Edited by Associate Editor Laurie Sehn, this 3-part Review Series covers rare, aggressive B-cell lymphomas. First, Olszewski et al discuss a recently-designated new diagnostic category of “high-grade B-cell lymphoma, not otherwise specified (HBCL, NOS).” While lacking a cardinal molecular feature, they share a gene expression profile signature, and 45% have MYC rearrangement. Heterogeneity of HBCL, NOS has impeded treatment standardization, but new insights into molecular pathophysiology may offer entry points for better therapy. In the second article, Savage provides an updated review of primary mediastinal large B-cell lymphoma, highlighting its distinct molecular signature that overlaps with nodular sclerosis Hodgkin lymphoma, including JAK-STAT and NF-κB signaling pathways leading to immune evasion. Treatment outcomes are variable, but recent data confirming the efficacy of PD1 inhibitors have led to studies using immunotherapy in first-line therapy. In the third article, Schaff and Grommes review the clinical presentation, evaluation, and treatment of primary central nervous system lymphoma. They highlight improvement in outcomes with the use of high-dose methotrexate chemotherapy; however, survival at 5 years is only 30-40%. More recent studies of targeted therapies have had promising preliminary results.
Visual Abstract
Abstract
Primary central nervous system lymphoma (PCNSL) is a rare extranodal lymphomatous malignancy that affects the brain, spinal cord, leptomeninges, or vitreoretinal space, without evidence of systemic involvement. The diagnosis of PCNSL requires a high level of suspicion because clinical presentation varies depending upon involved structures. Initiation of treatment is time sensitive for optimal neurologic recovery and disease control. In general, the prognosis of PCNSL has improved significantly over the past few decades, largely as a result of the introduction and widespread use of high-dose methotrexate (MTX) chemotherapy, which is considered the backbone of first-line polychemotherapy treatment. Upon completion of MTX-based treatment, a consolidation strategy is often required to prolong duration of response. Consolidation can consist of radiation, maintenance therapy, nonmyeloablative chemotherapy, or myeloablative treatment followed by autologous stem cell transplant. Unfortunately, even with consolidation, relapse is common, and 5-year survival rates stand at only 30% to 40%. Novel insights into the pathophysiology of PCNSL have identified key mechanisms in tumor pathogenesis, including activation of the B-cell receptor pathway, immune evasion, and a suppressed tumor immune microenvironment. These insights have led to the identification of novel small molecules targeting these aberrant pathways. The Bruton tyrosine kinase inhibitor ibrutinib and immunomodulatory drugs (lenalidomide or pomalidomide) have shown promising clinical response rates for relapsed/refractory PCNSL and are increasingly used for the treatment of recurrent disease. This review provides a discussion of the clinical presentation of PCNSL, the approach to work-up and staging, and an overview of recent advancements in the understanding of the pathophysiology and current treatment strategies for immunocompetent patients.
Introduction
Primary central nervous system lymphoma (PCNSL) is an extranodal non-Hodgkin lymphoma affecting the brain, spine, cerebrospinal fluid (CSF), or vitreoretinal space.1,2 It is a rare cancer with an annual incidence of 0.4 per 100 000 in all comers,3,4 but the incidence increases with age to as high as 4 per 100 000 in those older than 70 years.3 PCNSL accounts for 4% to 6% of all extranodal lymphomas, as well as for 4% of newly diagnosed malignant brain tumors.5 PCNSL can develop at any age (0.4% occur in patients younger than 9 years; 1.5% occur in patients younger than 19 years), but it has a median age of 56 to 61 years.3 Immunosuppressed patients, such as those with HIV/AIDS or patients posttransplant, are at an increased risk for PCNSL associated with Epstein-Barr virus.
The majority of PCNSL tumors are diffuse large B-cell lymphomas (DLBCLs). Although these tumors are highly chemo- and radioresponsive, relapse rates are high. Diagnosis is often made via biopsy of contrast-enhancing brain lesions, vitreous biopsy, or sampling of the CSF. High-dose methotrexate (MTX) chemotherapy regimens are the backbone of treatment, but consolidation therapy following MTX further improves duration of response. This review focuses on the clinical presentation, work-up, and treatment of immunocompetent patients with PCNSL.
Clinical presentation
The diagnosis of PCNSL requires a high level of suspicion because clinical presentations can vary widely. Only a proportion of patients with PCNSL (∼50% to 70%) will present with focal neurologic deficits.6 Almost as common, patients with PCNSL (∼40% to 50%) may develop nonspecific cognitive or behavioral changes over a matter of weeks to months. Signs of elevated intracranial pressure, such as headache, vomiting, or nausea, may also be seen (33%) but are not always readily identified as neurologic. Patients may have lymphoma isolated to the vitreoretinal space; as a result, their initial clinical presentation may be limited to subtle visual abnormalities, such as blurring, decreased acuity, or floaters. When the eye is the only site of disease, patients are diagnosed with primary intraocular lymphoma.7 Symptoms of primary intraocular lymphoma can be very indolent and may precede involvement of other central nervous system (CNS) compartments by years. Ocular abnormalities often require slit lamp examination for detection. Even when observed, these changes can be misdiagnosed because they often resemble uveitis. Further complicating matters, a majority of patients with PCNSL with ocular involvement have no visual symptoms.7 B symptoms that are commonly seen with systemic hematologic malignancy, such as fever, night sweats, and weight loss, are rare in PCNSL.
Diagnosis
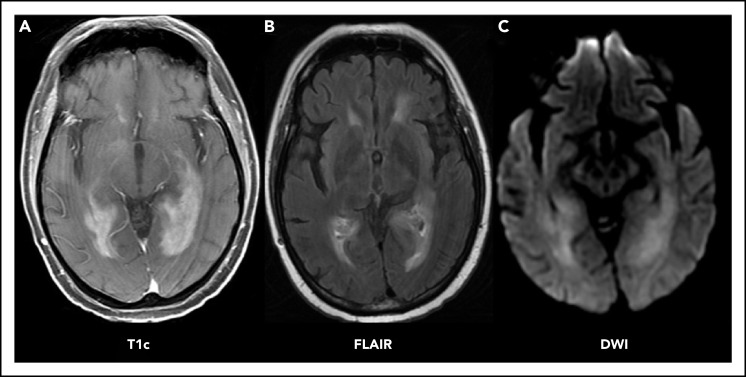
Patients with neurologic signs or symptoms as described above should immediately undergo brain imaging. The preferred imaging modality is magnetic resonance imaging (MRI) with and without contrast. MRI provides the best resolution for visualization and may be helpful with diagnosis of PCNSL, because it is often associated with characteristic radiographic features (Figure 1A). Lesions may be multifocal or unifocal. They are often periventricular (60%), affecting deep brain structures like the corpus callosum, the deep white matter, or the basal ganglia. On T2 sequencing, they tend to be iso- to hyperintense (Figure 1B) and are typically homogeneously enhancing with associated diffusion restriction (Figure 1C). In contrast to glioma or solid tumor metastases, PCNSL lesions tend to present with a mild amount of edema relative to their size.6 In patients who are unable to tolerate MRI scans, a computed tomography (CT) scan with and without contrast may be performed. The role of FDG–positron emission tomography (PET) brain imaging has not been established.8 PCNSL can present with isolated leptomeningeal or ocular involvement, although this is rare. Still, a normal-appearing MRI should not preclude lumbar puncture and ocular examinations when suspicion remains.
Figure 1.
PCNSL imaging pattern on MRI. Characteristic PCNSL imaging features on MRI. (A) T1 sequence with gadolinium contrast (T1c) demonstrates homogenously enhancing lesions affecting the deep white matter. (B) Fluid-attenuated inversion recovery (FLAIR) sequence demonstrates moderate edema surrounding the lesion. (C) Diffusion-weighted imaging sequence (DWI) demonstrates restricted diffusion within the lesions.
To establish a definitive diagnosis of PCNSL, a tissue sample is required. This often necessitates a brain biopsy, but CSF examination or vitrectomy may be diagnostic. Up to 20% of patients with PCNSL may have leptomeningeal involvement at diagnosis. As such, lumbar puncture is required for complete staging. CSF should be assessed by a basic cell count with differential, CSF glucose and protein levels, cytology, and flow cytometry. Immunoglobulin heavy chain gene rearrangement testing can be performed in the CSF, if available. Results may support cytology and flow cytometry findings. A slit-lamp evaluation to look for malignant cells in the vitreous humor or retina is necessary to complete staging. If found, biopsy may be performed, potentially obviating need for a brain biopsy. In general, however, brain biopsy should not be delayed while awaiting results of other investigations because PCNSL symptoms may progress rapidly and could lead to acute clinical deterioration if treatment is delayed.
When a diagnosis of PCNSL is being considered, it is important to defer corticosteroid administration until after pathologic confirmation. Corticosteroids are toxic to lymphoma cells and can cause a complete resolution of PCNSL lesions, resulting in poor diagnostic yield, potentially delaying definitive diagnosis and treatment. Therefore, corticosteroids should be reserved only for life-threatening circumstances. If it is necessary to start steroids, a brain biopsy should be performed within 24 to 48 hours of the first dose to optimize the diagnostic yield.
When a surgical procedure is required for diagnosis, biopsy is typically favored over a complete resection to minimize the potential for morbidity. Often PCNSL is multifocal and involves deep brain structures. Retrospective data conflict on the role of aggressive resection, with some studies finding no evidence of improved clinical outcomes6 and others suggesting a benefit in lower-risk patients.9 Notably, high-risk patients, defined as those with medical comorbidities, difficulties with activities of daily living, age > 55 years, and those with deep or multiple lesions, do not appear to benefit from resection; in practice, these parameters apply to the majority of patients with PCNSL. Most importantly, PCNSL is highly chemo and radio-responsive. Medical treatment alone can result in rapid and dramatic neurologic recovery, perhaps obviating the need for resection and supporting a more conservative surgical approach.
Pathology
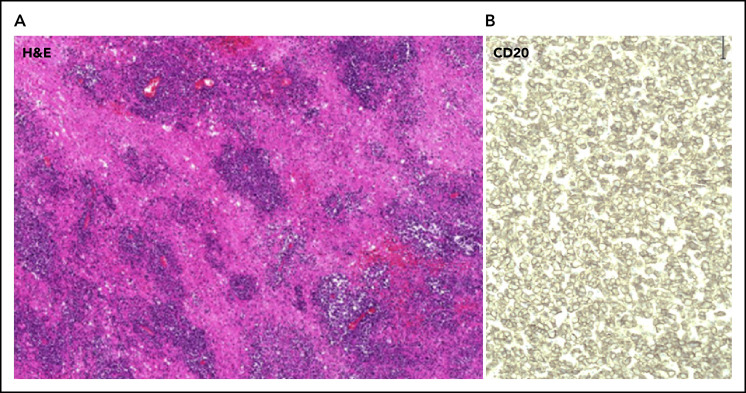
The majority of PCNSL tumors are DLBCLs (90%), with the vast majority of these categorized as the activated B-cell–like (ABC)/nongerminal center subtype.10,11 On histology, cells are typically highly proliferative, diffusely infiltrating the brain parenchyma and exhibiting an angiocentric growth pattern (Figure 2A). Neoplastic cells are identified by the high expression of the B-cell surface marker CD20 (Figure 2B). In systemic DLBCL, the ABC/nongerminal subgroup is associated with a poorer prognosis, as well as frequent recurrent mutations in the B-cell receptor (BCR) pathway.12 In PCNSL, this pathway is also affected by frequent recurrent mutations primarily affecting MYD88 and CD79B.11,13 Interestingly, and in contrast to non-CNS systemic DLBCL, these mutations are found in germinal and ABC center subtypes, suggesting that the BCR plays a central role in PCNSL. PCNSL demonstrates frequent copy number gains at 9p24.1, which includes loci of immunosuppressive genes programmed death ligand 1 (PD-L1) and PD-L2.14 Although PD-L1 expression is only found in 30%13 of PCNSL tumors, immune evasion might play a role in PCNSL pathogenesis.
Figure 2.
Effects of corticosteroids on imaging in PCNSL and histopathologic features of PCNSL. (A) Hematoxylin and eosin (H&E) staining (10× magnification) of PCNSL demonstrating angiocentric growth pattern and diffusely infiltrating PCNSL cells invading the brain parenchyma. (B) Cells are positive for the B-cell surface marker CD20 (40× magnification).
Rarely, lymphoma subtypes other than DLBCL may be the cause of isolated CNS lesions. More indolent B-cell lymphomas, including mantle cell, Burkett, and T-cell lymphomas, have all been described, as has Bing-Neel syndrome, or CNS involvement in patients with Waldenström’s macroglobulinemia.10 The underlying histology may dictate treatment and impact clinical outcomes. Five-year overall survival (OS) rates for CNS DLBCL are ∼30% compared with 79% in marginal zone lymphoma, 66% in follicular lymphoma, 45% in Hodgkin lymphoma, 38% in small lymphocytic lymphoma, 42% in Burkitt lymphoma, and 33% in peripheral T-cell lymphoma.15
Staging
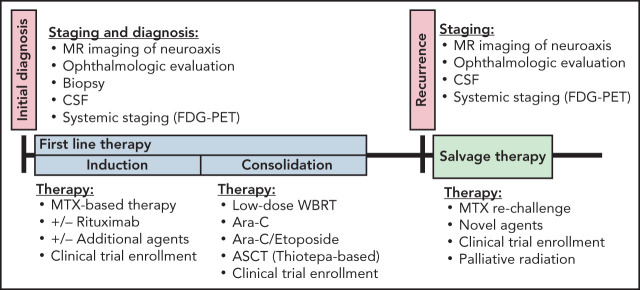
Once the diagnosis of PCNSL is confirmed, patients require systemic staging to rule out extra-CNS disease, which would change management and require the addition of chemotherapy agents used in systemic disease (eg, rituximab/cyclophosphamide/doxorubicin/vincristine/prednisone). Active systemic disease is ultimately discovered in 4% to 7%16 of patients with a newly diagnosed central lymphomatous lesion. The International PCNSL Collaborative Group17 recommends staging with a whole-body PET scan and bone marrow biopsy (Table 1). If PET cannot be performed, a CT tomography chest/abdomen/pelvis with and without contrast may suffice. Men who are unable to undergo PET or those with an abnormal scrotal examination should have a testicular ultrasound because there is a high frequency of CNS disease associated with testicular lymphoma. A basic laboratory evaluation should include a complete blood count with a differential, a complete metabolic panel to assess baseline renal and liver function, serum lactate dehydrogenase (LDH), hepatitis B and C serology, and HIV testing.
Table 1.
Baseline evaluations in newly diagnosed PCNSL (based on International PCNSL Collaborative Group guidelines)
Systemic staging/work-up
Neurological staging
|
Prognosis may be predicted with 2 commonly used models: (1) The Memorial Sloan Kettering Cancer Center model, which utilizes Karnofsky Performance Scale (≥70 vs <70) and age (≤50 vs >50 years) at diagnosis to predict median progression-free survival (PFS) and OS18 and (2) The International Extranodal Lymphoma Study Group model, which relies on Eastern Cooperative Oncology Group Performance Status (0-1 vs >1), age (<60 vs >60 years), LDH (normal vs elevated), involvement of deep brain structures, and CSF protein concentration (normal vs elevated).19
Treatment at first diagnosis
To prevent morbidity and optimize the chances of neurologic recovery, treatment of PCNSL should be initiated as soon as the diagnosis is confirmed. Postbiopsy recovery and wound healing are usually not a concern and should not delay treatment initiation. Treatment is typically administered in 2 stages: induction and consolidation. The purpose of induction is to induce a partial, or ideally, complete response, whereas consolidation eliminates residual, often microscopic disease, and helps to maintain remission. There is a paucity of phase 3 randomized clinical trials for PCNSL treatment; as such, there is no standard agreed upon induction or consolidation strategy. However, it is generally accepted that MTX-based chemotherapy is the standard of care for induction. The dose of MTX and concurrent therapies administered may differ based on regional and institutional preferences. Consolidation strategies are also varied and are often tailored to the specific patient. They can range from myeloablative chemotherapy with autologous stem cell transplant (ASCT) to high-dose nonmyeloablative chemotherapy, radiation, medical maintenance, or even observation.
Historically, patients with PCNSL were treated with radiation. Although radiation tends to induce dramatic imaging responses, focal radiation resulted in high rates of relapse outside of the radiation field. Whole-brain radiation therapy (WBRT) yields impressive 90% overall response rates (ORRs); however, responses are not durable, with an OS of only 12 to 18 months.20,21
The standard chemotherapy agents used for treatment of systemic lymphoma (rituximab/cyclophosphamide/doxorubicin/vincristine/prednisone) lack efficacy in PCNSL,22,23 likely as a result of poor penetration of the blood-brain barrier (BBB). MTX administered at doses > 1.5 g/m2 penetrates the BBB and achieves cytotoxic concentrations in the CSF. The addition of MTX to WBRT results in improved OS, with 5-year survival rates of 30% to 50%.24,25 Ferreri et al26 demonstrated that the addition of cytarabine to MTX/WBRT improved ORR and prolonged PFS from 3 to 18 months, suggesting that polychemotherapy is more effective than single-agent MTX, setting the stage for the regimens in current use.
Unfortunately, the combination of chemotherapy and radiation therapies has proven to be neurotoxic. Over time, patients may develop symptoms, such as psychomotor slowing, executive and memory dysfunction, progressive subcortical dementia, gait ataxia, behavioral changes, and incontinence. Imaging reveals diffuse white matter disease and cortical-subcortical atrophy. These changes are mainly attributed to WBRT, particularly at doses > 42 Gy, and are common in patients treated with chemoradiation, particularly those older than 60 years.27,28
To address whether WBRT improves OS, a large phase 3 study randomized patients to receive MTX, with or without ifosfamide; those with a complete response were then randomized to 45-Gy WBRT or observation.29 The study revealed a significant improvement in PFS with WBRT (18 vs 12 months), but this did not translate to a benefit in OS (32.4 months [WBRT] vs 37.1 months). RTOG 1114 is a randomized phase 2 study of low-dose (23.4-Gy) WBRT for consolidation after MTX-based chemotherapy. Early data suggest an improvement in PFS, but OS has not been reached in either arm. It also remains to be seen whether this regimen is less neurotoxic than higher doses of radiation.30 Based on current data, WBRT is often reserved for salvage therapy.
Increasingly, strategies of intensified chemotherapy have replaced WBRT in upfront treatment of PCNSL. Many regimens include the use of rituximab, a monoclonal antibody targeting the B-cell surface antigen CD20. Rituximab dramatically improves response and clinical outcomes in systemic DLBCL and, therefore, was incorporated into early PCNSL treatment regimens. Although rituximab is a large protein, it can be detected in the CSF after systemic administration in patients with PCNSL,31 particularly when the BBB was disrupted. Retrospective studies demonstrated an added benefit with the addition of rituximab to MTX-based chemotherapy. A meta-analysis identified a PFS, but no OS, benefit.32 The randomized International Extranodal Lymphoma Study Group 32 study showed that the addition of rituximab to MTX/cytarabine improved ORR (73% vs 53%) and median PFS (20 vs 6 months).33 In striking contrast, the randomized HOVON/ALLG phase 3 study did not demonstrate a survival benefit with the addition of rituximab to the combination MTX/teniposide/BCNU/prednisone (MVBP) chemotherapy regimen, followed by cytarabine and WBRT consolidation.34 As a result, the role of rituximab in PCNSL remains undefined. However, given prior evidence and its relatively low side effect profile, many regimens, including in the clinical trial setting, still incorporate its use.
Additional therapies administered in conjunction with MTX are still debated because head-to-head trials are lacking. A recent noncomparative study suggested improvement in ORR and PFS when thiotepa is added to rituximab/MTX/cytarabine (MATRix regimen).33 Evidence also supports the use of MTX in combination with other alkylators, such as procarbazine, temozolomide, or carmustine.
Common induction regimens include rituximab + MTX + vincristine + procarbazine (R-MVP),35 rituximab + MTX + temozolomide,36 MATRix,33 and rituximab + MTX + etoposide + carmustine + prednisone (R-MVBP)34 (Table 2).
Table 2.
Upfront treatment regimens in PCNSL
| Study | Regimen | ORR, % | 2-y PFS, % | Treatment-related deaths, % |
|---|---|---|---|---|
| DeAngelis et al, 200224 | MVP+WBRT+IT MTX | 94 | 50 | 8 |
| Morris et al, 201335 | R-MVP+Ara-C+low-dose WBRT | 95 | 77 | 6 |
| Ferreri et al, 200926 | MA+WBRT | 69 | 38 | 8 |
| Thiel et al, 201029 | MTX/WBRT vs MTX | 53 (in all patients) | 43.5 vs 30.7 | 5 |
| Rubenstein et al, 201336 | MT-R | 66 | 57 | 2 |
| Omuro et al, 201539 | R-MVP + ASCT | 96 | 79 | 9 |
| Omuro et al, 201537 | MT vs MVP | 71 vs 82 | 39 vs 58 | 10 vs 6 |
| Ferreri et al, 201633 | MA vs MARix vs MATRix | 40 vs 51 vs 65 | 36 vs 46 vs 61 | 6 |
| Bromberg et al, 201934 | R-MBVP vs MBVP | 81 vs 75 | 43 vs 37 | 2 vs 3 |
IT, intrathecal; MA, MTX/cytarabine(Ara-C); MARix, MTX/cytarabine/rituximab; MBVP, MTX/carmustine/teniposide/prednisone; MT, MTX/temozolomide; MT-R, rituximab/MTX/temozolomide; MVP, MTX/vincristine/procarbazine; R-MBVP, rituximab/MTX/carmustine/teniposide/prednisone.
Although most of these regimens have not been directly compared, 1 multicenter phase 2 trial randomized elderly patients (age ≥ 60 years) to receive MTX/temozolomide or MTX/vincristine/procarbazine/cytarabine. Toxicity profiles were comparable in both groups. ORR was 71% and 82% respectively; median OS was not significantly different at 14 and 31 months.37
Consolidation strategies vary and are typically tailored to the specific patient based on their age, medical comorbidities, and response of disease to induction. More aggressive high-dose myeloablative chemotherapy (HDC) regimens followed by ASCT(HDC-ASCT) are used in younger (<70 years) patients with few medical comorbidities. Recent phase 2 studies, using MATRix,38 R-MVP,39 or R-MVBP,40 in combination with HDC-ASCT as consolidation, demonstrated high ORR (>90%) and prolonged PFS (>74 months), supporting that HDC-ASCT is a promising consolidative strategy, especially for young otherwise healthy patients with PCNSL. Thiotepa-based conditioning regimens have yielded superior results over BCNU/etoposide/cytarabine/melphalan, a conditioning regimen that is commonly used in systemic lymphoma.38,39,41-43
Alternative consolidation strategies include nonmyeloablative high-dose chemotherapy with agents such as cytarabine with or without etoposide; maintenance therapy with temozolomide, rituximab, or MTX; WBRT (23.4 or 45 Gy); or even observation. Additionally, novel targeted agents like lenalidomide44 or ibrutinib45 have been used in clinical trials as maintenance strategies. Ongoing trials that randomize patients to different consolidation treatments will shed further light on the optimal consolidation regimen.
The role of site-directed therapy for lymphoma involving the leptomeningeal or vitreoretinal spaces remains unknown. In early polychemotherapy trials, intrathecal (IT) or intraventricular chemotherapy was considered an integral treatment component. There was concern that CSF could serve as a sanctuary reservoir for PCNSL cells, contributing to early treatment failure and relapses. In addition, it was thought that longer exposure to cytotoxic concentrations in the CSF could be achieved with IT drug delivery. However, high-dose MTX administered systemically achieves cytotoxic levels in the CSF, and retrospective studies did not demonstrate a benefit for patients with PCNSL who received additional IT chemotherapy. IT drug delivery is also associated with increased adverse events related to the Ommaya reservoir used for administration, as well as drug toxicity, such as leukoencephalopathy.1 Currently, no consensus has been reached regarding the role of IT therapy, and it is increasingly omitted from upfront treatment regimens. However, in situations of persistent lymphomatous leptomeningeal or CSF involvement, IT chemotherapy may be appropriate. Common IT chemotherapy selections include MTX, rituximab, or cytarabine.
High-dose systemic MTX may induce clearance of the vitreoretinal space when it is involved with disease. However, ocular radiation or intraocular chemotherapy should be considered when ocular disease persists after systemic treatment.
Toxicity observed with chemotherapy varies depending on the agents used in the treatment of PCNSL. In general, MTX is well tolerated, even in elderly patients and those with multiple medical comorbidities. It is administered in the inpatient setting with continuous hydration and close monitoring of urine pH to prevent MTX crystallization in the kidney and renal failure. Of note, an impaired renal function, characterized by a creatinine clearance < 30 mL/min, is a contraindication for the use of MTX. Caution should be applied in patients with fluid collections (pleural effusions, ascites), because MTX can accumulate in these fluid spaces, leading to prolonged MTX clearance and an increased risk for adverse events. Although MTX is generally well tolerated, patients can develop nausea, vomiting, diarrhea, and headache. Other MTX-associated adverse events include pneumonitis, rash, mucositis, and myelosuppression. Neurologic toxicity is not common, but stroke-like symptoms and acute or subacute encephalopathy can occur. A delayed multifocal leukoencephalopathy may also be seen in long-term follow up, particularly when MTX is combined with WBRT. The US Food and Drug Administration has approved glucarpidase, a bacterial recombinant enzyme that cleaves MTX to the inactive byproducts glutamate and 4-deoxy-4-amino-N10-methylpteroic acid, for patients with MTX toxicity in the setting of MTX-induced renal failure. To reduce MTX-related toxicity, the empiric use of glucarpidase to aid in rapid MTX clearance and prevent MTX toxicity is currently being investigated (NCT03684980). For patients who are unable to receive treatment with MTX, either because of renal failure or intolerance, agents used in the salvage setting should considered as next-line therapy. Age is not a contraindication to MTX. Treatment with MTX-based regimens is effective and safe among the oldest patients with PCNSL.46 However for patients who are especially frail, one could consider a reduction in the MTX dose, MTX-only treatment, or enrollment into clinical trials using MTX-free regimens built around novel targeted agents, such as single-agent ibrutinib (NCT02623010) or ibrutinib combination therapy (copanlisib/ibrutinib, NCT03581942).
Rituximab is generally well tolerated. It can be associated with infusion reactions, which can be severe. It can also increase the risk of a reactivation of viral hepatitis B. Therefore, hepatitis serologies should be collected prior to treatment initiation and in patients with past exposure; prophylactic antiviral agents need to be used.
Posttreatment monitoring
PCNSL has a high rate of recurrence, particularly in the first 2 years after treatment. Patients who are elderly or frail tend to develop early recurrence, possibly as the result of less aggressive upfront therapy. Although relapses occur usually within the first 2 years after the initial diagnosis, late relapses even after more than 10 years have been described. The National Comprehensive Cancer Network guideline recommends surveillance MRI brain imaging every 3 months during the first 2 years after completion of first-line treatment and then every 6 months thereafter. After 5 years, imaging can be performed annually, but it should continue indefinitely because of the risk of late relapses.1 Serial CSF collection and surveillance spine MRIs are only performed in patients with leptomeningeal and/or spinal disease at initial diagnosis. Patients with ocular involvement need to be assessed with serial ophthalmologic examinations.
Therapy at recurrence/salvage
Despite the efficacy of MTX-based therapy, 10% to 15% of PCNSL cases may prove to be refractory to MTX. In patients whose clinical course was complicated by delays in MTX administration, additional MTX therapy may still be useful. Relapse after initial response to MTX-based therapy is seen up to 50% of the time.47 The median time to relapse is 10 to 18 months, with most relapses occurring in the first 2 years. Prognosis for refractory disease and at relapse is poor, with an estimated OS of 2 months for patients with refractory disease and 3.7 months for those with relapsed disease.48 At the time of relapse, restaging should be performed and should include imaging of the neuroaxis (brain and spine), lumbar puncture, and another ophthalmologic examination. A whole-body PET scan should also be repeated, because, although rare, CNS lymphoma may relapse systemically.47
There is no single established approach to the treatment of patients with relapsed/refractory disease. Choice of treatment typically depends on patient age, performance status, response to prior therapy, and medical comorbidities. Rechallenge with MTX is reasonable, particularly for those with initial durable response following first-line MTX-based treatment. Retrospective studies have reported ORRs of 85% to 91% in this setting.49,50 Prospective trials of other agents, such as pemetrexed, temozolomide, topotecan, and rituximab, have demonstrated ORRs between 31% and 55% with a PFS of 1.6 to 5.7 months51 (Table 3). In patients who are radiation naive, WBRT remains an effective strategy for palliation and short-term control, although neurocognitive impairment remains a concern in patients with durable response.
Table 3.
Salvage regimens in PCNSL based on prospective trials
| Study | Agents | Patients, n | ORR* (%) | Median PFS, mo | Median OS, mo |
|---|---|---|---|---|---|
| Fischer et al, 200660 | Topotecan | 27 | 9/27 (33) | 2 | 8.4 |
| Reni et al, 200761 | Temozolomide | 36 | 11/36 (31) | 2.8 | 3.9 |
| Soussain et al, 200852 | CYVE+ASCT | 43 | 20/40 (50) | 11.6 | 18.3 |
| Batchelor et al, 201162 | Rituximab | 12 | 5/12 (42) | 1.9 (57 d) | 20.9 |
| Raizer et al, 201263 | Pemetrexed | 11 | 6/11 (55) | 5.7 | 10.1 |
| Rubenstein et al, 201336 | IT rituximab+IT MTX | 14 | 6/14 (43) | 1.2 | NR |
| Nayak et al, 201364 | Rituximab+TMZ+pred | 16 | 5/14 (36) | 1.6 (7 wk) | NR |
| Korfel et al, 201655 | Temsirolimus | 37 | 20/37 (54) | 2.1 | 3.7 |
| Grommes et al, 201711 | Ibrutinib | 13 | 10/13 (77) | 4.6 | 15 |
| Grommes et al, 201945 | Ibrutinib/MTX/rituximab | 15 | 8/15 (80) | 9.2 | NR |
| Soussain et al, 201956 | Ibrutinib | 52 | 27/52 (52) | 4.8 | 19.2 |
| Ghesquieres et al, 201944 | Lenalidomide/rituximab | 45 | 28/45 (62) | 7.8 | 17.7 |
| Tun et al, 201858 | Pomalidomide | 25 | 12/25 (48) | 5.3 | NR |
| Rubenstein et al, 201859,60 | Lenalidomide | 13 | 8/13 (62) | NR | NR |
CYVE, cytarabine + etoposide; NR, not reached; pred, methylprednisone; TMZ, temozolomide.
Partial response/complete response.
In younger patients, HDC-ASCT remains an option at relapse. A prospective multicenter trial of high-dose etoposide/cytarabine, followed by HDC-ASCT, demonstrated a median PFS of 11.6 months and a 2-year OS of 45%.52
Multiple new treatment strategies have been tested in the relapsed/refractory setting, including immunotherapy. Two recent case series of PD-1 antibody therapy in patients with relapsed/refractory PCNSL suggested a response. In 1 case series, 4 patients with PCNSL received the anti–PD-1 antibody nivolumab, and all demonstrated an objective radiographic response. PFS for each was >13 months, and all were alive at a median follow-up of 17 months.53 A second retrospective study reported on 6 patients with CNS lymphoma, including 3 with PCNSL, who were treated with anti–PD-1 therapy in combination with rituximab. In that series, 3 patients achieved complete response, including 2 of the patients with PCNSL.54 Prospective studies exploring the role of anti–PD-1 antibody as monotherapy and in combination with targeted or immunomodulatory drugs (IMiDs) are ongoing.
A better understanding of the pathophysiology of PCNSL has led to the development and exploitation of novel targeted therapies. The first targeted agent investigated was temsirolimus, a mammalian target of rapamycin inhibitor that induced high response rates (54%) that, unfortunately, were not durable (PFS, 2.1 months), suggesting rapid development of drug resistance.55
More promising, ibrutinib, an inhibitor of Bruton tyrosine kinase, a member of the BCR pathway, has been shown to induce significant responses when used alone11,56 (560 mg, ORR = 52%; 840 mg, ORR = 77%) or in combination with methotrexate/rituximab45 (ORR = 80%) in recurrent PCNSL. A phase 1 trial incorporated ibrutinib with temozolomide, etoposide, liposomal doxorubicin, dexamethasone, and rituximab. Patients were first treated with ibrutinib alone, prior to the addition of the remainder of the temozolomide, etoposide, liposomal doxorubicin, dexamethasone, and rituximab regimen. Imaging demonstrated radiographic reduction in tumor size in 94% of patients after only 2 weeks of monotherapy.57 Unfortunately, frequent serious and sometimes fatal complications of pulmonary or cerebral aspergillosis were seen in this trial, which is ongoing to better define treatment-associated toxicities. Therefore, caution should be used when ibrutinib is prescribed for patients with PCNSL, particularly in the setting of immunosuppression from chronic steroid use, prior chemotherapy, or prior transplant. Ibrutinib is under investigation in combination with other agents in the recurrent setting (rituximab/ibrutinib/lenalidomide [NCT03703167], ibrutinib/copanlisib [NCT03581942], ibrutinib/pembrolizumab/rituximab [NCT04421560]), as well as in the first-line treatment in combination with R-MVP (NCT02315326) or as single-agent maintenance for elderly patients with PCNSL (NCT02623010). A randomized French study will evaluate response to R-MVP/ibrutinib in comparison with R-MVP/lenalidomide (NCT04446962). Moreover, second-generation Bruton tyrosine kinase inhibitors are now also being investigated in the recurrent setting (tirabrutinib [NCT04947319], acalabrutinib alone [NCT04548648] or in combination with rituximab and durvalumab [NCT04688151]).
Another promising class of therapeutic agents are IMiDs. The IMiDs lenalidomide and pomalidomide have been used in patients with relapsed/refractory PCNSL, alone or in combination with rituximab. ORR was 43% with pomalidomide,58 62% with single-agent lenalidomide,59 and 63% with lenalidomide + rituximab.44 Lenalidomide is under investigation in combination with MTX and the checkpoint inhibitor nivolumab in the first-line setting (NCT04609046). Based on these results, the updated National Comprehensive Cancer Network guidelines for the treatment of relapsed/refractory PCNSL now include the use of single-agent ibrutinib, single-agent lenalidomide, and the combination of lenalidomide and rituximab.
Overall, these novel agents seem to induce frequent and significant radiographic responses. Unfortunately, responses are often not durable, suggesting early development of resistance. Combination therapy may be 1 way to improve outcomes, and trials combining novel targeted treatments with cytotoxic agents, immunotherapy, or other targeted treatments are ongoing.
Conclusions
There have been significant advances in the management of PCNSL, but relapses remain frequent and difficult to treat. MTX-based polychemotherapy is widely considered the standard of care in the upfront setting, but optimal treatment in the salvage setting has not been defined. New pathophysiologic insights into PCNSL have led to the recent introduction and more common use of novel targeted agents in the salvage setting. These agents are increasingly adopted from the clinical trial setting into routine clinical practice and are beginning to be studied in newly diagnosed patients. In the next few years, we hope to see the integration of these novel agents into first-line treatment regimens in the anticipation that they improve the frequency and duration of response and offer additional treatment options for patients at increased risk for a poor clinical outcome.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30-CA008748, as well as by grants from Cycle for Survival Equinox and the Leukemia and Lymphoma Society.
Contribution: L.R.S. and C.G. designed research, performed research, contributed vital new reagents or analytical tools, collected data, analyzed and interpreted data, performed statistical analysis and wrote the manuscript.
Conflict-of-interest disclosure: L.S. has received research support from BTG and has acted as a consultant for DebioPharm. C.G. has received research support from Pharmacyclics, Bayer, and Bristol Myers Squibb and has acted as a consultant for Kite, BTG, and ONO.
Correspondence: Christian Grommes, Department of Neurology, Memorial Sloan Kettering Cancer Center, 1275 York Ave, Box 71, New York, NY 10065; e-mail: grommesc@mskcc.org.
REFERENCES
- 1.Grommes C, DeAngelis LM. Primary CNS lymphoma. J Clin Oncol. 2017;35(21):2410-2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grommes C. Central nervous system lymphomas. Continuum (Minneap Minn). 2020;26(6):1476-1494. [DOI] [PubMed] [Google Scholar]
- 3.Mendez JS, Ostrom QT, Gittleman H, et al. The elderly left behind-changes in survival trends of primary central nervous system lymphoma over the past 4 decades. Neuro-oncol. 2018;20(5):687-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiels MS, Pfeiffer RM, Besson C, et al. Trends in primary central nervous system lymphoma incidence and survival in the U.S. Br J Haematol. 2016;174(3):417-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villano JL, Koshy M, Shaikh H, Dolecek TA, McCarthy BJ. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer. 2011;105(9):1414-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bataille B, Delwail V, Menet E, et al. Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg. 2000;92(2):261-266. [DOI] [PubMed] [Google Scholar]
- 7.Grimm SA, Pulido JS, Jahnke K, et al. Primary intraocular lymphoma: an International Primary Central Nervous System Lymphoma Collaborative Group Report. Ann Oncol. 2007;18(11):1851-1855. [DOI] [PubMed] [Google Scholar]
- 8.Krebs S, Barasch JG, Young RJ, Grommes C, Schöder H. Positron emission tomography and magnetic resonance imaging in primary central nervous system lymphoma-a narrative review. Ann Lymphoma. 2021;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rae AI, Iwamoto FM, Sonabend AM. In reply: craniotomy and survival for primary central nervous system lymphoma. Neurosurgery. 2018;83(4):E192. [DOI] [PubMed] [Google Scholar]
- 10.Camilleri-Broët S, Martin A, Moreau A, et al. Primary central nervous system lymphomas in 72 immunocompetent patients: pathologic findings and clinical correlations. Groupe Ouest Est d’étude des Leucénies et Autres Maladies du Sang (GOELAMS). Am J Clin Pathol. 1998;110(5):607-612. [DOI] [PubMed] [Google Scholar]
- 11.Grommes C, Pastore A, Palaskas N, et al. Ibrutinib unmasks critical role of Bruton tyrosine kinase in primary CNS lymphoma. Cancer Discov. 2017;7(9):1018-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasqualucci L, Dalla-Favera R. The genetic landscape of diffuse large B-cell lymphoma. Semin Hematol. 2015;52(2):67-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nayyar N, White MD, Gill CM, et al. MYD88 L265P mutation and CDKN2A loss are early mutational events in primary central nervous system diffuse large B-cell lymphomas. Blood Adv. 2019;3(3):375-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chapuy B, Roemer MG, Stewart C, et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood. 2016;127(7):869-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chihara D, Fowler NH, Oki Y, et al. Impact of histologic subtypes and treatment modality among patients with primary central nervous system lymphoma: a SEER database analysis. Oncotarget. 2018;9(48):28897-28902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malani R, Bhatia A, Wolfe J, Grommes C. Staging identifies non-CNS malignancies in a large cohort with newly diagnosed lymphomatous brain lesions. Leuk Lymphoma. 2019;60(9):2278-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abrey LE, Batchelor TT, Ferreri AJ, et al. ; International Primary CNS Lymphoma Collaborative Group . Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23(22):5034-5043. [DOI] [PubMed] [Google Scholar]
- 18.Abrey LE, Ben-Porat L, Panageas KS, et al. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol. 2006;24(36):5711-5715. [DOI] [PubMed] [Google Scholar]
- 19.Ferreri AJ, Blay JY, Reni M, et al. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21(2):266-272. [DOI] [PubMed] [Google Scholar]
- 20.Nelson DF, Martz KL, Bonner H, et al. Non-Hodgkin’s lymphoma of the brain: can high dose, large volume radiation therapy improve survival? Report on a prospective trial by the Radiation Therapy Oncology Group (RTOG): RTOG 8315. Int J Radiat Oncol Biol Phys. 1992;23(1):9-17. [DOI] [PubMed] [Google Scholar]
- 21.Shibamoto Y, Ogino H, Hasegawa M, et al. Results of radiation monotherapy for primary central nervous system lymphoma in the 1990s. Int J Radiat Oncol Biol Phys. 2005;62(3):809-813. [DOI] [PubMed] [Google Scholar]
- 22.Schultz C, Scott C, Sherman W, et al. Preirradiation chemotherapy with cyclophosphamide, doxorubicin, vincristine, and dexamethasone for primary CNS lymphomas: initial report of radiation therapy oncology group protocol 88-06. J Clin Oncol. 1996;14(2):556-564. [DOI] [PubMed] [Google Scholar]
- 23.O’Neill BP, Wang CH, O’Fallon JR, et al. Primary central nervous system non-Hodgkin’s lymphoma (PCNSL): survival advantages with combined initial therapy? A final report of the North Central Cancer Treatment Group (NCCTG) Study 86-72-52. Int J Radiat Oncol Biol Phys. 1999;43(3):559-563. [DOI] [PubMed] [Google Scholar]
- 24.DeAngelis LM, Yahalom J, Thaler HT, Kher U. Combined modality therapy for primary CNS lymphoma. J Clin Oncol. 1992;10(4):635-643. [DOI] [PubMed] [Google Scholar]
- 25.Glass J, Gruber ML, Cher L, Hochberg FH. Preirradiation methotrexate chemotherapy of primary central nervous system lymphoma: long-term outcome. J Neurosurg. 1994;81(2):188-195. [DOI] [PubMed] [Google Scholar]
- 26.Ferreri AJ, Reni M, Foppoli M, et al. ; International Extranodal Lymphoma Study Group (IELSG) . High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet. 2009;374(9700):1512-1520. [DOI] [PubMed] [Google Scholar]
- 27.Gavrilovic IT, Hormigo A, Yahalom J, DeAngelis LM, Abrey LE. Long-term follow-up of high-dose methotrexate-based therapy with and without whole brain irradiation for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2006;24(28):4570-4574. [DOI] [PubMed] [Google Scholar]
- 28.Doolittle ND, Korfel A, Lubow MA, et al. Long-term cognitive function, neuroimaging, and quality of life in primary CNS lymphoma. Neurology. 2013;81(1):84-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thiel E, Korfel A, Martus P, et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol. 2010;11(11):1036-1047. [DOI] [PubMed] [Google Scholar]
- 30.Omuro AMP, DeAngelis LM, Karrison T, et al. Randomized phase II study of rituximab, methotrexate (MTX), procarbazine, vincristine, and cytarabine (R-MPV-A) with and without low-dose whole-brain radiotherapy (LD-WBRT) for newly diagnosed primary CNS lymphoma (PCNSL). J Clin Oncol. 2020;38(15 suppl):2501 [Google Scholar]
- 31.Ruhstaller TW, Amsler U, Cerny T. Rituximab: active treatment of central nervous system involvement by non-Hodgkin’s lymphoma? Ann Oncol. 2000;11(3):374-375. [DOI] [PubMed] [Google Scholar]
- 32.Schmitt AM, Herbrand AK, Fox CP, et al. Rituximab in primary central nervous system lymphoma - a systematic review and meta-analysis. Hematol Oncol. 2019;37(5):548-557. [DOI] [PubMed] [Google Scholar]
- 33.Ferreri AJ, Cwynarski K, Pulczynski E, et al. ; International Extranodal Lymphoma Study Group (IELSG) . Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. 2016;3(5):e217-e227. [DOI] [PubMed] [Google Scholar]
- 34.Bromberg JEC, Issa S, Bakunina K, et al. Rituximab in patients with primary CNS lymphoma (HOVON 105/ALLG NHL 24): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2019;20(2):216-228. [DOI] [PubMed] [Google Scholar]
- 35.Morris PG, Correa DD, Yahalom J, et al. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol. 2013;31(31):3971-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubenstein JL, Hsi ED, Johnson JL, et al. Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol. 2013;31(25):3061-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Omuro A, Chinot O, Taillandier L, et al. Methotrexate and temozolomide versus methotrexate, procarbazine, vincristine, and cytarabine for primary CNS lymphoma in an elderly population: an intergroup ANOCEF-GOELAMS randomised phase 2 trial. Lancet Haematol. 2015;2(6):e251-e259. [DOI] [PubMed] [Google Scholar]
- 38.Illerhaus G, Kasenda B, Ihorst G, et al. High-dose chemotherapy with autologous haemopoietic stem cell transplantation for newly diagnosed primary CNS lymphoma: a prospective, single-arm, phase 2 trial. Lancet Haematol. 2016;3(8):e388-e397. [DOI] [PubMed] [Google Scholar]
- 39.Omuro A, Correa DD, DeAngelis LM, et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood. 2015;125(9):1403-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Houillier C, Taillandier L, Dureau S, et al. ; Intergroupe GOELAMS–ANOCEF and the LOC Network for CNS Lymphoma . Radiotherapy or autologous stem-cell transplantation for primary CNS lymphoma in patients 60 years of age and younger: results of the Intergroup ANOCEF-GOELAMS Randomized Phase II PRECIS Study. J Clin Oncol. 2019;37(10):823-833. [DOI] [PubMed] [Google Scholar]
- 41.Colombat P, Lemevel A, Bertrand P, et al. High-dose chemotherapy with autologous stem cell transplantation as first-line therapy for primary CNS lymphoma in patients younger than 60 years: a multicenter phase II study of the GOELAMS group. Bone Marrow Transplant. 2006;38(6):417-420. [DOI] [PubMed] [Google Scholar]
- 42.Montemurro M, Kiefer T, Schüler F, et al. Primary central nervous system lymphoma treated with high-dose methotrexate, high-dose busulfan/thiotepa, autologous stem-cell transplantation and response-adapted whole-brain radiotherapy: results of the multicenter Ostdeutsche Studiengruppe Hamato-Onkologie OSHO-53 phase II study. Ann Oncol. 2007;18(4):665-671. [DOI] [PubMed] [Google Scholar]
- 43.Abrey LE, Moskowitz CH, Mason WP, et al. Intensive methotrexate and cytarabine followed by high-dose chemotherapy with autologous stem-cell rescue in patients with newly diagnosed primary CNS lymphoma: an intent-to-treat analysis. J Clin Oncol. 2003;21(22):4151-4156. [DOI] [PubMed] [Google Scholar]
- 44.Ghesquieres H, Chevrier M, Laadhari M, et al. Lenalidomide in combination with intravenous rituximab (REVRI) in relapsed/refractory primary CNS lymphoma or primary intraocular lymphoma: a multicenter prospective ‘proof of concept’ phase II study of the French Oculo-Cerebral lymphoma (LOC) Network and the Lymphoma Study Association (LYSA). Ann Oncol. 2019;30(4):621-628. [DOI] [PubMed] [Google Scholar]
- 45.Grommes C, Tang SS, Wolfe J, et al. Phase 1b trial of an ibrutinib-based combination therapy in recurrent/refractory CNS lymphoma. Blood. 2019;133(5):436-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welch MR, Omuro A, Deangelis LM. Outcomes of the oldest patients with primary CNS lymphoma treated at Memorial Sloan-Kettering Cancer Center. Neuro-oncol. 2012;14(10):1304-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jahnke K, Thiel E, Martus P, et al. ; German Primary Central Nervous System Lymphoma Study Group . Relapse of primary central nervous system lymphoma: clinical features, outcome and prognostic factors. J Neurooncol. 2006;80(2):159-165. [DOI] [PubMed] [Google Scholar]
- 48.Langner-Lemercier S, Houillier C, Soussain C, et al. Primary CNS lymphoma at first relapse/progression: characteristics, management, and outcome of 256 patients from the French LOC network. Neuro-oncol. 2016;18(9):1297-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plotkin SR, Betensky RA, Hochberg FH, et al. Treatment of relapsed central nervous system lymphoma with high-dose methotrexate. Clin Cancer Res. 2004;10(17):5643-5646. [DOI] [PubMed] [Google Scholar]
- 50.Pentsova E, Deangelis LM, Omuro A. Methotrexate re-challenge for recurrent primary central nervous system lymphoma. J Neurooncol. 2014;117(1):161-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grommes C, Nayak L, Tun HW, Batchelor TT. Introduction of novel agents in the treatment of primary CNS lymphoma. Neuro-oncol. 2019;21(3):306-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soussain C, Hoang-Xuan K, Taillandier L, et al. ; Société Française de Greffe de Moëlle Osseuse-Thérapie Cellulaire . Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Société Française de Greffe de Moëlle Osseuse-Thérapie Cellulaire. J Clin Oncol. 2008;26(15):2512-2518. [DOI] [PubMed] [Google Scholar]
- 53.Nayak L, Iwamoto FM, LaCasce A, et al. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood. 2017;129(23):3071-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ambady P, Szidonya L, Firkins J, et al. Combination immunotherapy as a non-chemotherapy alternative for refractory or recurrent CNS lymphoma. Leuk Lymphoma. 2019;60(2):515-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Korfel A, Schlegel U, Herrlinger U, et al. Phase II trial of temsirolimus for relapsed/refractory primary CNS lymphoma. J Clin Oncol. 2016;34(15):1757-1763. [DOI] [PubMed] [Google Scholar]
- 56.Soussain C, Choquet S, Blonski M, et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: final analysis of the phase II ‘proof-of-concept’ iLOC study by the Lymphoma Study Association (LYSA) and the French Oculo-Cerebral Lymphoma (LOC) network. Eur J Cancer. 2019;117:121-130. [DOI] [PubMed] [Google Scholar]
- 57.Lionakis MS, Dunleavy K, Roschewski M, et al. Inhibition of B cell receptor signaling by ibrutinib in primary CNS lymphoma. Cancer Cell. 2017;31(6):833-843.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tun HW, Johnston PB, DeAngelis LM, et al. Phase 1 study of pomalidomide and dexamethasone for relapsed/refractory primary CNS or vitreoretinal lymphoma. Blood. 2018;132(21):2240-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rubenstein JL, Geng H, Fraser EJ, et al. Phase 1 investigation of lenalidomide/rituximab plus outcomes of lenalidomide maintenance in relapsed CNS lymphoma. Blood Adv. 2018;2(13):1595-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fischer L, Thiel E, Klasen H-A, et al. Prospective trial on topotecan salvage therapy in primary CNS lymphoma. Ann Oncol. 2006;17(7):1141–1145. [DOI] [PubMed] [Google Scholar]
- 61.Reni M, Zaja F, Mason W, et al. Temozolomide as salvage treatment in primary brain lymphomas. Br J Cancer. 2007;96(6):864–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Batchelor TT, Grossman SA, Mikkelsen T, Ye X, Desideri S, Lesser GJ. Rituximab monotherapy for patients with recurrent primary CNS lymphoma. Neurology. 2011;76(10):929–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raizer JJ, Rademaker A, Evens AM, et al. Pemetrexed in the treatment of relapsed/refractory primary central nervous system lymphoma. Cancer. 2012;118(15):3743–3748. [DOI] [PubMed] [Google Scholar]
- 64.Nayak L, Abrey LE, Drappatz J, et al. Multicenter phase II study of rituximab and temozolomide in recurrent primary central nervous system lymphoma. Leuk Lymphoma. 2013;54(1):58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.