Abstract
Current guidelines recommend neoadjuvant (NAC) and/or adjuvant chemotherapy for locally advanced gastric cancers (LAGCs). However, the choice and duration of NAC regimen is standardized, rather than personalized to biologic response, despite the availability of several different classes of agents for the treatment of gastric cancer (GC). The current trial will use a tumor-informed ctDNA assay (Signatera™) and monitor response to NAC. Based on ctDNA kinetics, the treatment regimen is modified. This is a prospective single center, single-arm, open-label study in clinical stage IB-III GC. ctDNA is measured at baseline and repeated every 8 weeks. Imaging is performed at the same intervals. The primary end point is the feasibility of this approach, defined as percentage of patients completing gastrectomy.
Keywords: : ctDNA, gastrectomy, gastric cancer, neoadjuvant chemotherapy
Introduction
Resectable, locally advanced gastric cancer (LAGC; i.e. stage II/III: T2+, any N; or any T, N+ by AJCC staging) accounts for approximately 30% of newly diagnosed patients. Despite best currently available treatments including chemotherapy, radiotherapy, surgery and immunotherapy, the prognosis remains poor with more than 50% of patients eventually developing recurrence [1]. Many perioperative strategies added to surgery proved systemic treatment with or without radiotherapy are superior to surgery alone. However, with few exceptions they were not directly compared to each other. Therefore, the result has been treatment heterogeneity based on geographical, institutional, and even individual physician preferences.
For gastric cancer (GC), 5FU, leucovorin, oxaliplatin and docetaxel (FLOT) for four cycles before and after surgery [2] was superior to the previous standard regimen, epirubicin, cisplatin, 5FU (ECF) [3]. Other options include surgery first followed by adjuvant capecitabine and oxaliplatin for 6 months [4]. A neoadjuvant approach (e.g., using FLOT) has several advantages, including tumor downstaging and improved complete resection (R0) rates, early treatment of possible micrometastatic disease, and improved selection for patients who will actually benefit from gastrectomy.
All available chemotherapy regimens have significant toxicity and the prognosis for LAGC remains poor. Hence, there is a large unmet need for improvement in the neoadjuvant space. The problem is highlighted even more by the fact that in the FLOT-4 study, only 50–60% of patients complete the adjuvant component of FLOT. The main reasons being poor performance post-operatively. Therefore, one option to address this issue is to complete all the therapy prior to surgery using total neoadjuvant therapy (TNT). This approach has been successfully applied to rectal cancers [5].
More recently, bespoke (i.e., tumor informed) ctDNA molecular residual disease (MRD) testing has been introduced in the management of many solid tumors, including gastroesophageal cancers [6].
The available ctDNA MRD data are consistent with mounting evidence that ctDNA-positivity at after neoadjuvant therapy and curative intent surgery is likely the most important negative prognostic biomarker. Recent prospective data in colon cancer show that ctDNA eradication with chemotherapy can improve outcomes, i.e. ctDNA also appears to be predictive [7]. In LACG, prospective data are needed to demonstrate whether ctDNA guided therapeutic decisions (i.e., continuation or changing therapy) can improve outcomes. An early lack of ctDNA response, similar to PET-directed therapy [8], and subsequent adjustment of the neoadjuvant regimen may each help personalize therapy and improve outcomes.
Background & rationale
Neoadjuvant systemic therapy (+/- radiation based on anatomic site) has several advantages over adjuvant treatment for LAGC. The treatment is better tolerated, patient compliance is higher, clinical response to treatment can be monitored in vivo, R0 resection rates (i.e. negative surgical margins) are higher, and potential eradication of micrometastases is initiated earlier [9]. Additionally, there is a potential benefit to facilitate the surgical approach, either by converting an inoperable cancer to one that is operable, or by converting a patient who is felt to be a candidate for total gastrectomy to one who might be treated successfully with partial stomach conserving therapy.
The potential concern is overtreatment of patients. However, current guidelines for LAGGC recommend adjuvant chemotherapy (mainly in Asia), peri-operative chemotherapy (mainly outside of Asia) or neoadjuvant chemoradiation followed by adjuvant nivolumab (for gastroesophageal junction adenocarcinoma) for all patients with at least cT2+ or any cN+ gastroesophageal adenocarcinoma [10].
Thus, the argument of overtreatment does not apply for LAGC since patients are clinically staged at diagnosis, and any stage IB or higher is committed to the entire treatment regimen. However, the choice and duration of peri-operative systemic treatment is standardized, rather than personalized to biologic response, despite the availability of several different classes of agents in the treatment of GC.
Hence, the unmet need in the treatment of stage IB-III LAGC is a personalized approach with real-time adaptation of the treatment regimen based on biologic response. The assessment of the stomach wall for radiographic response is inherently challenging and as a matter of fact not recommended as ‘measurable lesion’ for clinical trial enrollment. Similarly, locoregional lymphadenopathy might not exhibit any significant radiographic changes in the setting of neoadjuvant treatment, and thus it is not suitable for treatment monitoring. By definition, there are no other sites of visible disease in LAGC. Traditional tumor markers such as CEA and CA19-9 are not sensitive or specific enough to accurately reflect response to treatment, especially in patients with lower baseline levels of these markers [11].
The hypothesis is that using Signatera's ctDNA assay for molecular residual disease [12–15], it is possible to closely monitor response to treatment in a highly sensitive and specific manner and modify neoadjuvant treatment regimens based on response without negatively affecting curative intent surgery and surgical complications.
Study design
This is a prospective, single-arm, open-label, phase Ib feasibility study. The patients will be treated with one of the standard neoadjuvant protocols listed in Table 1. Staging laparoscopy will be done prior to enrollment. ctDNA will be measured at baseline and then every 8 weeks (i.e. four cycles for 14-day regimens). ctDNA levels are reported as tumor molecules per ml of plasma (MTM/ml). Any decline or increase of MTM/ml compared to the previous measurement is defined as decrease or increase. If no decline in ctDNA is detected after four cycles, treatment will be switched to a different chemotherapy backbone (e.g., irinotecan based regimen, or taxane based if not used upfront). ctDNA will be re-evaluated after three cycles (if a 21-day regimen) or four cycles (if a 14 day regimen) of the 2nd line regimen. If ctDNA is lower than the previous measurement, then the same regimen continues for three–four more cycles (depending on whether 14-day or 21-day cycle), followed by gastrectomy (Figure 1). Since the current standard peri-operative or adjuvant chemotherapy trials use at least 4 months of chemotherapy [2,4,16], all patients who remain on study will receive a minimum of eight cycles (or 4 months) of systemic treatment (i.e. even in patients with a negative ctDNA level after the initial four cycles [or 2 months] of treatment).
Table 1. . Chemotherapy regimens.
| First-line options: | |
| FLOT | Oxaliplatin 85 mg/m2 i.v. on day 1 Docetaxel 50 mg/m2 i.v. on day 1 Leucovorin 200 mg/m2 i.v. on day 1 Fluorouracil 2600 mg/m2 continuous infusion over 24 h daily on day 1 Every 14 days |
| FOLFOX | Oxaliplatin 85 mg/m2 i.v. on day 1 Leucovorin 400 mg/m2 i.v. on day 1 Fluorouracil 400 mg/m2 i.v. push on day 1 Fluorouracil 1200 mg/m2 continuous infusion over 24 h daily on days 1 and 2 Every 14 days |
| Second-line options: | |
| FOLFIRI | Irinotecan 180 mg/m2 i.v. on day 1 Leucovorin 400 mg/m2 i.v. on day 1 Fluorouracil 400 mg/m2 i.v. push on day 1 Fluorouracil 1200 mg/m2 continuous infusion over 24 h daily on days 1 and 2 Every 14 days |
| FOLFIRINOX | Oxaliplatin 85 mg/m2 i.v. on day 1 Irinotecan 150 mg/m2 i.v. on day 1 Leucovorin 200 mg/m2 i.v. on day 1 Fluorouracil 1200 mg/m2 continuous infusion over 24 h daily on days 1 and 2 Every 14 days |
| Paclitaxel with carboplatin | Paclitaxel 200 mg/m2 i.v. on day 1 Carboplatin AUC 5 i.v. on day 1 Every 21 days |
| Paclitaxel | Paclitaxel 80 mg/m2 i.v. on days 1, 8, 15 Every 28 days |
| Docetaxel and irinotecan† | Docetaxel 35 mg/m2 i.v. on days 1 and 8 Irinotecan 50 mg/m2 i.v. on days 1 and 8 Every 21 days |
| Docetaxel 75 mg/m2 i.v. on day 1 Every 21 days | |
| Docetaxel 150 mg/m2 i.v. on day 1 Every 14 days | |
| Additional therapy options: | |
| Nivolumab‡ | 240 mg i.v. on day 1 every 14 days, or 360 mg i.v. on day 1 every 21 days, or 480 mg i.v. on day 1 every 28 days |
| Pembrolizumab‡ | 200 mg i.v. on day 1 every 21 days, or 400 mg i.v. on day 1 every 42 days |
Alone or combined.
Alone or when added to a regimen above.
AUC: Area under the curve; FLOT: 5FU, leucovorin, oxaliplatin and docetaxel; h: Hour; i.v.: Intravenously.
Figure 1. . Study schema.
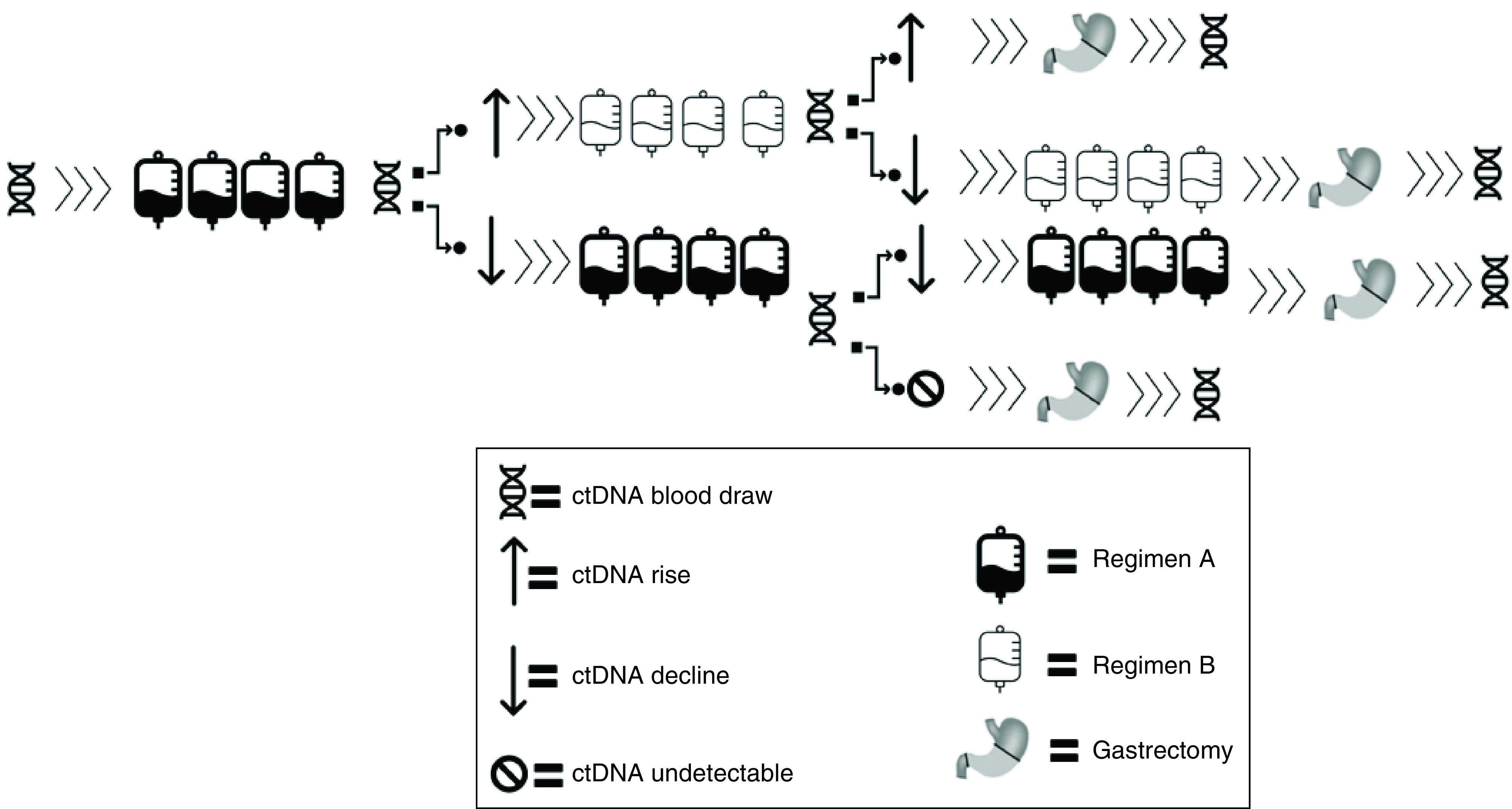
Patients who do not show a ctDNA decline to either first or second regimen are presumed refractory and proceed to gastrectomy.
However, if a ctDNA decline is seen after the first four cycles, then the initial regimen is continued for another four cycles, and if ctDNA is negative then the patient proceeds with gastrectomy. Patients with declining ctDNA on the first regimen after eight cycles proceed with another four cycles of the same prior to gastrectomy. If a post-operative ctDNA is negative, then the patient will be observed (since already treated with at least the standard 4 months or eight cycles of total chemotherapy). Adjuvant treatment is given based on the investigator's discretion.
Following surgery, ctDNA will be monitored in all patients every 3 months for up to 2 years, or clinical recurrence, whichever occurs first.
Imaging of the chest, abdomen and pelvis using computed tomography (CT) or MRI will be performed every 8 weeks. Patients with new metastatic disease while on treatment will come off protocol since not a candidate for gastrectomy anymore.
Radiation is allowed if deemed to be beneficial prior to resection based on the discretion of the investigator. If radiation is indicated, it can be incorporated any time after the first dose of study treatment. Systemic treatment can be delayed by up to 3 weeks in order to accommodate for radiation. Use of short course radiation is preferred (5–15 fractions). If indicated, concurrent single agent capecitabine or 5FU, or a combination of carboplatin and paclitaxel might be given as radio sensitizing agents. If needed, radiation would be given after all protocol-mandated systemic treatment has been completed and hence would not affect ctDNA measurements.
This study has been approved by the institutional review board at the University of California, Irvine (CA, USA).
Eligibility criteria
Inclusion Criteria
The inclusion criteria for this study are as follows:
-
1.
Histologically or cytologically confirmed adenocarcinoma of the stomach or gastroesophageal junction;
-
2.
Must have stage IB, II or stage III GC eligible for (neo)adjuvant doublet or triplet chemotherapy for up to 6 months;
-
3.
Must have baseline ctDNA positive assay (tested by Signatera™ MRD assay [17]) prior to initiation of neoadjuvant chemotherapy. Patients who are otherwise eligible may start per protocol treatment if ctDNA result is not available at the time of initiation of systemic therapy. However, once the result is available, they can only remain on study if the ctDNA is positive;
-
4.
Age ≥18 years;
-
5.
Performance status: ECOG performance status ≤2;
-
6.
Life expectancy of greater than 6 months;
-
7.Adequate organ and marrow function as defined below:
-
a.Leukocytes ≥3000/ul;
-
b.Absolute neutrophil count ≥1500/ul;
-
c.Platelets ≥80,000/ul;
-
d.Total bilirubin within normal institutional limits;
-
e.AST(SGOT)/ALT(SPGT) ≤5 × institutional upper limit of normal;
-
f.Creatinine <2 × institutional upper limit of normal.
-
a.
-
8.
Ability to understand and the willingness to sign a written informed consent.
Exclusion Criteria
The exclusion criteria for this study are as follows:
-
1.
Patients may not be receiving any other investigational agents;
-
2.
Patients with known metastases from GC;
-
3.
History of allergic reactions attributed to agents used in study;
-
4.
Uncontrolled intercurrent illness including, but not limited to, ongoing or active infection, symptomatic congestive heart failure, unstable angina pectoris, cardiac arrhythmia or psychiatric illness/social situations that would limit compliance with study requirements;
-
5.
History of another primary cancer which requires active treatment or is expected to require treatment within 12 months after enrollment;
-
6.
Inability to comply with study and follow-up procedures as judged by the investigator;
-
7.
Patients who are pregnant or nursing due to the potential for congenital abnormalities and the potential of this regimen to harm nursing infants.
Planned sample size & study period
Based on internal data at the study center and published data [6], it is expected that >75% of patients with LAGC have successfully detectable baseline ctDNA assays and hence be eligible for per protocol treatment. Thus, the accrual goal is up to 26 total patients and up to 20 evaluable participants, allowing for up to six patients (30%) drop-out rate due to lack of detectable ctDNA levels. The total duration of study participation is anticipated to be 36 months.
Study objectives
Primary Objective
To demonstrate the safety and feasibility of bespoke ctDNA response guided neoadjuvant treatment in LAGC.
Primary Endpoint
Percent patients completing per protocol treatment.
Secondary Objectives
To describe clinic-pathologic and molecular outcomes.
Secondary Endpoints
Percent patients completing gastrectomy, percent negative margins (i.e. R0 resection), 30-day post-operative complication rate, percent ctDNA clearance after neoadjuvant chemotherapy and post-operative (within 6–8 weeks), adverse events as determined by CTCAE v5 [18], efficacy as measured by recurrence-free survival (time from surgery to clinical recurrence).
Exploratory Objectives
Comprehensive Genomic Profiling. For patients with sufficient tissue available, whole exosome sequencing using Natera's ALTERA assay will be obtained (www.natera.com/oncology/signatera-advanced-cancer-detection/clinicians/altera/). The test will be requested at the same time as the baseline ctDNA test is sent (prior to cycle 1). The results are not used to modify patient treatment on protocol. Molecular alterations based on the Altera assay may be used as an exploratory platform for future research.
Conclusion
Despite the advances in implementing personalized medicine in metastatic gastrointestinal tumors, we have yet to see the adaptation to locoregional disease. In colon cancers, efforts to individualize adjuvant treatment based on MRD status are ongoing [7,19–21]. In gastroesophageal carcinomas, the ongoing TOPGEAR and CRITICS-II trials are emphasizing the trend of a total neoadjuvant approach and re-addressing the potential contribution of radiation in this setting [22,23]. However, both are randomized trials, which do not consider individual patient response to individualize the treatment regimen. The RANT-GC trial will focus on establishing a novel neoadjuvant treatment paradigm which emphasizes therapy development over drug development: i.e. the main question is not the efficacy of a specific cytotoxic regimen used, but rather whether the response to that specific treatment modality (with ctDNA kinetics as a molecular surrogate for clinical response) and if necessary its modification will improve outcomes.
Some of the limitations of this study include a relatively small sample size, the heterogeneity of the allowed neoadjuvant regimens, and a single institution non-randomized single arm design. There might also some concern regarding the fact that a ‘TNT’ approach is applied in this study, which is not fully consistent with the currently endorsed peri-operative chemotherapy recommendations based on previous studies [10]. While it would be desirable to have a larger multi-institutional and randomized study, we believe a smaller proof of principle feasibility study might provide some important initial insights with more limited resources and a within a shorter timeframe. These insights could then guide the design of a larger likely cooperative group led randomized trial. The perioperative approach was established based on comparison to surgery alone (MAGIC trial [3], accrual mainly from the UK, but also including patients from The Netherlands, Germany, Brazil, Singapore and New Zealand), and without further modification was adopted to compare two different chemotherapy regimens (FLOT-4 trial) rather than interrogating the optimal timing of the systemic treatment [2,3]. However, the already ongoing ‘next generation’ trials have already adopted the contemporary adaptation of a TNT approach in gastrointestinal malignancies (e.g., CRITICS-2 trial [22]) and likely will be incorporated as acceptable alternative in future iterations of national guidelines. We purposely allowed for inclusion of all evidence-based active GC regimens since the main objective of this study is ‘optimal therapy development’, rather than pure drug development. The goal is trying to maximize patient outcomes by using all available regimens needed to achieve ctDNA negativity. Hence, based on our design, a patient who achieves ctDNA negativity after four months (or eight cycles) of standard regimen (e.g. FLOT) will proceed with gastrectomy. While it is possible that variations of ctDNA levels occur independent of treatment response, for the quantitative assay used in our study, there is a well-described role for the quantification of the actual ctDNA levels, which correspond with response and prognosis [24,25].
Future perspective
Future trials based on the anticipated results of this trial are expected to further elucidate the optimal duration of neoadjuvant treatment, personalizing the choice of the systemic regimen based on the molecular profile of the tumor (including incorporation of targeted agents and immunotherapy), establish relevant cutoffs for ctDNA dynamics that correlate with benefit from therapy, and help further evaluate the role of radiation in the setting of molecular response assessment based on ctDNA.
Executive summary.
Tumor informed ctDNA & molecular residual disease testing
The Signatera™ bespoke mPCR NGS assay is a personalized and tumor-informed multiplex PCR assay for the detection and quantification of ctDNA.
RANT-GC study rationale
Current guidelines recommend (neo)adjuvant chemotherapy (gastric cancer [GC]) or neoadjuvant chemoradiation followed by adjuvant nivolumab (gastroesophageal junction) for all patients with at least cT2+ or any cN+ disease.
The specific regimen and duration of peri-operative systemic treatment is standardized, rather than personalized to biologic response, despite the availability of several different classes of agents in the treatment of GC.
Hence, then unmet need in the treatment of stage IB-III GC is a personalized approach with real-time adaptation of the treatment regimen based on biologic response.
The hypothesis is that using Signatera ctDNA assay, it is possible to closely monitor response to treatment in a highly sensitive and specific manner and modify neoadjuvant treatment regimens based on response without negatively affecting curative intent surgery and surgical complications.
RANT-GC study design
Prospective single center, single arm, open label study in clinical stage IB-III GC patients. ctDNA will be measured at baseline, and repeated every 8 weeks.
Patients will be treated with standard neoadjuvant regimen (i.e. 5FU, leucovorin, oxaliplatin and docetaxel or FOLFOX). Depending on ctDNA response the same regimen will either be maintained or switched to a different chemotherapy backbone (e.g. irinotecan based regimen, or taxane based if not used upfront).
Patients will procced to gastrectomy after a minimum of 4 and maximum of 6 months of total neoadjuvant treatment.
ctDNA will be monitored in all patients after gastrectomy every 3 months for up to 2 years, or clinical recurrence, whichever occurs later.
RANT-GC study objectives
The primary objective is testing the feasibility of approach. The primary endpoint is defined as percentage of patients completing per protocol treatment.
Secondary objectives include safety and efficacy. Secondary endpoints are post-operative complication rate, percent patients with ctDNA clearance after neoadjuvant treatment and after surgery, recurrence free survival and adverse events.
An explorative objective is to compare tissue whole exome sequencing to patient outcomes.
Footnotes
Author contributions
All authors met the criteria for authorship set forth by the International Committee of Medical Journal Editors, and were involved in conception, preparation and approval of the manuscript.
Financial & competing interests disclosure
This study is supported by Comprehensive Cancer Center Pilot Project Award and Natera, Inc. F Dayyani has received research grants (to the institution) from AstraZeneca, Bristol-Myers Squibb, Merck, Genentech/Roche, Taiho, Exelixis, Trishula, Leap Therapeutics, has received a speaker honorarium from Amgen, Eisai, Ipsen, Exelixis, Sirtex, Deciphera, Ipsen, Natera, and has received a consultancy honorarium from Natera, QED, Eisai, Exelixis, Genentech/Roche. MT Cho has received research grant (to the institution) from Bristol-Myers Squibb, a speaker honorarium from Pfizer, Natera, Taiho, BMS, AstraZeneca, a consultancy honorarium from Amgen, Incyte, Eisai, Ipsen, Astellas, Taiho, Exelixis, QED, I-Mab, Tempus, Seattle Genetics, HelioDx, Bayer, AstraZeneca, Genentech/Roche, Pfizer, Natera, Taiho, BMS, Basilea. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
This study has been approved by the institutional review board at the University of California, Irvine.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Shah MA, Kennedy EB, Catenacci DV et al. Treatment of locally advanced esophageal carcinoma: ASCO guideline. J. Clin. Oncol. 38(23), 2677–2694 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Al-Batran SE, Homann N, Pauligk C et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet (London, England). 393(10184), 1948–1957 (2019). [DOI] [PubMed] [Google Scholar]; •• This trial establishes the current standard of care for the peri-operative treatment of locally advanced gastric cancer (LAGC).
- 3.Cunningham D, Allum WH, Stenning SP et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 355(1), 11–20 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Bang YJ, Kim YW, Yang HK et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet (London, England). 379(9813), 315–321 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Liu S, Jian T, Xiao L et al. Total neoadjuvant therapy (TNT) versus standard neoadjuvant chemoradiotherapy for locally advanced rectal cancer: asystematic review and meta-analysis. Oncologist 26(9), e1555–e1566 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huffman B, Budde G, Chao J et al. Performance of a tumor-informed circulating tumor DNA assay from over 250 patients with over 600 plasma time points in esophageal and gastric cancer Ann. Oncol. (2021) (ePoster 1415). [Google Scholar]
- 7.Kotaka M, Shirasu H, Watanabe J et al. Association of circulating tumor DNA dynamics with clinical outcomes in the adjuvant setting for patients with colorectal cancer from an observational GALAXY study in CIRCULATE-Japan. J. Clin. Oncol. 40(Suppl. 4), 9 (2022). [Google Scholar]; •• This study is the largest prospective validation of the prognostic and predictive role of ctDNA in gastrointestinal malignancies.
- 8.Goodman KA, , Niedwiecki D, Hall N et al. Initial results of CALGB 80803 (Alliance): arandomized phase II trial of PET scan-directed combined modality therapy for esophageal cancer. J. Clin. Oncol. 35(Suppl. 4), 1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayes DF, Schott AF. Neoadjuvant chemotherapy: what are the benefits for the patient and for the investigator? JNCI Monogr. 2015(51), 36–39 (2015). [DOI] [PubMed] [Google Scholar]
- 10.McMillian NN et al. NCCN guidelines version 3.2021 gastric Cancer continue NCCN guidelines panel disclosures (2021). www.nccn.org/professionals/physician_gls/pdf/gastric.pdf
- 11.Holdenrieder S, Pagliaro L, Morgenstern D, Dayyani F. Clinically meaningful use of blood tumor markers in oncology. Biomed Res. Int. 2016, (2016) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reinert T, Vasterman Henriksen T, Christensen E et al. Analysis of plasma cell-free DNA by ultradeep sequencing in patients with stages I to III colorectal cancer. JAMA Oncol. 5(8), 1124–1131 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loaiza-Bonilla A, , Benson AB 3rd, Grothey A et al. Use of molecular assays and circulating tumor DNA in early-stage colorectal cancer: aroundtable discussion of the gastrointestinal cancer therapy expert group. Oncologist 26(8), 651–659 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbosh C, Birkbak NJ, Wilson GA et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature 545(7655), 446–451 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Introduction of the tumor-informed ctDNA assay in solid tumors.
- 15.Dayyani F, Boland PM, Dean A et al. Abstract B14: CA 19-9 levels in patients with metastatic pancreatic adenocarcinoma receiving first-line therapy with liposomal irinotecan plus 5-fluorouracil/leucovorin and oxaliplatin (NAPOX). Cancer Res. 79(24), B14 (2019). [Google Scholar]
- 16.de Steur WO, , van Amelsfoort RM, Hartgrink HH et al. Adjuvant chemotherapy is superior to chemoradiation after D2 surgery for gastric cancer in the per-protocol analysis of the randomized CRITICS trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 32(3), 360–367 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Signatera Clinicians | Natera (2022). www.natera.com/oncology/signatera-advanced-cancer-detection/clinicians/#pg-menu-tabs
- 18.NationalCancer Institute. Common terminology criteria for adverse events (CTCAE) common terminology criteria for adverse events (CTCAE) v5.0. Accessed: Jan. 03, 2021 Available: www.meddra.org/ (2017).
- 19.Gong J, Hendifar A, Gangi A et al. Clinical applications of minimal residual disease assessments by tumor-informed and tumor-uninformed circulating tumor DNA in colorectal cancer. Cancers (Basel). 13(18), 4547 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris VK, Yothers G, Kopetz S et al. NRG-GI005 (COBRA): phase II/III study of circulating tumor DNA as a predictive biomarker in adjuvant chemotherapy in patients with stage II colon cancer. J. Clin. Oncol. 38(Suppl. 4), TPS261 (2020). [Google Scholar]
- 21.Dasari A, Lin Y, Kopetz S et al. NRG-GI008: Colon adjuvant chemotherapy based on evaluation of residual disease (CIRCULATE-US). J. Clin. Oncol. 40(Suppl. 4), TPS212 (2022). [Google Scholar]
- 22.Slagter AE, Jansen EPM, van Laarhoven HWM et al. CRITICS-II: a multicentre randomised phase II trial of neo-adjuvant chemotherapy followed by surgery versus neo-adjuvant chemotherapy and subsequent chemoradiotherapy followed by surgery versus neo-adjuvant chemoradiotherapy followed by surgery in resectable gastric cancer. BMC Cancer. 18(1), 877 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study is one of the first to apply a total neoadjuvant approach in the treatment of LAGC.
- 23.Leong T, Smithers BM, Michael M et al. TOPGEAR: a randomised phase III trial of perioperative ECF chemotherapy versus preoperative chemoradiation plus perioperative ECF chemotherapy for resectable gastric cancer (an international, intergroup trial of the AGITG/TROG/EORTC/NCIC CTG). BMC Cancer 15(1), 532 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bratman SV, , Yang SYC, Iafolla MAJ et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat. Cancer 2020 19. 1(9), 873–881 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Henriksen TV, , Tarazona N, Frydendahl A et al. Circulating tumor DNA in stage III colorectal cancer, beyond minimal residual disease detection, toward Assessment of adjuvant therapy efficacy and clinical behavior of recurrences. Clin. Cancer Res. 28(3), 507–517 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• First study to describe the prognostic role of ctDNA kinetics in any solid tumor.


