Abstract
Mind wandering is a state in which our mental focus shifts toward task-unrelated thoughts. Although it is known that mind wandering has a detrimental effect on concurrent task performance (e.g., decreased accuracy), its effect on executive functions is poorly studied. Yet the latter question is relevant to many real-world situations, such as rapid stopping during driving. Here, we studied how mind wandering would affect the requirement to subsequently stop an incipient motor response. In healthy adults, we tested whether mind wandering affected stopping and, if so, which component of stopping was affected: the triggering of the inhibitory brake or the implementation of the brake following triggering. We observed that during mind wandering, stopping latency increased, as did the percentage of trials with failed triggering. Indeed, 67% of the variance of the increase in stopping latency was explained by increased trigger failures. Thus, mind wandering primarily affects stopping by affecting the triggering of the brake.
Keywords: response inhibition, mind wandering, cognitive processes, trigger failures, stop-signal reaction time, open data, preregistered
As we go about our jobs and responsibilities in our daily lives, our minds are not always focused on the task at hand. Sometimes they wander to task-unrelated thoughts (i.e., mind wandering), such as what to cook for dinner or worries about health and finances, and sometimes our minds may be blank and unfocused (i.e., off focus; Mittner et al., 2016). Although research is still required to determine the differences between mind wandering and being off focus, both these mental states reflect general task disengagement, and brain activity during these periods is different compared with on-task periods (for a review, see Christoff et al., 2016; Mittner et al., 2016). Mind wandering has been well studied in recent years and is thought to occur in up to half of our waking hours (Killingsworth & Gilbert, 2010). Unsurprisingly, studies have found that mind wandering is associated with poorer sustained attention on concurrent tasks, resulting in worse performance (e.g., increased variability in reaction times [RTs] and decreased accuracy; Arnau et al., 2020; Bastian & Sackur, 2013; Compton et al., 2019; Mittner et al., 2014). However, the effect of mind wandering might be quite consequential if during the mind-wandering episode there is a subsequent need for rapid executive control (i.e., the high-level processes that regulate other cognitive subprocesses and behavior; Miyake et al., 2000). Many daily activities, such as driving and handling machinery, require active engagement of executive control, especially response inhibition (i.e., the cognitive process that controls rapid behavioral action cancellation). For example, when one is driving and the traffic light suddenly turns red, one must quickly stop pressing the accelerator and switch to pressing the brake instead. Indeed, how mind wandering affects driving has been investigated in numerous studies (Albert et al., 2018; Baldwin et al., 2017; Galéra et al., 2012; Lin et al., 2016; Yanko & Spalek, 2014). How does mind wandering affect the executive requirement to rapidly stop an incipient action? In the present study, we specifically examined this question.
Although the literature has often suggested that mind wandering has a detrimental effect on response inhibition (Mooneyham & Schooler, 2013; Smallwood et al., 2007, 2008), the evidence supporting this notion is weak. Using the stop-signal task, a task that provides an unequivocal assessment of response inhibition (Bari & Robbins, 2013; Matzke et al., 2018; Verbruggen et al., 2019), one study found that response inhibition is unaffected by mind wandering (Mittner et al., 2014). On the other hand, several mind-wandering studies have used the go/no-go task (sometimes called the sustained-attention-to-response task) to demonstrate that no-go errors increase during mind wandering, which may reflect the failure to inhibit the prepotent response (Christoff et al., 2009; Groot et al., 2021; Smallwood et al., 2007, 2008; Stawarczyk et al., 2014). However, it has been suggested that most kinds of go/no-go tasks require action restraint (i.e., choosing to not “go” in the first place) rather than action cancellation (i.e., stopping an incipient action; Bari & Robbins, 2013; Eagle et al., 2008; Raud et al., 2020; Wessel, 2018). Go/no-go tasks can be made more likely to tax response inhibition by incorporating some modifications (e.g., having fast-paced trials, limiting the trial duration to < 1,500 ms, decreasing the probability of no-go trials to ~20%, and defining a response deadline to induce the urgency to respond; Wessel, 2018). Such changes increase prepotent motor activity: Participants try to initiate a response in every trial and then try to cancel it if the no-go cue is presented instead of waiting and then deciding whether or not to respond.
However, only one of the aforementioned mind-wandering studies using the go/no-go task satisfied the criteria of fast-paced trials and low no-go probability (Groot et al., 2021; there is no report of a response deadline having been used). Even then, when the task is well set up, it is debatable whether the go/no-go task assesses response inhibition: Prepotent motor activity is far lower in this task than in the stop-signal task (Raud et al., 2020). Indeed, numerous behavioral, neural, and pharmacological studies also support the notion that the go/no-go task requires action restraint rather than action cancellation (Bari & Robbins, 2013; Eagle et al., 2008; Krämer et al., 2013; Littman & Takács, 2017; Raud et al., 2020; Sebastian et al., 2012; Swick et al., 2011).
Thus, taken together, it is unclear whether these mind-wandering studies that used the go/no-go task actually tested the effect of mind wandering on response inhibition. Further, other tasks also do not clarify whether mind wandering affects response inhibition. For Stroop tasks, two studies found no effect of mind wandering on incongruent trials (Compton et al., 2019; Thomson et al., 2013), whereas one did (Kam & Handy, 2014). For switching tasks, three studies found no effect of mind wandering on the switch trials (Arnau et al., 2020; Kam & Handy, 2014; Thomson et al., 2014). And for flanker tasks, two studies found no effect of mind wandering on incongruent trials (Gonçalves et al., 2018; Thomson et al., 2014). Thus, taking these findings together, it remains unclear whether mind wandering affects response inhibition. Other factors might further explain such mixed results: Many of these studies were not specifically set up to test whether mind wandering affects response inhibition, many did not perform within-subjects comparisons of mind-wandering and on-task episodes, and many did not exclude participants who reported no or few mind-wandering episodes.
Statement of Relevance.
The ability to quickly control oneself is a key feature of everyday life. But how is that ability affected when our minds wander to task-unrelated thoughts? This has been poorly studied, and yet it has implications for real-world situations such as driving or handling machinery, which often demand rapid control. Using a task that measured the ability of people to rapidly control themselves, in this case to stop an action, we observed that participants were poorer at stopping during bouts of mind wandering compared with when they were focused. We further showed that this deficiency was largely due to the impact of mind wandering on the triggering of the stopping process rather than the way the stop was implemented, which suggests that mind wandering predominantly affects the neural circuitry that triggers stopping. These findings can inform future research and the development of new technologies that perform real-time detection of mind wandering in real-world situations to increase safety and productivity.
Learning from these issues, we designed our study with three key elements. First, we used a stop-signal task that gives an unequivocal measure of response inhibition (Bari & Robbins, 2013; Matzke et al., 2018; Verbruggen et al., 2019). Second, for each participant, we selected trials that require response inhibition during self-reported episodes of mind wandering and on-task behavior. We then performed within-subject comparisons of these two episodes. Third, we excluded participants from analysis who did not contribute enough data to the mind-wandering condition (i.e., had no or few mind-wandering episodes).
In the stop-signal task, participants are cued to make a response in every trial; however, in a minority of trials when a stop signal is presented, they must try to “brake” and stop the incipient response. Behaviorally, the stopping latency is measured as stop-signal RT (SSRT), and this is thought to indicate the time when the inhibitory brake is applied to stop the incipient response. As mentioned above, a previous mind-wandering study used this task and also had a within-subject design, but the results of that study are difficult to interpret because the authors did not report the typical SSRT metric (Mittner et al., 2014).
Using the stop-signal task instead of the other response-inhibition tasks mentioned above has two advantages. First, it taps into extensive research that has used this task to map out a prefrontal-basal-ganglia network that is critical for action cancellation (for a review, see Aron et al., 2016; Bari & Robbins, 2013; see also Jana et al., 2020). Second, this task provides a way to separate the response-inhibition process into two distinct stages, trigger and brake, and then investigate which stage is affected by mind wandering. It has been argued that successful action cancellation depends not only on a quick implementation of the brake to stop the response but also on how reliably the brake is triggered (Band et al., 2003; Matzke, Hughes, et al., 2017; Schachar & Logan, 1990; Sebastian et al., 2018). In other words, the application of the brake depends on how consistently it is triggered; if triggering is slow or inconsistent, then so will be the braking. This will result in poorer stopping, as measured by SSRT.
Triggering has recently been operationalized in a modeling framework for behavioral data by Matzke, Love, and Heathcote (2017). This model estimates a parameter called trigger failures—the percentage of trials in which there was a failure to trigger the brake. Subsequently, studies have demonstrated that the percentage of trigger failures is correlated with real-world measures of impulsivity (Skippen et al., 2019) and that some clinical populations who have response-inhibition deficits also have greater percentage of trigger failures (Matzke, Hughes, et al., 2017; Swick & Ashley, 2020), suggesting that the percentage of trigger failures indexes a core component of response inhibition. However, trigger failures have not yet been systematically investigated, and it may seem puzzling why the salient stop signal would not trigger the brake in some trials. One possibility is that trigger failures reflect periods of goal neglect or attentional failure in which the stop signal is processed by the sensory system but this encoded signal is not utilized to select the appropriate action (i.e., initiate the brake; Matzke, Hughes, et al., 2017; Skippen et al., 2019). Given that mind wandering is a mental state associated with attentional lapses (Mooneyham & Schooler, 2013; Smallwood & Schooler, 2015), an auxiliary aim in our study was to validate the attentional account of trigger failure by testing whether mind-wandering episodes are associated with a greater percentage of trigger failures.
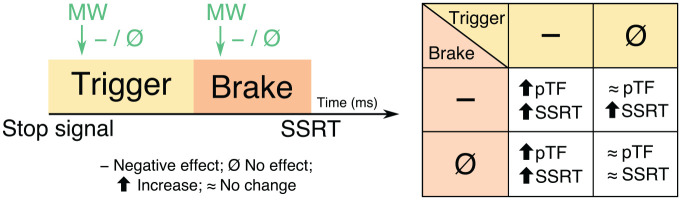
In two studies, participants performed the stop-signal task while they were intermittently probed to report their mental state. On the basis of the probe responses, we classified trials just prior to the probe as either on-task or mind-wandering episodes. We then performed within-subject comparisons of the stopping performance between on-task and mind-wandering episodes. We envisioned four possible modes in which mind wandering might affect stopping. First, because mind wandering often has a detrimental effect on task performance (Mooneyham & Schooler, 2013), mind wandering could negatively affect both trigger and brake (this will be referred to below as “Mode 1”). This would result in a greater percentage of trigger failures and make braking slower and/or more variable, (i.e., increase the mean and/or standard deviation of the SSRT distribution). Second, given that attention is directed away from the task during mind wandering, mind wandering could negatively affect the trigger without affecting the brake (this will be referred to below as “Mode 2”). This would result in a greater percentage of trigger failures, but would it change SSRT? We reasoned that because SSRT is the total time taken to stop the response, it includes both the trigger and brake stages, so any effect on the trigger should be reflected in SSRT. Hence, we hypothesized that even if mind wandering affects just the trigger, it would increase both the percentage of trigger failures and SSRT (this is the same outcome as in Mode 1; we will discuss how to distinguish these below). Third, mind wandering could specifically affect the brake without affecting the trigger, resulting in increased mean and standard deviation of SSRT but no change in the percentage of trigger failures. Last, mind wandering might not affect either the trigger or the brake, which would result in no change in the percentage of trigger failures or SSRT (see Fig. 1 for all hypothesized outcomes).
Fig. 1.
Hypothesized effects of mind wandering (MW) on the trigger and brake stages and how that might change the behavioral metrics: percentage of trigger failures (pTFs) and stop-signal reaction time (SSRT). We hypothesized that response inhibition proceeds in two stages: trigger and brake (as shown on the left). SSRT is the total time taken to stop the response and includes both the trigger and brake stages. Mind wandering can have either no effect or a negative effect on either of these stages. The possible changes in the behavioral metrics if mind wandering has no or a negative effect on the trigger and/or brake stage are shown on the right. First, if mind wandering negatively affects both the trigger and brake, then there will be an increase in the percentage of trigger failures and an increase in the mean and/or standard deviation of the SSRT distribution. Second, if mind wandering negatively affects the trigger without affecting the brake, then there will also be an increase in both the percentage of trigger failures and SSRT because SSRT encompasses both the trigger and brake stages. Third, if mind wandering affects the brake alone, there will be an increase in the mean and standard deviation of SSRT, but there will be no change in the percentage of trigger failures. Last, if mind wandering does not affect either the trigger or brake stages, then there will be no change in the percentage of trigger failures or SSRT.
Now note, as above, that for Modes 1 and 2, the behavioral outcome of mind wandering affecting both the trigger and brake (vs. affecting just the trigger) is the same: increased percentage of trigger failures and SSRT. To distinguish between these two modes, we quantified how much the change in the percentage of trigger failures relates to the change in SSRT. As suggested by Bissett et al. (2021), we considered that triggering is graded (i.e., worse and slower in some trials) and not absolute (i.e., the brake is triggered or not triggered). On this interpretation, the percentage of trigger failures reflects the weakly triggered trials (i.e., longer trigger stage) and captures some aspect of the duration of the trigger stage. Hence, an increased percentage of trigger failures might relate to a longer and more variable trigger stage in some trials. This change in the trigger stage would in turn be reflected in SSRT as an increased mean or standard deviation of the SSRT distribution. We reasoned that if mind wandering affects both the trigger and brake (i.e., Mode 1), then only a minority of the variance of the increase in the mean or standard deviation of SSRT should be explained by the increase in the percentage of trigger failures. On the other hand, if mind wandering affects only the trigger (i.e., Mode 2), then a majority of the variance of the increase in the mean or standard deviation of SSRT should be explained by the increase in the percentage of trigger failures. Thus, this investigation will inform whether and how mind wandering affects response inhibition: by directly affecting the braking mechanism, by affecting the triggering mechanism, or both. Further, at the end of the task, participants completed the Mind-Wandering Questionnaire (Mrazek et al., 2013). This allowed us to test whether trait-level mind wandering was related to state-level mind wandering, as demonstrated by Mrazek et al. (2013), and thus whether probe responses could be trusted.
Method
Participants
Participants were healthy adults recruited using Prolific, an online recruitment platform. They were monetarily compensated for their time. The study was covered by a protocol from the institutional review board of the University of California San Diego.
Study 1
Thirty-two participants took part in Study 1. Two participants were rejected because of poor behavior (one subject failed to stop in all trials, and another had a bimodal go-trial RT distribution). Thus, 30 participants were included in analyses (six female; 24 right-handed, five left-handed, one did not report handedness; age: M = 34 years, SEM = 2, minimum = 18, maximum = 64). Eleven of 30 participants reported a sufficient number of mind-wandering episodes (see below).
Study 2
On the basis of the results of Study 1, we used the software G*Power (Version 3.1.9.3; Faul et al., 2007) to calculate the sample size. Assuming a power of 90% and an α of .05, the required number of participants was 31 and 18, respectively. 1 As noted below, we anticipated that many participants would report no or few episodes of mind wandering. Hence, we decided to run the study until there were 40 participants who had good task performance and also had more than five episodes of mind wandering. We preregistered our replication study on OSF at https://osf.io/n24m9/ (data and analysis scripts are provided at https://osf.io/9v3gk/; see Fig. S9 in the Supplemental Material to see how effect sizes changed with number of participants).
Two hundred seven participants performed the study. Sixty-two were rejected because they did not satisfy the behavioral criteria (mean go-trial RT > mean failed stop-trial RT by 10 ms, accuracy in go trials > 90%, unimodal go-trial RT, probability of stopping = .3–.7). Thus, 145 participants were included in analyses (68 females, 64 males, 13 participants did not report gender; 122 right-handed, 23 left-handed; age: M = 34 years, SD = 1, range = 19–68). Of the 145 participants, 40 reported a sufficient number of mind-wandering episodes (see below).
Study design
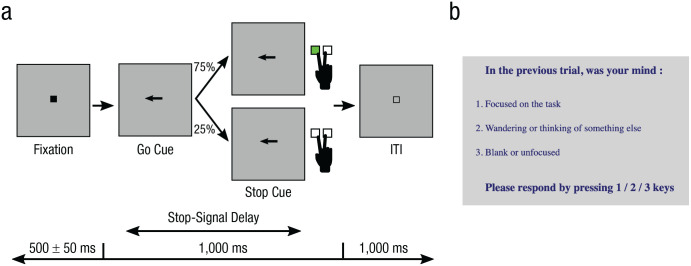
Participants performed a browser-based stop-signal task with an auditory tone as the stop signal (Fig. 2a). In each trial, an arrow pointing to the left or right was presented, and participants had to press the corresponding left or right arrow on the keypad with their right hand within a 1,000-ms response deadline. In 25% of trials, after a stop-signal delay, a tone was presented, and participants had to try to stop their responses in these trials. The stop-signal delays were tracked separately for the left and right directions. We reduced the delay by 50 ms after a failed stop response and increased it by 50 ms after a successful stop response, so participants could achieve successful stopping in roughly 50% of trials. Intermittently, after 16 to 22 trials (40–55 s), participants received a mental-state probe: “In the previous trial, was your mind: 1. Focused on the task, 2. Wandering or thinking of something else, 3. Blank or unfocused.” They responded by pressing the 1, 2, or 3 key with their left hand (Fig. 2b). Participants were encouraged to maintain (a) a mean go-trial RT of 300 ms to 600 ms, (b) a go-trial accuracy greater than 90%, and (c) a successful stopping rate of 25% to 75%.
Fig. 2.
Task design. An example trial from the stop-signal task is shown in (a). In each trial, participants saw an arrow pointing either to the left or the right. In 75% of trials, participants had to press the corresponding arrow key on the keyboard. In 25% of trials, an auditory tone was played after a stop-signal delay; in these trials, participants had to try to stop their response. A mental-state probe (b) was presented after every 16 to 22 trials. Participants responded to the probe by pressing 1, 2, or 3 on the number keypad to indicate their mental state (on task, mind wandering, or off focus, respectively). ITI = intertrial interval.
Briefly, the study had six sections—sound test, go-trial practice (at least 20 trials), stop-trial practice (at least 40 trials), stop-trial practice with mental-state probe (at least 45 trials), experiment, and questionnaire (see the Supplemental Material available online for more details). Participants had to satisfy the criteria of all practice sections to perform the experiment. During practice, the stop-signal delay started at 200 ms and was tracked. The final stop-signal delay during practice served as the starting stop-signal delay during the experiment. The experiment consisted of eight blocks of 80 trials each (32 mental-state probes, 160 stop trials). At the end of the study, participants completed the Mind-Wandering Questionnaire (Mrazek et al., 2013) and the Barratt Impulsiveness Scale (BIS; Stanford et al., 2009).
Analyses
On-task versus mind-wandering episodes
Most of our analyses were focused on the six-trial period (15 s) prior to the mental-state probe. Each six-trial period always included at least one stop trial (but when the probe appeared and when the stop signal was presented were not predictable). This duration is similar to that used in previous studies (Bastian & Sackur, 2013; Christoff et al., 2009; Kam et al., 2011, 2012). On the basis of the probe response, we classified the six-trial period as an on-task, a mind-wandering, or an off-focus episode. For each participant, analyses were performed on the data pooled across all episodes of a particular type. We also performed supplemental analyses with a 10-s (four-trial) period and confirmed that the main results still held (see Fig. S2 in the Supplemental Material).
Stop-signal reaction time
SSRT was computed using the integration method (with replacement of go-trial failures by maximum RT), as described in Verbruggen et al. (2019). Briefly, this estimation requires integrating the RT distribution and determining the point at which the integral reaches p(respond|stop). The RT distribution includes all go trials with a response (including go trials with response errors and go trials with premature responses), go omissions (i.e., go trials in which participants did not respond before the response deadline, which were then assigned the maximum RT), and premature responses on failed stop trials (i.e., responses executed before the stop signal was presented).
Estimation of trigger failures and SSRT using the Bayesian estimation of ex-Gaussian stop-signal (BEESTS) model
The percentage of trigger failures and the mean and standard deviation of SSRT were estimated using the BEESTS model developed by Matzke, Love, and Heathcote (2017). The model produced by this software assumes a race between two stochastically independent processes—a go process and a stop process. It estimates the SSRT distribution by using the go-trial RT distribution and by considering the failed-stop RT as a censored go-trial RT distribution. On each stop trial, the censoring points are sampled randomly from the SSRT distribution. The RT distribution underlying the go and stop process is assumed to be ex-Gaussian with a Gaussian and an exponential component and is characterized by three parameters (µgo, σgo, τgo, and µstop, σstop, τstop). For such distributions, the mean and variance of the RT distribution are determined as µ + τ and σ2 + τ2, respectively. The model also determines the probability of trigger failures for each participant. The model uses Bayesian parametric estimation to estimate the parameters of the distributions. We used a hierarchical Bayesian parametric estimation, in which individual participant parameters are modeled with the group-level distributions. This approach is thought to be more accurate than fitting the data of individual participants and is effective when there is less data per participant (Matzke et al., 2013). The priors were bounded uniform distributions—µgo, µstop: U(0, 2); σgo, σstop: U(0, 0.5); τgo, τstop: U(0, 0.5); percentage of trigger failures: U(0, 1). The posterior distributions were estimated using Metropolis-within-Gibbs sampling, and we ran multiple chains. We ran the model for 5,000 samples with a thinning of 5. The Gelman-Rubin (Rˆ) statistic was used to estimate the convergence of the chain. Chains were considered converged if (Rˆ) was less than 1.1. For further details about the model, refer to Heathcote et al. (2019).
Statistical analysis
Statistical analyses were performed in MATLAB (The MathWorks, Natick, MA) and JASP (Version 0.14.1; Jasp Team, 2020). For pairwise comparisons, the data were first checked for normality using the Lilliefors test. If the data were normally distributed, a two-tailed paired-samples t test was performed; otherwise a two-tailed Wilcoxon signed-rank test was used (z statistic). For unpaired comparisons of normally distributed data, a two-tailed unpaired-samples t test was performed; otherwise, a two-tailed Wilcoxon rank-sum test was used (z statistic). For parametric tests, the effect size was computed as Cohen’s d, whereas for nonparametric tests, effect size was calculated as where N is the number of observations. We interpreted the effect sizes as small (d = 0.2–0.5, r = .2–.5), medium (d = 0.5–0.8, r = .5–.8), and large (d > 0.8, r > .8). For comparisons across multiple levels, a repeated measures analysis of variance (ANOVA) or Friedman’s test (for nonparametric data) was used. We also report Bayes factors (BFs) from Bayesian repeated measures ANOVA posterior model odds (BFM). This was followed by pairwise comparisons using Bonferroni-Holm-corrected t tests or Wilcoxon signed-rank tests (Bonferroni-Holm-corrected p value [pBH]). The Greenhouse-Geisser correction was applied where the assumption of sphericity in the ANOVA was violated. Effect sizes for parametric and nonparametric ANOVAs were interpreted as small (η p 2 = .01–.06, Kendall’s W = 0.1–0.3), medium (η p 2 = .06–.14, Kendall’s W = 0.3–0.5), and large (η p 2 > .14, Kendall’s W > 0.5). For all correlational analyses for ordinal data, we report Kendall’s correlation coefficient (rk); otherwise, we report Pearson’s correlation coefficient (rp). Additionally, we report the BF in favor of the alternate over the null hypothesis (BF10) and 95% credible intervals (CIs).
Results
Study 1: behavior
Depending on the stop-signal delay, participants successfully stopped or failed to stop roughly half the time. Overall, behavioral performance was typical (Table 1). The mean SSRT was 289 ms (SEM = 11 ms), and the mean rate of successful stopping was 50% (SEM = 1%). Our core hypothesis was that the six-trial period (15 s) preceding mind-wandering reports would show slower SSRTs and a greater percentage of trigger failures compared with on-task reports. Thus, on the basis of the probe response, we classified six trials prior to all mental-state reports (32 probes in total) as on-task, mind-wandering, or off-focus episodes. However, not all participants reported having mind-wandering episodes, and still fewer reported having off-focus episodes (Table 2), so for the following analyses we considered only the 11 participants who had more than five instances of mind wandering. This cutoff was arbitrary, but the successful-stop percentage was within the 25% to 75% range for this cutoff (see Table S1 in the Supplemental Material), suggesting that SSRT could be properly estimated (Verbruggen et al., 2019). Further, the results also held up for other cutoffs (see Fig. S3 in the Supplemental Material). Because there were few off-focus reports, we focused on the mind-wandering episodes (see the Supplemental Material for off-focus results).
Table 1.
Mean Behavioral Responses Across All Trials
| Parameter | Study 1 |
Study 2 |
||
|---|---|---|---|---|
| Mind-wandering
group (n = 11) |
All
participants (N = 30) |
Mind-wandering
group (n = 40) |
All
participants (N = 145) |
|
| RT for correct responses on go trials (ms) | 505 (18) | 514 (12) | 517 (12) | 530 (6) |
| RT for failed responses on stop trials (ms) | 464 (16) | 474 (11) | 478 (10) | 489 (5) |
| Correct responses on go trials (%) | 99 (0) | 99 (0) | 98 (0) | 98 (0) |
| Errors on go trials (%) | 1 (0) | 1 (0) | 2 (0) | 1 (0) |
| Successful stopping (%) | 49 (1) | 50 (1) | 48 (1) | 49 (0) |
| Stop-signal RT (ms) | 300 (16) | 289 (11) | 307 (8) | 311 (5) |
| Stop-signal delay (ms) | 190 (17) | 209 (14) | 197 (13) | 207 (7) |
Note: Standard errors of the mean are given in parentheses. Participants were included in the mind-wandering group if they had more than five mind-wandering episodes. RT = reaction time.
Table 2.
Percentage of Mental-State Reports
| Parameter | Study 1 |
Study 2 |
||
|---|---|---|---|---|
| Mind-wandering
group (n = 11) |
Non-mind-wandering
group (n = 19) |
Mind-wandering group (n = 40) | Non-mind-wandering group (n = 105) | |
| On-task reports | 63 (3) | 95 (2) | 57 (3) | 92 (1) |
| Mind-wandering reports | 31 (3) | 5 (1) | 35 (2) | 5 (1) |
| Off-focus reports | 5 (2) | 1 (0) | 8 (1) | 3 (1) |
Note: Standard errors of the mean are given in parentheses. Participants were included in the mind-wandering group if they had more than five mind-wandering episodes.
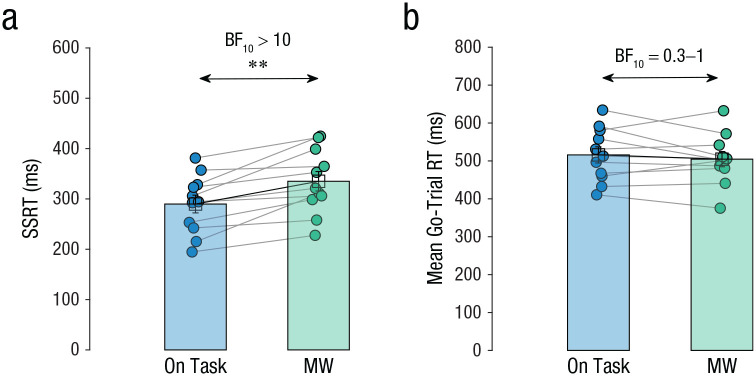
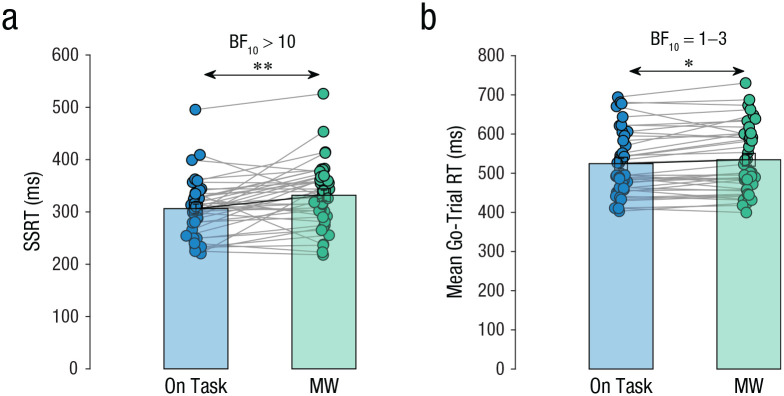
We started by comparing SSRT between mind-wandering and on-task episodes. The main result of interest was that SSRT in mind-wandering episodes (M = 335 ms, SEM = 19 ms, 95% CI = [292, 378]), was significantly slower than in on-task episodes (M = 290, SEM = 18 ms, 95% CI = [250, 329]), t(10) = 4.4, p = .001, d = 1.3, BF10 = 32.3 (see Fig. 3a). This occurred in the context of no significant change in mean go-trial RT between mind-wandering episodes (M = 502 ms, SEM = 21 ms, 95% CI = [456, 549]) and on-task episodes (M = 514, SEM = 21 ms, 95% CI = [468, 560]), t(10) = 0.9, p = .400, d = 0.3, BF10 = 0.4 (see Fig. 3b). Thus, mind wandering affected the executive function of stopping an incipient response without affecting general task performance.
Fig. 3.
Behavioral responses in Study 1. Mean stop-signal reaction time (SSRT; a) and go-trial reaction time (RT; b) are shown separately for on-task and mind-wandering (MW) episodes. Each dot represents the mean for an individual participant, and lines connect individual participants’ data between episode types. Data bars represent group means, and error bars represent standard errors of the group means. Asterisks indicate a significant difference between episode types (**p ≤ .01). A Bayes factor favoring the alternative over the null hypothesis (BF10) offers strong evidence for the alternative hypothesis when over 10 and weak evidence in favor of the null hypothesis when between 0.3 and 1.
Study 1: BEESTS
Next, we used the BEESTS model to estimate whether the percentage of trigger failures increased during mind-wandering compared with on-task episodes (see Fig. S4 in the Supplemental Material for the correlation between behavioral measures and BEESTS estimates). Because there were fewer mind-wandering than on-task episodes, we wanted to ensure that any observed difference could not be attributed to just the different number of trials used to estimate the parameters. Thus, we trial-matched the on-task episodes (called on-matched henceforth). For each participant, from all on-task episodes, we randomly selected the same number of episodes as there were mind-wandering episodes and estimated the BEESTS parameters. For each participant, this process was performed 10 times, and the estimates were averaged.
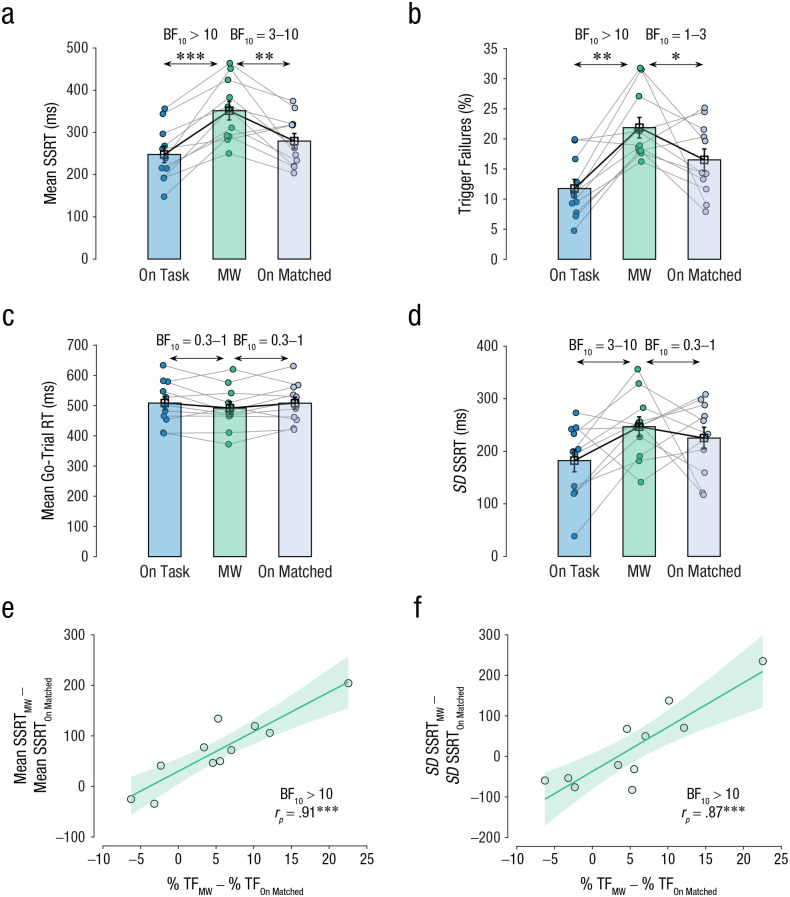
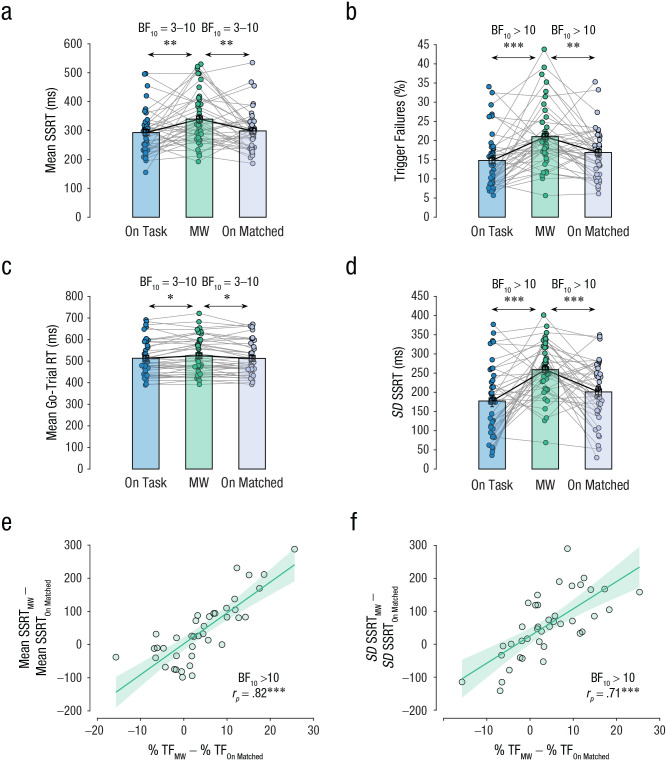
During mind wandering, the reliability of triggering decreased and the mean SSRT increased (i.e., response-inhibition ability became worse). A one-way repeated measures ANOVA with episode type (on task, mind wandering, on-matched) as the independent variable and mean SSRT as the dependent variable revealed that there was a significant effect of episode, Greenhouse-Geisser corrected F(1.3, 12.9) = 21.4, p < .001, η p 2 = .7, BFM > 100 (see Fig. 4a). Further, post hoc multiple comparisons revealed that mean SSRT in mind-wandering episodes (M = 351 ms, SEM = 22 ms, 95% CI = [303, 400]) was significantly slower than in on-task episodes (M = 248 ms, SEM = 20, 95% CI = [204, 292]), t(10) = 6.4, pBH < .001, d = 1.9, BF10 > 100, and in on-matched episodes (M = 279 ms, SEM = 18, 95% CI = [239, 320]), t(10) = 3.5, pBH = .012, d = 1.0, BF10 = 9.0. Similarly, a Friedman test with episode type (on task, mind wandering, on-matched) as the independent variable and percentage of trigger failures as the dependent variable revealed a significant effect of episode, c2(2, N = 20) = 12.2, p = .002, Kendall’s W = 0.6, BFM > 100 (see Fig. 4b). Further, post hoc multiple comparisons revealed that trigger failures in mind-wandering episodes (M = 22%, SEM = 2%, 95% CI = [18%, 26%]) were significantly greater than in both on-task episodes (M = 12%, SEM = 2%, 95% CI = [8%, 15%]), z(11) = 2.8, pBH = .013, r = .9, BF10 > 100, and on-matched episodes (M = 17%, SEM = 2%, 95% CI = [12%, 21%]), z(11) = 2.0, pBH = .041, r = .6, BF10 = 1.7. However, there was no significant effect of episode type on the estimated mean go-trial RT (on task: M = 508 ms, SEM = 22 ms, 95% CI = [459, 557]; mind wandering: M = 492 ms, SEM = 21 ms, 95% CI = [445, 539]; on-matched: M = 508, SEM = 20 ms, 95% CI = [464, 552]), F(2, 20) = 1.8, p = .190, η p 2 = .2, BFM = 0.6 (see Fig. 4c).
Fig. 4.
Bayesian estimation of ex-Gaussian stop-signal (BEESTS) estimates in Study 1. The graphs show (a) mean stop-signal reaction time (SSRT), (b) mean percentage of trigger failures, (c) mean go-trial reaction time (RT), and (d) the standard deviation of the SSRT distribution, separately for on-task, mind-wandering (MW), and on-matched episodes. Each dot represents the mean for an individual participant, and lines connect individual participants’ data between episode types. Data bars represent group means, and error bars represent standard errors of the group means. The scatterplots show the correlation (Pearson’s r [rp]) between the percentage of trigger failures between the MW and on-matched episodes (%TFMW – %TFOn-Matched) and (e) the change in SSRT between MW and on-matched episodes (SSRTMW – SSRTOn-Matched) and (f) the change in the standard deviation of the SSRT distribution between mind-wandering and on-matched episodes (SSRTMW – SSRTOn-Matched). In each scatterplot, the diagonal line represents the best-fitting linear regression, and the shaded region represents the 95% confidence interval. Asterisks indicate a significant difference between episode types (*p ≤ .05, **p ≤ .01, ***p ≤ .001). A Bayes factor favoring the alternative over the null hypothesis (BF10) offers strong evidence for the alternative hypothesis when over 10, moderate evidence between 3 and 10, and weak evidence when between 1 and 3. It offers weak evidence in favor of the null hypothesis when between 0.3 and 1.
Beside the change in mean stopping latency, increased variability of stopping latency can also impact one’s ability to effectively stop responses (Band et al., 2003; Matzke et al., 2018; Swick & Ashley, 2020; Weigard et al., 2019). Hence, we tested whether mind wandering increased the estimated standard deviation of the SSRT distribution. A Friedman test revealed that episode type did not have a significant effect, but there was weak evidence for the alternate hypothesis using a Bayesian repeated measures ANOVA, c2(2, N = 20) = 4.9, p = .086, Kendall’s W = 0.5, BFM = 1.8 (see Fig. 4d), with the highest standard deviation in mind-wandering episodes (mind wandering: M = 247 ms, SEM = 19 ms, 95% CI = [205, 289]; on task: M = 182 ms, SEM = 21 ms, 95% CI = [135, 230]; on-matched: M = 225 ms, SEM = 21 ms, 95% CI = [179, 271]).
The change in percentage of trigger failures between the mind-wandering and on-matched episodes was highly correlated to the change in mean SSRT, rp = .91, 95% CI = [.56, .98], p < .001, BF10 > 100 (see Fig. 4e). This correlation is unlikely to be a simulation artifact because we also observed a positive correlation between the percentage of trigger failures and the behavioral estimate of SSRT (see Fig. S5 in the Supplemental Material). This is consistent with the interpretation that triggering is graded and that trigger failure captures some aspect of the duration of the trigger stage. Similarly, there was a strong positive correlation between the change in percentage of trigger failures and the change in the standard deviation of the SSRT distribution, rp = .87, 95% CI = [.44, .96], p < .001, BF10 = 67.4 (see Fig. 4f). Thus, across participants, the change in the percentage of trigger failures during mind wandering could explain 82% and 75% of the variance of the change in the mean and variability of the SSRT distribution, respectively.
Taken together, our results were consistent with our predictions that the percentage of trigger failures and SSRT increased specifically during mind wandering compared with on-task episodes (i.e., response-inhibition ability worsened). Further, the change in the percentage of trigger failures explained a large proportion of the variance of the change in the mean and variability of the SSRT distribution, suggesting that the detrimental effect of mind wandering on response inhibition was largely due to the hit on the trigger stage. Given that mind wandering is associated with increased attentional lapses (Mooneyham & Schooler, 2013; Smallwood & Schooler, 2015), this supports the attentional account of trigger failure—that is, that trigger failures reflect attentional failures in which the stop signal is not utilized to select the appropriate action of initiating the brake (see Fig. S6 in the Supplemental Material to see how the percentage of go-trial failures varied across the three episodes).
We noticed that many participants reported no or few instances of mind wandering. This is consistent with other studies in which some subjects did not report mind-wandering episodes (Arnau et al., 2020; Compton et al., 2019). Perhaps these participants had episodes of mind wandering when they were not probed, or perhaps were low in trait mind wandering. Indeed, there was a moderate but significant correlation between the percentage of mind-wandering reports (i.e., state-level mind wandering) and the trait mind wandering assessed on the basis of the Mind-Wandering Questionnaire, rk = .29, 95% CI = [.03, .49], p = .037, BF10 = 2.5 (see Fig. S7a in the Supplemental Material), consistent with the findings of Mrazek et al. (2013). This suggests that participants reported their mental state accurately and that the probe reports could be trusted. Because using a cutoff of more than five episodes of mind wandering to select participants provided results consistent with our predictions, we continued with the same criterion in our replication study.
Study 2: behavior
As in Study 1, roughly one third of participants (40/145) reported more than five episodes of mind wandering (Table 2). Behavioral performance was typical (SSRT: M = 311 ms, SEM = 5 ms; successful stopping: M = 49%, SEM = 0%; see Table 1). Replicating Study 1, results showed that SSRT in mind-wandering episodes (M = 332 ms, SEM = 10 ms, 95% CI = [312, 352]) was significantly slower than in on-task episodes (M = 306 ms, SEM = 9 ms, 95% CI = [289, 324]), t(39) = 3.2, p = .003, d = 0.5, BF10 = 12.7 (see Fig. 5a). This time, mean go-trial RT for mind-wandering episodes (M = 531 ms, SEM = 14 ms, 95% CI = [503, 558]) was slower than for on-task episodes (M = 522 ms, SEM = 13 ms, 95% CI = [496, 548]), W(40) = 247, z(40) = 2.2, p = .029, r = .3, BF10 = 1.3 (see Fig. 5b), suggesting a decline in general task performance during mind wandering.
Fig. 5.
Behavioral responses in Study 2. Stop-signal reaction time (SSRT; a) and mean go-trial reaction time (RT; b) are shown separately for on-task and mind-wandering (MW) episodes. Each dot represents the value for an individual participant, and lines connect individual participants’ data between episode types. Data bars represent group means, and error bars represent standard errors of the group means. Asterisks indicate a significant difference between episode types (*p ≤ .05, **p ≤ .01). A Bayes factor favoring the alternative over the null hypothesis (BF10) offers strong evidence for the alternative hypothesis when over 10 and weak evidence between 1 and 3.
Study 2: BEESTS
Next, we estimated the BEESTS parameters. A Friedman test with episode (on task, mind wandering, on-matched) as the independent variable and mean SSRT as the dependent variable showed that the effect of episode was not significant. However, a Bayesian repeated measures ANOVA showed that there was strong evidence in favor of the alternate hypothesis and, further, the effect size was large, c2(2, N = 20) = 4.9, p = .089, Kendall’s W = 0.7, BFM = 31.4 (see Fig. 6a). Further, post hoc multiple comparisons revealed that mean SSRT in mind-wandering episodes (M = 339 ms, SEM = 15 ms, 95% CI = [310, 369]) was significantly slower than in both on-task episodes (M = 293 ms, SEM = 12 ms, 95% CI = [268, 318]), z(40) = 2.5, pBH = .035, r = .4, BF10 = 5.7, and on-matched episodes (M = 299 ms, SEM = 12 ms, 95% CI = [275, 322]), z(40) = 2.3, pBH = .043, r = .4, BF10 = 4.3. Next, a Friedman test with episode type (on task, mind wandering, on-matched) as the independent variable and percentage of trigger failures as the dependent variable revealed that there was a significant effect of episode type, c2(2, N = 20) = 18.7, p < .001, Kendall’s W = 0.6, BFM > 100 (Fig. 6b). Further, post hoc multiple comparisons revealed that trigger failures in mind-wandering episodes (M = 21%, SEM = 1%, 95% CI = [18%, 24%]) were significantly greater than in both on-task episodes (M = 15%, SEM = 1%, 95% CI = [12%, 17%]), z(40) = 2.9, pBH = .001, r = .5, BF10 > 100, and on-matched episodes (M = 17, SEM = 1%, 95% CI = [15%, 19%]), z(40) = 3.6, pBH = .008, r = .6, BF10 = 11.6. Thus, the main results of the increase in percentage of trigger failures and SSRT during mind wandering, as seen in Study 1, held up in the larger sample.
Fig. 6.
Bayesian estimation of ex-Gaussian stop-signal (BEESTS) estimates in Study 2. The graphs show (a) mean stop-signal reaction time (SSRT), (b) mean percentage of trigger failures, (c) mean go-trial reaction time (RT), and (d) the standard deviation of the SSRT distribution, separately for on-task, mind-wandering (MW), and on-matched episodes. Each dot represents the mean for an individual participant, and lines connect individual participants’ data between episode types. Data bars represent group means, and error bars represent standard errors of the group means. The scatterplots show the correlation (Pearson’s r [rp]) between the percentage of trigger failures between the MW and on-matched episodes (%TFMW – %TFOn-Matched) and (e) the change in SSRT between MW and on-matched episodes (SSRTMW – SSRTOn-Matched) and (f) the change in the standard deviation of the SSRT distribution between mind-wandering and on-matched episodes (SSRTMW – SSRTOn-Matched). In each scatterplot, the diagonal line represents the best-fitting linear regression, and the shaded region represents the 95% confidence interval. Asterisks indicate a significant difference between episode types (*p ≤ .05, **p ≤ .01, ***p ≤ .001). A Bayes factor favoring the alternative over the null hypothesis (BF10) offers strong evidence for the alternative hypothesis when over 10 and moderate evidence between 3 and 10.
Unlike Study 1, in which we observed no effect of mind wandering on mean go-trial RT, here results showed that mind wandering significantly slowed down responses. There was a significant effect of episode type on the estimated mean go-trial RT, F(2, 20) = 7.0, p = .002, η p 2 = .2, BFM = 18.6 (see Fig. 6c). Mean go-trial RT during mind-wandering episodes (M = 525 ms, SEM = 14 ms, 95% CI = [497, 552]) was significantly slower than during on-task episodes (M = 513 ms, SEM = 13 ms, 95% CI = [486, 540]), t(39) = 2.6, pBH = .027, d = 0.4, BF10 = 3.6, and on-matched episodes (M = 513 ms, SEM = 13 ms, 95% CI = [487, 539]), t(39) = 2.8, pBH = .027, d = 0.4, BF10 = 4.4. Thus, mind wandering affected the executive function of stopping an incipient response and also affected general task performance. Similar slowing of responses during mind wandering has also been reported previously (Kam et al., 2021; Stawarczyk et al., 2014).
Next, we tested whether mind wandering affected the variability of stopping latency. There was a significant effect of episode on the standard deviation of the SSRT distribution, F(2, 20) = 14.4, p < .001, η p 2 = .3, BFM > 100 (see Fig. 6d), and the standard deviation of SSRT distribution was significantly greater in the mind-wandering episodes (M = 259 ms, SEM = 11 ms, 95% CI = [236, 282]), compared with both on-task episodes (M = 177 ms, SEM = 15 ms, 95% CI = [148, 207]), t(39) = 4.6, pBH < .001, d = 0.7, BF10 > 100, and on-matched episodes (M = 201 ms, SEM = 13 ms, 95% CI = [175, 227]), t(39) = 3.8, pBH < .001, d = 0.6, BF10 = 60.8 (see Fig. S8 in the Supplemental Material for a comparison of the parameters σstop and τstop between the three types of episodes). Thus, during mind wandering, the percentage of trigger failures and the mean and variability of stopping latency increased.
As before, we correlated the change in percentage of trigger failures between mind-wandering and on-matched episodes with the change in the mean and variability of the SSRT distribution between the two episodes. Again, we observed a high positive correlation with the change in mean SSRT, rp = .82, 95% CI = [.66, .89], p < .001, BF10 > 100 (see Fig. 6e). Further, the change in the percentage of trigger failures was also well correlated with the change in the standard deviation of the SSRT distribution, rp = .71, 95% CI = [.49, .83], p < .001, BF10 > 100 (see Fig. 6f). Thus, the change in the percentage of trigger failures explained 66% and 50% of the variance of the change in the mean and variability of the SSRT distribution, respectively, replicating the results of Study 1. Taken together, this suggests that during mind wandering, stopping becomes slower and more variable, and this is largely because of the hit on the trigger stage.
Pooled Studies 1 and 2: correlation with age
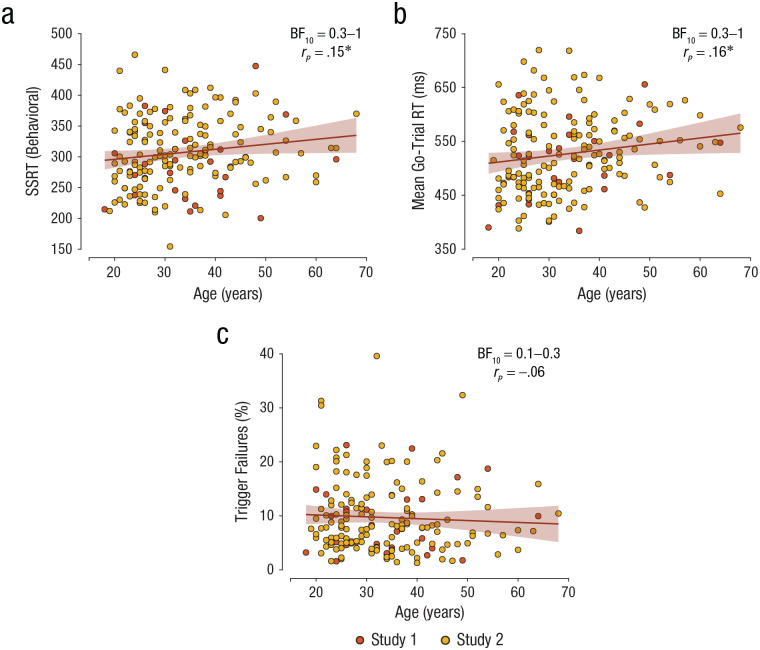
We performed an exploratory analysis of the effect of age on SSRT, mean go-trial RT, and percentage of trigger failures (across all trials). Previous studies have found that SSRT and mean go-trial RT increase with age (Hsieh & Lin, 2017; Williams et al., 1999). Consistent with this, results showed a weak positive correlation between age and behavioral measures of SSRT (rp = .15, 95% CI = [0.003, 0.29], p = .045, BF10 = 0.7; see Fig. 7a) and between age and BEESTS estimates of SSRT (rp = .13, 95% CI = [−0.02, 0.27], p = .095, BF10 = 0.4), as well as a weak positive correlation between age and mean go-trial RT (rp = .16, 95% CI = [0.01, 0.30], p = .032, BF10 = 0.9; see Fig. 7b). As mentioned above, SSRT includes both the trigger and brake stages, so if this increase in SSRT is due to worse triggering, then one would also expect a positive relationship between age and percentage of trigger failures. However, there was no significant correlation between age and percentage of trigger failures, rp = −.06, 95% CI = [−.20, .09], p = .468, BF10 = 0.1 (Fig. 7c). This suggests that age affects the brake and not the trigger stage.
Fig. 7.
Relationship between age, behavioral measures, and model measures. Scatterplots show the correlation between age and (a) mean behavioral stop-signal reaction time (SSRT), (b) mean go-trial reaction time (RT), and (c) mean percentage of trigger failures, separately for each participant in Study 1 and Study 2. In each scatterplot, the diagonal line represents the best-fitting linear regression, and the shaded region represents the 95% confidence interval. An asterisk indicates a significant Pearson’s correlation coefficient (*p ≤ .05). A Bayes factor favoring the alternative over the null hypothesis (BF10) offers weak evidence for the null hypothesis when between 0.3 and 1 and moderate evidence when between 0.1 and 0.3.
To further explore this possibility, we tested whether the correlation between age and percentage of trigger failures was significantly different from the correlation between age and SSRT using the web utility provided by Lee and Preacher (2013). Briefly, this utility converts each correlation coefficient to a z score using Fisher’s r-to-z transformation and then uses Steiger’s equations (1980) to compute the difference between the two correlations. The two correlations were significantly different from each other (z score = 2.5, p = .011). This raises the possibility that the factors (mind wandering and age) affect response inhibition in dissociable ways: Mind wandering may primarily affect the trigger stage, whereas age may primarily affect the brake stage. This could be investigated in future studies.
Pooled Studies 1 and 2: BIS ratings
At the end of each study, participants also filled out the BIS (Stanford et al., 2009). Using the BIS ratings, we aimed to replicate previous results that have shown a correlation between BEESTS outputs and BIS ratings (Skippen et al., 2019). The reported BIS ratings (Study 1: M = 56, SEM = 2; Study 2: M = 58, SEM = 1; pooled: M = 58, SEM = 1) were similar to those in Skippen et al.’s (2019) study. However, contrary to that study, our results did not show any significant relationship between BIS ratings and the behavioral and BEESTS results (Table 3). Although it is hard to interpret nonsignificant results, there are two possibilities. One is that the relationship between BIS ratings and BEESTS outputs requires more careful scrutiny, and another is that participants did not rate the BIS scale accurately in our study. Future studies could investigate this further.
Table 3.
Correlation Between Barratt Impulsiveness Scale (BIS) Ratings, Behavioral Measures, and Bayesian Estimation of Ex-Gaussian Stop-Signal (BEESTS) Estimates
| Parameter | SSRT
(Behavioral) |
SSRT
(BEESTS) |
Percentage of
trigger failures |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rk | p | 95% CI | BF10 | rk | p | 95% CI | BF10 | rk | p | 95% CI | BF10 | |
| BIS (attention) | −.05 | .476 | [−.20, .09] | 0.12 | −.06 | .465 | [−.20, .09] | 0.12 | −0.04 | .637 | [−.18, .11] | 0.11 |
| BIS (motor) | .12 | .120 | [−.03, .26] | 0.31 | .11 | .134 | [−.04, .26] | 0.29 | 0.11 | .132 | [−.04, .26] | 0.29 |
| BIS (nonplanning) | .01 | .868 | [−.14, .16] | 0.10 | .04 | .598 | [−.11, .19] | 0.11 | 0.02 | .768 | [−.13, .17] | 0.10 |
| BIS (total) | .03 | .696 | [−.012, .18] | 0.10 | .04 | .599 | [−.11, .19] | 0.10 | 0.04 | .596 | [−.11, .19] | 0.11 |
Note: SSRT = stop-signal reaction time. rk = Kendall’s correlation coefficient. BF10 = Bayes factor (BF) favoring the alternative over the null hypothesis; CI = credible interval.
Discussion
In two online studies on healthy adult participants, we tested how mind wandering affects response inhibition (i.e., the ability to rapidly stop actions). Participants were intermittently probed to report their mental state while they performed the stop-signal task, a task that measures response inhibition. On the basis of the probe responses, we classified trials prior to the probe as on-task, mind-wandering, or unfocused/blank episodes. We then performed paired comparisons of stopping performance between the episodes. Using this within-subjects design, we observed that during mind-wandering episodes, compared with on-task episodes, (a) SSRT, an estimate of the stopping latency, was slower and more variable; (b) the percentage of trigger failures, an estimate of the percentage of trials in which the “brake” mechanism that stops actions was weakly or not triggered, was greater; and (c) the increase in the percentage of trigger failures was well correlated with the slower and more variable stopping latency. Taken together, these studies provide strong evidence that mind wandering has a detrimental effect on response inhibition and suggest that this is largely due to the failure to trigger the inhibitory brake rather than to a deficit in the brake itself. Nevertheless, future studies should investigate how well the results of these online studies generalize to controlled lab settings and to real-world situations, such as driving, that require rapid stopping of actions.
Our key finding was that percentage of trigger failures and SSRT increased during mind-wandering episodes compared with on-task episodes and that across participants this change in percentage of trigger failures explained 67% and approximately 52% of the variance in the change in the mean and variability of SSRT, respectively. This suggests that poorer action cancellation during mind wandering is primarily due to a failure to trigger the inhibitory brake and not a failure of the brake per se. This result has several important implications. First, whereas previous studies provided mixed results on the effect of mind wandering on response inhibition, this was probably because of methodological differences or because many of these studies were not set up to answer this specific question. Our study provides a clear demonstration that response inhibition is affected during mind wandering and provides a mechanism by which this is mediated. Relatedly, some clinical populations, such as patients with attention-deficit/hyperactivity disorder, who have slower SSRT (Alderson et al., 2007) also have a greater percentage of trigger failures (Weigard et al., 2019). Interestingly, these patients also have increased mind wandering (Seli et al., 2015), and on the basis of our results, we hypothesize that poorer response inhibition in these patients might be related to their increased mind wandering. Future studies could test this.
Second, our results have theoretical importance in validating trigger failures. Whereas some researchers have argued that trigger failures may not point to separate trigger and brake stages (Bissett et al., 2021), here we found that the percentage of trigger failures changed with the mental state (on task vs. mind wandering) and that this partly explains the change in SSRT. Together, this suggests that the percentage of trigger failures really picks out a distinct stage (see also Fig. 7, which shows that age affects SSRT but not trigger failure—dissociating the two stages). Other neurophysiological evidence also supports the veracity of trigger failures. For example, the frontocentral auditory N1 potential (which is sensitive to attentional manipulations; Näätänen & Picton, 1987; Woods, 1995) is smaller in participants with higher trigger failures (Skippen et al., 2020). Third, our demonstration that state-level mind wandering related to trait-level mind wandering suggests a strategy for reducing the variability of response-inhibition studies. Potentially, one could acquire trait-level mind wandering at the end of the study and use it as a covariate.
We also observed a weak positive correlation between age and SSRT: Older people had longer SSRTs (consistent with the findings of Hsieh & Lin, 2017; Williams et al., 1999) but no significant correlation between age and percentage of trigger failures. This suggests that age affects braking but not triggering. Although the lack of significant correlation between age and percentage of trigger failures might be due to undersampling of older adults (our study was not designed to test this effect), it raises the possibility that when used in conjunction, the factors age and mind wandering may tease apart the contribution of the trigger and brake stages. Mind wandering would primarily affect the trigger, whereas age would primarily affect the brake without affecting the trigger. This could be investigated in future studies.
We also observed that, similar to mind-wandering episodes, in off-focus episodes participants had longer SSRTs and greater trigger failures (see Fig. S1 in the Supplemental Material). Because there were very few participants who had more than five instances of both mind-wandering and off-focus episodes, we could not specifically compare the stopping metrics within the same participants. However, when comparing the 40 participants who had more than five mind-wandering episodes and the 11 participants who had more than five off-focus episodes (i.e., unpaired comparisons), we did not observe any significant difference between the two mental states. Thus, on the basis of these results, we cannot speak to the debate about whether these two mental states are one and the same: Although many previous studies have often placed mind-wandering and off-focus episodes under the same heading, Mittner et al. (2016) have suggested that the off-focus and mind-wandering state might be distinct because of the level of locus coeruleus-norepinephrine activity and the pattern of brain connectivity. Nonetheless, note that the off-focus state occurred rarely: Only 11 of 175 participants reported more than five instances of off-focus episodes. The off-focus state was reported in approximately 3% of all probes, and stopping metrics tended to worsen during off-focus episodes. Future within-subject studies could investigate whether the rare off-focus state reflects a state of greater task disengagement than mind wandering.
In conclusion, in two studies, we observed that stopping latency was longer and more variable during self-reported periods of mind wandering compared with periods when participants were focused on the task. This increased stopping latency was predominantly a result of decreased reliability in triggering the brake. This clearly elucidates the impact of mind wandering on one important aspect of executive control and helps to validate trigger failures as a component of executive failures. More generally, the methodological approach we pioneered here could set the stage for studies that are focused on the real-world impact of mind wandering on response inhibition—for example, in relation to driving or handling machinery. Future studies could also test whether it is possible to measure mind wandering in real time and to use it to guide better behavior.
Supplemental Material
Supplemental material, sj-pdf-1-pss-10.1177_09567976211055371 for Mind Wandering Impedes Response Inhibition by Affecting the Triggering of the Inhibitory Process by Sumitash Jana and Adam R. Aron in Psychological Science
After the Study 1 preregistration, we realized that we had erroneously calculated SSRT in Study 1 and had used a parametric test on the difference between percentage of trigger failures. We then rectified these errors. However, following our preregistration, we enlisted 40 participants who had more than five mind-wandering episodes and used all the selection criteria as outlined in our preregistered report. Furthermore, we performed additional analyses to confirm effect sizes (see Fig. S9 in the Supplemental Material).
Footnotes
ORCID iD: Sumitash Jana  https://orcid.org/0000-0003-3742-3958
https://orcid.org/0000-0003-3742-3958
Supplemental Material: Additional supporting information can be found at http://journals.sagepub.com/doi/suppl/10.1177/09567976211055371
Transparency
Action Editor: Barbara Knowlton
Editor: Patricia J. Bauer
Author Contributions
S. Jana conceived and designed the research, performed the experiments, analyzed the data, interpreted the results, prepared the figures, and drafted the manuscript. S. Jana and A. R. Aron revised the manuscript and approved the final version for submission.
Declaration of Conflicting Interests: The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
Funding: This work was supported by the National Institute of Mental Health (Grant No. MH-020002) and by the James S. McDonnell Foundation (Grant No. 220020375).
Open Practices: All data and analysis scripts have been made publicly available via OSF and can be accessed at https://osf.io/9v3gk/. The design and analysis plans for Study 2 were preregistered at https://osf.io/n24m9/. This article has received the badge for Open Data and Preregistration. More information about the Open Practices badges can be found at http://www.psychologicalscience.org/publications/badges.


References
- Albert D. A., Ouimet M. C., Jarret J., Cloutier M. S., Paquette M., Badeau N., Brown T. G. (2018). Linking mind wandering tendency to risky driving in young male drivers. Accident Analysis and Prevention, 111, 125–132. 10.1016/j.aap.2017.11.019 [DOI] [PubMed] [Google Scholar]
- Alderson R. M., Rapport M. D., Kofler M. J. (2007). Attention-deficit/hyperactivity disorder and behavioral inhibition: A meta-analytic review of the stop-signal paradigm. Journal of Abnormal Child Psychology, 35(5), 745–758. 10.1007/s10802-007-9131-6 [DOI] [PubMed] [Google Scholar]
- Arnau S., Löffler C., Rummel J., Hagemann D., Wascher E., Schubert A.-L. (2020). Inter-trial alpha power indicates mind wandering. Psychophysiology, 57(6), Article e13581. 10.1111/psyp.13581 [DOI] [PubMed] [Google Scholar]
- Aron A. R., Herz D. M., Brown P., Forstmann B. U., Zaghloul K. (2016). Frontosubthalamic circuits for control of action and cognition. The Journal of Neuroscience, 36(45), 11489–11495. 10.1523/jneurosci.2348-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin C. L., Roberts D. M., Barragan D., Lee J. D., Lerner N., Higgins J. S. (2017). Detecting and quantifying mind wandering during simulated driving. Frontiers in Human Neuroscience, 11, Article 406. 10.3389/fnhum.2017.00406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band G., van der Molen M. W., Logan G. D. (2003). Horse-race model simulations of the stop-signal procedure. Acta Psychologica, 112(2), 105–142. 10.1016/S0001-6918(02)00079-3 [DOI] [PubMed] [Google Scholar]
- Bari A., Robbins T. W. (2013). Inhibition and impulsivity: Behavioral and neural basis of response control. Progress in Neurobiology, 108, 44–79. 10.1016/J.PNEUROBIO.2013.06.005 [DOI] [PubMed] [Google Scholar]
- Bastian M., Sackur J. (2013). Mind wandering at the fingertips: Automatic parsing of subjective states based on response time variability. Frontiers in Psychology, 4, Article 573. 10.3389/fpsyg.2013.00573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissett P. G., Jones H. M., Poldrack R. A., Logan G. D. (2021). Severe violations of independence in response inhibition tasks. Science Advances, 7(12), Article 4355. 10.1126/sciadv.abf4355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K., Gordon A. M., Smallwood J., Smith R., Schooler J. W. (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences, USA, 106(21), 8719–8724. 10.1073/pnas.0900234106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K., Irving Z. C., Fox K. C. R., Spreng R. N., Andrews-Hanna J. R. (2016). Mind-wandering as spontaneous thought: A dynamic framework. Nature Reviews Neuroscience, 17(11), 718–731. 10.1038/nrn.2016.113 [DOI] [PubMed] [Google Scholar]
- Compton R. J., Gearinger D., Wild H. (2019). The wandering mind oscillates: EEG alpha power is enhanced during moments of mind-wandering. Cognitive, Affective and Behavioral Neuroscience, 19(5), 1184–1191. 10.3758/s13415-019-00745-9 [DOI] [PubMed] [Google Scholar]
- Eagle D. M., Bari A., Robbins T. W. (2008). The neuropsychopharmacology of action inhibition: Cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology, 199, 439–456. 10.1007/s00213-008-1127-6 [DOI] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.-G., Buchner A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191. [DOI] [PubMed] [Google Scholar]
- Galéra C., Orriols L., M’Bailara K., Laborey M., Contrand B., Ribéreau-Gayon R., Masson F., Bakiri S., Gabaude C., Fort A., Maury B., Lemercier C., Cours M., Bouvard M.-P., Lagarde E. (2012). Mind wandering and driving: Responsibility case-control. BMJ, 345(7888), Article e8105. 10.1136/bmj.e8105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves Ó. F., Rêgo G., Conde T., Leite J., Carvalho S., Lapenta O. M., Boggio P. S. (2018). Mind wandering and task-focused attention: ERP correlates. Scientific Reports, 8(1), Article 7608. 10.1038/s41598-018-26028-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot J. M., Boayue N. M., Csifcsák G., Boekel W., Huster R., Forstmann B. U., Mittner M. (2021). Probing the neural signature of mind wandering with simultaneous fMRI-EEG and pupillometry. NeuroImage, 224, Article 117412. 10.1016/j.neuroimage.2020.117412 [DOI] [PubMed] [Google Scholar]
- Heathcote A., Lin Y. S., Reynolds A., Strickland L., Gretton M., Matzke D. (2019). Dynamic models of choice. Behavior Research Methods, 51(2), 961–985. 10.3758/s13428-018-1067-y [DOI] [PubMed] [Google Scholar]
- Hsieh S., Lin Y. C. (2017). Stopping ability in younger and older adults: Behavioral and event-related potential. Cognitive, Affective and Behavioral Neuroscience, 17(2), 348–363. 10.3758/s13415-016-0483-7 [DOI] [PubMed] [Google Scholar]
- Jana S., Hannah R., Muralidharan V., Aron A. R. (2020). Temporal cascade of frontal, motor and muscle processes underlying human action-stopping. Elife, 9, Article 50371. 10.7554/elife.50371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasp Team. (2020). JASP (Version 0.14.1) [Computer software]. https://jasp-stats.org/
- Kam J. W. Y., Dao E., Blinn P., Krigolson O. E., Boyd L. A., Handy T. C. (2012). Mind wandering and motor control: Off-task thinking disrupts the online adjustment of behavior. Frontiers in Human Neuroscience, 6, Article 329. 10.3389/fnhum.2012.00329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam J. W. Y., Dao E., Farley J., Fitzpatrick K., Smallwood J., Schooler J. W., Handy T. C. (2011). Slow fluctuations in attentional control of sensory cortex. Journal of Cognitive Neuroscience, 23, 460–470. [DOI] [PubMed] [Google Scholar]
- Kam J. W. Y., Handy T. C. (2014). Differential recruitment of executive resources during mind wandering. Consciousness and Cognition, 26(1), 51–63. 10.1016/j.concog.2014.03.002 [DOI] [PubMed] [Google Scholar]
- Kam J. W. Y., Irving Z. C., Mills C., Patel S., Gopnik A., Knight R. T. (2021). Distinct electrophysiological signatures of task-unrelated and dynamic thoughts. Proceedings of the National Academy of Sciences, USA, 118(4), Article 2011796118. 10.1073/pnas.2011796118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killingsworth M. A., Gilbert D. T. (2010). A wandering mind is an unhappy mind. Science, 330(6006), 932. 10.1126/science.1192439 [DOI] [PubMed] [Google Scholar]
- Krämer U. M., Solbakk A. K., Funderud I., Løvstad M., Endestad T., Knight R. T. (2013). The role of the lateral prefrontal cortex in inhibitory motor control. Cortex, 49(3), 837–849. 10.1016/j.cortex.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I. A., Preacher K. J. (2013). Calculation for the test of the difference between two dependent correlations with one variable in common [Computer software]. http://www.quantpsy.org/corrtest/corrtest2.htm
- Lin C. T., Chuang C. H., Kerick S., Mullen T., Jung T. P., Ko L. W., Chen S.-A., King J.-T., McDowell K. (2016). Mind-wandering tends to occur under low perceptual demands during driving. Scientific Reports, 6, Article 21353. 10.1038/srep21353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littman R., Takács Á. (2017). Do all inhibitions act alike? A study of go/no-go and stop-signal paradigms. PLOS ONE, 12(10), Article e0186774. 10.1371/journal.pone.0186774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke D., Dolan C. V., Logan G. D., Brown S. D., Wagenmakers E.-J. (2013). Bayesian parametric estimation of stop-signal reaction time distributions. Journal of Experimental Psychology: General, 142(4), 1047–1073. 10.1037/a0030543 [DOI] [PubMed] [Google Scholar]
- Matzke D., Hughes M., Badcock J. C., Michie P., Heathcote A. (2017). Failures of cognitive control or attention? The case of stop-signal deficits in schizophrenia. Attention, Perception, & Psychophysics, 79(4), 1078–1086. 10.3758/s13414-017-1287-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke D., Love J., Heathcote A. (2017). A Bayesian approach for estimating the probability of trigger failures in the stop-signal paradigm. Behavior Research Methods, 49(1), 267–281. 10.3758/s13428-015-0695-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke D., Verbruggen F., Logan G. D. (2018). The stop-signal paradigm. In Wagenmakers E.-J. (Ed.), Stevens’ handbook of experimental psychology and cognitive neuroscience (Vol. 5). Wiley. 10.1002/9781119170174.epcn510 [DOI]
- Mittner M., Boekel W., Tucker A. M., Turner B. M., Heathcote A., Forstmann B. U. (2014). When the brain takes a break: A model-based analysis of mind wandering. The Journal of Neuroscience, 34(49), 16286–16295. 10.1523/JNEUROSCI.2062-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittner M., Hawkins G. E., Boekel W., Forstmann B. U. (2016). A neural model of mind wandering. Trends in Cognitive Sciences, 20(8), 570–578. 10.1016/j.tics.2016.06.004 [DOI] [PubMed] [Google Scholar]
- Miyake A., Friedman N. P., Emerson M. J., Witzki A. H., Howerter A., Wager T. D. (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41(1), 49–100. 10.1006/cogp.1999.0734 [DOI] [PubMed] [Google Scholar]
- Mooneyham B. W., Schooler J. W. (2013). The costs and benefits of mind-wandering: A review. Canadian Journal of Experimental Psychology, 67(1), 11–18. 10.1037/a0031569 [DOI] [PubMed] [Google Scholar]
- Mrazek M. D., Phillips D. T., Franklin M. S., Broadway J. M., Schooler J. W. (2013). Young and restless: Validation of the Mind-Wandering Questionnaire (MWQ) reveals disruptive impact of mind-wandering for youth. Frontiers in Psychology, 4, Article 560. 10.3389/fpsyg.2013.00560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näätänen R., Picton T. (1987). The N1 wave of the human electric and magnetic response to sound: A review and an analysis of the component structure. Psychophysiology, 24(4), 375–425. 10.1111/j.1469-8986.1987.tb00311.x [DOI] [PubMed] [Google Scholar]
- Raud L., Westerhausen R., Dooley N., Huster R. J. (2020). Differences in unity: The go/no-go and stop signal tasks rely on different mechanisms. NeuroImage, 210, Article 116582. 10.1016/j.neuroimage.2020.116582 [DOI] [PubMed] [Google Scholar]
- Schachar R., Logan G. D. (1990). Impulsivity and inhibitory control in normal development and childhood psychopathology. Developmental Psychology, 26(5), 710–720. [Google Scholar]
- Sebastian A., Forstmann B. U., Matzke D. (2018). Towards a model-based cognitive neuroscience of stopping – a neuroimaging perspective. Neuroscience and Biobehavioral Reviews, 90, 130–136. 10.1016/j.neubiorev.2018.04.011 [DOI] [PubMed] [Google Scholar]
- Sebastian A., Gerdes B., Feige B., Klöppel S., Lange T., Philipsen A., Tebartz van Elst L., Lieb K., Tüscher O. (2012). Neural correlates of interference inhibition, action withholding and action cancelation in adult ADHD. Psychiatry Research, 202(2), 132–141. 10.1016/j.pscychresns.2012.02.010 [DOI] [PubMed] [Google Scholar]
- Seli P., Smallwood J., Cheyne J. A., Smilek D. (2015). On the relation of mind wandering and ADHD symptomatology. Psychonomic Bulletin and Review, 22, 629–636. 10.3758/s13423-014-0793-0 [DOI] [PubMed] [Google Scholar]
- Skippen P., Fulham W. R., Michie P. T., Matzke D., Heathcote A., Karayanidis F. (2020). Reconsidering electrophysiological markers of response inhibition in light of trigger failures in the stop-signal task. Psychophysiology, 57(10), Article e13619. 10.1111/psyp.13619 [DOI] [PubMed] [Google Scholar]
- Skippen P., Matzke D., Heathcote A., Fulham W. R., Michie P., Karayanidis F. (2019). Reliability of triggering inhibitory process is a better predictor of impulsivity than SSRT. Acta Psychologica, 192, 104–117. 10.1016/j.actpsy.2018.10.016 [DOI] [PubMed] [Google Scholar]
- Smallwood J., McSpadden M., Luus B., Schooler J. (2008). Segmenting the stream of consciousness: The psychological correlates of temporal structures in the time series data of a continuous performance task. Brain and Cognition, 66(1), 50–56. 10.1016/j.bandc.2007.05.004 [DOI] [PubMed] [Google Scholar]
- Smallwood J., McSpadden M., Schooler J. W. (2007). The lights are on but no one’s home: Meta-awareness and the decoupling of attention when the mind wanders. Psychonomic Bulletin and Review, 14, 527–533. 10.3758/BF03194102 [DOI] [PubMed] [Google Scholar]
- Smallwood J., Schooler J. W. (2015). The science of mind wandering: Empirically navigating the stream of consciousness. Annual Review of Psychology, 66(1), 487–518. 10.1146/annurev-psych-010814-015331 [DOI] [PubMed] [Google Scholar]
- Stanford M. S., Mathias C. W., Dougherty D. M., Lake S. L., Anderson N. E., Patton J. H. (2009). Fifty years of the Barratt Impulsiveness Scale: An update and review. Personality and Individual Differences, 47(5), 385–395. 10.1016/j.paid.2009.04.008 [DOI] [Google Scholar]
- Stawarczyk D., Majerus S., Catale C., D’Argembeau A. (2014). Relationships between mind-wandering and attentional control abilities in young adults and adolescents. Acta Psychologica, 148, 25–36. 10.1016/j.actpsy.2014.01.007 [DOI] [PubMed] [Google Scholar]
- Steiger J. H. (1980). Tests for comparing elements of a correlation matrix. Psychological Bulletin, 87(2), 245–251. [Google Scholar]
- Swick D., Ashley V. (2020). The specificity of inhibitory control deficits in post-traumatic stress disorder: A dissociation between the speed and reliability of stopping. Journal of Anxiety Disorders, 75, Article 102278. 10.1016/j.janxdis.2020.102278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D., Ashley V., Turken U. (2011). Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. NeuroImage, 56(3), 1655–1665. 10.1016/J.NEUROIMAGE.2011.02.070 [DOI] [PubMed] [Google Scholar]
- Thomson D. R., Besner D., Smilek D. (2013). In pursuit of off-task thought: Mind wandering-performance trade-offs while reading aloud and color naming. Frontiers in Psychology, 4(1), Article 360. 10.3389/fpsyg.2013.00360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson D. R., Seli P., Besner D., Smilek D. (2014). On the link between mind wandering and task performance over time. Consciousness and Cognition, 27(1), 14–26. 10.1016/j.concog.2014.04.001 [DOI] [PubMed] [Google Scholar]
- Verbruggen F., Aron A. R., Band G. P., Beste C., Bissett P. G., Brockett A. T., Brown J. W., Chamberlain S. R., Boehler C. N. (2019). A consensus guide to capturing the ability to inhibit actions and impulsive behaviors in the stop-signal task. Elife, 8, Article e46323. 10.7554/eLife.46323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigard A., Heathcote A., Matzke D., Huang-Pollock C. (2019). Cognitive modeling suggests that attentional failures drive longer stop-signal reaction time estimates in attention deficit/hyperactivity disorder. Clinical Psychological Science, 7(4), 856–872. 10.1177/2167702619838466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel J. R. (2018). Prepotent motor activity and inhibitory control demands in different variants of the go/no-go paradigm. Psychophysiology, 55(3), Article e12871. 10.1111/psyp.12871 [DOI] [PubMed] [Google Scholar]
- Williams B. R., Ponesse J. S., Schachar R. J., Logan G. D., Tannock R. (1999). Development of inhibitory control across the life span. Developmental Psychology, 35(1), 205–213. 10.1037/0012-1649.35.1.205 [DOI] [PubMed] [Google Scholar]
- Woods D. L. (1995). The component structure of the N1 wave of the human auditory evoked potential. Electroencephalography and Clinical Neurophysiology-Supplement, 44, 102–109. [PubMed] [Google Scholar]
- Yanko M. R., Spalek T. M. (2014). Driving with the wandering mind: The effect that mind-wandering has on driving performance. Human Factors, 56(2), 260–269. 10.1177/0018720813495280 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-pss-10.1177_09567976211055371 for Mind Wandering Impedes Response Inhibition by Affecting the Triggering of the Inhibitory Process by Sumitash Jana and Adam R. Aron in Psychological Science