Abstract
Multiple de novo brain arteriovenous malformations (bAVM) have been reported in the literature, raising questions about the contended purely congenital nature of these lesions. We present the 15-year course of a pediatric patient, who initially presented at age 5 with a thalamic cavernous malformation and was treated with radiosurgery, and then subsequently developed a thalamic de novo bAVM immediately adjacent to the initial lesion location, discovered 2 years later. Treatment of the bAVM entailed two transarterial embolizations and one radiosurgery session which ultimately led to complete angiographic resolution. Finally, this patient’s course was complicated by intraparenchymal hemorrhage and acute obstructive hydrocephalus, and further imaging revealed two newly formed cavernous malformations, also associated with the initial lesion’s location, that have remained stable since their formation. This case likely represents the second-hit model for the formation of vascular malformations, as sparsely supported by the current literature. According to this, genetically aberrant, yet quiescent, brain areas might promote the de novo formation of vascular malformations after brain injury, including radiation.
Keywords: (MeSH): arteriovenous malformation, familial cerebral cavernous malformation, de novo
Case description
We report a case of de novo brain arteriovenous malformation (bAVM) formation following stereotactic radiosurgery targeting a thalamic cavernous malformation in a pediatric patient. The patient originally presented at 5 years of age with right upper extremity (UE) tremor and was found to have a left thalamic lesion with MR characteristics suggestive of a cerebral cavernous malformation (CCM), as well as an associated developmental venous anomaly (DVA) draining into the internal cerebral vein (Figure 1). There was no evidence of acute hemorrhage at that time. Cerebral digital subtraction angiography performed at the time of initial diagnosis with cavernous malformation showed no evidence of bAVM or any other shunting vascular lesion (Figure 2). With the intention of treating the cavernous malformation, the patient underwent gamma knife surgery (14Gy) which resulted in right upper extremity weakness. He presented again 1 year later with a small intraparenchymal hemorrhage at the site of the cavernous malformation and was treated with corticosteroids for 6 months.
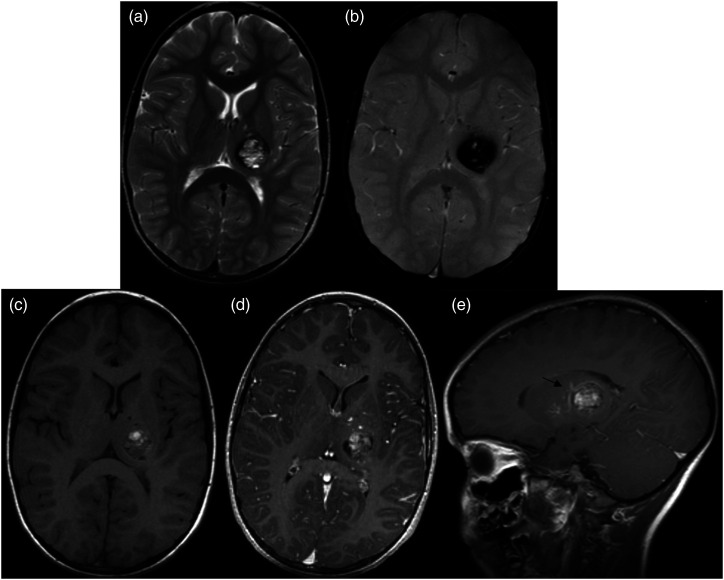
Figure 1.
MRI at initial presentation in the patient at 5 years of age. (A) Axial T2-weighted acquisition centered at the level of the basal ganglia demonstrates a well-circumscribed lesion with mixed hypo/hyperintense T2 signal centrally. Corresponding susceptibility weighted image (B) shows significant associated blooming artifact. Central heterogenous hyperintensity on non-contrast T1 (C) suggests the presence of subacute blood products. Post-contrast T1 MPRAGE images (D) and (E) demonstrated scattered central enhancement and an enhancing curvilinear structure draped over the superior margin of the lesion, suggestive of cavernous malformation with an associated developmental venous anomaly (arrow).
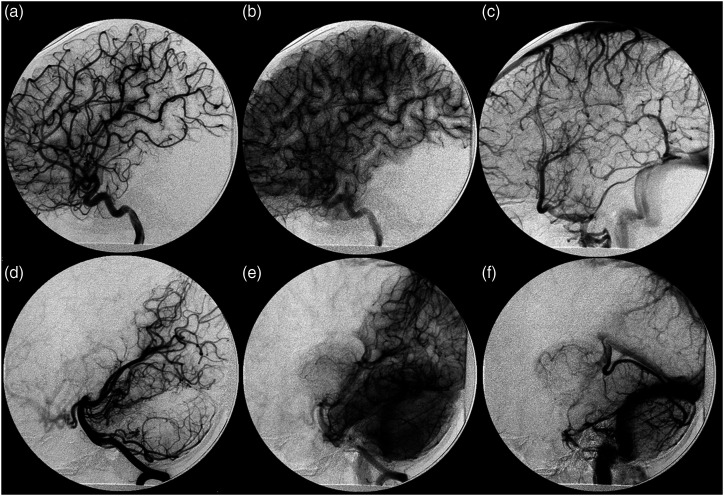
Figure 2.
Digital subtraction angiography at initial presentation. Lateral views of left ICA (A)–(C) and vertebral artery (D)–(F) depicting arterial, capillary, and venous phases (left to right) demonstrate no evidence of arteriovenous shunting. Alternate views (not shown) demonstrated DVA seen on corresponding MRI.
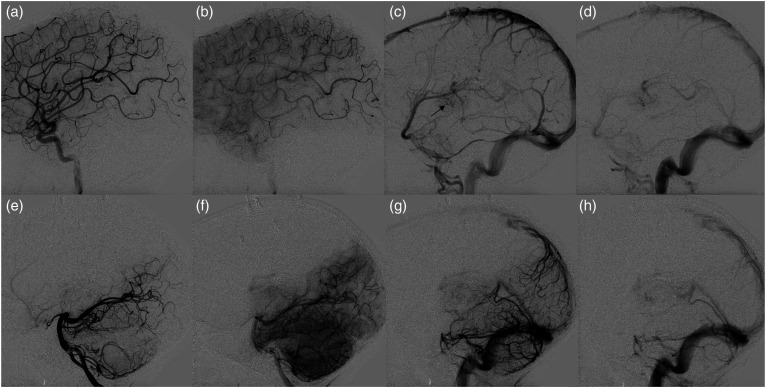
Two years after initial presentation, at the age of 7 years, follow-up MRI and catheter angiogram (Figure 3) demonstrated formation of a de novo bAVM in left thalamus, just medial to the previously treated lesion. Angiography confirmed the presence of a left thalamic bAVM draining into a dilated pouch of the previously observed DVA. The bAVM nidus was supplied by the left anterior choroidal artery, left posterior choroidal artery, lateral lenticulostriate arteries, thalamoperforating, and MCA trans-sylvian feeders. Physical exam at that point demonstrated right arm spastic weakness and right-hand tremor.
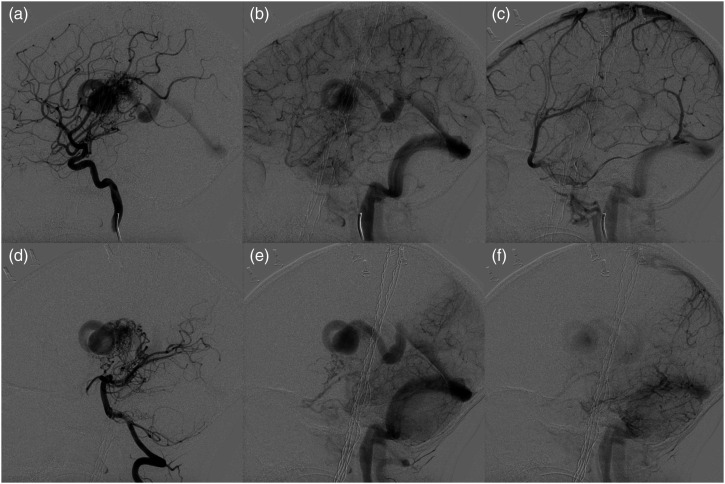
Figure 3.
Digital subtraction angiography following radiosurgery for left basal ganglia cavernous malformation 2 years after initial presentation. Lateral views of left ICA (A)–(C) and vertebral (D)–(F) injections demonstrates interval formation of an arteriovenous shunting lesion draining into the internal cerebral vein through an intervening nidus associated with a large ectatic venous pouch, compatible with a bAVM.
The bAVM was treated by embolization in two stages followed by radiosurgery. In the first procedure, two feeders from the left medial posterior choroidal artery were occluded with n-BCA injection, yielding a decrease in the posterior portion of the bAVM nidus. In a second embolization procedure, n-BCA was injected into posterior choroidal arterial feeders followed by Onyx into lenticulostriate arterial feeders, resulting in minimal residual opacification of the malformation. Follow-up studies demonstrated a decrease in the bAVM’s size, with no sign of additional vascular malformations (Figure 4). Two years after the second embolization, a third embolization attempt was unsuccessful and after consideration of the risk-benefit profile, a gamma knife surgery was performed to definitively cure the bAVM. A second radiosurgery was considered to be the best option, despite risks of adjacent brain injury or new lesion development, given the prior hemorrhage, location, and age of the patient.
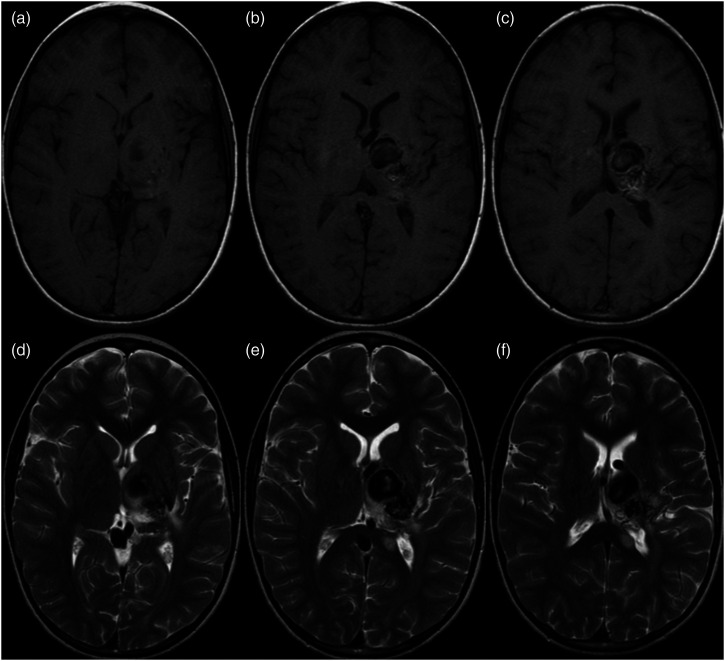
Figure 4.
Axial non-contrast T1 (A)–(C) and T2 (D)–(F) centered on basal ganglia at three levels acquired after embolization and prior to radiosurgery for left thalamic bAVM. The large ectatic venous lesion seen in the previous angiogram is again demonstrated. Central T1 hyperintensity, T2 flow void, and pulsation artifact suggests slow flow through the draining vein.
Nearly 1 year after this second radiosurgery, at the age of 9 years, angiography demonstrated complete resolution of the bAVM (Figure 5). There was no filling within the ectatic venous pouch that had previously drained the bAVM, though contrast stagnation was noted within the previously observed DVA, which was not fully thrombosed. Venous drainage was otherwise normal. A neurologic exam demonstrated mild right hemiparesis.
Figure 5.
Digital subtraction angiography following radiosurgery on previously embolized left thalamic AVM. Lateral views of left ICA (A)–(D) and vertebral artery (E)–(H) depicting arterial, capillary, early venous, and late venous phases (left to right) demonstrate no evidence of arteriovenous shunting, consistent with cure. Slow drainage within previously observed developmental venous anomaly is indicated by the black arrow (C).
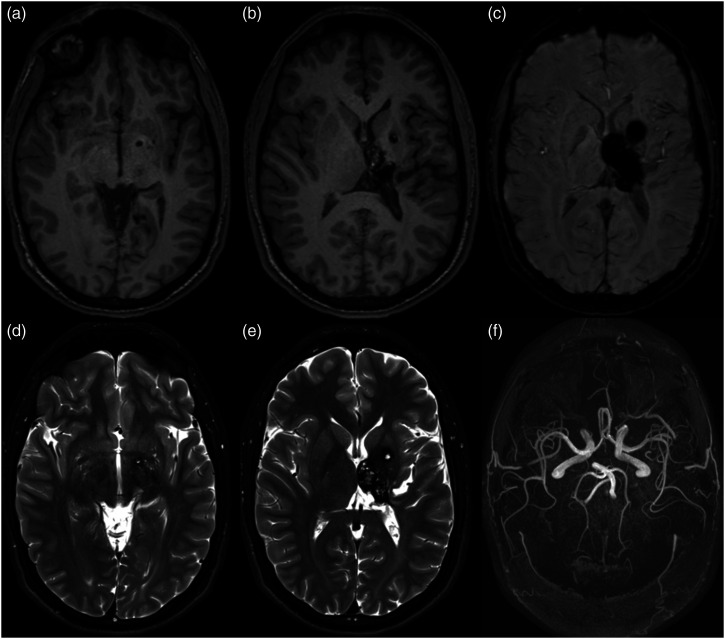
Three years after angiographic cure of the bAVM was demonstrated, at the age of 12, the patient presented again with intraparenchymal hemorrhage and acute obstructive hydrocephalus, and MRI demonstrated the presence of two newly formed cavernous malformations in the left putamen and inferior globus pallidus, in addition to the thalamic malformation that has been noted at first presentation. The patient recovered following medical management, and the cavernous malformations have remained stable through his most recent MRI follow-up at the age of 19 years old (Figure 6). Informed consent was sought and obtained from the patient and his family.
Figure 6.
Long-term MRI follow-up at age 19. Axial non-contrast T1 (A)–(B), SWI (C), and T2 (D)–(E) centered on the basal ganglia at two demonstrate lesions with T1 and T2 signal characteristics consistent with cavernous malformations located in the inferior globus pallidus, medial putamen, and thalamus. Susceptibility weighted imaging demonstrates significant associated blooming artifact. Axial maximum intensity projection of time of flight MR angiography (f) shows no evidence of residual shunting lesion.
Discussion
Brain arteriovenous malformation is defined as an abnormal direct arterial to venous anastomosis without capillaries and with no interposed brain parenchyma. Development of the vascular system, and consequently its anomalies, takes place during the third to eighth gestational weeks. Traditionally, bAVMs were contended to be congenital malformations. However, due to multiple reports of de novo bAVMs tracing back to Morioka et al. in 1988, this concept of bAVMs has largely been replaced by a more dynamic concept that considers the interplay between genetic, mechanical, and environmental factors.1–7 Due to limited cases in the literature, the natural history and prognosis of bAVMs is not well documented; however, based on a review of 38 cases published in 2018, most de novo bAVMs are discovered either incidentally (36.8%) or as a result of symptomatic hemorrhage (31.6%). 8 In this review, the mean time between bAVM presentation and the last normal angiographic study was 6.6 years (SD: 4.9; range: 2–25). 8 Similar to primary bAVMs, de novo malformations are usually found in the supratentorial compartment, while infratentorial cases are rarer. 7 To the best of our knowledge, this is the first report of a de novo bAVM after radiotherapy for a CCM.
Though the precise pathophysiology of bAVMs is not clearly understood, multiple genetic and environmental culprits serve as triggering factors. In a review of de novo bAVMs conducted by Nagai et al. in 2020, 40 cases were identified during the last two decades, all of which had a prior history of either a preexisting vascular abnormality (including cavernous malformation) or nonvascular condition (including radiation). 2 In addition, they presented two cases of theirs, one of which had no previous intracranial pathology and the other had Bell’s palsy (second de novo AVM associated with Bell’s palsy ever reported). These insults could have acted according to a second-hit model2,5 in an already aberrant genome, thus promoting the appearance of a de novo bAVM by triggering abnormal angiogenesis in genetically abnormal yet quiescent brain areas (usually over the course of years). The second-hit model has also been supported by Park et al., who filmed in real time the development of a subdermal de novo bAVM in a mouse with an activin-like kinase 1 (Alk1) deletion after imposing a vascular injury. 9 Additionally, shear stress could trigger the release of growth factors by the endothelial cells, thus allowing the development of a preexisting but occult bAVM. 6 Ultimately, normally suppressed angiogenesis can be reactivated in light of the aforementioned second hits, 4 especially in the background of an aberrant genome.
The presence of a DVA has been supported to promote the development of other shunting lesions. 10 Mullan et al. presented four bAVM cases with certain cerebral venous malformation characteristics, supporting the notion that these cases were “transitioning” forms of venous malformations that fistulized over time becoming bAVMs. 3 The authors contended that the DVA (“venous star”-like) cluster of deep draining veins may represent an aberrant vessel network that can promote the development of an AVM. 3 In a review by Rinaldo et al., venous hypertension was identified as a possible culprit for the release of vascular endothelial growth factors and subsequent neoangiogenesis, leading to arteriovenous connections within DVA radicles. 10 In our case, radiosurgery of the CCM, with resultant obliteration of that lesion, could have inadvertently affected the adjacent DVA, changing hemodynamics resulting in the release of factors contributing to the AVM formation. This could explain the formation of a de novo bAVM draining into a previously identified DVA, at the periphery of an initial abnormality treated with radiosurgery. Furthermore, we believe that the presence of a DVA played a vital role in the pathogenesis of the future vascular malformations. Two radiosurgery treatments to the same area may have increased the susceptibility to form new vascular lesions.
Importantly, bAVM tissue in both human and animal studies has demonstrated upregulated VEGF, 11 TGF, 11 NOTCH1 and NOTCH4, 12 and FABP4. 13 Differentiation of pericytes induce capillary formation suppression, through angiopoietin-1, ephrin-B2, and metalloproteinases. 4 It is highly possible that a second hit could cause dysregulation of these angiogenic-suppressor effects thus favoring the development of a bAVM. Other candidate triggers for bAVM development include upregulation of IL-6, 14 myeloperoxidase, 14 vascular endothelial growth factor (VEGF) 15 and hypoxia-inducible-factor 1 (HIF1), 15 as well as downregulation of angiopoietin-1. 15 VEGF and angiopoietin-2 (ANG-2) upregulation have been associated with angiogenic effects mediated through matrix metalloproteinases, which, when taken in conjunction with the abundance of inflammatory cells found in bAVM stroma, may suggest a possible inflammatory contribution at some point during the course of the disease. 14 No genetic tests were conducted for the patient of the index case. Multiple genes have been identified as culprits, so a negative result would not rule out an aberrant genetic background.
The case presented in this report offers further support for the phenomena of de novo bAVM formation as a result of the second-hit effect on already aberrant cerebrovascular tissue. Its uniqueness lies in the demonstration of the tendency of a possibly genetically susceptible individual who had radiation exposure to form multiple cerebrovascular lesions over the course of a 15-year follow-up period.
Footnotes
Author contributor statement: JF and SM conceptualized the project. SM and DB drafted and significantly edited the manuscript and figures. All authors made final edits to the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Stavros Matsoukas https://orcid.org/0000-0001-5902-0637
Devin V Bageac https://orcid.org/0000-0002-4183-2829
References
- 1.Morioka T, Nishio S, Hikita T, et al. Marked growth of an angiographically occult arteriovenous malformation: case report. Neurosurgery 1988; 23(1): 101–103. [DOI] [PubMed] [Google Scholar]
- 2.Nagai Y Anan Mand Fujiki M. Cerebral arteriovenous malformations as acquired lesions: case reports and review of the literature. J Stroke Cerebrovasc Dis 2020; 29(10): 105157. [DOI] [PubMed] [Google Scholar]
- 3.Mullan S, Mojtahedi S, Johnson DL, et al. Cerebral venous malformation-arteriovenous malformation transition forms. J Neurosurg 1996; 85(1): 9–13. [DOI] [PubMed] [Google Scholar]
- 4.Dalton A, Dobson G, Prasad M, et al. De novo intracerebral arteriovenous malformations and a review of the theories of their formation. Br J Neurosurg 2018; 32(3): 305–311. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Rivera V, Sheriff FG, Sandberg DI, et al. De novo thalamic arteriovenous malformation in a boy with a brainstem cavernous malformation. J Clin Neurosci 2020; 76: 226–228. [DOI] [PubMed] [Google Scholar]
- 6.Jeffree RLand Stoodley MA. Postnatal development of arteriovenous malformations. Pediatr Neurosurg 2009; 45(4): 296–304. [DOI] [PubMed] [Google Scholar]
- 7.Kilbourn KJ, Spiegel G, Killory BD, et al. Case report of a de novo brainstem arteriovenous malformation in an 18-year-old male and review of the literature. Neurosurg Rev 2014; 37(4): 685–691. [DOI] [PubMed] [Google Scholar]
- 8.Lv Xand Wang G. Review of de novo cerebral arteriovenous malformation: haemorrhage risk, treatment approaches and outcomes. Neuroradiol J 2018; 31(3): 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park SO, Wankhede M, Lee YJ, et al. Real-time imaging of de novo arteriovenous malformation in a mouse model of hereditary hemorrhagic telangiectasia. J Clin Invest 2009; 119(11): 3487–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rinaldo L, Lanzino G, Flemming KD, et al. Symptomatic developmental venous anomalies. Acta Neurochir (Wien) 2020; 162: 1115–1125. DOI: 10.1007/s00701-020-04213-z. [DOI] [PubMed] [Google Scholar]
- 11.Kiliç T, Pamir MN, Küllü S, et al. Expression of structural proteins and angiogenic factors in cerebrovascular anomalies. Neurosurgery 2000; 46(5): 1179–1191, discussion 1191-2. [DOI] [PubMed] [Google Scholar]
- 12.Murphy PA, Lu G, Shiah S, et al. Endothelial Notch signaling is upregulated in human brain arteriovenous malformations and a mouse model of the disease. Lab Invest 2009; 89(9): 971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cataltepe S, Arikan MC, Liang X, et al. Fatty acid binding protein 4 expression in cerebral vascular malformations: implications for vascular remodelling. Neuropathol Appl Neurobiol 2015; 41(5): 646–656. [DOI] [PubMed] [Google Scholar]
- 14.Kim H, Su H, Weinsheimer S, et al. Brain arteriovenous malformation pathogenesis: a response-to-injury paradigm. Acta Neurochir Suppl 2011; 111: 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Storkebaum E, Quaegebeur A, Vikkula M, et al. Cerebrovascular disorders: molecular insights and therapeutic opportunities. Nat Neurosci 2011; 14(11): 1390–1397. [DOI] [PubMed] [Google Scholar]