Abstract
Objective: This study aims to estimate the cost-effectiveness of combined physical and cognitive programs designed to prevent community-dwelling healthy young-old adults from developing dementia. Methods: The analysis was conducted from a public healthcare and long-term care payer’s perspective. Quality-adjusted life years (QALYs) and expenses for health services and long-term care services were described in terms of effectiveness and cost, respectively. A thousand community-dwelling healthy adults aged 65 years were generated through simulation and analyzed. The incremental cost-effectiveness ratio (ICER) of adults with preventive program intervention compared to those with nonintervention was simulated with a 10-year cycle Markov model. The data sources for the parameters to build the Markov models were selected with priority given to higher levels of evidence. The threshold for assessing cost-effectiveness was set as less than 5,000,000 Japanese yen/QALY. Results: The ICER was estimated as −5,740,083 Japanese yen (US$−57,400)/QALY. Conclusion: A program targeting community-dwelling healthy young-old adults could be cost-effective.
Keywords: Cost-effectiveness analysis, Prevention, Dementia, Combined physical and cognitive exercises, Older adults
The rapid aging of populations is being seen in many parts of the world1). The same trend is also seen in Japan, where in 2018, the population aged 65 years and older was 35.89 million, making up 28.4% of the total population2). Aging induces an increase in the number of frail older adults and the cost of their healthcare and long-term care3).
One of the typical and problematic diseases related to aging is dementia, which not only induces deterioration of health but also increases the cost of medical and long-term care and makes it difficult to sustain the health and long-term care systems of Japan4,5); Japanese society’s total costs from dementia have been estimated to reach 24.3 trillion Japanese yen by 2060. Therefore, there has been a call for the introduction of preventive programs to avoid the risk of cognitive decline and dementia6).
It has been suggested that people who experience multiple-domain mild cognitive impairment (MCI) have a high risk of developing dementia7). MCI has been indicated to be highly reversible to a healthy state if the appropriate intervention is received as early as possible7–9). Exercise has been suggested as an effective intervention to prevent dementia8,9), and combined physical and cognitive programs10,11) have been suggested to be especially effective if introduced early, when young-old adults are in a healthy or MCI state before developing dementia7).
To achieve dementia-free communities7), introducing a sufficiently cost-effective public health program might be beneficial to society. A Markov model has been suggested to be useful for health-economic analyses because, for example, it can incorporate risks that are continuous over time12). Indeed, it has been applied to health-economic analyses for health-related preventive services in international cases13) and in Japan14). In the same way, a health-economic evaluation of primary prevention programs for dementia has also been recommended15). Although there has been a study of health-economic analysis for pharmacological interventions for people who have already developed dementia16), to our knowledge, no studies have applied health-economic analysis to primary prevention programs for dementia using a Markov model.
Understanding the value of the cost-effectiveness of a prevention program might aid in making a decision on introducing it into the community.
This study therefore aims to estimate to what extent combined physical and cognitive programs designed to prevent community-dwelling healthy young-old adults from developing dementia would be cost-effective using a Markov modeling analysis.
Methods
The analysis was conducted from a public healthcare and long-term care payer’s perspective17). Quality-adjusted life years (QALYs) were described in terms of effectiveness, and expenses in terms of cost, for health services and long-term care services. The target population was Japanese community-dwelling healthy young-old adults; the subjects were aged 65 years. The data sources for the parameters to build the Markov models17) were selected with priority given to those with higher levels of evidence, such as systematic reviews, and those developed in Japan, as recommended elsewhere17).
The analysis was conducted with TreeAge Pro Healthcare version 2021 R1.1 (TreeAge Software, Williamstown, MA, USA). This study was approved by the Ethics Committee of the Tokyo Professional University of Health Science (TPU-20-002). The sources of the parameters are shown in Table 17,17–26).
Table 1.
Parameters related to the transition probability, effectiveness, costs, and discount rate
| Parameter | Subgroup | Unit | Basic value | Range (lowest − highest) | Type of range | Distribution | Source | |
|---|---|---|---|---|---|---|---|---|
| MCI, mild cognitive impairment; CI, confidence interval; QALY, quality-adjusted life year | ||||||||
| Transition probabilities and effectiveness | ||||||||
| Transition probability from state of well to dementia | 65−69 years old | %/year | 2.18 | 1.44 | 14.91 | 95%CI | Triangular | Ninomiya (2015)18) |
| 70−74 years old | %/year | 4.84 | 95%CI | Triangular | Ninomiya (2015)18) | |||
| 75− years old | %/year | 10.75 | 95%CI | Triangular | Ninomiya (2015)18) | |||
| Transition probability from MCI to dementia | %/year | 3.93 | 1.14 | 5.90 | 95%CI | Triangular | Shimada (2017)7) | |
| Probability of severity in dementia | Mild | % | 38.50 | 30.80 | 46.20 | ±20% | Dirichlet | Asada (2013)19) |
| Moderate | % | 24.10 | 19.28 | 28.92 | ±20% | Dirichlet | Asada (2013)19) | |
| Severe | % | 37.40 | 29.92 | 44.88 | ±20% | Dirichlet | Asada (2013)19) | |
| Transition probability from MCI to well | %/year | 10.98 | 5.69 | 19.02 | 95%CI | Triangular | Shimada (2017)7) | |
| Transition probability from well to MCI | %/year | 13/15 times of transition probability from state of well to dementia | 95%CI | Triangular | Ninomiya (2015)18) Asada (2013)19) |
|||
| Relative risk for death in the state of dementia | %/year | 2.80 | 1.85 | 4.24 | 95%CI | Triangular | July (2021)20) | |
| Effect of combined physical and cognitive exercises to prevent the progression of MCI | All age | rate/year | 0.65 | 0.55 | 0.76 | 95 % CI | Triangular | Blondell (2014)21) |
| Effect of combined physical and cognitive exercises to prevent the progression of dementia | All age | rate/year | 0.86 | 0.76 | 0.97 | 95 % CI | Triangular | Blondell (2014)21) |
| Effectiveness | Well | QALY/year | 1.00 | 1.00 | 1.00 | 95% CI | Triangular | Estimated by authors |
| MCI | QALY/year | 0.75 | 0.16 | 1.00 | 95% CI | Triangular | Landeiro (2020)22) | |
| Dementia (Mild) | QALY/year | 0.61 | 0.00 | 1.00 | 95% CI | Triangular | Landeiro (2020)22) | |
| Dementia (Moderate) | QALY/year | 0.41 | 0.00 | 1.00 | 95% CI | Triangular | Landeiro (2020)22) | |
| Dementia (Severe) | QALY/year | 0.21 | 0.00 | 0.74 | 95% CI | Triangular | Landeiro (2020)22) | |
| Dead | QALY/year | 0.00 | 0.00 | 0.00 | 95% CI | Triangular | Estimated by authors | |
| Costs related to the program | ||||||||
| Cost for screening | Well MCI | yen/year | 6,000 | 4,800 | 7,200 | ± 20 % | Triangular | Expert opinion by Shimada (2017)7) |
| Number of individuals in a session | Well MCI | n/session | 15 | 12 | 18 | ± 20 % | Triangular | Expert opinion by Shimada (2017)7) |
| Number of physical therapists for a session | Well MCI | n/session | 2 | 1 | 3 | Estimated | Triangular | Shimada (2017)7) |
| Number of instructors for a session | Well MCI | n/session | 5 | 4 | 6 | ± 20 % | Triangular | Expert opinion by Shimada (2017)7) |
| Cost for a physical therapist for a session | Well MCI | yen/session | 8,396 | 6,716 | 10,075 | ± 20 % | Triangular | Ministory of Health, Labour and Welfare (2020)23) |
| Cost for a instructors for a session | Well MCI | yen/ session | 3,000 | 2,400 | 3,600 | ± 20 % | Triangular | Expert opinion by Shimada (2017)7) |
| Numbers of sessions held in a year | Well MCI (First year) | sessions/year | 40 | 32 | 48 | ± 20 % | Triangular | Shimada (2017)7) |
| Well MCI (From 2nd year) | sessions/year | 2 | 2 | 2.4 | ± 20 % | Triangular | Estimated by authors | |
| Cost for a public space to conduct the session | Well MCI | yen/ session | 900 | 720 | 1,080 | ± 20 % | Triangular | Obu city (2021)24) |
| Cost for maintaining the program | Well MCI | yen/year | 6,000 | 4,800 | 7,200 | ± 20 % | Triangular | Expert opinion by Shimada (2017)7) |
| Costs for medical and long-term care | ||||||||
| Cost for day service for dementia | Dementia (Mild/Moderate) | yen/year | 1,530,000 | 1,224,000 | 1,836,000 | ± 20 % | Triangular | Ministory of Health, Labour and Welfare (2019)25) |
| Cost for preventive day service for dementia | Dementia (Mild) | yen/year | 614,400 | 491,520 | 737,280 | ± 20 % | Triangular | Ministory of Health, Labour and Welfare (2019)25) |
| Cost for preventive short stay for dementia | Dementia (Mild) | yen/year | 471,600 | 377,280 | 565,920 | ± 20 % | Triangular | Ministory of Health, Labour and Welfare (2019)25) |
| Cost for short stay use for dementia | Dementia (Mild/Moderate) | yen/year | 1,315,200 | 1,052,160 | 1,578,240 | ± 20 % | Triangular | Ministory of Health, Labour and Welfare (2019)25) |
| Cost for stay in community-based facility | Dementia (Severe) | yen/year | 3,448,800 | 2,759,040 | 4,138,560 | ± 20 % | Triangular | Ministory of Health, Labour and Welfare (2019)25) |
| Cost for medical treatment for dementia | Dementia (Mild) | yen/year | 527,344 | 421,875.2 | 632,812.8 | ± 20 % | Triangular | Tomata (2014)26) |
| Dementia (Moderate) | yen/year | 841,974 | 673,579.2 | 1,010,368.8 | ± 20 % | Triangular | Ministory of Health, Labour and Welfare (2019)25) | |
| Dementia (Severe) | yen/year | 1,035,384 | 828,307.2 | 1,242,460.8 | ± 20 % | Triangular | Ministory of Health, Labour and Welfare (2019)25) | |
| Discount rate | %/year | 2.00 | 0.00 | 4.00 | estimated | Triangular | Shiroiwa (2017)17) | |
The group assumed not to receive the preventive exercise program was defined as the nonintervention group (NIG); the others who participated in the program were defined as the intervention group (IG). The incremental cost-effectiveness ratio (ICER) of the IG to the NIG was analyzed using a 10-year Markov model. One year was set as one cycle. The threshold for assessing cost-effectiveness was set as less than 5,000,000 yen/QALY27). Additionally, the incremental net monetary benefit (INMB)28) was calculated by equating 1 QALY to 5,000,000 yen to consider the cost-effectiveness of the preventive exercise program from a restricted societal perspective17). The INMB was used to consider the 10-year productivity loss when the subjects did not receive the intervention. In this study, 100 yen was considered equal to 1 United States dollar (US$) to understand the results from an international perspective.
Structure of the Markov models
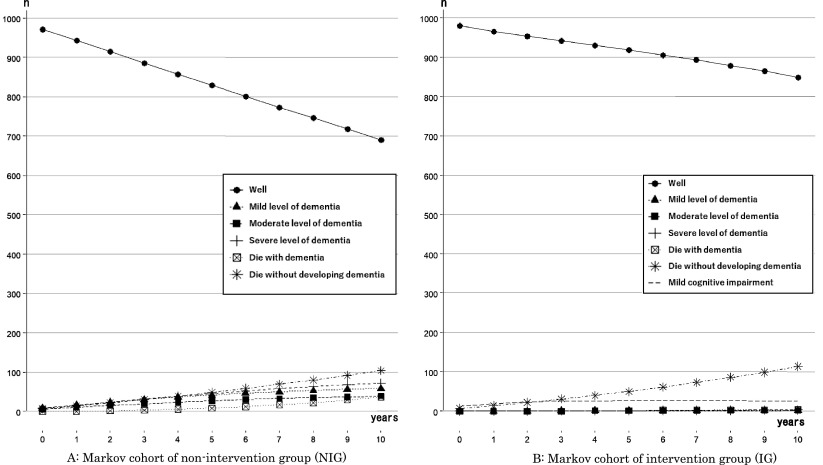
Two 10-year cycle Markov models for NIG (Fig. 1A) and IG (Fig. 1B) were built. One thousand healthy community-dwelling adults aged 65 years in the first year of the simulation were selected for the analysis.
Fig. 1.
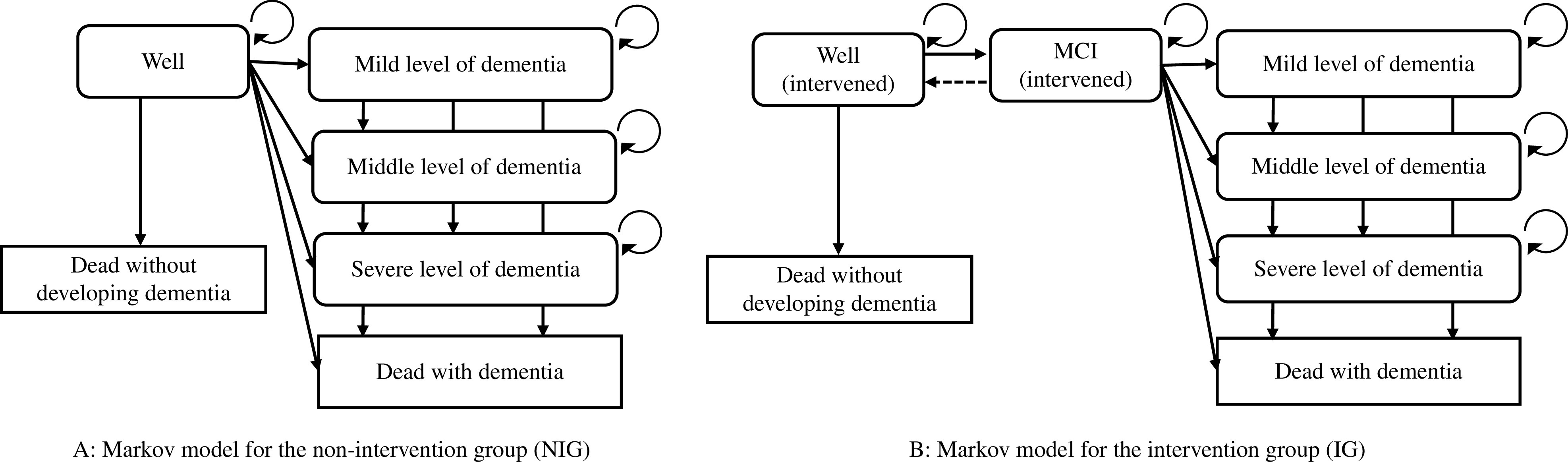
Markov models
NIG, non-intervention group; IG, intervention group; MCI, mild cognitive impairment
The six transition states indicated in a previous study20) to categorize people with predementia, MCI, or dementia were applied. The NIG (Fig. 1A) had the states (a) well, (b) mild level of dementia, (c) middle level of dementia, (d) severe level of dementia, (e) dead with dementia, and (f) dead without developing dementia. Similarly, seven transition states for the IG (Fig. 1B) were defined by adding the MCI state to those of the NIG.
The states of death were set as the endpoints of the models. Those who were in a state of dementia were assumed to receive medical treatment. The severity of dementia was classified into each care needs level of the Japanese long-term care system29) by a physical therapist who was certified to give a specialist opinion on care needs level; this method, asking for a specialist opinion for the classification, follows a previous cost-effectiveness study for dementia conducted in Japan16). Those who were in the state of a mild level of dementia were assumed to be in support levels 1 or 2, or care need level 1. They were assumed to receive preventive day service for dementia or day service for dementia, and preventive short stay for dementia or short stay for dementia from the long-term care system. Those who were in the middle level of dementia were assumed to be in care need levels 2 or 3. They were assumed to receive day service for dementia and short stay for dementia. Those who were in the state of severe dementia were assumed to be in care need levels 4 or 5. They were assumed to stay in community-based facilities. The possibility of reversion from the MCI state to the well state was only present in the IG, while reversion after dementia was not considered possible. QALY and costs were calculated with a half-cycle correction13) and translated to the current value with a 2% per year discount rate17).
The individuals in the IG were assumed to receive screening for MCI and dementia once a year, and the program with combined physical and cognitive exercises involved a 90-minute weekly session focused on physical and cognitive activities, which was conducted 40 times in the first year (details are reported elsewhere11)). Based on a previous study11), 15 individuals were assumed to participate in each session conducted by 2 geriatric physical therapists and 5 instructors in public halls. As a note, the number of participants in each session for this study was smaller than that of the previous study (15 individuals vs. 16–32 individuals). This was because the space of public halls for the intervention in this study was assumed to be narrower than that of the fitness facilities in the previous study. From the second year, the individuals who were in the well or MCI states were assumed to receive the screening once a year and the program twice a year.
Parameters
The basic values of the parameters were used to produce a base case of the results from a Markov cohort analysis. The range of values and distribution were applied for sensitivity analysis. The mean values among subgroups, such as sex and type of dementia, were used as needed to produce the base case, while the lowest and highest values among subgroups were used for the sensitivity analysis. Multiple years (n) of transition probabilities were converted to values for 1 year (p) using the following formula as needed30):
Transition probability
The basic values of the transition probabilities are shown in Table 1. The probability of newly developing dementia in a year was substituted with the estimated prevalence of dementia, following age groups in Japan18). The probabilities of newly developing dementia in a year for 66–69-year-old, 70–74-year-old, and 75-year-old adults were set at 2.18%, 4.84%, and 10.75%, respectively18). The mean transition probability from the well state to the MCI state was set at 13/15 times the probability of newly developing dementia per year. This was because the prevalence of dementia was 15%, while that of MCI was 13%, according to a study that analyzed 104,785 subjects from 10 areas in Japan19). The transition probability from the well state to death was referenced from the Japanese simple life table in 201931). The mean transition probabilities of those 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, and 75 years old were calculated as 0.73%, 0.81%, 0.89%, 0.98%, 1.09%, 1.19%, 1.30%, 1.42%, 1.56%, 1.73%, and 1.92%, respectively.
Effectiveness
The utility values of subjects were used to indicate effectiveness in this study. The mean values of health-related quality of life scaled for predementia Alzheimer’s disease, MCI, or dementia with EuroQol-5 Dimension (EQ-5D)32) reported by Heßmann et al.27,33) were used for the effectiveness and treated as QALY values (Table 1). The proxy-rated values, not self-rated values, were adapted to the model to maintain validity and take into account the caregivers’ productivity loss27). The mean values for the QALY values per year for the states of well, MCI, mild severity of dementia, moderate severity of dementia, severe severity of dementia, and dead (with or without dementia) were 1.00, 0.75, 0.61, 0.41, 0.21, and 0.00, respectively. The values could not be below 0.00 or higher than 1.00.
Costs
The cost of one hour for a physical therapist, 2798.5 yen, was referenced from the basic statistical survey of wage structure in Japan in 202023). This was an average of the values for a temporary physical therapist’s hourly salary in organizations with 100–999 workers (4104 yen) and in organizations with 10–99 workers (1493 yen). The cost of a physical therapist for a session was calculated as (physical therapist’s salary paid per hour × working hours × numbers of physical therapists in a session) ÷ numbers of individuals. The physical therapists were set to contribute three hours each to a session. The working hours included not only conducting exercise but also the preparation of and finishing up for the session.
The screening cost, 6000 yen, and an hourly cost of 1000 yen for an instructor to support the program were set by the authors who conducted the program11,34). As a screening test, the Mini-Mental State Examination (MMSE)35), the Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS cog)36), and the Wechsler Memory Scale-Revised32) were set. The instructors were set to contribute three hours each to a session.
The annual medical cost for treating dementia was calculated based on a study that reported the monthly medical costs for treating dementia, depending on the care needs levels26). The mean values of these, depending on the severity of dementia, were used for the models. The medical costs for dementia with care support levels 1 and 2 were referenced from the value of noncertified long-term care needs from a previous study26).
Analysis
The individual cohorts for the analysis were generated via simulation to calculate the base case of the ICER. The sample size of the cohorts for the simulation was set at 1000 individuals, following a previous study15,38); the result was said to be unchanged by the sample size28). The individual cohorts were assumed to follow the parameters of the basic values shown in Table 1. The base case of the ICER was calculated by inputting the basic values for the model parameters and analyzing them using Markov cohort analysis. The mean cumulative 10-year costs and QALYs were calculated based on a cohort analysis of 1000 individuals. These results were then used to calculate the base case of the ICER.
Next, a one-way sensitivity analysis was conducted to scale the size of the impact of the parameters on the base case and investigate the direction of them by changing the parameters within the ranges between the low values and the high values shown in Table 1. To avoid confusion when the ICER showed negative values, instead of reporting ICERs, INMB39) was reported to consider the effect of the uncertainty of the parameters on the base case. If the values of the parameters increased with the increased values of the INMBs, the associations were considered to have improved; otherwise, they were considered to have worsened. The proportion of the size of impact (%) was calculated to measure how much of the total uncertainty of the base case of the INMB was represented by the specified parameters by dividing the squared spread value by the total sum of each parameter’s squared spread value of INMBs.
Finally, a set of Monte Carlo probabilistic simulations for 1000 individuals and microsimulations with 1000 trials were conducted to check the robustness of the base case. The simulated results were plotted, and a 95% confidence ellipse and the willingness to pay (WTP) line were drawn; the plots on the WTP line were equal to 5,000,000 yen; therefore, the scatter plots and the area of the 95% confidence ellipse under the WTP line were interpreted as cost-effective ICER values.
In conducting the sensitivity analyses, the parameters were estimated to follow the distribution shown in Table 1. The range of the discount rate was set as 0.00%–4.00%. The low value and the high value of transition probability from well to death were set as 0.73% and 1.92%, respectively.
Results
The base case of the ICER
Simulated trends of the numbers in each state among 1000 individuals for 10 years in the NIG and the IG are shown in Fig. 2A and 2B, respectively. The Markov cohort model simulated with the passage of 10 years the numbers in each state in the NIG and the IG, respectively; after 10 years, there were 690/849 in the state of well, 58/2 in the state of a mild level of dementia, 39/2 in the state of the middle level of dementia, 72/3 in the state of severe level of dementia, 37/4 in the state of dead with dementia, and 104/114 in the state of dead without developing dementia. After 10 years, the number in the state of MCI was 26 in the IG.
Fig. 2.
Ten years simulated trends of the number of states among 1000 individuals
NIG, non-intervention group; IG, intervention group
The cumulative QALYs and costs for 10 years in the NIG/IG were 8.13 QALY/8.62 QALY, and 3,022,498 yen/203,666 yen, respectively. As such, the incremental effectiveness, incremental costs, ICER (base case), and INMB were estimated as 0.49 QALY, –2,818,833 yen, –5,736,166 yen (US$–57400)/QALY, and 5,275,903 yen, respectively (Table 2).
Table 2.
Base case values simulated by 10 years Markov cohort analysis
| Cumulativeeffectiveness(QALY) | Incrementaleffectiveness(QALY) | Cumulativecost (yen) | Incrementalcost (yen) | ICER(yen/QALY) | INMB(yen/10 years) | |
|---|---|---|---|---|---|---|
| QALY, quality-adjusted life year; ICER, incremental cost-effectiveness ratio; INMB, incremental net monetary benefit | ||||||
| Non-intervention model | 8.13 | 3,022,498 | ||||
| Intervention model | 8.62 | 0.49 | 203,666 | –2,818,833 | –5,736,166 | 5,275,903 |
One-way sensitivity analysis
The ICER showed negative values; therefore, the INMB was analyzed. The most impactive parameter on the base case of INMB was the transition probability from well to dementia (risk percentage of 97.44%); the direction of the impact was improved (Table 3). The second-highest parameter was the transition probability from well to MCI (0.97%); the direction of the impact was worsened. The least impactive parameters on the base case ranked from 21 to 29 were the costs of the preventive programs (Table 3).
Table 3.
Results of one-sensitivity analysis
| Parameters | Unit of parameters | Low value of parameters | High value of parameters | Incremental cost, yen (Low value) | Incremental cost, yen (High value) | Incremental effectiveness, QALY (Low value) | Incremental effectiveness, QALY (High value) | Direction of impact on INMB | INMB, yen (Low value) | INMB, yen (High value) | Spread | Size of impact | Rank |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PTs, physical therapists; MCI, mild cognitive impairment; QALY, quality-adjusted life years; INMB, incremental net monetary benefit | |||||||||||||
| Transition probability from well to dementia | %/year | 1.44 | 14.91 | −1,857,147 | −15,097,902 | 0.33 | 2.53 | Improved | 3,508,401 | 27,725,220 | 24,216,819 | 97.44% | 1 |
| Transition probability from well to MCI | %/year | 1.25 | 9.32 | −2,850,307 | −2,480,181 | 0.53 | 0.12 | Worsened | 5,482,745 | 3,070,710 | 2,412,034 | 0.97% | 2 |
| Utility of mild severity of dementia | QALY/year | 0.00 | 1.00 | −2,818,833 | −2,818,833 | 0.68 | 0.37 | Worsened | 6,204,850 | 4,681,985 | 1,522,865 | 0.39% | 3 |
| Utility of severe severity of dementia | QALY/year | 0.00 | 0.74 | −2,818,833 | −2,818,833 | 0.56 | 0.31 | Worsened | 5,633,536 | 4,375,007 | 1,258,529 | 0.26% | 4 |
| Discount rate | %/year | 0.00 | 4.00 | −3,169,289 | −2,518,118 | 0.55 | 0.44 | Worsened | 5,936,051 | 4,710,194 | 1,225,857 | 0.25% | 5 |
| Probability to be severe level of dementia | % | 29.92 | 44.88 | −3,086,784 | −2,548,013 | 0.44 | 0.54 | Improved | 4,737,488 | 5,808,704 | 1,071,216 | 0.19% | 6 |
| Utility of moderate severity of dementia | QALY/year | 0.00 | 1.00 | −2,818,833 | −2,818,833 | 0.57 | 0.37 | Worsened | 5,682,240 | 4,691,174 | 991,066 | 0.16% | 7 |
| Utility of MCI | QALY/year | 0.16 | 1.00 | −2,818,833 | −2,818,833 | 0.39 | 0.57 | Improved | 4,786,017 | 5,649,672 | 863,655 | 0.12% | 8 |
| Probability to be mild level of dementia | % | 30.80 | 46.20 | −2,954,314 | −2,681,953 | 0.46 | 0.52 | Improved | 4,988,577 | 5,560,434 | 571,857 | 0.05% | 9 |
| Probability to be middle level of dementia | % | 19.28 | 28.92 | −2,676,455 | −2,960,412 | 0.46 | 0.52 | Improved | 5,001,310 | 5,549,048 | 547,738 | 0.05% | 10 |
| Cost for stay in community-based facility for dementia | yen/year | 2,759,040 | 4,138,560 | −2,583,898 | −3,053,767 | 0.49 | 0.49 | Improved | 5,040,968 | 5,510,837 | 469,869 | 0.04% | 11 |
| Transition probability from well to die | %/year | 0.73 | 1.92 | −2,917,401 | −2,538,425 | 0.49 | 0.48 | Worsened | 5,375,925 | 4,931,448 | 444,477 | 0.03% | 12 |
| Transition probability of reversing from MCI to well | %/year | 20.90 | 57.00 | −2,793,731 | −2,838,896 | 0.46 | 0.52 | Improved | 5,083,098 | 5,416,020 | 332,922 | 0.02% | 13 |
| Effect of combined physical and cognitive exercises to prevent the progression of MCI | rate/year | 0.55 | 0.76 | −2,833,451 | −2,803,828 | 0.51 | 0.47 | Worsened | 5,371,929 | 5,177,414 | 194,515 | 0.01% | 14 |
| Transition probability from MCI to dementia | %/year | 1.14 | 5.90 | −2,885,101 | −2,773,405 | 0.50 | 0.49 | Worsened | 5,386,981 | 5,199,350 | 187,631 | 0.01% | 15 |
| Cost for day service for dementia | yen/year | 1,224,000 | 1,836,000 | −2,727,113 | −2,910,552 | 0.49 | 0.49 | Improved | 5,184,183 | 5,367,622 | 183,439 | 0.01% | 16 |
| Cost for short stay use for dementia | yen/year | 1,052,160 | 1,578,240 | −2,739,990 | −2,897,675 | 0.49 | 0.49 | Improved | 5,197,060 | 5,354,746 | 157,686 | 0.00% | 17 |
| Cost for preventive day service for dementia | yen/year | 491,520 | 737,280 | −2,793,882 | −2,843,783 | 0.49 | 0.49 | Improved | 5,250,952 | 5,300,853 | 49,901 | 0.00% | 18 |
| Cost for preventive short stay use for dementia | yen/year | 377,280 | 565,920 | −2,799,681 | −2,837,984 | 0.49 | 0.49 | Improved | 5,256,751 | 5,295,054 | 38,303 | 0.00% | 19 |
| Effect of combined physical and cognitive exercises to prevent the development of dementia | rate/year | 0.76 | 0.97 | −2,829,313 | −2,807,434 | 0.49 | 0.49 | Worsened | 5,293,468 | 5,256,792 | 36,675 | 0.00% | 20 |
| Cost for screening for cognitive impairment | yen | 4,800 | 7,200 | −2,829,254 | −2,808,411 | 0.49 | 0.49 | Worsened | 5,286,324 | 5,265,481 | 20,842 | 0.00% | 21 |
| Cost for maintaining the program | yen/year | 4,800 | 7,200 | −2,828,077 | −2,809,588 | 0.49 | 0.49 | Worsened | 5,285,147 | 5,266,658 | 18,489 | 0.00% | 22 |
| Number of individuals in a session | n/ session | 12 | 18 | −2,814,810 | −2,821,231 | 0.49 | 0.49 | Improved | 5,271,880 | 5,278,301 | 6,421 | 0.00% | 23 |
| Number of physical therapists (PTs) for a session | n/ session | 1 | 3 | −2,820,871 | −2,816,794 | 0.49 | 0.49 | Worsened | 5,277,941 | 5,273,864 | 4,076 | 0.00% | 24 |
| Cost of a physical therapist for a session | yen/ session | 6,716 | 10,075 | −2,819,648 | −2,818,017 | 0.49 | 0.49 | Worsened | 5,276,718 | 5,275,087 | 1,631 | 0.00% | 25 |
| Cost for a public space to conduct the session | yen/ session | 720 | 1,080 | −2,819,488 | −2,818,177 | 0.49 | 0.49 | Worsened | 5,276,558 | 5,275,247 | 1,311 | 0.00% | 26 |
| Cost for instructors for a session | yen/ session | 2,400 | 3,600 | −2,818,833 | −2,818,833 | 0.49 | 0.49 | Improved | 5,275,903 | 5,275,903 | 0 | 0.00% | 27 |
| Number of instructors for a session | instructors/session | 4 | 6 | −2,818,833 | −2,818,833 | 0.49 | 0.49 | Improved | 5,275,903 | 5,275,903 | 0 | 0.00% | 28 |
| Numbers of sessions held in a year | sessions/year | 32 | 48 | −2,818,833 | −2,818,833 | 0.49 | 0.49 | Improved | 5,275,903 | 5,275,903 | 0 | 0.00% | 29 |
Probabilistic sensitivity analysis
Figure 3 shows the scatter plots and the 95% confidence ellipse from the results of the Monte Carlo probabilistic simulation for 1000 individuals and microsimulation with 1000 trials. No plots (0%) were located in the upper area of the WTP line; a large area of the 95% confidence ellipse was under the WTP line. The mean (95% confidence interval) of the simulated ICER was –6,874,807 (–14,075,713 and –3,729,394) yen/QALY.
Fig. 3.
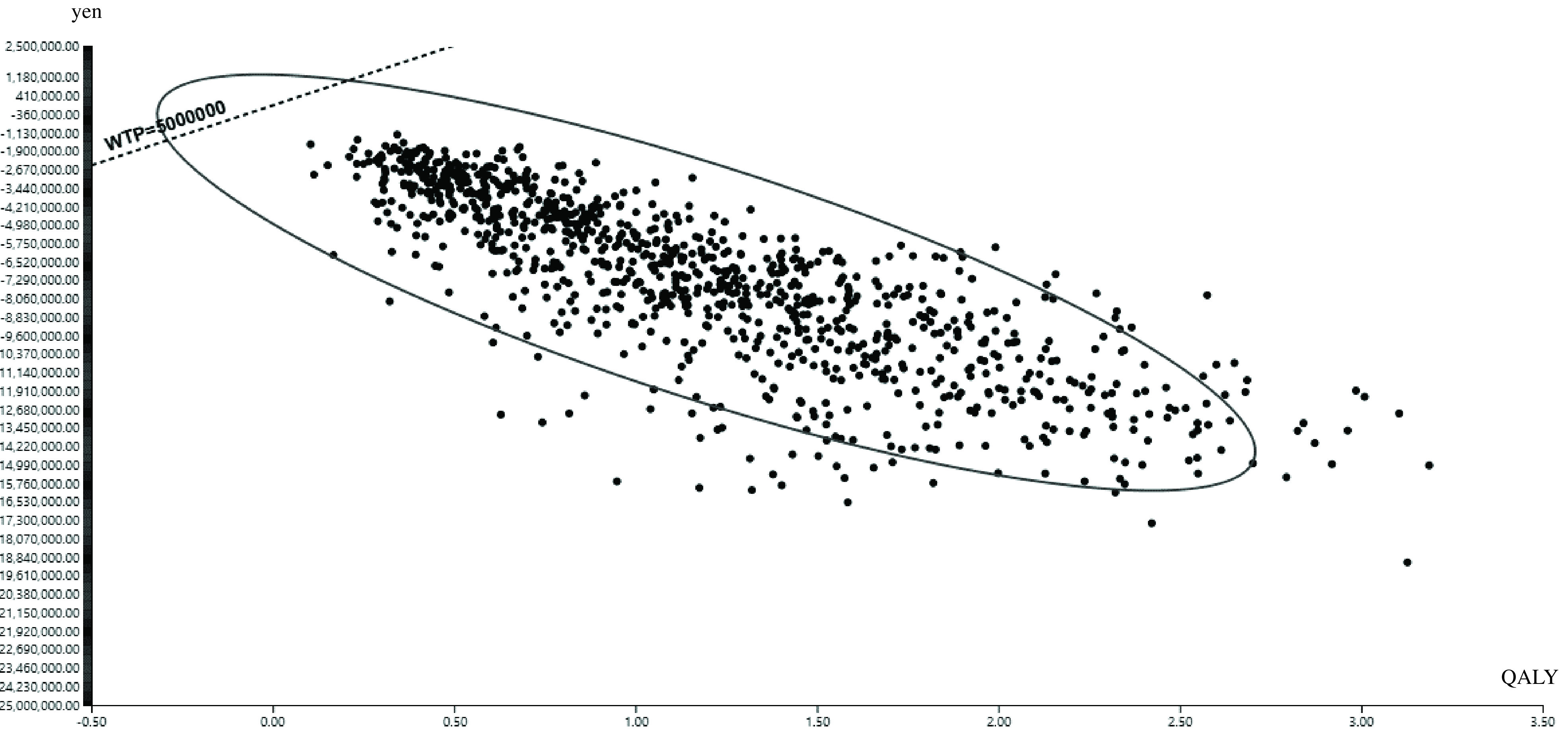
Scatter plots and 95% confidence eclipse from the results of the Monte Carlo probabilistic simulation for 1000 individuals and microsimulation with 1000 trials
QALY, quality adjusted life years; WTP, willingness to pay
Discussion
This study attempted to estimate the cost-effectiveness of combined physical and cognitive programs designed to prevent community-dwelling healthy young-old adults from developing dementia using a Markov cohort modeling simulation.
The Markov cohort modeling revealed that the base case of the ICER for 10 years was less than 5,000,000 yen/QALY, and furthermore, the value was negative. This supports the idea that introducing prevention programs might have much better cost-effectiveness than that of nonintervention from a public healthcare and long-term care payer’s perspective. Furthermore, the INMB showed a positive value of 5,275,903 yen; this indicates that the prevention program was also cost-effective from a restricted societal perspective.
A previous study revealed that dementia prevention intervention was cost-effective over a short period40), whereas our study suggested that the program might be cost-effective over longer periods.
Although our study showed good cost-effectiveness, a previous study41) that investigated the cost-effectiveness of an exercise program for older adults who had already developed mild to moderate dementia reported that the program was not cost-effective. Two reasons might be considered for this. First, adults who develop dementia lose their chance to recover to a healthy state, while adults with MCI still have a chance to revert to a healthy state. Therefore, older adults who receive a preventive exercise program have a better chance of improving their health and saving expenses for their future healthcare and long-term care. Our study results (Fig. 2) showed a trend of the numbers in the well state in the IG, demonstrating a lower negative slope than that of the NIG. Moreover, the trends in the number of states concerning dementia showed horizontal slopes in comparison with the NIG, which had positive slopes. As such, the preventive exercise program for older adults in healthy and MCI states might result in more health-related utility gain and a reduction in expenses concerning the development of dementia than nonintervention. In other words, the suggested introduction of preventive programs to young-old adults in a healthy state or MCI state might not only be effective7), but also economically beneficial for the health system and in the long term for Japan. Second, an intervention program for adults with dementia might cost more than a preventive program. Therefore, the program for adults with dementia would need to provide an individually tailored frequent exercise program, with 20–30 minutes of exercise at least five times per week42); our proposed program could be provided collectively and less frequently, at most once per week. As our results indicate, the costs for the preventive programs showed the lowest impact, 0% of the size of the impacts on the INMB (Table 3). Hence, our study supported the idea that collective exercise for dementia prevention programs represented a lower cost43,44) than individual exercise programs for adults with dementia41).
According to the results of the one-way sensitivity analysis, there was no parameter range indicating the effect of ICER values exceeding 5,000,000 yen/QALY. Furthermore, the probabilistic sensitivity analysis showed that no simulated values (0%) were over 5,000,000 yen/QALY; certainly, the mean value and the range were not over 5,000,000 yen/QALY. Combined, the sensitivity analysis suggested that the base case had high robustness.
To consider the most cost-effective way to deliver a preventive program to the community, interpreting the results of the one-way sensitivity analysis might be useful. First, the transition probability from well to dementia is worth paying attention to because of its notable impact on INMB. The results indicate that a higher transition probability from well to dementia improved INMB. This suggests that early effective screening to find community-dwelling young-old adults who might have a high risk of developing dementia might contribute to the impactive improvement of the cost-effectiveness of the preventive exercise program. Second, the transition probability from well to MCI might be worth paying attention to because the results indicate that a higher transition probability from well to MCI worsened INMB. Thus far, there has been little evidence that older adults can be prevented from developing dementia without diet changes, exercise, and cognitive stimulation8). Hence, our study suggested that early intervention, including exercise programs, such as the combined physical and cognitive exercises programs designed for preventing dementia in community-dwelling healthy young-old adults before they progress to the MCI state, might increase the cost-effectiveness in the community from a public healthcare and long-term care perspective.
Limitations
This study has three main limitations. First, we classified the severity of dementia into care needs levels of long-term care with specialist opinion. Since the classification system has been revised, it was not possible to make a simple comparison between a previous study16) and our study; however, our classification had the possibility of overestimating the cost of long-term care services. The one-way sensitivity analysis confirmed that when the costs related to long-term care services decreased, the INMB value became small, which meant that the cost-effectiveness worsened. However, even if the cost for a stay in a community-based facility for dementia service, which was the most expensive and had the largest impact on INMB value among long-term care services, decreased in the range of 20%, the INMB value was still 5,040,968 yen, and the impact was as small as 0.04% (Table 3). Our conclusion therefore might not be significantly changed. Further research is needed to clarify this.
Second, proxy-rated utility instead of self-rated utility was used to define the effectiveness values. The self-rated values among people with dementia were reported to be higher than the proxy-rated values27). According to our one-way sensitivity analysis, parameters related to utilities of the state of dementia induced a worsening impact on INMB, which indicate that the cost-effectiveness of the preventive program might be worsened. However, we found that the size of the impact was small, ranging from 0.16% to 0.39%; hence, our conclusion might not be changed even if the self-rated utility was used in the model.
Finally, this study used the data from Japan as a priority and was conducted with assumptions about the background of Japanese healthcare and the long-term care system. Other international perspectives might be necessary to adapt our results to other countries.
Conclusion
The present results indicate that the combined physical and cognitive programs designed to prevent community-dwelling healthy young-old adults from developing dementia might have good cost-effectiveness with high robustness.
Acknowledgments
The work was funded by Health, Labour and Welfare Policy Research Grants (20CA20) by Ministry of Health, Labour and Welfare of Japan.
Conflict of Interest
The author reports no conflicts of interest in this work.
References
- 1). Beard JR, Officer AM, et al. : The World Report on Ageing and Health. Gerontologist. 2016; 56: S163– S166. [DOI] [PubMed] [Google Scholar]
- 2).Cabinet Office, Government of Japan [Internet] . Annual Report on the Ageing Society. [Summary] FY 2020 [cited 2021 Oct 1]. Available from: https://www8.cao.go.jp/kourei/english/annualreport/2020/pdf/2020.pdf (in Japanese)
- 3). Makizako H, Shimada H, et al. : Physical frailty and future costs of long-term care in older adults: results from the NCGG-SGS. Gerontology. 2021; 67: 695– 704. [DOI] [PubMed] [Google Scholar]
- 4). Okamoto Y: Health care for the elderly in Japan: medicine and welfare in an aging society facing a crisis in long term care. BMJ. 1992; 305: 403– 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Sado M, Ninomiya A, et al. : The estimated cost of dementia in Japan, the most aged society in the world. PLoS One. 2018; 13: e0206508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Toman J, Klímová B, et al. : Multidomain lifestyle intervention strategies for the delay of cognitive impairment in healthy aging. Nutrients. 2018; 10: 1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Shimada H, Makizako H, et al. : Conversion and reversion rates in Japanese older people with mild cognitive impairment. J Am Med Dir Assoc. 2017; 18: 808.e1– 808.e6. [DOI] [PubMed] [Google Scholar]
- 8). Sanford AM: Mild cognitive impairment . Clin Geriatr Med. 2017; 33: 325– 337. [DOI] [PubMed] [Google Scholar]
- 9). Jongsiriyanyong S, Limpawattana P: Mild cognitive impairment in clinical practice: a review article. Am J Alzheimers Dis Other Demen. 2018; 33: 500– 507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Suzuki T, Shimada H, et al. : A randomized controlled trial of multicomponent exercise in older adults with mild cognitive impairment. PloS One. 2013; 8: e61483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Shimada H, Makizako H, et al. : Effects of combined physical and cognitive exercises on cognition and mobility in patients with mild cognitive impairment: a randomized clinical trial. J Am Med Dir Assoc. 2018; 19: 584– 591. [DOI] [PubMed] [Google Scholar]
- 12). Sonnenberg FA, Beck JR: Markov models in medical decision making: a practical guide. Med Decis Making. 1993; 13: 322– 338. [DOI] [PubMed] [Google Scholar]
- 13). Stollenwerk B, Bartmus T, et al. : Cost-effectiveness of hip protector use on a geriatric ward in Germany: a Markov model. Osteoporos Int. 2015; 26: 1367– 1379. [DOI] [PubMed] [Google Scholar]
- 14). Kato G, Kurachi Y: Cost-effective analysis of exercise programs designed for fall prevention among healthy younger old community-dwelling adults. Physical Therapy Japan. 2020; 47: 420– 430. (in Japanese) [Google Scholar]
- 15). Handels R, Wimo A: Challenges and recommendations for the health-economic evaluation of primary prevention programmes for dementia. Aging Ment Health. 2019; 23: 53– 59. [DOI] [PubMed] [Google Scholar]
- 16). Ikeda S, Yamada Y, et al. : Economic evaluation of Donepezil treatment for Alzheimer’s disease in Japan. Iryo To Shakai. 2000; 10: 27– 38. (in Japanese) [DOI] [PubMed] [Google Scholar]
- 17). Shiroiwa T, Fukuda T, et al. : Development of an official guideline for the economic evaluation of drugs/medical devices in Japan. Value Health. 2017; 20: 372– 378. [DOI] [PubMed] [Google Scholar]
- 18). Ninomiya, T[Internet] . Research on future estimation of the elderly population with dementia in Japan 2014 Summary / Shared Research Report (Nihonniokeru ninchishono koreishajinkono shoraisuikeinikansurukenkyu heisei26nenndo soukatu · bunntankenkyu hokokusho). [cited 2021 Jul 5]. Available from: https://mhlw-grants.niph.go.jp/system/files/2014/141031/201405037A/201405037A0001.pdf (in Japanese)
- 19). Asada T[Internet] . Prevalence of dementia in urban areas and coping with dementia living dysfunction (Toshibuniokeru ninnchishoyubyorituto ninnchishono seikatukinoshogaiheno taio). [cited 2021 Jul 5]. Available from: http://www.tsukuba-psychiatry.com/wp-content/uploads/2013/06/H24Report_Part1.pdf (in Japanese) [Google Scholar]
- 20). July J, Pranata R: Prevalence of dementia and its impact on mortality in patients with coronavirus disease 2019: a systematic review and meta-analysis. Geriatr Gerontol Int. 2021; 21: 172– 177. [DOI] [PubMed] [Google Scholar]
- 21). Blondell SJ, Hammersley-Mather R, et al. : Does physical activity prevent cognitive decline and dementia? a systematic review and meta-analysis of longitudinal studies. BMC Public Health. 2014; 14: 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Landeiro F, Mughal S, et al. : Health-related quality of life in people with predementia Alzheimer’s disease, mild cognitive impairment or dementia measured with preference-based instruments: a systematic literature review. Alzheimers Res Ther. 2020; 12: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Ministry of Health, Labour and Welfare[Internet] . Basic statistical survey of wage structure in FY2020 (Reiwagannnenn chinnginn kozo kihonn tokei chosa). [cited 2021 Jul 5]. Available from: https://www.e-stat.go.jp/stat-search/file-download?statInfId=000031919933&fileKind=0 (in Japanese)
- 24). Obu city [Internet] . Cost for a public space of Obu (Obu kominkan shiyo ryo) [cited 2021 July 5]. Available from: https://www.city.obu.aichi.jp/bunka/manabi/kouminkan_shisetsu/1006956/1006958.html (in Japanese)
- 25). Ministry of Health, Labour and Welfare [Internet] . Overview of actual statistics of long-term care benefit costs in FY2018 (Heisei30nenndo kaigokyufu tojittai tokei no gaikyo) [cited 2021 July 5]. Available from: https://www.mhlw.go.jp/toukei/saikin/hw/kaigo/kyufu/18/dl/03.pdf (in Japanese)
- 26). Tomata Y, Tsuji I, et al. : Prediction of future cost savings in long-term care and medical care if Japan achieves the health expectancy target of Health Japan 21 (second term). Nihon Koshu Eisei Zasshi (JAPANESE JOURNAL OF PUBLIC HEALTH). 2014; 61: 679– 685. (in Japanese) [PubMed] [Google Scholar]
- 27). Shiroiwa T, Sung Y-K, et al. : International survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ. 2010; 19: 422– 437. [DOI] [PubMed] [Google Scholar]
- 28). Gray AM, Clarke PM, et al. : Applied Methods of Cost-effectiveness Analysis in Healthcare. Oxford University Press, Oxford, 2010, pp. 282– 283. [Google Scholar]
- 29). Iwagami M, Tamiya N: The long-term care insurance system in Japan: past, present, and future. JMA J. 2019; 2: 67– 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Gidwani R, Russell LB: Estimating transition probabilities from published evidence: a tutorial for decision modelers. Pharmacoeconomics. 2020; 38: 1153– 1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Ministry of Health, Labour and Welfare[Internet] . Overview of the simple life table for the first year of Reiwa (Ryō wa gan’nen kan’i seimei-hyō no gaikyō) [cited 2021 Jul 5]. Available from: https://www.mhlw.go.jp/toukei/saikin/hw/life/life19/index.html (in Japanese)
- 32). Sullivan PW, Slejko JF, et al. : Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making. 2011; 31: 800– 804. [DOI] [PubMed] [Google Scholar]
- 33). Heßmann P, Seeberg G, et al. : Health-related quality of life in patients with Alzheimer’s disease in different German Health Care Settings. J Alzheimers Dis. 2016; 51: 545– 561. [DOI] [PubMed] [Google Scholar]
- 34). Doi T, Shimada H, et al. : Multicomponent exercise to improve cognition in older adults with mild cognitive impairment. Research in Exercise Epidemiology. 2017; 19: 102– 109. (in Japanese) [Google Scholar]
- 35). Folstein MF, Folstein SE, et al. : “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975; 12: 189– 198. [DOI] [PubMed] [Google Scholar]
- 36). Rosen WG, Mohs RC, et al. : A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984; 141: 1356– 1364. [DOI] [PubMed] [Google Scholar]
- 37). Elwood RW: The Wechsler Memory Scale-Revised: psychometric characteristics and clinical application. Neuropsychol Rev. 1991; 2: 179– 201. [DOI] [PubMed] [Google Scholar]
- 38). Gulliford M, Charlton J, et al. : Social and material deprivation and the cost-effectiveness of an intervention to promote physical activity: cohort study and Markov model. J Public Health (Oxf). 2014; 36: 674– 683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39). Vreman RA, Geenen JW, et al. : The application and implications of novel deterministic sensitivity analysis methods. Pharmaco Economics. 2021; 39: 1– 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). McRae I, Zheng L, et al. : Cost-effectiveness of dementia prevention interventions. J Prev Alzheimers Dis. 2021; 8: 210– 217. [DOI] [PubMed] [Google Scholar]
- 41). Khan I, Petrou S, et al. : Does structured exercise improve cognitive impairment in people with mild to moderate Dementia? a cost-effectiveness analysis from a confirmatory randomised controlled trial: the Dementia and Physical Activity (DAPA) trial. Pharmacoecon Open. 2019; 3: 215– 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). D’Amico F, Rehill A, et al. : Cost-effectiveness of exercise as a therapy for behavioural and psychological symptoms of dementia within the EVIDEM-E randomised controlled trial. Int J Geriatr Psychiatry. 2016; 31: 656– 665. [DOI] [PubMed] [Google Scholar]
- 43). Blanchet S, Chikhi S, et al. : The benefits of physical activities on cognitive and mental health in healthy and pathological aging. Geriatr Psychol Neuropsychiatr Vieil. 2018; 16: 197– 205. [DOI] [PubMed] [Google Scholar]
- 44). Kramer A: An overview of the beneficial effects of exercise on health and performance. Adv Exp Med Biol. 2020; 1228: 3– 22. [DOI] [PubMed] [Google Scholar]