Abstract
Background
Studies comparing mental disorder risks between women with breast cancer and cervical cancer are lacking. This study compared risks of developing anxiety and depression between women with breast cancer (BC cohort) and women with cervical cancer (CC cohort) using insurance claims data of Taiwan.
Methods
From the 2000 to 2016 data, we identified a BC cohort and BC controls (N = 96,862) and a CC cohort and CC controls (N = 26,703), matched by propensity scores. Incident mental disorders and the Cox method estimated the related cancer cohort to control cohort hazard ratios (HRs), and 95% confidence intervals (CIs) were estimated by the end of 2016.
Results
Compared to the CC cohort, the BC cohort had slightly higher incident anxiety (15.9 versus 15.5 per 1,000 person-years) and depression (6.92 vs. 6.28 per 1,000 person-years). These mental disorders were higher in respective cancer cohorts than controls. The BC cohort to BC control adjusted HRs of anxiety and depression were 1.29 (95% CI = 1.25–1.33) and 1.78 (95% CI = 1.69–1.87), respectively. The corresponding adjusted HRs for the CC cohort were 1.12 (95% CI = 1.06–1.18) and 1.29 (95% CI = 1.18–1.41). The combined incidence rates of both disorders were 1.4-fold greater in the BC cohort than in BC controls (22.8 vs. 15.8 per 1,000 person-years), and 1.2-fold greater in the CC cohort than in the CC controls (21.7 vs. 18.3 per 1,000 person-years).
Conclusion
Women with breast cancer or cervical cancer are at an elevated likelihood of developing anxiety and depression disorders. These incident disorders are slightly higher in those with breast cancer.
Keywords: anxiety, breast cancer, cervical cancer, depression, retrospective cohort study
Introduction
Patients with anxiety and/or depression are in depressed mood and at aversion to social activity (1, 2). With more than 264 million people of all ages being affected, depression has become an important burden in medical care and public health worldwide (3, 4). These disorders can also lead to subsequent health disorders and shortened life expectancy (4). Depression and anxiety may result from biological or psychological factors with a complex interaction of socioeconomic factors. People who have health ailments or gone through adverse life events are at an elevated risk to develop anxiety and depression. In fact, depressed mood is frequently developed as a reaction to a catastrophic disorder perceived to threaten the life and wellbeing, such as in patients with cancer (5–10). Patients may suffer from cancer treatment side effects, chronic pain, self-esteem and body image, changes in quality of life and family life, and fears of recurrence, subsequent disorders, and death (11–15). Anxiety, depression, and other mood disorders are thus prevalent in cancer patients (6–8, 16–18).
Breast cancer and cervical cancer rank as the first and fourth common female cancers, respectively, with disparities in incidence and mortality worldwide (19, 20). Breast cancer is more prevalent in women in Western countries than in women in developing counties (19), whereas nearly 90% of women with cervical cancer were identified in low-income and middle-income populations (20). The breast and cervix represent the image of appearance and femininity of women. Treatments for cancer can have a negative impact on their self-image. Women with breast cancer and cervical cancer are at a high risk to be dissatisfied by changes in body image during and after the treatment procedure. Patients may also suffer from changes of sexual function and fertility, which can lead to not only sexual disfunction but also psychological adaptation (21, 22). Concurrent treatments for both cancers have been highly effective. However, survival rates have large differences internationally (23). The breast cancer survival may range from 40% in South Africa to 90% or higher in high-income countries (24). The gap of survival rates was even greater for cervical cancer, ranging from 50% to 70% (25). The 5-year survival rates also vary by the stage of the disease at diagnosis and sociodemographic status of patients. The racial disparity of survival rates was greater for cervical cancer than for breast cancer (59.8%–73.7% versus 82.2%–91.5%) in 2011–2017 in the US (26). The psychological adaptation may differ between women with breast cancer and women with cervical cancer and is associated with the disparities. Anxiety, depression, and other mood disorders developed in women with breast cancer and cervical cancer may vary by prevalence and survival among populations.
A systemic review based on seven studies found that the depression could last for years after treatment in women with CC (27). A German study interviewed a nationwide random sample of 2,141 patients with cancer and found that patients with breast cancer had the highest prevalence of mental disorder. A systematic review based on 17 studies found that the prevalence rates of anxiety ranged from 17.9% to 33.3% and of depression from 9.4% to 66.1% in breast cancer survivors (9). Studies have also associated depression with elevated cancer mortality (10).
Studies comparing the psychological adaptation between women with breast cancer and women with cervical cancer are in demand for Asian women. Cervical cancer and breast cancer ranked earlier as the first and second most common female cancers in Taiwan, with age-adjusted incidence rates of 26.27 and 20.95 per 100,000 in 1988–1993, respectively (28). Breast cancer overtook cervical cancer in 1993–1997 with incidence rates of 28.99 versus 26.82 per 100,000, respectively. The incidence gap between the two cancers increased consistently, shifting to 71.91 and 8.72 per 100,000, respectively, in 2013–2016, with cervical cancer ranking the eighth common female cancer. We suspected that the risk of developing mental disorders might be greater in women with breast cancer than women with cervical cancer, although the 5-year survival rate for breast cancer was greater than that for cervical cancer (86.8% versus 72.5%) (29). Thus, the purpose of this study was to compare the risk of developing anxiety and depression between women with breast cancer and women with cervical cancer using the insurance claims data of Taiwan.
Methods and materials
Data source
We used insurance claims databases and cancer registry databases from 1996 to 2016 available at the Health and Welfare Data Science Center, Ministry of Health and Welfare of Taiwan. The insurance claims data consisted of information on demographic status of insured population, longitudinal medical records of outpatient and inpatient cares, including treatments and medications provided, and costs of cares. More than 99% of residents in Taiwan have been covered in this compulsory single-payer healthcare program (30). Diseases were coded with International Classification of Diseases, Clinical Modification Ninth Revision, (ICD-9-CM), before 2016 and Tenth Revision (ICD-10-CM) since 2016. All identifications of all three data sets had been changed into the same surrogate numbers before the databases were released to users. This study was approved by the Ethical Research Committee at China Medical University and Hospital (H107257). Because personal identifications in the data files had been scrambled to protect privacy, patient consents were waived.
Study design
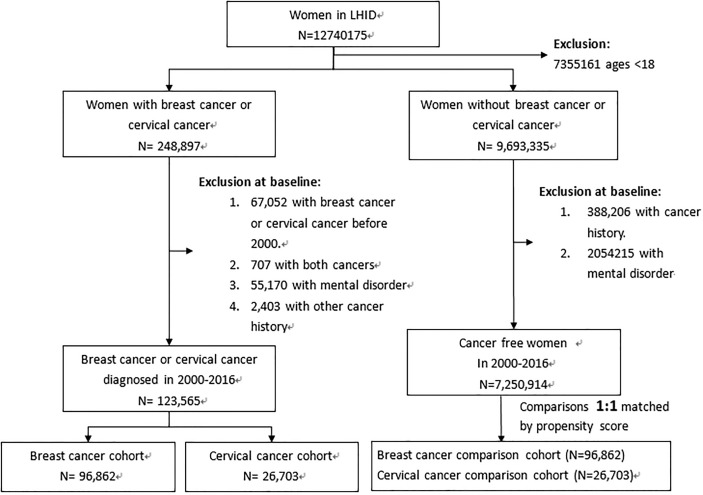
From claims data with healthcare records in the period of 2000–2016, all women aged 18 and above were identified to establish 2 pairs of cancer cohorts and control cohorts. After excluding women with cancer history and mental disorders diagnosed before 2000, we identified 96,862 women with breast cancer and 26,703 women with cervical cancer as the breast cancer cohort (BC cohort) and the cervical cancer cohort (CC cohort), respectively ( Figure 1 ). The date with the cancer diagnosed was defined as the index date. Among 7,250,914 women without the history of cancer and mental disorders, we randomly selected 96,862 women as the BC cohort’s controls (BC controls) and 26,703 women as the CC cohort’s controls (CC controls), matched by the propensity score. Multivariable logistic regression estimated the propensity score for each woman with variables of age, income, urbanization level of residential areas, diagnosis year, and Charlson comorbidity index (CCI). We estimated the CCI with the sum of weighted values of comorbidities: one point was scored for myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, ulcer disease, mild liver disease, and diabetes; two points for hemiplegia, moderate or severe renal disease, diabetes with end organ damage, leukemia, and lymphoma; three points for moderate or severe liver disease; and six points for AIDS (31).
Figure 1.
Flowchart for establishing study cohorts.
Outcome
From the databases, we identified mental health disorders of anxiety (ICD-9: 300.0; ICD-10-CM: 110 F40 and F41) and depression: ICD-9-CM: 296.0-296.8, 300.4, and 311.X; ICD-10-CM: F32.9, F30-F33, F34.8-F34.9, and F39), which appeared in the outpatient records for at least twice or in the inpatient records for at least once. Follow-up time in person-years was calculated for each woman from the index date until the mental health disorder diagnosis, withdrawal from the insurance, death, or the end of 2016.
Data analysis
This study used SAS Software 9.4 in Windows (SAS Institute, Cary, NC, USA) to analyze data, and 118 used P < 0.05 to indicate the significance level in comparisons. Data analysis first compared the baseline distributions, between the BC cohort and BC controls, and between the CC cohort and CC controls, including age, occupation, income, urbanization level of residential area, and CCI. The standardized mean difference of each variable between each pair of cancer cohort and control cohort was calculated to indicate the significance level. The Kaplan–Meier method was used to estimate the combined cumulative incident anxiety and depression between each pair of the cancer cohort and the control cohort. Differences were examined by the log-rank test. R software (R Foundation for Statistical Computing, Vienna, Austria) was used to plot the cumulative incidence. We calculated the incidence number and rate of each type of mental disorder for each cohort ( Table 2 ). Cox proportional hazard regression analysis was used to calculate the cancer cohort to the control cohort aHR of each type of disorder. Adjustment was performed by the matched pair. The BC cohort to the CC cohort aHRs was also calculated for the two types of disorder, controlling for age, occupation, income, urbanization, and CCI score. Incidence rates of anxiety and depression (per 1,000 person-years) were then pooled as the overall rate calculated for each cohort by the baseline variables. Cox proportional hazard regression analysis was also used to calculate the cancer cohort to the control cohort adjusted hazard ratios (aHRs) and 95% confidence intervals (CI) by these variables. Adjustment was performed by the matched pair.
Table 2.
Incidence rates of anxiety and depression and cancer cohort to comparisons hazard ratio.
| Breast cancer | BC control | Hazard ratio (95% confidence interval) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | n | PY | Rate | n | PY | Rate | Crude | Adjusted | |
| Anxiety | 8,724 | 549,703 | 15.9 | 7,617 | 632,488 | 12.0 | 1.33 (1.30-1.39) | 1.29 (1.25-1.33) | |
| Depression | 3,806 | 549,703 | 6.92 | 2,385 | 632,488 | 3.77 | 1.83 (1.79-1.89) | 1.78 (1.69-1.87) | |
| Cervical cancer | CC control | ||||||||
| Anxiety | 2,584 | 167,182 | 15.5 | 2,754 | 203,189 | 13.6 | 1.14 (1.09-1.21) | 1.12 (1.06-1.18) | |
| Depression | 1,050 | 167,182 | 6.28 | 960 | 203,189 | 4.72 | 1.33 (1.25-1. 41) | 1.29 (1.18-1.41) | |
PY, person-years; Rate, per 1,000 person-years; Adjusted hazard ratio, adjusted for matched pair.
p < 0.001 for each hazard ratio.
Results
Table 1 shows that distributions of all baseline variables were not different between the BC cohort and BC controls and between the CC cohort and CC controls. The cancer cohort was slightly older than their controls in both pairs (mean ages 52.3 versus 51.7 years for the BC pair and 56.6 versus 56.1 for the CC pair). Nearly 30% of women in the CC pairs and 15% of women in the BC pairs were the elderly. Compared to the BC cohort, women in the CC cohort had less white-collar jobs (18% versus 32%) with more lower incomes (52% versus 36%), living in less urbanized areas (52% versus 40%) and having a higher portion of women with a CCI score of 1 and above (14.0% versus 9.20%).
Table 1.
Distributions of baseline characteristics compared between breast cancer cohort and BC control cohort and between cervical cancer cohort and CC control cohort.
| Variable | Breast cancer N = 96,862 | BC control N=96,862 | Standardization difference | Cervical cancer N = 26,703 | CC control N = 26,703 | Standardization difference | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age, years | n | % | n | % | n | % | n | % | ||
| 18-39 | 11,988 | 12.4 | 11,964 | 12.4 | 0.001 | 2,915 | 10.9 | 2,908 | 10.9 | 0.001 |
| 40-49 | 31,919 | 33.0 | 32,199 | 33.2 | 0.006 | 6,555 | 24.6 | 6,592 | 24.7 | 0.003 |
| 50-64 | 37,843 | 39.1 | 37,906 | 39.1 | 0.001 | 9,270 | 34.7 | 9,151 | 34.3 | 0.009 |
| 65-74 75+ |
11,485 3,627 |
11.9 3.74 |
11,243 3,550 |
11.6 3.67 |
0.009 0.010 |
5,335 2,628 |
20.0 9.84 |
5,234 2,818 |
19.6 10.5 |
0.010 0.012 |
| Mean (SD) | 52.3 | (11.9) | 51.7 | (13.8) | 0.046 | 56.6 | (14.4) | 56.1 | (16.0) | 0.034 |
| Occupation | ||||||||||
| Homemaker | 26,383 | 27.2 | 26,122 | 27.0 | 0.006 | 8,923 | 33.4 | 9,026 | 33.8 | 0.008 |
| White collar | 20,911 | 31.9 | 30,913 | 31.9 | 0.000 | 4,881 | 18.3 | 4,782 | 17.9 | 0.010 |
| Blue collar | 28,101 | 29.0 | 28,346 | 29.3 | 0.006 | 9,452 | 35.4 | 9,485 | 35.5 | 0.003 |
| Other | 11,467 | 11.8 | 11,481 | 11.9 | 0.000 | 3,447 | 12.9 | 3410 | 12.8 | 0.004 |
| Income, NTD | ||||||||||
| ≤20,000 | 35,152 | 36.3 | 35,141 | 36.3 | 0.000 | 13,777 | 51.6 | 13,845 | 51.9 | 0.005 |
| 20,001-39,999 | 40,279 | 41.6 | 40,387 | 41.7 | 0.002 | 9,669 | 36.2 | 9,669 | 36.2 | 0.000 |
| 40,000+ | 21,431 | 22.1 | 21,334 | 22.0 | 0.002 | 3,257 | 12.2 | 3,189 | 11.9 | 0.008 |
| Urbanization | ||||||||||
| Urban | 57,723 | 59.6 | 58,045 | 59.9 | 0.007 | 12,811 | 48.0 | 12,857 | 48.2 | 0.003 |
| Suburban | 30,659 | 31.7 | 3,791 | 31.8 | 0.003 | 10,118 | 37.9 | 10,215 | 38.3 | 0.007 |
| Rural | 8,480 | 8.75 | 8,026 | 8.29 | 0.017 | 3,774 | 14.1 | 3,631 | 13.6 | 0.015 |
| CCI score | ||||||||||
| 0 | 87,948 | 90.8 | 89,857 | 92.8 | 0.072 | 22,961 | 86.0 | 23,408 | 87.7 | 0.050 |
| 1 | 6,053 | 6.25 | 5,106 | 5.27 | 0.042 | 2,281 | 8.54 | 2,086 | 7.81 | 0.027 |
| 2+ | 2,861 | 2.95 | 1,899 | 1.96 | 0.064 | 1,461 | 5.47 | 1,209 | 4.53 | 0.043 |
SD, standard deviation; Other: unemployed or retired; CCI, Charlson comorbidity index.
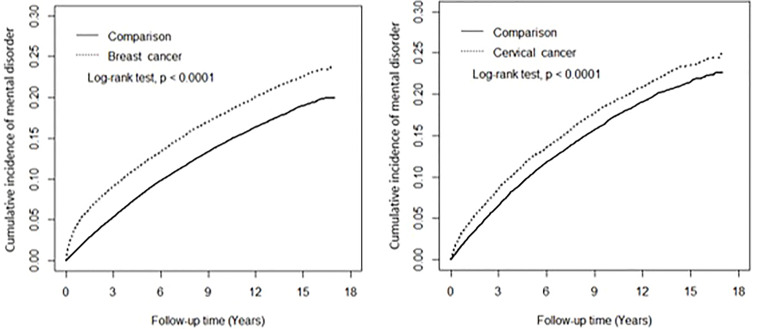
Figure 2 shows that after the 17-year follow-up, the cumulative incidence of the two mental disorders combined in the BC cohort was approximately 3.9% higher than the BC control cohort (23.9% versus 20.0%) (log-rank test p < 0.0001), and that in the CC cohort was about 2.2% higher than in the CC control cohort (24.7% versus 22.5%) (log-rank test p < 0.0001).
Figure 2.
Cumulative incident anxiety and depression combined among study cohorts.
Table 2 shows that the incidence of anxiety was higher than that of depression in each cohort. The incidence of anxiety was 1.3-fold higher in the BC cohort than in the BC controls (15.9 versus 12.0 per 1,000 person-years) with an aHR of 1.29 (95% CI = 1.25–1.33). The incidence of depression was 1.8-fold higher in the BC cohort than in the BC controls (6.92 versus 3.77 per 1,000 person-years) with an aHR of 1.78 (95% CI = 1.69–1.87). The incidence rates of both anxiety and depression were slightly lower in the CC cohort than in the BC cohort, whereas these incidence rates were slightly higher in the CC controls than in the BC controls. The CC cohort to CC control aHRs for these two disorders were 1.12 (95% CI = 1.06–1.18) and 1.29 (95% CI = 1.18–1.41), respectively. Compared to the CC cohort, the incidence rates of both anxiety and depression were slightly higher in the BC cohort, but significant for depression (aHR = 1.09, 95% CI = 1.01–1.17) not for anxiety (aHR = 1.04, 95% CI 158 = 0.99–1.09) ( Table 3 ).
Table 3.
Breast cancer cohort to cervical cancer cohort adjusted hazard ratio of mental disorder by type.
| Outcome | Crude HR (95% CI) | Adjusted hazard ratio (95% CI) |
|---|---|---|
| Anxiety | 1.01 (0.94-1.03) | 1.04 (0.99-1.09) |
| Depression | 1.08 (1.04-1.11) | 1.09 (1.02-1.17) |
Adjusted for age, occupation, income, urbanization, and CCI score.
During the study period, the overall incidence of anxiety and depression was nearly 1.44-fold higher in breast cancer women than in the comparisons (22.8 versus 15.8 per 1,000 person-years, or 12,534 versus 10,005 cases), with an aHR of 1.40 (95% CI = 1.37–1.44) ( Table 4 ). The incidence increased with age in both cohorts and peaked in the 65–74-year age, whereas the BC cohort to BC control cohort aHRs decreased with age, from 1.91 at 18–39 years of age to 1.15 in 65–74 years of age. The incidence decreased with income in both cohorts, whereas the HR for the BC cohort increased with income to 1.51 for those with higher incomes. Comorbidity had no contribution to the risk for the BC cohort but contributed an increased incidence for BC controls from 15.6 per 1,000 person-years in those without comorbidity to 20.7 per 1,000 person-years in those with comorbidity. The increased hazard remained significant for the BC cohort.
Table 4.
Combined incidence of anxiety and depression and breast cancer cohort to comparison cohort adjusted hazard ratio by age, occupation, income, urbanization, and Charlson comorbidity index score.
| Variable | BC control | Breast cancer | Adjusted HR (95% CI) | ||||
| Event, n | PY | Rate | Event, n | PY | Rate | ||
| Overall | 10,002 | 632,488 | 15.8 | 12,530 | 549,703 | 22.8 | 1.40 (1.37-1.44)*** |
| Age, years | |||||||
| 18-39 | 854 | 95,003 | 8.99 | 1,400 | 78,297 | 17.9 | 1.91 (1.75-2.07)*** |
| 40-49 | 3,438 | 234,562 | 14.7 | 4,348 | 19,962 | 21.8 | 1.44 (1.38-1.51)*** |
| 50-64 | 4,182 | 231,962 | 18.0 | 5,039 | 201,127 | 25.0 | 1.36 (1.30-1.41)*** |
| 65-74 75+ |
983 545 |
44,085 26,876 |
22.3 20.3 |
1,332 411 |
51,600 19,069 |
25.8 21.6 |
1.15 (1.06-1.25)*** 1.07 (0.94-1.22) |
| Occupation | |||||||
| Homemaker | 2,862 | 164,289 | 17.4 | 3,480 | 145,502 | 23.9 | 1.34 (1.28-1.41)*** |
| White collar | 2,633 | 209,800 | 12.6 | 3,563 | 183,478 | 19.4 | 1.51 (1.43-1.58)*** |
| Blue collar | 3,370 | 190,807 | 17.7 | 4,147 | 164,392 | 25.4 | 1.40 (1.34-1.46)*** |
| Other | 1,137 | 67,592 | 16.8 | 1,340 | 57,232 | 23.4 | 1.35 (1.25-1.46)*** |
| Income | |||||||
| ≤20,000 | 4,844 | 270,388 | 17.9 | 5,472 | 230,279 | 23.8 | 1.29 (1.24-1.34)*** |
| 20,001-39,999 | 3,409 | 232,941 | 14.6 | 4,639 | 203,872 | 22.7 | 1.51 (1.45-1.58)*** |
| 40,000+ | 1,749 | 129,159 | 13.5 | 2,419 | 11,553 | 20.9 | 1.51 (1.42-1.60)*** |
| Urbanization | |||||||
| Urban | 6,008 | 382,364 | 15.7 | 7,462 | 334,402 | 22.3 | 1.39 (1.34-1.43)*** |
| Suburban | 3,119 | 197,532 | 15.8 | 3,859 | 167,820 | 23.0 | 1.41 (1.35-1.48)*** |
| Rural | 875 | 52,592 | 16.6 | 1,209 | 47,481 | 25.5 | 1.48 (1.36-1.62)*** |
| CCI score | |||||||
| 0 | 9,380 | 602,452 | 15.6 | 11,644 | 510,899 | 22.8 | 1.42 (1.39-1.46)*** |
| 1+ | 622 | 30,036 | 20.7 | 886 | 38,804 | 22.8 | 1.12 (1.01-1.24)* |
PY, person-years; Rate, per 1,000 person-years; CCI, Charlson comorbidity index.
Adjusted for matched pair. *p < 0.05, ***p < 0.001.
The overall combined incidence of anxiety and depression was near 1.2-fold higher in the CC cohort than in the comparisons (21.7 versus 18.3 per 1,000 person-years), with an aHR of 1.17 (95% CI =1.11-1.22) ( Table 5 ). The incidence increased with age in both cohorts, whereas the aHR decreased with age from 1.63 (95% CI = 1.39-1.89) at 18-39 years-old to 1.07 (95% CI = 0.96-1.19) in those aged 65-74. The comorbidity associated incidence was higher in the CC cohort than in controls (24.3 versus 21.7 per 1,000 person-years), with an aHR of 1.12 (95% CI = 0.96-1.29) for CC patients, which was not significant.
Table 5.
Combined incidence of anxiety and depression and cervical cancer cohort to comparison cohort adjusted cohort hazard ratio by age, occupation, income, urbanization, and Charlson comorbidity index score.
| Variable | CC control | Cervical cancer | HR (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Event, n | PY | Rate | Event, n | PY | Rate | ||
| Overall | 3,714 | 203,189 | 18.3 | 3,634 | 167,182 | 21.7 | 1.17 (1.11-1.22)*** |
| Age, years | |||||||
| 18-39 | 300 | 27,120 | 11.1 | 401 | 21,653 | 18.5 | 1.63 (1.40-1.89)*** |
| 40-49 | 973 | 59,991 | 16.2 | 952 | 48,342 | 19.7 | 1.18 (1.08-1.29)** |
| 50-64 | 1,385 | 70620 | 19.6 | 1,285 | 58,275 | 22.0 | 1.10 (1.02-1.19)* |
| 65-74 75+ |
691 365 |
28,134 17,324 |
24.6 21.1 |
672 324 |
25,667 13,245 |
25.6 24.5 |
1.07 (0.96-1.19) 1.15 (0.99-1.34) |
| Occupation | |||||||
| Homemaker | 1,206 | 65,362 | 18.5 | 1,152 | 52,531 | 21.9 | 1.16 (1.08-1.26)*** |
| White collar | 576 | 40,548 | 14.2 | 627 | 34,288 | 18.3 | 1.26 (1.12-1.40)*** |
| Blue collar | 1,496 | 73,822 | 20.3 | 1,393 | 62,784 | 22.2 | 1.08 (1.00-1.16)* |
| Other | 436 | 23,458 | 18.6 | 462 | 17,579 | 26.3 | 1.37 (1.20-1.56)*** |
| Income, NTD | |||||||
| ≤20,000 | 2,318 | 117,010 | 19.8 | 2,233 | 95,869 | 23.3 | 1.15 (1.09-1.22)*** |
| 20,001-39,999 | 1,043 | 63868 | 16.3 | 1,011 | 52,493 | 19.3 | 1.16 (1.06-1.26)** |
| 40,000+ | 353 | 22,311 | 15.8 | 390 | 18,820 | 20.7 | 1.27 (1.10-1.47)*** |
| Urbanization | |||||||
| Urban | 1,765 | 98,506 | 17.9 | 1,740 | 80,132 | 21.7 | 1.18 (1.11-1.26)*** |
| Suburban | 1,439 | 77,461 | 18.6 | 1,308 | 63,790 | 20.5 | 1.09 (1.01-1.17)* |
| Rural | 510 | 27,223 | 18.7 | 586 | 23,260 | 25.2 | 1.32 (1.17-1.49)*** |
| CCI score | |||||||
| 0 | 3,379 | 187,765 | 18.0 | 3,282 | 152,701 | 21.5 | 1.17 (1.12-1.23)*** |
| 1+ | 335 | 15,424 | 21.7 | 352 | 14,481 | 24.3 | 1.12 (0.96-1.29) |
PY, person-years; Rate, per 1,000 person-years; CCI, Charlson comorbidity index.
Adjusted for matched pair. *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
Mental disorder as the consequence of reaction to a catastrophic disorder may vary by type and severity of the disorder, sociodemographic variation, and stage of disorder diagnosed (8, 9, 32, 33). A population study using Japanese medical claims data evaluating mental disorders in women found that 16.9% breast cancer patients and 2.7% cervical cancer patients developed major depressive disorder (MDD) after being diagnosed with the cancers. This contrast indicates that the risk of developing the mood disorder was greater for Japanese women with breast cancer than for those with cervical cancer (33). Disorders of anxiety and depression may go hand in hand associated with the risk and severity of diseases. An anxiety disorder may trigger the occurrence of depression with a strong association with the risk level of disease. In our propensity score-matched cohort study, proportions of patients with depression developed were similar in both BC cohort and CC cohort (3.9%), and anxiety developed was slightly lower in the BC cohort than in the CC cohort (9.0 versus 9.7%). The data showed similar absolute risks of developing these mental disorders in women with BC and in women with CC, although the sample size of the BC cohort was 3.6-fold greater than that of the CC cohort, demonstrating that women in Taiwan are at a higher risk of developing BC than developing CC. The HR of developing anxiety was slightly greater for women with breast cancer than for those with cervical cancer, but not significant, featuring that both types of cancer could trigger anxiety at a similar level. The HR of developing depression was also slightly greater for women with breast cancer than with cervical cancer, but significant, featuring that BC has a slightly stronger impact than CC in triggering depression. This contrast is much slighter than that in Japanese women.
An earlier study using a smaller randomly selected database of insurance claims data of Taiwan found that women with breast cancer had significant incidence rate ratios of 1.94 for MDD and 1.22 for anxiety, relative to controls (34). Another study using a similar database found that women with cervical cancer were prominent for developing depression with an incidence rate ratio of 1.35 (35). These risk estimates are consistent with our findings. The smallish risk variations among these three studies might be associated with databases used.
Our study showed that the occurrence of both depression and anxiety in BC patients and CC patients shared similar risk characteristics associated with age: both incidence rates increased with age, but the HRs were the greatest for patients of the youngest group. The development of psychiatric disorder has been associated with comorbidities. A meta-analysis based on 40 articles showed that patients with multimorbidity could be up to three times more likely depressed than persons without chronic disorders (36). Our data failed to show this relationship in breast cancer patients, but a higher CCI was associated with slightly increased HR of combined incidence of disorders in cervical cancer patients. Comorbidities generally increase with age and are more prevalent in the elderly, explaining that the combined incidence of these two psychiatric disorders increased with age in all our cohorts. However, the subgroup analysis for HRs among age groups showed that, relative to the same age group of the respective control cohort, younger cancer patients had higher HRs than those of older age, indicating that BC or CC is the major risk factor associated with developing psychiatric disorders in younger patients, probably because comorbidities are less prevalent in younger patients than in the elderly. Therefore, the metal disorder reaction to the catastrophic diseases is stronger for younger patients.
Research has shown that social inequalities can contribute to the severity of diseases at which disadvantaged patients might be more likely to experience mood disorders (37–40). An earlier meta-analysis reported that individuals with the lowest socioeconomic position had an odds ratio of 1.81 being depressed relative to those with the highest socioeconomic position (38). The European Health Interview Survey in Spain showed that MDD in women is strongly associated with socioeconomic disadvantage, including those retired and homemakers (39). A recent meta-analysis based on 40 studies in China reported that the lifetime prevalence of MDD was the highest in participants with the lowest education or those living in rural areas (40). Our data also showed that women from disadvantaged backgrounds experienced greater incident rates of anxiety and depression, the highest for women with blue collar jobs or with a lower income in both the BC cohort and the BC controls. In the CC cohort and CC controls, CC patients of unemployed or retired or of low income were at a higher risk, and these patients are more likely older.
This study had the advantage of using a large population-based health insurance data, from which we could perform a robust cohort study design to randomly select controls matched by propensity score. The matching capacity helped minimize potential bias. The large sample sizes allowed multivariate analyses to assess the mental disorder risks associated with the sociodemographic category. There were also some limitations in this study. The claims data provided no information on the cancer stage. We therefore were unable to examine the incidence of mental disorder by the severity of cancers. Information on lifestyle was also unavailable to be included in data analysis. However, the propensity matching design and CCI use in this study could reduce potential bias, in addition to being controlled by occupation, income, and residential area. Furthermore, based on clinical diagnoses in the claims data, cancer patients with mild mental disorder symptoms might not be diagnosed. The risks of anxiety and depression may be underestimated for both cancer cohorts and comparison cohorts.
Conclusion
Our study found that women in Taiwan are at a much higher risk of developing breast cancer than developing cervical cancer. The risks of further developing anxiety and depression are slightly higher in women with breast cancer than in women with cervical cancer, but the risk difference is significant for depression, but not for anxiety. Risks of both disorders increase with age, but relatively the hazards of developing the mental disorders were greater for youngers. Women with a less well-off economic status are also at a relatively elevated risk.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by the Ethical Research Committee at China Medical University and Hospital (H107257). Because Personal identifications in the data files had been scrambled to protect privacy, Patient consents were waived.
Author contributions
Conception: C-MY, F-CS, S-SH. Design: C-MY, F-CS, C-HM, C-HL. Data analysis: C-HM. Results interpretation: C-MY, C-HM, F-CS. Data evaluation: C-HL, P-HW, S-SH. Drafting the article: C-MY, F-CS, S-SH. Manuscript revision: all authors. C-HM, F-CS, and S-SH had access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the article and approved the submitted version.
Funding
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW110-TDU-B-212-124004), China Medical University Hospital (DMR-111-228) and China Medical University (CMU110-S-18), Taiwan. The funders had no role in the study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
Acknowledgments
We are grateful to the Health Data Science Center and China Medical University Hospital for providing administrative, technical and funding support for using the data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Yang X, Fang Y, Chen H, Zhang T, Yin X, Man J, et al. Global, regional and national burden of anxiety disorders from 1990 to 2019: results from the global burden of disease study 2019. Epidemiol Psychiatr Sci (2021) 30:e36. doi: 10.1017/S2045796021000275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kupferberg A, Bicks L, Hasler G. Social functioning in major depressive disorder. Neurosci Biobehav Rev (2016) 69:313–32. doi: 10.1016/j.neubiorev.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 3. WHO Depression . World health organization, Geneva (2021). Available at: https://www.who.int/news-room/fact-sheets/detail/depression (Accessed September 13, 2020).
- 4. Gilman SE, Sucha E, Kingsbury M, Horton NJ, Murphy JM, Colman I. Depression and mortality in a longitudinal study: 1952-2011. CMAJ (2017) 189:E1304–10. doi: 10.1503/cmaj.170125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koch-Gallenkamp L, Bertram H, Eberle A, Holleczek B, Schmid-Hopfner S, Waldmann A, et al. Fear of recurrence in long-term cancer survivors-do cancer type, sex, time since diagnosis, and social support matter? Health Psychol (2016) 35:1329–33. doi: 10.1037/hea0000374 [DOI] [PubMed] [Google Scholar]
- 6. Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr (2004) 32:57–71. doi: 10.1093/jncimonographs/lgh014 [DOI] [PubMed] [Google Scholar]
- 7. Mehnert A, Brahler E, Faller H, Harter M, Keller M, Schulz H, et al. Four-week prevalence of mental disorders in patients with cancer across major tumor entities. J Clin Oncol (2014) 32:3540–6. doi: 10.1200/JCO.2014.56.0086 [DOI] [PubMed] [Google Scholar]
- 8. Carreira H, Williams R, Muller M, Harewood R, Bhaskaran K. Adverse mental health outcomes in breast cancer survivors compared to women who did not have cancer: a systematic review. JNCI (2018) 110:djy177. doi: 10.1186/s13643-017-0551-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maass SW, Roorda C, Berendsen AJ, Verhaak PF, de Bock GH. The prevalence of long-term symptoms of depression and anxiety after breast cancer treatment: A systematic review. Maturitas (2015) 82:100–8. doi: 10.1016/j.maturitas.2015.04.010 [DOI] [PubMed] [Google Scholar]
- 10. Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med (2010) 40:1797–810. doi: 10.1017/S0033291709992285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hartl K, Janni W, Kastner R, Sommer H, Strobl B, Rack B, et al. Impact of medical and demographic factors on long-term quality of life and body image of breast cancer patients. Ann Oncol (2003) 14:1064–71. doi: 10.1093/annonc/mdg289 [DOI] [PubMed] [Google Scholar]
- 12. Gartner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA (2009) 302:1985–92. doi: 10.1001/jama.2009.1568 [DOI] [PubMed] [Google Scholar]
- 13. DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol (2013) 14:500–15. doi: 10.1016/S1470-2045(13)70076-7 [DOI] [PubMed] [Google Scholar]
- 14. Kim Y, Kashy DA, Wellisch DK, Spillers RL, Kaw CK, Smith TG. Quality of life of couples dealing with cancer: dyadic and individual adjustment among breast and prostate cancer survivors and their spousal caregivers. Ann Behav Med (2008) 35(2):230–8. doi: 10.1007/s12160-008-9026-y [DOI] [PubMed] [Google Scholar]
- 15. Inhestern L, Bergelt C. When a mother has cancer: strains and resources of affected families from the mother's and father's perspective - a qualitative study. BMC Womens Health (2018) 18:72. doi: 10.1186/s12905-018-0562-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, Johansen C, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol (2011) 12:160–74. doi: 10.1016/S1470-2045(11)70002-X [DOI] [PubMed] [Google Scholar]
- 17. Krebber AM, Buffart LM, Kleijn G, Riepma IC, de Bree R, Leemans CR, et al. Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psychooncology (2014) 23:121–30. doi: 10.1002/pon.3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zahid JA, Grummedal O, Madsen MT, Gögenur I. Prevention of depression in patients with cancer: A systematic review and meta-analysis of randomized controlled trials. J Psychiatr Res (2020) 120:113–23. doi: 10.1016/j.jpsychires.2019.10.009 [DOI] [PubMed] [Google Scholar]
- 19. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 20. Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet (2019) 393:169–82. doi: 10.1016/S0140-6736(18)32470-X [DOI] [PubMed] [Google Scholar]
- 21. Ye S, Yang J, Cao D, Lang J, Shen KA. Systematic review of quality of life and sexual function of patients with cervical cancer after treatment. Int J Gynecol Cancer (2014) 24:1146–57. doi: 10.1097/IGC.0000000000000207 [DOI] [PubMed] [Google Scholar]
- 22. Boquiren VM, Esplen MJ, Wong J, Toner B, Warner E, Malik N. Sexual functioning in breast cancer survivors experiencing body image disturbance. Psychooncology (2016) 25:66–76. doi: 10.1002/pon.3819 [DOI] [PubMed] [Google Scholar]
- 23. Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet (2018) 391:1023–75. doi: 10.1016/S0140-6736(17)33326-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. WHO . Breast cancer (2021). Geneva: World Health Organization. Available at: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (Accessed July 25, 2021). [Google Scholar]
- 25. WHO . Cervical cancer (2018). Geneva: World Health Organization. Available at: http://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer/en/ (Accessed July 25, 2021). [Google Scholar]
- 26. SEER cancer statistics review (2020). Available at: https://seer.cancer.gov/csr/1975_2018/.
- 27. Tsatsou I, Parpa E, Tsilika E, Katsaragakis S, Batistaki C, Dimitriadou E, et al. A systematic review of sexuality and depression of cervical cancer patients. J Sex Marital Ther (2019) 45:739–54. doi: 10.1080/0092623X.2019.1610125 [DOI] [PubMed] [Google Scholar]
- 28. Huang YC, Chen YH. Cancer incidence characteristic evolution based on the national cancer registry in Taiwan. J Oncol (2020) 2020:1408793. doi: 10.1155/2020/1408793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Health promotion administration, ministry of health and welfare, in: Cancer registry report-annual report . Available at: https://cris.hpa.gov.tw/pagepub/Home.aspx?itemNo=cr.a.10 (Accessed July 4, 2022).
- 30. Hsing AW, Ioannidis JP. Nationwide population science: Lessons from the taiwan national health insurance research database. JAMA Internal Med (2015) 175:1527–9. doi: 10.1001/jamainternmed.2015.3540 [DOI] [PubMed] [Google Scholar]
- 31. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classification of prognostic comorbidity for longitudinal studies: development and validation. J Chron Dis (1987) 40:373–83. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 32. Lu D, Andersson TM, Fall K, Hultman CM, Czene K, Valdimarsdóttir U, et al. Clinical diagnosis of mental disorders immediately before and after cancer diagnosis: a nationwide matched cohort study in Sweden. JAMA Oncol (2016) 2:1188–96. doi: 10.1001/jamaoncol.2016.0483 [DOI] [PubMed] [Google Scholar]
- 33. Akechi T, Mishiro I, Fujimoto S, Murase K. Risk of major depressive disorder in Japanese cancer patients: A matched cohort study using employer-based health insurance claims data. Psychooncology (2020) 29:1686–94. doi: 10.1002/pon.5509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hung YP, Liu CJ, Tsai CF, Hung MH, Tzeng CH, Liu CY, et al. Incidence and risk of mood disorders in patients with breast cancers in Taiwan: a nationwide population-based study. Psychooncology (2013) 22:2227–34. doi: 10.1002/pon.3277 [DOI] [PubMed] [Google Scholar]
- 35. Shyu IL, Hu LY, Chen YJ, Wang PH, Huang BS. Risk factors for developing depression in women with cervical cancer: a nationwide population-based study in Taiwan. Int J Womens Health (2019) 11:135–41. doi: 10.2147/IJWH.S193003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Read JR, Sharpe L, Modini M, Dear BF. Multimorbidity and depression: a systematic review and meta-analysis. J Affect Disord (2017) 15:36–46. doi: 10.1016/j.jad.2017.06.009 [DOI] [PubMed] [Google Scholar]
- 37. Lorant V, Deliège D, Eaton W, Robert A, Philippot P, Ansseau M, et al. Socioeconomic inequalities in depression: a meta-analysis. Am J Epidemiol (2003) 157:98–112. doi: 10.1093/aje/kwf182 [DOI] [PubMed] [Google Scholar]
- 38. Muntaner C, Eaton WW, Miech R, O’Campo P. Socioeconomic position and major mental disorders. Epidemiol Rev (2004) 26:53–62. doi: 10.1093/epirev/mxh001 [DOI] [PubMed] [Google Scholar]
- 39. Arias-de la Torre J, Vilagut G, Martín V, Molina AJ, Alonso J. Prevalence of major depressive disorder and association with personal and socio-economic factors. results for Spain of the European health interview survey 2014-2015. J Affect Disord (2018) 15:239. doi: 10.1016/j.jad.2018.06.051 [DOI] [PubMed] [Google Scholar]
- 40. Zhao YJ, Jin Y, Rao WW, Zhang QE, Zhang L, Jackson T, et al. Prevalence of major depressive disorder among adults in china: a systematic review and meta-analysis. Front Psychiatry (2021) 12:659470. doi: 10.3389/fpsyt.2021.659470 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.