Abstract
This literature review examines the need to develop appropriate policies specific to the oral health needs of older people that are individualised, cost-effective, and sustainable. Poor oral health and impaired oral function negatively affect the health and quality of life of older adults. Developing care systems that aim to meet patients’ normative needs as well as their perceived needs and expectations is one factor in successful delivery of appropriate dental care. Cost is another significant driver of utilisation, and many older adults worldwide lack adequate resources for dental care. Failure to address these issues results in poor outcomes and increased costs of dental and medical care. Disease prevention and control at early stages can preserve public and private financial resources as well as quality of life and well-being for older adults at any stage of life.
Key words: Older adults, Oral systemic connection, Frailty, Expenditures, Prevention, Policy
Introduction
Oral health is a critical but neglected component of healthy ageing.1 The FDI World Dental Federation defines oral health as “multi-faceted and includes the ability to speak, smile, smell, taste, touch, chew, swallow and convey a range of emotions through facial expressions with confidence and without pain, discomfort and disease of the craniofacial complex (head, face, and oral cavity).”2 Oral problems such as missing teeth, dental decay, mobile teeth, denture problems, oral lesions, and xerostomia may cause pain and discomfort; impair chewing, speaking, swallowing, smiling, and communicating; and diminish quality of life. A growing body of evidence has shown numerous associations, some of them bidirectional, between oral and systemic diseases that are prevalent in older adults such as diabetes, cardiovascular disease, dementia, and respiratory diseases.1,2
Ideally at any age, oral diseases are prevented or treated at an early stage, but this becomes more critical as dependency increases due to either systemic illness or very advanced age. These individuals may be less able to tolerate or benefit from even routine dental care, and discrepancies can arise amongst dentist, other health care providers, and patient perceptions and expectations for treatment as needs become more complex and patients become frail and dependent.3
Oral health is a person's right, but it often presents a hidden problem. And just as provider and patient points of view may differ chairside, they may also differ around the kind of research and policies that are needed to improve experiences and outcomes of care. Therefore, older adults themselves should be active participants when policy and resources for oral health care are established. Information about the oral-systemic connection, the oral health needs and demands of older adults, and the costs and neglect of care should be shared broadly as policy is set. There is also evidence for increased expenditures associated with tooth loss and medical management as a result of acute dental disease that arises from neglect of oral health. Unmet oral health needs in older adults are a problem worldwide, and models and policies that have effectively moved the needle forward to maintain or improve oral health as an element of healthy ageing should be sought out and shared. Whilst each country will have unique challenges and opportunities, sharing lessons learned and strategies to reduce disparities to improve outcomes of care and integrate oral health care in health care systems will be required to reap the benefits of good oral health on total health and well-being over a lifetime. This literature review examines the need for countries to develop appropriate policies that support individualised, cost-effective, sustainable care that is appropriate to meet the unique oral health needs of older adults.
The oral systemic connection in older adults
There is increasing evidence of an association between oral and general health in older people, mediated through common risk factors including nutrition, systemic inflammation, infection, and psychosocial factors, but causal links are still under investigation.4, 5, 6, 7, 8, 9 A confirmed bidirectional association exists between periodontal disease and diabetes mellitus. Poor control of diabetes results in increased severity of periodontal disease, whilst periodontitis is associated with elevated risk of dysglycaemia and insulin resistance in patients with diabetes as well as increased incidence of diabetes.10 Therefore, it is recommended that physicians consider the risk of periodontal disease in patients with diabetes and refer them to a dentist for evaluation and treatment. And dentists may consider the possibility that patients with periodontal disease could have undiagnosed diabetes and refer accordingly as well as monitor disease control in patients with known diabetes, whether or not they have periodontal disease.10 There is still lack of evidence on whether poorly controlled diabetes mellitus can significantly reduce clinical nonsurgical periodontal treatment outcomes.11 Moreover, some studies have shown that periodontal treatment may reduce the haemoglobin A1c levels within a few months,12 whilst others did not find any association between the treatment of periodontitis and the management of diabetes.13
Many studies have also shown an association between periodontal disease and cardiovascular disease.7,8,14, 15, 16 Periodontal treatment may benefit some biochemical parameters associated with cardiovascular risk,17 but more studies are necessary to clarify its effect on the prevention and progress of cardiovascular disease in patients with chronic periodontitis.5,14
There is also increasing evidence of an association between aspiration pneumonia and poor oral health amongst frail older people, including the presence of periodontal pathogens, dental and denture plaque, and denture wearing at night.18 In a prospective study amongst community-dwelling older old people in Japan, perceived swallowing difficulties and denture wearing during sleep were associated with a 2.3-fold higher risk of pneumonia.4 Oral hygiene and improvement of oral function may help in the prevention of aspiration pneumonia in hospitalised or institutionalised older patients, but more research is necessary on this topic as well as on the most effective preventive practices.19 Based on randomised controlled trials, oral care interventions to reduce biofilm, when provided by dental professionals, can reduce mortality from nosocomial-associated pneumonia in frail older adults in hospitals or nursing homes.20 A European consensus from dentists and physicians on oral care in care-dependent older people suggested that dentures should be always removed at night, cleaned with a denture brush, immersed in a cleansing tablet solution, and stored dry overnight to reduce the risk of systemic infections.21
Various systematic conditions may deteriorate oral health, whilst poor oral health may negatively affect general health status in older adults. The association between oral health and frailty specifically is also currently under investigation. Frailty is associated with increased vulnerability related to ageing in which the homeostatic balance is disrupted, resulting in an increased risk of adverse health-related outcomes even after exposure to a minor stressor.22 Various oral health indicators including number of teeth, chewing performance, oral diadochokinesis, tongue pressure, swallowing disorders, and dry mouth have been investigated in association with physical frailty, but the evidence is scarce and there is a need for a consensus on the definitions and the methodologies applied.23, 24, 25
Tooth loss and increased tooth mobility have been associated with poor masticatory performance in older people.26, 27, 28 Poor masticatory performance has been one of the most important predictors of poor adherence to the Mediterranean Diet, known to reduce morbidity and mortality amongst older community-dwelling people.29, 30, 31 However, the effect of poor oral health on food selection and nutritional intake is still under investigation due to the large variety of factors affecting nutrition in older people.32, 33, 34
There is currently no consensus on the association between periodontal disease and oral health–related quality of life (OHRQoL).35 OHRQoL is defined as “a multidimensional construct that reflects (amongst other things) people's comfort when eating, sleeping and engaging in social interaction; their self-esteem; and their satisfaction with respect to their oral health.”36 Efficient functioning, freedom from pain and discomfort, satisfaction with orofacial aesthetics, and maintaining self-esteem and social interaction are different dimensions of OHRQoL. Two systematic reviews in adult populations have shown that periodontal disease may affect OHRQoL.37,38 Severe periodontal disease negatively impacts function and aesthetics, whilst gingivitis negatively impacts comfort and can cause pain.38 However, a UK study of 1277 older people did not reveal any association between OHRQoL and periodontal disease, in contrast to the significant effects of active caries, dental pain, and wearing dentures.39 Other studies did not reveal an association between caries and OHRQoL.35 Oral dryness is also associated with impairment in OHRQoL and particularly with functional limitations and psychological disability35,40 as well as pain and chewing problems.35
The large variety of the QoL dimensions explains the unique, personalised experience of health problems and the discrepancy between perceived and normative needs for oral care that is often recorded amongst older people. For example, older persons usually report better subjective oral health compared to younger people, despite the higher burden of oral disease, a phenomenon associated with various cohort and psychosocial characteristics.41 In old age, apart from oral health, various other factors affect OHRQoL such as general health, personal traits, and demographic, social, and environmental factors.42 Therefore, the effect of oral health on the QoL is not only population-specific but also highly individualised. That is why the effect of tooth loss on restrictions in social activities varies in different cultures and appears to be age-dependent, pointing to the need for individualised dental care approaches.9
Appropriate resources for preventive and restorative care for older adults can reduce the need for more complex oral health care in frail individuals
Frail individuals are less able to handle stressors. Even minor dental procedures can present a challenge, and at the same time poor or declining oral health can exacerbate systemic decline in these older adults. Providing dental care in earlier stages of dependency—when patients are first diagnosed with diseases or prescribed medications that have the potential to impact their oral health—will mean that if or when they enter stages of greater dependency, they would do so in good oral health. Preventive plans should then be revised to address any new risk factors associated with changes in physical or cognitive status as they arise. For individuals who are frail or dependent on others for basic daily activities of living, such as bathing, dressing, and eating, and/or require extensive medical care and management as a result of disease or dependence, the goals of care would ideally shift to a more preventive and palliative approach.2,3,43 Ideally, individuals with a high level of dependency or frailty, or those at the end of life, would reach this stage with good oral health so that the focus can be solely on prevention and comfort. Measures such as salivary substitutes or more frequent prophylaxis and higher fluoride intake to provide relief of discomfort and increase protection from oral disease and pathology should be a standard during this time with heightened risk. This treatment philosophy has been established though an evidence-based approach to the goals of care for older adults based on dependency rather than age, as described in the Seattle Care Pathway (SCP).2,3,43
SCP and the Lucerne Pathway43 delineate what kind of assessment, prevention, treatment, and communication are required to develop treatment plans for older adults through all levels of dependency. At each stage, the risks to oral health are considered as well as the challenges to providing dental care in the presence of systemic diseases. However, many older adults have limited resources for oral health care, challenges accessing care, and perceptions of need that are different from their actual needs.6,43,44 Their resources may be further strained by the costs and time spent managing their systemic diseases. Additionally, some providers may lack confidence or training to appropriately treat a frail older adult with a complex health history or to work effectively with an interprofessional team.3,44, 45, 46
Whether intensive preventive and comfort measures are needed or more invasive or definitive care is indicated, dental providers must clearly communicate with the rest of the interdisciplinary team caring for these frail and highly or moderately dependent individuals.2,3,47 The health care team must understand which elements of their patient's health status present a risk to their oral health, such as an inability of the patient to complete oral hygiene due to a physical or cognitive issue; oral side effects of medications such as gingival enlargement or xerostomia; Candida infections associated with poor denture hygiene; or an inability of the patient to identify or report a problem.2,3,43,48 Caregivers must understand the importance of a daily oral hygiene plan and their role in that element of care.3,44 Further, for patients who are unable to express a complaint, other health care providers and caregivers must know signs and symptoms to look for that may indicate a problem, such as refusing food or oral hygiene or changes in behaviour.43,48 Since these individuals may not be able to get to a dentist regularly or at all, the availability of mobile or teledentistry to assess frail adults at home or in places that they frequent is critical. Better utilisation of the interprofessional team to deliver frequent and timely care is also key to improving access and outcomes and avoiding the need for extensive dental care in the most vulnerable older adults whilst still maintaining oral health and quality of life.3,43, 44, 45
Many times there is the added challenge of the perception that pain is the primary reason to proceed with dental care, but pain may not be the primary presentation of disease in an older adult, even in the presence of frank disease such as extensive caries or teeth with excessive mobility.43,48 Older adults may decline treatment themselves without a proper understanding of the course of their disease if left untreated. Surrogate decision-makers may also decline on a patient's behalf if they do not perceive the person to be in pain or discomfort. Primary care providers may also fail to reinforce to their patients the importance of seeking and receiving dental care. Patient education, interprofessional education and collaboration, and public health campaigns to improve oral health literacy are needed so that older adults and those who care for them understand the importance of maintaining their oral health as they age and that decline of oral health is not a forgone conclusion.2,45, 46, 47
The reality for individuals struggling with access to care, concomitant comorbidities that have compromised their ability to maintain their oral health, and a lack of awareness about their oral health needs or the importance of prevention and treatment is that many reach the most dependent stages with extensive oral disease.1,2,45 What may have once been routine care for a healthy older adult can come with more risk as dependency increases. In the absence of treatment, those chronic oral diseases can become acute conditions that become more complicated, expensive, and risky to treat. Where once a restoration or periodontal procedure may have addressed the issue, antibiotics, pain relievers and extractions may become necessary.49 For those who are most frail and least able to receive or access care, palliative care and a perpetually increased risk of an acute condition from untreated dental disease may be their only recourse.
Delivery of individualised dental care
Dental delivery systems for ageing and older populations should address their specific needs and demands and promote OHRQoL. The 8020 National Health Promotion Programme in Japan encourages citizens to keep 20 healthy natural teeth into their 80s.50 An observational study has shown that shortened dental arches can last for 27 years or more.51 A 2-year follow-up randomised controlled trial in partially dentate elders compared the effect of rehabilitation either with shortened dental arches or removable partial dentures on OHRQoL and revealed that although both treatments improved the OHRQoL, the improvements of the shortened dental arch approach was maintained throughout the study period, in contrast to the denture group in which the improvements diminished after 6 months.52 However, although shortened dental arches may offer adequate functioning and patient satisfaction and can be considered as cost-effective approaches, many dentists hesitate to offer this as a treatment option.53
An extensive evaluation of the patient's beliefs and expectations before treatment could help to improve treatment outcomes. This is particularly important considering the discrepancies between the dentist's point of view and that of patient in terms of expectations and post-treatment perceived improvement.54 In completely edentulous patients, rehabilitation with complete dentures may improve quality of life in the long term and replacement of unsatisfactory dentures may have a positive patient outcome.55 Rehabilitation with complete maxillary dentures has been shown to improve patient satisfaction that is mainly associated with esthetics and speech,56 whilst more problems are anticipated with the mandibular denture associated with lower retention and functional problems.50,57 The common problems associated with the conventional mandibular dentures use may be addressed by rehabilitation with implant-supported mandibular overdentures that lead to higher OHRQoL scores and patient satisfaction compared to dentures relining even amongst frail older old individuals.58 However, significant improvement in the objectively recorded masticatory performance using a 2-colour mixing ability test has not been demonstrated.58 Whilst a 2015 systematic review amongst fully edentulous patients treated with mandibular implant overdentures showed significant improvements in satisfaction from their oral health, the effect on general QoL was inconclusive.59
All studies on the effect of oral factors on quality of life indicate that the patient's opinion is important to deliver individualised dental care and develop care systems that meet their expressed needs. Apart from patient perceptions and the high cost of dental care, particularly when not covered by insurance or public funds, accessing dental care is another major barrier to care for many older adults. The specific challenges of ageing societies, combined with unpredictable outcomes of many current treatments and rising health care costs, support the need for personalised dentistry based on tailor-made prevention and treatment strategies that are based on individual risk assessment.60
To improve dental utilisation, treatment outcomes, and cost-effectiveness of dental care, collaborative research and design of dental delivery systems is necessary to meet the needs of older patients.61 Several methodological approaches can be used to design patient-centred dental services, such as priority setting partnerships; discrete choice experiments; core outcome sets; and experience-based co-design that aim build partnerships amongst professionals, various stakeholders, and patients.61
Few studies based on this approach to oral care have been conducted to identify the perceived need of older patients. A priority-setting partnership experiment conducted in the UK aimed to enable older people to prioritise research to improve their oral health.62 Patients, caregivers, health care specialists, and third-sector organisations such as care home staff were invited to contribute. They identified 3 research priorities: identify best practices in the prevention and treatment of oral diseases for older people, identify the training needs for dentists, and understand the key issues for oral care from the older patients’ point of view.62 A discrete choice experiment conducted in community-dwelling older people in Switzerland measured preferences for dental examinations and treatment when they become care-dependent. The study showed that amongst these subjects, there was a preference to be treated by the family dentist in the dental office instead of receiving domiciliary care. This finding revealed the need to develop appropriate health policies in Switzerland to reduce barriers for accessing dental care in patients’ preferred settings.63
Economic impact of oral health care for older adults on total dental and medical expenditure
Dental expenditure
Determining and comparing global dental expenditures and utilisation across countries is difficult in general and in particular to hone down differences in older adults specifically. This is due to a multitude of reasons including the complexity of comparing monetary value across countries, varying resources dedicated to or available for oral health care, differing economies, and variable methods of managing oral disease from more conservative preventive approaches achieved primarily through public health measures or preventive measures offered ahead of more extensive surgical and restorative approaches.64 Many times, these approaches are driven by reimbursement issues as well as cultural and societal norms or other social determinants of health. Ringholt et al examined the global, regional, and country costs of all dental disease in 2010 and again in 2015 using data available from 73 countries and cost estimates for another 122 countries. Despite inherent limitations in data sources, they estimated that in 2015 $356.80 billion US dollars was spent on direct dental treatment worldwide and $187.61 billion was consumed by losses in productivity due to oral diseases, totaling $544.41 billion expended for oral health care. They determined expenditures on oral health care to account for 4.6% of total expenditures on health globally. Increases in these expenditures appear to be equivalent to increases in other health care expenditures since 2010, but there appears not to have been growth in the percentage of dental costs within total costs from 2010 to 2015.64
An estimated 3.5 billion people suffer with untreated oral diseases.65 It is unknown how much of this can be attributed to older adults specifically; however, individuals 65 and older currently compose 10% of the global population and more than 20% of the population in 17 countries. By 2050, the older adult population is anticipated to grow to 16%, or 1 in 6 people, worldwide. At the age of 65, life expectancy is expected to rise from about 82 to 84 years by 2050.66 The 2015 Global Burden of Disease Study identified conditions found in high prevalence amongst older adults to be common across the world. Untreated caries in permanent teeth are the most prevalent oral disease worldwide, experienced by 2.5 billion people. Severe periodontal disease affects 538 million people, and more than a quarter billion people were determined to be edentulous.67 The worldwide average dental expenditure per capita was $49 in US dollars. The 5 countries with the highest expenditures on direct dental care in 2015 were Iceland (US$458), Germany (US$417), Norway (US$381), Australia (US$378), and the United States (US$370).64
High-income countries have demonstrated significantly greater economic burdens of dental diseases. This is potentially due to a longer life expectancy with common risk factors for systemic and oral diseases, increases in retained dentition, and possibly greater access to more expensive procedures and different expectations for care. Interestingly, Luxembourg and Switzerland, which had the highest GDPs, had lower expenditures than those countries listed above with the highest expenditures; usually those countries with the highest GDP also have the greatest expenditures.64 There can be many reasons for this. As one example, the average cost of an emergency department visit for a nontraumatic dental emergency in the United States is US$900 and usually results in only palliative care until definitive care can be provided elsewhere.49
In the United States, a 2016 survey showed more than a third of adults aged older than 65 continued to work,68 so the potential for increased spending amongst those who may have some additional income or insurance benefits as a result of working as well as the potential for losses due to missed work as a result of untreated oral diseases should also be considered in examining the impact of older adults on dental expenditures. There are many reasons people chose to work past retirement, some of which include a financial need to do so, likely precluding available funds for routine dental care. Others may wish to continue working, but a lack of dentition or obviously poor dentition may present a challenge to securing some types of employment.45
Severe tooth loss accounted for 67% of losses in productivity, 21% due to severe periodontitis and 11% to untreated caries worldwide. The 5 countries with the highest productivity losses were the United States (US$45.90 billion), followed distantly by Japan (US$16.57 billion), China (US$16.15 billion), Germany (US$14.07 billion), and the United Kingdom (US$8.38 billion). Worldwide average productivity loss per capita due to untreated caries, severe periodontitis, and severe tooth loss (ie, fewer than 9 remaining teeth) combined was US$25.82. The highest per-person costs due to lost productivity were in Switzerland (US$339), Norway (US$278), the Netherlands (US$209), Austria (US$193), Luxembourg (US$184), and Denmark (US$182).64,69
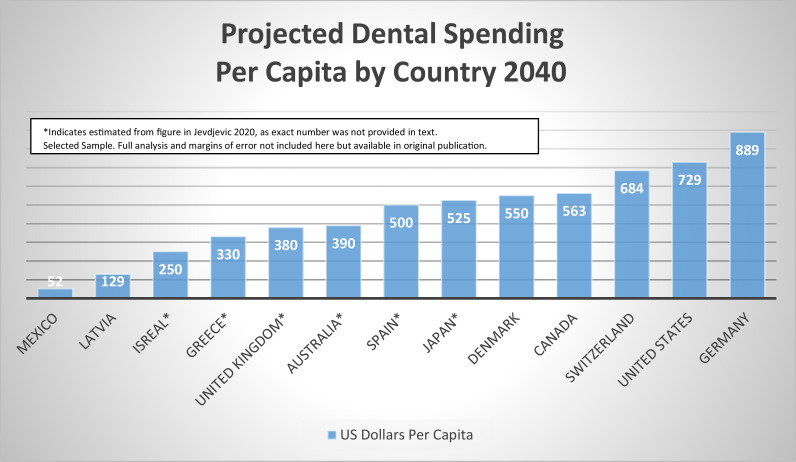
Jevdjevic et al developed models for future forecasting of expenditures and forecasted total dental expenditures of the 32 Organisation for Economic Cooperation and Development (OECD) countries to rise from US$316 billion in 2020 to US$434 billion in 2030 and US$594 billion in 2040.69 Forecasts were based on percent of GDP not including losses in productivity, as were the Ringholt analysis.64,69 Of the countries included in the modeling, per capita spending in 2040 was forecasted to be highest in Germany at US$889, followed by the United States at US$729, Switzerland at US$684, Canada at US$563, and Denmark at US$550, and lowest in Mexico at US$52 per capita.69 See Figure 1 for sample projected spending in 2040 per capita by country.
Fig. 1.
Projected dental spending per capita by country, 2040.69
The impact on dental expenditures and lost productivity from older adults working past retirement may be insignificant in countries such as Spain and France, where only an estimated of 5% to 6% of retirement-age adults continue to work and there are provisions for oral health care in their health care systems.68 The impact could be more significant in countries where a larger percentage continue to work and guaranteed dental benefits are lacking, such as in the United States.
More information about the economic impacts of unmet dental needs in adults 65 years and older on individual out-of-pocket and private insurance spending, the costs to public health for those without means, and the loss of productivity in the workplace for those who continue to work are needed to help shape policy and programmes that reduce total expenditures for oral health care by addressing those unmet needs. Understanding and predicting the costs of dental care across populations to identify and create opportunities to increase access, improve outcomes of care, and reduce cost should also reduce the pain, suffering, and embarrassment that can occur when these diseases—which are largely preventable—go untreated.1
For many around the world, the cost and lack of dental insurance are the primary barriers to dental care, and for many in low- and middle- income countries, paying for dental care may push them into poverty.45,70,71 Increased expenditures in preventive and basic restorative care that is better integrated with health care systems could improve outcomes of care and reduce dental expenditures overall.45,72,73 Policymakers need current and accurate data about the oral health needs of older adults and clarity around their financial needs in order to anticipate future expenditures whilst improving access, prevention, and systems of care to reduce the financial impact of untreated and acute oral diseases on individuals and communities.
Medical expenditure
Increased morbidity, mortality, and medical expenditures have been specifically associated with tooth loss. Studies have reported better health outcomes and reduced cost when oral health care is provided and further when it is integral to the rest of the health care system. Management of unmet dental needs in emergency and acute care medical settings has also been shown to be significant driver of cost when individuals lack resources for routine care.45
A preponderance of evidence indicating a relationship between oral and general health has accumulated globally. At the same time, population aging is increasing the financial burden of universal health coverage in countries attempting to provide it. Clarifying the effect of oral health on medical expenditures requires the preventive effects of oral health care to be quantified with respect to systemic health outcomes. Policymakers are likely to direct more resources towards preventive efforts, resulting in actual and long-term reduction of medical expenditures if the systemic health benefits of dental care and oral health maintenance become clearer. Because preventive dental and oral care is less expensive than treatment of systemic diseases such as noncommunicable diseases (NCDs), which are strongly associated with poor oral health45 and geriatric syndrome, this shift would be financially advantageous.
There has not yet been extensive research on the effect of oral health on medical expenditures, but recently a number of researchers have investigated this relationship using the following methods: (1) analysing medical and dental expenditure data obtained from the national government74, 75, 76, 77 and insurers78,79; (2) tracking the medical expenditure data of patients (obtained from insurers) after dental examinations80, 81, 82, 83; and (3) collecting medical expense receipts from patients after dental examinations.84 In these studies, oral health has been assessed via number of teeth,74,76,77,82,83 periodontal disease,81,83 subjective oral health status,84 or frequency of dental care procedures such as scaling and dental maintenance.75,77,78 In some studies, total medical expenditure is calculated,74,81,84 whilst in other studies only the expenditures for certain diseases such as such as diabetes,76,78, 79, 80 heart disease,74,78,79 cerebrovascular disease,78,79,82 and dementia77,83 are reported. For example, Tsuneishi et al conducted a cross-sectional study of patients aged 50 to 79 years using Japan's National Database of Health Insurance Claim Information and Specified Medical Checkups.74 The samples were 327,689; 610,087; and 654,410 eligible patients in their 50s, 60s, and 70s, respectively. To describe associations between number of teeth (independent variable) and medical expenditure (primary outcome) whilst controlling for sex, they also constructed generalised linear models with a gamma distribution and log-link function by using maximum likelihood estimation. The models, which were conducted by age group, showed that medical expenditure increased by 2.4% per each tooth lost in patients in their 50s (multiplicative effect, 0.98; 95% confidence interval [CI], 0.97–0.98). The association was also found in patients in their 60s (multiplicative effect, 0.98; 95% CI, 0.98–0.99) and in those in their 70s (multiplicative effect, 0.99; 95% CI, 0.99–0.99).74
In order to analyse the effects of periodontal therapy on medical costs amongst individuals diagnosed with type 2 diabetes (T2D), coronary artery disease (CAD), and cerebral vascular disease (CVD), Jeffcoat et al used insurance claim data from 338,891 individuals with both medical and dental insurance coverage.79 Patients were categorised according to whether they had completed treatment for periodontal disease in the baseline year. The outcome variable was total approved medical costs per subscriber per year. Statistically significant reductions in expenses (P < 0.05) were found in patients who had completed periodontal disease treatment amongst patients with T2D, CVD, or CAD, for which costs were reduced by 40.2%, 40.9%, and 10.7%, respectively.87 Further accumulation of evidence in this area is warranted; however, the results of these studies provide compelling evidence that preventive dental care is an efficient use of financial resources, and they provide strong justification for covering preventive dental care under public health insurance systems.79
Policies and strategies to improve access to dental care for older adults
The resources and attitudes towards oral health care for older adults varies from country to country, and the degree to which older adults have resources to support oral health is variable. Many countries are actively engaged in research to better understand the needs of their older citizens and the barriers to achieving and maintaining good oral health. They are exploring and developing novel approaches to improve oral health status along with overall health and well-being. Common themes are inadequate knowledge about the importance of oral health as people age, lacking financial resources for oral health care, poor integration with medical care, and the importance of interprofessional care. To illustrate the variability across the world, 3 case studies are presented here that describe the availability of resources and policies around oral health and older adults. There may be lessons learned from each that providers, health care systems, and policymakers could draw from to improve access and outcomes in their own countries.
Case study 1: USA—access to public and private dental benefits for older adults45,85, 86, 87
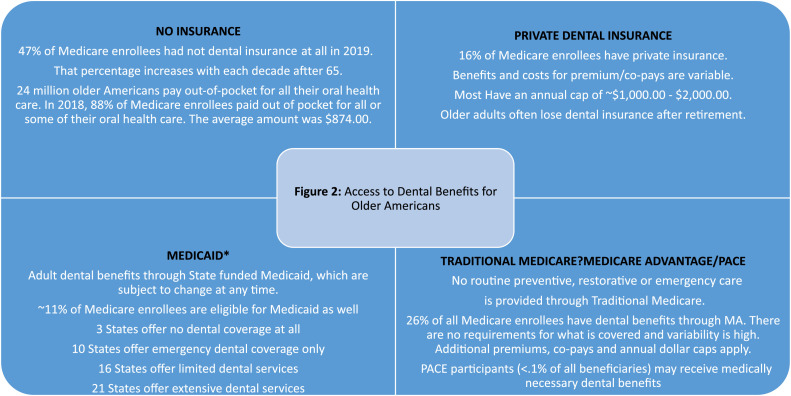
The Centers for Medicare and Medicaid Services (CMS) administers federal health care coverage. Americans aged 65 and older may receive benefits through Medicare, Medicaid, and PACE (Program of All Inclusive Care for Elders). Hospital and medical benefits are provided through Medicare for all Americans at age 65. When Medicare was enacted in 1965, dental care was expressly excluded from the benefits: “where such expenses are for services in connection with the care, treatment, filling, removal, or replacement of teeth or structures directly supporting teeth” (Section 1862 (a)(12) of the Social Security Act). The only exceptions are when hospitalisation is required to complete the dental treatment or for dental extractions in preparation for radiation therapy and inpatient oral exams prior to kidney transplant or in a Rural/Federally Qualified Health Center prior to heart valve replacement. Enrollees may choose to enroll in Medicare Advantage, which may offer a dental benefit at an additional cost. Nearly half of all Medicare enrollees do not have dental insurance and most pay some or all dental expenses out of pocket. See Figure 2. Medicaid eligibility is based on income, and individual states determine benefits. Individuals or families whose income is less than 100% of the federal poverty level (FPL) qualify for Medicaid, and many states have expanded coverage to those with income 138% of the FPL. In 2020, this was an annual income of $12,880 for one person and $17,420 for a family of two. Most PACE participants are dually eligible for Medicare and Medicaid. They must be 55 and older, eligible for skilled nursing care but able to live in the community with PACE support, and live in a defined service area. PACE programmes provide medically necessary oral health care as determined by a PAC E interdisciplinary team.
Fig. 2.
Access to dental benefits for older Americans.
The National Institute of Dental and Craniofacial Research at the National Institutes of Health recently published Oral Health in America: Advances and Challenges. The state of oral health and oral health care for older Americans was specifically examined over the last 20 years to identify areas of progress, areas that require more work, and those that hold promise in Section 3: Oral Health Across the Lifespan: Older Adults.45 The following call to action resulted from the review: “A policy that mandates dental coverage in Medicare would reduce health inequities for older adults by assuring access to preventive and other oral health services for all, including those who are place bound or in need of caregiver assistance.” The most recent legislative efforts to realise a dental benefit in Medicare were not successful, but a growing and diverse body of advocates continue to pursue such legislation and one survey found that 93% of older respondents supported the addition of a benefit. Inclusion of a universal dental benefit in Medicare for all enrollees would raise awareness of the importance of oral health to general health and pave the way for meaningful integration of dental and medical care to improve the health and well-being of older adults in the United States.45
Case study 2: Europe—recommendations for oral health promotion in older adults
Financing oral health care in Europe differs amongst different countries, but in most cases direct patient payments are more common than in general health care. Oral care may be financed by national social security systems or health services, state-recognised or compulsory health insurance, voluntary private insurances, and out-of-pocket expenses.88 In many countries, children and patients who are unemployed, disabled, older, medically compromised, or low-income may receive some form of free dental services in public clinics.
Based on the close association between oral and general health and the multiple barriers in oral health promotion met in older age, the European College of Gerodontology (ECG) and the European Geriatric Medicine Society (EuGMS) have published European Policy Recommendations on oral health promotion in older adults. These recommendations include educational interventions for dental and nondental health care providers, development of appropriate oral health policies, and citizens’ empowerment and involvement.47 Geriatric oral health education amongst dental44,89, 90, 91, 92 and nondental health care professionals47,93 should be included at all educational levels, including undergraduate, postgraduate/specialty, and continuing education, and be based on specific learning objectives as described in the recommendations. Emphasis should be placed on interprofessional collaborative education and practice as a prerequisite for high-quality geriatric care.46,94, 95, 96 Oral health literacy should be promoted not only for self-care but also for those who are dependent.49
In terms of health policies, the EGG/EuGMS recommendations stress the need to integrate oral health assessment into comprehensive geriatric assessment, preventive oral care into routine medical care, and oral health promotion into general health campaigns.47 Dental care is expensive and cannot be covered by private funding for the majority of the (older) population, so it is imperative to integrate at least basic oral care into general health care coverage schemes.47,73 Other specific issues highlighted in the recommendations are ensuring access to domiciliary care, promoting research on effective oral health interventions in communities and institutions, and encouraging companies to develop oral care products aligned with the needs of older adults.47
Specific protocols for oral health prevention and promotion in institutional care have been recommended, such as the following: incorporate oral health assessment upon entry; provide regular oral screenings using validated tools; ensure access to emergency and routine oral health care; ensure efficient daily oral hygiene either by prompting residents to self-care or by staff-performed practices; ensure availability of daily oral care products; and provide a healthy diet that protects oral health.47 To ensure collaboration of staff and quality of care, consistent training in oral care practices is necessary.47,97,98
Case study 3: Japan—Dental and Oral Health Promotion Act
Japanese health policy began placing markedly greater emphasis on oral health in 2011, when the Dental and Oral Health Promotion Act was established.99 Article 1 of this act included the statement, “oral health plays an absolutely necessary role in achieving individual health and well-being.”
Although the main role of dental professionals remains the prevention and control of dental disease to prevent the decline of oral function, recently implemented evidence-based health policies have shifted towards seeing dental care as an important contributing factor to general health, particularly in areas such as NCD and frailty/dependency prevention. This portends a meaningful expansion of the role of dental clinics in the context of the larger health care system. The following is a list of current and planned nationally funded programmes that expressly prescribe the linking of dental and oral health services with general medical services.100
-
(1)
Health Japan 21: The goal of Phase II of Health Japan 21, Japan's overarching health promotion policy (2013-2022), is to improve health longevity and close the health gap. Concrete goals include NCD prevention and control and health maintenance of elderly people. The specific activities and behaviours targeted to achieve these goals include maintenance of dental and oral health alongside exercise, nutrition, smoking cessation, avoidance of alcohol abuse, and rest.
-
(2)
Cancer health policy: Cancer treatments such as surgery, chemotherapy, and radiotherapy can cause serious side effects such as pneumonia and stomatitis, and these side effects can in some cases prevent further treatment. For example, stomatitis can disturb food intake, leading to a weakened physical condition. With an eye to preventing unwanted side effects, it is now recommended that patients with cancer undergo dental treatment by a dentist before beginning their cancer treatment. The dentist must be certified in precancer dental care (using a nationally issued textbook) This has been included in the government-issued recommendations for cancer specialists since 2013.
-
(3)
Dementia policy: In the dementia policy paper called the “New Orange Plan (2015–2025)” and also in the national dementia policy outline established in 2019, it is clearly stated that community-based dental care plays a role in early diagnosis and prevention of cognitive decline and dementia. In addition, the first-ever government-issued set of dental care guidelines for older people with dementia was published in 2019.
-
(4)
Diabetes policy: Since 2016, the national policy for the prevention and control of diabetic nephropathy (an NCD) has included an oral health programme designed to control periodontal disease and improve eating behaviour. Medical clinical guidelines for the treatment of diabetes include periodontal disease as a risk factor, and dental clinical guidelines for the treatment of periodontal disease include diabetes as a risk factor. It is quite rare for medical clinical guidelines to include such specific dental-related information. This illustrates just how well accepted this association is in both the medical and dental communities. These guidelines have the beneficial effect of promoting further cooperation between dental and medical professionals and institutions, particularly in community settings.
-
(5)
Prevention of frailty and reduction of dependence on long-term care: The importance of frailty prevention was explicitly stated in the national guidelines for elderly health promotion. Prevention of oral functional decline has been included as a key factor in frailty prevention in this document since 2018. In addition, in 2016, dental clinics began offering nationally funded dental checkups and health guidance for elderly persons aged 75 and older. This programme aims not only to prevent decline in oral health and function but also to prevent frailty and reduce dependence on long-term care.
-
(6)
Prevention and control of metabolic syndrome: A national system of specific health checkups and health guidance for the prevention of metabolic syndrome, which targets people older than 40 years, was implemented in April 2008. As part of this system, health education materials designed to prevent metabolic syndrome (diabetes, obesity, and heart disease), as well as related risk factors such as periodontal disease and chewing function decline, have been developed. This national system of specific health checkups and specific health guidance is reviewed and updated once every 5 years. In 2018, question items regarding subjective chewing function were added because a decline in chewing function is indicative of dental disease and tooth loss.
The rapidly accumulating evidence showing the links between oral health and general health signals the need for a completely new framework of medical and dental collaboration on practice and policy. The policies outlined above represent a clear and growing trend towards positioning dental and oral health as an essential element in all relevant areas of Japan's health policy, including NCDs and frailty prevention. This trend represents a significant step towards translating evidence into practice, and it calls for a large-scale shift towards multisectorial and interprofessional cooperation.
Conclusions
Poor oral health has been shown to have strong associations with other noncommunicable chronic diseases such as diabetes, respiratory diseases, and dementia, which in turn can increase the risk of oral disease and increase the need for oral health care. Poor oral health and impaired oral function negatively affect older patients’ quality of life and can exacerbate systemic decline in frail older adults. Improved access and appropriate resources for preventive and restorative care are needed for well to frail older adults so that more complex curative or restorative care is not needed when individuals are most frail. Delivering appropriate dental care also necessitates incorporating the patients’ perspectives on what aspects of oral health are important to them and developing care systems that meet perceived, expressed, and normative needs.
Numerous studies have reported better health outcomes and reduced costs of medical care when regular, preventive oral health care is provided as a fundamental part of health care. Management of unmet dental needs in emergency and acute care medical settings has also been shown to be a significant driver of cost in countries where older adults lack resources for routine dental care. Policies and strategies that support oral health literacy, integration of oral health care with medical care, availability of resources to address inequities in access to care, and appropriate expenditures with improved utilisation are needed worldwide. Oral disease prevention and treatment at early stages of oral disease can support systemic health, preserve private and public resources, and maintain quality of life and well-being for older adults at every stage of life.
Conflict of interest
None disclosed.
Acknowledgments
Funding
This article is published as part of a supplement sponsored by GC International AG and the Nakao Foundation.
REFERENCES
- 1.World Health Organization . WHO Press; Geneva, Switzerland: 2015. World report on ageing and health. [Google Scholar]
- 2.FDI World Dental Federation, Oral Health for an Ageing Population: Roadmap for Healthy Aging, 2018. Available from:http://www.fdiworlddental.org/sites/default/files/2020-11/ohap-2018-roadmap_ageing.pdf. Accessed 27 April 2022.
- 3.Pretty IA, Ellwood RP, Lo EC, et al. The Seattle Care Pathway for securing oral health in older patients. Gerodontology. 2014;31((Suppl 1)):77–87. doi: 10.1111/ger.12098. [DOI] [PubMed] [Google Scholar]
- 4.Linuma T, Arai Y, Abe Y, et al. Denture wearing during sleep doubles the risk of pneumonia in the very elderly. J Dent Res. 2015;94(3 Suppl):28S–36S. doi: 10.1177/0022034514552493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li C, Lv Z, Shi Z, et al. Periodontal therapy for the management of cardiovascular disease in patients with chronic periodontitis. Cochrane Database Syst Rev. 2017;11 doi: 10.1002/14651858.CD009197.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kossioni AE, Hajto-Bryk J, Janssens B, et al. Practical guidelines for physicians in promoting oral health in frail older adults. J Am Med Dir Assoc. 2018;19(12):1039–1046. doi: 10.1016/j.jamda.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Yang S, Zhao LS, Cai C, et al. Association between periodontitis and peripheral artery disease: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2018;18(1):141. doi: 10.1186/s12872-018-0879-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van der Putten G-J. In: Gerodontology essentials for health care professionals. Kossioni AE, editor. Springer Nature; Switzerland AG: 2020. The association between oral and general health; pp. 49–65. editor. [Google Scholar]
- 9.Konstantopoulou K, Kossioni A. Mechanisms linking oral health and frailty in older adults: a narrative review. Stoma Edu J. 2021;8(3):195–204. [Google Scholar]
- 10.Sanz M, Ceriello A, Buysschaert M, et al. Scientific evidence on the links between periodontal diseases and diabetes: consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J Clin Periodontol. 2018;45(2):138–149. doi: 10.1111/jcpe.12808. [DOI] [PubMed] [Google Scholar]
- 11.Hsu YT, Nair M, Angelov N, et al. Impact of diabetes on clinical periodontal outcomes following non-surgical periodontal therapy. J Clin Periodontol. 2019;46(2):206–217. doi: 10.1111/jcpe.13044. [DOI] [PubMed] [Google Scholar]
- 12.Madianos PN, Koromantzos PA. An update of the evidence on the potential impact of periodontal therapy on diabetes outcomes. J Clin Periodontol. 2018;45:188e195. doi: 10.1111/jcpe.12836. [DOI] [PubMed] [Google Scholar]
- 13.Engebretson SP, Hyman LG, Michalowicz BS, et al. The effect of nonsurgical periodontal therapy on hemoglobin A1c levels in persons with type 2 diabetes and chronic periodontitis: a randomized clinical trial. JAMA. 2013;310(23):2523–2532. doi: 10.1001/jama.2013.282431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lockhart PB, Bolger AF, Papapanou PN, et al. American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, Council on Epidemiology and Prevention, Council on Peripheral Vascular Disease, and Council on Clinical Cardiology. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association? A scientific statement from the American Heart Association. Circulation. 2012;125:2520e2544. doi: 10.1161/CIR.0b013e31825719f3. [DOI] [PubMed] [Google Scholar]
- 15.Hansen GM, Egeberg A, Holmstrup P, et al. Relation of periodontitis to risk of cardiovascular and all-cause mortality (from a Danish nationwide cohort study) Am J Cardiol. 2016;118(4):489–493. doi: 10.1016/j.amjcard.2016.05.036. [DOI] [PubMed] [Google Scholar]
- 16.Furuta M, Fukai K, Aida J, et al. Periodontal status and self-reported systemic health of periodontal patients regularly visiting dental clinics in the 8020 Promotion Foundation Study of Japanese Dental Patients. J Oral Sci. 2019;61(2):238–245. doi: 10.2334/josnusd.18-0128. [DOI] [PubMed] [Google Scholar]
- 17.Roca-Millan E, González-Navarro B, Sabater-Recolons MM, et al. Periodontal treatment on patients with cardiovascular disease: systematic review and meta-analysis. Med Oral Patol Oral Cir Bucal. 2018;23(6):e681–e690. doi: 10.4317/medoral.22725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Müller F. Oral hygiene reduces the mortality from aspiration pneumonia in frail elders. J Dent Res. 2015;94(3 Suppl):14S–16S. doi: 10.1177/0022034514552494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tada A, Miura H. Prevention of aspiration pneumonia (AP) with oral care. Arch Gerontol Geriatr. 2012;55(1):16–21. doi: 10.1016/j.archger.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 20.Sjögren P, Wårdh I, Zimmerman M, et al. Oral care and mortality in older adults with pneumonia in hospitals or nursing homes: systematic review and meta-analysis. J Am Geriatr Soc. 2016;64(10):2109–2115. doi: 10.1111/jgs.14260. [DOI] [PubMed] [Google Scholar]
- 21.Charadram N, Maniewicz S, Maggi S, et al. Development of a European consensus from dentists, dental hygienists and physicians on a standard for oral health care in care-dependent older people: an e-Delphi study. Gerodontology. 2021;38(1):41–56. doi: 10.1111/ger.12501. [DOI] [PubMed] [Google Scholar]
- 22.Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka T, Takahashi K, Hirano H, et al. Oral frailty as a risk factor for physical frailty and mortality in community-dwelling elderly. J Gerontol A Biol Sci Med Sci. 2018;73(12):1661–1667. doi: 10.1093/gerona/glx225. [DOI] [PubMed] [Google Scholar]
- 24.Hakeem FF, Bernabé E, Sabbah W. Association between oral health and frailty: a systematic review of longitudinal studies. Gerodontology. 2019;36(3):205–215. doi: 10.1111/ger.12406. [DOI] [PubMed] [Google Scholar]
- 25.Dibello V, Zupo R, Sardone R, et al. Oral frailty and its determinants in older age: a systematic review. The Lancet Healthy Longevity. 2021;2:e507–e520. doi: 10.1016/S2666-7568(21)00143-4. [DOI] [PubMed] [Google Scholar]
- 26.Schimmel M, Memedi K, Parga T, et al. Masticatory performance and maximum bite and lip force depend on the type of prosthesis. Int J Prosthodont. 2017;30(6):565–572. doi: 10.11607/ijp.5289. [DOI] [PubMed] [Google Scholar]
- 27.Barbe AG, Javadian S, Rott T, et al. Objective masticatory efficiency and subjective quality of masticatory function among patients with periodontal disease. J Clin Periodontol. 2020;47(11):1344–1353. doi: 10.1111/jcpe.13364. [DOI] [PubMed] [Google Scholar]
- 28.Bousiou A, Konstantopoulou K, Polychronopoulou A, et al. Sociomedical and oral factors affecting masticatory performance in an older population. Clin Oral Investig. 2022;26(4):3477–3486. doi: 10.1007/s00784-021-04316-6. [DOI] [PubMed] [Google Scholar]
- 29.Trichopoulou A, Costacou T, Bamia C, et al. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348(26):2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 30.Psaltopoulou T, Sergentanis TN, Panagiotakos DB, et al. Mediterranean diet, stroke, cognitive impairment, and depression: a meta-analysis. Ann Neurol. 2013;74(4):580–591. doi: 10.1002/ana.23944. [DOI] [PubMed] [Google Scholar]
- 31.Bousiou A, Konstantopoulou K, Martimianaki G, et al. Oral factors and adherence to Mediterranean diet in an older Greek population. Aging Clin Exp Res. 2021;33(12):3237–3244. doi: 10.1007/s40520-021-01861-8. [DOI] [PubMed] [Google Scholar]
- 32.Kossioni AE. The association of poor oral health parameters with malnutrition in older adults: a review considering the potential implications for cognitive impairment. Nutrients. 2018;10(11):1709. doi: 10.3390/nu10111709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Keeffe M, Kelly M, O'Herlihy E, et al. Potentially modifiable determinants of malnutrition in older adults: a systematic review. Clin Nutr. 2019;38(6):2477–2498. doi: 10.1016/j.clnu.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Volkert D, Beck AM, Cederholm T, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38(1):10–47. doi: 10.1016/j.clnu.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 35.van de Rijt LJM, Stoop CC, Weijenberg RAF, et al. The influence of oral health factors on the quality of life in older people: a systematic review. Gerontologist. 2020;60(5):e378–e394. doi: 10.1093/geront/gnz105. [DOI] [PubMed] [Google Scholar]
- 36.FDI World Dental Federation, Oral health and quality of life. Available from:https://www.fdiworlddental.org/resources/policy-statements-and-resolutions/oral-health-and-quality-of-life. Accessed 27 April 2022.
- 37.Buset SL, Walter C, Friedmann A, et al. Are periodontal diseases really silent? A systematic review of their effect on quality of life. J Clin Periodontol. 2016;43(4):333–344. doi: 10.1111/jcpe.12517. [DOI] [PubMed] [Google Scholar]
- 38.Ferreira MC, Dias-Pereira AC, Branco-de-Almeida LS, et al. Impact of periodontal disease on quality of life: a systematic review. J Periodontal Res. 2017;52(4):651–665. doi: 10.1111/jre.12436. [DOI] [PubMed] [Google Scholar]
- 39.Masood M, Newton T, Bakri NN, et al. The relationship between oral health and oral health related quality of life among elderly people in United Kingdom. J Dent. 2017;56:78–83. doi: 10.1016/j.jdent.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Skośkiewicz-Malinowska K, Malicka B, Ziętek M, et al. Does oral dryness influence quality of life? Current perspectives in elderly dental care. Adv Clin Exp Med. 2019;28(9):1209–1216. doi: 10.17219/acem/104601. [DOI] [PubMed] [Google Scholar]
- 41.Slade GD, Sanders AE. The paradox of better subjective oral health in older age. J Dent Res. 2011;90(11):1279–1285. doi: 10.1177/0022034511421931. [DOI] [PubMed] [Google Scholar]
- 42.Niesten D, McKenna G. In: Gerodontology essentials for health care professionals. Kossioni AE, editor. Springer Nature; Switzerland AG;: 2020. Quality of life and oral health in older people; pp. 101–112. editor. [Google Scholar]
- 43.Calabrese JM, Rawal K. Demographics and oral health care utilization for older adults. Dent Clin North Am. 2021;65(2):241–255. doi: 10.1016/j.cden.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Ghezzi EM, Niessen LC, Jones JA. Innovations in geriatric oral health care. Dent Clin North Am. 2021;65(2):393–407. doi: 10.1016/j.cden.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 45.National Institutes of Health . US Department of Health and Human Services, National Institutes of Health, National Institute of Dental and Craniofacial Research; Bethesda, MD: 2021. Oral health in America: advances and challenges.https://www.nidcr.nih.gov/sites/default/files/2021-12/Oral-Health-in-America-Advances-and-Challenges.pdf#page=411 Available from: Accessed 27 April 2022. [Google Scholar]
- 46.Kossioni A, McKenna G, Müller F, et al. Higher education in gerodontology in European universities. BMC Oral Health. 2017;17:71. doi: 10.1186/s12903-017-0362-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kossioni AE, Hajto-Bryk J, Maggi S, et al. An expert opinion from the European College of Gerodontology and the European Geriatric Medicine Society: European policy recommendations on oral health in older adults. J Am Geriatr Soc. 2018;66(3):609–613. doi: 10.1111/jgs.15191. [DOI] [PubMed] [Google Scholar]
- 48.Chávez EM, Wong LM, Subar P, et al. Dental care for geriatric and special needs populations. Dent Clin N Am. 2018;62(2):245–267. doi: 10.1016/j.cden.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 49.Amen TB, Kim I, Peters G, Gutiérrez-Sacristán A, Palmer N, Simon L. Emergency department visits for dental problems among adults with private dental insurance: a national observational study. Am J Emerg Med. 2021;44:166–170. doi: 10.1016/j.ajem.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 50.Papadaki E, Anastassiadou V. Elderly complete denture wearers: a social approach to tooth loss. Gerodontology. 2012;29(2):e721–e727. doi: 10.1111/j.1741-2358.2011.00550.x. [DOI] [PubMed] [Google Scholar]
- 51.Gerritsen AE, Witter DJ, Bronkhorst EM, et al. An observational cohort study on shortened dental arches–clinical course during a period of 27-35 years. Clin Oral Investig. 2013;17(3):859–866. doi: 10.1007/s00784-012-0765-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McKenna G, Allen PF, Hayes M, et al. Impact of oral rehabilitation on the quality of life of partially dentate elders in a randomized controlled clinical trial: 2 year follow-up. PLoS One. 2018;13(10) doi: 10.1371/journal.pone.0203349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan H, Peres KG, Peres MA. Retention of teeth and oral health-related quality of life. J Dent Res. 2016;95(12):1350–1357. doi: 10.1177/0022034516657992. [DOI] [PubMed] [Google Scholar]
- 54.Marachlioglou CR, Dos Santos JF, Cunha VP, et al. Expectations and final evaluation of complete dentures by patients, dentist and dental technician. J Oral Rehabil. 2010;37(7):518–524. doi: 10.1111/j.1365-2842.2010.02072.x. [DOI] [PubMed] [Google Scholar]
- 55.Martins AMC, Guimarães LS, Campos CH, et al. The effect of complete dentures on edentulous patients’ oral health-related quality of life in long-term: a systematic review and meta-analysis. Dent Res J (Isfahan) 2021;18:65. [PMC free article] [PubMed] [Google Scholar]
- 56.Thalji G, McGraw K, Cooper LF. Maxillary complete denture outcomes: a systematic review of patient-based outcomes. Int J Oral Maxillofac Implants. 2016;31(Suppl):s169–s181. doi: 10.11607/jomi.16suppl.g5.1. [DOI] [PubMed] [Google Scholar]
- 57.Komagamine Y, Kanazawa M, Kaiba Y, et al. Association between self-assessment of complete dentures and oral health-related quality of life. J Oral Rehabil. 2012;39(11):847–857. doi: 10.1111/joor.12004. [DOI] [PubMed] [Google Scholar]
- 58.Müller F, Duvernay E, Loup A, et al. Implant-supported mandibular overdentures in very old adults: a randomized controlled trial. J Dent Res. 2013;92(12 Suppl):154S–160S. doi: 10.1177/0022034513509630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boven GC, Raghoebar GM, Vissink A, et al. Improving masticatory performance, bite force, nutritional state and patient's satisfaction with implant overdentures: a systematic review of the literature. J Oral Rehabil. 2015;42(3):220–233. doi: 10.1111/joor.12241. [DOI] [PubMed] [Google Scholar]
- 60.European Comission. Personalised medicine. Available from:https://ec.europa.eu/info/research-and-innovation/research-area/health-research-and-innovation/personalised-medicine_en. Accessed 27 April 2022.
- 61.Brocklehurst PR, McKenna G, Schimmel M, et al. How do we incorporate patient views into the design of healthcare services for older people: a discussion paper. BMC Oral Health. 2018;18(1):61. doi: 10.1186/s12903-018-0513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brocklehurst PR, Mackay L, Goldthorpe J, et al. Older people and oral health: setting a patient-centred research agenda. Gerodontology. 2015;32(3):222–228. doi: 10.1111/ger.12199. [DOI] [PubMed] [Google Scholar]
- 63.Chebib N, Abou-Ayash S, Maniewicz S, et al. Exploring older Swiss people's preferred dental services for when they become dependent. Swiss Dent J. 2020;130(11):876–884. doi: 10.61872/sdj-2020-11-690. [DOI] [PubMed] [Google Scholar]
- 64.Righolt AJ, Jevdjevic M, Marcenes W, et al. Global-, regional-, and country-level economic impacts of dental diseases in 2015. J Dent Res. 2018;97(5):501–507. doi: 10.1177/0022034517750572. [DOI] [PubMed] [Google Scholar]
- 65.Global Burden of Disease Collaborative Network . Institute of Health Metrics and Evaluation (IHME); Seattle: 2020. Global Burden of Disease Study 2019 (GBD 2019)http://ghdx.healthdata.org/gbd-results-tool Available from: Accessed 17 June 2022. [Google Scholar]
- 66.United Nations, Department of Economic and Social Affairs, Population Division. 2019. World population ageing 2019: highlights (ST/ESA/SER.A/430). Available from:https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf. Accessed 27 April 2022.
- 67.Kassebaum NJ, Smith AGC, Bernabé E, et al. GBD 2015 Oral Health Collaborators. Global, regional, and national prevalence, incidence, and disability-adjusted life years for oral conditions for 195 countries, 1990-2015: a systematic analysis for the global burden of diseases, injuries, and risk factors. J Dent Res. 2017;96(4):380–387. doi: 10.1177/0022034517693566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCarthy N. Where people are working beyond 65 [infographic]. 2017. Available from:https://www.forbes.com/sites/niallmccarthy/2017/12/08/where-people-are-working-beyond-65-infographic/?sh=649a83233680. Accessed 27 April 2022.
- 69.Jevdjevic M, Listl S, Beeson M, et al. Forecasting future dental health expenditures: development of a framework using data from 32 OECD countries. Community Dent Oral Epidemiol. 2021;49(3):256–266. doi: 10.1111/cdoe.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bernabé E, Masood M, Vujicic M. The impact of out-of-pocket payments for dental care on household finances in low and middle income countries. BMC Public Health. 2017;17(1):109. doi: 10.1186/s12889-017-4042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Palm W, Webb E, Hernández-Quevedo C, et al. Gaps in coverage and access in the European Union. Health Policy. 2021;125(3):341–350. doi: 10.1016/j.healthpol.2020.12.011. [DOI] [PubMed] [Google Scholar]
- 72.Lamster IB, Malloy KP, DiMura PM, et al. Dental services and health outcomes in the New York State Medicaid Program. J Dent Res. 2021;100(9):928–934. doi: 10.1177/00220345211007448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aida J, Fukai K, Watt RG. Global neglect of dental coverage in universal health coverage systems and Japan's broad coverage. Int Dent J. 2021;71(6):454–457. doi: 10.1016/j.identj.2020.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsuneishi M, Yamamoto T, Okumura Y, et al. Number of teeth and medical care expenditure. Health Sci Health Care. 2017;17:36–37. [Google Scholar]
- 75.Kao YW, Shia BC, Chiang HC, et al. Association of tooth scaling with acute myocardial infarction and analysis of the corresponding medical expenditure: a nationwide population-based study. Int J Environ Res Public Health. 2021;18(14):7613. doi: 10.3390/ijerph18147613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suzuki S, Noda T, Nishioka Y, et al. Evaluation of public health expenditure by number of teeth among outpatients with diabetes mellitus. Bull Tokyo Dent Coll. 2021;62(1):55–60. doi: 10.2209/tdcpublication.2020-0035. [DOI] [PubMed] [Google Scholar]
- 77.Tsuneishi M, Yamamoto T, Yamaguchi T, et al. Association between number of teeth and Alzheimer's disease using the National Database of Health Insurance Claims and Specific Health Checkups of Japan. PLoS One. 2021;16(4) doi: 10.1371/journal.pone.0251056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Albert DA, Sadowsky D, Papapanou P, et al. An examination of periodontal treatment and per member per month (PMPM) medical costs in an insured population. BMC Health Serv Res. 2006;6:103. doi: 10.1186/1472-6963-6-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jeffcoat MK, Jeffcoat RL, Gladowski PA, et al. Impact of periodontal therapy on general health: evidence from insurance data for five systemic conditions. Am J Prev Med. 2014;47(2):166–174. doi: 10.1016/j.amepre.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 80.Iwasaki M, Sato M, Yoshihara A, et al. Effects of periodontal diseases on diabetes-related medical expenditure. Curr Oral Health Rep. 2016;3:7–13. [Google Scholar]
- 81.Sato M, Iwasaki M, Yoshihara A, et al. Association between periodontitis and medical expenditure in older adults: a 33-month follow-up study. Geriatr Gerontol Int. 2016;16(7):856–864. doi: 10.1111/ggi.12569. [DOI] [PubMed] [Google Scholar]
- 82.Iwasaki M, Sato M, Yoshihara A, et al. Association between tooth loss and medical costs related to stroke in healthy older adults aged over 75 years in Japan. Geriatr Gerontol Int. 2017;17(2):202–210. doi: 10.1111/ggi.12687. [DOI] [PubMed] [Google Scholar]
- 83.Saito M, Shimazaki Y, Nonoyama T, et al. Association between oral health and the medical costs of dementia: a longitudinal study of older Japanese. Am J Alzheimers Dis Other Demen. 2021;36 doi: 10.1177/1533317521996142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harada E, Moriya S, Murata A, et al. Relationship between subjective assessment of oral health and medical expenses in community-dwelling elderly persons. Gerodontology. 2012;29(2):e246–e252. doi: 10.1111/j.1741-2358.2011.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaiser Family Foundation . 2021. Medicare and dental coverage: a closer look.https://www.kff.org/medicare/issue-brief/medicare-and-dental-coverage-a-closer-look/ Available from: Accessed 27 April 2022. [Google Scholar]
- 86.Centers for Medicare & Medicaid Services. History: CMS’ program history. Available from: https://www.cms.gov/about-cms/agency-information/history. Accessed 27 April 2022.
- 87.Simon L, Giannobile WV. Is it finally time for a Medicare dental benefit? N Engl J Med. 2021;385(23):e80. doi: 10.1056/NEJMp2115048. [DOI] [PubMed] [Google Scholar]
- 88.Kravitz AS, Bullock A, Cowpe J. Manual of dental practice 2015 (edition 5.1). Council of European Dentists. 2015. https://www.omd.pt/content/uploads/2017/12/ced-manual-2015-completo.pdf
- 89.Kitagawa N, Sato Y, Komabayashi T. Graduate and undergraduate geriatric dentistry education in a selected dental school in Japan. Eur J Dent Educ. 2011;15(4):231–235. doi: 10.1111/j.1600-0579.2010.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ettinger RL, Goettsche ZS, Qian F. Predoctoral teaching of geriatric dentistry in U.S. dental schools. J Dent Educ. 2017;81(8):921–928. doi: 10.21815/JDE.017.043. [DOI] [PubMed] [Google Scholar]
- 91.Ettinger RL, Goettsche ZS, Qian F. Postdoctoral teaching of geriatric dentistry in U.S. dental schools. J Dent Educ. 2017;81(10):1220–1226. doi: 10.21815/JDE.017.079. [DOI] [PubMed] [Google Scholar]
- 92.Marchini L, Ettinger R, Chen X, et al. Geriatric dentistry education and context in a selection of countries in 5 continents. Spec Care Dentist. 2018;38(3):123–132. doi: 10.1111/scd.12281. [DOI] [PubMed] [Google Scholar]
- 93.Hummel J, Phillips KE, Holt B, et al. Oral health: an essential component of primary care. White paper. 2015. Available from: http://www.niioh.org/sites/default/files/Oral_Health_white_paper_final.pdf. Accessed 27 April 2022.
- 94.World Health Organization Department of Human Resources for Health . WHO; Geneva, Switzerland: 2010. Framework for action on interprofessional education & collaborative practice. [Google Scholar]
- 95.Kossioni AE, Marchini L, Childs C. Dental participation in geriatric interprofessional education courses: a systematic review. Eur J Dent Educ. 2018;22(3):e530–e541. doi: 10.1111/eje.12348. [DOI] [PubMed] [Google Scholar]
- 96.Partnership for Health in Aging Workgroup on Interdisciplinary Team Training in Geriatrics Position statement on interdisciplinary team training in geriatrics: an essential component of quality health care for older adults. J Am Geriatr Soc. 2014;62(5):961–965. doi: 10.1111/jgs.12822. [DOI] [PubMed] [Google Scholar]
- 97.Janssens B, De Visschere L, van der Putten GJ, et al. Effect of an oral healthcare protocol in nursing homes on care staffs’ knowledge and attitude towards oral health care: A cluster-randomized controlled trial. Gerodontology. 2016;33:275–286. doi: 10.1111/ger.12164. [DOI] [PubMed] [Google Scholar]
- 98.Konstantopoulou K, Kossioni A, Karkazis H, et al. Implementation and evaluation of an oral health education programme for caregivers in nursing homes. Spec Care Dentist. 2021;41(2):154–163. doi: 10.1111/scd.12558. [DOI] [PubMed] [Google Scholar]
- 99.Fukai K. Oral health for achieving healthy longevity in an aging society: evidence and policy. Int J Oral Health. 2017;13:52–57. [Google Scholar]
- 100.Fukai K. Oral health for healthy aging society-evidence and health policy. JJHEP. 2019;27:360–368. [Google Scholar]