Abstract
Human monkeypox (MPX) is a rare zoonotic infection characterized by smallpox-like signs and symptoms. It is caused by monkeypox virus (MPXV), a double stranded DNA virus belonging to the genus Orthopoxvirus. MPX was first identified in 1970 and mostly prevailed in the rural rainforests of Central and West Africa in the past. Outside Africa, MPX was reported in the United Kingdom, the USA, Israel, and Singapore. In 2022, the resurgence of MPX in Europe and elsewhere posed a potential threat to humans. MPXV was transmitted by the animals-human or human-human pathway, and the symptoms of MPXV infection are similar to that of smallpox, but in a milder form and with lower mortality (1%–10%). Although the smallpox vaccination has been shown to provide 85% protection against MPXV infection, and two anti-smallpox virus drugs have been approved to treat MPXV, there are still no specific vaccines and drugs against MPXV infection. Therefore it is urgent to take active measures including the adoption of novel anti-MPXV strategies to control the spread of MPXV and prevent MPX epidemic. In this review, we summarize the biological features, epidemiology, pathogenicity, laboratory diagnosis, and prevention and treatment strategies on MPXV. This review provides the basic knowledge for prevention and control of future outbreaks of this emerging infection.
Keywords: Monkeypox virus (MPXV), Orthopoxvirus, Smallpox vaccine, Antiviral drugs
Highlights
-
•
The resurgence of monkeypox in Europe and elsewhere posed a potential threat to humans.
-
•
There is still no specific vaccine or drug against MPXV infection.
-
•
It is urgent to take active measures control the spread of MPXV and prevent MPX epidemic.
1. Introduction
Monkeypox (MPX) is a rare zoonotic infectious disease caused by the monkeypox virus (MPXV). The earlier outbreaks happened mostly in Central and West Africa, commonly known as “monkey smallpox”. Its symptoms are similar to that of smallpox, but the disease is milder, mainly manifested as high fever, headache, lymphadenopathy, and systemic blisters and pustules, with a case fatality rate of about 1%–10% (Doshi et al., 2019; Ogoina et al., 2019). Recently, MPX has occurred in Europe and North America. Since MPX cases were first reported in Europe in early May 2022, more than 400 confirmed or suspected cases have emerged in at least 20 non-African nations (Kozlov, 2022b). The UK's first case of MPV in 2022 had traveled to Nigeria before its diagnosis, but many of the new confirmed cases had no history of travel to Nigeria or Africa, suggesting that MPXV had begun community transmission (Mahase, 2022). The continuous emergence of the MPX epidemic has attracted widespread attention around the world and has been suspected to be a potential threat to wider populations. Although smallpox vaccine has been reported to provide 85% protection against MPXV (Fine et al., 1988), the smallpox virus vaccination has been discontinued since 1980 (Jezek et al., 1987), when the WHO announced the eradication of smallpox virus. And there is a lack of specific drugs and vaccines to MPXV. Therefore, to curb the spread of MPX epidemics, it is necessary to have an in-depth understanding of the biological characteristics and pathogenicity of MPXV. Here, we reviewed the current research progress of MPXV and provided clues for prevention and control of MPX outbreak.
2. Biological features of MPXV
MPXV belongs to the genus Orthopoxvirus, family Poxviridae. The virus particles are oval or brick-shaped and approximately 200 × 250 nm in size (Cho and Wenner, 1973). Poxvirus produces two infectious viral particles during replication: intracellular mature virus (MV) and extracellular enveloped virus (EV). The outer layer of MV has a lipoprotein envelope that encloses the viral core and lateral body containing some proteins. MV is released upon cell lysis and it is relatively stable in the external environment. It is mainly used for transmission between animals. EV is released by exocytosis and formed by a lipid membrane wrapped around MVs. It is derived from the transport Golgi apparatus or endosomes (Pickup, 2015). The MPXV genome is a linear, double-stranded DNA, approximately 197 kb (Kugelman et al., 2014), with inverted terminal repeats (ITRs) at its ends, and more than 190 ORFs were encoded by some MPXV strains (e.g. Congo_2003_358). The non-conserved genes of the virus are generally located in ITRs at both ends, which are poxvirus species and host-specific. They are mostly associated with the immune escape of the poxvirus, such as inhibiting apoptosis, interfering with antigen presentation and recognition, and overcoming interferon (IFN) influence and disturbing with other signal paths (Esposito and Knight, 1985), etc. Like all orthopoxviruses, genes encoding viral replication enzymes and structural proteins are relatively conservative, mostly located in the central region of the genome. They encode all the proteins needed for viral DNA replication, transcription, assembly, and release (Kugelman et al., 2014).
MPXV completes its replication process in the cytoplasm. The invasion of host cells by poxvirus is mainly completed by three steps: adsorption, membrane fusion and core invasion. Specific cell receptors for poxvirus have not been identified, but for vaccinia virus (VACV), which is most in likely MPXV, four viral proteins (D8, A27, A26 and H3) were found to mediate MV adsorption on the cell surface. D8 binds to chondroitin (Matho et al., 2018), A27 and H3 bind to heparan (Singh et al., 2016), and A26 binds to laminin (Chiu et al., 2007). Knockout of genes encoding A27 and H3 significantly reduces the infectivity of VACV. MPXV E8, A29, A28 and H3 proteins are the VACV D8, A27, A26 and H3 orthologs respectively, which share the same functions as those in VACV (Hughes et al., 2014). For VACV, membrane fusion and core invasion of MV are mediated by 11 viral proteins, including A16, A21, A28, F9, G3, G9, H2, J5, L1, L5, and O3, which together form an entry fusion complex (EFC) mediating the invasion of MV and EV after viral adsorption (Schin et al., 2021; Senkevich et al., 2005). Except for the O3 protein, the remaining 10 proteins are necessary for poxvirus replication, but the deletion of the gene encoding O3 protein can also seriously affect VACV replication. EFC is known to have three subcomponents, A28-H2, A16-G9, and G3-L5. A16-G9 sub-complex interacts with the viral A56-K2 complex to inhibit cell fusion under repeated infections and neutral pH conditions, and can also interact with A26 proteins on the surface of MV to prevent fusion under neutral pH conditions after adsorption (Chang et al., 2012). L1 and A28 are VACV envelope proteins which are essential for cellular entry (Foo et al., 2009). A28 binds to the H2 protein and its immunogenicity is enhanced (Kaever et al., 2014). After the membrane of MV is fused with cytomembrane or the membrane of endosome, the viral core enters the cytoplasm and is de-hulled under the action of several viral proteins, such as A16L, A21L, A28L, F9L, G3L, G9R, H2R, J5L, and L5R, initiating the process of viral biosynthesis (Brown et al., 2006; Senkevich et al., 2005). Due to the high homology between the genome of MPXV and VACV, they may share the same features in virus entry-fusion step (Senkevich et al., 2005).
Poxviruses encode host range factor (Hrf) that regulates certain cell-specific antiviral responses to ensure the virus can replicate in some cells. The gene encoding Hrf is generally located in a reverse repeat sequence at both ends of the poxvirus genome, called host range gene (Hrg). MPXV encodes a variety of host range proteins, such as the virulence protein BR-203, which exerts antiviral-infected apoptosis effects, and BR-209 protein is an IL-1β-binding protein that inhibits IL-1β and IL-1 receptor binding. These two proteins are not present in smallpox virus (Weaver and Isaacs, 2008). In addition, MPXV F3 protein encoded by host genes F3L, a homologue of the VACV E3 protein, was shown to inhibit the cellular antiviral immune response (Arndt et al., 2015). MPXV A29L, M1R and B6R, the orthologous to A27L, L1R and B5R VACV antigens respectively, were selected as MPXV vaccine candidates and were shown to be able to elicit a protective immune response against lethal MPXV challenge (Franceschi et al., 2015). MPXV has a wide range of tissue tropism. Osorio et al. found that in severe combined immunodeficiency (SCID) mice infected with MPXV, MPXV antigens were detected in multiple tissues such as ovarian, brain, heart, kidney, liver, pancreas, and lung; and the viral titer in ovarian tissue was higher than other tissues, suggesting that ovarian was highly sensitive to MPXV (Osorio et al., 2009). Histopathological studies by Zaucha et al. on Macca fasillaris have shown that lymphoid tissue is the principal target for MPXV, and viral antigens have been detected in salivary epithelium, follicles, lip sebaceous tissue, and many other tissues (Zaucha et al., 2001).
MPXV can be cultured in a variety of cells such as Vero (Realegeno et al., 2017), A549 (Realegeno et al., 2017), HAP1 cells (Lopera et al., 2015), HeLa (Arndt et al., 2016), RK-13 (Arndt et al., 2015), and typical inclusion bodies are visible in the cytoplasm of infected cells. Several small animals such as rabbits, rats, squirrels, prairie dogs (Hutson and Damon, 2010), mice (Earl et al., 2015; Sergeev et al., 2016) and non-human primates (NHPs) are all susceptible to MPXV. Rabbits challenged with MPXV by intravenous route will develop acute illness and generalized rash (Hutson and Damon, 2010). The prairie dog model is remarkably similar to human MPXV incubation/presentation, so it is widely utilized in the characterization of MPX disease and evaluation of medical countermeasures (MCMs) against MPXV (Hutson et al., 2011, 2015, 2021). NHPs aerosol challenged with MPXV can produce expected disease progression and 67%–100% lethality (Nalca et al., 2010; Zaucha et al., 2001). Infected-NHPs such as rhesus and cynomolgus macaques with MPXV can serve as models to study MPXV pathogenesis and to test vaccines and antiviral drug candidates (Barnewall et al., 2012; Hatch et al., 2013; Russo et al., 2020).
MPXV is not heat-resistant, and can be inactivated after 30 min of treatment at 56 °C. The virus is easily inactivated by organic solvents such as formaldehyde, methanol, sodium dodecyl sulfonate (SDS), phenol, and chloroform. MPXV is resistant to drying and low temperature, and it can maintain vitality for a long time at 4 °C (Cho and Wenner, 1973).
3. Epidemiologic characteristics of MPXV
MPXV currently has evolved into two distinct clades: West African and Congo Basin (Likos et al., 2005). The epidemiological and clinical features of the disease caused by the two MPXV clades are different. Congo Basin has a case fatality rate of up to 10% (Doshi et al., 2019), while West African has a case fatality rate of about only 1%, with a higher mortality rate in patients with HIV co-infection (Ogoina et al., 2019). MPXV was first identified in 1958, and the first human MPX case was found in Democratic Republic of the Congo (DRC) in 1970 (Breman et al., 1980). Since then, MPX has become endemic to DRC and has spread to other African countries, mainly in Central and West Africa. From 1970 to 1979, there were 47 human MPX cases reported in five Central and West African countries, of which 38 cases were reported from DRC, all occurring in tropical rain forest areas and associated with animal contact (Breman et al., 1980). After the extinction of smallpox, a total of 338 cases of human MPX were found in DRC from 1981 to 1986. The mortality rate is as high as 9.8% in people who have not been vaccinated against smallpox. Seventy two percent of the cases are zoonotic transmission and most of the cases occur in children, with an average age of 4.4 years (Damon, 2011). Since the end of the WHO monitoring project in 1986, reports of persistent occurrence of MPX in human have decreased. From 1986 to 1992, only 13 cases were reported, and no cases were reported from 1993 to 1995 (Heymann et al., 1998). However, in 1996, there was a sudden increase in the number of human MPXV infected cases reported in DRC, and by 1997, a total of 88 people had been confirmed to have MPX infection (Heymann et al., 1998; Hutin et al., 2001). In 2003, MPX broke out in the United States. This is the first reported MPX outbreak outside Africa and has been related to the carriage of MPXV by marmots imported from Africa in the United States, resulting in a total of 47 people being diagnosed in five states (Reed et al., 2004; Sale et al., 2006). In 2005, an outbreak of MPX in Sudan reported a total of 10 confirmed cases and 9 suspected cases of MPXV from September to December 2005 (Formenty et al., 2010). Between 2006 and 2007, Human MPX infection was found again in DRC. MPX transmission had increased 20-fold since the 1980s, and smallpox-vaccinated people have a 21-fold lower risk of infection than unvaccinated people (Rimoin et al., 2010). Zoonotic transmission occurred in most cases. Since September 2017, MPX have broken out in Nigeria, with a total of 183 confirmed cases reported in 18 states as of November 2019. The outbreak was also the largest epidemic on record in West African (Alakunle et al., 2020; Nguyen et al., 2021; Yinka-Ogunleye et al., 2018). Subsequently, imported cases of MPX were reported in Israel (Erez et al., 2019), the United Kingdom (Vaughan et al., 2018), Singapore (Ng et al., 2019) and other countries. Up to May 2022, MPX outbreaks have occurred in several countries around the world, which has aroused the strong vigilance of scientists in many countries (Kozlov, 2022a).
4. Pathogenicity
4.1. Transmission of MPXV to humans
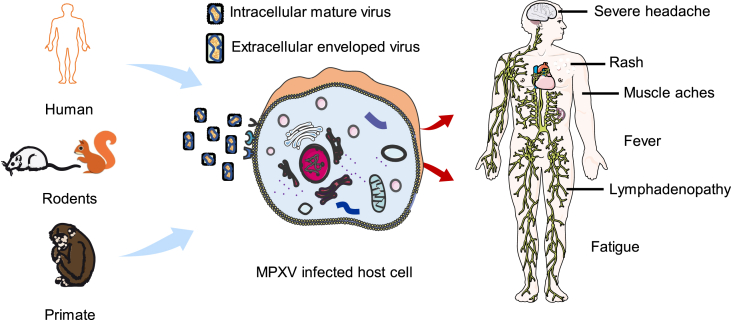
The host reservoir of MPVX is not fully defined, and to date, MPXV has only been isolated from Funisciurus anerythrus (Khodakevich et al., 1986) and Cercocebusatys (Radonic et al., 2014). A variety of rodents such as squirrels, Gambian rats, and other primates are considered to be the natural hosts for MPVX (Durski et al., 2018). MPVX is usually transmitted from animals to humans. Human infection with MPVX is mainly caused by bites from infected animals or direct contact through blood, body fluids, and MPX lesions of infected animals, and eating infected animals improperly cooked can also lead to the spread of the virus to human (Ellis et al., 2012; Ihekweazu et al., 2020). After the eradication of smallpox, the population's immunity to orthopoxvirus is gradually reduced, and occasional human-to-human transmission of MPVX can occur, usually in direct, long-term face-to-face contact, or through a large number of respiratory droplets containing the virus. It can also be transmitted through direct contact with the infected person's body fluids or virus-contaminated items, such as clothing and bedding (Hutson et al., 2011), and transmitted from mother to fetus through the placenta. In addition, there is a possibility of sexual transmission of MPVX (Alakunle et al., 2020; Ogoina et al., 2019). In the recent outbreak, most cases were among young man who have sex with man (MSM) with genital lesions which may constitutes close contact (Kozlov, 2022b). People who have not been vaccinated against smallpox are generally susceptible to MPXV. Workers slaughtering wild game, pet lovers, staff at animal breeding facilities, and the direct contacts of MPX patients may be at high risk (Fig. 1).
Fig. 1.
Schematic illustration of the transmission, infection of monkeypox virus (MPXV) and clinical features of monkeypox (MPX).
4.2. Clinical features of monkeypox
The symptoms of MPX are very similar to those of smallpox patients, but not as severe. The incubation period for MPX is usually 7–14 days, with a maximum of 21 days. Sufferers often have a history of exposure to animals or people infected with MPXV, initially showing symptoms similar to “influenza”, followed by herpes on the skin, experiencing pustules, and scarring after scabs. The process of MPVX infection is mainly divided into two phases: the prodromal phase (lasting about 0–2 days): fever, fatigue, severe headache, lymphadenopathy, muscle aches, and the rash phase (lasting 7–21 days). The rash usually begins to appear within 1–5 days after fever, and the patient is contagious when the rash appears. The rash is concentrated on the face and extremities, affecting the face (95%), palms and soles of the feet (75%), oral mucosa (70%), genitals (30%) and conjunctiva (20%). The rash lasts about 2–4 weeks and evolves from plaque to papules, blisters, pustules, scabs and then shedding. Lesions can occur in locations ranging from a few to several thousands (Petersen et al., 2019c). In severe cases, the areas of lesions can merge and cause large patches of skin to fall off. Patients often present with characteristic lymphadenopathy, most commonly in the groin, and may also be accompanied by a range of complications such as secondary bacterial infection, respiratory distress, bronchopneumonia, encephalitis, corneal infection with vision loss, and dehydration due to vomiting and diarrhea (Brown and Leggat, 2016; Petersen et al., 2019c). MPX is a self-limiting disease, and the severity of the disease is related to the degree of exposure to the virus, the patient's health conditions and the nature of its complications. Severe cases occur more commonly in children, and also lead to death, with a case fatality rate of 1%–10% (Doshi et al., 2019; Ogoina et al., 2019) (Fig. 1).
5. Laboratory diagnosis
Rapid diagnosis plays an essential role in controlling the outbreak and epidemic of MPX. The clinical manifestations of MPXV infection are difficult to distinguish from the other poxvirus caused diseases. Hence, laboratory tests are critical for diagnosing MPXV infection (Di Giulio and Eckburg, 2004; Macneil et al., 2009).
5.1. Nucleic acid testing
Currently, a variety of methods have been developed for MPXV nucleic acid detection, among which real-time PCR (RT-PCR) is the preferred method for routine diagnosis. Generally, the conserved regions of extracellular envelope protein gene (B6R), DNA polymerase gene E9L (Li et al., 2006; Yinka-Ogunleye et al., 2019), DNA-dependent RNA polymerase subunit 18 (RPO18) gene (Orba et al., 2015), and complement binding protein C3L (Li et al., 2010), F3L and N3R (Kulesh et al., 2004) genes are usually selected as targets for PCR amplification. The whole genome sequencing is the gold standard for distinguishing MPXV from other orthopoxvirus (Cohen-Gihon et al., 2020; Farlow et al., 2010). At present, the genome sequencing of the current MPXV that caused the global outbreak has been carried out. Phylogenetic analyses from the first genome sequence of the current MPX outbreak suggest the virus from this outbreak belongs to the mild West African clade and is closely related to the MPX virus isolated from the 2018–2019 UK, Singapore, and Israel outbreak (Thakur et al., 2022). Compared with the 2018–2019 viral genome, an average of 50 single-nucleotide diversity locus (SNPs) mutations appeared (Isidro et al., 2022). However, due to the high cost, the sequencing technology is limited in some areas. In addition, recombinase polymerase amplification (RPA) (Davi et al., 2019), loop-mediated isothermal amplification (LAMP) technology (Iizuka et al., 2009) and restriction length fragment polymorphism (RFLP) (Dumont et al., 2014) have all been developed for MPXV DNA detection.
5.2. Serological testing
Enzyme-linked immunosorbent assay (ELISA) can be used to detect the specific IgM and IgG antibodies in the serum of MPX patients after 5- and 8-days infection, respectively. A 4-fold increase in serum antibodies at both acute and convalescent stages can be used in the diagnosis of MPXV infection. Due to the antigenic cross reaction between MPXV and other poxvirus, the specificity is insufficient. Therefore, this method cannot accurately identify MPXV and is often used in epidemiological investigation (Alakunle et al., 2020).
5.3. Electron microscope observation
Electron microscopy could be used to assist diagnosis according to the morphological characteristics of MPXV. Since, MPXV and other poxviruses are not distinguishable in morphology, this method can't confirm the diagnosis, and can only provide clues that the virus belongs to poxvirus family. Moreover, the sensitivity of electron microscope is not high, and the preparation electronic sample is complicated and the period is long. Meanwhile, the electron microscopy is expensive and the operation is extremely complex, which limit its application in the practical detection.
5.4. Other tests
Immunochemistry analysis and multiplexed immunofluorescence imaging could be used for MPXV antigen detection (Doellinger et al., 2015). Viral isolation and culture that live virus is grown and characterized from a patient specimen are also required to establish a definitive diagnosis (Petersen et al., 2019c).
6. Prevention and treatment
6.1. Vaccines
At present, there is no specific vaccine against MPXV infection. Smallpox vaccination has been reported to provide 85% protection against MPXV (Brown and Leggat, 2016; Nasir et al., 2018). Epidemiological investigations indicated that approximately 90% of confirmed MPXV cases had not been infected with other poxviruses, and most cases were born after the end of the smallpox virus eradication program, very likely having not been vaccinated with smallpox vaccine (Brown and Leggat, 2016). There are currently two approved vaccines for preventing smallpox virus and MPXV: the second-generation vaccine ACAM 2000 and the third-generation vaccine IMVAMUNE. During the MPXV epidemic in the United States in 2003, ACAM 2000 was demonstrated to reduce the symptoms of MPX (Brown and Leggat, 2016), but side effects may occur in patients with atopic dermatitis and immunocompromised persons. This vaccine is not available to the public and is not used in MPXV endemic areas. IMVAMUNE is a replication-deficient, attenuated, third-generation modified vaccinia Ankara (MVA) vaccine that has also been approved by the Food and Drug Administration (FDA) and the European Medicine Agency (EMA) for the prevention of smallpox virus and MPXV in adults aged 18 years or older of high-risk population. Unlike ACAM 2000, IMVAMUNE can be used in patients with atopic dermatitis and immunodeficiency persons (Petersen et al., 2019a). So far, neither ACAM2000 nor IMVAMUNE is approved for use in the general population. Therefore, whether these approved smallpox vaccines could be effective in preventing MPX diseases in the MPXV endemic areas remains to be determined (Brown and Leggat, 2016; McCollum and Damon, 2014; Petersen et al., 2019b) (Table 1).
Table 1.
Candidate of vaccines and antiviral drugs for prevention and treatment of human poxvirus.
| Categories | Names | Features | Anti-poxvirus | Reference |
|---|---|---|---|---|
| Vaccines | ACAM 2000 | Second-generation vaccine | Smallpox virus, MPXV | Brown and Leggat (2016) |
| IMVAMUNE | Third-generation vaccine | Smallpox virus, MPXV | Petersen et al. (2019a) | |
| Antiviral drugs | Tecovirimat (ST-246) | Small molecule virus inhibitor | Smallpox virus, MPXV, and cowpox virus. |
Yang et al. (2005), Thakur et al. (2022) |
| Cidofovir Brincidofovir 289 derivative (CMX001) |
Viral DNA polymerase inhibitors | MPXV |
Magee et al. (2008) Magee et al. (2005) Delaune and Iseni (2020) |
|
| Nioch-14 | Nucleoside analogues inhibitor | MPXV and vaccinia virus | Delaune and Iseni (2020) | |
| Ribavirin, Tiazofurin | Inosine monophosphate dehydrogenase inhibitors | All of poxviruses | Baker et al. (2003) | |
| C-CA3-ADO, C3-NPC A | S-adenosylhomocysteine hydrolase inhibitors | All of poxviruses | Baker et al. (2003) | |
| HPMA, Adenosine N1 oxide (ANO) | DNA polymerase inhibitors | All of poxviruses | Baker et al. (2003) | |
6.2. Antiviral drugs
Until now, there are no specific antiviral drugs for the treatment of MPX, and most of the treatments are symptomatic and supportive therapies. Anti-smallpox virus drugs can play a role in anti-MPXV.
Tecovirimat (ST-246) is a small molecule virus inhibitor, which has a strong activity against orthopoxvirus, such as smallpox virus, MPXV, and cowpox virus. It can prevent virus spread by inhibiting the function of the major envelope protein (F13L), thereby preventing the virus from leaving an infected cell (Yang et al., 2005). It was approved in Europe in 2022 for the treatment of MPX (Thakur et al., 2022). Cidofovir and Brincidofovir derivative (CMX001) are both viral DNA polymerase inhibitors. Cidofovir is an acyclic nucleoside phosphate. When the CMX001 is ingested by the host cells, the lipid wrap of the drug can be cleaved to release free Cidofovir, which will be phosphorylated into Cidofovir-diphosphate (CDV-PP). CDV-PP inhibits the synthesis of viral DNA polymerase in the form of a substitute matrix, and eventually blocks viral DNA synthesis at the DNA polymerase level (Magee et al., 2005, 2008). Both Cidofovir and Brincidofovir have demonstrated that they can inhibit MPXV replication in vitro and in vivo (Delaune and Iseni, 2020). The nucleoside analogues inhibitor, Nioch-14, has strong antiviral activity against many orthopoxviruses, and its anti-MPXV and VACV effects are comparable to those of Tecovirimat. Nioch-14 is considered as a potential anti-MPXV drug since it can be easy produced (Delaune and Iseni, 2020). Ribavirin and Tiazofurin are inosine monophosphate dehydrogenase (IMP) inhibitors, that can reduce the replication of all poxviruses, and variola virus (VARV) and MPXV are more sensitive to them (Baker et al., 2003). S-adenosylhomocysteine (SAH) hydrolase inhibitors C-CA3-ADO and C3-NPC A, DNA polymerase inhibitors HPMA and adenosine N1 oxide (ANO) all showed well anti-poxvirus activity and could be used as potential anti-MPXV drugs (Baker et al., 2003) (Table 1).
7. Conclusions
The outbreak of MPX has become endemic in more than 20 countries since May 2022. The infectivity of MPXV is relative low and MPX may not become a pandemic as stated by WHO officer (Centor and Adalja, 2022). However, as the largest and most widespread MPX epidemic outside Africa so far, this international outbreak with an infrequently high number of cases has raised the alarms of international health authorities (Cabanillas et al., 2022). Therefore, we should pay high attention to scientific arrangement, strengthen the basic research on zoonotic poxvirus disease. To control the spread of MPX, it is critical to strengthen awareness and surveillance. The early diagnosis is key to prevent the epidemic of MPX, and the technical international cooperation is essential to reduce the risk of MPX.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
This work was partially funded by grants from the Natural Science Foundation of Hebei Province, China (no. H2020206352), the National Natural Science Foundation of China (no. 81902026), Science and Technology Project of Hebei Education Department (no. BJ2020018), Project for the Introduction overseas students of Hebei Provincial Department of Human Resources and Social Security (no. C20200344).
Contributor Information
Xia Chuai, Email: chuaixiahb@126.com.
Sandra Chiu, Email: qiux@ustc.edu.cn.
References
- Alakunle E., Moens U., Nchinda G., Okeke M.I. Monkeypox virus in Nigeria: infection biology, epidemiology, and evolution. Viruses. 2020;12:1257. doi: 10.3390/v12111257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt W.D., Cotsmire S., Trainor K., Harrington H., Hauns K., Kibler K.V., Huynh T.P., Jacobs B.L. Evasion of the innate immune type I interferon system by monkeypox virus. J. Virol. 2015;89:10489–10499. doi: 10.1128/JVI.00304-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt W.D., White S.D., Johnson B.P., Huynh T., Liao J., Harrington H., Cotsmire S., Kibler K.V., Langland J., Jacobs B.L. Monkeypox virus induces the synthesis of less dsRNA than vaccinia virus, and is more resistant to the anti-poxvirus drug, IBT, than vaccinia virus. Virology. 2016;497:125–135. doi: 10.1016/j.virol.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R.O., Bray M., Huggins J.W. Potential antiviral therapeutics for smallpox, monkeypox and other orthopoxvirus infections. Antivir. Res. 2003;57:13–23. doi: 10.1016/S0166-3542(02)00196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnewall R.E., Fisher D.A., Robertson A.B., Vales P.A., Knostman K.A., Bigger J.E. Inhalational monkeypox virus infection in cynomolgus macaques. Front. Cell. Infect. Microbiol. 2012;2:117. doi: 10.3389/fcimb.2012.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breman J.G., Kalisa R., Steniowski M.V., Zanotto E., Gromyko A.I., Arita I. Human monkeypox, 1970-79. Bull. World Health Organ. 1980;58:165–182. [PMC free article] [PubMed] [Google Scholar]
- Brown E., Senkevich T.G., Moss B. Vaccinia virus F9 virion membrane protein is required for entry but not virus assembly, in contrast to the related L1 protein. J. Virol. 2006;80:9455–9464. doi: 10.1128/JVI.01149-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K., Leggat P.A. Human monkeypox: current state of knowledge and implications for the future. Trav. Med. Infect. Dis. 2016;1:8. doi: 10.3390/tropicalmed1010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabanillas B., Valdelvira R., Akdis C. Monkeypox outbreak in Europe, UK, North America, and Australia: a changing trend of a zoonotic disease. Allergy. 2022 doi: 10.1111/all.15393. [DOI] [PubMed] [Google Scholar]
- Centor R., Adalja A. Annals on call - monkeypox: should we worry about another pandemic? Ann. Intern. Med. 2022;175:OC1. doi: 10.7326/A21-0014. [DOI] [PubMed] [Google Scholar]
- Chang S.J., Shih A.C., Tang Y.L., Chang W. Vaccinia mature virus fusion regulator A26 protein binds to A16 and G9 proteins of the viral entry fusion complex and dissociates from mature virions at low pH. J. Virol. 2012;86:3809–3818. doi: 10.1128/JVI.06081-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu W.L., Lin C.L., Yang M.H., Tzou D.L., Chang W. Vaccinia virus 4c (A26L) protein on intracellular mature virus binds to the extracellular cellular matrix laminin. J. Virol. 2007;81:2149–2157. doi: 10.1128/JVI.02302-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho C.T., Wenner H.A. Monkeypox virus. Bacteriol. Rev. 1973;37:1–18. doi: 10.1128/br.37.1.1-18.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Gihon I., Israeli O., Shifman O., Erez N., Melamed S., Paran N., Beth-Din A., Zvi A. Identification and whole-genome sequencing of a monkeypox virus strain isolated in Israel. Microbiol Resour Announc. 2020;9:e01524–19. doi: 10.1128/MRA.01524-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damon I.K. Status of human monkeypox: clinical disease, epidemiology and research. Vaccine. 2011;29(Suppl. 4):D54–D59. doi: 10.1016/j.vaccine.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Davi S.D., Kissenkotter J., Faye M., Bohlken-Fascher S., Stahl-Hennig C., Faye O., Faye O., Sall A.A., Weidmann M., Ademowo O.G., Hufert F.T., Czerny C.P., Abd El Wahed A. Recombinase polymerase amplification assay for rapid detection of Monkeypox virus. Diagn. Microbiol. Infect. Dis. 2019;95:41–45. doi: 10.1016/j.diagmicrobio.2019.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaune D., Iseni F. Drug development against smallpox: present and future. Antimicrob. Agents Chemother. 2020;64:e01683–19. doi: 10.1128/AAC.01683-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giulio D.B., Eckburg P.B. Human monkeypox: an emerging zoonosis. Lancet Infect. Dis. 2004;4:15–25. doi: 10.1016/S1473-3099(03)00856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doellinger J., Schaade L., Nitsche A. Comparison of the cowpox virus and vaccinia virus mature virion proteome: analysis of the species- and strain-specific proteome. PLoS One. 2015;10 doi: 10.1371/journal.pone.0141527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi R.H., Guagliardo S.A.J., Doty J.B., Babeaux A.D., Matheny A., Burgado J., Townsend M.B., Morgan C.N., Satheshkumar P.S., Ndakala N., Kanjingankolo T., Kitembo L., Malekani J., Kalemba L., Pukuta E., N'Kaya T., Kangoula F., Moses C., McCollum A.M., Reynolds M.G., Mombouli J.V., Nakazawa Y., Petersen B.W. Epidemiologic and ecologic investigations of monkeypox, likouala department, republic of the Congo, 2017. Emerg. Infect. Dis. 2019;25:281–289. doi: 10.3201/eid2502.181222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont C., Irenge L.M., Magazani E.K., Garin D., Muyembe J.J., Bentahir M., Gala J.L. Simple technique for in field samples collection in the cases of skin rash illness and subsequent PCR detection of orthopoxviruses and varicella zoster virus. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durski K.N., McCollum A.M., Nakazawa Y., Petersen B.W., Reynolds M.G., Briand S., Djingarey M.H., Olson V., Damon I.K., Khalakdina A. Emergence of monkeypox - west and central Africa, 1970-2017. MMWR Morb. Mortal. Wkly. Rep. 2018;67:306–310. doi: 10.15585/mmwr.mm6710a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P.L., Americo J.L., Moss B. Genetic studies of the susceptibility of classical and wild-derived inbred mouse strains to monkeypox virus. Virology. 2015;481:161–165. doi: 10.1016/j.virol.2015.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis C.K., Carroll D.S., Lash R.R., Peterson A.T., Damon I.K., Malekani J., Formenty P. Ecology and geography of human monkeypox case occurrences across Africa. J. Wildl. Dis. 2012;48:335–347. doi: 10.7589/0090-3558-48.2.335. [DOI] [PubMed] [Google Scholar]
- Erez N., Achdout H., Milrot E., Schwartz Y., Wiener-Well Y., Paran N., Politi B., Tamir H., Israely T., Weiss S., Beth-Din A., Shifman O., Israeli O., Yitzhaki S., Shapira S.C., Melamed S., Schwartz E. Diagnosis of imported monkeypox, Israel, 2018. Emerg. Infect. Dis. 2019;25:980–983. doi: 10.3201/eid2505.190076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito J.J., Knight J.C. Orthopoxvirus DNA: a comparison of restriction profiles and maps. Virology. 1985;143:230–251. doi: 10.1016/0042-6822(85)90111-4. [DOI] [PubMed] [Google Scholar]
- Farlow J., Ichou M.A., Huggins J., Ibrahim S. Comparative whole genome sequence analysis of wild-type and cidofovir-resistant monkeypoxvirus. Virol. J. 2010;7:110. doi: 10.1186/1743-422X-7-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine P.E., Jezek Z., Grab B., Dixon H. The transmission potential of monkeypox virus in human populations. Int. J. Epidemiol. 1988;17:643–650. doi: 10.1093/ije/17.3.643. [DOI] [PubMed] [Google Scholar]
- Foo C.H., Lou H., Whitbeck J.C., Ponce-de-Leon M., Atanasiu D., Eisenberg R.J., Cohen G.H. Vaccinia virus L1 binds to cell surfaces and blocks virus entry independently of glycosaminoglycans. Virology. 2009;385:368–382. doi: 10.1016/j.virol.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formenty P., Muntasir M.O., Damon I., Chowdhary V., Opoka M.L., Monimart C., Mutasim E.M., Manuguerra J.C., Davidson W.B., Karem K.L., Cabeza J., Wang S., Malik M.R., Durand T., Khalid A., Rioton T., Kuong-Ruay A., Babiker A.A., Karsani M.E., Abdalla M.S. Human monkeypox outbreak caused by novel virus belonging to Congo Basin clade, Sudan, 2005. Emerg. Infect. Dis. 2010;16:1539–1545. doi: 10.3201/eid1610.100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi V., Parker S., Jacca S., Crump R.W., Doronin K., Hembrador E., Pompilio D., Tebaldi G., Estep R.D., Wong S.W., Buller M.R., Donofrio G. BoHV-4-Based vector single heterologous antigen delivery protects STAT1(-/-) mice from monkeypoxvirus lethal challenge. PLoS Neglected Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch G.J., Graham V.A., Bewley K.R., Tree J.A., Dennis M., Taylor I., Funnell S.G., Bate S.R., Steeds K., Tipton T., Bean T., Hudson L., Atkinson D.J., McLuckie G., Charlwood M., Roberts A.D., Vipond J. Assessment of the protective effect of Imvamune and Acam2000 vaccines against aerosolized monkeypox virus in cynomolgus macaques. J. Virol. 2013;87:7805–7815. doi: 10.1128/JVI.03481-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann D.L., Szczeniowski M., Esteves K. Re-emergence of monkeypox in Africa: a review of the past six years. Br. Med. Bull. 1998;54:693–702. doi: 10.1093/oxfordjournals.bmb.a011720. [DOI] [PubMed] [Google Scholar]
- Hughes L.J., Goldstein J., Pohl J., Hooper J.W., Lee Pitts R., Townsend M.B., Bagarozzi D., Damon I.K., Karem K.L. A highly specific monoclonal antibody against monkeypox virus detects the heparin binding domain of A27. Virology. 2014;464–465:264–273. doi: 10.1016/j.virol.2014.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutin Y.J., Williams R.J., Malfait P., Pebody R., Loparev V.N., Ropp S.L., Rodriguez M., Knight J.C., Tshioko F.K., Khan A.S., Szczeniowski M.V., Esposito J.J. Outbreak of human monkeypox, democratic republic of Congo, 1996 to 1997. Emerg. Infect. Dis. 2001;7:434–438. doi: 10.3201/eid0703.010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson C.L., Carroll D.S., Gallardo-Romero N., Drew C., Zaki S.R., Nagy T., Hughes C., Olson V.A., Sanders J., Patel N., Smith S.K., Keckler M.S., Karem K., Damon I.K. Comparison of monkeypox virus clade kinetics and pathology within the prairie dog animal model using a serial sacrifice study design. BioMed Res. Int. 2015;2015:965710. doi: 10.1155/2015/965710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson C.L., Carroll D.S., Gallardo-Romero N., Weiss S., Clemmons C., Hughes C.M., Salzer J.S., Olson V.A., Abel J., Karem K.L., Damon I.K. Monkeypox disease transmission in an experimental setting: prairie dog animal model. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson C.L., Damon I.K. Monkeypox virus infections in small animal models for evaluation of anti-poxvirus agents. Viruses. 2010;2:2763–2776. doi: 10.3390/v2122763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson C.L., Kondas A.V., Mauldin M.R., Doty J.B., Grossi I.M., Morgan C.N., Ostergaard S.D., Hughes C.M., Nakazawa Y., Kling C., Martin B.E., Ellison J.A., Carroll D.S., Gallardo-Romero N.F., Olson V.A. Pharmacokinetics and efficacy of a potential smallpox therapeutic, Brincidofovir, in a lethal monkeypox virus animal model. mSphere. 2021;6:e00927–20. doi: 10.1128/mSphere.00927-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihekweazu C., Yinka-Ogunleye A., Lule S., Ibrahim A. Importance of epidemiological research of monkeypox: is incidence increasing? Expert Rev. Anti Infect. Ther. 2020;18:389–392. doi: 10.1080/14787210.2020.1735361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka I., Saijo M., Shiota T., Ami Y., Suzaki Y., Nagata N., Hasegawa H., Sakai K., Fukushi S., Mizutani T., Ogata M., Nakauchi M., Kurane I., Mizuguchi M., Morikawa S. Loop-mediated isothermal amplification-based diagnostic assay for monkeypox virus infections. J. Med. Virol. 2009;81:1102–1108. doi: 10.1002/jmv.21494. [DOI] [PubMed] [Google Scholar]
- Isidro J., Borges V., Pinto M., Sobral D., Santos J.D., Nunes A., Mixao V., Ferreira R., Santos D., Duarte S., Vieira L., Borrego M.J., Nuncio S., de Carvalho I.L., Pelerito A., Cordeiro R., Gomes J.P. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat. Med. 2022 doi: 10.1038/s41591-022-01907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezek Z., Khodakevich L.N., Wickett J.F. Smallpox and its post-eradication surveillance. Bull. World Health Organ. 1987;65:425–434. [PMC free article] [PubMed] [Google Scholar]
- Kaever T., Meng X., Matho M.H., Schlossman A., Li S., Sela-Culang I., Ofran Y., Buller M., Crump R.W., Parker S., Frazier A., Crotty S., Zajonc D.M., Peters B., Xiang Y. Potent neutralization of vaccinia virus by divergent murine antibodies targeting a common site of vulnerability in L1 protein. J. Virol. 2014;88:11339–11355. doi: 10.1128/JVI.01491-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodakevich L., Jezek Z., Kinzanzka K. Isolation of monkeypox virus from wild squirrel infected in nature. Lancet. 1986;1:98–99. doi: 10.1016/S0140-6736(86)90748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov M. Monkeypox goes global: why scientists are on alert. Nature. 2022;606:15–16. doi: 10.1038/d41586-022-01421-8. [DOI] [PubMed] [Google Scholar]
- Kozlov M. Monkeypox outbreaks: 4 key questions researchers have. Nature. 2022;606:238–239. doi: 10.1038/d41586-022-01493-6. [DOI] [PubMed] [Google Scholar]
- Kugelman J.R., Johnston S.C., Mulembakani P.M., Kisalu N., Lee M.S., Koroleva G., McCarthy S.E., Gestole M.C., Wolfe N.D., Fair J.N., Schneider B.S., Wright L.L., Huggins J., Whitehouse C.A., Wemakoy E.O., Muyembe-Tamfum J.J., Hensley L.E., Palacios G.F., Rimoin A.W. Genomic variability of monkeypox virus among humans, Democratic Republic of the Congo. Emerg. Infect. Dis. 2014;20:232–239. doi: 10.3201/eid2002.130118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesh D.A., Loveless B.M., Norwood D., Garrison J., Whitehouse C.A., Hartmann C., Mucker E., Miller D., Wasieloski L.P., Jr., Huggins J., Huhn G., Miser L.L., Imig C., Martinez M., Larsen T., Rossi C.A., Ludwig G.V. Monkeypox virus detection in rodents using real-time 3'-minor groove binder TaqMan assays on the Roche LightCycler. Lab. Invest. 2004;84:1200–1208. doi: 10.1038/labinvest.3700143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Olson V.A., Laue T., Laker M.T., Damon I.K. Detection of monkeypox virus with real-time PCR assays. J. Clin. Virol. 2006;36:194–203. doi: 10.1016/j.jcv.2006.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhao H., Wilkins K., Hughes C., Damon I.K. Real-time PCR assays for the specific detection of monkeypox virus West African and Congo Basin strain DNA. J. Virol. Methods. 2010;169:223–227. doi: 10.1016/j.jviromet.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likos A.M., Sammons S.A., Olson V.A., Frace A.M., Li Y., Olsen-Rasmussen M., Davidson W., Galloway R., Khristova M.L., Reynolds M.G., Zhao H., Carroll D.S., Curns A., Formenty P., Esposito J.J., Regnery R.L., Damon I.K. A tale of two clades: monkeypox viruses. J. Gen. Virol. 2005;86:2661–2672. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]
- Lopera J.G., Falendysz E.A., Rocke T.E., Osorio J.E. Attenuation of monkeypox virus by deletion of genomic regions. Virology. 2015;475:129–138. doi: 10.1016/j.virol.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macneil A., Reynolds M.G., Braden Z., Carroll D.S., Bostik V., Karem K., Smith S.K., Davidson W., Li Y., Moundeli A., Mombouli J.V., Jumaan A.O., Schmid D.S., Regnery R.L., Damon I.K. Transmission of atypical varicella-zoster virus infections involving palm and sole manifestations in an area with monkeypox endemicity. Clin. Infect. Dis. 2009;48:e6–8. doi: 10.1086/595552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee W.C., Aldern K.A., Hostetler K.Y., Evans D.H.J.A.A., Chemotherapy Cidofovir and (S)-9-[3-Hydroxy-(2-Phosphonomethoxy)Propyl]Adenine are highly effective inhibitors of vaccinia virus DNA polymerase when incorporated into the template strand. 2008;52:586. doi: 10.1128/AAC.01172-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee W.C., Hostetler K.Y., Evans D.H.J.A.A., Chemotherapy Mechanism of inhibition of vaccinia virus DNA polymerase by. Cidofovir Diphosphate. 2005;49:3153–3162. doi: 10.1128/AAC.49.8.3153-3162.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahase E. Monkeypox: what do we know about the outbreaks in Europe and North America? BMJ. 2022;377:o1274. doi: 10.1136/bmj.o1274. [DOI] [PubMed] [Google Scholar]
- Matho M.H., Schlossman A., Gilchuk I.M., Miller G., Mikulski Z., Hupfer M., Wang J., Bitra A., Meng X., Xiang Y., Kaever T., Doukov T., Ley K., Crotty S., Peters B., Hsieh-Wilson L.C., Crowe J.E., Jr., Zajonc D.M. Structure-function characterization of three human antibodies targeting the vaccinia virus adhesion molecule D8. J. Biol. Chem. 2018;293:390–401. doi: 10.1074/jbc.M117.814541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum A.M., Damon I.K. Human monkeypox. Clin. Infect. Dis. 2014;58:260–267. doi: 10.1093/cid/cit703. [DOI] [PubMed] [Google Scholar]
- Nalca A., Livingston V.A., Garza N.L., Zumbrun E.E., Frick O.M., Chapman J.L., Hartings J.M. Experimental infection of cynomolgus macaques (Macaca fascicularis) with aerosolized monkeypox virus. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasir I.A., Dangana A., Ojeamiren I., Emeribe A.U. Reminiscing the recent incidence of monkeypox in Nigeria: its ecologic-epidemiology and literature review. P. H. Med. J. 2018;12:1–9. [Google Scholar]
- Ng O.T., Lee V., Marimuthu K., Vasoo S., Chan G., Lin R.T.P., Leo Y.S. A case of imported Monkeypox in Singapore. Lancet Infect. Dis. 2019;19:1166. doi: 10.1016/S1473-3099(19)30537-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen P.Y., Ajisegiri W.S., Costantino V., Chughtai A.A., MacIntyre C.R. Reemergence of human monkeypox and declining population immunity in the context of urbanization, Nigeria, 2017-2020. Emerg. Infect. Dis. 2021;27:1007–1014. doi: 10.3201/eid2704.203569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogoina D., Izibewule J.H., Ogunleye A., Ederiane E., Anebonam U., Neni A., Oyeyemi A., Etebu E.N., Ihekweazu C. The 2017 human monkeypox outbreak in Nigeria-Report of outbreak experience and response in the Niger Delta University Teaching Hospital, Bayelsa State, Nigeria. PLoS One. 2019;14 doi: 10.1371/journal.pone.0214229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orba Y., Sasaki M., Yamaguchi H., Ishii A., Thomas Y., Ogawa H., Hang'ombe B.M., Mweene A.S., Morikawa S., Saijo M., Sawa H. Orthopoxvirus infection among wildlife in Zambia. J. Gen. Virol. 2015;96:390–394. doi: 10.1099/vir.0.070219-0. [DOI] [PubMed] [Google Scholar]
- Osorio J.E., Iams K.P., Meteyer C.U., Rocke T.E. Comparison of monkeypox viruses pathogenesis in mice by in vivo imaging. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen B.W., Kabamba J., McCollum A.M., Lushima R.S., Wemakoy E.O., Muyembe Tamfum J.J., Nguete B., Hughes C.M., Monroe B.P., Reynolds M.G. Vaccinating against monkeypox in the democratic republic of the Congo. Antivir. Res. 2019;162:171–177. doi: 10.1016/j.antiviral.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen E., Abubakar I., Ihekweazu C., Heymann D., Ntoumi F., Blumberg L., Asogun D., Mukonka V., Lule S.A., Bates M., Honeyborne I., Mfinanga S., Mwaba P., Dar O., Vairo F., Mukhtar M., Kock R., McHugh T.D., Ippolito G., Zumla A. Monkeypox - enhancing public health preparedness for an emerging lethal human zoonotic epidemic threat in the wake of the smallpox post-eradication era. Int. J. Infect. Dis. 2019;78:78–84. doi: 10.1016/j.ijid.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen E., Kantele A., Koopmans M., Asogun D., Yinka-Ogunleye A., Ihekweazu C., Zumla A. Human monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infect. Dis. Clin. 2019;33:1027–1043. doi: 10.1016/j.idc.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickup D.J. Extracellular virions: the advance guard of poxvirus infections. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1004904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radonic A., Metzger S., Dabrowski P.W., Couacy-Hymann E., Schuenadel L., Kurth A., Matz-Rensing K., Boesch C., Leendertz F.H., Nitsche A. Fatal monkeypox in wild-living sooty mangabey, Cote d'Ivoire, 2012. Emerg. Infect. Dis. 2014;20:1009–1011. doi: 10.3201/eid2006.131329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Realegeno S., Puschnik A.S., Kumar A., Goldsmith C., Burgado J., Sambhara S., Olson V.A., Carroll D., Damon I., Hirata T., Kinoshita T., Carette J.E., Satheshkumar P.S. Monkeypox virus host factor screen using haploid cells identifies essential role of GARP complex in extracellular virus formation. J. Virol. 2017;91:e00011–17. doi: 10.1128/JVI.00011-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K.D., Melski J.W., Graham M.B., Regnery R.L., Sotir M.J., Wegner M.V., Kazmierczak J.J., Stratman E.J., Li Y., Fairley J.A., Swain G.R., Olson V.A., Sargent E.K., Kehl S.C., Frace M.A., Kline R., Foldy S.L., Davis J.P., Damon I.K. The detection of monkeypox in humans in the Western Hemisphere. N. Engl. J. Med. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- Rimoin A.W., Mulembakani P.M., Johnston S.C., Lloyd Smith J.O., Kisalu N.K., Kinkela T.L., Blumberg S., Thomassen H.A., Pike B.L., Fair J.N., Wolfe N.D., Shongo R.L., Graham B.S., Formenty P., Okitolonda E., Hensley L.E., Meyer H., Wright L.L., Muyembe J.J. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc. Natl. Acad. Sci. U. S. A. 2010;107:16262–16267. doi: 10.1073/pnas.1005769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo A.T., Berhanu A., Bigger C.B., Prigge J., Silvera P.M., Grosenbach D.W., Hruby D. Co-administration of tecovirimat and ACAM2000 in non-human primates: effect of tecovirimat treatment on ACAM2000 immunogenicity and efficacy versus lethal monkeypox virus challenge. Vaccine. 2020;38:644–654. doi: 10.1016/j.vaccine.2019.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale T.A., Melski J.W., Stratman E.J. Monkeypox: an epidemiologic and clinical comparison of African and US disease. J. Am. Acad. Dermatol. 2006;55:478–481. doi: 10.1016/j.jaad.2006.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schin A.M., Diesterbeck U.S., Moss B. Insights into the organization of the poxvirus multicomponent entry-fusion complex from proximity analyses in living infected cells. J. Virol. 2021;95 doi: 10.1128/JVI.00852-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkevich T.G., Ojeda S., Townsley A., Nelson G.E., Moss B. Poxvirus multiprotein entry-fusion complex. Proc. Natl. Acad. Sci. U. S. A. 2005;102:18572–18577. doi: 10.1073/pnas.0509239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeev A.A., Kabanov A.S., Bulychev L.E., Sergeev A.A., Pyankov O.V., Bodnev S.A., Galahova D.O., Zamedyanskaya A.S., Titova K.A., Glotov A.G., Taranov O.S., Omigov V.V., Shishkina L.N., Agafonov A.P., Sergeev A.N. The possibility of using the ICR mouse as an animal model to assess antimonkeypox drug efficacy. Transbound Emerg Dis. 2016;63:e419–430. doi: 10.1111/tbed.12323. [DOI] [PubMed] [Google Scholar]
- Singh K., Gittis A.G., Gitti R.K., Ostazeski S.A., Su H.P., Garboczi D.N. The vaccinia virus H3 envelope protein, a major target of neutralizing antibodies, exhibits a glycosyltransferase fold and binds UDP-glucose. J. Virol. 2016;90:5020–5030. doi: 10.1128/JVI.02933-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur V., Thakur P., Srivastava S., Kumar P. Monkeypox virus (MPX) in humans a concern: trespassing the global boundaries - Correspondence. Int. J. Surg. 2022;104:106703. doi: 10.1016/j.ijsu.2022.106703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan A., Aarons E., Astbury J., Balasegaram S., Beadsworth M., Beck C.R., Chand M., O'Connor C., Dunning J., Ghebrehewet S., Harper N., Howlett-Shipley R., Ihekweazu C., Jacobs M., Kaindama L., Katwa P., Khoo S., Lamb L., Mawdsley S., Morgan D., Palmer R., Phin N., Russell K., Said B., Simpson A., Vivancos R., Wade M., Walsh A., Wilburn J. Two cases of monkeypox imported to the United Kingdom, September 2018. Euro Surveill. 2018;23:1800509. doi: 10.2807/1560-7917.ES.2018.23.38.1800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver J.R., Isaacs S.N. Monkeypox virus and insights into its immunomodulatory proteins. Immunol. Rev. 2008;225:96–113. doi: 10.1111/j.1600-065X.2008.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Pevear D.C., Davies M.H., Collett M.S., Bailey T., Rippen S., Barone L., Burns C., Rhodes G., Tohan S., Huggins J.W., Baker R.O., Buller R.L., Touchette E., Waller K., Schriewer J., Neyts J., DeClercq E., Jones K., Hruby D., Jordan R. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus Challenge. J. Virol. 2005;79:13139–13149. doi: 10.1128/JVI.79.20.13139-13149.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yinka-Ogunleye A., Aruna O., Dalhat M., Ogoina D., McCollum A., Disu Y., Mamadu I., Akinpelu A., Ahmad A., Burga J., Ndoreraho A., Nkunzimana E., Manneh L., Mohammed A., Adeoye O., Tom-Aba D., Silenou B., Ipadeola O., Saleh M., Adeyemo A., Nwadiutor I., Aworabhi N., Uke P., John D., Wakama P., Reynolds M., Mauldin M.R., Doty J., Wilkins K., Musa J., Khalakdina A., Adedeji A., Mba N., Ojo O., Krause G., Ihekweazu C., Team C.D.C.M.O. Outbreak of human monkeypox in Nigeria in 2017-18: a clinical and epidemiological report. Lancet Infect. Dis. 2019;19:872–879. doi: 10.1016/S1473-3099(19)30294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yinka-Ogunleye A., Aruna O., Ogoina D., Aworabhi N., Eteng W., Badaru S., Mohammed A., Agenyi J., Etebu E.N., Numbere T.W., Ndoreraho A., Nkunzimana E., Disu Y., Dalhat M., Nguku P., Mohammed A., Saleh M., McCollum A., Wilkins K., Faye O., Sall A., Happi C., Mba N., Ojo O., Ihekweazu C. Reemergence of human monkeypox in Nigeria, 2017. Emerg. Infect. Dis. 2018;24:1149–1151. doi: 10.3201/eid2406.180017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaucha G.M., Jahrling P.B., Geisbert T.W., Swearengen J.R., Hensley L. The pathology of experimental aerosolized monkeypox virus infection in cynomolgus monkeys (Macaca fascicularis) Lab. Invest. 2001;81:1581–1600. doi: 10.1038/labinvest.3780373. [DOI] [PMC free article] [PubMed] [Google Scholar]