Abstract
Itch is one of the most primal sensations, being both ubiquitous and important for the well-being of animals. For more than a century, a desire to understand how itch is encoded by the nervous system has prompted the advancement of many theories. Within the past 15 years, our understanding of the molecular and neural mechanisms of itch has undergone a major transformation, and this remarkable progress continues today without any sign of abating. Here I describe accumulating evidence that indicates that itch is distinguished from pain through the actions of itch-specific neuropeptides that relay itch information to the spinal cord. According to this model, classical neurotransmitters transmit, inhibit and modulate itch information in a context-, space- and time-dependent manner but do not encode itch specificity. Gastrin-releasing peptide (GRP) is proposed to be a key itch-specific neuropeptide, with spinal neurons expressing GRP receptor (GRPR) functioning as a key part of a convergent circuit for the conveyance of peripheral itch information to the brain.
Interest in itch and scratch-reflex mechanisms is more than a century old1–7, and it has long been appreciated that itch and pain appear to be intricately entangled. Pain inhibits itch, and opioids correspondingly inhibit pain but induce itch8,9. In addition, many pain-sensing sensory neurons (nociceptors, also known as C-nociceptive fibres) can also transmit itch2,7. Historically, therefore, the focus of investigation of itch has been its relationship with pain1,5,7,10–13.
Different types of itch can be classified in various ways. Depending on whether histamine release is involved, itch can be broadly divided into histaminergic itch (such as acute allergic itch) and non-histaminergic itch, both of which are mediated by a wide variety of itch-related receptors14. Itch can also be elicited by various external physical and non-physical stimuli, which can be used as a method of classification. For example, chemical itch is evoked by pruritogens that penetrate the epidermis of the skin, whereas mechanical itch is induced by innocuous light touch (such as that of a wool fibre) on the skin1,5,15. Seeing others scratch or merely viewing an image of a bug crawling on the skin may elicit the urge to scratch16,17, referred to as ‘contagious itch’. As a form of interoception18, itch can also occur spontaneously and can be modulated by internal states or homeostatic demands19. Such spontaneous itch can be physiological (many animals scratch spontaneously to clean their fur or maintain their body temperature1,20), psychological (triggered by stress or anxiety, for example)21 or pathological (such as neuropathic or systemic itch)1,2,22,23. Finally, itch can be classified as acute or chronic according to the duration of itch sensation: acute itch is transient, whereas chronic itch lasts for more than 6 weeks in humans14,24, or more than 1 hour (and up to months) in mice (in which chronic itch is induced via periodic chemical sensitization of the skin for several days25,26 or neural tissue injury26–28). The lack of effective therapeutics for chronic itch, most types of which are refractory to antihistamines, poses a significant burden to patients and may compromise their quality of life to a degree often comparable to that for chronic pain29.
A fundamental question in the somatosensory field is how different types of sensory modalities are encoded and represented in the brain. With respect to itch, most research has focused on how primary sensory neurons and/or the spinal cord differentiate between itch and pain; however, the answer to this century-old puzzle continues to elude researchers. In this Perspective, I describe a neuropeptide code hypothesis of itch, with an emphasis on the neuropeptides, signalling mechanisms and spinal neural circuits that encode itch in mice. I critically appraise the current state of knowledge of the spinal circuitry of itch and propose a model in which classical neurotransmitters and neuropeptides play distinct roles in conveying itch information to the CNS. Along the way, I discuss technical and conceptual challenges. Specifically, I explore alternative explanations for some conflicting results and revisit several commonly held views. The peripheral neuroimmune mechanisms of itch are not the focus of this Perspective, which are well covered in recent reviews14,30,31. Brain mechanisms of itch and chronic itch are tangentially touched upon whenever relevant.
Coding theories of itch
Somatosensory information — including that important for itch, pain, temperature sensing and touch — is relayed from the skin to various categories of primary sensory neurons with cell bodies residing in the dorsal root ganglia (DRG) and trigeminal ganglia. These sensory neurons project to second-order interneurons or directly synapse with projection neurons in the dorsal horn of the spinal cord (FiG. 1). The spinal dorsal horn is divided into several morphologically distinguishable laminae. Laminae I and II receive direct inputs from slowly conducting unmyelinated C fibres and faster-conducting myelinated Aδ fibres, together encoding pain, itch, pleasant touch and temperature, whereas laminae III and IV are innervated mainly by Aβ low-threshold mechanoreceptors (LTMRs) that convey touch and proprioceptive information32,33 (FIG. 1a).
Fig. 1 |. Dorsal horn architecture and coding theories for itch.
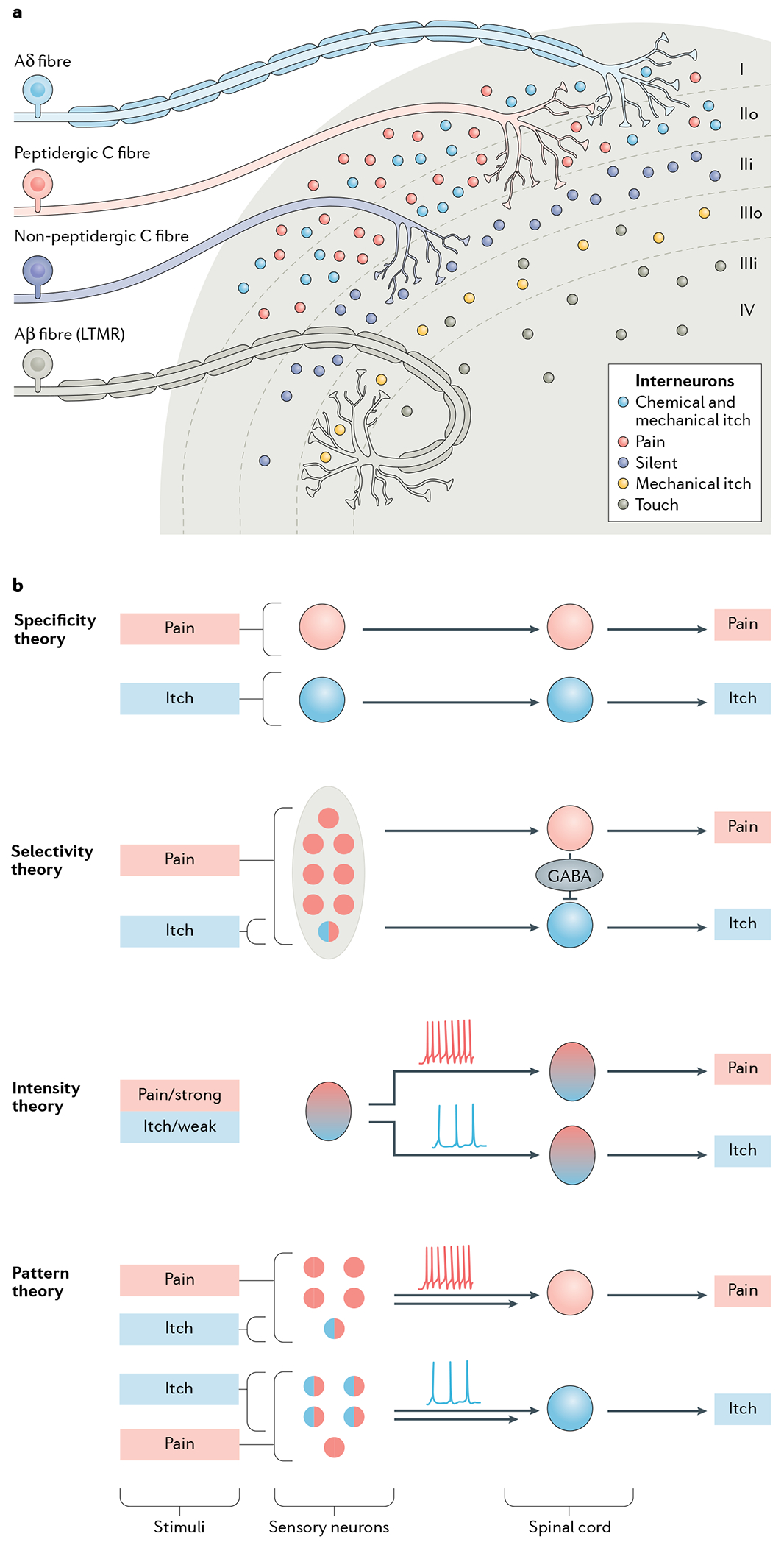
a | The dorsal horn, illustrating its division into several distinct laminae (I–IV). Laminae I and II are innervated by unmyelinated C fibres and fast-conducting myelinated Aδ fibres, which together convey information relevant to pain, itch, pleasant touch and temperature sensing. Interneurons in laminae I and II additionally receive mechanical itch information from Aβ low-threshold mechanoreceptors (LTMRs) via an indirect route (not shown)58,188. Lamina II can be subdivided into two layers: unmyelinated peptidergic C fibres terminate mainly in lamina I and the outer layer of lamina II and convey information that requires slow conduction velocities, such as that important for chemical itch and chemical pain33,247. The inner layer of lamina II is innervated mainly by unmyelinated non-peptidergic C fibres that convey pain, itch and other somatosensory information. Laminae III and IV are typically innervated by myelinated Aβ LTMR fibres that convey mechanical touch, mechanical itch and proprioceptive information32,33,58,188,193. Lamina III can be further divided into two layers. The outer layer contains TAC2 neurons, which mediate mechanical itch58. Dorsal horn projection neurons in lamina I and possibly laminae III and IV, integrate and relay itch information to the somatosensory cortex (not shown)61,75,248. b | The main current coding theories of itch. According to the specificity (or labelled line) theory, information about itch-inducing and painful stimuli is conveyed via distinct neural pathways from the skin to the spinal cord3. There are itch-specific neurons in the periphery, which are activated by itch stimuli exclusively.The selectivity (or population coding) theory proposes that pruriceptors (represented by a circle filled half in red and half in blue) are a small subset of nociceptive neurons. Pruriceptors respond to both itch-inducing and painful stimuli, whereas a much larger population of nociceptors (red) convey pain information exclusively3,19. This theory suggests that distinct labelled lines for pain and itch exist in the spinal cord and that pain transmission can activate spinal inhibitory neurons to inhibit or occlude itch by crosstalk between the two labelled lines34,35. According to the intensity theory, weak activation of nociceptors provokes itch, whereas strong activation of the same population of neurons evokes pain, via different modes of firing pattern (tonic for weak and burst for strong stimuli)3,10,12,13. Like the selectivity theory, the pattern theory3,34 postulates that within the larger population of sensory nociceptive neurons (red) there is a subset of pruriceptors (half red, half blue). However, it suggests that the distribution of these pruriceptors may be different in the epidermis and dermis (not shown). Therefore, depending on the nature and location of a given stimulus, a mixture of pruriceptors and nociceptors will be differentially activated, leading to different population firing patterns (tonic versus burst) and thereby encoding itch or pain specificity34,106. This theory does not explain how itch and pain information is encoded in the spinal cord. However, it can be assumed that itch and pain are conveyed separately in the spinal cord after the encoding by primary sensory neurons.
Many theories have been proposed to explain how itch is encoded and distinguished from pain in the sensory neurons and/or spinal cord3,34–37 (FIG. 1b). For many, the most influential and attractive theory (perhaps for its simplicity) is the labelled line (or specificity) theory, which is deeply rooted in Johannes Müller’s ‘law of specific nerve energies’, first published in 1835 (REF.38). It postulates that itch and pain are conveyed by dedicated and distinct neural pathways that begin in specialized peripheral detectors and continue within the spinal cord3. In the past decade, genetic and chemical ablation approaches, combined with behavioural assessment, have been used to try to identify itch-specific neurons (TABLE 1). However, as discussed in more detail later, extensive searching has yet to conclusively identify the components of a labelled line for itch in peripheral sensory neurons39,40. It could be argued that this theory is incompatible with the intrinsic properties of the primary sensory neurons, which are typically polymodal41,42.
Table 1 |.
Approaches used to define itch-specific neuropeptides and neurons
| Manipulation | Criteria | Advantages | Disadvantages |
|---|---|---|---|
| Assessment of behavioural correlates of itch | |||
| Loss of neuropeptide function in mice (via genetic KO or siRNA-mediated knockdown) | Impaired itch behaviour and normal pain behaviour | Direct evidence Sensory neuron-specific KO is feasible |
Compensation: multiple related neuropeptides and receptors Time consuming to generate double-KO or triple-KO mice and CKO mice Spinally restricted CKO may not be feasible |
| Chemogenetic or optogenetic inhibition or viral, chemical or intersectional genetic ablation of neurons | Impaired itch behaviour and normal pain behaviour | Spinally restricted inhibition/ablation possible Sensory neuron-specific inhibition/ablation possible Ablation can be fast and efficient |
Low level of fidelity of a Cre line Neurotoxin approach may have off-target effects Most acute pain behaviour cannot be appropriately evaluated after cervical inhibition or ablation Genetic compensation Indirect effects |
| Loss of neuropeptide function via pharmacological approaches | Impaired itch behaviour and normal pain behaviour | Acute and convenient Agonists may be available Dose-dependent scratching behaviour can be assessed |
Off-target effects Potential presynaptic effects originating in primary afferents Pharmacological artefacts |
| Gain of neuropeptide function via pharmacological approaches | Induced itch behaviour and normal pain behaviour | Acute and convenient Agonists may be available Dose-dependent scratching behaviour can be assessed |
Off-target effects Potential presynaptic effects originating in primary afferents Pharmacological artefacts Ceiling or floor effects |
| Chemogenetic or optogenetic activation of neurons | Induced itch behaviour and normal pain behaviour | Spinal cervical cord-restricted activation Validation using BB-sap possible Sensory neuron-specific activation of central or peripheral fibres possible |
Low level of fidelity of a Cre line Chemogenetic or optogenetic artefacts Artificial behaviour can only suggest the capacity of fibres and is not necessarily an indicator of an endogenous function of manipulated neurons |
| Assessment of neural correlates of itch | |||
| Electrophysiological recording of neuronal responses to chemical itch, pain, cooling or scratching | Inhibited by pain stimuli | Single-cell resolution Cell-type recording |
Low level of fidelity of Cre or eGFP mouse lines Lack of specificity of some chemicals (e.g. capsaicin) May not simulate neuropeptide release in freely behaving animals |
BB-sap, bombesin–saporin; CKO, conditional knockout; eGFP, enhanced GFP; KO, knockout; siRNA, small interfering RNA.
A competing theory is the selectivity (or population coding) theory, which assumes that most nociceptors are insensitive to pruritogenic stimuli and that the activity of this large population of nociceptors can inhibit that of the small subset of nociceptors that are also pruriceptors (itch-sensing neurons)3,35,43. In other words, a painful stimulus will simultaneously activate both itch-insensitive nociceptors and pruriceptors (but will mask the sensation of itch through cross-inhibition within the spinal cord)35: itch will be transmitted only when pain is absent. However, in light of the polymodality of most sensory neurons42,44, it remains unclear whether itch-insensitive nociceptors exist. Moreover, cooling-sensitive neurons, which account for fewer than 10% of the DRG neurons in rodents45–47, are efficacious in inhibiting itch48,49 and even pain50. This suggests that one sensory modality may inhibit another on non-quantitative grounds and that other factors, such as synaptic strength and wiring, may also play a part.
Several other theories have been proposed to explain itch coding (FIG. 1). The intensity coding theory posits that itch and pain are transmitted by the same fibres, but are distinguished by the different firing intensities that they induce3,10,12,13. This theory was first proposed by von Frey, who used a plant bristle to stimulate the skin and found that a weak mechanical touch evoked itch, whereas a strong one led to pain4. However, this theory is not supported by more recent findings that, for some cutaneous fibres, either electrical or optogenetic stimulation can evoke itch in an intensity-dependent manner but does not evoke pain at any intensity tested51–53.
The pattern theory hypothesizes that distinct patterns of firing evoked in sensory neuron populations (nociceptors and pruriceptors) that display partially overlapping innervation patterns in the skin by itch-inducing or painful stimuli may encode these two sensations3,34 (FIG. 1b). A variation of this theory is the spatial contrast theory37, which postulates that itch arises from selective activation of pruriceptors, but that surrounding nociceptors must also be quiescent, or their inhibition of pruriceptors reduced, for itch to be conveyed37.
These coding theories have focused primarily on the coding of itch versus pain by the primary sensory neurons. However, as I will outline herein, it is now becoming clear that how itch and pain information is distinctly relayed to the spinal cord may be more important for our understanding of itch coding.
Chemical itch coding
Chemical itch (a common cause of acute itch) occurs as a result of a cascade of inflammatory and immune responses that involve receptors in skin, immune and nerve cells14,30. A major breakthrough in our understanding of the coding of chemical itch over the past decade was the identification of several itch-specific neuropeptides and receptors.
The GRP–GRPR neural pathway.
Studies in which genome-wide mapping and behavioural screening were performed in search of spinal dorsal horn lamina-specific nociceptive genes54 led, in 2007, to the serendipitous discovery of gastrin-releasing peptide (GRP) receptor (GRPR), a G-protein-coupled receptor (GPCR), as the first itch-specific receptor in the mouse spinal cord55. GRPR-expressing neurons (hereafter referred to as GRPR neurons) make up approximately 20% of the excitatory interneurons in laminae I and II and receive inputs from C fibres, Aδ fibres, Aβ LTMRs and other spinal itch neurons56–58. They convey itch information to the parabrachial nuclei via projection neurons59–62. The neurotoxin bombesin–saporin (BB-sap) binds to and is internalized by GRPR neurons; however, it is not internalized by those expressing the related receptor neuromedin B (NMB) receptor (NMBR)63, to which it also binds64. This thereby affords a highly selective method to chemically ablate GRPR neurons in mice65. By this method, ablation of spinal GRPR neurons abolished itch transmission without impairing pain behaviours65. It could be argued that the normal pain behaviour observed in mice treated with BB-sap could be due to genetic compensation: this might occur if the population of neurons that were ablated contained cells sensitive to a mixture of sensory modalities, each of which accounted for only a small proportion of the total number of neurons sensitive to a given modality (as is the case for spinal Grp-expressing neurons (see later))53. Additionally, the potential for rewiring of the dorsal horn circuitry as a result of developmental and genetic ablation of a given population of neurons may make it difficult to assess their functional requirement in the adult spinal cord66. However, these confounding factors should be considered together with the results of gain-of-function studies. Pharmacological or optogenetic activation of GRPR neurons evokes itch-related but not pain-related scratching behaviour55,67, suggesting that these neurons are dedicated to the transmission of itch alone. In my view, these combined findings rule out the possibility of genetic compensation or developmental rewiring, and effectively separate itch from pain at the spinal level (TABLE 1).
The search for itch-specific neurons or receptors is complicated by the fact that, in humans, some commonly used experimental pruritogens, such as histamine and cowhage (a non-histaminergic pruritogen), can also evoke pain and some algogens (such as capsaicin) can elicit itch68,69. Likewise, in mice, intraplantar injection of non-histaminergic pruritogens (such as chloroquine, an antimalaria drug that induces pruritus in mice and in some African patients70, bovine adrenal medulla 8-22 peptide (BAM8-22), PAR2 and SLIGRL) induces mechanical and thermal pain that is dependent on the cation channel TRPA1 (REFS71,72). Thus, what might be thought to be a ‘pure’ pruritogen may activate a putative itch-specific neuron to transmit nociceptive information rather than inducing itch-related scratching behaviour. To overcome this limitation, an alternative approach takes advantage of the fact that itch can be inhibited by pain and cooling. This idea was first postulated by Sherrington6, who suggested that this inhibition was mediated through central mechanisms57,73–75. However, it is only recently that it has been adopted as a criterion to define itch-specific neurons; electrophysiological recording confirmed that both chemical pain (induced using mustard oil) and a cooling agent (menthol) inhibit the activity of GRPR neurons57. Thus, converging evidence has demonstrated that GRPR neurons fulfil three key criteria for defining itch-specific neurons: they transmit itch information but not pain information (as demonstrated by a loss-of-function approach), their activation evokes itch-related but not pain-related behaviour and they are inhibited by pain and cooling (as shown by electrophysiological recording) (TABLE 1).
GRP, a 27 amino acid peptide, is the ligand for GRPR and was for a long time considered to be a mammalian homologue of the amphibian neuropeptide bombesin64,76–78. Amphibians, however, were later discovered to also possess GRP79,80, indicating that this peptide is evolutionarily conserved in vertebrates. Grp mRNA has been shown to be abundantly expressed in lamina II of the mammalian dorsal horn, and GRP-immunoreactive fibres have been detected in the dorsal horn of the mammalian spinal cord53,54,59,81–83. The origin of these fibres has recently attracted renewed attention, and several studies have investigated potential sources of GRP and, in particular, whether the neuropeptide is produced by spinal or primary sensory (DRG) neurons.
GRP and/or its mRNA have been detected via in situ hybridization, reverse transcription–PCR, western blot and immunohistochemical staining in DRG neurons across various animal species, including rodents, dogs, monkeys and humans53,55,59,81,82,84–93. By contrast, several large-scale RNA-sequencing studies have reported that Grp mRNA was not detected in these neurons94,95, which has been considered by some researchers to provide evidence for an absence of Grp expression in DRGs. However, absence of evidence is not evidence of absence, and it is possible that the low transcript levels of Grp mRNA could simply fall below the empirical detection threshold of these methods, a recognized limitation of such high-throughput assays96,97. Indeed, an earlier study suggested that the level of Grp mRNA transcripts in DRGs is at least ten times lower than that of MAS-related G-protein-coupled receptor A3 gene (Mrgpra3) mRNA transcripts59. It is also important to note that the relative level of mRNA detected for a given gene should not be equated with that of the corresponding protein nor with its functional importance98. For instance, the mRNA for a given neuropeptide might be barely detectable because there is already a large quantity of its bioactive form stored in large dense-core vesicles sufficient for activity-dependent release99. It has long been recognized that some neuropeptides, whose expression is below a detectable level, become detectable only when prolonged or repeated environmental stimuli are encountered99. Taking these factors into account, I consider that there is strong evidence for the presence of Grp mRNA in DRGs.
In line with this suggestion, two research groups that performed dorsal root rhizotomy followed by immunostaining with a GRP antibody concluded that the GRP-positive fibres present in the mouse dorsal horn are predominantly of primary afferent origin26,81, corroborating several studies conducted independently almost 40 years ago93,100. However, two other groups using the same approach pointed to a spinal cord origin for these fibres82,83. The reason for these discrepancies remains unclear. However, several methodological issues need to be considered regarding the detection of Grp mRNA and GRP, as discussed previously59. In particular, there is a widespread notion that the GRP antibody used in these experiments is not specific owing to some background immunostaining problems associated with immunohistochemical staining59,83. However, it is worth emphasizing that — using a variety of approaches that included western blotting59, the creation of Grp-knockout (KO) mice26, peptide preabsorption testing81,82 and appropriate dilution of the antibody83 — all four of the teams examining the source of GRP using the GRP antibody confirmed its specificity26,53,59,81–83. Moreover, the GRP antibody is known to be one of the most reliable antibodies for cell type classification in the suprachiasmatic nucleus (SCN)101–104. Although the level of Grp mRNA in the SCN is very low104, immunohistochemical staining indicates that GRP is abundant in the central axonal terminals of GRP neurons104, similar to the findings in DRG26,53. I hypothesize that the low transcriptional activity of Grp and the rapid onset of transcription, translation and axonal transportation of GRP in response to itchy stimuli could all be important mechanisms by which the level of GRP is maintained. From the findings taken together, I believe that there is compelling evidence that some DRG neurons express GRP and that the amount of GRP present in the central terminals of primary afferents is adequate for an animal’s daily use.
To examine the functional role of GRP, the phenotype of mice in which there is a conditional KO (CKO) of Grp in sensory neurons has been analysed53. Grp-CKO mice displayed deficits in non-histaminergic itch but not in histamine-evoked itch or pain behaviours53, which phenocopies observations in mice with global KO of either Grp53,56,105 or Grpr55,65 and is further corroborated by studies that induced a pharmacological blockade of spinal GRPR87. A knock-in GrpiCre mouse line that recapitulates endogenous Grp expression has also been generated and used to show that optogenetic stimulation of cutaneous GRP-expressing fibres at frequencies up to 20 Hz evokes itch-related scratching behaviour, a finding that is consistent with the results of chemogenetic studies53. Of note, the broad arborization of GRP fibres in the epidermis is in line with the finding that cutaneous itch arises from the epidermis or the dermoepidermal junction but not the dermis5,106.
One could interpret these results as evidence that GRP neurons are part of a labelled line for itch, in support of the specificity theory. However, for GRP neurons to qualify as part of an itch-specific circuit, they should not respond to painful stimuli. It has long been recognized that some GRP neurons also express substance P (SP)59,84,92, a nociceptive neuropeptide that activates several neurokinin receptors with different affinities107,108. NK3R is expressed in lamina II excitatory and inhibitory neurons109, and during nociceptive transmission, SP is released to activate spinal NK3R neurons110–113. It is notable that SP has also been implicated in itch transmission at the spinal level87,114; however, determining its site of action is complicated by its crosstalk with other neurokinin receptors and its peripheral action in itch115 (TABLE 1). Conceivably, a subset of neurons expressing both GRP and SP may therefore participate in nociceptive transmission via NK3R, in addition to transmission of itch via GRPR, meaning that GRP neurons cannot be considered part of a labelled line for itch. In the same vein, a recent study showed that a subset of primary sensory neurons expressing Mrgpra3 — previously thought to be itch-specific neurons39 — can also transmit nociceptive information40, further arguing against the existence of an itch-specific circuit in primary sensory neurons.
A neuropeptide code for chemical itch.
As noted earlier herein, the key question of how itch information is conveyed to the spinal cord has attracted relatively little attention compared with the coding by primary sensory neurons. Cutaneous sensory neurons possess two cardinal features. First, they have a certain level of modality specificity, manifesting itself in their topographic projection patterns in the spinal cord32,33,42,116,117 (FIG. 1a). Second, they possess a high degree of plasticity and diversity and can respond to different types of environmental stimulus33,41,42. By contrast, electrophysiological studies indicate that the modular organization of the dorsal horn circuitry discriminates between modality-specific inputs and conveys this information to the brain118,119. This prompts the proposition that the connectivity between the primary afferent fibres and their central targets dictates modality-specific transmission.
How does the coding of itch fit into this general schema? The identification of spinal GRPR neurons provides one of the first pieces of evidence supporting the existence of itch-specific circuitry in the spinal cord. Another clue comes from an important observation that there is a significant reduction of GRP immunoreactivity in neuronal fibres within the mouse spinal cord after robust scratching elicited by chloroquine53. Chloroquine-evoked itch is mediated by primary sensory neurons that innervate spinal GRPR neurons53,59,87. The reduction in the intensity of GRP immunoreactive fibres in the superficial dorsal horn after robust scratching suggests a depletion of GRP from the axonal terminals as a result of its release (and is presumably accompanied by activation of GRPR53). By contrast, central SP immunoreactivity remains unaffected by scratching53, implying that there is a lack of, or negligible, SP release during the itch–scratch cycle. Immuno-electron microscopy has revealed that, in some cases, both GRP and SP are stored in the axonal terminals that directly synapse with GRPR neurons, consistent with their partial colocalization in DRG neurons59,84,87,92, and evidence indicates that SP is released in the spinal cord in response to a variety of noxious thermal, mechanical and chemical stimuli108. This suggests that some peripheral nociceptors expressing both SP and GRP will be activated by pruritogenic stimuli, but that the nociceptive information encoded by SP is occluded from accessing the dorsal horn pain circuits because only GRP is released into the spinal cord.
In light of recent advances, I propose here a neuropeptide code model that incorporates the merits of various coding theories (FIG. 2). It posits that it is the neuropeptides that encode the specificity of itch.
Fig. 2 |. Hypothetical model for the neuropeptide coding of itch and pain.
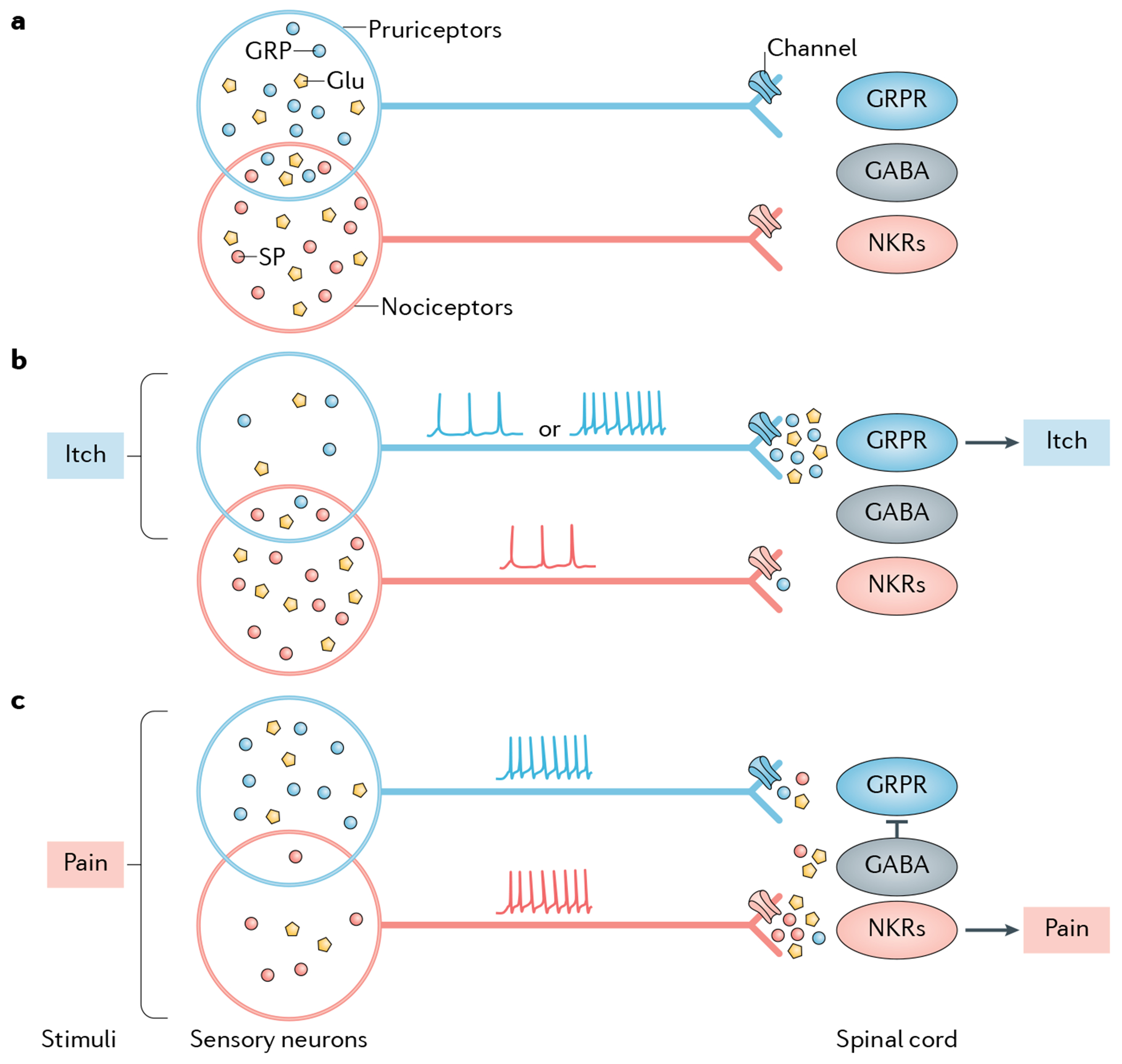
The schematics illustrate the neuropeptide code model, which suggests that itch and pain are encoded and transmitted separately through discrete neuropeptides. Substance P (SP) is shown as an example of a pain-specific neuropeptide transmitter, whereas gastrin-releasing peptide (GRP) is shown as an example of an itch-specific neuropeptide transmitter. Other types of itch-specific or pain-specific neuropeptides may exist but are not shown for illustrative purposes (see also FiG. 3). a | Pruriceptors and nociceptors partially overlap, which is manifested in co-expression of GRP and SP. In the absence of itch or pain stimuli, neither GRP nor SP is released from its terminal, resulting in a lack of itch or pain transmission. For simplistic purposes, neurotransmitters are shown only in the cell bodies of nociceptors and pruriceptors. b | Under itchy conditions, pruriceptors are activated either spontaneously (resulting in tonic firing) or in response to itch stimuli (resulting in bursting firing). This activation is proposed to release GRP and glutamate into the spinal cord, activating GRP receptor (GRPR)-expressing neurons (GRPR neurons), which transmit itch information exclusively to the brain. A smaller number of nociceptors that co-express GRP and SP (indicated as the overlap of the ovals representing these two cell populations) are also activated but fail to release SP, owing to tight control of presynaptic neurotransmitter release via channels such as sodium or calcium channels. Therefore, no pain information is transmitted. This subset of neurons may also release a small amount of GRP at synapses with spinal nociceptive neurons expressing NK receptors (NKRs), but will fail to activate them owing to the lack of GRPR in these neurons. The GABAergic inhibitory interneurons that innervate itch-specific spinal neurons remain inactive. c | Under painful conditions, a population of nociceptors that contain some pruriceptors are concomitantly activated via a bursting firing pattern that provokes the release of SP and glutamate from these neurons132,249,250, which activate spinal nociceptive neurons expressing NKRs. A small amount of GRP may be co-released but fails to activate these neurons owing to the lack of GRPR expression. Meanwhile, a smaller subset of pruriceptors expressing both GRP and SP or GRP alone (not shown) (indicated as the overlap of the ovals representing these two cell populations) is also activated and releases GRP and glutamate, which marginally activate GRPR neurons. A small amount of SP may also be co-released from pruriceptors/nociceptors via the central terminals that innervate GRPR neurons, but may fail to activate them owing to the lack of NKRs in these neurons. A concurrent activation of spinal GABAergic inhibitory neurons by SP and/or glutamate, however, inhibits the activity of GRPR neurons and thereby blocks itch transmission (see also Supplementary Fig. 1). Depending on the properties and location of the stimuli encountered, therefore, a balance between the activation of pruriceptors, nociceptors and spinal inhibitory neurons dictates whether the output of itch is completely or partially blocked. In the latter scenario, both itch information and pain information can be transmitted to the brain (not shown).
A signature of chemical itch is the relatively slow onset of scratching behaviour after exposure to the stimulus5,55, perhaps due to the time required for the pruritogens to initiate a cascade of allergic and inflammatory events7,14,120. For example, there is a latency of several minutes between chloroquine injection and the onset of scratching behaviour in mice55. Chloroquine triggers the release of a plethora of proinflammatory mediators70,121,122, many of which have pruritogenic properties and may, in turn, activate their respective receptors (including, but not limited to, MRGPRA3, Toll-like receptor 3 (TLR3), TLR7 and arginine vasopressin receptor 1A (AVPR1A))90,91,122,123, to increase the excitability of pruriceptors. In line with this, the percentage of dissociated DRG neurons that responded to chloroquine, as measured by calcium imaging, is approximately two to three times that of those expressing what is proposed to be the major itch receptor for chloroquine, MRGPRA3 (approximately 4–5%)87,122,124–126, indicating that additional receptors mediate chloroquine-evoked itch. It remains unclear to what extent MRGPRA3 contributes to itch elicited by chloroquine and other pruritogens, since only mice in which the genes encoding a cluster of MAS-related G-protein coupled receptors (a family that includes other itch-related or pain-related receptors) are knocked out has been examined for changes in chloroquine-evoked itch122. Furthermore, chloroquine is a promiscuous ligand that binds to a number of receptors127. Likewise, no single GPCR can account for formalin-induced or mustard oil-induced chemical pain. These examples suggest that a repertoire of receptors and/or channels in primary sensory neurons (instead of one receptor for one pruritogen as widely presumed) synergistically orchestrate sequential inflammatory responses to a pruritogenic stimulus72. Logically, this makes sense: if one receptor (or one subset of pruriceptors) were dedicated to the transmission of only one pruritogenic stimulus, sensory neurons would quickly exhaust their arsenal, given the potentially vast number of natural pruritogens that exist. Instead, multiple skin, immune and neural pathways are thought to be involved in the transmission of itch evoked by one pruritogen30,31,128.
Although this argument suggests that diversity in peripheral signalling mechanisms is required to coordinate the detection of varied and numerous pruritogenic stimuli, I suggest that it is not necessary or economical for dorsal horn interneurons to match this with multiple microcircuits to process the itch inputs. Itch is a subjective perception that (in humans, at least) may have a variety of qualities; however, all types of itch give rise to an unpleasant sensation that provokes an urge to scratch to counteract itch. To maximize the efficiency and accuracy of the coding that manifests the same motor output, I therefore argue that a process of integration and convergence of diverse peripheral itch inputs must occur and that this happens in the spinal dorsal horn.
The neuropeptide code model, therefore, postulates that a prerequisite for a subset of sensory neurons to participate in transmitting itch information is that the neurons contain itch-specific neuropeptides, such as GRP (FIG. 2). This hypothesis suggests that the activation of these neurons by pruriceptive stimuli would provoke them to release itch (but not pain) neuropeptides, which would bind to their receptors through monosynaptic connections59,122. Thereby, itch information will be transmitted, while irrelevant, non-specific information will be filtered out or blocked (FIG. 2). Any leaky release of pain neuropeptides (under certain burst firing conditions; see later) would be rendered non-functional by the fact that the spinal itch-specific neurons lack the receptors for nociceptive neuropeptides. This is known as a mismatch phenomenon: the peptide release site and corresponding receptor expression are anatomically distinct129, likely reflecting changes that have occurred in their evolutionary or ontogenetic past. For instance, GRPR neurons cannot be activated by SP to evoke pain-related scratching and/or biting behaviour130, or by chemical pain or cooling57, even though fibres containing both GRP and SP or those containing SP alone may directly innervate GRPR neurons59.
A central question for the neuropeptide code model is how an itch neuropeptide is released independently of a pain neuropeptide. While recent studies suggest that SP is not released during the itch–scratch cycle, when some of these afferents co-expressing GRP and SP are presumably active53,59, some confusion arose when researchers attempted to extrapolate the results obtained from electrophysiological recordings of spinal cord slice preparations to freely behaving mice. For example, it has been shown that burst firing or high-frequency stimulation of primary afferents, or the dorsal horn neurons they innervate, can evoke GRP or SP release under in vitro recording conditions131,132. An earlier study found that the response of the dorsal horn GRP-sensitive neurons to monosynaptic C fibre stimulation could be completely blocked by 6-cyano-7-nitroquinoxaline-2,3-dione, a glutamatergic AMPA and kainate antagonist, under in vitro recording conditions, and thus claimed that it is glutamate instead of GRP that functions as an itch transmitter in C fibres133. However, recent optogenetic stimulation of cutaneous GRP fibres of freely behaving mice even at a low frequency (1–5 Hz) could evoke itch-related scratching behaviour53. Even contagious itch induced by a rather weak visual stimulus in the absence of physical stimulation corresponds to GRP release in the SCN104. Moreover, rodents are known to engage in frequent self-scratching and/or grooming behaviours that enable them to maintain various physiological functions20, which may imply that there is a tonic release of itch-related and grooming-related neuropeptides at the baseline level in the absence of external stimuli in these animals. Therefore, repeated stimulation of primary afferents at high frequencies, which is normally required for neuropeptide release under in vitro electrophysiological recording conditions134, is not a prerequisite for GRP release from primary afferents in freely behaving mice.
Although it is difficult to evoke neuropeptide release from primary afferents using an electrophysiological recording approach, presynaptic glutamate release could be readily evoked in spinal slice preparations57,133,135,136. However, pain is not frequently experienced unless a stimulus reaches certain noxious thresholds that enable both glutamate release and SP release from primary nociceptive afferents137–139. These inconsistencies reflect limitations of electrophysiological recording of spinal slice preparations in detecting the slow activation of GPCR responses evoked by neuropeptide binding, contrasting with that of fast glutamatergic transmission at postsynaptic sites140. It can be envisioned that the brake mechanism that is in place in the central terminals of primary afferents to gate the release of pain transmitters must be much tighter than the machinery that gates the release of itch neurotransmitters. For example, discrete channels and/or Ca2+ sensors expressed in the central nerve terminals of primary afferents may subserve as gatekeepers for these two types of stimulus141,142 that distinguish between resting and stimulated states143.
Since itch (GRP) and pain (SP) neurotransmitters partially overlap in sensory neurons (FIG. 2) and since the gating of itch transmitter release is supposed to be weaker than that of pain transmitter release, it could be predicted that the release of pain transmitters in response to noxious stimuli will be accompanied by that of itch transmitters (FIG. 2). To override itch information that may coincidentally travel from the spinal cord to the brain, the neuropeptide code model suggests that spinal interneurons that inhibit itch transmission are activated by pain transmission (FIG. 2). This model postulates that SP, GRP and glutamate simultaneously co-activate spinal pain neurons, itch neurons and inhibitory circuits for itch, respectively.
The neuropeptide code may shed new light on the development of vicious itch–scratch cycles under certain pathological itch conditions. In these conditions, primary nociceptors that are usually involved in the detection and transmission of non-itchy stimuli would be recruited to express itch neuropeptides26,59. This could make the nociceptive apparatus that otherwise could be recruited for efficacious inhibition of itch ineffective. Furthermore, the spinal circuitry responsible for inhibiting itch might be disabled144,145 (see the discussion later), in conjunction with an augmented release of GRP that sensitizes and recruits some ‘silent’ GRPR neurons into action26,59. All these mechanisms could underlie the observations that painful mechanical, thermal, chemical and electrical stimuli induce itch but not pain in patients with chronic itch146,147.
In summary, the neuropeptide code model proposes that, in the somatosensory system, the specificity of itch is encoded by the selective release of slow-acting itch neuropeptides rather than by the activation of itch stimulus-specific receptors or a specific subset of sensory neurons. Itch stimuli predominantly activate pruriceptors but also marginally activate nociceptors in the skin; however, the central terminals of nociceptors are unable to release pain neurotransmitters, and the spinal pain circuits thereby remain quiescent (FIG. 2).
Neuropeptide coding of histaminergic versus non-histaminergic itch.
Human and animal studies have shown that histamine-dependent and histamine-independent itch are conveyed through parallel peripheral pathways37,56,126,148,149, indicating that distinct neuropeptides may be used to convey information about these types of itch. However, the question of how histaminergic and non-histaminergic itch information is relayed to the spinal cord — and, more specifically, which neuropeptides are involved — has been insufficiently considered. As mentioned earlier, evidence suggests that GRP encodes only non-histaminergic itch53. An important question is, therefore, whether there is an equivalent neuropeptide that encodes histamine-evoked itch.
NMB, another mammalian bombesin-like neuropeptide, binds to NMBR with high affinity but also cross-activates GRPR64,150. NMB has several biological and pharmacological properties that are analogous to those of GRP, including its capacity to induce immediate dose-related scratching behaviours when injected into the spinal cord at picomolar concentrations56,64,76,151, an indicator that it is directly activating receptors involved in itch transmission. Comprehensive genetic studies in mice have demonstrated that NMB and NMBR are required for histaminergic itch but not for chloroquine-evoked itch56,105. Consistent with this notion, a recent study found that sneezing reflex, a histamine-dependent acute allergic response152, is mediated by NMB153. Interestingly, normal histaminergic itch is observed in Nmbr-KO mice; however, it was shown that this is not due to compensatory effects but rather is due to a cross-activation of GRPR neurons by NMB in the absence of NMBR105. By contrast, Nmb-KO mice exhibited deficits in histaminergic itch, even in the presence of NMBR, indicating that NMB is required for itch evoked by histamine53,56. In support of these findings, Grpr-KO mice exhibit normal histaminergic itch, owing to the presence of normal NMB-NMBR signalling65,105. Because NMBR neurons can be weakly activated by GRP, they additionally relay some non-histaminergic itch information56, with this information converging in GRPR neurons through glutamatergic transmission56,105 (FIG. 3a). These studies suggest that GRP–GRPR and NMB–NMBR signalling are parallel peripheral pathways that encode itch information in a non-complementary manner53,56,105. The partial overlap of NMB expression and GRP expression that has been observed by immunohistochemical staining105 suggests that NMB and GRP may also be co-released, leading to competition between histaminergic and non-histaminergic itch transmission, resulting from the binding of NMB to GRPR neurons, where it acts as a functional antagonist, and the binding of GRP to NMBR neurons, where it acts as a weak agonist56,105. This may explain why chloroquine-mediated itch manifests itself in a histamine-independent manner at the behavioural level, even though chloroquine induces the degranulation of mast cells (releasing histamine), and neurons expressing the chloroquine receptor MRGPRA3 are required for histamine-mediated itch39,122.
Fig. 3 |. Neuropeptide coding in peripheral and central itch pathways.
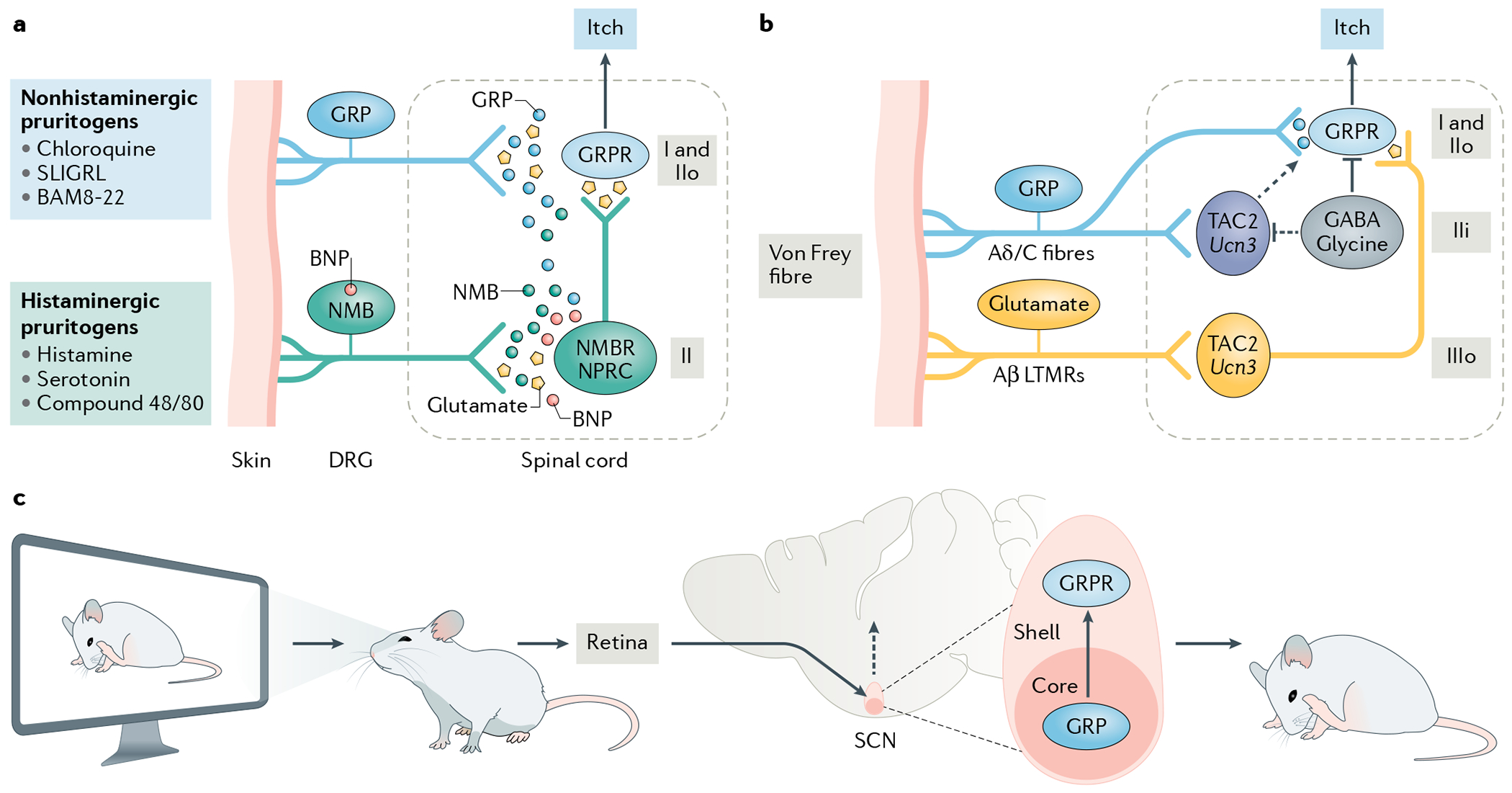
a | Chemical itch pathways. A schematic illustration of the encoding of histaminergic and non-histaminergic itch according to the neuropeptide code model. According to this model, itch induced by histaminergic and non-histaminergic pruritogens is encoded by either gastrin-releasing peptide (GRP) or neuromedin B (NMB), but not both. GRP and NMB work not only in a complementary manner but also in synergy, with GRP acting as a partial agonist for NMB receptor (NMBR)105. GRP and NMB neurons release GRP and NMB, respectively, to activate GPR receptor (GRPR)-expressing neurons (GRPR neurons) in laminae I and II of the spinal dorsal horn or NMBR neurons in lamina II (REFS55,56,105. NMBR neurons send predominantly histaminergic and marginally non-histaminergic itch information to GRPR neurons via glutamate56,105. B-type, or brain, natriuretic peptide (BNP) is co-released with NMB, binds to natriuretic peptide receptor C (NPRC) in the spinal cord and facilitates NMB-mediated histaminergic itch via NPRC-NMBR crosstalk155. BNP and NMB are co-expressed in some neurons155. Since NMB and GRP expression partially overlaps56, some NMB may be co-released with GRP; however, this NMB will act as a functional antagonist to compete with GRP for binding to GRPR neurons. Conversely, a small amount of GRP is co-released with NMB and binds to NMBR, where it acts as a weak agonist to convey non-histaminergic itch information. b | The mechanical itch pathway as described by the neuropeptide code model. Neurons in the outer layer of lamina III (IIIo) of the dorsal horn that express TAC2 or originate from progenitors expressing the urocortin 3 gene (Ucn3) are proposed to be activated by innocuous tactile information (such as that arising from the touch of a von Frey fibre) via Aβ low-threshold mechanoreceptors (LTMRs) and to relay this information to GRPR neurons via glutamatergic transmission58,188. The convergence of inputs from these IIIo neurons and from neurons arising from the same lineage but located in the inner layer of lamina II (IIi) may be a prerequisite for converting this innocuous touch into itch58. The TAC2 neurons in IIi receive direct inputs from C fibres and/or Aδ fibres but are hypothesized to be silent under naive conditions owing to tonic inhibition by local GABAergic neurons. Under chronic itch conditions, they may be activated to send itch information to GRPR neurons (dashed arrow). The neuropeptides expressed by the GABA/glycine inhibitory neurons may differ and are not shown to avoid confusion. c | The contagious itch pathway according to the neuropeptide code model. In a contagious itch test, the observer watches the scratching of conspecifics displayed on a screen. The retina receives the visual itch information and conveys it to the suprachiasmatic nucleus (SCN)104. It is hypothesized that GRP neurons in the retinorecipient zone or the core of the SCN release GRP to activate GRPR neurons, which send the information to downstream neural circuits to trigger a scratching behaviour104. BAM8-22, bovine adrenal medulla 8-22 peptide; DRG, dorsal root ganglion; IIo, outer layer of lamina II.
B-type, or brain, natriuretic peptide (BNP), an inhibitory neuropeptide expressed in sensory neurons and encoded by the gene Nppb, has also been implicated in itch25,154–156; however, its contribution to itch remains unclear and controversial. Mice with a global KO of Nppb exhibited deficits in histaminergic and non-histaminergic itch154. However, it is possible that these deficits may be attributable to the loss of BNP expression in skin and immune cells156 in these mice. The neurotoxin BNP–saporin was initially reported to exclusively ablate neurons expressing the BNP receptor natriuretic peptide receptor A (NPRA), encoded by Npr1 (REF.154), and to abolish itch responses in mice153. However, older evidence suggests that BNP binds to both NRPA and NPRC, encoded by Npr3, with equal affinity157. This was recently supported by the findings reported in a preprint in which it was reported that both Npr1 and Npr3 spinal neurons were ablated with use of BNP–saporin155. Most natriuretic peptide receptors that act as guanylyl cyclases (including NRPA and NRPC) inhibit the activity of the neurons in which they are expressed158,159. Indeed, intrathecal injection of BNP inhibits nociceptive transmission160, while exogenous application of BNP inhibits the excitability of primary nociceptors161–163, suggesting that BNP may be a negative modulator of nociceptive transmission under certain inflammatory pain conditions. Furthermore, intrathecal or intradermal injection of BNP at picomolar or nanomolar concentration fails to induce immediate scratching behaviour in mice, indicating that administration of BNP alone at a physiologically relevant concentration does not stimulate pruriceptive transmission154,155,160. The findings reported in the recent preprint mentioned above suggest that BNP is instead involved in facilitating histaminergic itch that is mediated by NMB through NPRC–NMBR crosstalk155. This finding is in agreement with the observations that Nppb is markedly upregulated upon histamine release from mast cells in mice with allergic contact dermatitis25,158,164. By contrast, the preprint suggests that Nppb is downregulated in DRGs of mice with a dry skin itch condition155 that is devoid of a histaminergic component165. Consistent with these findings, a study that used a behavioural screening approach to identify anti-Npr1 compounds used an antihistamine effect as a validation tool158. These preliminary findings indicate that the role of BNP in DRGs may be limited to the facilitation of histamine-mediated itch and that NPRC–NMBR crosstalk may switch the otherwise inhibitory action of BNP to an excitatory one. Although BNP-expressing neurons were proposed to be a peripheral itch-specific neural pathway154,164, they also express genes important for mediating mechanical pain, such as those encoding PAR2 (REFS72,166) and neuropeptide Y (NPY) receptor type 2 (NPY2R)167,168. A receptor may function differently in different tissues. For example, in contrast to DRGs, PAR2 acts as an itch receptor in the skin169,170 Notably, CKO of Sst, the gene encoding somatostatin (SST), an inhibitory neuropeptide that is largely co-expressed in BNP neurons, in TRPV1-expressing sensory neurons in mice (Trpv1Cre;Sstf/f mice) increased mechanical pain but not itch171, providing another example that disputes the idea that the specificity of itch is encoded by a subpopulation of sensory neurons.
Together, these findings suggest that GRP and NMB encode histaminergic itch and non-histaminergic itch, respectively. By contrast, preliminary findings suggest that BNP acts as a neuromodulator that facilitates histaminergic itch transmission. Thus, the neuropeptide code model hypothesizes that distinct modes of neuropeptide action confer the specificity of different types of itch (FIG. 3a).
Spinal Grp neurons in chemical itch.
In recent years, there has been considerable interest in and debate on the possible role of spinal Grp neurons, which express Grp mRNA in itch59,83,154,172,173. It was proposed that spinal Grp neurons exclusively express NPRA and release GRP to activate GRPR neurons in response to BNP release by primary sensory neurons154. According to this model, BNP transmits itch, irrespective of whether the itch is histaminergic or non-histaminergic154. From an evolutionary perspective, however, I believe that it is difficult to envisage why the dorsal horn would expend the metabolic costs required to support an additional layer of intralaminar transmission or a detour in the wiring to perform a function that is identical to that of GRPR neurons. In principle, spinal GPCRs — which are predominantly activated by neuropeptides released from sensory neurons — are responsible for mediating relatively slow transmission, such as that involved in the transmission of chemical itch and inflammatory pain, typically conducted by slowly conducting C fibres174. However, once the information that encodes a warning signal has arrived at and been processed by neural circuits defined by GPCRs in the spinal cord, it is highly desirable to ensure its timely transfer to the brain. Local intralaminar communication between tightly apposed interneurons of laminae I and II, using a neuropeptide rather than fast-acting glutamate, would delay the body’s defensive response. Thus, I argue that neuropeptides synthesized within the dorsal horn are dispensable for modality-specific excitation during normal reflexive responses. Instead, a more sustained slow release of neuropeptides and their slower activation of GPCRs (within seconds to minutes) conducted by C fibres is optimally suited for mediating chemical itch and for tonic modulation of itch99,134,140.
Several research groups have attempted to examine the possible role of spinal Grp neurons and have obtained contradictory results. They all used bacterial artificial chromosome (BAC)-based Cre and/or enhanced GFP (eGFP) mouse lines produced by the Gene Expression Nervous System Atlas (GENSAT) project175. One group used the global ablation approach and found that mice lacking GrpCre neurons showed reduced itch but increased thermal pain behaviour. In addition, it was shown that stronger activation of these neurons recruits opioid signalling to inhibit pain, while weaker activation induces itch172. On the assumption that abnormal itch and pain originated from the spinal ablation of GrpCre neurons, it was proposed that spinal GrpCre neurons constitute a ‘leaky gate’ for itch and pain172. However, these mice also exhibited an ectopic ablation of lamina II nociceptive neurons (including nearly half of the population of neurons expressing protein kinase Cγ (PKCγ) neurons, which do not overlap with spinal Grp neurons53). Therefore, the most parsimonious explanation for these findings, in my view, is that the reduced itch and enhanced thermal pain deficits stem from the brain, considering that there are numerous Grp neurons in the brain regions potentially involved in itch and pain transmission and modulation53,61,173. For example, the decreased itch behaviour can be explained by the ablation of Grp neurons in the parabrachial nuclei implicated in itch transmission61. By contrast, a subsequent study that used biting behaviour as a behavioural surrogate of itch observed a rather modest reduction in the biting behaviour induced by chloroquine, histamine and serotonin (also known as 5-hydroxytryptamine (5-HT)), but not SLIGRL, nor noxious stimuli, after ablation of GrpCre neurons at the lumbar level, and concluded that spinal Grp neurons are required for itch but not pain transmission173. It is now known that these BAC-based Cre and/or eGFP lines not only fail to express Cre and/or eGFP in sensory neurons but also capture no more than a quarter of Grp neurons in the spinal cord83,154,172,176. Thus, the existing BAC Grp lines preclude functional analysis of a vast majority of spinal Grp neurons.
To circumvent this problem, a knock-in GrpiCre mouse line has been generated that recapitulates the endogenous Grp expression in both sensory neurons and the spinal cord53. An intersectional genetic approach that involves the use of two genetic lines expressing different recombinases (one of which is expressed in a region-specific manner177) was used to ablate all spinal Grp neurons of GrpiCre mice so that intradermal injection in the nape — a gold standard approach for investigating itch behaviour in mice — could be used. No pain and itch deficits were found after ablation of spinal Grp neurons53. Molecular analysis, however, revealed that spinal Grp neurons express a myriad of itch-related and pain-related genes, such as those encoding NMBR, NPY receptor type 1 (NPY1R), NPR3 and NK3R53,178. Interestingly, the transgenic spinal Grp neurons that are directly innervated by MRGPRA3 fibres are potently activated in the BAC Cre and/or eGFP lines — rather than inhibited, as would be the case if they were itch-specific neurons57 — by nociceptive stimuli172 but not by chloroquine179, in line with the suggestion that MRGPRA3 fibres also convey nociceptive information40. Furthermore, they can be inhibited by a μ-opioid receptor (MOR) agonist176 or endogenous opioids172. Collectively, these findings strongly indicate that the BAC GrpCre transgenic lines might have captured a subpopulation of spinal nociceptive neurons expressing MOR that can be directly activated by TRPV1/MRGPRA3 nociceptive fibres40,109,172.
These controversies raise the important question of how to define an operational microcircuit that is physiologically meaningful for chemical itch. It may be difficult to believe that the Grp mRNA found in the dorsal horn has no role in itch. However, it has been proposed that many neuropeptides present in the dorsal horn may be vestigial remnants of ontogenetic development or past evolution180. It is not surprising that none of the neuropeptides contained by dorsal horn neurons has been convincingly demonstrated to be required for acute somatosensory transmission or inhibition. Furthermore, numerous genes share overlapping expression with GRPR or NMBR95, suggesting that the ablation of neurons expressing these genes may result in partial deficits in itch and pain. For instance, neuronal nitric oxide synthase (nNOS) is expressed not only in GABAergic inhibitory neurons implicated in pain inhibition181 but also in some GRPR neurons67,181,182. Conceivably, therefore, activation of nNOS neurons in a BAC Cre line might result in more itch and fewer pain behaviours67. In this case, if the expression of nNOS in GRPR neurons were unknown, these results might be interpreted as arguing against the intensity or differential coding theory.
In spite of the invaluable role of GENSAT’s Cre lines in advancing neuroscience, their limitations have become increasingly evident (TABLE 1). In the spinal cord, most BAC-based Cre lines probably label a subset of neurons with a mixture of sensory modalities and often lack fidelity. Several well-known factors, such as partial gene regulatory elements contained in the BACs, irregular genomic integration sites and variable copy number of transgenes, have similarly hampered the functional classification of other types of sensory neuron (such as retina ganglion cells183). Perhaps linked to these difficulties and despite their fundamental roles as sensors and their therapeutic value, spinal GPCRs and their circuits remain understudied. This negligence similarly exists for circuit analysis in the brain134. For instance, functional neural circuits in the extended amygdala and the SCN are typically classified according to neuropeptide expression rather than GPCR expression102,184. That GPCRs have largely escaped attention can be attributed to technical difficulties in detecting these receptors in the CNS owing to their very low expression levels98, as well as a paucity of specific antibodies. Maintaining the basal level expression of GPCRs, however, could be a mechanism whereby an accidental switch of the itch and pain circuits from a quiescent state to a pathological one is prevented. A real challenge, therefore, will be to identify a few truly modality-specific Cre lines defined by GPCRs.
Mechanical and contagious itch
Mechanical and contagious itch are idiosyncratic sensations (occurring only under special circumstances), with certain traits possibly unique to humans185. For example, only a few facial regions are sensitive to mechanical itch15, whereas certain populations are prone to contagious itch17,185. Mechanical itch is modulated by PIEZO2, an ion channel present in Merkel cells in the skin, which are involved in discriminating light touch186. Contagious itch is a type of automatic motor mimicry, a primitive and pervasive phenomenon present in most vertebrates that enables social animals to act in synchrony to promote survival and social bonding185,187. There are a number of differences and similarities between the coding of mechanical, chemical and contagious itch.
Mechanical itch.
In the mouse spinal cord, neurons of the Ucn3 lineage (that is, neurons derived from progenitors transiently expressing Ucn3 during development) and Tac2-expressing neurons (labelled with use of a Cre reporter) have been independently demonstrated to constitute the first relay station for Aβ-LTMR inputs that carry information about mechanical itch but not about pain or chemical itch58,188. Of note, while lineage neurons — tracked through permanent genetic reporters such as tdTomato fluorescent protein — are commonly used as a surrogate for circuit analysis, they may not necessarily represent a true functional circuit in their entirety. These two excitatory interneuron populations, which are found in the outer layer of lamina III of the dorsal horn, are likely to overlap since they share several remarkable features58,188. It was recently shown that Tac2-expressing neurons convey light touch information to GRPR neurons (FIG. 3), and that ablation of either Tac2-expressing neurons or GRPR neurons abolishes mechanical itch58, indicating that mechanical itch transmission requires GRPR neurons. Several studies, however, indicated that mechanical itch is transmitted through NPY1R neurons (a group of excitatory interneurons in laminae I and II that were previously implicated in chronic pain189,190 and were recently implicated in chemical itch191,192) and that this transmission is not affected by the ablation of GRPR neurons using BB-sap188,193,194. However, there was still a certain amount of chloroquine-induced scratching behaviours (about 50 scratches) in mice after BB-sap treatment. I therefore hypothesize that there was an incomplete ablation of GRPR neurons in these studies and that the remaining GRPR neurons are sufficient for mediating mechanical itch behaviour188,193,194. Although one study reported that NPY1R and GRPR are not colocalized in the spinal cord, the specificity of the GRPR antibody used remains unclear194. In contrast, other studies have reported substantial overlapping expression between Npy1r and Grpr in the dorsal horn58,195. It can, therefore, be concluded that NPY1R neurons are a mixture of excitatory interneurons (including GRPR neurons) involved in both itch and pain transmission. Furthermore, this emerging evidence supports the notion that GRPR neurons convert innocuous light touch into the sensation of itch.
Is the coding of mechanical itch exclusively mediated by fast-conducting myelinated Aβ-LTMR inputs and/or through glutamatergic transmission or does neuropeptide coding play a role in this type of itch? To answer this question, it is worth considering that not all scratches evoked by innocuous light touch reflect mechanical itch. Rodents are well equipped with a repertoire of stereotyped reflexive behaviours mediated via Aβ-LMTR fibres32 — such as avoidance, body shivering, wiping and scratching — that allow them to distance themselves from or remove both non-itchy stimuli and irritants. This begs the question of how we may reliably distinguish between scratching behaviours evoked by non-itchy touch versus those evoked by itchy touch, an important but overlooked confounding factor that may lead to some controversial interpretations of results. It has long been appreciated that a certain degree of skin sensitization (manifesting itself in tactile insensitivity and tonic activation of C tactile fibres) is necessary for converting touch into itch1,4,5,7. Consistent with this, mechanical itch can be elicited by application of von Frey hairs to the skin surrounding an area sensitized by histamine or bordering an itchy region186,196, or to nape skin that has been sensitized by shaving in naive mice58,186,193,194. This suggests that unmyelinated or histamine-sensitive C fibres are involved in mechanical itch5,15,197, in a manner reminiscent of their role in mechanical allodynia197,198, a painful mechanical hypersensitivity to innocuous touch mediated by synergistic inputs from Aβ and C fibres199. Researchers may easily mistake non-itchy light touch-evoked scratching behaviour, which is mediated, independently of GRPR neurons, exclusively through Aβ-LTMR input58, for mechanical itch. Neuropeptide signalling could be an integral part of the code that distinguishes non-itchy touch from itchy-touch input5,7,15 (FIG. 3b; Supplementary Table 1).
Contagious itch.
Contagious itch, first reported in humans and non-human primates17,187,200, was serendipitously discovered in mice in 2017, when it was found that wild-type mice mistakenly housed in the same cage as BrafV600E mice (a genetic model of neuropathic itch26) exhibited robust spontaneous scratching behaviour104. Modelling contagious itch in naive mice is, however, challenging owing to the poor visual acuity of mice, the presence of spontaneous scratching, the fact that the visual itch stimulus is subtle and the randomness of the ‘looking’ behaviour of a freely moving observer towards a scratching conspecific. These technical nuances may explain why some researchers failed to observe contagious itch behaviour in mice using a paradigm that involved a weak pruritogen, such as histamine201,202. In a study in which contagious itch was induced by exposure of an observer mouse to a visual recording of a demonstrator mouse scratching, it was shown that the probability of the observer mouse noticing a scratching demonstrator is maximized when the speed of scratching motion displayed on the computer monitor is 1 Hz: that is, one scratching bout (or four scratching strokes) per second104,202. The discovery of contagious itch in mice suggests that recognizing and copying a scratching motion has an adaptive value and probably evolved millions of years ago. A recent study meticulously replicated the contagious itch protocol and discovered that a mouse model of autism spectrum disorders failed to imitate scratching behaviour without impacting spontaneous scratching behaviour203. The study is important, in light of a degree of scepticism about the existence of contagious itch in mice. Socially contagious pain and empathic behaviours, however, have been known to exist between familiar rodents for decades204,205. Since contagious itch in mice does not require familiarity or prior training, it could be a primitive form of emotional contagion and could have arisen earlier than pain contagion in evolution206.
The development of a contagious itch test in mice has enabled the elucidation of the mechanisms involved in coding contagious itch. Mapping expression of FOS after contagious itch had been induced revealed that the most robustly active area is the SCN, where GRP is also expressed104. GRP neurons in the ventrolateral ‘core’ of the SCN project to GRPR neurons in the dorsomedial ‘shell’ of the SCN104. Importantly, the scratching behaviour evoked by observing the scratching motion of a conspecific was abolished through SCN-specific deletion of GRPR104. Analogous to the reduced spinal GRP immunohistochemical staining observed after chloroquine-evoked scratching53, GRP immunostaining was reduced in the SCN after the contagious itch test, suggesting a release of GRP and the activation of GRPR104. These findings were surprising because the SCN receives direct inputs from the retina through intrinsic photosensitive retina ganglion cells (ipRGCs), which were thought to be involved only in non-image-forming responses207. Nevertheless, this demonstrated that SCN GRP encodes contagious itch information and suggested that the retina–ipRGC–SCN pathway may be an evolutionarily ancient visual pathway that has been repurposed to encode itch (FIG. 3c). Duplicating and repurposing older circuits and molecules for a novel function is a fundamental mechanism underlying the expansion of the complexity of organisms throughout evolution208.
It is clear that, despite profound differences in the properties of the stimuli (visual versus physical) and the principal neural circuits involved (the SCN versus the spinal cord), the coding of contagious, chemical and mechanical itch is remarkably similar. Their slow kinetics, the skin sensitization required for mechanical itch and the dependence on GRPR neurons all support the importance of the neuropeptide code model in these types of itch.
Modulation of itch
Itch evokes the urge to scratch. While scratching inhibits the initial cause of the itch, it may (by driving the release of inflammatory mediators) provoke a further urge to scratch128. The neuropeptide code model posits that this rhythmic itch–scratch cycle is driven by the sequential release of itch neuropeptides and fast-acting neurotransmitters. As described earlier herein, the model suggests that, upon exposure to a pruritogenic stimulus, itch neuropeptides and glutamate activate GRPR neurons, directly or indirectly. It further proposes that subsequent scratching behaviour drives the release of glutamate from primary afferents, which activates spinal inhibitory neurons, which, in turn, release GABA and glycine to inhibit GRPR neurons. The inhibition of GRPR neurons must be both rapid and transient so that the brain, while being warned, can continue monitoring the environment and skin condition from moment to moment. How this extraordinarily intricate balance between promoting and inhibiting itch transmission is seamlessly executed in a state-specific and spatio-temporal-specific manner is a fascinating question for scientific enquiry.
Inhibitory neural circuits.
Approximately 25–30% of neurons of dorsal horn laminae I–III are functionally heterogeneous GABAergic and/or glycinergic interneurons182. These neurons are broadly classified according to their expression of neuropeptides such as galanin (GAL), dynorphin (DYN; encoded by Pdyn), NPY and parvalbumin (PV). DYN neurons are additionally composed of some excitatory neurons implicated in chronic pain209. An overriding question in the field is whether these spinal inhibitory neurons gate itch and pain separately. Since most transgenic Cre mouse lines used for spinal circuit research also express Cre in the brain, it is necessary to use an intersectional genetic strategy to achieve spinally restricted ablation of particular cell populations. Alternatively, virus-mediated manipulation of neural circuits should be performed within the cervical spinal cord (rather than the lumbar cord) to allow scratching behaviour evoked at the nape to be used to assess itch using BB-sap, as scratching behaviour may also reflect pain or non-itchy touch as discussed earlier67,210. Intersectional ablation of spinal PdynCre lineage neurons in mice revealed that these neurons gate mechanical pain but not chemical itch211,212. In contrast, chemogenetic inhibition and caspase-based ablation of GalCre lineage neurons that also express DYN213 in the cervical cord increase chemical itch but not spontaneous itch or pain behaviour67. These seemingly conflicting results suggest that the PdynCre mouse line does not faithfully capture all adult spinal DYN inhibitory neurons, complicating the interpretation of results derived from the use of this line. Together, these studies indicate that DYN neurons are functionally heterogeneous and that subpopulations of these neurons exert anti-itch (GalCre inhibitory neurons), anti-nociceptive (DYN inhibitory neurons) and pro-nociceptive (DYN excitatory neurons) functions214,215. More recently, a group of Cre lineage neurons that transiently express Ptf1a (which encodes a transcription factor that specifies the fate of GABAergic neurons in the dorsal horn216,217) were shown to partially gate spontaneous itch through the inhibition of GRPR neurons; however, they did not inhibit chemical itch28. Since only 40% of Ptf1aCre lineage neurons were ablated in that study28, it is unclear whether Ptf1aCre lineage neurons may additionally gate spontaneous pain. Nonetheless, these studies suggest that chemical and spontaneous itch may be differentially gated and that there may be distinct types of GRPR neurons that mediate chemical and spontaneous itch, respectively28,57,67.
GalCre lineage neurons have been found to inhibit less than one-third of GRPR neurons via GABA-mediated monosynaptic connections67. This is less than the proportion of GRPR neurons (~60%) that are inhibited by GABAergic and/or glycinergic inputs in response to primary afferent stimulation57 but similar to the proportion of GRPR neurons that are inhibited by cooling and chemical pain57. Interestingly, PVCre lineage neurons in the inner layer of lamina II and in lamina III of the dorsal horn, which gate certain types of mechanical pain transmission218, do not gate chemical itch and rarely synapse with GRPR neurons67. This implies that the synaptic connectivity between local inhibitory neurons dedicated to gating nociceptive transmission and those gating GRPR neurons is distinct, stringent and hard-wired. By paired recordings, it was shown that there is a low connection probability between inhibitory neurons and other neurons in lamina II of the dorsal horn (11%)118. Importantly, monosynaptic connections between excitatory neurons within this region are also rather sparse119. Thus, synaptic connections between excitatory neurons of laminae I and II of different sensory modalities could also be stringent. This suggests that rapid transmission and convergence of submodality inputs, such as that occurring at the synaptic connections between NMBR neurons and GRPR neurons that convey histaminergic itch, are hard-wired. Such a strategy could allow efficient inhibition of itch through the exclusive inhibition of GRPR neurons, a convergent node for transmitting discrete types of itch. Together with the observation that chemical pain and cooling inhibit rather than excite GRPR neurons57, the evidence of low connection probabilities within the spinal cord is in favour of the notion that the excitatory neural pathways that transmit pain and cooling do not undergo crosstalk with GRPR neurons directly in the spinal cord. Correspondingly, inhibitory modulation of itch and pain is modality specific and hard-wired.
NpyCre lineage neurons, widespread in the dorsal horn, receive direct monosynaptic inputs from C fibres and Aβ fibres and can be activated by mechanical, thermal and chemical stimuli193,219. They were initially proposed to gate mechanical itch but not chemical itch on the basis of observations that mice with ablation of NpyCre lineage neurons developed spontaneous scratching behaviour and skin lesions and were sensitive to innocuous touch, which characteristics have been interpreted as a manifestation of mechanical itch193,194. However, given that mechanical itch (like chemical itch) can be gated at the level of GRPR neurons (and possibly by inner layer of lamina II TAC2 and/or Ucn3 lineage neurons58,188) (FIG. 3), it seems unlikely that the scratching evoked by chemical itch versus mechanical itch would activate two distinct populations of spinal inhibitory neurons (GalCre lineage neurons versus NpyCre lineage neurons) if the intention of mechanical itch-induced scratching is also to inhibit itch. Instead, it is possible that scratching evoked by mechanical itch may mainly help an animal to remove irritating light touch on the skin, but not to inhibit the sensation of itch, thus resembling the scratching evoked by non-itchy touch (see earlier); if so, it would not be necessary to entail the major spinal inhibitory circuits to inhibit this type of mechanical touch unless such irritating light touch becomes persistent, possibly to the degree that might lead to an allergic skin response. In the latter scenario, the spontaneous scratching/biting behaviour that resulted from ablation of NpyCre lineage neurons193,194 may be an indicator of spontaneous or neuropathic pain due to a loss of tonic gating, akin to that resulting from the spinal perturbation of NPY2R signalling210. Moreover, the increased scratching behaviour evoked by von Frey fibre stimulation applied to the nape of mice lacking NPY neurons may be an indicator of mechanical hypersensitivity due to ongoing neuropathic pain. These rodents showed normal basal pain responses189,193,194, supporting the notion that acute and fast withdrawal nocifensive behaviour does not require spinal inhibitory peptidergic gating58. Consistently, there is ample evidence demonstrating the primary role of spinal NPY signalling in tonic gating of chronic and spontaneous pain via NPY1R and/or NPY2R189,190,210,220. Thus, some of the spontaneous scratching, licking or biting behaviours and skin lesions that arise following the ablation of various populations of inhibitory neurons, which are typically considered as manifestations of itch rather than pain, could actually be due to spontaneous pain210. Moreover, because inhibitory neuropeptides are not involved in fast inhibition of itch, the observations that exogenous NPY or NPY1R agonists inhibit chemical itch are likely to be pharmacological artefacts191,192 (TABLE 1) that are attributable to NPY1R expression in GRPR neurons58. A pharmacological artefact refers to the behavioural or neuronal response elicited by exogenously administered substances that otherwise would not occur under natural conditions (TABLE 1). For example, intrathecal injection of SST could induce itch-related scratching behaviour155. However, neither spinal cord-specific (Lbx1Cre;Sstf/f mice) nor DRG-specific (Trpv1Cre;Sstf/f mice) deletion of Sst alters itch transmission171, indicating that endogenous SST in these two areas is not involved in itch modulation. Although GRPR neurons are subject to both local tonic and phasic GABAergic inhibition221, tonic inhibition of these neurons appears to be the principal mechanism for gating the flow of itch information.
In summary, while spontaneous pain has been mostly ignored owing to overemphasis on itch, recent studies on functional characterization of various populations of spinal inhibitory neurons lend considerable support to anatomically segregated inhibitory circuits for itch and pain, respectively. Although the components of these inhibitory circuits are usually identified through their neuropeptide or transcription factor expression, they invariably use fast-acting GABA and/or glycine, rather than neuropeptides, to rapidly inhibit acute itch transmission57,58,67,74,188,222. Given that some of the inhibitory circuits examined to date may overlap, further delineation of inhibitory microcircuits defined by physiologically relevant ligand–receptor interactions will be required to understand their activation mechanisms.
The dichotomy of glutamate: excitation and inhibition.
The role of glutamate in itch is also controversial and confusing because of conflicting claims in large part resulting from methodological differences and limitations57,60,87,133,223,224. Two comprehensive genetic studies showed that mice lacking vesicular glutamate transporter 2 (VGLUT2), which regulates the probability of glutamate release from the central terminals225 in Nav1.8Cre lineage and TRPV1 DRG neurons (Vglut2-CKO mice; Nav1.8 is also known as Scn10a and Vglut2 is also known as Slc17a6), exhibited enhanced itch but reduced pain behaviour223,224. These studies cast doubt on the role of glutamate as a co-transmitter of itch in primary C fibres; however, it remains possible that glutamate released by a subset of C fibres in Vglut2-CKO mice may have been sufficient to transmit itch. Indeed, Vglut2-CKO mice exhibited only a 50% reduction of fast glutamatergic transmission between primary afferents and the spinal cord, as well as normal or partial attenuation of certain types of acute thermal and mechanical pain223,224.
Within the spinal cord, however, it is clear that glutamate plays an essential role in local intralaminar itch transmission. It relays chemical itch from NMBR neurons56 and mechanical itch from TAC2 neurons58 to GRPR neurons, and relays chemical itch and mechanical itch from GRPR neurons to projection neurons56,60,61. The finding that co-application of glutamate receptor, GRPR and NK1R antagonists into the spinal cord dramatically attenuates histaminergic and non-histaminergic itch may result from the combinatory effect of these antagonists acting at discrete sites within the itch transmission pathways114.
At first sight, the impaired pain behaviour and enhanced itch observed in Vglut2-CKO mice223,224 appear to indicate a loss of the inhibition of itch mediated by pain. However, the deficits in itch and pain are actually fully dissociable; when pruritogenic responses were tested, the nociceptive transmission pathway was quiescent in both transgenic mice and control mice because of the endogenous tonic gating even without peripheral noxious input (as shown previously). Conversely, the pain deficits observed in Vglut2-CKO mice were shown to result from glutamate deficiency and to be independent of itch transmission57,65. Given this evidence of distinct pain and itch circuits in the spinal cord, how could the loss of glutamate in C fibres of Vglut2-CKO mice give rise to augmented itch?
A long-held belief is that scratching activates mild noxious mechanical pain to inhibit itch2,10,73. However, scratching — or rubbing or stroking in animals that cannot scratch — commonly induces a pleasant and hedonic sensation4,10,226,227, which is likely to be relayed through some interneurons of laminae I and II innervated by C LTMRs (Supplementary Fig. 1) and neurons of laminae III and IV targeted by Aβ LTMRs to the brain via spinal projection neurons32,228,229. Under normal circumstances, if scratching induces mild pain to inhibit itch, this would imply that pain neurotransmitters can be easily released and that spinal GalCre lineage neurons are activated indirectly by a spinal nociceptive circuit instead of directly by primary afferents, which is inconsistent with the finding that GAL neurons also receive monosynaptic inputs from both C fibres and Aβ fibres182. Further arguing against the view is that scratching an itch evokes pleasant and relieves aversive sensation; in accordance with this, activation of C LTMRs inhibits, rather than activates, nociceptive C fibres32,118. Accordingly, the enhanced scratching behaviour of Vglut2-CKO mice might be explained by the disinhibition of GRPR neurons due to a loss of the feedforward inhibition that is mediated by the spinal inhibitory neurons (which in turn results from a lack of glutamate release from C fibres). In this context, it can be hypothesized that glutamate may have a much less appreciated role in the inhibition of itch transmission. Scratching may release glutamate to instantaneously activate GalCre neurons. Because diminished glutamate release from C fibres could weaken the activation of these spinal neurons, Vglut2-CKO mice might have to compensate by scratching harder. Furthermore, GRP expression or release might be enhanced to compensate for a deficiency of glutamate that otherwise is tonically co-released with GRP, leading to increased acute itch or the development of chronic itch. Thus, glutamate released from C fibres could be essential for facilitating GRP-GRPR signalling.
In short, disentangling peripheral and central neural pathways involved in the itch–scratch cycle is important for understanding the neuropeptide code for itch in context. Scratching an itch can simultaneously activate multiple spinal excitatory and inhibitory pathways in parallel. The genetic studies described above have uncovered an unexpected functional dichotomy in the action of glutamate released by C fibres. First, glutamate from primary afferents excites spinal itch neurons as a co-transmitter of itch neuropeptides. Subsequently, it inhibits itch through a different subset of primary afferents that activate spinal inhibitory neurons. A rapid alternation of glutamate-mediated excitation and inhibition of itch manifests itself in a temporally distinct site-dependent and state-dependent manner (Supplementary Fig. 1). Because of its opposing role in itch, glutamate release into the spinal cord must be made through discrete types of primary afferents shielded from each other at their central synaptic targets to safeguard modality-specific excitation and inhibition (Supplementary Fig. 1).
Descending modulation.
In addition to spinal inhibitory circuits, spinal itch transmission is under descending modulation via several parallel pathways originating in the periaqueductal grey and travelling to the spinal cord indirectly via the rostral ventromedial medulla (RVM) pathway67,105,230–232. Serotonin (5-HT) derived from the dorsal raphe nucleus of the RVM has opposing functions in itch and pain modulation: it inhibits nociceptive neurons expressing 5-HT receptor 1A (5-HT1A), an inhibitory GPCR, in lamina II of the spinal dorsal horn, while concomitantly facilitating itch via crosstalk between 5-HT1A and GRPR, which form a heteromeric complex233 (FIG. 4). Although the effect of 5-HT itself on GRPR neurons is inhibitory because 5-HT1A is an inhibitory GPCR under experimental conditions233, it becomes facilitatory when it is released in conjunction with GRP230,233. TAC1 glutamatergic neurons in the periaqueductal grey, which synapse directly onto 5-HT neurons in the RVM, modulate the function of GRPR neurons indirectly via several descending pathways, including those involving 5-HT and GABA67. Recently, a population of non-serotonergic GABAergic neurons that co-express G-protein-coupled oestrogen receptor (GPER) and MOR in the RVM was shown to inhibit spinal itch via regulation of opioid signalling by GPER232.
Fig. 4 |. GRPR neural and signalling pathways.
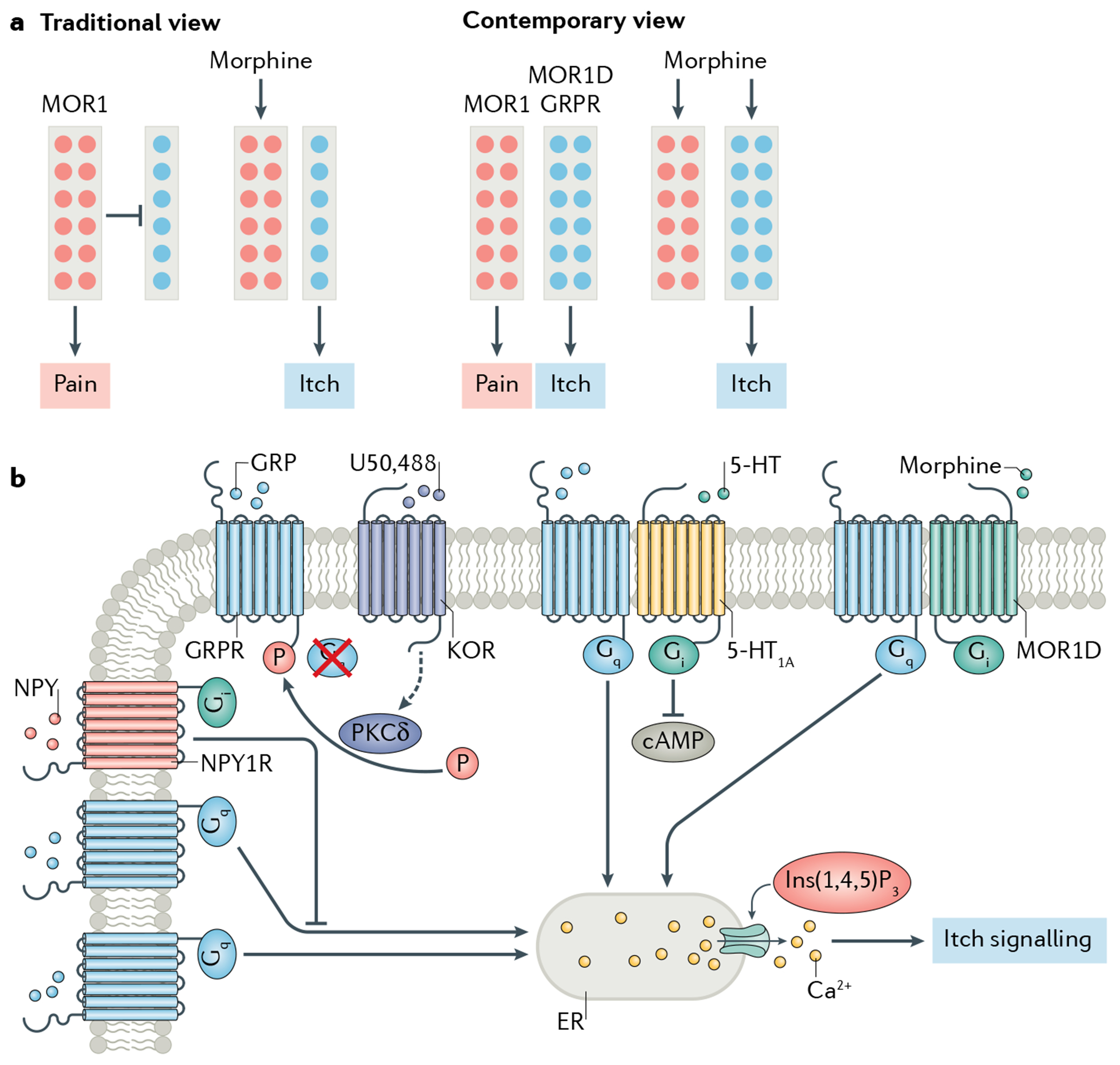
a | The neural pathways mediating morphine-induced itch and analgesia. The rectangles represent the itch-specific and pain-specific neural pathways. The coloured circles represent μ-opioid receptor 1 (MOR1)-expressing neurons (MOR1 neurons; red) and neurons expressing the MOR1 isoform MOR1D and gastrin-releasing peptide (GRP) receptor (GRPR) (blue). According to the traditional view, there are more nociceptors (expressing MOR1) than pruriceptors (expressing MOR1D and GRPR) in the spinal cord. Therefore, pain transmission masks or occludes itch2,22, and morphine inhibits pain by activating MOR1, thereby unmasking itch transmission. The contemporary view, however, states that two non-overlapping populations of excitatory interneurons in the spinal cord mediate morphine-induced itch and analgesia, respectively. MOR1 neurons convey nociceptive information, whereas MOR1D and GRPR neurons transmit itch. Morphine simultaneously activates MOR1 neurons to induce analgesia and MOR1D/GRPR neurons to induce itch8. b | Diverse GRPR signalling pathways. GRPR can be activated directly by GRP released from primary afferents to open the endoplasmic reticulum (ER) Ca2+ store and induce itch signalling8,57,144,233. In addition, it can be indirectly activated by opioids through a unidirectional MOR1D–GRPR cross-signalling in a GRP-independent manner. GRPR can also be inhibited directly by neuropeptide Y (NPY) or by NPY receptor type 1 (NPY1R) agonists58,191 and indirectly by κ-opioid receptor (KOR) agonists through KOR-activated protein kinase Cδ (PKCδ)-mediated phosphorylation (P) of GRPR144. Lastly, GRPR is subject to descending modulation via 5-hydroxytryptamine (5-HT) receptor 1A (5-HT1A)-GRPR crosstalk upon concurrent activation by their respective agonists, GRP and 5-HT. 5-HT1A-GRPR crosstalk converts the inhibitory 5-HT1A-mediated cAMP pathway into an excitatory pathway, thereby facilitating GRPR signalling233. These GPCR cross-signalling events may occur in distinct microdomains within the same GRPR neurons or in different subpopulations of GRPR neurons. Ins(1,4,5)P3, inositol 1,4,5-trisphosphate.
In addition to the descending input from the RVM and the periaqueductal grey, the release of noradrenaline from the locus coeruleus into the spinal cord also exerts a potent inhibitory effect on spinal itch transmission234,235. Spinal dorsal horn-projecting noradrenergic neurons inhibit acute and chronic itch, and noradrenaline may facilitate inhibitory input into GRPR neurons via the α1A-adrenergic receptor235. Therefore, multiple descending systems may have a powerful role in the modulation of spinal GRPR neurons via long-range neurotransmitter-mediated signalling.
Opposing opioid signalling.
Besides endogenous modulation of itch, exogenous drugs and chemicals can also modulate itch transmission. One example often cited in support of the notion that pain and itch are inherently related is opioid-induced pruritus, one of the most prevalent side effects of epidural analgesia9. Research on the molecular and neural mechanisms by which opioids induce itch in the spinal cord of mice has further demonstrated that spinal itch and pain circuits are distinct. Most strikingly, morphine-induced itch is completely lost in Grpr-KO mice8, indicating an indispensable role of GRPR in opioid-induced itch. How could morphine activate GRPR in the spinal cord?
Pioneering work identified dozens of MOR spliced isoforms, all encoded by the gene Oprm1, in rats, mice and humans236. Of these, MOR1D — a spliced isoform and an inhibitory GPCR first identified in the mouse and rat spinal cord237 — was later identified in GRPR neurons as an important receptor for mediating morphine-induced itch (but not analgesia) in mice8. To examine MOR1D in situ, a rabbit antibody was generated with use of the same MOR1D amino acid sequence that had been used to generate the original rat antibody237, and it was found that MOR1D is expressed in some mouse GRPR neurons8. This replication of the original findings made in rats and mice237 should help dispel the doubt regarding the presence of MOR1D in mouse spinal cord, which, akin to Grp in DRGs, could be difficult to detect if the technique lacks sensitivity. A spinal isoform-specific knockdown indicated that a MOR1D isoform is required for morphine-induced itch but not analgesia8. Cross-activation of GRPR by MOR1D (but not MOR1) through heterodimerization involving seven amino acids of the MOR1D carboxy terminus was demonstrated in both the mouse dorsal horn and HEK 293 cells expressing these receptors, showing that GRPR can be internalized by morphine only in the presence of MOR1D but not MOR1 (REF.8). By contrast, MOR1, which is not expressed in GRPR neurons in lamina II, was shown to mediate morphine analgesia but not itch8. This study thus revealed a cell type-specific double dissociation between opioid-induced analgesia and itch (FIG. 4a). Importantly, MOR1Y, an orthologue of MOR1D, was recently isolated from human spinal cords and shown to similarly cross-activate GRPR238, indicating a conserved mechanism between rodents and humans. Thus, the transmission of itch and pain can be simultaneously and independently modulated by opioids, further demonstrating the dissociation between itch and pain circuits in the spinal cord.
In contrast to the findings that MOR1D/MOR1Y–GRPR crosstalk is important for mediating opioid-induced itch, recent studies have shown that deletion of Oprm1 using Oprm1f/f mice in VgatCre (Vgat is also known as Scl32a1) or PdynCre neurons in the CNS abolishes opioid-induced itch192,239, indicating the involvement of disinhibition in this type of itch. Although the deficit is ascribed to the spinal Oprm1 activated by morphine, the deletion is not limited to the spinal cord. Importantly, this disinhibition mechanism, which occurs at the circuit level, fails to explain observations made at the molecular level, including a loss of morphine-induced itch in Grpr-KO mice, after spinal knockdown of GRPR or its downstream Ca2+ signalling molecules such as phospholipase Cβ (PLCβ), and disruption of GRPR-MOR1D interactions8. It can be predicted that a disruption of any component in the canonical Gq-protein-mediated Ca2+ signalling pathway would attenuate opioid-induced itch. Several possibilities may explain these discrepancies. First, VgatCre or PdynCre could be transiently expressed in developing GRPR neurons. It was shown that some Grpr neurons express Vgat178, while nNOS neurons partially express Pdyn, Oprm1 (REF.239) and GRPR67. Conceivably, CKO of Oprm1 in these mice could have deleted MOR1D in Grpr neurons. Second, Oprm1 is broadly expressed in GABAergic neurons in discrete brain regions, and its deletion in these neurons is known to abolish morphine analgesia240,241. By analogy, the brain Oprm1 in GABAergic neurons could be involved in opioid-induced itch. For instance, a loss of Oprm1 in RVM GABAergic neurons might attenuate descending inhibition of itch232. However, given the crucial role of spinal GRPR, neither the spinal disinhibition mechanism nor the brain disinhibition mechanism is likely to contribute to spinal opioid-induced itch. Nonetheless, evidence suggests that the transmission of itch and pain can be simultaneously and independently modulated by opioids, further demonstrating the dissociation between itch and pain circuits in the spinal cord.
Unlike MOR agonists, κ-opioid receptor (KOR) agonists such as nalfurafine have strong anti-itch properties and have been used in clinical trials to treat uraemic pruritus242. Exogenous KOR agonists initiate a non-canonical PKC signalling pathway within GRPR neurons, leading to inhibition of itch via PKC-mediated phosphorylation of GRPR for up to 3 days144. Such a potent and long-lasting inhibitory effect indicates that the type of inhibitory signalling induced by exogenous KOR agonists (like that induced by DYN or by NPY1R agonists) is unlikely to occur under natural conditions, such as during scratching, as it would impede the warning function of itch. Indeed, the onset of inhibition of the spinal neuronal firing activity induced by scratching is rapid and transient, and ends instantaneously with the cessation of scratching74,75, which is typical of fast synaptic inhibition of GRPR neurons mediated by GABA and glycine (on the order of milliseconds)57,67,74,188,221,243,244. Consistent with this, mice lacking inhibitory neuropeptides such as DYN and GAL showed normal acute itch behaviours144,245, whereas spinal transplantation of the precursors of cortical GABAergic inhibitory neurons could attenuate neuropathic itch behaviours in mice lacking Bhlhb5 (also known as Bhlhe22) (REFS27,243).
Summary.
GRPR has emerged as an extremely versatile receptor that interacts with diverse GPCRs to modulate itch transmission under several well-known physiological, pharmacological, and pathological conditions (FIG. 4b). The presence of perhaps dozens of distinct GPCRs on GRPR neurons makes them amenable to pharmacological perturbation of itch signalling independently of GRPR, which conceivably could lead to a plethora of behavioural outcomes.
Concluding remarks
While itch has rapidly emerged as a popular topic that attracts researchers from several different disciplines, the field is — perhaps unavoidably — replete with competing claims and conflicting results. Here, I have attempted to show that many of these issues can be reconciled and made sense of through the consideration of alternative hypotheses. This discussion has underscored the importance of experiments that involve functional perturbation of neural circuits/molecules at the cervical spinal cord level in adult animals as well as the necessity of rigorous validation of evoked or spontaneous scratching behaviours, whose complexity is inadequately appreciated. Despite the challenges, powerful mouse genetics, ethologically relevant animal models and unprecedented genetic and pharmacological access to pivotal itch circuits have allowed the basic architecture of the spinal itch circuitry and the organizing principles that govern spinal itch transmission to be uncovered. Under the disguise of redundancy and the intermingling of numerous molecularly diverse interneurons of varied modalities lies a constellation of intricate networks, which are wired through highly efficient and unidirectional transmission programmes. These microcircuits — shaped over hundreds of millions of years of evolution — are endowed with precision and specificity and can be defined functionally according to the ligand–receptor interactions through which they operate under different physiological conditions.
The emerging properties of these microcircuits and the underlying coding logic of itch described here provide a coherent framework whereby neuropeptides and classic neurotransmitters play differing yet concerted roles in a highly spatio-temporal-specific pattern during the itch–scratch cycle. Moving forward, a more focused approach to substantiate these ideas — some remain speculative — is necessary. For example, how primary afferents control the differential release of itch neuropeptides versus pain neuropeptides is an enigma. Whether GRPR neurons are composed of subtypes that mediate histaminergic itch versus non-histaminergic itch or mechanical itch versus chemical itch is also unclear. After separating itch from pain in the spinal cord, we do not understand how itch information is relayed through projection neurons to the brain. Finally, it is logical to presume that the neuropeptide code outlined here can be extended to the coding of other sensory modalities with slow response kinetics, such as chemical pain and pleasant touch. With the advent of technologies such as monosynaptic rabies virus tracing246 and an emphasis on circuits defined by GPCR expression, future endeavours to decipher modality-specific connectivity between primary afferents and spinal microcircuits may prove fruitful. Crucially, the advances in illuminating the neuropeptide code for itch support the idea that how sensory information flows from primary afferents to the brain via the spinal cord can be approached and understood, a quest once thought beyond our reach.
Supplementary Material
Acknowledgements
The author thanks X. Liu for help with the figure preparation, and R. Bardoni and B. Kim for constructive comments. The research was supported by NIH grants 1R01 AR056318-06, R01NS094344 and R01 DA037261-01 A1 to Z.-F.C.
Footnotes
Competing interests
The author declares no competing interests.
Supplementary information
The online version contains supplementary material available at https://doi.org/10.1038/s41583-021-00526-9.
References
- 1.Rothman S Physiology of itching. Physiol. Rev 21, 357–381(1941). [Google Scholar]
- 2.Ikoma A, Steinhoff M, Stander S, Yosipovitch G & Schmelz M The neurobiology of itch. Nat. Rev. Neurosci 7, 535–547 (2006). [DOI] [PubMed] [Google Scholar]
- 3.McMahon SB & Koltzenburg M Itching for an explanation. Trends Neurosci. 15,497–501 (1992). [DOI] [PubMed] [Google Scholar]
- 4.von Frey M Zur Physiologie der Juckempfindung. Arch. Need and. Physiol 7, 142–145 (1922). [Google Scholar]
- 5.Shelley WB & Arthur RP The neurohistology and neurophysiology of the itch sensation in man. AMA Arch. Dermatol 76, 296–323 (1957). [DOI] [PubMed] [Google Scholar]
- 6.Sherrington CS The Integrative Action of the Nervous System (Scribner, 1906). [Google Scholar]
- 7.Keele C & Armstrong D Substances Producing Pain and Itch (Williams & Wilkins, 1964). [Google Scholar]
- 8.Liu XY et al. Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell 147, 447–458 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballantyne JC, Loach AB & Carr DB Itching after epidural and spinal opiates. Pain 33, 149–160 (1988). [DOI] [PubMed] [Google Scholar]
- 10.Bishop GH The skin as an organ of senses with special reference to the itching sensation. J. Invest. Dermatol 11, 143–154 (1948). [PubMed] [Google Scholar]
- 11.Bickford RG Experiments relating to the itch sensation, its peripheral mechanism, and central pathways. Clin. Sci 3, 377–386 (1938). [Google Scholar]
- 12.Graham DT, Goodell H & Wolff HG Neural mechanisms involved in itch, itchy skin, and tickle sensations. J. Clin. Invest 30, 37–49 (1951). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis T & Zotterman Y Vascular reactions of the skin to injury: part VIII. The resistance of the human skin to constant currents, in relation to injury and vascular response. J. Physiol 62, 280–288 (1927). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang F & Kim BS Itch: a paradigm of neuroimmune crosstalk. Immunity 52, 753–766 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Fukuoka M, Miyachi Y & Ikoma A Mechanically evoked itch in humans. Pain 154, 897–904 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Lloyd DM, Hall E, Hall S & McGlone FP Can itch-related visual stimuli alone provoke a scratch response in healthy individuals? Br. J. Dermatol 168, 106–111 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Holle H, Warne K, Seth AK, Critchley HD & Ward J Neural basis of contagious itch and why some people are more prone to it. Proc. Natl Acad. Sci. USA 109, 19816–19821 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craig AD How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci 3, 655–666 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Patel T, Ishiuji Y & Yosipovitch G Nocturnal itch: why do we itch at night? Acta Derm. Venereol 87, 295–298 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Spruijt BM, van Hooff JA & Gispen WH Ethology and neurobiology of grooming behavior. Physiol. Rev 72, 825–852 (1992). [DOI] [PubMed] [Google Scholar]
- 21.Sanders KM & Akiyama T The vicious cycle of itch and anxiety. Neurosci. Biobehav. Rev 87, 17–26 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paus R, Schmelz M, Biro T & Steinhoff M Frontiers in pruritus research: scratching the brain for more effective itch therapy. J. Clin. Invest 116, 1174–1186 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yosipovitch G & Samuel LS Neuropathic and psychogenic itch. Dermatol. Ther 21,32–41 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Stander S et al. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm. Venereol 87, 291–294 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Liu XT et al. Spinal GRPR and NPRA contribute to chronic itch in a murine model of allergic contact dermatitis. J. Invest. Dermatol 140, 1856–1866 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Zhao ZQ et al. Chronic itch development in sensory neurons requires BRAF signaling pathways. J. Clin. Invest 123,4769–4780 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross SE et al. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron 65, 886–898 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Escalante A & Klein R Spinal inhibitory Ptf1a-derived neurons prevent self-generated itch. Cell Rep. 33, 108422 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Kini SP et al. The impact of pruritus on quality of life: the skin equivalent of pain. Arch. Dermatol 147, 1153–1156 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Dong XT & Dong XZ Peripheral and central mechanisms of itch. Neuron 98, 482–494 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinhoff M, Buddenkotte J & Lerner EA Role of mast cells and basophils in pruritus. Immunol. Rev 282, 248–264 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Abraira VE & Ginty DD The sensory neurons of touch. Neuron 79, 618–639 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basbaum AI, Bautista DM, Scherrer G & Julius D Cellular and molecular mechanisms of pain. Cell 139, 267–284 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carstens E Responses of rat spinal dorsal horn neurons to intracutaneous microinjection of histamine, capsaicin, and other irritants. J. Neurophysiol 77, 2499–2514 (1997). [DOI] [PubMed] [Google Scholar]
- 35.Ma Q Labeled lines meet and talk: population coding of somatic sensations. J. Clin. Invest 120, 3773–3778(2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LaMotte RH, Dong X & Ringkamp M Sensory neurons and circuits mediating itch. Nat. Rev. Neurosci 15, 19–31 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Namer B et al. Separate peripheral pathways for pruritus in man. J. Neurophysiol 100, 2062–2069 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Müller J Elements of Physiology (Taylor & Walton, 1840). [Google Scholar]
- 39.Han L et al. A subpopulation of nociceptors specifically linked to itch. Nat. Neurosci 16, 174–182 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharif B, Ase AR, Ribeiro-da-Silva A & Seguela P Differential coding of itch and pain by a subpopulation of primary afferent neurons. Neuron 106, 940–951 (2020). [DOI] [PubMed] [Google Scholar]
- 41.Perl ER Cutaneous polymodal receptors: characteristics and plasticity. Prog. Brain Res 113, 21–37 (1996). [DOI] [PubMed] [Google Scholar]
- 42.Snider WD & McMahon SB Tackling pain at the source: new ideas about nociceptors. Neuron 20, 629–632 (1998). [DOI] [PubMed] [Google Scholar]
- 43.Carstens E, Follansbee T & Iodi Carstens M The challenge of basic itch research. Acta Derm. Venereol 100, adv00023 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumazawa T The polymodal receptor: bio-warning and defense system. Prog. Brain Res 113,3–18 (1996). [DOI] [PubMed] [Google Scholar]
- 45.Wang F et al. Sensory afferents use different coding strategies for heat and cold. Cell Rep. 23, 2001–2013 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Lynn B & Carpenter SE Primary afferent units from the hairy skin of the rat hind limb. Brain Res. 238, 29–43 (1982). [DOI] [PubMed] [Google Scholar]
- 47.Takashima Y et al. Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of transient receptor potential melastatin 8 neurons. J. Neurosci 27, 14147–14157 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bromm B, Scharein E, Darsow U & Ring J Effects of menthol and cold on histamine-induced itch and skin reactions in man. Neurosci. Lett 187, 157–160 (1995). [DOI] [PubMed] [Google Scholar]
- 49.Palkar R et al. Cooling relief of acute and chronic itch requires TRPM8 channels and neurons. J. Invest. Dermatol 138, 1391–1399 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Proudfoot CJ et al. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr. Biol 16, 1591–1605 (2006). [DOI] [PubMed] [Google Scholar]
- 51.Tuckett RP Itch evoked by electrical stimulation of the skin. J. Invest. Dermatol 79, 368–373 (1982). [DOI] [PubMed] [Google Scholar]
- 52.Torebjork HE & Ochoa JL Specific sensations evoked by activity in single identified sensory units in man. Acta Physiol. Scand 110, 445–447 (1980). [DOI] [PubMed] [Google Scholar]
- 53.Barry DM et al. Exploration of sensory and spinal neurons expressing gastrin-releasing peptide in itch and pain related behaviors. Nat. Commun 11,1397 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li MZ et al. Molecular mapping of developing dorsal horn-enriched genes by microarray and dorsal/ventral subtractive screening. Dev. Biol 292, 555–564 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Sun YG & Chen ZF A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 448, 700–703 (2007). [DOI] [PubMed] [Google Scholar]
- 56.Wan L et al. Distinct roles of NMB and GRP in itch transmission. Sci. Rep 7, 15466 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bardoni R et al. Pain inhibits GRPR neurons via GABAergic signaling in the spinal cord. Sci. Rep 9, 15804 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen S et al. A spinal neural circuitry for converting touch to itch sensation. Nat. Commun 11,5074 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barry DM et al. Critical evaluation of the expression of gastrin-releasing peptide in dorsal root ganglia and spinal cord. Mol. Pain 12, 1744806916643724 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aresh B et al. Spinal cord interneurons expressing the gastrin-releasing peptide receptor convey itch through VGLUT2-mediated signaling. Pain 158, 945–961 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mu D et al. A central neural circuit for itch sensation. Science 357, 695–698(2017). [DOI] [PubMed] [Google Scholar]
- 62.Wang X et al. Excitatory superficial dorsal horn interneurons are functionally heterogeneous and required for the full behavioral expression of pain and itch. Neuron 78, 312–324 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mishra SK, Holzman S & Hoon MA A nociceptive signaling role for neuromedin B. J. Neurosci 32, 8686–8695 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jensen RT, Battey JF, Spindel ER & Benya RV International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol. Rev 60, 1–42 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun YG et al. Cellular basis of itch sensation. Science 325, 1531–1534 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen ZF et al. The paired homeodomain protein DRG11 is required for the projection of cutaneous sensory afferent fibers to the dorsal spinal cord. Neuron 31,59–73 (2001). [DOI] [PubMed] [Google Scholar]
- 67.Liu MZ et al. Synaptic control of spinal GRPR+ neurons by local and long-range inhibitory inputs. Proc. Natl Acad. Sci. USA 116, 27011–27017 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Atanassoff PG et al. Enhancement of experimental pruritus and mechanically evoked dysesthesiae with local anesthesia. Somatosens. Mot. Res 16, 291–298 (1999). [DOI] [PubMed] [Google Scholar]
- 69.Sikand P, Shimada SG, Green BG & LaMotte RH Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain 144, 66–75 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aghahowa SE, Obianwu HO, Isah AO & Arhewoh IM Chloroquine-induced pruritus. Indian. J. Pharm. Sci 72, 283–289 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsagareli MG et al. Thermal hyperalgesia and mechanical allodynia elicited by histamine and non-histaminergic itch mediators: respective involvement of TRPV1 and TRPA1. Neuroscience 449, 35–45 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hassler SN et al. The cellular basis of protease-activated receptor 2-evoked mechanical and affective pain. JCI Insight 5, e137393 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ward L, Wright E & McMahon SB A comparison of the effects of noxious and innocuous counterstimuli on experimentally induced itch and pain. Pain 64, 129–138(1996). [DOI] [PubMed] [Google Scholar]
- 74.Akiyama T, Carstens MI & Carstens E Transmitters and pathways mediating inhibition of spinal itch-signaling neurons by scratching and other counterstimuli. PLoS ONE 6, e22665 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davidson S, Zhang X, Khasabov SG, Simone DA & Giesler GJ Jr. Relief of itch by scratching: state-dependent inhibition of primate spinothalamic tract neurons. Nat. Neurosci 12, 544–546 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kroog GS, Jensen RT & Battey JF Mammalian bombesin receptors. Med. Res. Rev 15, 389–417 (1995). [DOI] [PubMed] [Google Scholar]
- 77.Erspamer V, Erpamer GF & Inselvini M Some pharmacological actions of alytesin and bombesin. J. Pharm. Pharmacol 22, 875–876 (1970). [DOI] [PubMed] [Google Scholar]
- 78.Anastasi A, Erspamer V & Bucci M Isolation and structure of bombesin and alytesin, 2 analogous active peptides from the skin of the European amphibians Bombina and Alytes. Experientia 27, 166–167 (1971). [DOI] [PubMed] [Google Scholar]
- 79.Nagalla SR, Gibson BW, Tang DZ, Reeve JR & Spindel ER Gastrin-releasing peptide (GRP) Is not mammalian bombesin. Identification and molecular-cloning of a true amphibian GRP distinct from amphibian bombesin in Bombina orientalis. J. Biol. Chem 267, 6916–6922 (1992). [PubMed] [Google Scholar]
- 80.Wang P et al. Characterization of GRP as a functional neuropeptide in basal chordate amphioxus. Int. J. Biol. Macromol 142, 384–394 (2020). [DOI] [PubMed] [Google Scholar]
- 81.Takanami K et al. Distribution of gastrin-releasing peptide in the rat trigeminal and spinal somatosensory systems. J. Comp. Neurol 522, 1858–1873 (2014). [DOI] [PubMed] [Google Scholar]
- 82.Fleming MS et al. The majority of dorsal spinal cord gastrin releasing peptide is synthesized locally whereas neuromedin B is highly expressed in pain- and itch-sensing somatosensory neurons. Mol. Pain 8, 52 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Solorzano C et al. Primary afferent and spinal cord expression of gastrin-releasing Peptide: message, protein, and antibody concerns. J. Neurosci 35, 648–657 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fuxe K et al. Immunohistochemical indications of gastrin releasing peptide–bombesin-like immunoreactivity in the nervous system of the rat. Codistribution with substance P-like immunoreactive nerve terminal systems and coexistence with substance P-like immunoreactivity in dorsal root ganglion cell bodies. Neurosci. Lett 37, 17–22 (1983). [DOI] [PubMed] [Google Scholar]
- 85.Nattkemper LA et al. Overexpression of the gastrinreleasing peptide in cutaneous nerve fibers and its receptor in the spinal cord in primates with chronic itch. J. Invest. Dermatol 133, 2489–2492 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Timmes TR et al. Gastrin-releasing peptide-expressing nerves comprise subsets of human cutaneous Adelta and C fibers that may sense pruritus. J. Invest. Dermatol 133, 2645–2647 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Akiyama T et al. Roles for substance P and gastrin-releasing peptide as neurotransmitters released by primary afferent pruriceptors. J. Neurophysiol 109, 742–748 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wheeler JJ, Lascelles BD, Olivry T & Mishra SK Itch-associated neuropeptides and their receptor expression in dog dorsal root ganglia and spinal cord. Acta Derm. Venereal 99, 1131–1135 (2019). [DOI] [PubMed] [Google Scholar]
- 89.Alemi F et al. The TGR5 receptor mediates bile acid-induced itch and analgesia. J. Clin. Invest 123, 1513–1530 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu T et al. TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice. J. Clin. Invest 122, 2195–2207 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu T, Xu ZZ, Park CK, Berta T & Ji RR Toll-like receptor 7 mediates pruritus. Nat. Neurosci 13, 1460–1462 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Panula P, Hadjiconstantinou M, Yang HY & Costa E Immunohistochemical localization of bombesin/gastrin-releasing peptide and substance P in primary sensory neurons. J. Neurosci 3, 2021–2029 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Panula P, Yang HY & Costa E Neuronal location of the bombesin-like immunoreactivity in the central nervous system of the rat. Regul. Pept 4, 275–283 (1982). [DOI] [PubMed] [Google Scholar]
- 94.Goswami SC et al. Itch-associated peptides: RNA-Seq and bioinformatic analysis of natriuretic precursor peptide B and gastrin releasing peptide in dorsal root and trigeminal ganglia, and the spinal cord. Mol. Pain 10, 44 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Usoskin D et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci 18, 145–153 (2015). [DOI] [PubMed] [Google Scholar]
- 96.Sha Y, Phan JH & Wang MD Effect of low-expression gene filtering on detection of differentially expressed genes in RNA-seq data. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc 2015, 6461–6464 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Conesa A et al. A survey of best practices for RNA-seq data analysis. Genome Biol. 17, 13 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Regard JB, Sato IT & Coughlin SR Anatomical profiling of G protein-coupled receptor expression. Cell 135, 561–571 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hokfelt T et al. Neuropeptides–an overview. Neuropharmacology 39, 1337–1356 (2000). [DOI] [PubMed] [Google Scholar]
- 100.Massari VJ et al. Distribution and origin of bombesin, substance P and somatostatin in cat spinal cord. Peptides 4, 673–681 (1983). [DOI] [PubMed] [Google Scholar]
- 101.Mikkelsen JD, Larsen PJ, O’Hare MM & Wiegand SJ Gastrin releasing peptide in the rat suprachiasmatic nucleus: an immunohistochemical, chromatographic and radioimmunological study. Neuroscience 40, 55–66 (1991). [DOI] [PubMed] [Google Scholar]
- 102.Abrahamson EE & Moore RY Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res. 916, 172–191 (2001). [DOI] [PubMed] [Google Scholar]
- 103.Karatsoreos IN, Yan L, LeSauter J & Silver R Phenotype matters: identification of light-responsive cells in the mouse suprachiasmatic nucleus. J. Neurosci 24, 68–75 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yu YQ, Barry DM, Hao Y, Liu XT & Chen ZF Molecular and neural basis of contagious itch behavior in mice. Science 355, 1072–1076 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao ZQ et al. Cross-inhibition of NMBR and GRPR signaling maintains normal histaminergic itch transmission. J. Neurosci 34, 12402–12414 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tuckett RP in Itch: Mechanisms and Management of Pruritus (ed. Bernhard JD) 1–22 (McGraw-Hill, 1994). [Google Scholar]
- 107.Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C & Bunnett NW Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol. Rev 94, 265–301 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Otsuka M & Yoshioka K Neurotransmitter functions of mammalian tachykinins. Physiol. Rev 73, 229–308 (1993). [DOI] [PubMed] [Google Scholar]
- 109.Ding YQ et al. Two major distinct subpopulations of neurokinin-3 receptor-expressing neurons in the superficial dorsal horn of the rat spinal cord. Eur. J. Neurosci 16, 551–556 (2002). [DOI] [PubMed] [Google Scholar]
- 110.Seybold VS et al. Relationship of NK3 receptor-immunoreactivity to subpopulations of neurons in rat spinal cord. J. Comp. Neurol 381,439–448 (1997). [DOI] [PubMed] [Google Scholar]
- 111.McCarson KE & Krause JE NK-1 and NK-3 type tachykinin receptor mRNA expression in the rat spinal cord dorsal horn is increased during adjuvant or formalin-induced nociception. J. Neurosci 14, 712–720 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schmid G, Carita F, Bonanno G & Raiteri M NK-3 receptors mediate enhancement of substance P release from capsaicin-sensitive spinal cord afferent terminals. Br. J. Pharmacol 125, 621–626 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zaratin P et al. NK3 receptor blockade prevents hyperalgesia and the associated spinal cord substance P release in monoarthritic rats. Neuropharmacology 39, 141–149 (2000). [DOI] [PubMed] [Google Scholar]
- 114.Akiyama T, Tominaga M, Takamori K, Carstens MI & Carstens E Roles of glutamate, substance P, and gastrin-releasing peptide as spinal neurotransmitters of histaminergic and nonhistaminergic itch. Pain 155, 80–92 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Azimi E et al. Dual action of neurokinin-1 antagonists on Mas-related GPCRs. JCI Insight 1, e89362 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Swett JE & Woolf CJ The somatotopic organization of primary afferent terminals in the superficial laminae of the dorsal horn of the rat spinal cord. J. Comp. Neurol 231, 66–77 (1985). [DOI] [PubMed] [Google Scholar]
- 117.Light AR & Perl ER Reexamination of the dorsal root projection to the spinal dorsal horn including observations on the differential termination of coarse and fine fibers. J. Comp. Neurol 186, 117–131 (1979). [DOI] [PubMed] [Google Scholar]
- 118.Lu Y & Perl ER A specific inhibitory pathway between substantia gelatinosa neurons receiving direct C-fiber input. J. Neurosci 23, 8752–8758 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lu Y & Perl ER Modular organization of excitatory circuits between neurons of the spinal superficial dorsal horn (laminae I and II). J. Neurosci 25, 3900–3907 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shim WS & Oh U Histamine-induced itch and its relationship with pain. Mol. Pain 4, 29 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Green KB & Lim HW Effects of chloroquine on release of mediators from mast cells. Skin Pharmacol. 2, 77–85 (1989). [DOI] [PubMed] [Google Scholar]
- 122.Liu Q et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 139, 1353–1365 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li R et al. Intradermal injection of oxytocin aggravates chloroquine-induced itch responses via activating the vasopressin-1a receptor/nitric oxide pathway in mice. Front. Pharmacol 10, 1380 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Than JYXL, Li L, Hasan R & Zhang X Excitation and modulation of TRPA1, TRPV1, and TRPM8 channel-expressing sensory neurons by the pruritogen chloroquine. J. Biol. Chem 288, 12818–12827 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim S et al. Facilitation of TRPV4 by TRPV1 is required for itch transmission in some sensory neuron populations. Sci. Signal 9, ra71 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wilson SR et al. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat. Neurosci 14, 595–602 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Deshpande DA et al. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat. Med 16, 1299–1304 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mack MR & Kim BS The Itch-scratch cycle: a neuroimmune perspective. Trends Immunol. 39, 980–991 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Herkenham M Mismatches between neurotransmitter and receptor localizations in brain: observations and implications. Neuroscience 23, 1–38 (1987). [DOI] [PubMed] [Google Scholar]
- 130.Hylden JL & Wilcox GL Intrathecal substance P elicits a caudally-directed biting and scratching behavior in mice. Brain Res. 217, 212–215 (1981). [DOI] [PubMed] [Google Scholar]
- 131.Pagani M et al. How gastrin-releasing peptide opens the spinal gate for itch. Neuron 103, 102–117 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Marvizon JCG, Martinez V, Grady EF, Bunnett NW & Mayer EA Neurokinin 1 receptor internalization in spinal cord slices induced by dorsal root stimulation is mediated by NMDA receptors. J. Neurosci 17, 8129–8136 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Koga K et al. Glutamate acts as a neurotransmitter for gastrin releasing peptide-sensitive and insensitive itch-related synaptic transmission in mammalian spinal cord. Mol. Pain 7, 47 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Baraban SC & Tallent MK Interneuron diversity series: interneuronal neuropeptides–endogenous regulators of neuronal excitability. Trends Neurosci. 27, 135–142 (2004). [DOI] [PubMed] [Google Scholar]
- 135.Yoshimura M & Nishi S Blind patch-clamp recordings from substantia gelatinosa neurons in adult rat spinal cord slices: pharmacological properties of synaptic currents. Neuroscience 53, 519–526 (1993). [DOI] [PubMed] [Google Scholar]
- 136.Bardoni R, Magherini PC & MacDermott AB. NMDA EPSCs at glutamatergic synapses in the spinal cord dorsal horn of the postnatal rat. J. Neurosci 18, 6558–6567 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cao YQ et al. Primary afferent tachykinins are required to experience moderate to intense pain. Nature 392, 390–394 (1998). [DOI] [PubMed] [Google Scholar]
- 138.Lagerstrom MC et al. A sensory subpopulation depends on vesicular glutamate transporter 2 for mechanical pain, and together with substance P, inflammatory pain. Proc. Natl Acad. Sci. USA 108, 5789–5794 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Scherrer G et al. VGLUT2 expression in primary afferent neurons is essential for normal acute pain and injury-induced heat hypersensitivity. Proc. Natl Acad. Sci. USA 107, 22296–22301 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.van den Pol AN Neuropeptide transmission in brain circuits. Neuron 76, 98–115 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Waxman SG & Zamponi GW Regulating excitability of peripheral afferents: emerging ion channel targets. Nat. Neurosci 17, 153–163 (2014). [DOI] [PubMed] [Google Scholar]
- 142.Bourinet E et al. Calcium-permeable ion channels in pain signaling. Physiol. Rev 94, 81–140 (2014). [DOI] [PubMed] [Google Scholar]
- 143.Verhage M et al. Differential release of amino acids, neuropeptides, and catecholamines from isolated nerve terminals. Neuron 6, 517–524 (1991). [DOI] [PubMed] [Google Scholar]
- 144.Munanairi A et al. Non-canonical opioid signaling inhibits itch transmission in the spinal cord of mice. Cell Rep. 23, 866–877 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li HP et al. 100 Hz electroacupuncture alleviated chronic itch and GRPR expression through activation of kappa opioid receptors in spinal dorsal horn. Front. Neurosci 15, 625471 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ikoma A et al. Painful stimuli evoke itch in patients with chronic pruritus: central sensitization for itch. Neurology 62, 212–217 (2004). [DOI] [PubMed] [Google Scholar]
- 147.Hosogi M, Schmelz M, Miyachi Y & Ikoma A Bradykinin is a potent pruritogen in atopic dermatitis: a switch from pain to itch. Pain 126, 16–23 (2006). [DOI] [PubMed] [Google Scholar]
- 148.Roberson DP et al. Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons. Nat. Neurosci 16, 910–918 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Johanek LM et al. Psychophysical and physiological evidence for parallel afferent pathways mediating the sensation of itch. J. Neurosci 27, 7490–7497 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ohki-Hamazaki H Neuromedin B. Prog. Neurobiol 62, 297–312 (2000). [DOI] [PubMed] [Google Scholar]
- 151.Sukhtankar DD & Ko MC Physiological function of gastrin-releasing peptide and neuromedin B receptors in regulating itch scratching behavior in the spinal cord of mice. PLoS ONE 8, e67422 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kokumai S, Imamura T, Masuyama K, Kambara T & Ishikawa T Effect of capsaicin as a neuropeptide-releasing substance on sneezing reflex in a type I allergic animal model. Int. Arch. Allergy Immunol 98, 256–261 (1992). [DOI] [PubMed] [Google Scholar]
- 153.Li F et al. Sneezing reflex is mediated by a peptidergic pathway from nose to brainstem. Cell 184, 3762–3773 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mishra SK & Hoon MA The cells and circuitry for itch responses in mice. Science 340, 968–971 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Meng QT et al. BNP facilitates NMB-mediated histaminergic itch via NPRC-NMBR crosstalk. Preprint at bioRxiv 10.1101/2021.01.26.428310 (2021). [DOI] [PMC free article] [PubMed]
- 156.Meng J et al. New mechanism underlying IL-31-induced atopic dermatitis. J. Allergy Clin. Immunol 141, 1677–1689 (2018). [DOI] [PubMed] [Google Scholar]
- 157.Koller KJ et al. Selective activation of the B-natriuretic peptide receptor by C-type natriuretic peptide (CNP). Science 252, 120–123 (1991). [DOI] [PubMed] [Google Scholar]
- 158.Solinski HJ et al. Inhibition of natriuretic peptide receptor 1 reduces itch in mice. Sci. Transl. Med 11, eaav5464 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Potter LR, Abbey-Hosch S & Dickey DM Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr. Rev 27, 47–72 (2006). [DOI] [PubMed] [Google Scholar]
- 160.Liu XY et al. B-type natriuretic peptide is neither itch-specific nor functions upstream of the GRP-GRPR signaling pathway. Mol. Pain 10, 4 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang FX et al. Inhibition of inflammatory pain by activating B-type natriuretic peptide signal pathway in nociceptive sensory neurons. J. Neurosci 30, 10927–10938 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Vilotti S, Marchenkova A, Ntamati N & Nistri A B-type natriuretic peptide-induced delayed modulation of TRPV1 and P2X3 receptors of mouse trigeminal sensory neurons. PLoS ONE 8, e81138 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li ZW, Wu B, Ye P, Tan ZY & Ji YH Brain natriuretic peptide suppresses pain induced by BmK I, a sodium channel-specific modulator, in rats. J. Headache Pain 17, 90 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Solinski HJ et al. Nppb neurons are sensors of mast cell-induced itch. Cell Rep. 26, 3561–3573 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Miyamoto T, Nojima H, Shinkado T, Nakahashi T & Kuraishi Y Itch-associated response induced by experimental dry skin in mice. Jpn. J. Pharmacol 88, 285–292 (2002). [DOI] [PubMed] [Google Scholar]
- 166.Poole DP et al. Protease-activated receptor 2 (PAR2) protein and transient receptor potential vanilloid 4 (TRPV4) protein coupling is required for sustained inflammatory signaling. J. Biol. Chem 288, 5790–5802 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Arcourt A et al. Touch receptor-derived sensory information alleviates acute pain signaling and fine-tunes nociceptive reflex coordination. Neuron 93, 179–193 (2017). [DOI] [PubMed] [Google Scholar]
- 168.Qi L et al. Nuclear factor I/A controls A-fiber nociceptor development. Neurosci. Bull 36, 685–695 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhao J et al. PAR2 mediates itch via TRPV3 signaling in keratinocytes. J. Invest. Dermatol 140, 1524–1532 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Steinhoff M et al. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J. Neurosci 23, 6176–6180 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Huang J et al. Circuit dissection of the role of somatostatin in itch and pain. Nat. Neurosci 21, 707–716 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sun S et al. Leaky gate model: intensity-dependent coding of pain and itch in the spinal cord. Neuron 93, 840–853 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Albisetti GW et al. Dorsal horn gastrin-releasing peptide expressing neurons transmit spinal itch but not pain signals. J. Neurosci 39, 2238–2250 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Geppetti P, Veldhuis NA, Lieu T & Bunnett NW G protein-coupled receptors: dynamic machines for signaling pain and itch. Neuron 88, 635–649 (2015). [DOI] [PubMed] [Google Scholar]
- 175.Gong S et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425, 917–925 (2003). [DOI] [PubMed] [Google Scholar]
- 176.Dickie AC et al. Morphological and functional properties distinguish the substance P and gastrin-releasing peptide subsets of excitatory interneuron in the spinal cord dorsal horn. Pain 160, 442–462 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dymecki SM, Ray RS & Kim JC Mapping cell fate and function using recombinase-based intersectional strategies. Method. Enzymol 477, 183–213 (2010). [DOI] [PubMed] [Google Scholar]
- 178.Haring M et al. Neuronal atlas of the dorsal horn defines its architecture and links sensory input to transcriptional cell types. Nat. Neurosci 21, 869–880 (2018). [DOI] [PubMed] [Google Scholar]
- 179.Bell AM, Gutierrez-Mecinas M, Polgar E & Todd AJ Spinal neurons that contain gastrin-releasing peptide seldom express Fos or phosphorylate extracellular signal-regulated kinases in response to intradermal chloroquine. Mol. Pain 12, 1744806916649602 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Marti E et al. Ontogeny of peptide- and amine-containing neurones in motor, sensory, and autonomic regions of rat and human spinal cord, dorsal root ganglia, and rat skin. J. Comp. Neurol 266, 332–359 (1987). [DOI] [PubMed] [Google Scholar]
- 181.Freire MA, Guimaraes JS, Leal WG & Pereira A Pain modulation by nitric oxide in the spinal cord. Front. Neurosci 3, 175–181 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Todd AJ Identifying functional populations among the interneurons in laminae I-III of the spinal dorsal horn. Mol. Pain 13, 1–19 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sanes JR & Masland RH The types of retinal ganglion cells: current status and implications for neuronal classification. Annu. Rev. Neurosci 38, 221–246 (2015). [DOI] [PubMed] [Google Scholar]
- 184.Giardino WJ & Pomrenze MB Extended amygdala neuropeptide circuitry of emotional arousal: waking up on the wrong side of the bed nuclei of stria terminalis. Front. Behav. Neurosci 15, 613025 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Provine RR Curious Behavior (Belknap Press of Harvard University Press, 2012). [Google Scholar]
- 186.Feng J et al. Piezo2 channel-Merkel cell signaling modulates the conversion of touch to itch. Science 360, 530–533 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Schut C, Grossman S, Gieler U, Kupfer J & Yosipovitch G Contagious itch: what we know and what we would like to know. Front. Hum. Neurosci 9, 57 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pan H et al. Identification of a spinal circuit for mechanical and persistent spontaneous itch. Neuron 103, 1135–1149 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nelson TS et al. Facilitation of neuropathic pain by the NPY Y1 receptor-expressing subpopulation of excitatory interneurons in the dorsal horn. Sci. Rep 9, 7248 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Solway B, Bose SC, Corder G, Donahue RR & Taylor BK Tonic inhibition of chronic pain by neuropeptide Y. Proc. Natl Acad. Sci. USA 108, 7224–7229 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gao TL et al. The neuropeptide Y system regulates both mechanical and histaminergic itch. J. Invest. Dermatol 138, 2405–2411 (2018). [DOI] [PubMed] [Google Scholar]
- 192.Wang Z et al. Central opioid receptors mediate morphine-induced itch and chronic itch via disinhibition. Brain 144, 665–681 (2021). [DOI] [PubMed] [Google Scholar]
- 193.Bourane S et al. Gate control of mechanical itch by a subpopulation of spinal cord interneurons. Science 350, 550–554 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Acton D et al. Spinal neuropeptide Y1 receptor-expressing neurons form an essential excitatory pathway for mechanical itch. Cell Rep. 28, 625–639 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zeisel A et al. Molecular architecture of the mouse nervous system. Cell 174, 999–1014 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Akiyama T et al. Mouse model of touch-evoked itch (alloknesis). J. Invest. Dermatol 132, 1886–1891 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Schemlz M & Handwerker HO in Itch; Basic Mechanisms and Therapy (eds Yosipovitch G, Greaves MW, Fleischer AB Jr & McGlone F) 13–20 (Marcel Dekker, 2004). [Google Scholar]
- 198.Sandkuhler J Models and mechanisms of hyperalgesia and allodynia. Physiol. Rev 89, 707–758 (2009). [DOI] [PubMed] [Google Scholar]
- 199.Hill RZ & Bautista DM Getting in touch with mechanical pain mechanisms. Trends Neurosci 43, 311–325 (2020). [DOI] [PubMed] [Google Scholar]
- 200.Feneran AN et al. Monkey see, monkey do: contagious itch in nonhuman primates. Acta Derm. Venereal 93, 27–29 (2013). [DOI] [PubMed] [Google Scholar]
- 201.Liljencrantz J, Pitcher MH, Low LA, Bauer L & Bushnell MC Comment on “Molecular and neural basis of contagious itch behavior in mice”. Science 357, eaan4749 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Barry DM, Yu YQ, Hao Y, Liu XT & Chen ZF Response to Comment on “Molecular and neural basis of contagious itch behavior in mice”. Science 357, eaan5000 (2017). [DOI] [PubMed] [Google Scholar]
- 203.Gonzales-Rojas R et al. The mouse model of fragile X syndrome exhibits deficits in contagious itch behavior. Sci. Rep 10, 17679 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Langford DJ et al. Social modulation of pain as evidence for empathy in mice. Science 312, 1967–1970 (2006). [DOI] [PubMed] [Google Scholar]
- 205.Chen J Empathy for distress in humans and rodents. Neurosci. Bull 34, 216–236 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.de Waal FBM & Preston SD Mammalian empathy: behavioural manifestations and neural basis. Nat. Rev Neurosci 18, 498–509 (2017). [DOI] [PubMed] [Google Scholar]
- 207.Schmidt TM, Chen SK & Hattar S Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 34, 572–580 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.True JR & Carroll SB Gene co-option in physiological and morphological evolution. Annu. Rev. Cell Dev. Biol 18, 53–80 (2002). [DOI] [PubMed] [Google Scholar]
- 209.Podvin S, Yaksh T & Hook V The emerging role of spinal dynorphin in chronic pain: a therapeutic perspective. Annu. Rev. Pharmacol. Toxicol 56, 511–533 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Chen S, Liu XY, Jiao Y, Chen ZF & Yu W NPY2R signaling gates spontaneous and mechanical, but not thermal, pain transmission. Mol. Pain 15, 1744806919887830 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhang Y et al. Timing mechanisms underlying gate control by feedforward inhibition. Neuron 99, 941–955 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Duan B et al. Identification of spinal circuits transmitting and gating mechanical pain. Cell 159, 1417–1432 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sardella TCP et al. Dynorphin is expressed primarily by GABAergic neurons that contain galanin in the rat dorsal horn. Mol. Pain 7, 76 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wang Z et al. Pronociceptive actions of dynorphin maintain chronic neuropathic pain. J. Neurosci 21 , 1779–1786 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Xu M et al. Neuropathic pain activates the endogenous kappa opioid system in mouse spinal cord and induces opioid receptor tolerance. J. Neurosci 24, 4576–4584 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Glasgow SM, Henke RM, Macdonald RJ, Wright CV & Johnson JE Ptf1a determines GABAergic over glutamatergic neuronal cell fate in the spinal cord dorsal horn. Development 132, 5461–5469 (2005). [DOI] [PubMed] [Google Scholar]
- 217.Wildner H et al. Genome-wide expression analysis of Ptf1a- and Ascl1-deficient mice reveals new markers for distinct dorsal horn interneuron populations contributing to nociceptive reflex plasticity. J. Neurosci 33, 7299–7307 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Petitjean H et al. Dorsal horn parvalbumin neurons are gate-keepers of touch-evoked pain after nerve Injury. Cell Rep. 13, 1246–1257 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Polgar E et al. Functional differences between neurochemically defined populations of inhibitory interneurons in the rat spinal dorsal horn. Pain 154, 2606–2615 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Naveilhan P et al. Reduced antinociception and plasma extravasation in mice lacking a neuropeptide Y receptor. Nature 409, 513–517 (2001). [DOI] [PubMed] [Google Scholar]
- 221.Foster E et al. Targeted ablation, silencing, and activation establish glycinergic dorsal horn neurons as key components of a spinal gate for pain and itch. Neuron 85, 1289–1304 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Freitag FB, Ahemaiti A, Jakobsson JET, Weman HM & Lagerstrom MC Spinal gastrin releasing peptide receptor expressing interneurons are controlled by local phasic and tonic inhibition. Sci. Rep 9, 16573 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Liu Y et al. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron 68, 543–556 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lagerstrom MC et al. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron 68, 529–542 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hnasko TS & Edwards RH Neurotransmitter corelease: mechanism and physiological role. Annu. Rev. Physiol 74, 225–243 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Papoiu AD et al. Brain’s reward circuits mediate itch relief. a functional MRI study of active scratching. PLoS ONE 8, e82389 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Su XY et al. Central processing of itch in the midbrain reward center. Neuron 102, 858–871 (2019). [DOI] [PubMed] [Google Scholar]
- 228.McGlone F, Wessberg J & Olausson H Discriminative and affective touch: sensing and feeling. Neuron 82, 737–755 (2014). [DOI] [PubMed] [Google Scholar]
- 229.Choi S et al. Parallel ascending spinal pathways for affective touch and pain. Nature 587, 258–263 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Gao ZR et al. Tac1-expressing neurons in the periaqueductal gray facilitate the itch-scratching cycle via descending regulation. Neuron 101, 45–59 (2019). [DOI] [PubMed] [Google Scholar]
- 231.Samineni VK, Grajales-Reyes JG, Sundaram SS, Yoo JJ & Gereau RWT Cell type-specific modulation of sensory and affective components of itch in the periaqueductal gray. Nat. Commun 10, 4356 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Gao T et al. GPER in the rostral ventromedial medulla is essential for mobilizing descending inhibition of itch. J. Neurosci 4, 7727–7741 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhao ZQ et al. Descending control of itch transmission by the serotonergic system via 5-HT1A-facilitated GRP-GRPR signaling. Neuron 84, 821–834 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Gotoh Y, Omori Y, Andoh T & Kuraishi Y Tonic inhibition of allergic itch signaling by the descending noradrenergic system in mice. J. Pharmacol. Sci 115, 417–420 (2011). [DOI] [PubMed] [Google Scholar]
- 235.Koga K et al. Intrinsic braking role of descending locus coeruleus noradrenergic neurons in acute and chronic itch in mice. Mol. Brain 13, 144 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Pasternak GW & Pan YX Mu opioids and their receptors: evolution of a concept. Pharmacol. Rev 65, 1257–1317 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Abbadie C, Pan Y, Drake CT & Pasternak GW Comparative immunohistochemical distributions of carboxy terminus epitopes from the mu-opioid receptor splice variants MOR-1D, MOR-1 and MOR-1C in the mouse and rat CNS. Neuroscience 100, 141–153 (2000). [DOI] [PubMed] [Google Scholar]
- 238.Liu XY, Ginosar Y, Yazdi J, Hincker A & Chen ZF Cross-talk between human spinal cord mu-opioid receptor 1Y isoform and gastrin-releasing peptide receptor mediates opioid-induced scratching behavior. Anesthesiology 131, 381–391 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Nguyen E et al. Morphine acts on spinal dynorphin neurons to cause itch through disinhibition. Sci. Transl. Med 13, eabc3774 (2021). [DOI] [PubMed] [Google Scholar]
- 240.Zhang XY et al. Different neuronal populations mediate inflammatory pain analgesia by exogenous and endogenous opioids. eLife 9, e55289 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Charbogne P et al. Mu opioid receptors in gamma-aminobutyric acidergic forebrain neurons moderate motivation for heroin and palatable food. Biol. Psychiatry 81, 778–788 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Cowan A, Kehner GB & Inan S Targeting itch with ligands selective for kappa opioid receptors. Handb. Exp. Pharmacol 226, 291–314 (2015). [DOI] [PubMed] [Google Scholar]
- 243.Braz JM, Juarez-Salinas D, Ross SE & Basbaum AI Transplant restoration of spinal cord inhibitory controls ameliorates neuropathic itch. J. Clin. Invest 124, 3612–3616 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zeilhofer HU, Wildner H & Yevenes GE Fast synaptic inhibition in spinal sensory processing and pain control. Physiol. Rev 92, 193–235 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Holmes FE, Vanderplank P & Wynick D Galanin-expression and galanin-dependent sensory neurons are not required for itch. Mol. Pain 8, 87 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Luo L, Callaway EM & Svoboda K Genetic dissection of neural circuits: a decade of progress. Neuron 98, 256–281 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Todd AJ Neuronal circuitry for pain processing in the dorsal horn. Nat. Rev. Neurosci 11, 823–836 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Andrew D & Craig AD Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nat. Neurosci 4, 72–77 (2001). [DOI] [PubMed] [Google Scholar]
- 249.Lever IJ et al. Brain-derived neurotrophic factor is released in the dorsal horn by distinctive patterns of afferent fiber stimulation. J. Neurosci 21, 4469–4477 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ueda M, Kuraishi Y, Sugimoto K & Satoh M Evidence that glutamate Is released from capsaicin-sensitive primary afferent-fibers in rats - study with online continuous monitoring of glutamate. Neurosci. Res 20, 231–237 (1994). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


