Abstract
Background:
Cervical cancer screening programs are facing a programmatic shift where screening protocol based on human papillomavirus testing (HPV-Screening protocol) is replacing the liquid-based cytology (LBC-Screening protocol). For safe technology transfer within the nationwide screening programme in Norway, HPV-Screening protocol was implemented randomized to compare the real-world effectiveness of HPV-Screening protocol and LBC-Screening protocol at the first screening round.
Methods:
Among 302,295 women ages 34 to 69 years scheduled to attend screening from February 2015 to June 2017, 157,447 attended. A total of 77,207 were randomly allocated to the HPV-Screening protocol and 80,240 were allocated to the LBC-Screening protocol. All women were followed up for 18 months.
Results:
The HPV-Screening protocol resulted in a relative increase of 60% in the detection of cervical intraepithelial neoplasia (CIN) grade 2 or worse [risk ratio (RR) = 1.6, 95% confidence interval (CI) = 1.5–1.7], 40% in CIN grade 3 or worse (RR = 1.4, 95% CI = 1.3–1.6), 40% in cancer (RR = 1.4, 95% CI = 1.0–2.1), and 60% in colposcopy referrals (RR = 1.6, 95% CI = 1.5–1.6) compared with LBC-Screening. The performance of both protocols was age dependent, being more effective in women ages under 50 years.
Conclusions:
The HPV-Screening protocol was well accepted by women in Norway and detected more CIN2, CIN3, and cancers compared with the LBC-Screening protocol.
Impact:
A randomized implementation of the HPV-Screening protocol with real-world assessment enabled a gradual, quality assured, and safe technology transition. HPV-based screening protocol may further be improved by using HPV genotyping and age-specific referral algorithms.
Introduction
Cervical cancer screening programmes that employ repeated cytology testing of the adult female population with appropriate follow-up and treatment of cervical preinvasive abnormalities have reduced cervical cancer incidence significantly (1). The large difference in cervical cancer incidence between countries with and without established screening programmes represents indirect evidence of their effectiveness (2, 3).
The discovery of the causal role of high-risk human papillomavirus (HPV) infection in cervical cancer pathogenesis (4) has led to the development of molecular tests for HPV detection (5). Compared with cytology, HPV testing has demonstrated a higher sensitivity to detect prevalent and incipient high-grade cervical abnormalities, cervical intraepithelial neoplasia (CIN) grade 2 (CIN2), grade 3 (CIN3), adenocarcinoma in situ (ACIS), and cervical cancer (6–8). As a consequence of its higher sensitivity, a negative HPV test predicts greater safety against high-grade cervical abnormalities and frank invasive cervical cancer over the next 10 years than cytology (7–11), which permits screening intervals to be extended safely. Furthermore, HPV testing offers consistent, reproducible clinical performance that can be transferred between labs (12). Available results from randomized controlled trials and observational studies strongly support adoption of HPV testing in screening programmes for women over 30 years of age to reduce costs while improving efficiency (13, 14).
The replacement of cytology with HPV testing in primary cervical cancer screening will result in substantial modifications to existing screening protocols. These changes will affect activity volumes (15), necessitate adjustments in staff training, and require changes in the data flow and communication systems used for reporting and evaluation. Because cervical cancer screening programmes target a large number of women, between 110 and 150 million women in Europe alone, and generate a large volume of health care services (13), the introduction of screening protocols based on HPV testing must be carefully designed to maintain, and hopefully increase, the effectiveness of cervical cancer screening programmes and their acceptability in the population.
To compare the real-world effectiveness of two cervical cancer screening protocols at the first screening round (baseline), one that started with HPV testing (HPV-Screening protocol) and one that started with liquid-based cytology (LBC; LBC-Screening protocol) in a Norwegian Cervical Cancer Screening Programme (NCCSP) we conducted a pilot programme evaluation during the transition from LBC-based to HPV testing–based primary screening.
Materials and Methods
The NCCSP
The organized NCCSP started in 1995, when national guidelines recommended cytology screening every 3 years for women ages 25 to 69 years. Since 2004, conventional cytology was gradually replaced by LBC, which was the prevailing technology in 2016. The NCCSP is part of the Cancer Registry of Norway, and contains almost 100% of cervical cancer screening-related diagnostics, as all public and private laboratories are legally required to report all results for cervical cytology (from 1991), histology (from 2002), and HPV testing (from 2005; ref. 16). For each of the approximately 1.4 million women of target screening age in Norway, the date of the most recent screening examination is systematically identified, so that a reminder of the next screening examination can be sent in 3 years’ time. The NCCSP sends a first reminder to women with no screening results registered in the last 34 months, and a second reminder to those with no results in the last 46 months.
Pilot programme for the transition to a primary HPV testing–based screening protocol
On the basis of the strength of existing evidence, including randomized clinical trials of HPV testing–based screening protocols conducted in Europe and recommendations in the European guidelines for quality assurance in cervical cancer screening (7, 13, 17–21) in 2013, Norwegian health authorities decided to conduct a pilot programme to implement a similar protocol in the NCCSP for women ages 34–69 years. NCCSP identified three counties that would be included in the pilot programme: Trøndelag (previously Sør- and Nord-Trøndelag), Hordaland, and Rogaland. These counties include about one-fourth of the target screening population in Norway and are comparable in size and population density, including both urban and rural population. The primary screening method in these counties in 2014 was LBC. Form of consent was not obtained from the women as the pilot was classified as part of a quality assurance programme.
Enrolment in the pilot programme started in 2015, and the implementation of the programme was overseen by an Academic Panel (M. Nygård, B. Engesæter, P.E. Castle, J.M. Berland, M.L. Eide, O.E. Iversen, C.M. Jonassen, I.K. Christiansen, O.K. Vintermyr, and A. Tropé) made up of representatives from collaborating laboratories and national and international experts. The randomized implementation of the HPV-Screening protocol was registered as a health service trial, 006_2014_10_RHS, after approval by the National Council for Quality and Priority in Health Services in the fall 2013 (22, 23). To clearly explain the design, conduct, analysis, and interpretation, we report our results according to the Consolidated Standards of Reporting Trials (CONSORT).
Procedures
During each screening examination, family doctors and gynecologists collected exfoliated cervical cells using a standard dry brush, which was eluted into PreservCyt medium (Hologic, Inc.). Vials were then transported for testing to laboratories at St. Olav Hospital in Trondheim, Haukeland University Hospital in Bergen, and Stavanger University Hospital in Stavanger, for the counties of Trøndelag, Hordaland, and Rogaland, respectively. All participating laboratories followed the same allocation, testing, and follow-up procedures, as per the instructions of the NCCSP's Advisory Board.
Following national tendering, the aforementioned laboratories performed all HPV testing using the cobas 4800 HPV Test (Roche Molecular Diagnostics), an automated, real-time PCR assay targeting the HPV L1 region of 14 HPV types in a single assay. The test detects HPV types 16 and 18 individually, while 12 other high-risk HPV types (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) are detected in aggregate. All three participating laboratories demonstrated a high interlaboratory reproducibility of HPV testing (12).
LBC was performed using the ThinPrep 5000 System (Hologic, Inc.). LBC results were interpreted according to the Bethesda System (24) as cancer, ACIS, high-grade squamous intraepithelial lesion (HSIL), atypical squamous cells cannot rule out HSIL (ASC-H), atypical glandular cells (AGC), low-grade squamous intraepithelial lesion (LSIL), atypical squamous cells of undetermined significance (ASC-US), and negative for intraepithelial lesions or malignancy (NILM).
Colposcopy and histology
Colposcopic examinations included the collection of biopsies or cone specimens. These samples were examined at local pathology labs, where the histologic results were categorized as NILM, CIN grade 1 (CIN1), CIN2, CIN3, ACIS, and cervical cancer (25). All women diagnosed with CIN2, CIN3, ACIS, or cancer were treated according to national clinical guidelines (26).
Description of the HPV-Screening and LBC-Screening protocols
HPV-Screening protocol
Women allocated to the HPV-Screening protocol underwent primary HPV testing at the first screening round (baseline) of the pilot programme. Women with inadequate HPV test results at baseline were recommended to undergo another examination within 1 to 3 months, and those with negative HPV test results at baseline were referred to screening in 5 years (Supplementary Fig. S1). Women with positive HPV test results at baseline had LBC done on the residual cervical specimen. These women were referred to colposcopy if their LBC results came back as ASC-US or worse (ASC-US+, including cancer, ACIS, HSIL, ASC-H, AGC, and LSIL), and to increased surveillance (i.e., follow-up HPV testing at 12 months) if their LBC results came back as NILM. HPV-positive and NILM cytology women with negative HPV test results at 12-month follow-up were referred to screening in 5 years, while women with positive results were referred to colposcopy.
LBC-Screening protocol
Women allocated to the LBC-Screening protocol underwent primary LBC at baseline. Women with inadequate LBC results at baseline were recommended to undergo another examination within 1 to 3 months, and those with LBC results of NILM at baseline were referred to screening in 3 years (Supplementary Fig. S1). Women whose LBC results came back as high-grade cytologic abnormalities (cancer, ACIS, HSIL, ASC-H, or AGC) were referred to colposcopy. Women with LBC results of LSIL or ASC-US had HPV testing performed on the residual cervical specimen. Those with negative HPV test results were referred to screening in 3 years; women with positive HPV test results were referred to increased surveillance (i.e., follow-up LBC and HPV testing within 6–12 months). Women who had follow-up LBC results of NILM or ASC-US and negative HPV test results were referred to screening in 3 years. Those with follow-up LBC results of LSIL or worse and/or positive HPV test results were referred to colposcopy.
Participants and randomization
Women ages 34 to 69 years in Trøndelag, Hordaland, and Rogaland counties who were scheduled to attend screening in the NCCSP from February 2015 to June 2017 were eligible for inclusion in the analysis. Women were randomly allocated to one of the two screening protocols (1:1): those with an odd date of birth were allocated to the HPV-Screening protocol, and those with an even date of birth to the LBC-Screening protocol. Women randomized to the HPV-Screening protocol reserved the right to change to the LBC-Screening protocol, but this choice to change protocols was not offered to women randomized to the LBC-Screening protocol.
Women randomized to the HPV-Screening protocol received a modified reminder letter from the NCCSP stating that HPV testing would be used for primary screening, while women randomized to the LBC-Screening protocol received the standard NCCSP reminder letter. Family doctors and gynecologists were also responsible for informing women randomized to the HPV-Screening protocol that their sample would be sent for primary HPV testing. HPV test results were sent to women by mail, along with all relevant follow-up information and instructions. Information about the implementation of the HPV-Screening protocol was also communicated to the public through local newspapers. Randomization to the HPV-Screening protocol began in Trøndelag on February 1, 2015, in Hordaland on March 15, 2015, and in Rogaland on April 1, 2015.
Statistical analyses
Information on the dates and results of screening and follow-up examinations within 18 months of baseline examination, and the dates and types of reminders was retrieved from the Cancer Registry of Norway. Individual timelines were then constructed for each participant (database lock on January 1, 2019), and analyses were stratified by the HPV-Screening and LBC-Screening protocols. The following programmatic key performance indicators were calculated: (i) screening results at baseline; screening outcomes; (ii) screening attendance within 1 year of the first and second reminder letter; (iii) number and rate of histologically confirmed CIN2, CIN2 or worse (CIN2+), CIN3, CIN3 or worse (CIN3+), and cervical cancer overall; (iv) number and rate of referral to colposcopy with the proportion of those diagnosed with CIN2+, CIN3+, and cervical cancer, that is, positive predictive value (PPV).
Screening outcomes were classified as inadequate, screen-negative, and screen-positive. Screening outcomes for all women with inadequate baseline screening results were classified as “inadequate”. Women allocated to the HPV-Screening protocol with negative HPV test result at baseline were classified as “screen-negative,” as were women allocated to the LBC-Screening protocol with LBC results of NILM at baseline, or LBC results of ASC-US or LSIL and negative HPV test results. Women allocated to the HPV-Screening protocol with positive HPV test results at baseline were classified as “screen-positive,” as were women allocated to the LBC-Screening protocol with LBC results of cancer, ACIS, HSIL, ASC-H, AGC, or LBC results of LSIL or ASC-US with positive HPV test results.
Differences in the distribution of age, county of residence, attendance by type of reminder, and screening outcome between women randomized to the HPV-Screening and LBC-Screening protocols were calculated with χ2 tests.
Attendance within 1 year of the first and second reminder letter was estimated as the proportion of women who attended a screening examination within 1 year of issuing of the letter among those to whom a first and second reminder letter was mailed. For 2015, attendance was calculated separately for women allocated to the HPV-Screening protocol and the LBC-Screening protocol. Screening attendance in the counties included in the pilot programme was compared with attendance in the rest of Norway by combining attendance in both investigated protocols in the pilot counties for 2015. Screening attendance in the three pilot counties was also compared with that in the rest of Norway in 2014, that is, before the pilot programme began, to understand historical attendance.
We compared the overall diagnostic yields of referrals to colposcopy for those allocated to the HPV-Screening and LBC-Screening protocols. The analyses included all randomized women, regardless of adherence to screening protocols (i.e., whether their baseline screening results warranted referral to colposcopy) to comply with the intention-to-screen principle (27). The number, percentage, risk ratios (RR), and PPV with 95% confidence intervals (CI; Wald intervals) of referral to colposcopy, and histology outcomes for women randomized to the HPV-Screening and LBC-Screening protocols, overall and stratified by age group, were calculated. For RR calculations, women allocated to the LBC-Screening protocol were used as the reference and those allocated to the HPV-Screening protocol as the exposed group.
Finally, we estimated diagnostic yields among referrals to colposcopy after baseline screening and among colposcopy exams after increased surveillance separately for women allocated to the HPV-Screening and LBC-Screening protocols. To compare the performance of referral to colposcopy after baseline and after increased surveillance, the RR for different outcomes was calculated using referral to colposcopy after baseline as the reference and referral to colposcopy after increased surveillance as the exposed group. All analyses were performed in STATA version 16 (StataCorp).
Ethical approval
Women randomized to the HPV-Screening protocol preserved their right to change to the LBC-Screening protocol. Specific information supporting personal choice at the baseline was provided through a personal letter issued by the NCCSP or/and by smear takers who were responsible for informing women that their sample would be sent for primary HPV testing. To increase public awareness, information about the implementation of the HPV-Screening protocol was also communicated through local newspapers. This randomized implementation of HPV-Screening protocol was approved by the National Council for Quality and Priority in Health Services in the fall of 2013 (13/200) and by the privacy Ombudsman at the Oslo University Hospital (2013/7918). Health care is delivered in accordance with ethical guidelines based on societal values and current legislation of the respective health regions in Norway. The early results were communicated to the stakeholders and based on this pilot study, Norwegian cervical cancer screening program recommended in 2017 a nationwide gradual implementation of primary HPV screening.
Data availability statement
The dataset presented in this article is not readily available based on the principles and conditions on the protection of natural persons with regard to the processing of their personal dataset out in the Regulation (EU) 2016/679 (General Data Protection Regulation). The legal basis for processing the data is Articles 6 (1) (e) and 9 (2) (j) of the General Data Protection Regulation with supplementary provisions in Act of April 14, 2000 no. 31 relating to the processing of personal data (Personal Data Act). The purpose of the processing is according to Paragraph 6 of Act of June 20, 2014 No. 43 on Personal Health Data Filing Systems and the Processing of Personal Health Data (Personal Health Data Filing System Act), that is, quality improvement, planning, management in the health and care administration and the health and care service. Project data used in the analyses will be available on request, given that the recipient has necessary approval and legal basis in Articles 6 and 9 of the General Data Protection Regulation and applicable national law. Requests to access the datasets should be directed to the Cancer Registry of Norway, email: Datautlevering@kreftregisteret.no.
Results
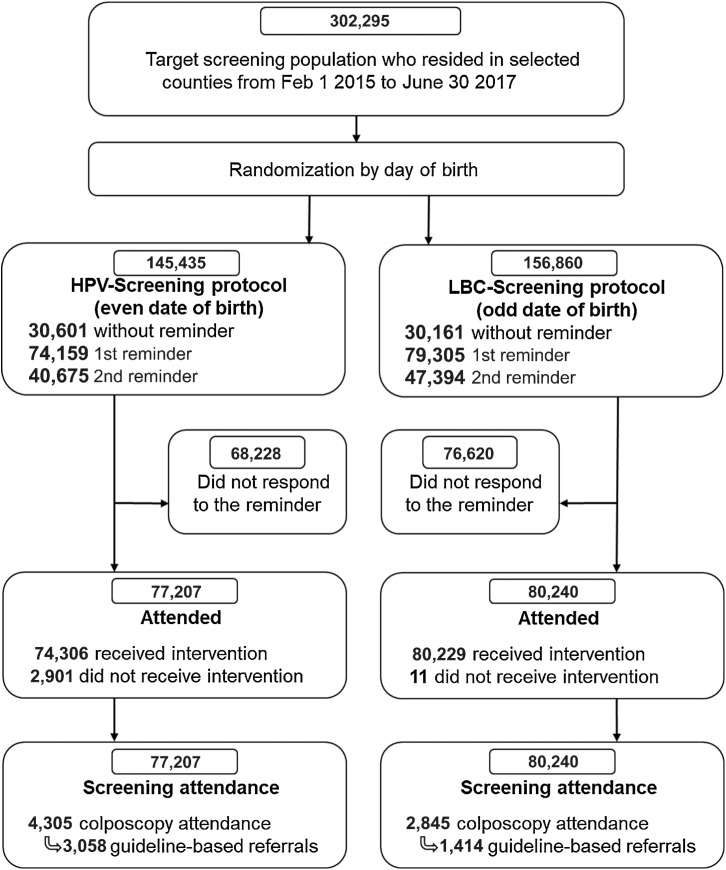
In total, there were 302,295 women ages 34 to 69 years who resided in the selected counties and were scheduled to attend screening in the NCCSP during the enrolment period. Of these, 60,762 (20.1%) attended screening without a reminder. The NCCSP issued a reminder letter to the remaining 241,533, and of those, 96,685 attended (73,267 attended screening within 1 year of the first reminder and 23,418 attended within 1 year of the second reminder). Thus, of the 157,447 women who attended screening, 77,207 with an even date of birth were randomized to the HPV-Screening protocol and 80,240 with an odd date of birth were randomized to the LBC-Screening protocol (Fig. 1).
Figure 1.
CONSORT flow diagram. During the period February 1, 2015 to June 30, 2017, the NCCSP identified approximately 302,000 women ages 34 to 69 years residing in Rogaland, Hordaland, and Trøndelag counties from the National Registry (40). Examination of cervical cancer screening history for each women in the target population identified 153,464 women with a screening visit due, that is, eligible for the first reminder, while 88,069 were eligible for second reminder (these women did not have a screening examination after the first reminder was sent to them 12 months earlier). A total of 302,295 women were eligible for pseudorandomization (1:1) to the HPV-Screening and LBC-Screening protocols based on odd or even day of birth. The analyses were performed on the intention-to-screen population, including women who did not received the intended screening modality. Of the 77,207 women who had a registered screening test after randomization to the HPV-Screening protocol 2,901 (3.8%) had LBC results at baseline, while only 11 women (0.1%) randomized to the LBC-Screening protocol received HPV testing at baseline.
Women allocated to the HPV-Screening protocol had a median and mean age of 49 and 49.8 years, respectively, while corresponding ages in the LBC-Screening protocol were 49 and 49.9 years. Considering screening results at baseline, younger women randomized to the HPV-Screening and LBC-Screening protocols were more likely to have positive HPV test results and abnormal LBC results, respectively, than older women (Ptrend < 0.001 for both; Supplementary Fig. S2). Screening outcomes among women allocated to the HPV-Screening protocol were: 475 (0.6%) inadequate, 71,948 (93.2%) screen-negative, and 4,784 (6.2%) screen-positive (Table 1). Among screen-positives, 1,968 (2.5% of women allocated to the HPV-Screening protocol and 41.1% of all screen-positives) were referred to colposcopy and 2,816 (3.6% of women allocated to the HPV-Screening protocol and 58.9% of all screen-positives) to increased surveillance. Screening outcomes among women allocated to the LBC-Screening protocol were: 4,502 (5.6%) inadequate and 73,904 (92.1%) screen-negatives (Table 1), including 71,397 (89.0%) women with LBC results of NILM and 2,507 (3.1%) women with a negative HPV test after LBC results of ASC-US or LSIL at baseline. In total, 2,917 (3.6%) women allocated to the LBC-Screening protocol had LBC results of ASC-US and 459 (0.6%) had LSIL at baseline. The 1,834 (2.3%) screen-positives from the LBC-Screening protocol comprised 965 (1.2%) women with high-grade cytologic abnormalities, 68 women with ASC-US or LSIL with inadequate or missing HPV test results, and 801 (1.0%) women with a positive HPV test result, of whom 510 (0.6%) had ASC-US and 291 (0.4%) had LSIL at baseline. Women allocated to the HPV-Screening protocol were more likely to be screen-positive and less likely to have an inadequate screening outcome than women randomized to the LBC-Screening protocol (P < 0.001 for both).
Table 1.
Distribution of age, county of residence, attendance by type of reminder, screening outcome, management of screen-positives, and outcomes after increased surveillance among women randomized to the HPV-Screening and LBC-Screening protocols in Norway.
| HPV-Screening | LBC-Screening | ||
|---|---|---|---|
| N = 77,207 | N = 80,240 | ||
| N (%) | N (%) | P | |
| Age group (years) | |||
| 34–39 | 14,847 (19.2) | 15,123 (18.8) | 0.23 |
| 40–44 | 12,361 (16.0) | 12,804 (16.0) | |
| 45–49 | 12,565 (16.3) | 13,033 (16.2) | |
| 50–54 | 11,133 (14.4) | 11,737 (14.6) | |
| 55–59 | 10,029 (13.0) | 10,341 (12.9) | |
| 60–64 | 8,775 (11.4) | 9,375 (11.7) | |
| 65–69 | 7,497 (9.7) | 7,827 (9.8) | |
| County of residence | |||
| Rogaland | 25,063 (32.5) | 26,056 (32.5) | 0.99 |
| Hordaland | 27,405 (35.5) | 28,497 (35.5) | |
| Trøndelag | 24,739 (32.0) | 25,687 (32.0) | |
| Attendance by type of reminder | |||
| None | 30,601 (39.6) | 30,161 (37.6) | <0.001 |
| 1st | 35,604 (46.1) | 37,663 (46.9) | |
| 2nd | 11,002 (14.3) | 12,416 (15.5) | |
| Screening outcome after increased surveillance | |||
| Inadequate | 475 (0.6) | 4,502 (5.6) | <0.001 |
| Screen-negative | 71,948 (93.2) | 73,904 (92.1) | |
| Screen-positive | 4,784 (6.2) | 1,834 (2.3) | <0.001 |
| Management of screen-positives | |||
| Colposcopy after baseline screeninga | 1,968 (2.5) | 965 (1.2) | |
| Increased surveillanceb | 2,816 (3.6) | 869 (1.1) | |
| Increased surveillance outcome | |||
| Surveillance positivec | 1,451 (1.9) | 563 (0.7) | |
| No follow-up | 371 (0.5) | 78 (0.01) | |
| Surveillance negative | 994 (1.3) | 228 (0.3) | |
Abbreviations: HPV, human papillomavirus; LBC, liquid-based cytology.
aClinical management guidelines recommended colposcopy for HPV-positive women who had a cytology diagnoses of atypical squamous cells of undetermined significance or worse at baseline in the HPV-Screening protocol; for the LBC-Screening protocol, colposcopy was recommended for women with cytology diagnoses of cancer, adenocarcinoma in situ, high-grade squamous intraepithelial lesion, atypical glandular cells, and atypically squamous cells cannot rule out high-grade squamous intraepithelial lesion.
bIncreased surveillance include HPV-positive women with LBC results of negative for intraepithelial lesions or malignancy in the HPV-Screening protocol; women with atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion at baseline and a positive or inadequate HPV exam in the LBC-Screening protocol.
cClinical management guidelines recommended colposcopy after increased surveillance for women with a positive HPV test at 12 months in the HPV-Screening protocol and for women with a positive HPV test and/or LSIL or worse cytology in 6 to 12 months in the LBC-Screening protocol.
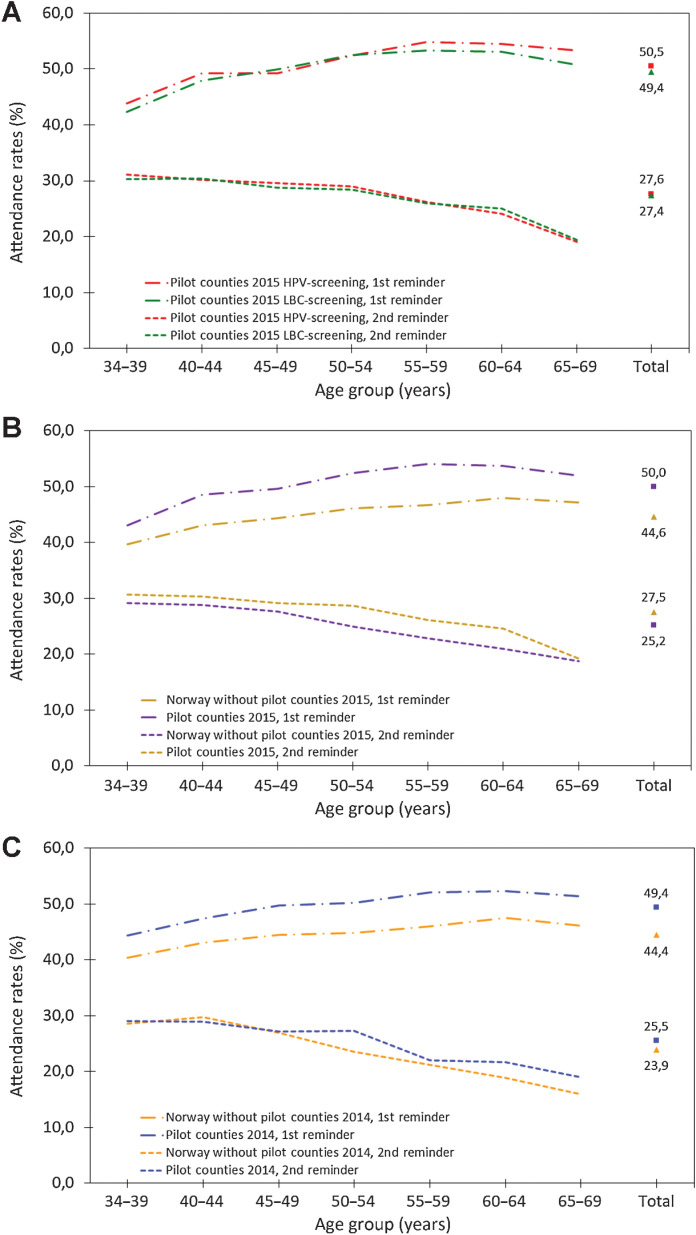
Although slightly more women from the HPV-Screening protocol attended screening after the first reminder than those from the LBC-Screening protocol, the absolute difference was marginal (50.5% vs. 49.4%; Fig. 2A). Overall attendance among those who were sent the second reminder was lower than that after the first reminder, but it did not differ by screening protocol (27.6% for the HPV-Screening protocol vs. 27.4% for the LBC-Screening protocol). Attendance by age was comparable for both screening protocols, with older women more likely to attend than younger women after the first reminder, and the converse being true for the second reminder. Regardless of screening protocol, women living in the counties selected for pilot programme were more likely to attend cervical screening after receiving a reminder letter than those living in other counties in Norway, both in the first year of enrolment in the pilot programme (2015; Fig. 2B) and the preceding year (2014; Fig. 2C).
Figure 2.
Screening attendance after first and second reminders by age. A, Screening attendance after reminder in the HPV-Screening protocol (red lines) and the LBC-Screening protocol (green lines) in the pilot counties in 2015. B, Overall attendance after reminder in the pilot counties (yellow lines) and in the remaining counties in Norway (purple lines) in 2015. C, Overall attendance after reminder in the pilot counties (blue lines) and the remaining counties in Norway (orange line) in 2014.
Within the first 4 months of the pilot programme, we observed a lower percentage of NILM (20.8%) and a higher percentage of ASC-US (47.6%) among screen-positive women randomized to the HPV-Screening protocol in one of participating labs (lab 2), while other labs reported corresponding values of 60.3% and 14.5%, and 61.7% and 18.8% (Supplementary Fig. S3; Supplementary Table S1). This observed alarming diagnostic shift was communicated immediately to lab 2 for rapid review of the cytologic diagnostic criteria. Over the subsequent 4-month period, lab 2 reported 25.0% of ASC-US and 52.0% of NILM in screen-positive women allocated to the HPV-Screening protocol, and a further reduction to 22.6% of ASC-US for the last 9 months of the enrolment period. The proportion of ASC-US in this subgroup of participants in the other participating labs was reduced below 13% for the last 9 months of the enrolment period.
In follow-up of the entire cohort (for screening outcomes inadequate, screen-positive, and screen-negative combined), 60% more colposcopies, 40% more normal biopsies, and 2.7 times more CIN1 were observed in women randomized to the HPV-Screening protocol when compared with the LBC-Screening protocol (Table 2). Among women with inadequate screening outcomes and screen-negatives in the HPV-Screening protocol and LBC-Screening protocol, eight cancers and seven cancers, respectively, were diagnosed that were presumably symptomatic. In total, 60% more CIN2+ (RR = 1.6, 95% CI = 1.5–1.7), 40% more CIN3+ (RR = 1.4, 95% CI = 1.3–1.6), and 40% more cervical cancer cases (risk ratio 1.4, 95% CI = 1.0–2.1) were detected with the HPV-Screening protocol compared with the LBC-Screening protocol (Table 3). Most CIN2+ were detected among screen-positives: 94.6% and 88.9% in the HPV-Screening protocol and LBC-Screening protocol, respectively. Notably, more CIN3/ACIS were diagnosed among screen-negatives in the LBC-Screening protocol than in the HPV-Screening protocol (43 vs. 14 cases, respectively, P < 0.001; Table 2). The HPV-Screening protocol was superior to the LBC-Screening protocol in detecting CIN2+ at all ages, and the difference became more evident with advancing age, with a RR = 2.3 (95% CI = 1.3–4.1) for CIN2+ in women ages 65–69 years compared 1.5 (95% CI = 1.3–1.7) in women ages 34–39 years. The prevalence of CIN3+ among women allocated to the HPV-Screening protocol declined with age and leveled off beyond age 59 years, with a CIN3+ prevalence of 2.7% and 0.4% of CIN3+ in women ages 34–39 and 64–69 years, respectively. Among those randomized to the LBC-Screening protocol, the prevalence of CIN3+ was 1.9% in women ages 34–39 years, and 0.2% in those ages 64–69 years. The PPV for CIN3+ was comparable for the two screening protocols and was highest in the youngest women, but it declined 3- to 4-fold with increasing age (Table 3).
Table 2.
Comparison of number of women, number of colposcopies, and histology diagnoses during 18-month follow-up period for women randomized to the HPV-Screening protocol (HPV) and the LBC-Screening protocol (LBC).
| Inadequate | Screen-negative | Screen-positive | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HPV | LBC | HPV | LBC | HPV | LBCa | HPV | LBC | ||
| Outcome | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | RR (95% CI) |
| Number of women | 475 (100) | 4,502 (100) | 71,948 (100) | 73,904 (100) | 4,784 (100) | 1,834 (100) | 77,207 (100) | 80,240 (100) | |
| Number of colposcopiesa | 130 (27.4) | 201 (4.5) | 1,061 (1.5) | 1,187 (1.6) | 3,114 (65.1) | 1,457 (79.4) | 4,305 (5.6) | 2,845 (3.5) | 1.6 (1.5–1.6) |
| NILM | 60 (12.6) | 157 (3.5) | 983 (1.4) | 1,059 (1.4) | 1,197 (25.0) | 490 (26.7) | 2,240 (2.9) | 1,706 (2.1) | 1.4 (1.3–1.5) |
| CIN1 | 27 (5.7) | 16 (0.4) | 45 (0.1) | 58 (0.1) | 719 (15.0) | 232 (12.6) | 791 (1.0) | 306 (0.4) | 2.7 (2.4–3.1) |
| CIN2 | 13 (2.7) | 6 (0.1) | 5 (0.0) | 14 (0.0) | 254 (5.3) | 91 (5.0) | 272 (0.4) | 111 (0.1) | 2.6 (2.0–3.2) |
| CIN3 | 26 (5.5) | 19 (0.4) | 14 (0.0) | 39 (0.1) | 827 (17.3) | 571 (31.1) | 867 (1.1) | 629 (0.8) | 1.4 (1.3–1.6) |
| ACIS | 1 (0.2) | 2 (0.0) | 0 (0.0) | 4 (0.0) | 57 (1.2) | 28 (1.5) | 58 (0.1) | 34 (0.0) | 1.8 (1.2–2.1) |
| Cancer | 3 (0.6) | 1 (0.0) | 5 (0.0) | 6 (0.0) | 58 (1.2) | 41 (2.2) | 66 (0.1) | 48 (0.1) | 1.4 (1.0–2.1) |
| Metastasis in cervixb | 0 (0.0) | 0 (0.0) | 9 (0.0) | 7 (0.0) | 2 (0.0) | 4 (0.2) | 11 (9.1) | 28 (0.6) | 1.0 (0.6–4.2) |
| CIN3 or ACIS | 27 (5.7) | 21 (0.5) | 14 (0.0) | 43 (0.1) | 884 (18.5) | 599 (32.7) | 925 (1.2) | 663 (0.8) | 1.5 (1.3–1.6) |
Abbreviations: ACIS, adenocarcinoma in situ; CIN, cervical intraepithelial neoplasia; CIN1, CIN grade 1; CIN2, CIN grade 2; CIN3, CIN grade 3; CI, confidence interval; HPV, human papillomavirus; LBC, liquid-based cytology; NILM, negative for intraepithelial lesions or malignancy; RR, risk ratio.
aColposcopy examination was always supplemented by tissue sampling which provided a histology diagnose.
bHistologically confirmed metastasis, not HPV-related malignancy.
Table 3.
Comparison of the number of colposcopy referrals and the number, percentage, and positive predictive values of CIN2+, CIN3+, and cancer, overall and by age, during the 18-month follow-up period, for all women randomized to the HPV-Screening protocol and the LBC-Screening protocol.
| Age groups (years) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 34–39 | 40–44 | 45–49 | 50–54 | 55–59 | 60–64 | 65–69 | Total | |
| Number of women | ||||||||
| HPV-Screening | 14,847 | 12,361 | 12,565 | 11,133 | 10,029 | 8,775 | 7,497 | 77,207 |
| LBC-Screening | 15,123 | 12,804 | 13,033 | 11,737 | 10,341 | 9,375 | 7,827 | 80,240 |
| CIN2+ N (%) | ||||||||
| HPV-Screening | 492 (3.3) | 307 (2.5) | 196 (1.6) | 109 (1.0) | 71 (0.7) | 48 (0,5) | 40 (0.5) | 1,263 (1.6) |
| LBC-Screening | 332 (2.2) | 196 (1.5) | 136 (1.0) | 67 (0.6) | 45 (0.4) | 28 (0.3) | 18 (0.2) | 822 (1.0) |
| RR (95% CI) | 1.5 (1.3–1.7) | 1.6 (1.4–1.9) | 1.5 (1.2–1.9) | 1.7 (1.3–2.3) | 1.6 (1.1–2.4) | 1.8 (1.2–2.9) | 2.3 (1.3–4.1) | 1.6 (1.5–1.7) |
| CIN3+ N (%) | ||||||||
| HPV-Screening | 397 (2.7) | 245 (2.0) | 152 (1.2) | 82 (0.7) | 52 (0.5) | 32 (0.4) | 31 (0.4) | 991 (1.3) |
| LBC-Screening | 293 (1.9) | 168 (1.3) | 120 (0.9) | 52 (0.4) | 37 (0.4) | 23 (0.2) | 18 (0.2) | 711 (0.9) |
| RR (95% CI) | 1.4 (1.2–1.6) | 1.5 (1.2–1.8) | 1.3 (1.0–1.7) | 1.7 (1.2–2.4) | 1.4 (1.0–2.2) | 1.5 (0.9–2.5) | 1.7 (1.0–3.2) | 1.4 (1.3–1.6) |
| Cancer | ||||||||
| HPV-Screening | 23 (0.2) | 13 (0.1) | 11 (0.1) | 9 (0.1) | 3 (0,0) | 2 (0.0) | 5 (0.1) | 66 (0.1) |
| LBC-Screening | 11 (0.1) | 10 (0.1) | 12 (0.1) | 7 (0.1) | 4 (0.0) | 2 (0.0) | 2 (0.0) | 48 (0.1) |
| RR (95% CI) | 2.1 (1.0–4.4) | 1.3 (0.6–3.0) | 1.0 (0.4–2.0) | 1.4 (0.5–3.4) | 0.8 (0.2–3.5) | 1.1 (0.2–7.6) | 2.6 (0.5–13.5) | 1.4 (1.0–2.1) |
| Colposcopy referrala | ||||||||
| HPV-Screening | 1,090 (7.3) | 857 (6.9) | 705 (5.6) | 531 (4.8) | 441 (4.4) | 359 (4.1) | 322 (4.3) | 4,305 (5.6) |
| LBC-Screening | 733 (4.8) | 533 (4.2) | 450 (3.5) | 356 (3.0) | 324 (3.1) | 263 (2.8) | 186 (2.4) | 2,845 (3.5) |
| RR (95% CI) | 1.5 (1.4–1.5) | 1.7 (1.5–1.9) | 1.6 (1.4–1.8) | 1.6 (1.4–1.8) | 1.4 (1.2–1.6) | 1.5 (1.2–1.7) | 1.8 (1.5–2.1) | 1.6 (1.5–1.6) |
| PPV CIN2+ % (95% CI) | ||||||||
| HPV-Screening | 45 (42–48) | 36 (33–39) | 28 (25–31) | 21 (17–24) | 16 (13–20) | 13 (10–17) | 12 (9–16) | 29 (28–31) |
| LBC-Screening | 45 (42–49) | 37 (33–41) | 30 (26–35) | 19 (15–23) | 14 (10–18) | 11 (7–14) | 10 (5–14) | 29 (27–31) |
| RR (95% CI) | 1.0 (0.9–1.1) | 1.0 (0.8–1.1) | 0.9 (0.8–1.1) | 1.1 (0.8–1.4) | 1.2 (0.8–1.6) | 1.3 (0.8–1.9) | 1.3 (0.8–2.2) | 1.0 (0.9–1.1) |
| PPV CIN3+ (95% CI) | ||||||||
| HPV-Screening | 36 (34–39) | 29 (26–32) | 22 (19–25) | 15 (12–19) | 12 (9–15) | 9 (6–12) | 10 (6–13) | 23 (21–24) |
| LBC-Screening | 40 (36–44) | 32 (28–36) | 27 (23–31) | 15 (11–18) | 11 (8–15) | 9 (5–12) | 10 (5–14) | 25 (23–27) |
| RR (95%CI)) | 0.9 (0.8–1.0) | 0.9 (0.8–1.1) | 0.8 (0.7–1.0) | 1.1 (0.8–1.5) | 1.0 (0.7–1.5) | 1.0 (0.6–1.7) | 1.0 (0.6–1.7) | 0.9 (0.8–1.0) |
Abbreviations: ACIS, adenocarcinoma in situ; CIN, cervical intraepithelial neoplasia; CIN1, CIN grade 1; CIN2, CIN grade 2; CIN3, CIN grade 3; CI, confidence interval; HPV, human papillomavirus; LBC, liquid-based cytology; PPV, positive predictive value; RR, risk ratio.
aColposcopy examination was always supplemented by tissue sampling which provided a histology diagnosis.
Altogether, 7,150 (4.5%) women underwent colposcopy. However, based on the HPV-Screening and LBC-Screening protocols, 2678 (37% of all colposcopies) of these women should not have been referred to colposcopy. Only 71.0%, (3,058 of 4,305) and 49.7% (1,414 of 2,845) of women allocated to the HPV-Screening and LBC-Screening protocols, respectively, were referred to colposcopy in accordance with the corresponding protocols (i.e., guideline-based referrals). Therefore, we restricted the analysis of colposcopy performance to guideline-based colposcopy exams (Table 4). A total of 50% fewer normal biopsies (RR = 0.5, 95% CI = 0.5–0.6), and 2.7 times more CIN3+ (RR = 2.7, 95% CI = 2.3–3.1) were diagnosed among guideline-based colposcopy exams after baseline, compared with colposcopy exams performed after increased surveillance in HPV-Screening. Furthermore, we observed a disproportional reduction in the PPV of CIN3+ by age among women with colposcopy at baseline and after increased surveillance. For the surveillance algorithm in HPV-Screening, a PPV for CIN 3+ gradually decreased from 24% in 34 to 39 years to 6% in 64 to 69 years. In comparison, a decline from 50% to 32% in respective age groups was documented in baseline screening algorithm (Supplementary Table S2). For the LBC-Screening protocol, we observed 60% less CIN1 (RR = 0.4, 95% CI = 0.3–0.5) and 60% more CIN3+ (RR = 1.6, 95% CI = 1.4–1.8) among guideline-based colposcopy exams after baseline compared with colposcopy exams performed after increased surveillance. Similarly to HPV-Screening, colposcopy after baseline was more effective to detect CIN2+ and CIN3+ than after increased surveillance in the LBC-Screening protocol, but the difference was less evident than that in the HPV-Screening protocol (Table 4).
Table 4.
Comparison of number of colposcopies and histology diagnoses among guideline-based colposcopy referrals after baseline screening and after increased surveillance in women randomized to the HPV-Screening protocol and the LBC-Screening protocol.
| Colposcopy referrals in HPV-Screening | Colposcopy referrals in LBC-Screening | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | After baseline screening | After increased surveillance | Total | After baseline screening | After increased surveillance | |||||||||||
| N | PPV (%) | N | PPV (%) | N | PPV (%) | RR | CI | N | PPV (%) | N | PPV (%) | N | PPV (%) | RR | CI | |
| Number of colposcopies | 3,058 | 1,853 | 1,205 | 1,414 | 923 | 491 | ||||||||||
| NILM | 1,162 | 38.0 | 514 | 27.7 | 648 | 53.8 | 0.5 | 0.5–0.6 | 477 | 33.7 | 320 | 34.7 | 157 | 32.0 | 1.1 | 0.9–1.3 |
| CIN1 | 708 | 23.2 | 412 | 22.2 | 296 | 24.6 | 0.9 | 0.8–1.0 | 222 | 15.7 | 98 | 10.6 | 124 | 25.3 | 0.4 | 0.3–0.5 |
| CIN2 | 250 | 8.2 | 172 | 9.3 | 78 | 6.5 | 1.4 | 1.1–1.9 | 89 | 6.3 | 36 | 3.9 | 53 | 10.8 | 0.4 | 0.2–0.5 |
| CIN3 | 822 | 26.9 | 657 | 35.5 | 165 | 13.7 | 2.6 | 2.2–3.0 | 554 | 39.2 | 404 | 43.8 | 150 | 30.5 | 1.4 | 1.2–1.7 |
| ACIS | 57 | 1.9 | 41 | 2.2 | 16 | 1.3 | 1.7 | 0.9–3.0 | 28 | 2.0 | 24 | 2.6 | 4 | 0.8 | 3.2 | 1.1–9.1 |
| Cancer | 57 | 1.9 | 55 | 3.0 | 2 | 0.2 | 17.9 | 4.4–73.2 | 40 | 2.8 | 37 | 4.0 | 3 | 0.6 | 6.5 | 2.0–21.2 |
| Metastasis in cervixa | 2 | 0.1 | 2 | 0.1 | 0 | 0 | — | — | 4 | 0.3 | 4 | 0.4 | 0 | 0 | — | — |
| CIN2+b | 1,186 | 38.8 | 925 | 49.9 | 261 | 21.7 | 2.3 | 2.1–2.6 | 711 | 50.3 | 501 | 54.3 | 210 | 42.8 | 1.3 | 1.1–1.4 |
| CIN3+c | 936 | 30.6 | 753 | 40.6 | 183 | 15.2 | 2.7 | 2.3–3.1 | 622 | 44.0 | 465 | 50.4 | 157 | 32.0 | 1.6 | 1.4–1.8 |
Abbreviations: ACIS, adenocarcinoma in situ; CIN, cervical intraepithelial neoplasia; CIN1, CIN grade 1; CIN2, CIN grade 2; CIN3, CIN grade 3; CI, confidence interval; HPV, human papillomavirus; LBC, liquid-based cytology; PPV, positive predictive value; RR, risk ratio.
aMetastasis detected in histology sample. Not included in CIN2+ and CIN3+.
bCIN2 or more severe diagnoses (not including metastasis in cervix).
cCIN3, ACIS, or cancer (not including metastasis in cervix).
Discussion
To our knowledge, this is the first randomized roll-out of primary HPV testing within an existing cytology-based cervical cancer screening programme, enabling a comparative effectiveness evaluation of these two routine practices. We demonstrated that the HPV-Screening protocol was more sensitive but less specific for the identification of CIN2, CIN3, and invasive cancer than the LBC-Screening protocol in women ages 34 to 69 years. This outcome could at least in part be attributed to two important aspects. First, the HPV-Screening protocol called for immediate colposcopy in HPV-positive women with abnormal LBC results (ASC-US+), whereas the LBC-Screening protocol called for increased surveillance within 6 to 12 months in women with LBC results of LSIL or ASC-US and a positive HPV test. Second, women randomized to the HPV-Screening protocol with positive HPV results and NILM cytology on triage were referred to increased surveillance, whereas women allocated to the LBC-Screening protocol who had NILM cytology were not further tested, instead they were returned to routine screening. Together, these differences in management likely contributed in a more sensitive and less specific algorithm in the HPV-Screening protocol as compared with the LBC-Screening protocol. In the future follow-up of these women, it will be important to confirm that equivalent or greater safety exists after 5 years for HPV-negative women allocated to the HPV-Screening protocol, compared with the safety after 3 years for cytology-negative women in the LBC-Screening protocol, as has been shown in several randomized controlled trials and observational studies (7, 18, 28).
The shift from the LBC-Screening to the HPV-Screening protocol in Norway did not influence screening attendance, which is in line with a recent publication from Norway that documented no increase in psychologic stress among women who received primary screening with HPV testing and strengthens our conclusion that implementation of the HPV-Screening protocol will not result in lower screening attendance (29). This is in contrast to a recent report from the Netherlands, which indicated lower attendance to primary screening with HPV testing than with LBC (30). We believe that the carefully orchestrated campaign to raise awareness regarding cervical cancer, which was launched in late 2014 and early 2015 in the pilot counties, is at least partially responsible for the overall good acceptance of the HPV-Screening protocol in Norway.
A meta-analysis of eight randomized controlled trials estimated that HPV testing was 27% more sensitive to detect CIN2+ than cytology at baseline (31). This difference was smaller than ours 60%, likely reflecting better cytology performance in randomized controlled trial settings as compared with real-world settings. Observational studies from England and Denmark have reported significant improvements of 49% and 47%, respectively, in detecting CIN2+ when comparing different geographical areas that used either HPV-based or cytology-based screening (28, 32). Our study design included the individual randomization of women to the HPV-Screening or LBC-Screening protocol and resulted in highly generalizable real-world evidence on the comparative effectiveness of protocols (22).
Admittedly, cervical cancer screening programmes inherently introduce harms related to imperfect screening tests and unnecessarily performed colposcopy procedures with normal or CIN1 histology diagnoses. In total, 5.6% of women allocated to the HPV-Screening protocol underwent colposcopy, which was 60% higher than the proportion who underwent colposcopy in the LBC-Screening protocol. With 60% more CIN2+ detected among those in the HPV-Screening protocol, the overall PPVs for CIN2+ and CIN3+ were similar which indicates a comparable balance of harms and benefits. However, when we restricted the analyses to guideline-based referrals, 2.2 times more colposcopies (3,058 vs. 1,414) and 1.7 times more CIN2+ (1,186 vs. 711) were registered in women allocated to the HPV-Screening than the LBC-Screening protocol, implying that the balance shifted toward increased harm when limiting evaluation for those adhered to the recommended management protocols. This difference was likely caused by the aggressive management of HPV-positive/NILM women after increased surveillance, which resulted in a PPV of 21.7% for CIN2+. In comparison, the PPV for CIN2+ among guideline-based referrals was 49.9% and 54.3% after baseline screening in the HPV-Screening protocol and the LBC-Screening protocol, respectively. A recent study from England demonstrated that the increase in referrals to colposcopy when using primary HPV testing is likely temporary, resulting in large part from the increased sensitivity of the HPV test to detect CIN2+ (28). This assumption can be evaluated in the current cohort in 2020 to 2022, when close to 71,000 screen-negative women from the HPV-Screening protocol will attend another screening round.
Although the performance of the two screening protocols to detect precancers and cancers was dependent on age, we demonstrated that the HPV-Screening protocol was more effective than LBC-Screening protocol. While the prevalence of high-risk HPV infection, abnormal LBC results, CIN2+, and the PPV for CIN2+ decreased with increasing age for women assigned to both protocols, the HPV-Screening protocol was superior in detecting precancerous lesions at all ages, with 2.3 times more CIN2+ detected in women ages 65 to 69 years. Yet, the absolute performance was poor, with a PPV for CIN3+ as low as 9% for women ages 60 to 64 years. Among guideline-based referrals after increased surveillance in the HPV-Screening protocol, the PPV for CIN3+ was only 6% (6/96) for women ages 65 to 69 years, again suggesting that a second positive HPV test result after 1 year might be a suboptimal threshold for referral to colposcopy. Although colposcopy and biopsy perform suboptimally in postmenopausal women because of atrophy and the migration of the transformation zone into the endocervical canal (33), it is not likely to explain the observed age gradient. Therefore, alternative biomarkers such as HPV 16 and 18 genotypes, viral load, transcriptional status, p16/Ki-67 dual staining, and host DNA methylation (34–38) should be considered to improve colposcopy referral algorithms in women 50 years for more equally balanced harm-benefit ratio in younger and older women. HPV genotyping was recently adopted in for the management of HPV-positive women in Norway and future evaluations will provide real-world evidence on age-specific performance (39). Management of women with remarkably different risk profiles, as exemplified with age in the presented dataset, has also been raised in connection with the screening of HPV-vaccinated women and requires further research.
The success of any screening programme, irrespective of the technologies used, relies on well-defined management guidelines, regular monitoring, and availability of resources. Individual randomization addressed several important quality assurance and operational aspects during the transition in Norway. First, stringent national quality control measures for cytology are necessary in the setting of HPV primary screening where information on positive HPV results can trigger diagnostic shift and unnecessary follow-up procedures. Second, the gradual implementation of the HPV-Screening protocol mitigated the overload of gynecologic and pathologic services that was expected because of an increase in colposcopies (17), as only 50% of women were allocated to the HPV-Screening protocol, and the other 50% received the LBC-Screening protocol, which has lower colposcopy referral rates. Third, the gradual implementation of the HPV-Screening protocol, as a consequence of randomization, will help level out the peak volume of screening samples in next screening round when shifting from a 3-year to a 5-year screening interval. Net benefits of randomized roll-out of changes in screening program provide a framework for safe technology transfer and will likely contribute to more speedy adoption of scientific advances.
Altogether 37% of referrals to colposcopy were not based on Norwegian management guidelines. Moreover, of the 1,702 CIN3+, 1,558 were diagnosed among women who had guideline-based colposcopy referrals, and 144 (8%) among women who did not; these women were referred to colposcopy following indications other than those stated in the protocols. We need to explore further whether these latter colposcopies were performed because of symptoms, abnormal cervical appearance during screening examination, or for any other reason. While the reasons for seemingly poor adherence to protocols may be diverse and well grounded, the effort should be made to understand why they were ignored and to what degree these protocols, or communication regarding these protocols, need to be improved.
Strengths and limitation
More than 300,000 participants were randomized individually with only 2% of women not receiving the allocated screening test (2,901 of 77,207 women in the HPV-Screening protocol and 11 of 80,240 women in the LBC-Screening protocol), which illustrate an effective randomization protocol for clinical setting. Individual randomization of the target population and an effective and accurate nationwide data capturing system resulted in data that could be verified to a level comparable with that expected in a prospective clinical trial. Women with the same combination of HPV status and ASC-US or LSIL diagnosis were managed differently in the HPV-Screening and LBC-Screening protocols. Also, clinical recommendations for increased surveillance differed in terms of time and type of follow-up test. This reflects typical, real-life challenges where new, emerging technology is followed up more carefully than existing routines. While we observed suboptimal adherence to management protocols with regard to referral to colposcopy, information on colposcopic evaluation is not systematically collected, and our assumption that colposcopic examination was always supplemented by tissue sampling might have underestimated the number of colposcopies performed. However, this most likely represents a nondifferential misclassification and would not affect the conclusion that 60% more referrals to colposcopy were performed in the HPV-Screening protocol.
Starting in 2015, HPV testing began to replace cytology as the primary method of cervical cancer screening in Norway for women ages 34 to 69 years. The HPV-Screening protocol detected more cervical precancers and cancers but had also higher rate of referral to colposcopy. HPV genotyping adopted in the management of HPV-positive women and age-specific referral algorithms may be needed to improve the overall performance of HPV-Screening program. The HPV-Screening protocol was introduced in Norway in three counties under randomized, controlled conditions. Gradual changes in established health services offer the opportunity to continuously compare the new service with the existing one. The introduction of new routines under randomized, controlled conditions can simplify and accelerate the decision-making processes.
Supplementary Material
Acknowledgments
This work was supported by the affiliating institutes (M. Nygård, B. Engesæter, J.M. Berland, M.L. Eide, O.E. Iversen, C.M. Jonassen, O.K. Vintermyr, A. Tropé) who were responsible for performing all clinical and research activities related to national cervical cancer screening program.
This work was supported by the affiliating institutes of the authors. Stavanger University Hospital funded J.M. Berland, Trondheim University Hospital funded M.L. Eide, Haukeland University Hospital funded O.E. Iversen, Center for Laboratory Medicine, Østfold Hostpital Trust funded C.M. Jonassen, the Gades Laboratory for Pathology, University of Bergen funded O.K. Vintermyr, Akershus University Hospital funded I.K. Christiansen and the Cancer Registry of Norway funded M. Nygård, B. Engesæter, and A. Tropé.
The guarantors, M. Nygård and A. Tropé accept full responsibility for the conduct of the study, declaring that the honest, accurate and transparent performance of the project is being reported, including all relevant aspects.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
This article is featured in Highlights of This Issue, p. 1669
Footnotes
Note: Supplementary data for this article are available at Cancer Epidemiology, Biomarkers & Prevention Online (http://cebp.aacrjournals.org/).
Authors' Disclosures
P.E. Castle reports receiving HPV tests and assays for research from Roche, Becton Dickinson, Arbor Vita Corporation, Cepheid, and Atila Biosystems at a reduced or no cost. No disclosures were reported by the other authors.
Authors' Contributions
M. Nygård: Conceptualization, resources, data curation, formal analysis, supervision, visualization, methodology, writing–original draft, project administration, writing–review and editing. B. Engesæter: Conceptualization, formal analysis, validation, visualization, methodology, writing–original draft, project administration, writing–review and editing. P.E. Castle: Conceptualization, formal analysis, supervision, validation, methodology, writing–original draft, project administration, writing–review and editing. J.M. Berland: Resources, funding acquisition, validation, project administration, writing–review and editing. M.L. Eide: Resources, funding acquisition, validation, methodology, project administration, writing–review and editing. O.E. Iversen: Resources, funding acquisition, validation, methodology, project administration, writing–review and editing. C.M. Jonassen: Resources, funding acquisition, validation, methodology, project administration, writing–review and editing. I.K. Christiansen: Resources, funding acquisition, validation, methodology, project administration, writing–review and editing. O.K. Vintermyr: Resources, funding acquisition, validation, methodology, project administration, writing–review and editing. A. Tropé: Conceptualization, resources, funding acquisition, validation, project administration, writing–review and editing.
References
- 1. Vaccarella S, Lortet-Tieulent J, Plummer M, Franceschi S, Bray F. Worldwide trends in cervical cancer incidence: impact of screening against changes in disease risk factors. Eur J Cancer 2013;49:3262–73. [DOI] [PubMed] [Google Scholar]
- 2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 3. von Karsa L, Arbyn M, De Vuyst H, Dillner J, Dillner L, Franceschi S, et al. European guidelines for quality assurance in cervical cancer screening. Summary of the supplements on HPV screening and vaccination. Papillomavirus Res 2015;1:22–31. [Google Scholar]
- 4. zur Hausen H. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J Natl Cancer Inst 2000;92:690–8. [DOI] [PubMed] [Google Scholar]
- 5. Arbyn M, Simon M, Peeters E, Xu L, Meijer C, Berkhof J, et al. 2020 list of human papillomavirus assays suitable for primary cervical cancer screening. Clin Microbiol Infect 2021;27:1083–95. [DOI] [PubMed] [Google Scholar]
- 6. Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM, et al. HPV screening for cervical cancer in rural India. N Engl J Med 2009;360:1385–94. [DOI] [PubMed] [Google Scholar]
- 7. Ronco G, Dillner J, Elfstrom KM, Tunesi S, Snijders PJ, Arbyn M, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet 2014;383:524–32. [DOI] [PubMed] [Google Scholar]
- 8. Ogilvie GS, van Niekerk D, Krajden M, Smith LW, Cook D, Gondara L, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL Randomized Clinical Trial. JAMA 2018;320:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dillner J, Rebolj M, Birembaut P, Petry KU, Szarewski A, Munk C, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008;337:a1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sherman ME, Lorincz AT, Scott DR, Wacholder S, Castle PE, Glass AG, et al. Baseline cytology, human papillomavirus testing, and risk for cervical neoplasia: a 10-year cohort analysis. J Natl Cancer Inst 2003;95:46–52. [DOI] [PubMed] [Google Scholar]
- 11. Thomsen LT, Frederiksen K, Munk C, Junge J, Iftner T, Kjaer SK. Long-term risk of cervical intraepithelial neoplasia grade 3 or worse according to high-risk human papillomavirus genotype and semi-quantitative viral load among 33,288 women with normal cervical cytology. Int J Cancer 2015;137:193–203. [DOI] [PubMed] [Google Scholar]
- 12. Engesaeter B, van Diermen Hidle B, Hansen M, Moltu P, Staby KM, Borchgrevink-Persen S, et al. Quality assurance of human papillomavirus (HPV) testing in the implementation of HPV primary screening in Norway: an inter-laboratory reproducibility study. BMC Infect Dis 2016;16:698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. von Karsa L, Arbyn M, De Vuyst H, Dillner J, Dillner L, Franceschi S, et al. European guidelines for quality assurance in cervical cancer screening. 2nd ed. Luxembourg: Office for Official Publications of the European Union; 2015. [Google Scholar]
- 14. Burger EA, Ortendahl JD, Sy S, Kristiansen IS, Kim JJ. Cost-effectiveness of cervical cancer screening with primary human papillomavirus testing in Norway. Br J Cancer 2012;106:1571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith MA, Gertig D, Hall M, Simms K, Lew JB, Malloy M, et al. Transitioning from cytology-based screening to HPV-based screening at longer intervals: implications for resource use. BMC Health Serv Res 2016;16:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leinonen MK, Hansen SA, Skare GB, Skaaret IB, Silva M, Johannesen TB, et al. Low proportion of unreported cervical treatments in the cancer registry of Norway between 1998 and 2013. Acta Oncol 2018;57:1663–70. [DOI] [PubMed] [Google Scholar]
- 17. Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol 2010;11:249–57. [DOI] [PubMed] [Google Scholar]
- 18. Rijkaart DC, Berkhof J, Rozendaal L, van Kemenade FJ, Bulkmans NW, Heideman DA, et al. Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol 2012;13:78–88. [DOI] [PubMed] [Google Scholar]
- 19. Leinonen M, Nieminen P, Kotaniemi-Talonen L, Malila N, Tarkkanen J, Laurila P, et al. Age-specific evaluation of primary human papillomavirus screening vs conventional cytology in a randomized setting. J Natl Cancer Inst 2009;101:1612–23. [DOI] [PubMed] [Google Scholar]
- 20. Kitchener HC, Gilham C, Sargent A, Bailey A, Albrow R, Roberts C, et al. A comparison of HPV DNA testing and liquid based cytology over three rounds of primary cervical screening: extended follow up in the ARTISTIC trial. Eur J Cancer 2011;47:864–71. [DOI] [PubMed] [Google Scholar]
- 21. Naucler P, Ryd W, Tornberg S, Strand A, Wadell G, Elfgren K, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 2007;357:1589–97. [DOI] [PubMed] [Google Scholar]
- 22. Hakama M, Malila N, Dillner J. Randomised health services studies. Int J Cancer 2012;131:2898–902. [DOI] [PubMed] [Google Scholar]
- 23. Finnish Cancer Registry. Randomised health services studies Registry Helsinki: Finnish Cancer Registry. Available from: https://cancerregistry.fi/research/randomised-health-services-studies/.
- 24. Solomon D, Nayar R. The Bethesda System for reporting Cervical Cytology. 2nd ed. New York, NY: Springer-Verlag New-York, Inc.; 2004. [Google Scholar]
- 25. Tavassoli F, Devilee P, editors.Pathology and genetics of tumours of the breast and female genital organs. Lyon: IARC Press; 2003. [Google Scholar]
- 26. Dørum A. Veileder gynekologisk onkologi 2015. Available from:https://www.legeforeningen.no/contentassets/9c5011955cc644a595cec42c01934c92/veileder-gynekologisk-onkologi.pdf.
- 27. Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res 2011;2:109–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rebolj M, Rimmer J, Denton K, Tidy J, Mathews C, Ellis K, et al. Primary cervical screening with high risk human papillomavirus testing: observational study. BMJ 2019;364:l240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Andreassen T, Melnic A, Figueiredo R, Moen K, Suteu O, Nicula F, et al. Attendance to cervical cancer screening among Roma and non-Roma women living in North-Western region of Romania. Int J Public Health 2018;63:609–19. [DOI] [PubMed] [Google Scholar]
- 30. Aitken CA, van Agt HME, Siebers AG, van Kemenade FJ, Niesters HGM, Melchers WJG, et al. Introduction of primary screening using high-risk HPV DNA detection in the Dutch cervical cancer screening programme: a population-based cohort study. BMC Med 2019;17:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arbyn M, Ronco G, Anttila A, Meijer CJ, Poljak M, Ogilvie G, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine 2012;30:F88–99. [DOI] [PubMed] [Google Scholar]
- 32. Thomsen LT, Kjaer SK, Munk C, Frederiksen K, Ornskov D, Waldstrom M. Clinical performance of human papillomavirus (HPV) testing versus cytology for cervical cancer screening: results of a large danish implementation study. Clin Epidemiol 2020;12:203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ren H, Jia M, Zhao S, Li H, Fan S. Factors correlated with the accuracy of colposcopy-directed biopsy: a systematic review and meta-analysis. J Invest Surg 2022;35:284–92. [DOI] [PubMed] [Google Scholar]
- 34. Molina MA, Carosi Diatricch L, Castany Quintana M, Melchers WJ, Andralojc KM. Cervical cancer risk profiling: molecular biomarkers predicting the outcome of hrHPV infection. Expert Rev Mol Diagn 2020;20:1099–120. [DOI] [PubMed] [Google Scholar]
- 35. Schmitz M, Eichelkraut K, Schmidt D, Zeiser I, Hilal Z, Tettenborn Z, et al. Performance of a DNA methylation marker panel using liquid-based cervical scrapes to detect cervical cancer and its precancerous stages. BMC Cancer 2018;18:1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lorincz AT, Brentnall AR, Scibior-Bentkowska D, Reuter C, Banwait R, Cadman L, et al. Validation of a DNA methylation HPV triage classifier in a screening sample. Int J Cancer 2016;138:2745–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Verhoef VM, Bosgraaf RP, van Kemenade FJ, Rozendaal L, Heideman DA, Hesselink AT, et al. Triage by methylation-marker testing versus cytology in women who test HPV-positive on self-collected cervicovaginal specimens (PROHTECT-3): a randomised controlled non-inferiority trial. Lancet Oncol 2014;15:315–22. [DOI] [PubMed] [Google Scholar]
- 38. Gustinucci D, Giorgi Rossi P, Cesarini E, Broccolini M, Bulletti S, Carlani A, et al. Use of cytology, E6/E7 mRNA, and p16INK4a-Ki-67 to define the management of human papillomavirus (HPV)-positive women in cervical cancer screening. Am J Clin Pathol 2016;145:35–45. [DOI] [PubMed] [Google Scholar]
- 39. Hashim D, Engesaeter B, Baadstrand Skare G, Castle PE, Bjorge T, Trope A, et al. Real-world data on cervical cancer risk stratification by cytology and HPV genotype to inform the management of HPV-positive women in routine cervical screening. Br J Cancer 2020;122:1715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. The Norwegian Tax Administration. National Registry 2019. The registry forms the basis for the tax register, the electoral register and population statistics. Available from:https://www.skatteetaten.no/en/person/national-registry/om/this-is-the-national-registry/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset presented in this article is not readily available based on the principles and conditions on the protection of natural persons with regard to the processing of their personal dataset out in the Regulation (EU) 2016/679 (General Data Protection Regulation). The legal basis for processing the data is Articles 6 (1) (e) and 9 (2) (j) of the General Data Protection Regulation with supplementary provisions in Act of April 14, 2000 no. 31 relating to the processing of personal data (Personal Data Act). The purpose of the processing is according to Paragraph 6 of Act of June 20, 2014 No. 43 on Personal Health Data Filing Systems and the Processing of Personal Health Data (Personal Health Data Filing System Act), that is, quality improvement, planning, management in the health and care administration and the health and care service. Project data used in the analyses will be available on request, given that the recipient has necessary approval and legal basis in Articles 6 and 9 of the General Data Protection Regulation and applicable national law. Requests to access the datasets should be directed to the Cancer Registry of Norway, email: Datautlevering@kreftregisteret.no.