Abstract
Background/purpose
Although many coronavirus disease 2019 (COVID-19) vaccine injections have been administered worldwide, the safety of this practice remains unclear. This study aimed to compare the rates of complications associated with COVID-19 vaccines administered by dentists with those of vaccines administered by nurses. This study aimed to evaluate the safety of a vaccination program delivered by dentists.
Materials and methods
This observational cohort study included 537 recipients of the second dose of the Pfizer COVID-19 vaccine, delivered as an intramuscular injection to the upper arm deltoid muscle by dentists or nurses at the study site. Vaccine recipients were divided into two groups according to the vaccination administrator (dentist vs. nurse groups). The rates of complications associated with intramuscular injection technique (numbness in the hand or arm at the time of the injection, vasovagal reflex at the time of the injection, vaccine-related shoulder injury, and prolonged numbness) were examined.
Results
A total of 125 vaccine recipients were included (nurse group, n = 84; dentist group, n = 41). The overall incidence rate of complications was lower in the dentist group (2.4%; 1/41) than in the nurse group (8.3%; 7/84). However, this difference was not statistically significant (P = 0.3).
Conclusion
This study suggests that the safety of COVID-19 vaccine administration is comparable between dentists and nurses.
Keywords: COVID-19, Complication, Dentist, Intramuscular injection, Novel coronavirus infection, Vaccinations
Introduction
On December 2019, an outbreak of unknown-cause pneumonia was observed among people associated with the Huanan Seafood Wholesale Market in Wuhan, Hubei Province, China.1,2 On January 8, 2020, the causative agent of this outbreak was identified as a novel coronavirus and the associated disease (COVID-19) was recognized. Approximately a month later, cases of COVID-19 were confirmed in Japan. The outbreak reached global proportions a few months thereafter, leading the World Health Organization to proclaim it a pandemic on March 11, 2020.
Vaccines for COVID-19 were developed in record time and continue to be administered worldwide, contributing to infection control and prevention. In Japan, vaccination began in February 2021. However, due to an overwhelmed healthcare system, securing enough physicians and nurses to deliver the vaccines was a challenge. In response, the Japanese government granted an extraordinary permission for dentists to deliver vaccines, aiming to support large-scale vaccination efforts without further compromising access to medical care.2 Similarly, some parts of the United States expanded the scope of dental practice to enable dentists to provide COVID-19 vaccines to community residents.3
Between May and July 2021 (3 months), a total of 12,727 dentists administered a total of 721,471 vaccines in Japan. Although many injections have been administered by dentists worldwide, the safety of this practice remains unclear. Given the requirement to continue to administer booster doses of the COVID-19 vaccines, which reduce the risk of severe disease and death, it is likely that dentists will remain involved in vaccination efforts. Consequently, understanding the safety profile of this practice is important to ensuring good outcomes. This study aimed to compare the rates of vaccination-related complications between individuals that received their injection from a nurse and those that received it from a dentist. To our best knowledge, this is the first study to appraise the safety of COVID-19 vaccine delivery by dentists.
Materials and methods
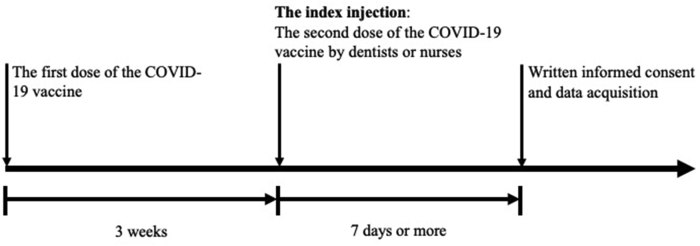
The study was approved by the relevant ethics committee and was conducted according to the principles of the Declaration of Helsinki. The study protocol is presented in Fig. 1. This observational cohort study included 537 recipients of the second dose (30 μg) of the Pfizer COVID-19 vaccine delivered to the deltoid muscle by dentists or nurses at the study site from May 12, 2021, to June 16, 2021. The injections were delivered at a point where the line connecting the top of the anterior and posterior axillary lines intersects perpendicularly with the line descending from the acromion (middle of the deltoid muscle located at a distance of two-to three-finger-width below the acromion).4,5 The skin was pierced in the perpendicular direction using 25-gauge (diameter) and 25-mm (length) needles.5 All second dose recipients had received their first dose of the Pfizer vaccine 3 weeks before the index injection; both injections were delivered in the same manner. All participants were vaccinated after a medical consultation with a physician. Seven dentists and 10 nurses were responsible for administering the second dose of vaccine. Whether the injection was delivered by a dentist or nurse was determined by convenience. Based on the guidance from the Ministry of Health, Labor and Welfare of Japan, ahead of delivering the vaccines, all dentists had to undergo e-learning training on the COVID-19 vaccines (including adverse effect information), injection site anatomy, vaccine delivery practicalities (including precautions recommended at the time of an injection), and allergic reaction/anaphylactic shock risks associated with the vaccine.2 All participating dentists were also required to undergo practical training on vaccination.2
Figure 1.
Study protocol.
Seven days or more after the index injection, vaccination recipients were asked to provide information on their demographic characteristics (sex, age, weight, high, and shoulder disorders) and any complications experienced in the aftermath of the injection (numbness in the hand or arm at the time of the injections, vasovagal reflex at the time of the injection, pain levels at the time of the injections, shoulder injury related to vaccine [SIRVA], and prolonged numbness lasting >7 days). The data were collected using Google Forms®. Written informed consent was obtained from the participants.
The vaccination recipients were divided based on vaccine administrators into the dentist group and nurse group. The primary endpoint was the overall incidence of vaccination complications related to the intramuscular injection technique. The duplications were excluded from the incidence of overall complications. The secondary study endpoint was the frequency of each complication. If nausea or syncope occurred immediately after the intramuscular injection, the recipient was classified as having a vasovagal reflex.6,7 SIRVA was diagnosed if shoulder pain and restricted range of motion began within 48 h of vaccination and persisted for more than 7 days.5 Finally, pain level at the time of the injection was measured using a numerical rating scale (0 points: no pain, 10 points: worst pain possible). Recipients with shoulder disorders were excluded from this study.
Statistical analysis
Age, height, weight, body mass index, and pain levels at the time of the injection were presented as mean values (±standard deviation); comparisons were made with the two-sample t-test. Sex and complication frequencies were presented as counts and percentages and were compared using the 2 × 2 χ2 test, Yates' 2 × 2 χ2 test, or Fisher's 2 × 2 χ2 test, as suitable. All tests of significance were two-sided, and P-values of <0.05 were considered indicative of statistically significant differences.
Results
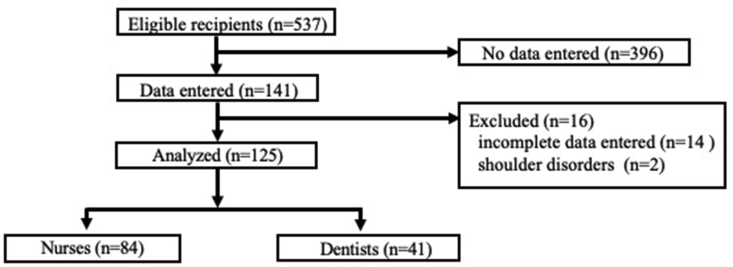
The details of participant recruitment and group assignment are presented in Fig. 2. Overall, 141 vaccine recipients returned follow-up questionnaires. Among them, 14 and 2 participants provided incomplete data and had shoulder disorders, respectively, and were excluded. Finally, a total of 125 participants were included in the study (nurse group, n = 84; dentist group, n = 41). Participant characteristics were comparable in both groups (Table 1).
Figure 2.
Vaccine recipient selection and group assignment.
Table 1.
Demographic data of the participants.
| Nurse (n = 84) |
Dentist (n = 41) |
|
|---|---|---|
| Age (yr) | 39.0 ± 9.4 | 36.7 ± 11.1 |
| Sex (male:female) | 36:48 | 8:33 |
| Hight (cm) | 163.4 ± 8.8 | 162.3 ± 7.8 |
| Weight (kg) | 60.0 ± 13.0 | 55.8 ± 10.8 |
| BMI (kg/m2) | 22.3 ± 3.7 | 21.1 ± 3.4 |
Data are expressed as number of participant or mean ± standard deviation. BMI, body mass index.
The incidence rates of complications associated with intramuscular injection technique are presented in Table 2. The overall incidence rate was lower in the dentist group (2.4%; 1/41) than in the nurse group (8.3%; 7/84); however, these differences were not statistically significant (P = 0.3). The incidence rates of numbness in the hand or arm at the time of the injection (4.8% vs. 2.4%; P = 1.0), vasovagal reflex at the time of the injection (2.4% vs. 0%; P = 1.0), and SIRVA (1.2% vs. 0%; P = 1.0), were also lower in the dentist group than in the nurse group; however, these differences were not statistically significant. Numbness in the hand or arm dissipated in both groups within 7 days of onset. Both groups experienced comparable levels of pain (3.2 ± 2.6 vs 2.9 ± 2.6 points; P = 0.5).
Table 2.
The incidence rates of complications associated with the intramuscular injection technique.
| Nurse (n = 84) |
Dentist (n = 41) |
P-value | |
|---|---|---|---|
| Incidence of complications [%(n)] | 8.3 (7) | 2.4 (1) | 0.3 |
| Complications immediately after vaccination | |||
| Numbness in the hand or arm [%(n)] | 4.8 (4) | 2.4 (1) | 1.0 |
| Vasovagal reflex [%(n)] | 2.4 (2) | 0 (0) | 1.0 |
| Pain levels at the time of injections [points] | 3.2 ± 2.6 | 2.9 ± 2.6 | 0.5 |
| SIRVA [%(n)] | 1.2 (1) | 0 (0) | 1.0 |
| Prolonged numbness in the hand or arm [%(n)] | 0 (0) | 0 (0) | 1.0 |
Data are expressed as percentage (number) or mean ± standard deviation. Duplicate data were excluded from incidence of overall complications. Pain level at the time of the injection was measured using a numerical rating scale. SIRVA, shoulder injury related to vaccine administration.
Discussion
This study suggests that COVID-19 vaccine administration by dentists is safe. In this study, the incidence of injection technique-related complications was lower in the dentist group than in the nurse group; however, this difference was not statistically significant. Although some Japanese media reports questioned the safety of vaccination by dentists, the present findings fail to support these claims. The present evidence suggests that vaccination safety is comparable between nurse and dentist providers.
Intramuscular injection into the deltoid muscle can injure the radial nerve and the anterior branch of the axillary nerve.4 The radial nerve branches off the posterior nerve bundle of the brachial plexus and travels wrapped around the humerus (spiral groove).4 Injury to the radial nerve may cause numbness and movement disorders from the upper arm to the fingers. The axillary nerve originates from the posterior nerve bundle of the brachial plexus. The anterior branch of the axillary nerve runs from the posterior to the anterior side of the humerus and distributes signals to the median deltoid muscle.4 Injury to the anterior branch of the axillary nerve may cause numbness in the deltoid region and weakness in shoulder abduction. All dentists and nurses who administered the vaccines were required to ask the recipient about any numbness in the hand or arm when the needle was inserted into the deltoid muscle. If the recipient complained of numbness in the hand or arm, the needle was removed, and the location of the injection was shifted before the vaccine was administered. In this study, numbness in the hand or arm at the time of the injection occurred in 4.8% (4/84) and 2.4% (1/41) of the recipients in the nurse and dentist groups, respectively, likely reflecting the rate of injury to radial or anterior axillary nerve branches or to the tissue surrounding those structures. However, the numbness occurred only at the time of injection and dissipated shortly thereafter in both groups. If a patient complains of numbness immediately after the injection, quickly changing the site of injection may help minimize the damage to the nerve axon and prevent prolonged numbness.
The vasovagal reflex is one of the most common systemic incidents in dentistry; it may occur in response to vaccination.6,7 Various factors are involved in the development of the vasovagal reflex, both in dentistry and in vaccination; however, it tends to be triggered by pain experienced under the conditions of anxiety or tension, which decrease blood flow to the brain, resulting in nausea or syncope.7 Vasovagal reflex occurs in approximately 2% of patients receiving local anesthesia in dental procedures;8 however, the corresponding rate in vaccination recipients is unclear. In this study, the incidence rates of the vasovagal reflex in the nurse and dentist groups were 2.4% and 0%, respectively; however, this difference was not statistically significant. The numerical rating scale of the dentist group was slightly lower than that of the nurse group; however, this difference was not statistically significant. This result suggests that an intramuscular injection delivered by a dentist may be less painful than that delivered by a nurse. The vasovagal reflex may have been absent in the dentist group because the intramuscular injections delivered by the dentists were less painful than those delivered by the nurses.
SIRVA may occur after vaccination; it is characterized by shoulder pain accompanied by a limited range of motion. SIRVA is a prolonged inflammatory reaction caused by an incorrect injection of the vaccine into the subdeltoid bursa.5 It tends to occur within minutes to 48 h after vaccination and may last for a week or longer.5 The prolonged inflammatory response associated with SIRVA distinguishes it from the local effect of vaccination that remits within 2–3 days.5 The most common cause of SIRVA is an incorrect injection technique. In this study, SIRVA occurred in only one case in the nurse group and did not occur at all in the dentist group, suggesting that incorrect injection of the vaccine into the subdeltoid bursa was successfully prevented. However, vaccine recipient characteristics may have affected the present results. A previous study has shown that obesity and older age are risk factors for SIRVA.9 These factors may also affect anatomical distances increasing the risk of incorrectly targeted injections. However, in this study, all participants were young Asians of normal stature. Consequently, these results may not be generalizable to other populations.
The limitations of the present study include its observational, single-center, and non-randomized study design and small sample size. Hence, a prospective, randomized, large-scale, multicenter study is necessary to validate the results of this study.
In conclusion, this study revealed that COVID-19 vaccine administration by dentists is as safe as that by nurses. This is the first study to support the safety of COVID-19 vaccine delivery by dentists.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgements
This study was supported by a Grant-in-Aid for 2021 Young Researcher Projects from Kanagawa Dental University Graduate School of Dentistry. The authors are greatly obliged to Prof. Satoshi Ino, director of the Kanagawa Dental University Hospital, for his valuable comments and suggestions.
References
- 1.Holshue M.L., DeBolt C., Lindquist S., et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanuki T. Dentists and COVID-19 vaccine administration. Kanagawa Shigaku. 2021;56:133–140. [In Japanese, English abstract] [Google Scholar]
- 3.Rojas-Ramirez M.V., DeVito D.M., McKee J.W., Miller C.S. Empowering dentists to administer COVID-19 vaccines. J Publ Health Dent. 2022 doi: 10.1111/jphd.12502. [DOI] [PubMed] [Google Scholar]
- 4.Cook I.F. Best vaccination practice and medically attended injection site events following deltoid intramuscular injection. Hum Vaccines Immunother. 2015;11:1184–1191. doi: 10.1080/21645515.2015.1017694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maliwankul K., Boonsri P., Klabklay P., Chuaychoosakoon C. Shoulder injury related to COVID-19 vaccine administration: a case series. Vaccines. 2022;10:588. doi: 10.3390/vaccines10040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention . CDC; Atlanta, GA: 2022. Fainting (syncope) after vaccination.https://www.cdc.gov/vaccinesafety/concerns/fainting.html Available at: [Date accessed: July 22, 2022] [Google Scholar]
- 7.Takase B., Hayashi K., Takei S., Hisada T., Masaki N., Nagata M. Delayed vasovagal reaction with reflex syncope following Covid-19 vaccination. Intern Med. 2022 doi: 10.2169/internalmedicine.9318-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arakeri G., Arali V. A new hypothesis of cause of syncope: trigeminocardiac reflex during extraction of teeth. Med Hypotheses. 2010;74:248–251. doi: 10.1016/j.mehy.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 9.Hesse E.M., Atanasoff S., Hibbs B.F., et al. Shoulder injury related to vaccine administration (SIRVA): petitioner claims to the national vaccine injury compensation program, 2010-2016. Vaccine. 2020;38:1076–1083. doi: 10.1016/j.vaccine.2019.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]