ac Hep | Steatohepatitis/Chol |
| 5 | Allopurinol | 2006 | 80 | M | 2 | 4 | 3 | Moderate | 4.0 | Mix Hep | |
| 6 | Telithromycin | 2006 | 83 | M | 3 | 4 | 3 | Severe | 2.0 | Chol Hep | |
| 7* | Nevirapine | 2005 | 61 | M | 1 | 1 | 5 | Severe | 4.8 | Mix Hep | Acute Hep/Chol |
| 8 | Dietary Suppl | 2006 | 59 | F | 2 | 1 | 5 | Moderate | 18.2 | Ac Hep | |
| 9 | Pravastatin | 2005 | 79 | M | 2 | 1 | 5 | Moderate | 6.2 | Ac Hep | |
| 10 | Levofloxacin | 2009 | 50 | M | 2 | 2 | 5 | Moderate | 7.0 | Ac Hep | |
| 11 | Lovastatin | 2009 | 76 | M | 2 | 2 | 5 | Moderate | 5.4 | Ac Hep | Steatohepatitis/Chol |
| 12 | Protein Shake | 2010 | 33 | M | 2 | 2 | 5 | Moderate | 10.2 | Ac Hep | |
| 13 | Atorvastatin | 2010 | 61 | F | 2 | 3 | 4 | Severe | 17.8 | Ac Hep | Acute Hep |
| 14 | Finasteride | 2011 | 71 | M | 1 | 2 | 4 | Moderate | 9.0 | Ac on Chr | Chol/Duct Injury |
| 15* | Isoniazid | 2013 | 63 | M | 1 | 2 | 4 | Moderate | 7.8 | Ac Hep | |
| 16 | CoEnzyme Q | 2015 | 71 | F | 2 | 2 | 5 | Moderate | 24.5 | Ac Hep | Acute Hep/Chol |
| 17 | Red Yeast Rice | 2015 | 53 | M | 2 | 2 | 4 | Moderate | 53.3 | Ac on Chr | Chol Hep |
| 18 | Doxycycline | 2019 | 76 | M | 1 | 2 | 5 | Severe | 34.5 | Ac Hep |
HEV Infection, HEV Causality and Drug Causality Scores are defined as 1 = definite (>95%), 2 = highly likely (75-95%), 3= probable (50-74%), 4= possible (25-49%) or 5=unlikely (<25%).
Severity was score as mild (bilirubin <2.5 mg/dL and INR <1.5), moderate (peak bilirubin ≥2.5 mg/dL without INR elevations ≥1.5; or vice versa), severe (bilirubin ≥2.5 and signs of hepatic failure), or fatal (death or liver transplantation for drug-induced liver injury within 6 months of onset).
Initial R ratio = (ALT/ULN) ÷ (Alk P/ULN) using lab values at presentation and ULN = upper limit of normal
Abbreviations: M, male; F, female; Hep, hepatitis; Ac, acute; Chr, chronic; Chol, cholestatic; Mix, mixed; Ac on Chr, acute on chronic as defined as presenting with acute injury superimposed on evidence of underlying chronic hepatitis or cirrhosis.
HIV positive.
Repeat adjudication was conducted on all 18 patients with all additional HEV test results provided to the reviewers (Table 2 and Supplementary Materials). The likelihood that HEV infection accounted for the liver injury episode was considered definite in 3 patients, highly likely in 9, and probable in 3. In the remaining 3 subjects, the liver injury was considered only possibly due to HEV infection with DILI still being considered probable or possible. These three subjects were thought to have experienced recent, incidental and subclinical HEV infection while the liver injury was considered at least partially due to the implicated medications (allopurinol, telithromycin and atorvastatin). Overall, the re-adjudication led to a downgrading of DILI causality score in 15 cases such that 9 were considered unlikely and 7 only possible while two were considered probable DILI. Comparison of the initial 9 patients enrolled during the first 4 years of the study to the subsequently enrolled 9 patients showed similar clinical features, disease severity and frequency of HIV infection (Table 2). Brief summaries with serial laboratory test results, liver biopsy findings and HEV serologies are provided in the Supplementary Material. Similar summaries of the initial 9 cases have been published (15).
HEV testing in HIV-positive patients.
Amongst the 55 subjects with HIV infection, 3 (5.6%) were anti-HEV IgM positive at the baseline visit and all of them had detectable HEV RNA. These 3 cases were all classified as having acute HEV infection (Table 2 cases 2, 7, 15). Testing for HEV RNA in the remaining 52 subjects without detectable anti-HEV IgG and anti-HEV IgM at the baseline visit demonstrated none with detectable HEV RNA alone. During follow-up, one of the 3 patients with HIV− HEV co-infection evolved into chronic hepatitis (Case #2).
Acute vs Resolved HEV infection.
A comparison of patients with acute versus those with resolved HEV infection is shown in Table 3. The 18 patients with acute HEV infection were significantly older (median age, 64.1 vs 59.4 years) and more likely to be male (83% vs 45%) compared to the previously infected patients. None of those with acute hepatitis E were below the age of 30. Those with acute HEV infection were also more likely to have HIV co-infection than those with resolved HEV (17% vs 2%), had higher levels of ALT and AST at presentation, and were more likely to have a hepatocellular pattern of enzyme elevation. While DILIN severity scores were similar in the two groups, none of the 18 patients with acute HEV infection died or required liver transplantation.
Table 3.
Clinical characteristics of patients with acute versus prior HEV infection
| Prior HEV infection (Anti-HEV IgG only) N= 389 |
Acute HEV infection (Anti-HEV IgG & IgM) N =18 |
P value | |
|---|---|---|---|
| Age (years) | 59.4 (8.4-88) | 64.1 (33.4-82.6) | 0.05 |
| Male (%) | 177 (45%) | 15 (83%) | <0.01 |
| Race | |||
| White | 314 (81%) | 16 (89%) | 0.33 |
| Black | 44 (11%) | 0 (0%) | |
| Asian | 15 (4%) | 1 (6%) | |
| Other/Mixed | 16 (4%) | 1 (6%) | |
| Hispanic/Latino | 32 (8%) | 1 (6%) | 0.99 |
| BMI (kg/m2) | 26.8 (12-57) | 24.4 (20-37) | 0.21 |
| Diabetes | 114 (29%) | 8 (44%) | 0.17 |
| HIV positive | 6 (2%) | 3 (17%) | <0.01 |
| Latency to injury onset (days) | 44 (1-4374) | 124 (6-4663) | 0.02 |
| Labs at onset | |||
| AST (U/L) | 347 (33-10,920) | 960 (92-2600) | 0.03 |
| ALT (U/L) | 462 (23-10,001) | 1231 (196-3838) | 0.05 |
| Alk P (U/L) | 220 (41-1952) | 196 (113-632) | 0.38 |
| Total bilirubin (mg/dL) | 4.6 (0.2-39.5) | 5.6 (0.4-17.3) | 0.35 |
| INR | 1.2(0.8, 6.6) | 1.3(1.2-2.0) | 0.48 |
| % Hospitalized | 257 (66%) | 13 (72%) | 0.59 |
| DILIN Severity Score | |||
| 1 (Mild) | 99 (25%) | 1 (6%) | |
| 2 (Moderate) | 63 (16%) | 4 (22%) | 0.128 |
| 3 (Moderate-Hospitalized) | 122 (31%) | 8 (44%) | |
| 4 (Severe) | 69 (18%) | 5 (28%) | |
| 5 (Death or Transplant) | 36 (9%) | 0 (0%) | |
| Death, all-cause | 46 (12%) | 0 (0%) | 0.24 |
| Death, liver-related | 26 (7%) | 0 (0%) | 0.62 |
| Chronic liver injury | 68/339 (20%) | 2/16 (12%) | 0.75 |
Data reported as median (range) or number (%)
Similar to the decrease in frequency of anti-HEV positivity over the 16 years of patient enrollment into DILIN, the frequency of acute hepatitis E also decreased. Thus, in the first 4 years of the prospective study, 9 cases of acute HEV infection were identified (2.4%). The numbers subsequently fell to 5 cases during the second (0.8%), 3 during the third (0.6%), and only 1 during the final four-year period (0.2%). Interestingly, we identified 2 of the last 3 enrollees with acute infection by local HEV test results, which resulted after enrollment but before the 6-month follow up period and centralized testing. Furthermore, one of these infections was clearly imported, occurring in a woman upon her return from a two month visit to India. The other 17 infections were believed to be locally acquired (autochthonous).
Liver histopathology.
Liver biopsies were available for central review in 8 patients with acute HEV infection (Table 2 and Supplementary Material). The biopsies were obtained at a median of 24 days (range, 3 to 127 days) after onset and were read centrally by a hepatopathologist (DEK) without accompanying clinical or HEV serologic data. Three subjects (#7, #13 and #16) showed typical features of acute hepatitis, two of whom had cholestasis. One patient (#2) with known HIV infection and clinical features of chronic hepatitis had biopsy findings of chronic hepatitis with duct injury and bridging fibrosis. Two patients (#4 and #11) had steatohepatitis with variable degrees of cholestasis (#4 with chronic cholestasis and #11 with marked bile stasis) and more than typical inflammation, both of whom had features of the metabolic syndrome (overweight or obesity, hyperlipidemia, hypertension or diabetes). One patient (#17), who had a history of alcoholism and clinical features of autoimmune hepatitis, had cholestatic hepatitis on liver biopsy and biochemical improvement with prednisone therapy. Finally, one patient (#14) had biopsy findings of cirrhosis with chronic cholestasis (pseudoxanthomatous change and copper deposition) and moderate bile stasis.
Discussion
Testing of serum samples from more than 2,000 patients with suspected DILI presenting between 2004 and 2020 in this multicenter study in the United States identified 18 (0.9%) with serological evidence of acute hepatitis E. All were positive for both IgG and IgM anti-HEV, and 8 had detectable HEV RNA. This rate is lower than previously described from this research network (14) and substantially lower than from studies in Spain and the United Kingdom (7-9). The data also showed that the number of cases of acute HEV infection appeared to be declining over the 16-year enrollment period from 3% initially to less than 1% in the last 4 years. Nonetheless, a consistent clinical phenotype of patients with acute HEV infection was seen that was similar to that of the first 9 patients and with other descriptions of typical cases of acute hepatitis E, mostly with acute, self-limited disease in elderly men. In addition, most cases were autochthonous; only one had a history of recent travel to an endemic area for HEV (3). For the others, there was no obvious source of infection, no reports of recent travel, exposure to contaminated water, ingestion of poorly cooked meats or wild game, blood transfusion, or temporal or geographic clustering.
Clinically, the 18 acute HEV patients presented with typical symptoms of acute viral hepatitis with fatigue, nausea, and abdominal pain followed by dark urine and jaundice. The predominant pattern of injury was hepatocellular (94%) while immunoallergic and autoimmune features were uncommon (Table 2). Several patients exhibited features of acute-on-chronic liver injury which is typical of HEV infection occurring in patients with chronic liver disease. Most patients were jaundiced (94%), many were hospitalized (72%), and 5 (28%) developed signs of hepatic failure (INR elevations, ascites or hepatic encephalopathy). However, no patient died of acute liver failure or required liver transplantation. Almost all patients recovered within 2 months with a median time for serum bilirubin falling to normal of 14.5 days, serum ALT of 60 days, and alk P of 63.5 days. Two patients (#2 and #17) had persistent evidence of liver injury 6 months after onset, one (#2) with suspected chronic hepatitis E and one (#17) with clinical features of autoimmune hepatitis.
This study also demonstrated a gradual decrease in the prevalence of anti-HEV over the 16-year period of enrollment. The overall rate of anti-HEV IgG without IgM was 19.3%. However, the rate appeared to decrease with time from 22% during the first four years and 18% during the last four years of the study. The decrease was also seen when shown as age-specific rates of anti-HEV with a shift to the right in prevalence suggesting that the rate was declining because of fewer new infections and persistence of antibody with aging of the cohort. Other explanations might be a change in the characteristics of patients over the duration of this study or changes in the average age of acquisition of antibody. Most persons with anti-HEV gave no history of jaundice or hepatitis, suggesting that the majority of cases were mild and subclinical. The epidemiology of hepatitis E remains unclear particularly with genotypes 3 and 4 infections, which tend to occur endemically and are likely zoonoses for which humans are an accidental host, as opposed to genotypes 1 and 2 infections which occur in outbreaks and epidemics in humans usually related to water contamination with human waste. While genotype 3 infections have been linked to eating undercooked pork and wild game, most patients with the disease give no clear history of such exposures. Epidemiologic data suggest that cases may be caused by contaminated well water. If so, the recent decline in acute cases of hepatitis E and its relative rarity in the United States may be related to improved water and food safety. However, a recent study demonstrated that 6.3% of market weight pigs had detectable HEV-RNA and 40% were anti-HEV IgG positive supporting the hypothesis that contaminated pork may be a source of authochonous HEV infection in humans (25)
The 19.3% rate of prior resolved HEV infection (positive HEV IgG and negative IgM) is similar to that described in a population-based study using NHANES data from 1988-1994 but much higher than a later NHANES analysis using an Italian commercial assay not approved in the US (17, 19). The prevalence of antibody varied markedly with age while no other clinical feature was independently associated with its presence. The rise in prevalence with age is likely due to the cumulative incidence of subclinical infection with HEV in the U.S. population. The data also suggest a gradual decrease in prevalence with a shift of rates to older age groups, a pattern suggestive of a cohort effect. Thus, as acute HEV infection becomes less common, the slope of the prevalence curve is likely to stay the same but shift to older age groups. Another explanation, however, is that HEV occurs in adulthood and at a steady rate. Regardless, the prevalence and risk factor for HEV in the United States are very different from other forms of viral hepatitis, that tend to occur in younger individuals and in high-risk groups (HBV, HCV, HDV) or as epidemics or small outbreaks (HAV).
While HEV infection can mimic DILI, it appears to be rare in the United States, making it difficult to recommend testing for HEV infection in all cases of liver disease of unclear origin. Furthermore, there are currently no commercially available, FDA-approved assays for either HEV antibody or HEV-RNA, and the only options are to rely upon commercial assays of uncertain sensitivity and specificity. Nevertheless, identification of HEV infection can be important. For one thing, it allows for the continuation of a medication that might have been unnecessarily discontinued. Additionally, HEV can become chronic and lead to significant liver disease, cirrhosis and end-stage liver disease, particularly in patients with immunodeficiencies or on immunosuppression. Clues that suggest HEV as the cause include acute hepatocellular liver injury with jaundice in elderly men with no apparent risk factors or other causes, suspected liver injury from an agent not previous associated with hepatotoxicity or with unusual clinical presentation or latency to onset, and acute hepatitis arising in patients with HIV infection or taking immunosuppressive agents. Strengths of our study include the use of the same assay and testing algorithm using anti-HEV IgG then anti-HEV IgM and PCR testing over a prolonged period of time in a large group of patients increasing the confidence and generalizability of our study results. The experience at the NIAID which helped develop the anti-HEV IgM and anti-HEV IgG serology tests, has indicated that no previous cases of acute HEV with detectable HEV RNA were anti-HEV IgM negative. Due to resource limitations and prior studies demonstrating that only 40 to 60% of acute HEV cases have detectable HEV-RNA, all of the serum samples in this study were not screened for HEV RNA (29). However, the serum of all 55 patients with HIV infection were tested and did not reveal any with detectable HEV RNA in the absence of anti-HEV IgM. We acknowledge that there is a potential for referral bias to the DILIN study. Over time, community physicians may be testing for occult HEV infection in their suspected DILI patients and not referring those with confirmed infection to the network.
In summary, the frequency of acute HEV infection masquerading as DILI is declining over time in the United States multicenter DILIN Prospective study. A prevalent phenotype of older, Caucasian men presenting with acute hepatitis and jaundice is noted in those with acute HEV infection that is initially misattributed to antibiotics or lipid-lowering agents, frequently with a prolonged drug latency. The declining incidence of acute and prior HEV infection in our large prospective study implies that the total burden and reservoir of HEV infection may be declining in the United States. Since sporadic acute HEV infection is a leading cause of acute viral hepatitis in several European countries, the European Liver Society guidelines recommend testing for HEV infection using anti-HEV IgM and HEV RNA by PCR in all patients with suspected DILI, particularly in those with atypical features or high serum ALT levels (9,30,31). In the United States, the American College of Gastroenterology recommends that assessment for HEV infection be limited to selected patients such as older men with unexplained acute hepatocellular injury with jaundice or HIV infection due to the lack of validated, widely available and authorized diagnostic assays (32).
Supplementary Material
Study Highlights.
What is known
Acute HEV infection is a rare cause of acute viral hepatitis in the general US population.
However, recent European studies have suggested that up to 10% of patients with suspected idiosyncratic drug induced liver injury (DILI) may have undiagnosed acute HEV.
What is new
The overall incidence of acute HEV infection among 2,012 consecutive American patients with suspected DILI was very low at 0.9%.
The 18 patients with acute HEV infection were largely older Caucasian men (median age 64 years, 83% male) that had moderately severe acute hepatocellular injury with jaundice but none died during follow-up.
The incidence of acute HEV infection is declining over time in this multicenter registry study of patients with suspected DILI but testing for HEV may be of value in selected individuals.
Financial Support:
This work performed by investigators of the Drug Induced Liver Injury Network (DILIN) is structured as an U01 cooperative agreement supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) with funds provided by the following grants: U01-DK065176 (Duke), U01-DK065201 (UNC), U01-DK065184 (Michigan), U01-DK065211 (Indiana), U01DK065193 (UConn), U01-DK065238 (UCSF/CPMC), U01-DK083023 (UTSW), U01-DK083027 (TJH/ UPenn), U01-DK082992 (Mayo), U01-DK083020 (USC). Additional support is provided by CTSA grants: UL1 RR025761 (Indiana), UL1TR000083 (UNC), UL1 RR024134 (UPenn), UL1 RR024986 (Michigan), UL1 RR024982 (UTSW), UL1 RR024150 (Mayo) and by the Intramural Research Program of the NIH, National Cancer Institute. R E. E, H.N., and P.F. are supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, 20892, USA. For further information regarding the DILIN network see https://dilin.org/ and for the NIDDK Central Repository see https://repository.niddk.nih.gov/home.
We are grateful to all patients and study coordinators who participated in the DILIN study.
Abbreviations
- ALF
Acute liver failure
- Alk P
Alkaline phosphatase
- ALT
Alanine aminotransferase
- ANA
Antinuclear antibody
- AST
Aspartate aminotransferase
- BMI
Body mass index
- DILI
Drug induced liver injury
- DILIN
Drug induced liver injury network
- GE
Genome equivalents
- HAV
Hepatitis A virus
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- HEV
Hepatitis E virus
- HIV
Human immunodeficiency virus
- SMA
Smooth muscle antibody
- ULN
Upper limit of normal
Footnotes
ClinicalTrials.gov number: NCT00345930.
Disclosures: Dr. Fontana has received research support from Gilead and Abbvie and consulted for Sanofi. Dr’s Barnhart, Hoofnagle, Kleiner, Farci, Engle, Nguyen and Gu have no conflict of interest to declare.
Disclaimer: Dr. Hayashi is employed by the US Food and Drug Administration (FDA), but conclusions in this paper do not reflect any opinion of the FDA. The FDA has not evaluated the findings, assessments or conclusions in this paper.
References
- 1.Fontana RJ, Seeff LB, Andrade RJ, et al. Standardization of Nomenclature and causality assessment in Drug-Induced Liver Injury: Summary of a Clinical Research Workshop. Hepatology 2010: 52: 7340–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalasani NP, Hayashi PH, Bonkovsky HL, et al. ACG Clinical Guideline: The Diagnosis and Management of Idiosyncratic Drug-Induced liver Injury. 2014; 109: 950–966. [DOI] [PubMed] [Google Scholar]
- 3.Hoofnagle JH, Bjornsson ES. Drug-induced liver Injury: Types and phenotypes. NEJM 2019; 381: 1396–1405. [DOI] [PubMed] [Google Scholar]
- 4.Drobeniuc J, Greene-Montfort T, Le NT, et al. Laboratory based surveillance for hepatitis E virus infection. United States, 2005-2012. Emerg Infect Dis 2013: 19: 218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoofnagle JH, Nelson KE, Purcell RH. Hepatitis E NEJM; 2012: 367: 1237–1244. [DOI] [PubMed] [Google Scholar]
- 6.Llovet LP, Gratacos-GInes J, Oswaldo O, et al. Higher seroprevalence of hepatitis E virus in autoimmune hepatitis: Role of false positive antibodies. Liver International 2020: 40: 558–564. [DOI] [PubMed] [Google Scholar]
- 7.Dalton HR, Fellows HJ, Stableforth W, et al. The role of hepatitis E virus testing in drug induced liver injury. Aliment Pharmacol Ther; 2007: 26: 1429–1435. [DOI] [PubMed] [Google Scholar]
- 8.Sanabria-Cabrera J, Sanjuan-imenez R, Clavinjo E, et al. Incidence and prevalence of acute HEV infection in patients with suspected drug-induced liver injury in the Spanish DILI Registry. Liver International 2021; 41: 1523–1531. [DOI] [PubMed] [Google Scholar]
- 9.Wallace SJ, Swann R, Donnelly M, et al. Mortality and morbidity of locally acquired hepatitis E in the National Scottish cohort: A multicenter, retrospective study. Aliment Pharmacol Ther 2020: 51: 974–986. [DOI] [PubMed] [Google Scholar]
- 10.Allaire M, Bazille C, Selves J, et al. Hepatitis E virus infection mimicking acute graft rejection in a liver transplant recipient. Clin Res Hepatol Gastroenterol 2018: 42(4): e68–e71. [DOI] [PubMed] [Google Scholar]
- 11.Fontana RJ, Engle RE, Gottfried M, , et al. Role of Hepatitis E Virus infection in North American Patients with severe acute Liver Injury. Clin Trans Gastro 2020: 11: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontana RJ, Engle RE, Scaglione S, et al. The role of Hepatitis E virus infection in Adult Americans with acute Liver Failure. Hepatology 2016; 64: 1870–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Love A, Bjornsdottir TB, Olafsson S, Bjornsson ES. Lower prevalence of hepatitis E in Iceland: A seroepidemiological study. Scand J Gastroenterol 2018; 53: 293–296. [DOI] [PubMed] [Google Scholar]
- 14.Fontana RJ, Watkins PB, Bonkovsky HL, et al. Drug-Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Saf. 2009; 32:55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davern TJ, Chalasani N, Fontana RJ, et al. Acute hepatitis E infection accounts for some cases of suspected drug-induced liver injury. Gastroenterology 2011: 14: 1665–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenggenhager D, Weber A. An update on the Clinicopathologic Features and Pathologic diagnosis of Hepatitis E in Liver Specimens. Adv Anat Pathol 2018: 25: 273–281. [DOI] [PubMed] [Google Scholar]
- 17.Ditah I, Ditah F, Devaki P, et al. Current epidemiology of hepatitis E virus infection in the US: Low seroprevalance in the National Health and Nutrition Evaluation Survey. Hepatology 2014: 64: 815–822. [DOI] [PubMed] [Google Scholar]
- 18.Holm DK, Moessner BK, Engle RE, et al. Declining prevalence of hepatitis E antibodies among Danish Blood donors. Transfusion 2015: 55: 1662–1667. [DOI] [PubMed] [Google Scholar]
- 19.Teshale EH, Denniston MM, Drobeniuc J, et al. Decline in hepatitis E virus antibody prevalence in the United States from 1988-1994 to 2009 −2010. J Infect Dis 2015: 211: 366. [DOI] [PubMed] [Google Scholar]
- 20.Kleiner DE, Chalasani NP, Lee WM, et al. Hepatic histological findings in suspected drug-induced liver injury: systematic evaluation and clinical associations. Hepatology. 2014; 59(2):661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engle RE, Yu C, Emerson SU, et al. Hepatitis E virus capsid antigens derived from viruses of human and swine origin are equally efficient for detecting anti-HEV by Enzyme immunoassay. J Clin Micriobiol 2002: 40: 4576–4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu C, Engle RE, Bryan JP, et al. Detection of immunoglobulin M antibodies to hepatitis E virus by class capture enzyme immunoassay. Clin Diagn Lab Immun 2003; 10: 579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johne R, Plenge-Bonig A, Hess M, et al. Detection of a novel hepatitis E-like virus in faeces of wild rates using a nested broad-spectrum RT-PCR. J Gen Virol 2010: 91: 750–758. [DOI] [PubMed] [Google Scholar]
- 24.Shukla P, Nguyen HT, Torian U, et al. Cross-species infections of cultured cells by hepatitis E virus and discovery of an infectious virus-host recombinant. PNAS 2011; 108: 2438–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sooryanarain H, Heffron CL, Hill DE, et al. Hepatitis E virus in pigs from Slaughterhouses, United States, 2017-2019. Emerg Inf Dis 2020; 26: 2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quickert S, Reuken PA, Rose M, et al. Acute hepatitis E is an under-reported cause of severe acute liver injury. Clin Gastro Hepatol 2018: 17: 1004–1007.24.5 [DOI] [PubMed] [Google Scholar]
- 27.Drebber U, Odenthal M, Aberle SW, et al. Hepatitis E in liver biopsies from patients with acute hepatitis of clinically unexplained origin. Front Physiol 2013: 4: 351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norder H, Galli C, Magnil E, Sikora P, et al. Hepatitis E virus genotype 3 genomes from RNA positive but serologically negative plasma donors have CUG as the start codon for ORF3. Intervirology 2018; 61: 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patra S, Kumar A, Trivedi SS, et al. Maternal and fetal outcomes in pregnant women with acute hepatitis E virus infection. Ann Intern Med 2007: 147: 28–33. [DOI] [PubMed] [Google Scholar]
- 30.EASL Clinical Practice Guidelines on Hepatitis E virus infection. J Hepatology 2018; 68 1256–1271. [DOI] [PubMed] [Google Scholar]
- 31.EASL Clinical Practice Guidelines: Drug-induced liver Injury. J Hepatology 2019; 70: 1222–1261. [DOI] [PubMed] [Google Scholar]
- 32.Chalasani N, Maddur H, Russo MW et al. ; Practice Parameters Committee of the American College of Gastroenterology. Diagnosis and Management of Idiosyncratic Drug- induced liver injury. Am J Gastroenterol 2021; 116: 878–898. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.