Key Points
Question
Do the 5-year outcomes of the PRODIGE 24/Canadian Cancer Trials Group PA6 trial confirm the survival benefits of treatment with adjuvant modified FOLFIRINOX vs gemcitabine for resected pancreatic ductal adenocarcinoma (PDAC), and which factors predict better survival?
Findings
The final results of this randomized clinical trial of 493 patients with PDAC confirmed significantly improved overall survival with treatment with modified FOLFIRINOX vs gemcitabine (median survival, 53.5 vs 35.5 months). Receipt of modified FOLFIRINOX, younger age, well-differentiated tumors, lower-stage tumors, larger-volume centers, and completing treatment predicted better survival; early disease relapse was an adverse prognostic factor for overall survival from relapse.
Meaning
The results of this randomized clinical trial found modified FOLFIRINOX to be a recommended adjuvant regimen following PDAC resection.
Abstract
Importance
Early results at 3 years from the PRODIGE 24/Canadian Cancer Trials Group PA6 randomized clinical trial showed survival benefits with adjuvant treatment with modified FOLFIRINOX vs gemcitabine in patients with resected pancreatic ductal adenocarcinoma; mature data are now available.
Objective
To report 5-year outcomes and explore prognostic factors for overall survival.
Design, Setting, and Participants
This open-label, phase 3 randomized clinical trial was conducted at 77 hospitals in France and Canada and included patients aged 18 to 79 years with histologically confirmed pancreatic ductal adenocarcinoma who had undergone complete macroscopic (R0/R1) resection within 3 to 12 weeks before randomization. Patients were included from April 16, 2012, through October 3, 2016. The cutoff date for this analysis was June 28, 2021.
Interventions
A total of 493 patients were randomized (1:1) to receive treatment with modified FOLFIRINOX (oxaliplatin, 85 mg/m2 of body surface area; irinotecan, 150-180 mg/m2; leucovorin, 400 mg/m2; and fluorouracil, 2400 mg/m2, every 2 weeks) or gemcitabine (1000 mg/m2, days 1, 8, and 15, every 4 weeks) as adjuvant therapy for 24 weeks.
Main Outcomes and Measures
Primary end point was disease-free survival. Secondary end points included overall survival, metastasis-free survival, and cancer-specific survival. Prognostic factors for overall survival were determined.
Results
Of the 493 patients, 216 (43.8%) were women, and the mean (SD) age was 62.0 (8.9) years. At a median of 69.7 months’ follow-up, 367 disease-free survival events were observed. In patients receiving chemotherapy with modified FOLFIRINOX vs gemcitabine, median disease-free survival was 21.4 months (95% CI, 17.5-26.7) vs 12.8 months (95% CI, 11.6-15.2) (hazard ratio [HR], 0.66; 95% CI, 0.54-0.82; P < .001) and 5-year disease-free survival was 26.1% vs 19.0%; median overall survival was 53.5 months (95% CI, 43.5-58.4) vs 35.5 months (95% CI, 30.1-40.3) (HR, 0.68; 95% CI, 0.54-0.85; P = .001), and 5-year overall survival was 43.2% vs 31.4%; median metastasis-free survival was 29.4 months (95% CI, 21.4-40.1) vs 17.7 months (95% CI, 14.0-21.2) (HR, 0.64; 95% CI, 0.52-0.80; P < .001); and median cancer-specific survival was 54.7 months (95% CI, 45.8-68.4) vs 36.3 months (95% CI, 30.5–43.9) (HR, 0.65; 95% CI, 0.51-0.82; P < .001). Multivariable analysis identified modified FOLFIRINOX, age, tumor grade, tumor staging, and larger-volume center as significant favorable prognostic factors for overall survival. Shorter relapse delay was an adverse prognostic factor.
Conclusions and Relevance
The final 5-year results from the PRODIGE 24/Canadian Cancer Trials Group PA6 randomized clinical trial indicate that adjuvant treatment with modified FOLFIRINOX yields significantly longer survival than gemcitabine in patients with resected pancreatic ductal adenocarcinoma.
Trial Registration
EudraCT: 2011-002026-52; ClinicalTrials.gov Identifier: NCT01526135
This randomized clinical trial examines 5-year outcomes and prognostic factors for overall survival for treatment with modified FOLFIRINOX for patients with pancreatic ductal adenocarcinoma.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a leading cause of cancer-related death worldwide.1,2,3,4,5 In Europe, mortality of PDAC is projected to rise by 42% by 2039.6,7,8 While surgical excision is potentially curative, only 15% to 20% of patients are eligible for up-front surgery,8 and resection alone is associated with a 5-year overall survival (OS) rate of approximately 10%.9,10
Adjuvant chemotherapy improves survival outcomes in patients with resected PDAC.9,10,11,12,13 In the phase 3 CONKO-001 randomized clinical trial, use of adjuvant gemcitabine for 6 months after PDAC resection significantly improved disease-free survival (13.4 vs 6.9 months; P < .001) and OS (22.8 vs 20.2 months; P = .01) compared with observation alone.10 The ESPAC-4 trial subsequently demonstrated that adjuvant combination of gemcitabine plus capecitabine was superior to gemcitabine alone in prolonging median overall survival (28.0 vs 25.5 months; P = .03).11 The multicenter, randomized, phase 3 PRODIGE 24/CCTG PA6 clinical trial evaluated the safety and efficacy of a modified FOLFIRINOX (mFOLFIRINOX) chemotherapy regimen compared with gemcitabine as adjuvant therapy in 493 patients with resected PDAC.14 At a median follow-up of 33.6 (IQR, 22.1-46.0) months, the primary end point of disease-free survival was significantly longer with treatment with mFOLFIRINOX compared with gemcitabine (median 21.6 [IQR, 9.9-nonestimable (NE)] vs 12.8 [IQR, 8.0-28.9] months; stratified hazard ratio [HR] of 0.58; 95% CI, 0.46-0.73; P < .001), as well as OS (median, 54.4 [IQR, 23.0-NE] vs 35.0 [IQR, 20.3-63.6] months; 3-year OS, 63.4% vs 48.6%). Treatment with mFOLFIRINOX is now a standard of adjuvant chemotherapy after resection of PDAC in eligible patients.15,16
The 3-year outcomes of PRODIGE 24/CCTG PA6 were based on a premature database lock following a recommendation in February 2018 by the independent data and safety monitoring committee to analyze and publish the data early in view of a large difference in efficacy between the treatment groups. The database was locked on April 13, 2018, at which time 91.8% of the expected events regarding disease-free survival had occurred. In this article, we report the updated 5-year survival outcomes from the final analysis of PRODIGE 24/CCTG PA6, with new results on prognostic factors for OS.
Methods
Trial Overview
Full details of the PRODIGE 24/CCTG PA6 trial design have been published previously (Supplement 1).14 This randomized, open-label, phase 3 clinical trial was conducted at 77 hospitals in France and Canada. Main inclusion criteria were age 18 to 79 years, histologically confirmed PDAC, complete macroscopic R0 or R1 resection within 12 weeks before randomization, no metastatic disease, World Health Organization performance status score of 0 or 1, and adequate hematologic, liver, and kidney functions. Patients were excluded in case of a serum cancer antigen (CA) 19-9 level of more than 180 U/mL (to convert to kU/L, multiply by 1) within 21 days before randomization, prior chemotherapy or radiotherapy, or coronary heart disease. Patients provided written informed consent before enrollment. The study protocol was approved by an independent ethics committee in France (Comité de Protection des Personnes Est III) and by ethics committees at participating centers in Canada.
Eligible patients were randomly assigned (1:1) to receive adjuvant mFOLFIRINOX (oxaliplatin, 85 mg/m2; leucovorin, 400 mg/m2; irinotecan, 150-180 mg/m2 [the dose was reduced from 180 mg/m2 to 150 mg/m2 after the enrollment of 162 patients, in accordance with a protocol-specified safety analysis]; and fluorouracil, 2400 mg/m2), every 14 days for 24 weeks (12 cycles), or adjuvant gemcitabine, 1000 mg/m2, on days 1, 8, and 15, every 28 days for 24 weeks (6 cycles).
The primary end point was disease-free survival, which was calculated from the date of randomization until date of first cancer-related event, second cancer, or death of any cause. Secondary end points included OS, metastasis-free survival, cancer-specific survival, and safety. Follow-up included clinical examination, CA 19-9 levels, and a computed tomography scan every 3 months for the first 2 years, and every 6 months thereafter. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
Statistical Analyses
Overall, 490 patients had to be included in the trial to observe 342 events and demonstrate a gain in 3-year disease-free survival of 10% (from 17% to 27%) and HR of 0.74, with a 80% power and 2-sided significance level of 5%.12 All efficacy analyses were performed in the intention-to-treat (ITT) population. Survival rate estimates were calculated using the Kaplan-Meier method, with comparison using a stratified log-rank test. A Cox proportional hazards model (stratified according to the stratification factors, except for trial center) was used to estimate HRs with 95% CIs.
In post hoc analyses, a Cox proportional hazards model was used to evaluate the effects of prognostic factors on OS in univariate and multivariable analyses. Prognostic factors explored included age; sex; World Health Organization performance status; excision procedure; tumor location, stage, and histological grade; lymph node ratio17; lymph nodes dissection (≤12 vs >12 nodes); lymphovascular invasion; perineural invasion; resection status (R0 vs R1); venous resection; and time between surgery and the start of chemotherapy. Clinically relevant factors or variables with P values of less than .20 were explored further in a multivariable analysis with the use of ascending or descending selection techniques. Hazard ratios and 95% CIs were calculated.
The effect on overall survival of factors collected at the end of adjuvant therapy was also explored using a landmark method.18 These included a dose intensity of 0.80 or greater for all drugs; duration of treatment (<6 vs ≥6 months), and completion of all treatment cycles. An 8-month landmark point was selected based on the ESPAC-3 trial.19 Patients lost to follow-up or who died within 8 months of randomization were not selected to avoid the potential immortal time bias. Sensitivity analyses using landmark of 9 to 12 months were performed. The median times to recurrence according to the type of recurrence were compared with a Kruskal-Wallis test.
A Cox proportional hazards model was used to explore the effect on OS of time between first relapse and relapse treatment according to center size (inclusion volume <10 vs ≥10 patients), as well as time between end of adjuvant chemotherapy and relapse (<6 vs >6 months, and <12 vs >12 months). The latter analysis included treated patients who had local or metastatic relapse after the treatment and excluded all patients who progressed during treatment, had a second cancer, or died as a first event. Hazard ratios and 95% CIs were calculated.
Results
Patients and Treatment
A total of 493 patients (mFOLFIRINOX: 247 [50.1%]; gemcitabine: 246 [49.9%]) were enrolled between April 16, 2012, and October 3, 2016, and formed the ITT population (eFigure 1 in Supplement 2). Patient baseline demographic characteristics were similar between treatment groups14; this remained the case following centralized review, with the only significant difference being a greater rate of lymphovascular invasion in the mFOLFIRINOX group (73.7% vs 63.1%; P = .02; eTable 1 in Supplement 2).
At this final analysis (cutoff date: June 28, 2021), the median follow-up was 69.7 months (95% CI, 67.1-73.9). The median duration of treatment was 24.6 weeks (range, 2.0-36.6) and 24.0 weeks (range, 3.0-36.0) in the mFOLFIRINOX and gemcitabine groups, respectively; 160 patients (67.2%) and 192 patients (79.0%), respectively, received all planned cycles of chemotherapy. The relative dose intensity was 0.80 or greater in 80 patients (33.6%) receiving mFOLFIRINOX and 192 patients (79.0%) receiving gemcitabine (P < .001). The relative dose intensity was 0.80 or greater during the first 3 months of treatment for 139 patients (58.4%) in the mFOLFIRINOX group vs 207 (85.2%) in the gemcitabine group. This was because of several factors, including delays in treatments administration, dose reductions, and early discontinuation of treatment with oxaliplatin. At least 1 delay in treatment administration occurred in 157 patients (66%) receiving mFOLFIRINOX vs 95 patients (39.1%) receiving gemcitabine. At least 1 dose reduction was reported for 82 patients (33.7%) receiving gemcitabine, and in 149 (62.6%), 132 (55.5%), and 81 (34.0%) patients receiving treatment with oxaliplatin, irinotecan, and fluorouracil, respectively. The relative dose intensity was 0.80 or greater in 100 patients (42%) for oxaliplatin, 141 (59%) for irinotecan, and 167 (70%) for fluorouracil. The median number of oxaliplatin cycles was 9 (range, 1-12), and 131 patients (55.0%) discontinued treatment with oxaliplatin at least once.
Survival Analyses
After a median follow-up of 69.7 (IQR, 59.4-84.1) months, 367 events had occurred in the ITT population (mFOLFIRINOX, 173 events [47.1%]; gemcitabine, 194 events [52.9%]). The median disease-free survival was 21.4 (IQR, 9.9-70.0) months in the mFOLFIRINOX group vs 12.8 (IQR, 7.9-29.8) months in the gemcitabine group (stratified HR for cancer-related event, second cancer, or death: 0.66; 95% CI, 0.54-0.82; P < .001; Figure 1A). The 5-year disease-free survival rate was 26.1% (95% CI, 20.5%-32.1%) with chemotherapy with mFOLFIRINOX compared with 19.0% (95% CI, 14.3%-24.3%) with gemcitabine. A total of 304 patients died (mFOLFIRINOX, 136 [44.7%]; gemcitabine, 168 [55.3%]). The median overall survival was 53.5 (IQR, 22.4-NE) months in the mFOLFIRINOX group compared with 35.5 (IQR, 20.3-80.8) months in the gemcitabine group (stratified HR for death, 0.68; 95% CI, 0.54-0.85; P = .001; Figure 1B). The 5-year overall survival rate was 43.2% (95% CI, 36.5%-49.7%) with chemotherapy with mFOLFIRINOX and 31.4% (95% CI, 25.5%-37.5%) with gemcitabine. A subgroup analysis showed no evidence of heterogeneity of the effect size of treatment on OS (Figure 2). Patients in the mFOLFIRINOX group vs gemcitabine group also had improved metastasis-free survival (median 29.4 months [IQR, 12.1-95.3] vs 17.7 months [IQR, 8.8-43.9]; stratified HR, 0.64; 95% CI, 0.52-0.80; P < .001; Figure 1C) and cancer-specific survival (54.7 months vs 36.6 months; stratified HR, 0.65; 95% CI, 0.51-0.82; P < .001; Figure 1D).
Figure 1. Kaplan-Meier Analysis of Survival in the Intention-to-Treat Population.
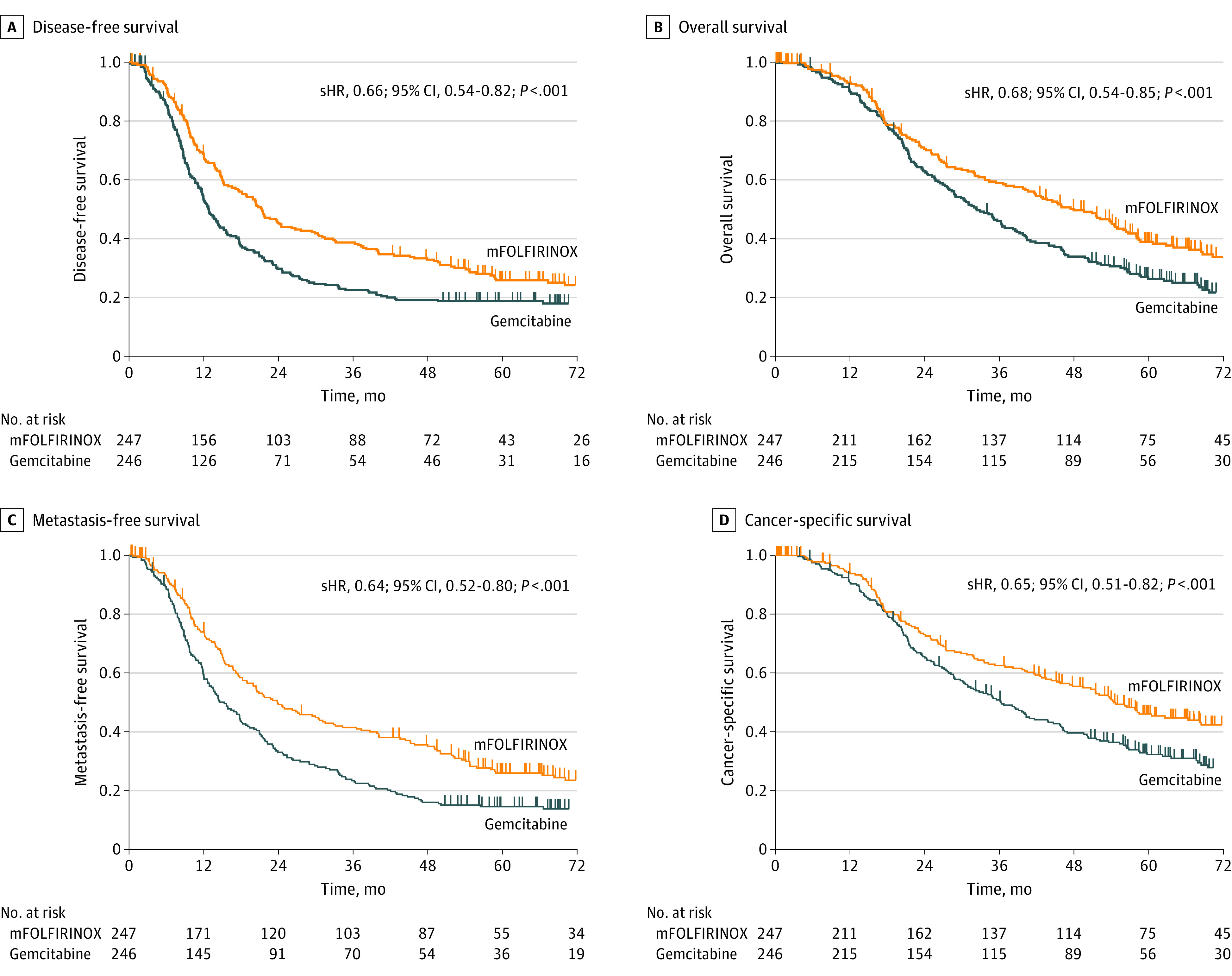
P values based on log rank test stratified by lymph node status, resection margins, and postoperative CA 19-9. mFOLFIRINOX indicates modified fluorouracil, leucovorin, irinotecan, and oxaliplatin; sHR, stratified hazard ratio.
Figure 2. Treatment Effect on Overall Survival in Subgroup Analyses.
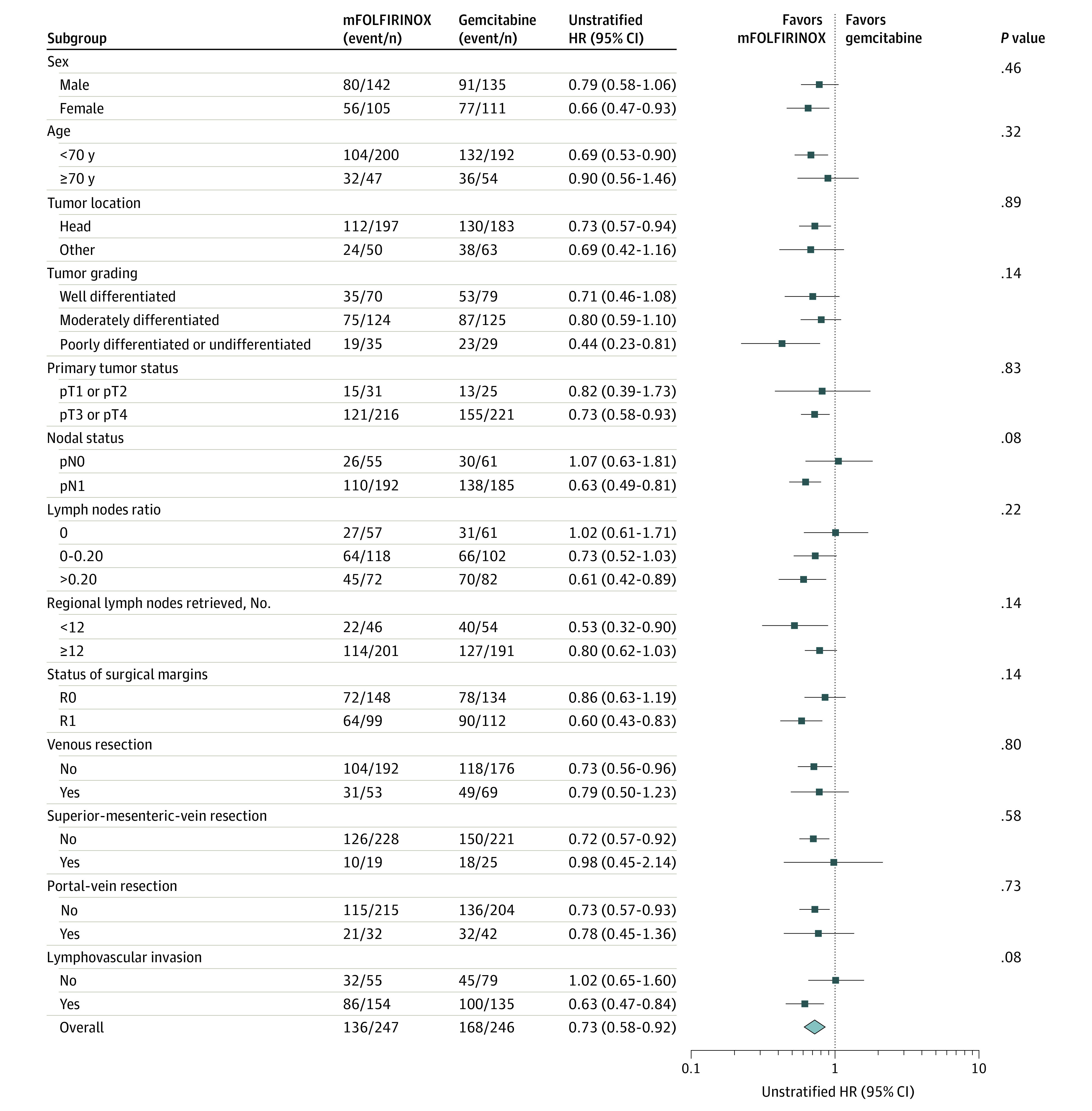
HR indicates hazard ratio; mFOLFIRINOX, modified fluorouracil, leucovorin, irinotecan, and oxaliplatin.
Patterns of Recurrence
The pattern of disease recurrence was similar between the treatment groups (eTable 2 in Supplement 2). Local recurrence as first event occurred at a median of 12.4 months (95% CI, 9.5-15.2 months), with no significant difference (P = .74) with the median times compared with metastatic recurrence (12.0 months; 95% CI, 10.4-14.2) or local and distant recurrences (10.2 months; 95% CI, 9.3-13.7). Median OS from relapse was 18.5 months in patients with only local recurrence (95% CI, 13.4-22.4), 14.0 months with only metastatic recurrence (95% CI, 11.6-18.0), and 11.4 months with local and metastatic recurrences (95% CI, 10.0-15.0), with no significant difference.
The most common first relapse event in both groups was the occurrence of metastases (mFOLFIRINOX, 54.3%; gemcitabine, 46.9%) with significantly more patients with single-site lung metastases (39.4% vs 24.6%) and fewer liver and peritoneal metastases as a single site in the mFOLFIRINOX group (P = .03). Polymetastatic, liver-only, and peritoneal-only metastatic disease occurred soonest, with a median of 9.2 months (95% CI, 7.9-14.2 months), 9.7 months (95% CI, 8.5-11.9 months), and 9.8 months (95% CI, 7.7-14.2 months), respectively, compared with lung metastases, which occurred after 18.7 months (95% CI, 13.8-21.4 months). In patients who had metastatic disease as a first event (eTable 3 in Supplement 2), the median overall survival from relapse was significantly longer in cases of lung-only metastases (26.0 months; 95% CI, 16.8-30.3) compared with multiple metastatic or other sites (11.5 months; 95% CI, 10.2-13.9) (HR, 0.50; 95% CI, 0.36-0.70; P < .001).
Relapse Treatment
Chemotherapy was administered at relapse in 107 of 160 patients (66.9%) in the mFOLFIRINOX group (of whom 72 [67.3%] received a gemcitabine-based regimen) and in 151 of 182 patients (83.0%) in the gemcitabine group (of whom 112 [74.2%] received FOLFIRINOX) (eTable 4 in Supplement 2). The time between first relapse and reintroduction of chemotherapy was 0.77 months (range, 0-14.0) and 0.80 months (range, 0.03-7.75), respectively (P = .57), with no difference according to center inclusion volume. The median postrelapse survival was 13.1 (95% CI, 10.43-18.3) and 15.0 (95% CI, 12.3-18.4) months in the mFOLFIRINOX and gemcitabine groups, respectively. Patients in the mFOLFIRINOX group received more frequent radiation therapy and/or surgery as part of treatment for relapse (27.2% vs 16.5%; P = .02). Three patients in the gemcitabine group had surgery for pulmonary metastases vs 8 patients in the mFOLFIRINOX group; 1 patient in each group was disease free.
Prognostic Factors for OS
In univariate analysis, prognostic factors present at the study baseline that were significantly associated with an improved survival included mFOLFIRINOX group, body/tail tumor location, well-differentiated tumor grade, pT1 to 2 stage, pN0 status, lower tumor stage (I, IIA, and IIB vs III/IV), lower lymph node ratio (0, 0-0.20, and 0.20-0.40 vs >0.40), R0 resection status, and no venous resection (eTable 5 in Supplement 2). In multivariable analysis, significant favorable baseline prognostic factors for OS were mFOLFIRINOX, being younger than 70 years, well-differentiated tumor grade, lower tumor stage, and larger-volume center (Table 1).
Table 1. Multivariable Analysis of Prognostic Factors for Overall Survival Present at Study Inclusion.
| Factor | HR (95% CI)a | P valueb |
|---|---|---|
| Adjuvant chemotherapy | ||
| Gemcitabine | 1 [Reference] | <.001 |
| mFOLFIRINOX | 0.65 (0.51-0.82) | |
| Center volume | ||
| Inclusion of ≥10 patients | 0.77 (0.61-0.98) | .03 |
| Inclusion of <10 patients | 1 [Reference] | |
| Age, y | ||
| <70 | 0.70 (0.52-0.93) | .02 |
| ≥70 | 1 [Reference] | |
| Tumor grading | ||
| Well differentiated | 0.69 (0.53-0.90) | .01 |
| Moderately/poorly/undifferentiated | 1 [Reference] | |
| Tumor staging | ||
| IA/IB | 0.10 (0.03-0.33) | .002 |
| IIA | 0.24 (0.09-0.60) | |
| IIB | 0.35 (0.17-0.72) | |
| III/IV | 1 [Reference] |
Abbreviations: HR, hazard ratio; mFOLFIRINOX, modified fluorouracil, leucovorin, irinotecan, and oxaliplatin.
Stratified Cox model and log-rank test on lymph node status, resection margins, and postoperative CA 19-9.
Likelihood ratio test.
To complete all treatment cycles was a significant positive predictor for OS for each regimen (data not shown) and for all patients (HR, 0.64; 95% CI, 0.49-0.84; P = .002; Table 2; eFigure 2 in the Supplement). A shorter time from end of adjuvant therapy to disease relapse was identified as an adverse prognostic factor for OS from relapse (HR, 1.03; 95% CI, 1.01-1.04; P < .001). A cutoff at 12 months was found optimal to dichotomize the time from end of adjuvant therapy to disease relapse (HR, 1.95; 95% CI, 1.49-2.54; P < .001; eFigure 3 in Supplement 2).
Table 2. Univariate Analysis of Prognostic Factors Collected at the End of Treatment for OS.
| Factor | No. event/No. total | 5-y OS, % | HR (95% CI) | P value |
|---|---|---|---|---|
| Dose intensity ≥0.80a | ||||
| No | 126/200 | 39.2 | 1 [Reference] | .93 |
| Yes | 159/249 | 38.2 | 1.01 (0.80-1.28) | |
| Duration of treatment, mo | ||||
| <6 | 260/407 | 38.0 | 1 [Reference] | .44 |
| ≥6 | 25/42 | 46.1 | 0.85 (0.57-1.29) | |
| All treatment cycles completedb | ||||
| No | 72/103 | 27.4 | 1 [Reference] | .002 |
| Yes | 213/346 | 41.9 | 0.64 (0.49-0.84) |
Abbreviations: HR, hazard ratio; mFOLFIRINOX, modified fluorouracil, leucovorin, irinotecan, and oxaliplatin; OS, overall survival.
Relative dose intensity of 0.80 or greater for all drugs.
Equates to 12 treatment cycles of mFOLFIRINOX (at least for fluorouracil) and 6 cycles of gemcitabine.
Safety
Safety data have been published in detail previously.14 The safety profile of the mFOLFIRINOX regimen was less favorable than that of gemcitabine but manageable.14 No new safety signals were identified with longer follow-up.
Discussion
This final 5-year analysis with mature data from the PRODIGE 24 randomized clinical trial indicates that adjuvant chemotherapy with a mFOLFIRINOX regimen significantly prolonged disease-free survival, OS, metastasis-free survival, and cancer-specific survival compared with treatment with gemcitabine. The OS benefit with mFOLFIRINOX was significant in most subgroups, including those with adverse prognostic factors (ie, poorly differentiated tumor, pT3-T4 tumor, positive lymph nodes, lymph node ratio >0.20, or R1 resection).
The median disease-free survival of 21.4 months with mFOLFIRINOX is to our knowledge one of the longest reported in randomized clinical trials of adjuvant therapy in resected PDAC (Table 39,10,11,12,13,20,21) and was 8.6 months longer than in gemcitabine group. It seems unlikely that the difference between treatment groups was because of a patient selection bias, as the median disease-free survival of 12.8 months in the gemcitabine group aligns with other trials (Table 3). Patient selection criteria were comparable with those in other trials,10,11,12,13,20,22 despite that the threshold value for CA 19-9 in PRODIGE 24 (180 U/mL) was higher than in CONKO-001 (100 U/mL) for patient inclusion.10
Table 3. Five-Year Outcomes From Randomized Clinical Trials of Adjuvant Chemotherapy in Patients With Resected PDAC.
| Source | Patients, No. | Adjuvant therapy | Median follow-up, mo (IQR) | DFS | OS | ||
|---|---|---|---|---|---|---|---|
| Median, mo (95% CI) | 5-y, % | Median, mo (95% CI) | 5-y, % | ||||
| ESPAC-19 | 147 | FU + leucovorin | 47.0 (33-62) | 15.3 (10.5-19.2) | NA | 20.1 (16.5-22.7) | 21.1a |
| 142 | Observation | 47.0 (33-62) | 9.4 (8.4-15.2) | NA | 15.5 (13.0-17.7) | 8.0a | |
| CONKO-00110 | 179 | Gemcitabine | 136 (104-144) | 13.4 (11.6-15.3) | 16.6 | 22.8 (NA) | 20.7 |
| 175 | Observation | 136 (104-144) | 6.7 (6.0-7.5) | 7.0 | 20.2 (NA) | 10.4 | |
| JSAP-0213 | 58 | Gemcitabine | 60.4 (40.6-77.1) | 11.4 (8.0-14.5) | NA | 22.3 (16.1-30.7) | 23.9 |
| 60 | Observation | 60.4 (40.6-77.1) | 5.0 (3.7-8.9) | NA | 18.4 (15.1-25.3) | 10.6 | |
| ESPAC-312 | 537 | Gemcitabine | 34.2 (27.1-43.4) | 14.3 (13.5-15.6)b | NA | 23.6 (21.4-26.4) | 17.5a |
| 551 | FU + leucovorin | 34.2 (27.1-43.4) | 14.1 (12.5-15.3)b | NA | 23.0 (21.1-25.0) | 15.9a | |
| JASPAC-0120 | 187 | S-1 | 79.3 (72.0-89.0) | 22.9 (NA)c | 33.3 | 46.5 (37.8-63.7) | 44.1a |
| 190 | Gemcitabine | 83.2 (71.8-88.5) | 11.3 (9.7-13.6)c | 16.8 | 25.5 (22.5-29.6) | 24.4a | |
| ESPAC-411 | 364 | Gemcitabine + capecitabine | 43.2 (39.7-45.5) | 13.9 (12.1-16.6) | 18.6 | 28.0 (23.5-31.5) | 28.8a |
| 366 | Gemcitabine | 43.2 (39.7-45.5) | 13.1 (11.6-15.3)c | 11.9 | 25.5 (22.7-27.9) | 16.3a | |
| APACT21 | 432 | Gemcitabine + nab-paclitaxel | 63.2 (NA)c | 16.6 (NA) | NA | 41.8 (NA)d | 38d |
| 434 | Gemcitabine | 63.2 (NA)c | 13.7 (NA) | NA | 37.7 (NA)d | 31d | |
| PRODIGE 24 | 247 | mFOLFIRINOX | 69.7 (59.4-84.1) | 21.4 (17.5-26.7) | 26.1 | 53.5 (22.4-NE) | 43.2 |
| 246 | Gemcitabine | 69.7 (59.4-84.1) | 12.8 (11.6-15.2) | 19.0 | 35.5 (20.3-80.8) | 31.4 | |
Abbreviations: DFS, disease-free survival; FU, fluorouracil; mFOLFIRINOX, modified fluorouracil, leucovorin, irinotecan, and oxaliplatin; NA, not available; NE, nonestimable; OS, overall survival; PDAC, pancreatic ductal adenocarcinoma.
Estimated 5-year survival.
Progression-free survival.
Recurrence-free survival.
Post hoc analysis; data cutoff date of April 9, 2021 (88% mature).
Noticeably, the gain in disease-free survival with treatment with mFOLFIRINOX translates into an OS benefit, with a 32% reduction in risk of death compared with the gemcitabine group. The median overall survival in PRODIGE 24 was 18 months longer with mFOLFIRINOX than with gemcitabine, with a 5-year overall survival rate of 43.2% compared with 31.4%. The median survival with treatment with gemcitabine (35.5 months) in the present trial was longer than in most other trials (22.3-26.0 months; Table 3). Also, the overall survival outcomes with gemcitabine in PRODIGE 24 were similar to those reported in the recent APACT trial after a similar length of follow-up (Table 3).21
Postrecurrence median survival rates (18.5 months with local recurrence and 14.0 months with metastatic recurrence) were relatively long compared with the ESPAC-4 data (9.4 and 8.9 months for local and distant recurrence, respectively).11 This may be because of an earlier diagnosis of recurrence using CA 19-9 levels and a computed tomography scan and the administration of subsequent treatments after tumor relapse. In the ESPAC-4 trial, only 35.7% of patients received treatment for relapse, vs 93.4% in PRODIGE 24. The use of mFOLFIRINOX at recurrence in 74.2% of patients of the gemcitabine group may explain their survival improvement. Fewer single-site liver and peritoneal metastases and more single-site lung metastases occurred in the mFOLFIRINOX group. As a short time to relapse following the end of adjuvant therapy constituted a significant adverse prognostic factor for OS, it is important to early detect and to treat promptly the relapse. In a nationwide Dutch cohort study, patients with asymptomatic recurrence detected by follow-up imaging after PDAC resection had a higher probability to receive recurrence treatment, which was significantly associated with improved survival.23
Defining prognostic factors may help to predict recurrence and poor survival outcomes. Completion of all cycles of adjuvant chemotherapy in the present study was associated with improved OS in both treatment groups, as previously reported.12,24 Other prognostic factors were mFOLFIRINOX treatment, tumor location, stage, grade, and being younger than 70 years. To our knowledge, this is the first demonstration of the effect of larger-volume centers (≥10 patients included) on survival after recurrence. Translational studies may help to identify tumor molecular signatures to better select chemotherapy regimens.
Limitations
This study has several limitations: quality of life was not planned as a secondary objective. Despite the fact that the upper age limit for inclusion in the trial was 80 years, only 20.5% of the patients were 70 years or older. More elderly patients are receiving diagnoses of pancreatic cancer and are now being considered for resection and adjuvant chemotherapy. Another limitation comes from inclusion criteria: selected patients had no cardiac ischemia and good performance status (World Health Organization 0 or 1). In routine practice, up to 30% of patients do not receive adjuvant chemotherapy because of the morbidity of pancreatectomy, slow recovery from surgery, comorbidities, development of early recurrence, or reduced performance status.24 This trial authorized inclusion until 12 weeks after resection to ensure adequate postoperative recovery and maximize the chance of receiving chemotherapy. Perioperative chemotherapy will deliver systemic chemotherapy earlier and may increase the completion rate of multimodal treatment. Ongoing randomized clinical trials will help define the role of preoperative chemotherapy, particularly mFOLFIRINOX, in the resectable setting.
Conclusions
With mature data and longer follow-up, the final results analysis of the PRODIGE 24/CCTG PA6 randomized clinical trial demonstrates that mFOLFIRINOX adjuvant chemotherapy significantly improves OS after complete resection of PDAC compared with single-agent adjuvant gemcitabine. The benefits were seen across all survival end points in all predefined subgroups. These findings confirm treatment with mFOLFIRINOX as the recommended adjuvant regimen in eligible patients.
Trial protocol
eTable 1. Baseline Demographic and Clinical Characteristics in the Intention-to-Treat Population
eTable 2. Disease-free survival events and sites of first metastases recurrences
eTable 3. Median overall survival from date of metastasis recurrence (OSr) according the site of first recurrence
eTable 4. Treatments administered at relapse
eTable 5. Univariate analysis of prognostic factors present at study inclusion for overall survival
eFigure 1. CONSORT Diagram of Participant Enrollment
eFigure 2. Kaplan-Meier analysis of Overall survival according to chemotherapy completion (Landmark method)
eFigure 3. Kaplan-Meier analysis of Overall survival from relapse according to the time from end of chemotherapy to relapse
Nonauthor collaborators
Data sharing statement
References
- 1.Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356-387. doi: 10.1016/j.ejca.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941-1953. doi: 10.1002/ijc.31937 [DOI] [PubMed] [Google Scholar]
- 3.Globocan 2020 . Pancreas fact sheet. Accessed February 21, 2022. https://gco.iarc.fr/today/data/factsheets/cancers/13-Pancreas-fact-sheet.pdf.
- 4.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 5.Rahib L, Wehner MR, Matrisian LM, Nead KT. Estimated projection of US cancer incidence and death to 2040. JAMA Netw Open. 2021;4(4):e214708. doi: 10.1001/jamanetworkopen.2021.4708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.GBD 2017 Pancreatic Cancer Collaborators . The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4(12):934-947. doi: 10.1016/S2468-1253(19)30347-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu J, Yang X, He W, Ye W. Burden of pancreatic cancer along with attributable risk factors in Europe between 1990 and 2019, and projections until 2039. Int J Cancer. 2021;149(5):993-1001. doi: 10.1002/ijc.33617 [DOI] [PubMed] [Google Scholar]
- 8.Ducreux M, Cuhna AS, Caramella C, et al. ; ESMO Guidelines Committee . Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v56-v68. doi: 10.1093/annonc/mdv295 [DOI] [PubMed] [Google Scholar]
- 9.Neoptolemos JP, Stocken DD, Friess H, et al. ; European Study Group for Pancreatic Cancer . A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200-1210. doi: 10.1056/NEJMoa032295 [DOI] [PubMed] [Google Scholar]
- 10.Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310(14):1473-1481. doi: 10.1001/jama.2013.279201 [DOI] [PubMed] [Google Scholar]
- 11.Neoptolemos JP, Palmer DH, Ghaneh P, et al. ; European Study Group for Pancreatic Cancer . Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011-1024. doi: 10.1016/S0140-6736(16)32409-6 [DOI] [PubMed] [Google Scholar]
- 12.Neoptolemos JP, Stocken DD, Bassi C, et al. ; European Study Group for Pancreatic Cancer . Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304(10):1073-1081. doi: 10.1001/jama.2010.1275 [DOI] [PubMed] [Google Scholar]
- 13.Ueno H, Kosuge T, Matsuyama Y, et al. A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br J Cancer. 2009;101(6):908-915. doi: 10.1038/sj.bjc.6605256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conroy T, Hammel P, Hebbar M, et al. ; Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group . FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379(25):2395-2406. doi: 10.1056/NEJMoa1809775 [DOI] [PubMed] [Google Scholar]
- 15.Khorana AA, McKernin SE, Berlin J, et al. Potentially curable pancreatic adenocarcinoma: ASCO Clinical Practice Guideline update. J Clin Oncol. 2019;37(23):2082-2088. doi: 10.1200/JCO.19.00946 [DOI] [PubMed] [Google Scholar]
- 16.European Society for Medical Oncology . Update—cancer of the pancreas treatment recommendations. Accessed February 21, 2022. https://www.esmo.org/guidelines/guidelines-by-topic/gastrointestinal-cancers/pancreatic-cancer
- 17.Slidell MB, Chang DC, Cameron JL, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol. 2008;15(1):165-174. doi: 10.1245/s10434-007-9587-1 [DOI] [PubMed] [Google Scholar]
- 18.Morgan CJ. Landmark analysis: a primer. J Nucl Cardiol. 2019;26(2):391-393. doi: 10.1007/s12350-019-01624-z [DOI] [PubMed] [Google Scholar]
- 19.Valle JW, Palmer D, Jackson R, et al. Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: ongoing lessons from the ESPAC-3 study. J Clin Oncol. 2014;32(6):504-512. doi: 10.1200/JCO.2013.50.7657 [DOI] [PubMed] [Google Scholar]
- 20.Uesaka K, Boku N, Fukutomi A, et al. ; JASPAC 01 Study Group . Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016;388(10041):248-257. doi: 10.1016/S0140-6736(16)30583-9 [DOI] [PubMed] [Google Scholar]
- 21.Tempero M, O’Reilly E, Van Cutsem E, et al. Phase 3 APACT trial of adjuvant nab-paclitaxel plus gemcitabine (nab-P + gem) vs gemcitabine (gem) alone in patients with resected pancreatic cancer (PC): updated 5-year overall survival. Ann Oncol. 2021;32(suppl 3):S226. doi: 10.1016/j.annonc.2021.06.009 [DOI] [Google Scholar]
- 22.Jones RP, Psarelli EE, Jackson R, et al. ; European Study Group for Pancreatic Cancer . Patterns of recurrence after resection of pancreatic ductal adenocarcinoma: a secondary analysis of the ESPAC-4 randomized adjuvant chemotherapy trial. JAMA Surg. 2019;154(11):1038-1048. doi: 10.1001/jamasurg.2019.3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daamen LA, Groot VP, Verkooijen HM, Molenaar IQ, van Santvoort HC. Detection, treatment, and survival of pancreatic cancer recurrence in the Netherlands: a nationwide analysis. Ann Surg. 2022;275:769-775. doi: 10.1097/SLA.0000000000004093 [DOI] [PubMed] [Google Scholar]
- 24.Altman AM, Wirth K, Marmor S, et al. Completion of adjuvant chemotherapy after upfront surgical resection for pancreatic cancer is uncommon yet associated with improved survival. Ann Surg Oncol. 2019;26(12):4108-4116. doi: 10.1245/s10434-019-07602-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eTable 1. Baseline Demographic and Clinical Characteristics in the Intention-to-Treat Population
eTable 2. Disease-free survival events and sites of first metastases recurrences
eTable 3. Median overall survival from date of metastasis recurrence (OSr) according the site of first recurrence
eTable 4. Treatments administered at relapse
eTable 5. Univariate analysis of prognostic factors present at study inclusion for overall survival
eFigure 1. CONSORT Diagram of Participant Enrollment
eFigure 2. Kaplan-Meier analysis of Overall survival according to chemotherapy completion (Landmark method)
eFigure 3. Kaplan-Meier analysis of Overall survival from relapse according to the time from end of chemotherapy to relapse
Nonauthor collaborators
Data sharing statement


