Abstract
Introduction
Presently, there are few population-level strategies to address SARS-CoV-2 infection except preventive measures such as vaccination. Micronutrient deficiency, particularly vitamin D and zinc deficiency, has been associated with dysregulated host responses, and may play an important role in COVID-19.
Methods and analysis
We have designed a 2×2 factorial, randomised, double-blind, multi-centre placebo-controlled trial to evaluate the effect of vitamin D and zinc on COVID-19 outcomes in Maharashtra, India. COVID-19 positive individuals are recruited from hospitals in Mumbai and Pune. Participants are provided (1) vitamin D3 bolus (180 000 IU) maintained by daily dose of 2000 IU and/or (2) zinc gluconate (40 mg daily), versus placebo for 8 weeks. Participants undergo a detailed assessment at baseline and at 8 weeks, and are monitored daily in hospital or every 3 days after leaving the hospital to assess symptoms and other clinical measures. A final follow-up telephone call occurs 12 weeks post-enrolment to assess long-term outcomes. The primary outcome of the study is to time to recovery, defined as time to resolution of all of fever, cough and shortness of breath. Secondary outcomes include: duration of hospital stay, all-cause mortality, necessity of assisted ventilation, change in blood biomarker levels and individual symptoms duration. Participant recruitment commenced on April 2021.
Ethics and dissemination
Ethical approval was obtained from institutional ethical committees of all participating institutions. The study findings will be presented in peer-reviewed medical journals.
Trial registration numbers
NCT04641195, CTRI/2021/04/032593, HMSC (GOI)-2021-0060.
Keywords: COVID-19, nutrition, clinical trials
Strengths and limitations of this study.
The setting of this study in India enables applicability of findings to the wider South Asia region, where evidence on this topic remains scarce despite a notable recent burden of COVID-19 and high prevalence of micronutrient deficiency.
As a double-blind factorial randomised controlled trial, this study enables an efficient assessment of the effect of vitamin D and zinc on COVID-19 symptoms that is less prone to confounding and bias than other observational studies on this topic.
With frequent follow-up of participants, this study collects information across a range of domains including sociodemographic and clinical measures, and biomarker data, which will allow for a detailed investigation of the effect of supplementation on disease progression.
One limitation to the study design is that with the current sample size, the statistical power to detect modification of the effects of each supplement by other factors may be limited.
Introduction
COVID-19 continues to be a problem globally, with over 16 million incident cases and 200 000 deaths reported in November 2021.1 Concerted global efforts have resulted in the development of vaccines, which may reduce the burden and impact of COVID-19, although suboptimal vaccine coverage and the rapid mutation of the virus continue to prolong the pandemic.2–5 Additionally, with limited proven treatment regimens for COVID-19 to date, it is essential to continue exploring low cost and commonly available effective interventions which can be implemented as standardised therapeutic treatment regimens at large.6 This is especially important in the context of low-income and middle-income countries in South Asia and Africa, which are particularly vulnerable given weak health systems and the coexistence of malnutrition and other comorbidities. This includes India, which continues to report a substantial number of COVID-19 cases.1
Observational and experimental evidence links vitamin D to an array of communicable and non-communicable diseases.7 Vitamin D deficiency (VDD; serum vitamin D <20 ng/mL)8 is common in urban and rural India despite the country’s sunny climate, due to environmental, sociological and biological factors,9 10 including skin pigmentation and cultural practices related to clothing and sun exposure. Countrywide studies suggest VDD may affect at least 70% of the Indian population. Vitamin D shows promise as a novel, cost-effective prevention and adjunctive treatment for respiratory infections. In laboratory studies, vitamin D metabolites support innate immune responses to rhinoviruses and respiratory syncytial virus.11–15 In participants with influenza, high-dose vitamin D supplementation shortened durations of fever, cough and wheezing, particularly among those with low vitamin D levels.16 In a recent systematic review and meta-analysis of randomised controlled trials, vitamin D supplementation was associated with decreased risk of acute respiratory infections and shortened duration of symptoms.17
Zinc is an essential mineral that plays critical roles in gene expression, cell division and immunity.18 In India, dietary predominance of micronutrient-sparse staples, limited consumption of animal foods and high consumption of zinc absorption inhibitors render the population at extremely high risk of inadequacy, which is exacerbated due to global climate change.19 About 25% of the Indian population is zinc inadequate, and 4.3 million child deaths (<5 years) were attributable to zinc deficiency in 2017.20 Multiple meta-analyses and pooled analyses of randomised controlled trials conducted in the USA and low-income and middle-income countries have shown that oral zinc supplementation reduces incidence of acute respiratory infections by 35%, shortens duration of symptoms and improves recovery rate.17 21–24 Zinc is a potential treatment in COVID-19, due to its immune modulatory effect, as well as direct antiviral effect.25 The mechanisms by which zinc may serve as adjunct therapy in COVID-19 has been recently reviewed by Skalny et al26 who note that Zn2+ cations, especially in combination with zinc ionophore pyrithione inhibit SARS-CoV RNA polymerase activity by decreasing replication.27
Vitamin D and zinc are safe, inexpensive and widely available therapies; therefore, experimental evidence that these nutrient supplements are effective against COVID-19 would readily support their inclusion in standard of care. Therefore, we are undertaking a randomised controlled trial to determine the effect of vitamin D and zinc supplementation on treatment outcomes among individuals with COVID-19 in India.
Objectives
The primary objectives of this trial are:
To determine the effect of vitamin D supplementation versus placebo on time to recovery among patients with COVID-19.
To determine the effect of zinc supplementation versus placebo on time to recovery among patients with COVID-19.
Secondary objectives include:
To determine the effect of vitamin D or zinc supplementation on duration of hospital stay, all-cause mortality, necessity for assisted ventilation and individual symptoms duration.
To examine the effect of vitamin D or zinc supplementation on key blood biomarkers, including serum vitamin D and zinc, and immunological and inflammatory markers.
Methods and analysis
Trial design, population, enrolment sites and time frame
This is a double-blind, placebo-controlled, randomised superiority trial with 2×2 factorial design and 1:1:1:1 allocation ratio, being conducted at two site hospitals in Mumbai and Pune, Maharashtra, India (figure 1). Maharashtra has the highest cumulative number of COVID-19 cases and fatalities out of all states in India.28 Within the state, both Pune and Mumbai have emerged as COVID-19 hotspots.29 30
Figure 1.
Map of India (grey) with Maharashtra highlighted in red, and Mumbai (black dot) and Pune (blue dot) identified. Map created with mapchart.net.
The two study sites (King Edward Memorial Hospital and Research Centre, Pune, and Saifee Hospital, Mumbai) are established medical institutions located within the cities of Pune and Mumbai. These hospitals have been designated as COVID-19 dedicated hospitals by local municipal corporations, where people can avail COVID-19-related treatment and services. The trial is targeting a sample size of 700. The study commenced in April 2021 and study activities are expected to continue until July 2022. While we initially targeted only hospitalised inpatients at each site for the study, we broadened our target population in June 2021 to include all hospital outpatients. This was done in order to increase generalisability of results and maintain enrolment in light of decreasing COVID-19 cases.31
Eligibility criteria
The original inclusion criteria for this study were as follows: (1) men and women aged ≥18 years, (2) RT-PCR-confirmed infection with SARS-CoV-2, (3) oxygen saturation level (SpO2)≥90 and (4) written informed consent.
The exclusion criteria were as following: (1) pregnant women, (2) individuals enrolled in other clinical trials, (3) daily use of multivitamins for the past 1 month.
To capture the greatest possible number and range of symptomatic COVID-19 cases and increase generalisability, we made the following alterations to our eligibility criteria from June 2021 (within 2 months of recruitment commencement): (1) added inclusion criterion of individuals with Rapid Antigen Test-confirmed SARS-CoV-2 infection (with confirmatory PCR tests performed subsequently on all such enrolled individuals), (2) removed inclusion criterion of SpO2≥90 and (3) removed exclusion criterion of recent daily multivitamin use. Since this change was made early, when few (<6% of target population) participants were enrolled in the trial, we anticipate that the majority of the final study population will have been enrolled under the updated, broader criteria.
Study procedures
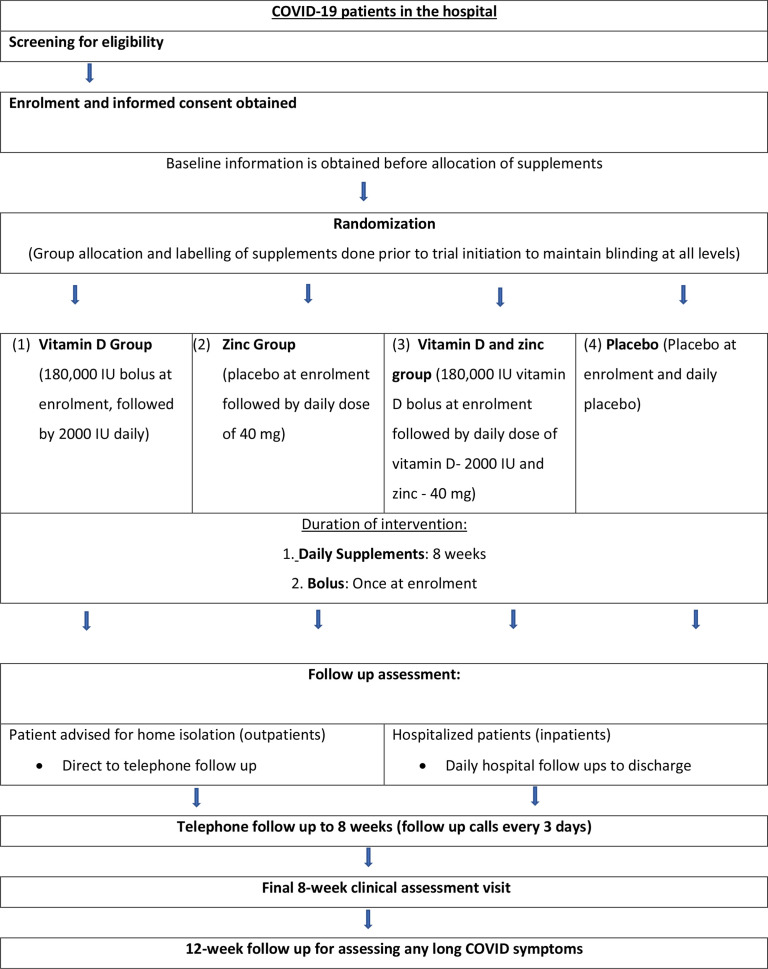
An overview of trial procedures is summarised in figure 2.
Figure 2.
Overview of trial procedures. RAT: rapid antigen test.
Recruitment and obtaining informed consent
Potential participants are approached by trained site hospital staff members when they present to site hospitals. Site hospital staff members undergo intensive training and refresher training in order to ensure that potential participants are able to make an informed decision regarding participation. These dedicated site hospital staff members determine their interest and eligibility, and provide a brief introduction including key details about the study and what participation involves. The staff members read out the participant information sheet in the appropriate conversational language (English, Hindi or Marathi), and discuss the trial components and the role of the participant in the study. Information provided includes a clear outline of potential benefits and harms, the length of the follow-up period, remuneration that can be expected, future use of information and samples, and resources available to the participant such as access to study clinics. Informed consent is obtained after responding to any raised queries. As part of the process, potential participants are informed that their participation is completely voluntary and they can withdraw any time at any stage of the study without providing any reasons. The informed consent process is completed once participants provide their signature on two copies of the consent document; one copy for the trial record and another provided to participants for their reference.
Information regarding eligibility of potential participants is collected on a secure electronic tablet using Open Data Kit (ODK),32 with questionnaires including built-in checks and data uploaded to a secure server. No identifiable data are collected until the participant has provided informed consent.
Baseline data and sample collection
Following informed consent, participants undergo baseline data and sample collection, including recording of key background and clinical information as follows:
Screening and background: the initial screening form is extended to collect information including participants’ demographic background, socioeconomic status, and health and prevention behaviours (smoking and drinking), and COVID-19 vaccination status.
Baseline dietary information: a food frequency questionnaire (FFQ) is administered, collecting information on dietary practices and habits in relation to 25 food groups. The FFQ is validated for use in India and has been adapted to the Maharashtra context.
Clinical baseline: clinical and physical measures are collected alongside information on COVID-19 symptoms, vital signs, blood investigations, medical conditions, treatment and medications including those prescribed for COVID-19, nutritional supplement use, complications and medical history.
A blood sample is also collected at baseline. All information is collected securely on electronic tablets, as described above.
Randomisation and blinding
Participants are assigned randomly to one of four groups: (1) vitamin D, (2) zinc, (3) vitamin D and zinc or (4) placebo. For randomisation, a computer-generated list was prepared by the study statistician, according to a randomisation sequence in blocks of 20 and stratified by follow-up clinic. The randomisation list assigns each participant randomisation identifier (ID) to a regimen code, with the actual regimen known only to the manufacturer and accessible to the statistician in a currently unopened, sealed envelope. Supplement bottles and envelopes are prelabelled with codes, and active tablets and placebo are indistinguishable, so that participants and all research staff including investigators remain blinded. At each site, each participant entering the trial is given the next available randomisation ID, and is provided their corresponding regimen based on the assigned regimen code.
Intervention
Participants are randomised to one of four groups:
Placebo–placebo group will receive a placebo vitamin D3 bolus at the hospital followed by placebo daily vitamin D3 maintenance doses and placebo daily zinc supplements.
Vitamin D–placebo group will receive an actual vitamin D3 bolus (180 000 IU) at the hospital followed by actual daily vitamin D3 maintenance doses (2000 IU daily) and daily placebo zinc supplements.
Placebo–zinc group will receive a placebo vitamin D3 bolus at the hospital followed by placebo daily vitamin D3 maintenance doses and actual daily zinc supplements (40 mg daily).
Vitamin D3–zinc group will receive an actual vitamin D3 bolus (180 000 IU) at the hospital followed by actual daily vitamin D3 maintenance doses (2000 IU daily) and actual daily zinc supplements (40 mg daily).
We selected vitamin D3 as it has been shown to be more effective in raising and maintaining high levels of circulating 25(OH)D than vitamin D2.33 34 A bolus dose followed by daily doses was chosen to boost vitamin D levels quickly and safely within the first few days and maintain levels thereafter. Previous studies have indicated the efficacy of large oral doses (>200 000 IU bolus and 1700–2000 IU per day) in increasing and sustaining blood 25(OH)D concentrations, with very low risk of side effects.35–41 The 40 mg dosage of zinc is understood to be sufficiently high to assess efficacy, while remaining within the Institute of Medicine’s tolerable upper intake level for adults.42 A placebo was chosen as the comparator group given that there is currently no widespread consensus on the use of any nutritional supplement as part of standard or routine treatment for COVID-19.17
Participants receive a prelabelled daily supplement bottle with 60 tablets, and an envelope which contains three vitamin D3/placebo bolus tablets to be consumed at baseline under supervision of site hospital staff. Following the bolus dose, participants are instructed to take supplements daily for 8 weeks. Participants are observed taking supplements daily while in hospital or contacted regularly via telephone after leaving the hospital to ensure compliance. Research staff identify barriers to compliance and aim to address these via appropriate counselling, and assess compliance at 8 weeks via direct questioning and pill count.
Supplement and placebo tablets were manufactured by Excellamed Laboratories Private Limited (Mumbai, India) with an external quality check done by an independent service provider (Bee Pharmo Labs Private Limited, Mumbai, India).
All participants are provided with care and treatment consistent with Indian national guidelines, and are encouraged to visit the study clinics 7 days a week for medical attention if they feel unwell. Indian national guidelines have evolved during the pandemic, and currently consist of appropriate treatment (which may include oxygen support, respiratory support, anti-inflammatory or immunomodulatory therapy and anticoagulation therapy) according to disease severity; discharge of admitted patients from the hospital on resolution of symptoms and sufficient oxygen saturation (SpO2>93%) for 3 days; and self-monitoring during home isolation.43–45
Study outcomes and follow-up
Following baseline assessment and provision of supplements, participants are regularly followed up as described below and in table 1:
Table 1.
Collection of data points in the trial
| Data category | Baseline (enrolment) | Follow-up | 8 weeks | 12 weeks |
| Demographic and background information | Age, gender, education, marital status, occupation, socioeconomic status, health and prevention behaviours, COVID-19 vaccination (self-reported by participant, assessed by staff or abstracted from participant record) |
COVID-19 vaccination (self-reported by participant) |
COVID-19 vaccination (self-reported by participant) |
|
| Dietary information | Food frequency questionnaire: consumption frequency of 25 diverse food groups in last 3 months (self-reported by participant) |
|||
| Clinical examination | Medical history, comorbidities, preadmission medications, non-intervention nutritional supplement use (self-reported by participant, assessed by staff or abstracted from participant record) Clinical symptoms* (Self-reported by participant) |
Hospital and telephone follow-up: clinical symptoms* (self-reported by participant) Hospital follow-up only: changes in medications, changes in non-intervention nutritional supplement use (assessed by staff or abstracted from participant record) |
Medical history, comorbidities, preassessment medications, non-intervention nutritional supplement use (Self-reported by participant, assessed by staff or abstracted from participant record) Clinical symptoms* (self-reported by participant) |
Clinical symptoms* (Self-reported by participant) |
| Clinical measurements | Respiratory rate, pulse, auxiliary temperature, SpO2, systolic and diastolic blood pressure, weight and height (assessed by staff or abstracted from participant record) |
Hospital follow-up only: Respiratory rate, pulse, auxiliary temperature, SpO2, systolic and diastolic blood pressure, weight, requirement for non-invasive ventilation or intubation/ventilator support, need for dialysis, lab investigations (assessed by staff or abstracted from participant record) |
Respiratory rate, pulse, auxiliary temperature, SpO2, systolic and diastolic blood pressure, weight and height (assessed by staff or abstracted from participant record) |
|
| Blood and other investigations and biomarkers | SARS-CoV-2 RT-PCR, chest X-ray, complete blood count, blood glucose, serum creatinine, CRP, LDH, serum ferritin, D-dimer, vitamin D, zinc, calcium, IgG, IgM, Ang2, IL-6 and sTREM-1 (assessed by laboratory or abstracted from participant record) |
CRP, LDH, serum ferritin, D-dimer, vitamin D, zinc, calcium, IgG, IgM, Ang2, IL-6 and sTREM-1 (assessed by laboratory or abstracted from participant record) |
||
| Other information |
Hospital and telephone follow-up: compliance, adverse events (self-reported by participant, assessed by staff or abstracted from participant record) |
Compliance (count of remaining pills) (assessed by staff) |
*Clinical symptoms include: fever, cough, shortness of breath, fatigue, headache, loss of smell, loss of taste, diarrhoea, anorexia, sore throat, nasal congestion, nausea and vomiting, and any other reported by the participant.
Ang2, angiopoietin-2; CRP, C reactive protein; IL-6, interleukin 6; LDH, lactate dehydrogenase; SpO2, oxygen saturation; sTREM-1, soluble triggering receptor expressed on myeloid cells-1.
Daily hospital follow-up: daily assessment of COVID-19 symptoms, vital signs, complications, medical conditions and study supplement compliance is recorded for hospitalised participants. Any new prescribed medications and supplements are also recorded alongside other interventions such as need for non-invasive ventilation or dialysis. Symptoms are specifically asked to participants; other measures are asked, observed, assessed or abstracted from the participants’ records. Clinical measurements are recorded in study-specific visits that are conducted independently after ward rounds, to minimise interference in care and ensure all relevant information for the day is noted.
Telephone follow-up: assessment of COVID-19 symptoms, supplement compliance and adverse events is conducted in a follow-up call every 3 days after leaving the hospital for all participants. All information is self-reported by participants.
8 week clinical assessment: after completion of study supplements at 8 weeks, information is gathered on results of a clinical and physical examination, COVID-19 symptoms, compliance with regimen (including direct questioning and pill count), vital signs, blood investigations (from a collected blood sample), medical conditions, treatment and medications, use of any other nutritional supplements, updates to COVID-19 vaccination status, complications and history. This assessment is conducted in person at the hospital, or at a location convenient to the participant where privacy can be ensured (including an option to collect some information via telephone if an in-person visit is not possible). Symptoms are specifically asked to participants; other measures are asked, observed, assessed or abstracted from the participants’ records.
12-week telephone follow-up: a final assessment is conducted of long-term COVID-19 symptoms, and any updates to COVID-19 vaccination status. All information is self-reported by participants.
All data are collected using standardised questionnaire forms on electronic tablets,32 as described above.
The primary outcome of the study is time to resolution of all of the following symptoms: (1) fever, (2) cough and (3) shortness of breath. These symptoms are most commonly reported among patients with COVID-19, including in Indian populations,46 47 and have also been assessed as part of studies examining vitamin D and zinc in respiratory illnesses.17 These and additional symptoms (including fatigue, headache, loss of smell and taste and sore throat) are captured on multiple time points, including baseline, daily hospital follow ups for admitted patients, telephone follow ups every 3 days after leaving the hospital until 8 weeks post-enrolment, the 8-week clinical assessment, and finally at a 12-week assessment call. Data on symptoms are collected using the same structured questions at each time point: (1) whether the participant has experienced X symptom today, and if so, (2) how many days in total including today the participant has experienced X symptom. Staff conducting in-person and telephone follow ups are trained uniformly using a standardised telephone script with regards to collecting this information. Metrics of individual symptoms and combination of symptoms are used to identify the time point of resolution symptoms from baseline.
Secondary outcomes include duration of hospital stay, need for assisted ventilation, individual symptoms duration, all-cause mortality and blood biomarker levels, including 25-hydroxy vitamin D, zinc, and other immunological and inflammatory biomarkers (including interleukin 6, angiopoietin-2, soluble triggering receptor expressed on myeloid cells-1, IgG and IgM). Biomarker levels are assessed using blood samples collected at baseline and at the 8-week clinical assessment. Occurrence of any other secondary endpoints between baseline to 8-week clinical assessment is recorded during follow-up calls or visits as described above. A list of collected data and blood investigations with time points at baseline, during follow-up visits or calls, and at 8 and 12 weeks is summarised in table 1.
Adverse events and reporting
Any undesirable circumstance or experiences reported by study participants during the study are categorised as adverse events. All adverse events which are possibly, probably or very likely related to administration of any supplement are monitored and reported to site institutional review boards (IRBs) within 72 hours (serious adverse events) or 1 month (all other adverse events), using a standardised reporting format. The trial Data and Safety Monitoring Board (DSMB) is also notified. Site principal investigators and independent physicians are responsible for assessing the causal relationship and making the conclusive decision about continuation of the trial for a particular participant. Additionally, medical insurance is provided to all study participants to take care of any progression of severe adverse events.
Data and sample management
All data collected as part of this trial are entered into password-protected android electronic tablets, with preprogrammed questionnaires using ODK.32 All data are automatically and directly uploaded from the tablets onto a secure electronic server, and entered into a password-protected database accessible only to authorised study team members. Data are stored in linked-anonymised form, with identifiable information and the linking key stored separately. All analyses and data checks are conducted on anonymised data only.
Blood samples collected as part of this trial are processed at the Foundation for Medical Research, Mumbai, and accredited laboratories in India including at the site hospitals. Specimens are linked-anonymised and are stored securely at the Foundation for Medical Research for a maximum of 3 years.
Data analysis
Planned analyses will initially be undertaken in blinded fashion (comparing coded treatment groups); unblinding of investigators and research staff with respect to treatment allocation will only occur once analyses are completed.
Planned analyses
An intent-to-treat analysis will be used as the primary analytic strategy. Time to primary outcome will be compared between participants randomised to vitamin D versus no vitamin D and zinc versus no zinc using Cox regression. We will investigate effect modification of either treatment effect by the other, and by third variables collected at baseline (including anthropometric status and vitamin D status). Effect modification will be assessed by including interaction terms in Cox regression models, and statistical significance assessed via likelihood ratio tests. There are no a priori effect modifiers hypothesised, and unless there is strong modification of a treatment effect, our power to detect these may be low. We will assess the success of randomisation by comparing baseline variables by treatment group using χ2 and t-tests and use multivariate modelling to adjust for imbalances if needed. Additional collected information, including data on prescribed medications and other treatments, will enable an assessment of whether important factors including non-protocol interventions are balanced across intervention groups.
The effect of vitamin D or zinc on dichotomous secondary outcomes will be analysed in a similar approach. The proportion of individuals experiencing hypercalcaemia will be compared between treatment groups using χ2 tests, and effects of the supplements on blood biomarkers will be compared via Wilcoxon and t-tests. This study will measure numerous risk factors for COVID-19 progression and severe treatment outcomes including haemoglobin; comorbidities; medications including chloroquine, hydroxychloroquine and ACE inhibitors; and socio-demographic, clinical, nutritional and lifestyle-related risk factors. We will examine relationships of these factors in the placebo group first, to avoid complex questions concerning interactions between risk factors and treatments. Once we find a satisfactory parsimonious model using principles of model selection as detailed by Greenland,48 we will test and modify it if needed in the whole study population, adjusting for treatment effects.
Analyses will consider sex and gender throughout, by disaggregating findings, and attempting to elucidate the roles of sex and gender in the clinical course and immune response by controlling for potential sociodemographic, nutritional, and immunological confounders.
Statistical power calculations
With a single endpoint for both interventions, the factorial design does not provide a ‘two-for-one’ power advantage, where the total number of participants required to test two treatments is lower using a single factorial trial compared with two parallel group trials.49 Power will decrease if each treatment has a moderate effect; we accounted for this in calculating the sample size. Assumptions related to treatment effects may be reasonably inferred from meta-analyses of well-designed randomised controlled trials studying these supplements in other acute respiratory illnesses.17 21–24 50 We based power analysis on the primary outcome of time from onset of disease to clinical recovery, using methodology for survival times, which assumes exponential distribution of the time to recovery.51 We calculated power for detecting specified hazard ratios associated with vitamin D or zinc given a specified true effect of the other treatment. Assuming average time to recovery of 22.2 days,52 and a low (5%) rate of loss to follow-up, enrolment of 700 patients will yield the statistical power estimates in table 2. This analysis indicates that we will have at least 80% power to detect a moderate (25–30%) effect of either treatment, given a maximum 30% true effect of the other treatment. We did not further adjust our power calculations and desired sample size following changes to our eligibility criteria, which may result in the inclusion of participants with symptoms that are both more severe (SpO2<90) and less severe (outpatients) at baseline.
Table 2.
Statistical power estimation
| True effect of treatment B | |||||||
| Effect of treatment A | 0% | 5% | 10% | 15% | 20% | 25% | 30% |
| 30% | 99 | 99 | 99 | 99 | 98 | 98 | 97 |
| 25% | 95 | 94 | 93 | 92 | 90 | 88 | 86 |
| 20% | 81 | 79 | 76 | 74 | 71 | 69 | 66 |
Patient and public involvement
Patients and the public were not involved in the design of this study.
Data and Safety Monitoring Board
The DSMB was established prior to commencement of the trial. It consists of independent experts in respiratory infection and communicable diseases, public health and nutrition, clinical research and biostatistics. The role of the board is to provide their inputs, recommendations, review the trial protocols and progress by ensuring the rights and safety of involving participants in the study through periodic trial review meetings.
The trial DSMB will examine efficacy endpoints by study arms when half of individuals are enrolled. In accordance with the Haybittle-Peto rule, if the difference in the primary outcome between study arms is <0.001, unblinding of the DSMB and stopping will be considered.53
Ethics and dissemination
This study is being conducted in the accordance with the Declaration of Helsinki 2013. The study was approved by the Institutional Review Board of the Harvard T.H. Chan School of Public Health (Protocol No. IRB20-1425), the University Health Network Research Ethics Board (20-5775), the Institutional Research Ethics Committee of the Foundation for Medical Research (IREC No. FMR/IREC/C19/02/2020), the Institutional Review Board of Saifee Hospital (Project No. EC/008/2020) and the KEM Hospital Research Centre Ethics Committee (KEMHRC ID No. 2027). Permission for the study was also obtained from the Health Ministry Screening Committee (HMSC), Government of India. Since the study intervention is related to micronutrient supplementation, endorsement from the Drugs Controller General of India was non-obligatory. The study findings will be presented in peer-reviewed medical journals.
Discussion
With continued high incidence of global cases, COVID-19 remains a global health challenge. Alongside vaccination and other preventative measures, low-cost and efficient interventions which may help minimise the occurrence of serious disease are needed. These would be particularly valuable in low-income and middle-income countries, where health systems are more overburdened and resources much fewer. In this context, and given previous evidence regarding the role of vitamin D and zinc in the development of and recovery from respiratory infections,17 21–24 50 there is a need to explore their potential value as part of therapeutic regimens for COVID-19.
We report here the protocol of a 2×2 factorial randomised controlled trial, designed to generate evidence on the effect of vitamin D and zinc on COVID-19 progression. The frequent follow-up of participants and collection of a range of sociodemographic, clinical and biomarker measures alongside blood samples will enable a detailed investigation of the effect of supplementation on disease progression, including potentially important immunological and inflammatory pathways. Importantly, in comparison with other vitamin D or zinc COVID-19 intervention studies currently registered on ClinicalTrials.gov, this would be the first conducted outside of the USA or Europe and other similar high-income countries. The location of this study in two large cities, alongside the broad eligibility criteria, increases the generalisability of study results. Given the current unpredictability of COVID-19 waves, one challenge to the study is to maintain recruitment during periods where cases may be on the decline. We have taken steps to mitigate any anticipated effects of this, including broadening our eligibility criteria as described previously, and rigorous training of site hospital staff to help improve recruitment of eligible individuals. Another limitation is that we may not be able to ascertain differences in distribution of sun exposure (as a source of vitamin D) across treatment groups, although we would expect this to be similar due to randomisation. Regardless, the findings of this study will have direct relevance to many settings in South Asia and sub-Saharan Africa with weak health systems and prevalent malnutrition. Ultimately, the evidence generated as part of this trial will enhance our understanding of the role of vitamin D and zinc in COVID-19 disease, and contribute high quality evidence on the potential value of supplementation of these micronutrients for the same.
Supplementary Material
Acknowledgments
We would like to thank all participants, doctors, nurses and site hospital staff at participating sites for their contribution in the trial implementation. We also thank all members of the DSMB and respective IRBs for their guidance and valuable inputs in the trial.
Footnotes
Contributors: WWF, KCK, YD and NM conceptualised the project and designed the study along with SB, KKS, ECH, YM and UP. YD, NM, PDC, GG, KKS, YM and SS are involved in data acquisition, and in study monitoring along with KCK, WWF and UP. MW provides statistical expertise. KKS and UP drafted the manuscript, and all authors reviewed and critically revised the draft and approved the final manuscript.
Funding: This trial is supported by the Canadian Institutes of Health Research, Operating Grant: COVID-19 Rapid Research Funding Opportunity—Therapeutics, application number: 447092 and the Canada Research Chair programme (to KCK). SB was supported by the National Institutes of Health (grant D43 TW010543).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020;20:533–4. 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogan A, Winksill P, Watson O. Report 33 - Modelling the allocation and impact of a COVID-19 vaccine.
- 3.Phillips N. The coronavirus is here to stay - here's what that means. Nature 2021;590:382–4. 10.1038/d41586-021-00396-2 [DOI] [PubMed] [Google Scholar]
- 4.Torjesen I. Covid-19 will become endemic but with decreased potency over time, scientists believe. BMJ 2021;372:n494. 10.1136/bmj.n494 [DOI] [PubMed] [Google Scholar]
- 5.Hockham C, Kotwal S, Wilcox A, et al. Protocol for the controlled evaLuation of angiotensin receptor blockers for COVID-19 respiratory disease (clarity): a randomised controlled trial. Trials 2021;22:573. 10.1186/s13063-021-05521-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teshome A, Adane A, Girma B, et al. The impact of vitamin D level on COVID-19 infection: systematic review and meta-analysis. Front Public Health 2021;9:624559. 10.3389/fpubh.2021.624559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouillon R, Marcocci C, Carmeliet G, et al. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocr Rev 2019;40:1109–51. 10.1210/er.2018-00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearce SHS, Cheetham TD. Diagnosis and management of vitamin D deficiency. BMJ 2010;340:b5664. 10.1136/bmj.b5664 [DOI] [PubMed] [Google Scholar]
- 9.Misra P, Srivastava R, Misra A, et al. Vitamin D status of adult females residing in Ballabgarh health and demographic surveillance system: a community-based study. Indian J Public Health 2017;61:194. 10.4103/ijph.IJPH_176_16 [DOI] [PubMed] [Google Scholar]
- 10.Suryanarayana P, Arlappa N, Sai Santhosh V, et al. Prevalence of vitamin D deficiency and its associated factors among the urban elderly population in Hyderabad metropolitan City, South India. Ann Hum Biol 2018;45:133–9. 10.1080/03014460.2018.1425479 [DOI] [PubMed] [Google Scholar]
- 11.Greiller CL, Martineau AR. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients 2015;7:4240–70. 10.3390/nu7064240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greiller CL, Suri R, Jolliffe DA, et al. Vitamin D attenuates rhinovirus-induced expression of intercellular adhesion molecule-1 (ICAM-1) and platelet-activating factor receptor (PAFR) in respiratory epithelial cells. J Steroid Biochem Mol Biol 2019;187:152–9. 10.1016/j.jsbmb.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 13.Telcian AG, Zdrenghea MT, Edwards MR, et al. Vitamin D increases the antiviral activity of bronchial epithelial cells in vitro. Antiviral Res 2017;137:93–101. 10.1016/j.antiviral.2016.11.004 [DOI] [PubMed] [Google Scholar]
- 14.Hansdottir S, Monick MM, Lovan N, et al. Vitamin D decreases respiratory syncytial virus induction of NF-kappaB-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J Immunol 2010;184:965–74. 10.4049/jimmunol.0902840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansdottir S, Monick MM, Hinde SL, et al. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol 2008;181:7090–9. 10.4049/jimmunol.181.10.7090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou J, Du J, Huang L, et al. Preventive effects of vitamin D on seasonal influenza A in infants: a multicenter, randomized, open, controlled clinical trial. Pediatr Infect Dis J 2018;37:749–54. 10.1097/INF.0000000000001890 [DOI] [PubMed] [Google Scholar]
- 17.Abioye AI, Bromage S, Fawzi W. Effect of micronutrient supplements on influenza and other respiratory tract infections among adults: a systematic review and meta-analysis. BMJ Glob Health 2021;6:e003176. 10.1136/bmjgh-2020-003176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown KH, Rivera JA, et al. , International Zinc Nutrition Consultative Group (IZiNCG) . International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr Bull 2004;25:S99–203. [PubMed] [Google Scholar]
- 19.Smith MR, DeFries R, Chhatre A, et al. Inadequate zinc intake in India: past, present, and future. Food Nutr Bull 2019;40:26–40. 10.1177/0379572118825176 [DOI] [PubMed] [Google Scholar]
- 20.GBD Compare . Institute for health metrics and evaluation, 2014. Available: https://www.healthdata.org/data-visualization/gbd-compare [Accessed 17 Dec 2021].
- 21.Hemilä H, Fitzgerald JT, Petrus EJ, et al. Zinc acetate Lozenges may improve the recovery rate of common cold patients: an individual patient data meta-analysis. Open Forum Infect Dis 2017;4:ofx059. 10.1093/ofid/ofx059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roth DE, Richard SA, Black RE. Zinc supplementation for the prevention of acute lower respiratory infection in children in developing countries: meta-analysis and meta-regression of randomized trials. Int J Epidemiol 2010;39:795–808. 10.1093/ije/dyp391 [DOI] [PubMed] [Google Scholar]
- 23.Bhatnagar S, Wadhwa N, Aneja S, et al. Zinc as adjunct treatment in infants aged between 7 and 120 days with probable serious bacterial infection: a randomised, double-blind, placebo-controlled trial. Lancet 2012;379:2072–8. 10.1016/S0140-6736(12)60477-2 [DOI] [PubMed] [Google Scholar]
- 24.Banupriya N, Bhat BV, Benet BD, et al. Short Term Oral Zinc Supplementation among Babies with Neonatal Sepsis for Reducing Mortality and Improving Outcome - A Double-Blind Randomized Controlled Trial. Indian J Pediatr 2018;85:5–9. 10.1007/s12098-017-2444-8 [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J Med Virol 2020;92:479–90. 10.1002/jmv.25707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skalny AV, Rink L, Ajsuvakova OP, et al. Zinc and respiratory tract infections: perspectives for COVID‑19 (review). Int J Mol Med 2020;46:17–26. 10.3892/ijmm.2020.4575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.te Velthuis AJW, van den Worm SHE, Sims AC, et al. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog 2010;6:e1001176. 10.1371/journal.ppat.1001176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.COVID19 STATEWISE status. MyGov.in, 2020. Available: https://mygov.in/corona-data/covid19-statewise-status/ [Accessed 17 Dec 2021].
- 29.Tambe MP, Parande MA, Tapare VS, et al. An epidemiological study of laboratory confirmed COVID-19 cases admitted in a tertiary care hospital of Pune, Maharashtra. Indian J Public Health 2020;64:S183–7. 10.4103/ijph.IJPH_522_20 [DOI] [PubMed] [Google Scholar]
- 30.Kodge BG. A review on current status of COVID19 cases in Maharashtra state of India using GIS: a case study. Spatial Information Research 2020:1–7. [Google Scholar]
- 31.India: who coronavirus disease (COVID-19) Dashboard with vaccination data. Available: https://covid19.who.int [Accessed 17 Dec 2021].
- 32.ODK - Collect data anywhere. Available: https://getodk.org [Accessed 17 Dec 2021].
- 33.Trang HM, Cole DE, Rubin LA, et al. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr 1998;68:854–8. 10.1093/ajcn/68.4.854 [DOI] [PubMed] [Google Scholar]
- 34.Armas LAG, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab 2004;89:5387–91. 10.1210/jc.2004-0360 [DOI] [PubMed] [Google Scholar]
- 35.Kearns MD, Binongo JNG, Watson D, et al. The effect of a single, large bolus of vitamin D in healthy adults over the winter and following year: a randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr 2015;69:193–7. 10.1038/ejcn.2014.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kearns MD, Alvarez JA, Tangpricha V. Large, single-dose, oral vitamin D supplementation in adult populations: a systematic review. Endocr Pract 2014;20:341–51. 10.4158/EP13265.RA [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vieth R, Bischoff-Ferrari H, Boucher BJ, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr 2007;85:649–50. 10.1093/ajcn/85.3.649 [DOI] [PubMed] [Google Scholar]
- 38.Dong Y, Stallmann-Jorgensen IS, Pollock NK, et al. A 16-week randomized clinical trial of 2000 international units daily vitamin D3 supplementation in black youth: 25-hydroxyvitamin D, adiposity, and arterial stiffness. J Clin Endocrinol Metab 2010;95:4584–91. 10.1210/jc.2010-0606 [DOI] [PubMed] [Google Scholar]
- 39.Schleithoff SS, Zittermann A, Tenderich G, et al. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 2006;83:754–9. 10.1093/ajcn/83.4.754 [DOI] [PubMed] [Google Scholar]
- 40.Pappa HM, Mitchell PD, Jiang H, et al. Treatment of vitamin D insufficiency in children and adolescents with inflammatory bowel disease: a randomized clinical trial comparing three regimens. J Clin Endocrinol Metab 2012;97:2134–42. 10.1210/jc.2011-3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abu-Mouch S, Fireman Z, Jarchovsky J, et al. Vitamin D supplementation improves sustained virologic response in chronic hepatitis C (genotype 1)-naïve patients. World J Gastroenterol 2011;17:5184–90. 10.3748/wjg.v17.i47.5184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Institute of Medicine (US) Panel on Micronutrients . Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academies Press (US), 2001. [PubMed] [Google Scholar]
- 43.AIIMS/ICMR National Task Force/Joint Monitoring Group . Clinical guidelines for management of adult COVID-19 patients: revised on 14/01/. New Delhi: Ministry of Health and Family Welfare, Government of India, 2022. https://www.mohfw.gov.in/pdf/ClinicalGuidanceforManagementofAdultCovid19Patientsupdatedason17thJanuary2022.pdf [Google Scholar]
- 44.MOHFW, GOI . Clinical Management Protocol for COVID-19 (In Adults) - Version 6 (24.05.21. New Delhi: Ministry of Health and Family Welfare, Government of India, 2021. https://www.mohfw.gov.in/pdf/UpdatedDetailedClinicalManagementProtocolforCOVID19adultsdated24052021.pdf [Google Scholar]
- 45.MOHFW, GOI . Revised discharge policy for COVID-19. New Delhi: Ministry of Health and Family Welfare, Government of India, 2022. https://www.mohfw.gov.in/pdf/RevisedDischargePolicyforCOVID19updatedon9thJanuary2022.pdf [Google Scholar]
- 46.Kumar N, Shahul Hameed SK, Babu GR, et al. Descriptive epidemiology of SARS-CoV-2 infection in Karnataka state, South India: transmission dynamics of symptomatic vs. asymptomatic infections. EClinicalMedicine 2021;32:100717. 10.1016/j.eclinm.2020.100717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laxminarayan R, B CM, G VT, et al. SARS-CoV-2 infection and mortality during the first epidemic wave in Madurai, South India: a prospective, active surveillance study. Lancet Infect Dis 2021;21:1665–76. 10.1016/S1473-3099(21)00393-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health 1989;79:340–9. 10.2105/ajph.79.3.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ellenberg SS, Finkelstein DM, Schoenfeld DA. Statistical issues arising in AIDS clinical trials. J Am Stat Assoc 1992;87:562–9. 10.1080/01621459.1992.10475240 [DOI] [Google Scholar]
- 50.Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 2017;356:i6583. 10.1136/bmj.i6583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schoenfeld DA, Richter JR. Nomograms for calculating the number of patients needed for a clinical trial with survival as an endpoint. Biometrics 1982;38:163–70. 10.2307/2530299 [DOI] [PubMed] [Google Scholar]
- 52.Dorigatti I, Okell L, Cori A. Report 4: severity of 2019-novel coronavirus (nCoV). Imperial College London, 2020. [Google Scholar]
- 53.Blenkinsop A, Parmar MK, Choodari-Oskooei B. Assessing the impact of efficacy stopping rules on the error rates under the multi-arm multi-stage framework. Clin Trials 2019;16:132–41. 10.1177/1740774518823551 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.