Abstract
The maltose transporter FGK2 complex of Escherichia coli was purified with the aid of a glutathione S-transferase molecular tag. In contrast to the membrane-associated form of the complex, which requires liganded maltose binding protein (MBP) for ATPase activity, the purified detergent-soluble complex exhibited a very high level of ATPase activity. This uncoupled activity was not due to dissociation of the MalK ATPase subunit from the integral membrane protein MalF and MalG subunits. The detergent-soluble ATPase activity of the complex could be further stimulated by wild-type MBP but not by a signaling-defective mutant MBP. Wild-type MBP increased the Vmax of the ATPase 2.7-fold but had no effect on the Km of the enzyme for ATP. When the detergent-soluble complex was reconstituted in proteoliposomes, it returned to being dependent on MBP for activation of ATPase, consistent with the idea that the structural changes induced in the complex by detergent that result in activation of the ATPase are reversible. The uncoupled ATPase activity resembled the membrane-bound activity of the complex also with respect to sensitivity to NaN3, as well as a mercurial, p-chloromercuribenzosulfonic acid. Verapamil, a compound that activates the ATPase activity of the multiple drug resistance P-glycoprotein, activated the maltose transporter ATPase as well. The activation of this bacterial transporter by verapamil suggests that a structural feature that is conserved among both eukaryotic and prokaryotic ATP binding cassette transporters is responsible for this activation.
The membrane components of the maltose transport system of Escherichia coli catalyze the intracellular accumulation of malto-oligosaccharides at the expense of ATP hydrolysis (5). They comprise two integral membrane proteins, MalF and MalG, and two copies of a cytoplasmic ATP binding subunit, MalK (36). In addition to these components directly involved in substrate translocation, there is a periplasmic maltose binding protein (MBP) and a maltoporin (LamB) located in the outer membrane (5). This transporter is a member of the ATP binding cassette (ABC) superfamily, and the MalK sequence shares significant similarity with the sequences of several other prokaryotic and eukaryotic transport proteins that belong to this family.
The FGK2 complex has been extensively characterized both genetically and biochemically. In most instances, the transporter has been studied either in whole cells, in subcellular vesicles, or in some cases following purification and reconstitution, in proteoliposomes. In all cases, the transport and ATPase activities of the FGK2 complex have been shown to be strongly dependent on MBP. Efforts to study the molecular basis of these transport and ATPase activities have been limited by the need to reconstitute the transporter in proteoliposomes. It has been generally assumed that a detergent-soluble form of this and other ABC transporters would not be a useful model for studying their molecular properties. Indeed, several publications have reported that detergent solubilization rendered both the maltose and histidine transporters inactive (7, 21). An alternative approach has been to study the water-soluble ABC subunit (e.g., MalK or HisP) in isolation (28, 39). Although these studies have provided information about the responses of the proteins to nucleotides, they cannot address the effects of signaling by their respective periplasmic binding proteins.
In the work described here, we found evidence that, in contrast to these previous studies, the detergent-soluble form of the wild-type FGK2 transporter is active and retains important characteristics, such as the ability to respond to signaling by the periplasmic MBP. In addition, we provide a quantitative comparison of the activities of (i) the reconstituted membrane-bound form of the purified wild-type complex, (ii) the detergent-soluble form of the wild-type and uncoupled mutant complexes, and (iii) the isolated MalK subunit. We also report that this bacterial periplasmic binding protein-dependent ABC transporter responds to verapamil, a drug that is known to reverse the MDR phenotype by uncoupling ATP hydrolysis from drug efflux (15). These results indicate that the structural features required for verapamil binding and activity are broadly conserved among prokaryotic and eukaryotic ABC transporters with diverse functions.
MATERIALS AND METHODS
Strains and plasmids.
All of the strains and plasmids used in this study are summarized in Table 1. Strain NT169 was used as a host for expression of the maltose complex, HS3309 was used as a host for expression of wild-type MBP and signaling-defective mutant MBP, and X90(DE3) (38) was used as a host for expression of the His6-MalK protein.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Source or reference |
|---|---|---|
| Strains | ||
| HS3309 | F−araD139 Δ(argF-lac)U169 relA1 rbsR flbB5301 ptsF25 thi-1 deoC1 malTp1Tp7 ΔmalE444zjb::Tn10-729 | Laboratory collection |
| NT169 | F−araD139 Δ(araABCD leu)7697 ΔlacX74 galU galK rpsL ΔmalB101 lacIq1rpoB | Laboratory collection |
| X90 (DE3) | F−ara Δ(lac pro) XIII gyrA argE(Am) thi rpoB | 38 |
| Plasmids | ||
| pMR31 | pACYC184 Ptac-malF+ | This work |
| pMR41 | pBR322 Ptac-gst-malG+-Ptac-malK+ | This work |
| pLH1 | pBR322 PmalB-malE+ | 17 |
| pLH38 | pBR322 PmalB-malE623 | 17 |
| pCP97 | pET15b PT7-his6-malK+ | 30 |
Construction of plasmids pMR41 and pMR31.
Plasmid pMR41 (ampicillin resistance [Ampr]) contains both the gst-malG fusion and the malK genes individually under control of the Ptac promoter. To create the gst-malG fusion, a BamHI restriction site was introduced immediately upstream from the ATG of the malG coding sequence in plasmid pYSF12. From this plasmid, a BamHI-BsaAI fragment containing the entire malG coding sequence was ligated into plasmid pGEX-2T (Pharmacia Biotech), which had been digested with BamHI and SmaI, to create plasmid pCP13. A BamHI fragment derived from plasmid pMR11 (32) containing the wild-type malK gene under control of the Ptac promoter was introduced into pCP13 by first filling in the BamHI sticky ends and then ligating the blunt-end fragment into the EcoRV site within the lacIq gene of plasmid pCP13.
Plasmid pMR31 contains the malF gene under control of the Ptac promoter on a derivative of pACYC184 (chloramphenicol resistance) and is compatible with pMR41. It was constructed by ligation of an NcoI-BclI fragment containing the malF gene into a derivative of pACYC184 (pLAW304) (40).
Overproduction of MalK, MalF, and GST-MalG.
NT169 cells were transformed with plasmids carrying the malF gene (pMR31) and the malK and gst-malG genes (pMR41). All genes were under control of the Ptac promoter. The bacteria were grown at 28°C to logarithmic phase in LB medium (10 g of tryptone per liter, 5 g of yeast extract per liter, 10 g of NaCl per liter) containing 100 μg of ampicillin per milliliter and 25 μg of chloramphenicol per ml. After induction with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 4 h, the cells were pelleted and membranes were prepared as described elsewhere (9). Briefly, the cell pellet was resuspended in 20 mM Tris-HCl (pH 7)–5 mM MgCl2–1 mM EDTA–50 mM NaCl–10 μg of phenylmethylsulfonyl fluoride per ml–1 mM dithiothreitol (DTT). The cells were passed twice through a French press at 16,000 lb/in2, and the unbroken cells were removed by centrifugation at 3,000 × g for 10 min. The supernatant fraction was centrifuged at 100,000 × g for 1 h at 4°C. The pellet was resuspended in the same buffer to a protein concentration of 20 mg/ml and stored at −80°C.
Solubilization of membrane protein.
Thawed membranes were solubilized in 20 mM Tris-HCl (pH 7)–5 mM MgCl2–10 μg of phenylmethylsulfonyl fluoride per ml–1 mM DTT–20% glycerol–1.6% dodecyl maltoside to a final protein concentration of 4 mg/ml. After 30 min of incubation on ice, the solubilized membranes were centrifuged at 100,000 × g for 1 h at 4°C and the supernatant was removed for purification of the maltose transporter on an affinity column.
Affinity purification of the maltose transporter complex.
A 2-ml volume of glutathione agarose (Pharmacia Biotech) resin was added to 10 ml of solubilized membrane supernatant and kept for 4 h at 4°C. The resin was transferred to a column and washed with 50 mM Tris-HCl (pH 8)–5 mM MgCl2–1 mM DTT–10% glycerol–0.01% dodecyl maltoside. To cleave the glutathione S-transferase (GST) from MalG, biotinylated thrombin (Novagen) was added to the resin, the mixture was subjected to 18 h of incubation at 4°C, and then the complex was eluted from the column. The eluted complex was incubated with 50 μl of a streptavidin agarose suspension for 30 min and then centrifuged for 5 min at 1,000 × g through a spin filter centrifuge tube to remove the thrombin-containing beads. If thrombin was not used to cleave the GST-MalG hybrid, the GST-MalG-containing complex was eluted with the same buffer containing 10 mM glutathione.
Assay of ATP hydrolysis.
ATP hydrolysis was measured as described elsewhere (19). Briefly, the purified complex (5 μg/ml) was added to a solution of 40 mM Tris-HCl (pH 7)–4 mM MgCl2–5% glycerol–0.01% dodecyl maltoside. ATP was added to a final concentration of 4 mM, and the reaction mixture was incubated in a 37°C bath. Aliquots of 20 μl were removed after 0 to 30 min into 160 μl of 0.033% malachite green in 1 N HCl. After 1 min, the reaction was stopped by addition of 20 μl of 34% citric acid and the A650 was read. When doing the assay in the presence of large amounts of protein, we used a variation of the method as described before (6). We established that the assay was linear over time and with respect to the concentration of the added complex up to 25 μg/ml. When added to ATPase assays, MBP was always saturated with maltose unless otherwise specified.
Overproduction and purification of MBP.
MBP was purified by affinity chromatography as described before (24). Briefly, fresh transformants of HS3309 were grown for 18 h in Terrific Broth medium (12 g of tryptone per liter, 24 g of yeast extract per liter, 0.4% glycerol, 2.31 g of KH2PO4, 12.54 g of K2HPO4 per liter), and the periplasmic fraction was loaded onto an amylose column. After washing with 50 mM Tris-HCl (pH 7.5), the purified MBP was eluted with the same buffer containing 20 mM maltose. A Centricon 30 concentrator (Amicon) was used to concentrate the purified MBP and to wash out the excess maltose.
Expression and purification of His6-MalK protein.
Expression and purification of His6-MalK was done as described before (30). Briefly, the his6-malK gene (pCP97) was transformed into strain X90/(DE3), which was grown to logarithmic phase in Terrific Broth medium and induced for 1 h with 1 mM IPTG. The cells were pelleted, resuspended in 10 mM Tris-HCl (pH 8)–200 mM NaCl–10% glycerol and passed twice through a French press at 16,000 lb/in2. The soluble fraction was recovered after a 30-min spin at 15,000 rpm in the ss-34 rotor of a Sorvall Re-5B centrifuge, added to Ni-nitrilotriacetic acid-agarose (QIAGEN), and incubated for 1 h at 4°C. The resin was transferred to a column and washed with 10 mM Tris-HCl (pH 8)–500 mM NaCl–20% glycerol. The His6-MalK protein was eluted in the same buffer containing 250 mM imidazole.
Reconstitution of the purified complex into proteoliposomes.
The dilution procedure described by Racker et al. (31) was used to reconstitute the purified maltose transporter into proteoliposomes. Dry, crude E. coli phospholipids (Avanti Polar Lipid, Inc.) were resuspended to a final concentration of 50 mg/ml in 50 mM Tris-HCl (pH 8)–2 mM 2-mercaptoethanol and stored at −80°C under nitrogen until use. Aliquots were thawed and sonicated to clarity in a bath sonicator.
Typically, 100 μl of sonicated lipids was mixed with 450 μl of the purified complex and with or without 5 mg of MBP per ml and incubated on ice for 15 min. The mixture was then diluted into 25 ml of 50 mM Tris (pH 7)–1 mM DTT. Proteoliposomes were isolated by centrifugation at 100,000 × g for 1 h at 4°C and resuspended in 250 μl of 50 mM Tris-HCl (pH 7)–1 mM DTT.
RESULTS
Overproduction and purification of FGK2 complex.
We used two compatible plasmids to express the transport complex. One plasmid, pMR31, encodes resistance to chloramphenicol and the MalF protein under control of the Ptac promoter on a P15A replicon. The other plasmid, pMR41, encodes ampicillin resistance, as well as the MalK protein and a hybrid GST-MalG protein, both under control of separate Ptac promoters. The GST-MalG hybrid protein retains the ability to participate in active transport. The fused gst-malG gene complements a malG null mutation (data not shown). In addition, the reconstituted complex containing the GST-MalG protein exhibited both transport and ATPase activities (C. Panagiotidis, unpublished results). These plasmids were introduced into strain NT169, which lacks the entire malB region and supplies the LacI repressor. The transformants were grown to logarithmic phase, and synthesis of the transport complex was induced by IPTG. Following harvesting of the cells and lysis in a French press, a crude membrane fraction was prepared and stored at −80°C. Aliquots of the membrane preparation were thawed and solubilized in buffer containing dodecyl maltoside. The detergent-soluble proteins were recovered following centrifugation and applied to a glutathione-agarose column. After extensive washing of the column, the transport complex was eluted either with glutathione or by incubation with the protease thrombin, which cleaves at a recognition site between the GST and MalG sequences. We routinely observed a small amount of MalK protein in the flowthrough of the glutathione agarose column. This is likely due to the presence of unassociated MalK subunits in the extract. The unassociated MalK may be the result of excess production of MalK subunits relative to MalF and GST-MalG subunits. Although we did not see any functional differences between the complex containing the GST-fused MalG protein and the thrombin-cleaved complex (data not shown), all of the experiments described in this report, unless stated otherwise, were done with the thrombin-cleaved FGK2 complex.
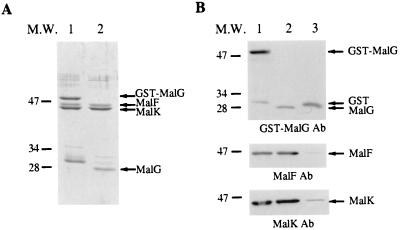
As shown in lane 1 of Fig. 1A, when the proteins are eluted by glutathione, the three complex proteins (GST-MalG, MalF, and MalK) are the most abundant species, along with a 30-kDa species that corresponds to GST protein that is present in the crude extract and presumably represents spontaneous cleavage of the GST-MalG hybrid protein by endogenous proteases. As shown in lane 2, when the proteins are eluted by thrombin cleavage, the major species correspond to the MalG (28 kDa), MalF (43 kDa), and MalK (40 kDa) proteins. The amino-terminal sequence of the MalG subunit was determined by Edman degradation and was found to correspond to the wild-type sequence plus two additional upstream amino acids, glycine and serine, encoded as a result of the gene fusion. Figure 1B shows Western immunoblots of the proteins bound to the column following extensive washing (lane 1), the proteins eluted by thrombin (lane 2), and the proteins retained on the column following thrombin cleavage (lane 3).
FIG. 1.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunodetection of the purified FGK2 complex. The FGK2 complex was purified as described in Materials and Methods. The purified complex was then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane for immunoblotting with specific antibodies (Ab). (A) Coomassie staining of the gel. Lanes: 1, proteins eluted by glutathione; 2, proteins eluted by thrombin cleavage. (B) Immunoblotting of the purified complex with specific antibodies to GST-MalG, MalF, and MalK individually. Lanes: 1, proteins bound to the glutathione agarose resin; 2, proteins eluted by thrombin cleavage; 3, proteins retained on the column following thrombin cleavage. The values on the left are molecular weights (M.W.) in thousands.
ATP hydrolysis by the soluble FGK2 complex.
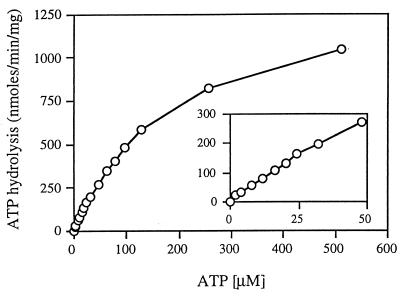
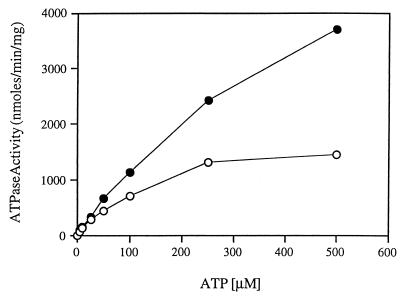
Although the ATPase activity of the wild-type FGK2 complex has been studied in detail, there is little information about its ability to hydrolyze ATP in detergent solution. We found that the material eluted from the glutathione agarose column exhibited very high rates of ATP hydrolysis in the absence of MBP. In order to characterize this activity, we measured the rates of ATP hydrolysis at different ATP concentrations (Fig. 2). We found that the results followed a simple Michaelis-Menten type of kinetics. Double-reciprocal transformation of the data yielded values for Km of 194 ± 10 μM and Vmax of 1,411 ± 49 nmol/min/mg. These values are similar to those reported by others for the membrane-bound transporter when activated by liganded MBP (11). We did not observe the cooperativity with respect to ATP concentrations reported by Davidson et al. (7), presumably because, as they reported, there is no cooperativity at neutral pH. The ability of the purified maltose transporter to hydrolyze ATP in the absence of MBP was also observed when, instead of dodecyl maltoside, octyl glucoside was used to solubilize and purify the complex (data not shown).
FIG. 2.
Saturation curve of ATP hydrolysis by the detergent-soluble complex. The purified FGK2 complex (final concentration, 5 μg/ml) was incubated in 40 mM Tris-HCl (pH 7)–4 mM MgCl2–5% glycerol–0.01% dodecyl maltoside and various concentrations of ATP. For each reaction, samples were removed at various times and the amount of inorganic phosphate was determined as described in Materials and Methods. The rates of inorganic phosphate production were linear over the course of the reaction. The apparent Km is 194 ± 10 μM, and the Vmax is 1,411 ± 49 nmol/min/mg. The inset is a plot of the first 10 data points.
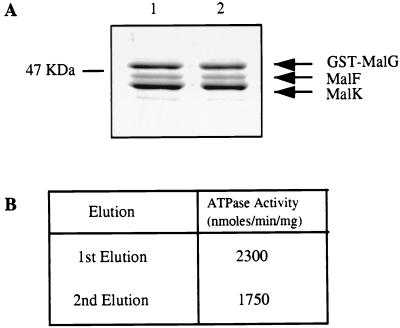
Because it has been reported that the isolated MalK subunit exhibits ATPase activity, we considered the possibility that the activity of the detergent-soluble complex is the result of dissociation of the MalK subunit from the MalF and MalG subunits. Two results indicate that this explanation is highly unlikely. First, the Vmax value of the complex is at least fivefold greater than that reported for the isolated MalK subunit of E. coli (27). Second, if the MalK subunit dissociates from the MalF and GST-MalG proteins, it would not be retained if the complex is rebound to glutathione agarose. As can be seen in Fig. 3A, when the purified detergent-soluble GST-MalGFK2 complex was dialyzed to remove glutathione, rebound to the glutathione resin, and re-eluted, the protein profiles of the two eluates were indistinguishable. In addition, 76% of the ATPase activity was recovered in the second eluate (Fig. 3B). We conclude that the MalF and MalK subunits do not dissociate from the GST-MalG subunit in detergent solution and that the ATPase activity is due to the entire complex.
FIG. 3.
The GST-fused FGK2 complex does not dissociate in detergent. A sample of the purified complex (50 μg), eluted by glutathione from the glutathione agarose column, was dialyzed, rebound to the column, and re-eluted. The proteins from both elutions were assayed for ATPase activity. (A) Sodium dodecyl sulfate-polyacrylamide gel of the first and second elutions. Lanes: 1, first elution; 2, second elution. (B) ATP hydrolysis rates of the complex.
MBP increases the activity of the purified maltose transporter.
In whole cells, as well as in the membrane, the ATPase and transport activities of the maltose transporter are increased in the presence of MBP (11, 35). We have shown, however, that the detergent-purified complex can hydrolyze ATP in the absence of MBP. Nevertheless, the detergent-soluble form of the complex retains the ability to interact with MBP. When MBP containing maltose is added to the complex, there is an increase of about threefold in the rate of ATP hydrolysis (Fig. 4). This increase is MBP specific, since addition of a signaling-defective mutant MBP (17) resulted in a much smaller ATPase activity increase. In addition, maltose in the absence of MBP did not affect the rate of ATP hydrolysis (data not shown). The dependence of the ATPase activity on the concentration of MBP is similar to that reported both in vivo (22) and in membrane vesicles (23).
FIG. 4.
Stimulation of the ATPase activity of the purified detergent-soluble complex by the wild type but not by a signaling-defective mutant MBP. The purified FGK2 complex was incubated in the absence and in the presence of different concentrations of maltose-saturated wild-type MBP or signaling-defective mutant MBP. The ATP hydrolysis rates of the mixtures were determined as described in legend to Fig. 2 in the presence of 4 mM ATP. Symbols: ●, wild-type MBP; ○, signaling-defective mutant MBP.
The increase in ATP hydrolysis in the presence of MBP could result either from a change in the affinity of the complex for ATP or a change in the maximal rate of hydrolysis. In order to distinguish these possibilities, we measured the rates of ATP hydrolysis by the soluble FGK2 complex as a function of ATP concentration in the absence or presence of 50 μM MBP containing maltose. As shown in Fig. 5, MBP mainly changes the maximal rate of ATP hydrolysis. In the absence of MBP, the apparent Km for ATP is 154 ± 22 μM and the Vmax is 1,733 ± 148 nmol/min/mg, whereas in the presence of 50 μM MBP, the apparent Km is 143 ± 24 μM and the Vmax is 4,697 ± 1,186 nmol/min/mg. (The specific activity does not take into account the protein contributed by the added MBP.) Thus, we conclude that MBP does not change the affinity of the complex for ATP but does increase the maximal rate of ATP hydrolysis. It is likely that larger increases in the rates of ATP hydrolysis by the soluble FGK2 complex could be achieved at higher MBP concentrations. It is estimated that in vivo, the concentration of MBP in the periplasm may reach close to 1 mM (22). These data argue strongly that although detergent solubilization stimulates the ATPase activity of the transporter, the FGK2 complex interacts with and is affected by MBP in a way that resembles the membrane-bound complex. The ability to study the FGK2-MBP interaction and the resulting changes in conformation that result in the activation of ATPase activity in a soluble system will facilitate many biophysical studies.
FIG. 5.
MBP increases the Vmax of the ATPase activity but does not change the apparent Km for ATP. The rate of ATP hydrolysis by the soluble FGK2 complex was measured at different ATP concentrations (as described in the legend to Fig. 2) in the absence and in the presence of 50 μM maltose-saturated wild-type MBP. Symbols: ○, soluble FGK2 complex; ●, soluble FGK2 complex in the presence of 50 μM MBP. In the absence of MBP, the apparent Km is 154 ± 22 μM and the Vmax is 1,733 ± 148 nmol/min/mg, whereas in the presence of 50 μM MBP, the apparent Km is 143 ± 24 μM and the Vmax is 4,697 ± 1,186 nmol/min/mg.
Davidson et al. (7) have reported that the wild-type FGK2 complex does not exhibit ATPase activity in detergent solution. In contrast, they found that mutant complexes that do not require MBP for transport or ATPase activity (e.g., F500GK2) did exhibit some low level of ATPase activity in dodecyl maltoside solution. Although it is difficult to reconcile the results presented above with those of Davidson et al., we examined the level of ATPase activity of the F500GK2 complex in the presence and absence of MBP. We found that the ATPase activity of the purified F500GK2 complex in dodecyl maltoside was higher (2,757 nmol/min/mg) than that of the wild-type complex (see above) but that addition of even a low concentration of MBP (25 μM) decreased the activity by 30% (1,883 nmol/min/mg). This is in marked contrast to the effects of MBP on the ATPase activity on the wild-type complex and closely resembles the results reported for the behavior of the F500GK2 mutant complexes in membranes (11).
The ATPase activity of the reconstituted FGK2 complex is MBP dependent.
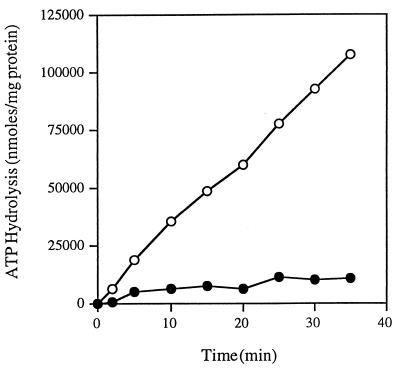
Several studies have shown that when the wild-type FGK2 complex is reconstituted from detergent solution, its ATPase activity is dependent on the presence of liganded MBP (7, 8). In order to test the hypothesis that either the presence of the GST moiety fused to MalG or some other aspect of the protocol that we used to prepare the detergent-soluble complex resulted in a permanent change in the properties of the FGK2 complex, we studied the ATPase activity of the soluble intact complex following reconstitution in proteoliposomes. We reconstituted the detergent-soluble purified FGK2 complexes with E. coli phospholipids into proteoliposomes. We compared the ATPase activities of complexes reconstituted with and without liganded MBP. If the GST moiety or other effects of the detergent irreversibly changed the dependence of the complex on MBP, we would expect that even following reconstitution into proteoliposomes, the FGK2 complex would exhibit high rates of ATP hydrolysis with only two- to threefold stimulation by liganded MBP. As shown in Fig. 6, the activity of the FGK2 complexes reconstituted with liganded MBP is more than 12 times as high as the activity of the complexes reconstituted in the absence of liganded MBP (3,026 versus 229 nmol/min/mg). These results are indistinguishable from those reported by others for the MBP dependence of ATP hydrolysis by the FGK2 complex (11). These results also indicate that the changes produced by dodecyl maltoside that result in activation of the ATPase are reversible upon reconstitution into proteoliposomes.
FIG. 6.
The ATPase activity of the reconstituted FGK2 complex is MBP dependent. The purified FGK2 complex was reconstituted into proteoliposomes (as described in Materials and Methods) in the absence and in the presence of wild-type MBP. The reconstituted complex (final protein concentration, 3 μg/ml) was incubated in 40 mM Tris-HCl (pH 7)–4 mM MgCl2–5% glycerol–4 mM ATP. For each reaction, samples were removed at various times and the amount of inorganic phosphate was determined as described in Materials and Methods. Symbols: ●, reconstituted FGK2 purified complex; ○, reconstituted FGK2 purified complex with wild-type MBP.
Purified MalK protein is less active than the intact complex and is not activated by MBP.
It was shown that the MalK subunit isolated from either Salmonella typhimurium (39) or E. coli (27) displays ATPase activity. Although we presented evidence that the ability of the soluble FGK2 complex to hydrolyze ATP is not due to free MalK subunits that had dissociated from the MalF and MalG subunits, we considered the possibility that detergent or MBP is able to stimulate the ATPase activity of the isolated MalK subunit. Finding that detergent stimulates the free MalK subunit would mean that the effect of the detergent is attributable to this subunit of the complex, and finding that MBP is able to stimulate the ATPase activity of the isolated MalK subunit would support the hypothesis that the ABC subunits are exposed to the periplasm and directly interact with the ligand-binding proteins (2). In contrast, finding that the activity of the isolated MalK subunit is not stimulated by the MBP would support the idea that the binding protein transmits a stimulatory signal via the integral membrane proteins that, in turn, activates ATP hydrolysis by the MalK subunits that form part of the complex.
We expressed the His6-MalK protein as described elsewhere (10, 30) in X90(DE3) bacteria and purified the water-soluble protein on an Ni2+ column. We then measured the rates of ATP hydrolysis by the purified His6-MalK protein at different ATP concentrations. The apparent Km for ATP is 150 ± 8 μM, and the Vmax is 217 ± 15 nmol/mg/min. These characteristics are very similar to what was reported for E. coli purified MalK protein (27). The affinity of MalK for ATP is very similar to that of the intact soluble complex, but the maximal rate of ATP hydrolysis is approximately 10% of that exhibited by the intact complex.
We then asked if detergent had any effect on the rate of ATP hydrolysis. Addition of dodecyl maltoside to the MalK protein resulted in a 40 to 50% increase in the rate of ATP hydrolysis (from 239 to 341 nmol/min/mg). It is unlikely that this modest increase accounts for the larger rates of hydrolysis exhibited by the intact complex in the presence of detergent.
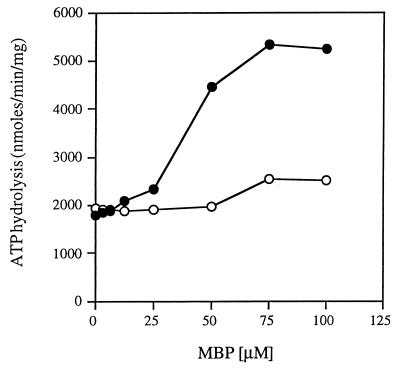
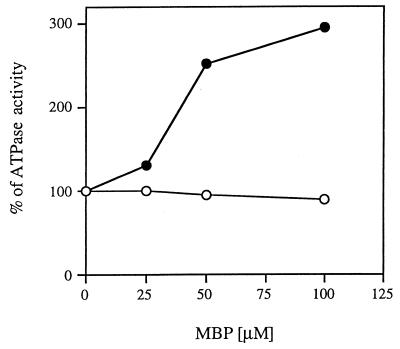
To examine the effect of MBP on the ability of the His6-MalK subunit to hydrolyze ATP, we added increasing concentrations of ligand-bound MBP to either the isolated His6-MalK subunit or the intact detergent-soluble complex and measured the rates of ATP hydrolysis. The results are shown in Fig. 7. It is clear that MBP has no discernible effect on the rate of ATP hydrolysis by the His6-MalK protein but increases the rate of the intact complex about threefold at the highest concentration. We conclude that there is no evidence to support the idea that the MBP directly influences the activity of the His6-MalK subunit. In contrast, these results support the hypothesis that the ligand binding proteins transmit a signal to the integral membrane proteins that, in turn, activate the ATPase activity of the MalK subunit.
FIG. 7.
Purified His6-MalK is not stimulated by MBP. The purified FGK2 complex and the purified His6-MalK protein were incubated in the absence and in the presence of different concentrations of maltose-saturated MBP. The ATP hydrolysis rates of the mixtures were determined as described in legend to Fig. 2. Symbols: ●, detergent-soluble FGK2 complex; ○, His6-MalK.
FGK2 complex is inhibited by Na-azide and PCMBS.
Most ABC transporters, including the FGK2 complex, are sensitive to inhibition by SH-specific compounds, as well as azide, which is known to covalently modify nucleotide binding sites. In order to find out if the intact detergent-soluble complex resembles the membrane-bound complex with respect to sensitivity to these compounds, we examined the effects of a polar, membrane-impermeant mercurial, p-chloromercuribenzosulfonic acid (PCMBS), and azide on the ATPase activity of the intact complex. We focused on PCMBS because preliminary results indicate that the wild-type complex is insensitive to PCMBS in whole cells but is rapidly inactivated at low PCMBS concentrations in everted membrane vesicles (C. Panagiotidis and M. Reyes, unpublished data). When the detergent-soluble complex was treated with various concentrations of PCMBS, the ATPase activity was inhibited by >95% (data not shown). Half-maximal inhibition was observed at 25 to 50 μM. Sodium azide also inhibited the activity of the purified complex by >90%, with half-maximal inhibition at 10 μM. Vanadate, although known to inhibit the activity of several ABC transporters, including the membrane-bound FGK2 complex at acidic pH, had no effect on the ATPase activity of the detergent-soluble complex (data not shown). The inability of vanadate to inhibit the MBP-independent F500GK2 complex at neutral pH has been previously reported (7). Vanadate may only inhibit specific conformations of ABC transporters achieved when ADP is bound at the nucleotide binding sites.
Verapamil increases the ATPase activity of the purified FGK2 complex and lowers its affinity for ATP.
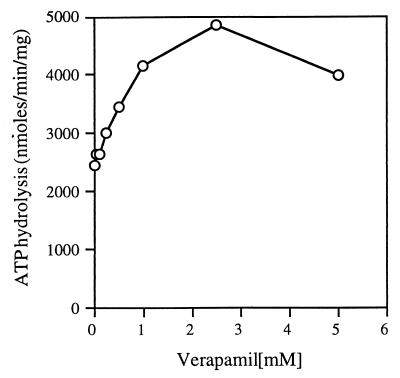
Verapamil is a known modulator of the activity of P-glycoprotein (15). In the presence of 10 to 50 μM verapamil, the ATPase activity of P-glycoprotein is increased by about 50 to 150% (3, 20, 29). Verapamil is a competitive inhibitor of P-glycoprotein (14). Recent work suggests that verapamil binds P-glycoprotein with different affinities, probably at two different binding sites (13, 33). Mutations that alter residues in the hydrophobic linker region change the sensitivity of the P-glycoprotein to verapamil and related compounds (3, 4). The same stimulation was observed with the bacterial protein LmrA from Lactococcus lactis, which shares a high degree of sequence similarity with P-glycoprotein (37). Because the bacterial importers including the FGK2 complex also have sequence similarity to the other members of the ABC transporter family, especially surrounding the ATP binding regions, including the hydrophobic linker region, we wanted to find out if verapamil would have a similar effect on the ATPase activity of the FGK2 complex. When we assayed ATP hydrolysis by the soluble intact complex in the presence of increasing concentrations of verapamil, we observed a 70 to 100% increase in the ATPase activity of the complex at 1 to 2.5 mM (Fig. 8), concentrations that are approximately 100-fold higher than that required to stimulate the activity of P-glycoprotein.
FIG. 8.
Concentration-dependent stimulation of detergent-soluble FGK2 complex ATPase activity by verapamil. The purified FGK2 complex was assayed for ATP hydrolysis activity (as described in the legend to Fig. 2) with 4 mM ATP in the absence and in the presence of increasing concentrations of verapamil.
It was shown that verapamil decreases the affinity of ATP for P-glycoprotein (34). In order to see whether verapamil influences the affinity of the FGK2 complex for ATP or the maximal rate of hydrolysis, we measured the rate of ATP hydrolysis by the soluble FGK2 complex as a function of ATP concentration in the absence and presence of 1 mM verapamil. We performed these measurements several times to get a more accurate measurement of the modest effects of verapamil on the FGK2 ATPase activity. As summarized in Table 2, while increasing the rate of ATP hydrolysis, verapamil lowers the affinity of the complex for ATP. Since we saw that MBP does not influence the affinity of ATP for the complex, it is clear that these two molecules act in different ways and probably at different sites. Although the magnitude of the stimulation by verapamil is somewhat less than that observed for P-glycoprotein, the fact that there is a concentration-dependent effect is likely indicative of the conservation of structure of this region among ABC transporters. It would be interesting to know how many different types of ABC transporters are affected by verapamil and related compounds.
TABLE 2.
Effect of verapamil on the affinity of the complex for ATP and the hydrolysis ratea
| Expt no. |
Km (μM)
|
Vmax (nmol/min/mg)
|
||||
|---|---|---|---|---|---|---|
| Without verapamil | With 1 mM verapamil | % Increase | Without verapamil | With 1 mM verapamil | % Increase | |
| 1 | 200–400 | 350–500 | 42 | 550–1,000 | 850–1,250 | 36 |
| 2 | 160–250 | 250–400 | 58 | 500–720 | 850–1,000 | 52 |
| 3 | 125–150 | 200 | 46 | 550–625 | 850 | 45 |
| 4 | 133–200 | 250–350 | 80 | 1,000 | 1,600–2,000 | 80 |
| 5 | 133–200 | 200–400 | 80 | 500–600 | 1,000 | 82 |
Assays were performed in duplicate with the purified detergent-soluble FGK2 complex as described in Materials and Methods.
DISCUSSION
ABC transporters comprise the largest single group of paralogous proteins. Although members of this family perform a variety of transport functions in diverse cell types, the transporters must share common mechanisms determined by the signature ABC sequence and similarity in overall structural organization (16). Efforts to understand the molecular basis of transport and the defects in medically important family members, such as the P-glycoprotein responsible for multiple drug resistance in tumors and the CFTR protein, which malfunctions in individuals with cystic fibrosis, are complicated by the complex nature of the cells and tissues in which they function. In contrast, bacterial ABC transporters offer the opportunity to study the mechanism of transport in simple unicellular organism that are amenable to both biochemical and genetic analyses. Characterization of the maltose and histidine transporters has taken advantage of the ability to both purify the transporters and study them genetically but is limited by the necessity to study their activities following reconstitution in proteoliposomes. This has been based on the observations that the detergent-soluble forms of the transporters are inactive.
Both the wild-type maltose and histidine transporters have been reported to be inactive in detergent solution. Davidson et al. studied a mutant form of FGK2, F500GK2, that, in contrast to the wild-type complex, hydrolyzes ATP in the absence of MBP. They reported that the mutant complex was able to hydrolyze ATP in detergent solution and concluded that the mutations responsible for the uncoupled phenotype were also responsible for the ability to retain activity in detergent solution (7). Our results are different and clearly show that both the wild-type and F500GK2 complexes are active in detergent solution. Indeed, the complexes differ in their responses to MBP signaling, with the wild type stimulated and the mutant inhibited by MBP. These results are consistent with studies that reported the activity of several eukaryotic ABC transporters in detergent solution (1, 12, 25, 34).
In Table 3, we provide a summary of the ATPase activities of the different forms of the FGK2 transporter, including a comparison with the purified isolated MalK subunit. In agreement with the results of others (27, 39), we found that the isolated MalK subunit exhibited low activity that was not affected by MBP. In addition, the membrane-bound form of the wild-type transporter showed the same low level of activity that was stimulated 15-fold by MBP. Detergent solution of the wild-type transporter resulted in an eightfold increase in specific activity, that could be further increased by MBP. In contrast, as reported for membrane preparations (11), the F500GK2 complex was more active than the wild type in detergent solution but was inhibited by MBP. These results indicate that the purified soluble FGK2 complex is an accurate model for studying signal transmission from MBP to the MalK subunit via the MalFG subunits.
TABLE 3.
Comparison of the ATPase activities of various forms of MalK
| Form of complex or MalK | ATPase activity (nmol/min/mg)
|
|
|---|---|---|
| Without MBP | With MBP | |
| FGK2 complex in proteoliposomes | 229 | 3,026 |
| FGK2 complex in detergent | 1,733 | 4,697 |
| F500GK2 complex in detergent | 2,757 | 1,883 |
| Dissociated MalK subunit | 217 | 186 |
In addition to these results, we found that verapamil, a compound that reverses the MDR phenotype and activates the ATPase activity of the P-glycoprotein (15), also activates the ATPase activity of the intact complex. We also found that, as was reported for P-glycoprotein (34), verapamil lowers the affinity of the complex for ATP. There is some information about the sites in the P-glycoprotein that may be important for binding of and/or stimulation by verapamil. Alterations of sequences N terminal to the Walker B motif appear to alter the ability of verapamil to activate the P-glycoprotein ATPase (3, 4). It should also be possible to study the effects of malK mutations on the ability of verapamil to stimulate FGK2 complex ATPase activity.
With the recent appearance of the three-dimensional structure of a related ABC transporter subunit, HisP (18), it is possible to speculate about how the membrane components and ligands control the ability of MalK to hydrolyze ATP. Although the free MalK subunit exhibits ATPase activity, it is not stimulated by MBP. There must be an intimate association between the MalK subunits and the MalF and MalG subunits that can serve to activate ATP hydrolysis (26). Data presented here suggest that the activation by MBP is due not to an increase in the affinity of the MalK subunit for ATP but to a change that increases the rate of hydrolysis. Although we do not understand the molecular basis of the activation of ATP hydrolysis by detergent, we speculate that the interaction of the detergent molecules with the membrane-spanning segments of the MalF and MalG proteins somehow promotes conformational changes that are transmitted to the MalK subunit. The ability to study these phenomena in solution with a combination of genetic and biophysical methods may provide more direct information about these proposed changes.
ACKNOWLEDGMENTS
We thank Carmen Rodriguez for excellent technical assistance.
This work was supported by award GM51118 from the National Institute for General Medical Sciences.
REFERENCES
- 1.Ambudkar S V, Lelong I H, Zhang J, Cardarelli C O, Gottesman M M, Pastan I. Partial purification and reconstitution of the human multidrug-resistance pump: characterization of the drug-stimulatable ATP hydrolysis. Proc Natl Acad Sci USA. 1992;89:8472–8476. doi: 10.1073/pnas.89.18.8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baichwal V, Liu D, Ames G F. The ATP-binding component of a prokaryotic traffic ATPase is exposed to the periplasmic (external) surface. Proc Natl Acad Sci USA. 1993;90:620–624. doi: 10.1073/pnas.90.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakos E, Klein I, Welker E, Szabo K, Muller M, Sarkadi B, Varadi A. Characterization of the human multidrug resistance protein containing mutations in the ATP-binding cassette signature region. Biochem J. 1997;323:777–783. doi: 10.1042/bj3230777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaudet L, Urbatsch I L, Gros P. Mutations in the nucleotide-binding sites of P-glycoprotein that affect substrate specificity modulate substrate-induced adenosine triphosphatase activity. Biochemistry. 1998;37:9073–9082. doi: 10.1021/bi972656j. [DOI] [PubMed] [Google Scholar]
- 5.Boos W, Shuman H. Maltose/maltodextrin system of Escherichia coli: transport, metabolism, and regulation. Microbiol Mol Biol Rev. 1998;62:204–229. doi: 10.1128/mmbr.62.1.204-229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chifflet S, Torriglia A, Chiesa R, Tolosa S. A method for the determination of inorganic phosphate in the presence of labile organic phosphate and high concentrations of protein: application to lens ATPases. Anal Biochem. 1988;168:1–4. doi: 10.1016/0003-2697(88)90002-4. [DOI] [PubMed] [Google Scholar]
- 7.Davidson A L, Laghaeian S S, Mannering D E. The maltose transport system of Escherichia coli displays positive cooperativity in ATP hydrolysis. J Biol Chem. 1996;271:4858–4863. [PubMed] [Google Scholar]
- 8.Davidson A L, Nikaido H. Overproduction, solubilization, and reconstitution of the maltose transport system from Escherichia coli. J Biol Chem. 1990;265:4254–4260. [PubMed] [Google Scholar]
- 9.Davidson A L, Nikaido H. Purification and characterization of the membrane-associated components of the maltose transport system from Escherichia coli. J Biol Chem. 1991;266:8946–8951. [PubMed] [Google Scholar]
- 10.Davidson A L, Sharma S. Mutation of a single MalK subunit severely impairs maltose transport activity in Escherichia coli. J Bacteriol. 1997;179:5458–5464. doi: 10.1128/jb.179.17.5458-5464.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson A L, Shuman H A, Nikaido H. Mechanism of maltose transport in Escherichia coli: transmembrane signaling by periplasmic binding proteins. Proc Natl Acad Sci USA. 1992;89:2360–2364. doi: 10.1073/pnas.89.6.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doige C A, Yu X, Sharom F J. ATPase activity of partially purified P-glycoprotein from multidrug-resistant Chinese hamster ovary cells. Biochim Biophys Acta. 1992;1109:149–160. doi: 10.1016/0005-2736(92)90078-z. [DOI] [PubMed] [Google Scholar]
- 13.Doppenschmitt S, Langguth P, Regardh C G, Andersson T B, Hilgendorf C, Spahn-Langguth H. Characterization of binding properties to human P-glycoprotein: development of a [3H]verapamil radioligand-binding assay. J Pharmacol Exp Ther. 1999;288:348–357. [PubMed] [Google Scholar]
- 14.Ford J M, Hait W N. Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol Rev. 1990;42:155–199. [PubMed] [Google Scholar]
- 15.Gottesman M M, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 16.Higgins C F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 17.Hor L I, Shuman H A. Genetic analysis of periplasmic binding protein dependent transport in Escherichia coli. Each lobe of maltose-binding protein interacts with a different subunit of the MalFGK2 membrane transport complex. J Mol Biol. 1993;233:659–670. doi: 10.1006/jmbi.1993.1543. [DOI] [PubMed] [Google Scholar]
- 18.Hung L W, Wang I X, Nikaido K, Liu P Q, Ames G F, Kim S H. Crystal structure of the ATP-binding subunit of an ABC transporter. Nature. 1998;396:703–707. doi: 10.1038/25393. [DOI] [PubMed] [Google Scholar]
- 19.Lanzetta P A, Alvarez L J, Reinach P S, Candia O A. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979;100:95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- 20.Litman T, Zeuthen T, Skovsgaard T, Stein W D. Structure-activity relationships of P-glycoprotein interacting drugs: kinetic characterization of their effects on ATPase activity. Biochim Biophys Acta. 1997;1361:159–168. doi: 10.1016/s0925-4439(97)00026-4. [DOI] [PubMed] [Google Scholar]
- 21.Liu C E, Ames G F. Characterization of transport through the periplasmic histidine permease using proteoliposomes reconstituted by dialysis. J Biol Chem. 1997;272:859–866. doi: 10.1074/jbc.272.2.859. [DOI] [PubMed] [Google Scholar]
- 22.Manson M D, Boos W, Bassford P J J, Rasmussen B A. Dependence of maltose transport and chemotaxis on the amount of maltose-binding protein. J Biol Chem. 1985;260:9727–9733. [PubMed] [Google Scholar]
- 23.Merino G, Boos W, Shuman H A, Bohl E. The inhibition of maltose transport by the unliganded form of the maltose-binding protein of Escherichia coli: experimental findings and mathematical treatment. J Theor Biol. 1995;177:171–179. doi: 10.1006/jtbi.1995.0236. [DOI] [PubMed] [Google Scholar]
- 24.Merino G, Shuman H A. Unliganded maltose-binding protein triggers lactose transport in an Escherichia coli mutant with an alteration in the maltose transport system. J Bacteriol. 1997;179:7687–7694. doi: 10.1128/jb.179.24.7687-7694.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer T H, van Endert P M, Uebel S, Ehring B, Tampe R. Functional expression and purification of the ABC transporter complex associated with antigen processing (TAP) in insect cells. FEBS Lett. 1994;351:443–447. doi: 10.1016/0014-5793(94)00908-2. [DOI] [PubMed] [Google Scholar]
- 26.Mourez M, Hofnung M, Dassa E. Subunit interactions in ABC transporters: a conserved sequence in hydrophobic membrane proteins of periplasmic permeases defines an important site of interaction with the ATPase subunits. EMBO J. 1997;16:3066–3077. doi: 10.1093/emboj/16.11.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mourez M, Jehanno M, Schneider E, Dassa E. In vitro interaction between components of the inner membrane complex of the maltose ABC transporter of Escherichia coli: modulation by ATP. Mol Microbiol. 1998;30:353–363. doi: 10.1046/j.1365-2958.1998.01070.x. [DOI] [PubMed] [Google Scholar]
- 28.Nikaido K, Liu P Q, Ames G F. Purification and characterization of HisP, the ATP-binding subunit of a traffic ATPase (ABC transporter), the histidine permease of Salmonella typhimurium. Solubility, dimerization, and ATPase activity. J Biol Chem. 1997;272:27745–27752. doi: 10.1074/jbc.272.44.27745. [DOI] [PubMed] [Google Scholar]
- 29.Orlowski S, Mir L M, Belehradek J, Jr, Garrigos M. Effects of steroids and verapamil on P-glycoprotein ATPase activity: progesterone, desoxycorticosterone, corticosterone and verapamil are mutually non-exclusive modulators. Biochem J. 1996;317:515–522. doi: 10.1042/bj3170515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panagiotidis C H, Boos W, Shuman H A. The ATP-binding cassette subunit of the maltose transporter MalK antagonizes MalT, the activator of the Escherichia coli mal regulon. Mol Microbiol. 1998;30:535–546. doi: 10.1046/j.1365-2958.1998.01084.x. [DOI] [PubMed] [Google Scholar]
- 31.Racker E, Violand B, O'Neal S, Alfonzo M, Telford J. Reconstitution, a way of biochemical research; some new approaches to membrane-bound enzymes. Arch Biochem Biophys. 1979;198:470–477. doi: 10.1016/0003-9861(79)90521-6. [DOI] [PubMed] [Google Scholar]
- 32.Reyes M, Shuman H A. Overproduction of MalK protein prevents expression of the Escherichia coli mal regulon. J Bacteriol. 1988;170:4598–4602. doi: 10.1128/jb.170.10.4598-4602.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romsicki Y, Sharom F J. The membrane lipid environment modulates drug interactions with the P-glycoprotein multidrug transporter. Biochemistry. 1999;38:6887–6896. doi: 10.1021/bi990064q. [DOI] [PubMed] [Google Scholar]
- 34.Shapiro A B, Ling V. ATPase activity of purified and reconstituted P-glycoprotein from Chinese hamster ovary cells. J Biol Chem. 1994;269:3745–3754. [PubMed] [Google Scholar]
- 35.Shuman H A. Active transport of maltose in Escherichia coli K12. Role of the periplasmic maltose-binding protein and evidence for a substrate recognition site in the cytoplasmic membrane. J Biol Chem. 1982;257:5455–5461. [PubMed] [Google Scholar]
- 36.Silhavy T J, Brickman E, Bassford P J, Casadaban M J, Shuman H A, Schwartz V, Guarente L, Schwartz M, Beckwith J R. Structure of the malB region in Escherichia coli K12. II. Genetic map of the malE,F,G operon. Mol Gen Genet. 1979;174:249–259. doi: 10.1007/BF00267797. [DOI] [PubMed] [Google Scholar]
- 37.van Veen H W, Callaghan R, Soceneantu L, Sardini A, Konings W N, Higgins C F. A bacterial antibiotic-resistance gene that complements the human multidrug-resistance P-glycoprotein gene. Nature. 1998;391:291–295. doi: 10.1038/34669. [DOI] [PubMed] [Google Scholar]
- 38.Waldburger C D, Sauer R T. Signal detection by the PhoQ sensor-transmitter. Characterization of the sensor domain and a response-impaired mutant that identifies ligand-binding determinants. J Biol Chem. 1996;271:26630–26636. doi: 10.1074/jbc.271.43.26630. [DOI] [PubMed] [Google Scholar]
- 39.Walter C, Honer zu Bentrup K, Schneider E. Large scale purification, nucleotide binding properties, and ATPase activity of the MalK subunit of Salmonella typhimurium maltose transport complex. J Biol Chem. 1992;267:8863–8869. [PubMed] [Google Scholar]
- 40.Wiater L A, Marra A, Shuman H A. Escherichia coli F plasmid transfers to and replicates within Legionella pneumophila: an alternative to using an RP4-based system for gene delivery. Plasmid. 1994;32:280–294. doi: 10.1006/plas.1994.1067. [DOI] [PubMed] [Google Scholar]