Abstract
Vibrio parahaemolyticus possesses two types of flagella, polar and lateral, powered by distinct energy sources, which are derived from the sodium and proton motive forces, respectively. Although proton-powered flagella in Escherichia coli and Salmonella enterica serovar Typhimurium have been extensively studied, the mechanism of torque generation is still not understood. Molecular knowledge of the structure of the sodium-driven motor is only now being developed. In this work, we identify the switch components, FliG, FliM, and FliN, of the sodium-type motor. This brings the total number of genes identified as pertinent to polar motor function to seven. Both FliM and FliN possess charged domains not found in proton-type homologs; however, they can interact with the proton-type motor of E. coli to a limited extent. Residues known to be critical for torque generation in the proton-type motor are conserved in the sodium-type motor, suggesting a common mechanism for energy transfer at the rotor-stator interface regardless of the driving force powering rotation. Mutants representing a complete panel of insertionally inactivated switch and motor genes were constructed. All of these mutants were defective in sodium-driven swimming motility. Alkaline phosphatase could be fused to the C termini of MotB and MotY without abolishing motility, whereas deletion of the unusual, highly charged C-terminal domain of FliM disrupted motor function. All of the mutants retained proton-driven, lateral motility over surfaces. Thus, although central chemotaxis genes are shared by the polar and lateral systems, genes encoding the switch components, as well as the motor genes, are distinct for each motility system.
Vibrio parahaemolyticus is an organism with two distinct motility systems, adapted for life in different circumstances (42). The polar flagellar system (Fla) propels the bacterium in liquid environments (swimming motility), and the lateral flagellar (Laf) system moves the bacterium through viscous environments and over surfaces (swarming motility). The polar flagellar filament of the swimmer cell comprises multiple flagellin subunits and is sheathed by what appears, in the electron microscope, to be an extension of the cell outer membrane (41). This flagellum is produced constitutively, i.e., it is found on liquid- and surface-grown bacteria. In liquid medium, the rotating flagellum can propel the bacterium at speeds as fast as 60 μm per s (3). Although an effective propulsive organelle in dilute liquid environments, the polar flagellum of V. parahaemolyticus does not work well in viscous layers. Growth on surfaces or in viscous environments leads to induction of the alternate motility system and elaboration of numerous peritrichous flagella.
It is hypothesized that the polar flagellum serves not only as a propulsive organelle but also as a tactile sensor, informing the bacterium of contact with surfaces or viscous environments. Conditions that impede rotation of the polar flagellum lead to induction of the second motility system (38). Lateral flagella are produced solely under conditions that restrict the function of the polar flagellum. The lateral filaments are unsheathed and polymerized from a single flagellin subunit, which is distinct from the polar flagellins (44). Lateral flagella enable the bacterium to move over and colonize surfaces or viscous layers.
Energy to drive flagellar rotation is derived from the transmembrane electrochemical potential of specific ions (20, 28, 36). Rotation of the flagellum appears tightly coupled to the flow of ions through the motor (45). Two kinds of motors, which are dependent on different coupling ions, have been described: H+- and Na+-driven motors. In V. parahaemolyticus, the proton motive force powers the lateral flagella, whereas the sodium motive force drives polar flagellar rotation (3).
Proton-type motors of Escherichia coli and Salmonella enterica serovar Typhimurium have been extensively characterized (reviewed in references 5, 6, 13, and 32). The stator of this motor comprises two cytoplasmic membrane proteins, MotA and MotB (11, 54). Together, these proteins possess five transmembrane domains, which form a proton-conducting channel (7, 8, 51, 55, 66, 68). MotB contains a C-terminal domain that may fasten the MotA-MotB complex to peptidoglycan (10, 12). Multiple MotA-MotB torque-generating units surround the flagellar basal body (25). Torque is transmitted from the MotA-MotB stator to the rotor. Although the mechanism of torque generation is not understood, electrostatic interactions between specific, charged residues in MotA and FliG have been demonstrated (29, 30, 67). FliG is found at the base of the flagellar basal body in the switch complex with FliM and FliN (15, 46). This complex is essential for torque generation, flagellar assembly, and control of the direction of flagellar rotation (21, 53, 56, 57, 61, 64, 65).
The architecture of sodium-type motors of two marine Vibrio species, V. parahaemolyticus and V. alginolyticus, is currently being dissected. Four components of the stator have been described: MotX, MotY, MotA, and MotB. Transposon insertions in motX or motY of V. parahaemolyticus produce flagellated but nonmotile bacteria (39, 40). Other paralyzed mutants, which were generated by chemical mutagenesis, were isolated in V. alginolyticus. The lesions identified a new locus containing two genes, designated pomA and pomB (2). For V. parahaemolyticus, a similar locus was discovered and designated motAB (GenBank accession no., AF069391) (22). The proteins derived from these two loci are greater than 96% identical. With respect to function and predicted protein topology, the Vibrio proteins resemble MotA and MotB of the proton-type motor, whereas MotX and MotY are unique to the sodium-type motor. The existence of mutants with alterations in motA or motB conferring resistance to sodium channel inhibitors that block motility provides strong evidence for roles for both of these proteins in Na+ translocation (22, 26). MotY possesses a potentially extensive C-terminal peptidoglycan interaction domain (39). The function of MotX is a mystery. The switch components of the sodium-type motor, although presumed to exist, have not been identified until now.
Thus, the lateral and polar flagellar filaments are different, and the torque-generating units are distinct; however, a common chemotaxis system directs both forms of motility. Defects in chemotaxis genes affect both swimming and swarming motility (48). At times the bacterium elaborates both forms of flagella. Chemotaxis, specifically the modulation of direction of flagellar rotation, is effected through interaction of chemotaxis proteins with the switch complex. In E. coli and many other bacteria, phosphorylated CheY interacts with FliM (53, 60, 61, 62). What determines specificity with respect to assembly, function, and coordination of movement has not been determined, i.e., it is not known at what level divergence between the two motility systems occurs. This work describes the isolation and characterization of three newly identified polar flagellar switch genes, fliG, fliM, and fliN. Although mutations causing altered V. parahaemolyticus MotA or MotB function have been previously described (22), insertional inactivation of these genes had not been performed. Therefore, a complete panel of loss-of-function mutants, with insertions in each of the known switch and motor genes, was constructed to probe motor function and specificity of the gene products with respect to swimming and swarming motility.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains and plasmids used in this work are described in Table 1. Vibrio strains were cultured at 30°C. All V. parahaemolyticus strains were derived from the wild-type strain BB22 (4). Strain LM1017 contains a mutation in the lateral flagellar hook gene and is unable to swarm (44). E. coli flagellar strains were derived from DFB9 and were provided by David Blair. HI broth contained 25 g of heart infusion broth (Difco) and 20 g of NaCl per liter. Solidified swarming medium was prepared by adding 15 g of Bacto agar (Difco) per liter to HI broth. Semisolid motility medium (M agar) contained 10 g of tryptone and 3.25 g of agar per liter; the medium also contained 20 g of NaCl/liter for Vibrio and 10 g of NaCl/liter for E. coli.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Genotype or description | Source and/or parent; reference |
|---|---|---|
| Strains | ||
| V. parahaemolyticus | ||
| BB22 | Wild type | R. Belas; 4 |
| LM1017 | lfgE313::lux | BB22; 44 |
| LM4170 | motX118 lfgE313::lux | LM1017; 40 |
| LM4171 | motY141::mini-Mu lac lfgE313::lux | LM1017; 39 |
| LM4262 | motX1699::TnphoA | BB22; 40 |
| LM4289 | motY1719::TnphoA lfgE313::lux | LM1017; 39 |
| LM4474 | motY141::mini-Mu lac | LM4171; this work |
| LM4652 | motA2::TnphoA | BB22; this work |
| LM4653 | motA3::TnphoA | BB22; this work |
| LM4654 | motB2::TnphoA | BB22; this work |
| LM4655 | motA1::TnphoA | BB22; this work |
| LM4656 | motB1::TnphoA | BB22; this work |
| LM4657 | motA2::TnphoA lfgE313::lux | LM1017; this work |
| LM4658 | motA3::TnphoA lfgE313::lux | LM1017; this work |
| LM4659 | motB2::TnphoA lfgE313::lux | LM1017; this work |
| LM4660 | motA1::TnphoA lfgE313::lux | LM1017; this work |
| LM4661 | motB1::TnphoA lfgE313::lux | LM1017; this work |
| LM4806 | fliG1::TnlacZ/in | BB22; this work |
| LM4807 | fliG1::TnlacZ/in lfgE313::lux | LM1017; this work |
| LM4808 | fliG2::TnlacZ/in | BB22; this work |
| LM4809 | fliG2::TnlacZ/in lfgE313::lux | LM1017; this work |
| LM4810 | fliG3::TnlacZ/in | BB22; this work |
| LM4811 | fliG3::TnlacZ/in lfgE313::lux | LM1017; this work |
| LM4812 | fliN1::TnlacZ/in lfgE313::lux | LM1017; this work |
| LM4813 | fliM2::TnlacZ/in | BB22; this work |
| LM4814 | fliM2::TnlacZ/in lfgE313::lux | LM1017; this work |
| LM4815 | fliM1::TnlacZ/in lfgE313::lux | LM1017; this work |
| LM4889 | fliM1::TnlacZ/in | BB22; this work |
| LM4890 | fliN1::TnlacZ/in | BB22; this work |
| E. coli | ||
| DFB9 | thr(Am)1 leuB6 his4 metF(Am)159 thi1 rpsL136 Sm(R) lacY1 ara14 xyl5 mtl1 tsx78 tonA31 eda50 | D. Blair |
| DFB210 | ΔmotAB | DFB9, D. Blair |
| DFB223 | ΔfliN (in frame) | DFB9, D. Blair |
| DFB225 | ΔfliG (in frame) | DFB9, D. Blair |
| DFB228 | ΔfliM (in frame) | DFB9, D. Blair |
| DFB232 | ΔfliMN (in frame) | DFB9, D. Blair |
| Plasmids | ||
| pBBR1MCS | Camr; broad-host-range vector | M. Kovach; 27 |
| pLAFRII | Tetr; broad-host-range cosmid vector | 16 |
| pMMB66EH | Apr; broad-host-range expression vector carrying lacIq and Ptac | 17 |
| pRK415 | Tetr; broad-host-range vector | 24 |
| pUCGM | Genr | H. Schweizer; 49 |
| pLM1778 | Tetr; pLAFRII-derived cosmid carrying lfgE locus | This work |
| pLM1796 | Tetr; pLAFRII-derived cosmid carrying lafTU locus | 44 |
| pLM1877 | Genr Apr; broad-host-range expression vector | pMMB66EH; this work |
| pLM2058 | Tetr;pLAFRII-derived cosmid carrying motAB locus | 22 |
| pLM2047 | Tetr; pLAFRII-derived cosmid carrying fliF locus | This work |
| pLM2192 | Tetr; fliG+H+ | pRK415 and pLM2047; this work |
| pLM2293 | Camr; fliL+M+N+O+P+ | pBBR1MCS and LM2047; this work |
| pLM2294 | Genr; fliM+N+ | pLM1877 and PCR product; this work |
| pLM2296 | Genr; fliM+ | pLM1877 and PCR product; this work |
| pLM2297 | Genr; fliMshort | pLM1877 and PCR product; this work |
Mutant isolation.
Isolation of mini-Mu mutant strain ML199, which is nonmotile and which produces no flagella, has been described previously (38). Specific mutations were created on plasmids in E. coli and then transferred to V. parahaemolyticus by allelic replacement. Plasmids were mutagenized with λTnphoA (35) or λTnlacZ/in (34). The precise point of insertion was defined by DNA sequencing. The procedures for conjugation and gene replacement in V. parahaemolyticus have been described elsewhere (52). General DNA manipulations were adapted from the methods of Sambrook et al. (47). All strain constructions were confirmed by Southern blot analysis of restricted chromosomal DNA. Chromosomal DNA was prepared according to the protocol of Woo et al. (63).
Retrieval of clones and plasmid construction.
The fliF locus was identified by cloning a tetracycline-resistant transposon from the mini-Mu-induced Fla− mutant strain ML199 according to procedures described previously (52). The segment of chromosomal DNA contiguous with the transposon was sequenced and then used as a probe to retrieve cosmid pLM2047 from a V. parahaemolyticus library constructed using DNA prepared from strain BB22. Because cosmid pLM2047 contains more than 25 kb of V. parahaemolyticus DNA, the switch genes were subcloned for mutagenesis. For product identification and complementation, the switch genes were amplified by high-fidelity PCR (Boehringer Mannheim) and cloned into the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible expression vector pLM1877. Gentamicin-resistant plasmid pLM1877 is a broad-host-range, low-copy-number vector that was derived from vector pMMB66EH (17). PCR-generated clones were sequenced to verify fidelity.
E. coli minicell system.
Minicells were made from strain P678-54 as described by Engebrecht and Silverman (14). Plasmids were introduced by transformation. Minicells were prepared from 400 ml of an overnight culture grown in Luria broth with the appropriate antibiotic. The final pellet of purified minicells was suspended in 0.5 ml. Minicells (0.25 ml) were incubated for 20 min at 37°C, and then 20 μCi (1 Ci = 38.7 GBq) of [35S]methionine with a specific activity of ∼1,000 Ci/mmol (Amersham Life Sciences) was added. After continued incubation for 20 min, cells were pelleted and suspended in electrophoresis sample buffer. Details of Tris-glycine sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) have been described previously (43). Fixed and stained gels were incubated with Amplify reagent (Amersham) before drying and autoradiography. The resolving gel was 10.5% acrylamide. Broad-range SDS-PAGE protein standards were purchased from Bio-Rad (Hercules, Calif.).
Immunoblot analysis.
SDS-PAGE was conducted as described above. Resolving gels contained 12% acrylamide. Gels were transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore Corp.) in transfer buffer containing 12.5 mM Tris base, 96 mM glycine, and 20% methanol for 90 min at 30 V. After being blocked in TBST buffer (10 mM Tris-Cl [pH 8], 0.15 M NaCl, 0.05% Tween 20) containing 5% nonfat dry milk, blots were incubated in TBST buffer with pooled antiflagellin (polar and lateral) antibodies. Production of antibodies to polar and lateral flagellins has been described previously (41, 44). The secondary antibody was anti-rabbit immunoglobulin conjugated to horseradish peroxidase (Amersham Life Sciences). It was incubated with the blot at a dilution of 1:20,000 in TBST for 1 h. Development of the immunoblot utilized the chemiluminescent Super Signal substrate (Pierce) according to the manufacturer's instructions.
Motility assays and flagellum preparation.
The effect of mutations on swimming motility was assessed by examining movement in M agar. Swarming motility was examined after inoculation on the surface of solidified swarming medium and overnight incubation. To examine swimming motility, plates were inoculated with 2 μl of cells normalized to an optical density at 600 nm of 2.0. Plates were incubated for the times indicated in legends to Fig. 4 to 6 and then refrigerated until photographed using a Kodak digital imaging system. Rates of radial expansion (in millimeters per hour) were determined in triplicate by measuring the diameter of expansion as a function of time. The slope was determined, and only lines with an R2 value greater than 0.9 were used to calculate rates. All expansion rates were normalized to the rate of control strain LM1017, which was inoculated on the same plate. Flagella were isolated after shearing in a Virtis homogenizer, as described earlier (38).
FIG. 4.
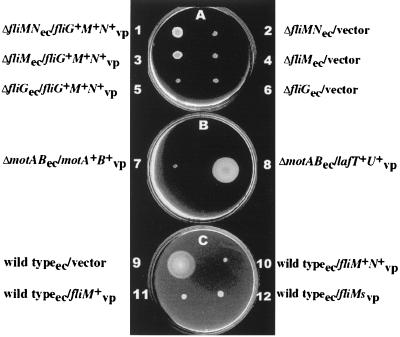
Complementation experiments of E. coli (ec) proton-type motor and switch mutants with V. parahaemolyticus (vp) sodium-type genes. Strains: 1, DFB232 (ΔfliMN)/pLM2047 (containing V. parahaemolyticus fliF locus); 2, DFB232/pLAFRII (parental vector control); 3, DFB228 (ΔfliM)/pLM2047; 4, DFB228/pLAFRII; 5, DFB225 (ΔfliG)/pLM2047; 6, DFB225/pLAFRII; 7, DFB210 (ΔmotAB)/pLM2058 (containing V. parahaemolyticus motAB); 8, DFB210/pLM1796 (containing the V. parahaemolyticus lafTU locus, which contains the lateral, proton-type motor genes); 9, DFB9 (wild type)/pLM1877; 10, DFB9/pLM2294; 11, DFB9/pLM2296; 12, DFB9/pLM2297. Plates A to C were incubated at 37°C for 60, 48, and 8 h, respectively. M agar in plates A and B contained 10 μg of tetracycline/ml for maintenance of the plasmids. M agar in plate C contained 40 μg of gentamicin/ml and 0.5 mM IPTG for induction of transcription of the fli genes contained on the expression vector pLM1877. fliMs, fliMshort.
FIG. 6.
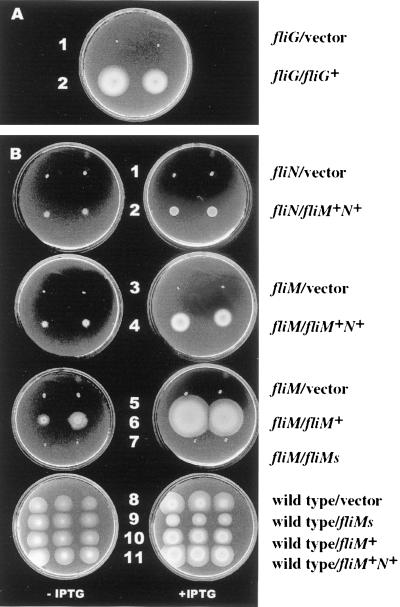
Complementation of swimming motility defects in V. parahaemolyticus switch mutants with plasmids carrying V. parahaemolyticus switch genes. (A) Strains: 1, LM4811 (fliG)/pRK415 (vector); 2, LM4811 (fliG)/pLM2192 (fliG+H+). M agar was supplemented with 10 μg of chloramphenicol and 10 μg of tetracycline/ml. The plate was incubated for 16 h at 30°C. (B) Strains: 1, LM4812 (fliN)/pLM1877 (vector); 2, LM4812 (fliN)/pLM2294 (fliM+N+); 3, LM4815 (fliM)/pLM1877; 4, LM4815 (fliM)/pLM2294 (fliM+N+); 5, LM4815 (fliM)/pLM1877; 6, LM4815 (fliM)/pLM2296 (fliM+); 7, LM4815 (fliM)/pLM2297 (fliMshort+); 8, LM1017/pLM1877 (vector); 9, LM1017/pLM2297 (fliMshort+); 10, LM1017/pLM2296 (fliM+); 11, LM1017/pLM2294 (fliM+N+). M agar was supplemented with 10 μg of chloramphenicol/ml, 40 μg of gentamicin/ml, and 0.5 mM IPTG as indicated. Plates, from top to bottom, were incubated at 30°C for 14, 14, 19, and 10 h, respectively.
Sequence analysis.
Sequence determination was performed by the DNA Core Facility of the University of Iowa. Sequence assembly was accomplished using the Genetics Computer Group (GCG) software package. Searches for homology were performed at the National Center for Biotechnology Information with the BLAST network service (1). Multiple sequence alignments were performed using the CLUSTAL W program (58).
Nucleotide sequence accession number.
The sequence obtained from clone pLM2047 for the fliF locus has been deposited with GenBank under accession no. AF069392.
RESULTS
Identification of genes encoding polar flagellar switch components.
Strain ML199 is nonmotile and fails to produce a polar flagellum due to an insertion of the transposon mini-Mu (Tetr). To determine the nature of the mutated gene, a restriction fragment containing part of the transposon encoding tetracycline resistance and the contiguous chromosomal DNA was cloned from strain ML199. Sequence analysis of the clone revealed that the transposon was inserted in a gene whose product was homologous to a protein involved in flagellar export, FlhA. Strain ML199 also contains a lux fusion in the lateral flagellar hook gene lfgE; thus it cannot swim or swarm. To test the effect of the flhA mutation on swarming, the lateral defect in ML199 was repaired using a cosmid carrying the lfgE locus. The resultant strain was competent for lateral flagellum production and swarming over solid surfaces but was unable to swim in semisolid motility medium (data not shown). Thus, the gene is pertinent to polar, but not lateral, flagellar assembly.
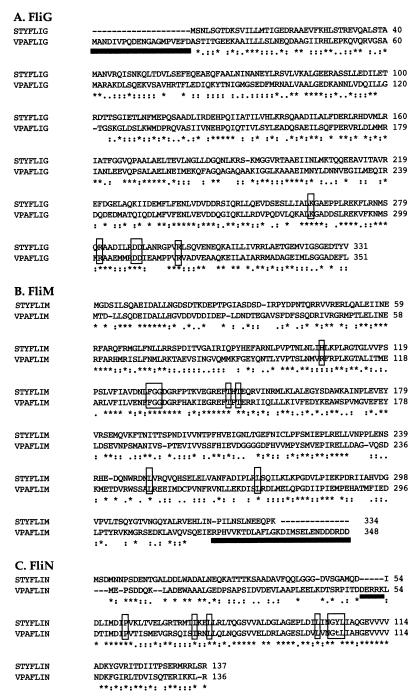
Sequencing upstream of the flhA gene revealed a large flagellar gene cluster, which included the three flagellar switch genes fliG, fliM, and fliN. The locus contains 15 potential flagellar genes. As depicted in Fig. 1, the genes are transcribed in the same direction and are tightly linked and the coding regions for many of the reading frames overlap with one another. The sodium-type switch components resemble their proton-type homologs. For comparison, alignments with the S. enterica serovar Typhimurium gene products are shown in Fig. 2. Although the V. parahaemolyticus FliG protein possesses 20 additional amino acids at its amino terminus, the FliG proteins from the two organisms align without the introduction of significant gaps and display 55% similarity and 40% identity on the basis of a GCG BestFit alignment. The highest similarity occurs in the C-terminal domain: amino acids beyond residue 200 display 45% identity with the S. enterica serovar Typhimurium C-terminal domain. A number of residues in the C-terminal domain have been identified by mutational analysis of E. coli and S. enterica serovar Typhimurium to be functionally important for motor function (19, 29, 37), and these amino acids are conserved in V. parahaemolyticus FliG. The specific charged residues that have been shown to be critical for torque generation in E. coli FliG (29) and the positionally identical residues in V. parahaemolyticus FliG are indicated in Fig. 2A.
FIG. 1.
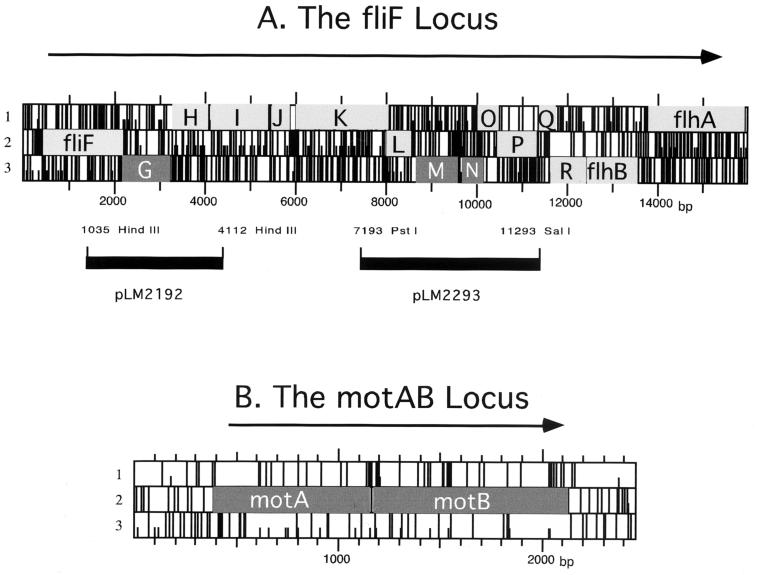
The fliF and motAB loci. The physical maps are derived from the nucleotide sequences. Gene designations, which are adopted from the closest E. coli homolog, are superimposed on the open reading frame (ORF) map; full bars indicate stop codons, and small bars indicate ATG codons. ORFs coding for switch and motor genes are dark. Arrows indicate the direction of transcription. The coding sequence upstream of fliF codes for a potential transcriptional regulator resembling E. coli GcvA. The sequence upstream of motA codes for a potential polypeptide with homology to the small subunit of E. coli exodeoxyribonuclease (type VII). The sequence downstream of motB codes for a potential homolog of S. enterica serovar Typhimurium ThiI. Regions containing the fliG and fliMN genes were subcloned using the restriction sites indicated to make plasmids pLM2192 and pLM2293.
FIG. 2.
Sequence alignment of sodium-type flagellar switch components of V. parahaemolyticus (VPA) with proton-type switch components of S. enterica serovar Typhimurium (STY). Amino acids are represented by the single-letter code. Gaps introduced to facilitate alignment are indicated by dashes. The consensus line below the sequence alignment indicates identity (*), strong conservation (:), and weak conservation (.) of amino acid matches. The open boxes outline conserved amino acids, and the lowercase letter indicates a nonconserved amino acid with respect to residues known to be critical for torque generation in S. enterica serovar Typhimurium or E. coli. Black bars underline the unusual domains of the V. parahaemolyticus proteins.
The predicted size of the fliM gene product is 348 residues. By using GCG BestFit analysis, it was found to be 52% similar and 37% identical with S. enterica serovar Typhimurium FliM over the first 320 amino acids. In particular, the N-terminal region, which for S. enterica serovar Typhimurium FliM has been shown to be critical for the interaction with phosphorylated CheY (61), is well conserved. Eight amino acid substitutions that produce motor-defective, i.e., paralyzed, flagella have been isolated in S. enterica serovar Typhimurium FliM (53). All of these amino acids are conserved between S. enterica serovar Typhimurium and V. parahaemolyticus (Fig. 1B). Seven of the eight are identical in the two organisms; the eighth is conserved with respect to charge (Arg in V. parahaemolyticus substituted for His in S. enterica serovar Typhimurium). In contrast to the conserved N-terminal region, the C terminus of the V. parahaemolyticus protein seems unusual. It is longer and more highly charged than any of the FliM sequences deposited in GenBank. The alignments of these proteins are without significant gaps over amino acids 1 through 320. A comparison of V. parahaemolyticus FliM with S. enterica serovar Typhimurium FliM is shown in Fig. 2B. Of the remaining 28 C-terminal amino acids beyond residue 320 of V. parahaemolyticus FliM, 14 possess charge, whereas only 3 of the remaining 12 C-terminal amino acids of S. enterica serovar Typhimurium are charged residues.
The predicted fliN gene product is 136 amino acids in length. It is 73% similar and 60% identical with S. enterica serovar Typhimurium FliN. Seven single-amino-acid substitutions that resulted in the paralyzed-Mot phenotype have been isolated within the S. enterica serovar Typhimurium gene (21). All of these residues (Fig. 2C) are identical between S. enterica serovar Typhimurium and V. parahaemolyticus except one (Thr substituted for Tyr). As was observed for E. coli (56), BLAST database searches with V. parahaemolyticus FliN reveal similarity with type III translocation proteins of the SpaO family (e.g., YopQ; GenBank accession no., 1176912). There is one significant gap in the alignment with S. enterica serovar Typhimurium FliN sequences. The predicted V. parahaemolyticus protein sequence possesses an insertion of five amino acids (Fig. 2C). All five of the inserted amino acids are charged residues.
Switch gene product identification.
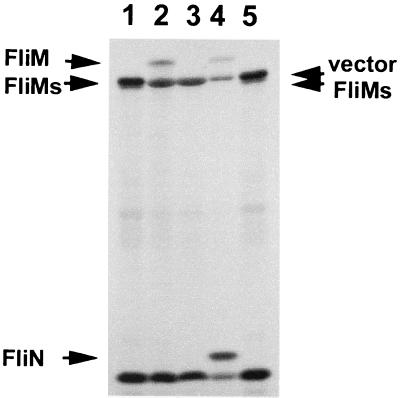
We were particularly interested in examining the protein product of fliM because of the unusual predicted C-terminal extension beyond residue 320. To do this, a plasmid was designed to encode a truncated FliM with 27 C-terminal amino acids deleted, FliMshort, which terminates at Arg-321. For S. enterica serovar Typhimurium, deletion of residues 321 to 334 produced a truncated FliM that fully supported motility (60). The E. coli minicell system was used to identify plasmid-encoded products. The plasmids contained coding sequence for FliM, FliN, and FliMshort, and the predicted molecular masses were 39,777, 14,964, and 36,709 Da, respectively. The autoradiogram of 35S-labeled proteins is shown in Fig. 3. The FliM and FliN proteins migrated with apparent molecular masses of 43,500 and 21,500 Da, respectively. Both FliM and FliN are acidic proteins with predicted pIs of ∼4.5, which may account for the apparently anomalous migration with respect to predicted mass in SDS-PAGE. FliMshort migrated similarly to a protein product produced by the vector with an apparent molecular mass of ∼41,200 Da.
FIG. 3.
FliM and FliN synthesis in minicells. Autoradiogram (12-h exposure) of 35S-labeled proteins synthesized in minicells containing plasmids. Lanes: 1, pLM2297 (fliMshort); 2, pLM2296 (fliM+); 3, pLM1877 (vector); 4, pLM2294 (fliM+N+); 5, pLM2297 (fliMshort). Arrows indicate polypeptides encoded by the fli genes and a vector-encoded product. The resolving gel contained 10.5% acrylamide. FliMs, the product of truncated fliMshort.
Complementation of proton-type defective motor and switch mutants with sodium-type genes.
The similarity between the sodium-type and proton-type switch components prompted investigation of protein function and interactions between heterologous motor parts. Clones containing V. parahaemolyticus motor and switch genes were transferred to E. coli strains with nonpolar defects in motor and switch genes. After extended incubation times, differences could be observed in M agar among E. coli strains DFB223 (ΔfliN), DFB228 (ΔfliM), and DFB232 (ΔfliMN) carrying the fliF locus on clone pLM2047 compared with those carrying a control vector. Figure 4A shows the effect of pLM2047 in DFB228 and DFB232 (strains 1 and 3 compared to strains 2 and 4), and the movement of strain DFB223 with pLM2047 was similar, but is not shown. Observation in the light microscope of strains with fliM and/or fliN defects and plasmid pLM2047 revealed cells that twitched or rotated in place without significant translocation. In contrast, no effect on motility could be observed in M agar or in the microscope for a strain with a fliG defect and pLM2047 (Fig. 4A, strain 5 compared to strain 6). Flagella could be isolated after shearing and high speed centrifugation for fliM or fliN, but not fliG, mutants carrying pLM2047. Immunoblots containing whole-cell samples probed with antiserum directed against E. coli flagellin revealed that E. coli fliM or fliN mutants containing cosmid pLM2047 produced approximately as much flagellin as a control E. coli strain that was wild type for motility, whereas no flagellin was detected in the fliG mutant containing pLM2047 (data not shown). Similarly, the polar mot gene products do not appear to compensate for motAB defects in E. coli: no motility was observed in M agar or in the microscope (Fig. 4B, strain 7). This was not an unexpected finding, for the degrees of similarity between the sodium-type MotA and MotB predicted polypeptides and the E. coli homologs were lower than those among the switch proteins. For comparison, complementation of the E. coli ΔmotAB strain with the lateral, proton-type homologs of V. parahaemolyticus is shown (Fig. 4B, strain 8).
Additional evidence supports the idea that certain proton- and sodium-type switch parts can interact, albeit not productively. The IPTG-inducible expression clones were introduced into motile strain DFB9. Induction of expression of V. parahaemolyticus fliM, fliMshort, or fliMN interfered with motility compared to what was found for DFB9 carrying the expression vector pLM1877 (Fig. 4C). Induction of expression had no effect on growth rates.
Transposon mutagenesis of switch and motor genes.
Insertion mutations were isolated in each of the V. parahaemolyticus switch and motor genes, i.e., fliG, fliM, fliN, motA, and motB. Previously, transposon insertions had been isolated in the other identified motor genes, motX and motY (39, 40). The motAB locus was mutagenized with the transposon TnphoA, which can serve as a probe for protein topology. Five insertions in motA or motB were analyzed. One in-frame fusion that produced a hybrid MotB-alkaline phosphatase protein was obtained. The fusion occurred at amino acid 308, which is very close to the end of the 316-amino-acid MotB polypeptide. The fliG subclone pLM2192 and fliMN subclone pLM2193 were mutagenized with a transposon suitable for isolating protein fusions to β-galactosidase, TnlacZ/in. The precise location of each insertion, defined by DNA sequencing, is provided in Table 2.
TABLE 2.
Insertion mutations in motor and switch genes
| Allele | Comments | Location of insertionb
|
|
|---|---|---|---|
| bp | aa | ||
| motA1::TnphoA | 576 | 58 | |
| motA2::TnphoA | 627 | 75 | |
| motA3::TnphoA | 1094 | 230 | |
| motB1::TnphoA | 2093 | 304 | |
| motB2::TnphoA | Blue fusiona, partial function | 2103 | 308 |
| fliG1::TnlacZ/in | Blue fusion | 2860 | 228 |
| fliG2::TnlacZ/in | Blue fusion | 2878 | 234 |
| fliG3::TnlacZ/in | 2536 | 120 | |
| fliM1::TnlacZ/in | 8613 | 23 | |
| fliM2::TnlacZ/in | Blue fusion | 9391 | 83 |
| fliN1::TnlacZ/in | Blue fusion | 9740 | 33 |
Blue fusion, insertion produced an active alkaline phosphatase or β-galactosidase fusion protein that was capable of converting the color of a chromogenic substrate to blue.
Location of insertion is given according to the base pairs (bp) on the physical map presented in Fig. 1, which corresponds to the sequence deposited in GenBank, and to the amino acid codon (aa) perturbed by the position of the transposon. The predicted total numbers of amino acids in the polypeptides encoded by each gene are 253 (motA), 316 (motB), 351 (fliG), 248, (fliM), and 136 (fliN).
Gene disruption via allelic replacement and effects on swimming.
The transposons in mot and fli genes were transferred to the chromosome of V. parahaemolyticus strain LM1017 via allelic exchange. Strain LM1017 contains a lfg::lux fusion in the lateral flagellar hook operon. Thus, movement of LM1017 in M agar is solely the result of propulsion by the polar motility system. Movement in M agar of a set of representative mutants is shown in Fig. 5. For comparison, the previously constructed motX and motY mutant strains are included. Insertions in motA, motB, motX, motY, fliG, fliM, or fliN caused complete loss of motility.
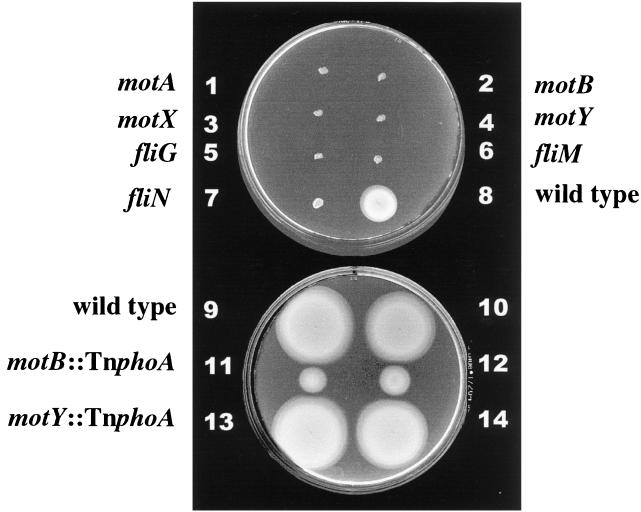
FIG. 5.
Swimming motility of V. parahaemolyticus mutant strains with flagellar switch or motor defects in M agar. Strains: 1, LM4657 (motA); 2, LM4661 (motB); 3, LM4170 (motX); 4, LM4171 (motY); 5, LM4811 (fliG); 6, LM4815 (fliM); 7, LM4812 (fliN); 8, LM1017; 9 and 10, LM1017; 11 and 12, LM4659 (motB2::TnphoA); 13 and 14, LM4289 (motY1719::TnphoA). Semisolid motility plates were incubated at 30°C for 8 (top) and 9.5 h (bottom). All mutant strains are derivatives of LM1017. Strain LM1017 fails to produce lateral flagella; therefore, there is no contribution to motility from the lateral motility system.
One mutant strain displayed some movement. The motility in M agar of LM4659, which contains the phoA fusion near the end of motB, is shown in Fig. 5, strain 11 and 12. The rate of radial expansion in M agar normalized to the rate of the parental strain, LM1017, was 0.38 ± 0.03. Previously, we constructed the mutant strain LM4289 using a TnphoA insertion in motY that created a fusion to alkaline phosphatase after amino acid 289. Like the MotB hybrid protein, which lacks eight C-terminal amino acids, the MotY-alkaline phosphatase hybrid, which lacks four N-terminal amino acids, is functional and permits motility (Fig. 5, strain 13 and 14). The rate of radial expansion for LM4289 was indistinguishable from that for LM1017 (Fig. 5, strains 9 and 10). Thus, in terms of protein function within the motor complex, both MotB and MotY can accommodate C-terminal extensions of fused alkaline phosphatase. Both of these fusion proteins retain their putative peptidoglycan interaction domains.
To distinguish loss of specific gene function from potentially polar effects on downstream flagellar assembly genes, the fli mutants were analyzed for complementation. Plasmid pLM2192, containing only intact fliG and fliH genes, restored the swimming motility of the fliG mutant strains LM4807, LM4809, and LM4811 in motility plates containing chloramphenicol to select for retention of the chromosomal mutation and tetracycline to select for maintenance of the plasmid. Complementation of LM4811 is shown in Fig. 6A. To confirm that the observed motility was due to complementation and not recombination, segregation analysis after serial passage of the complemented, motile swarms in the absence of an antibiotic demonstrated that the cells that had lost the plasmid concomitantly lost motility. IPTG-inducible motility was observed after the introduction of the fliMN expression plasmid pLM2294 into fliN strain LM4812 (Fig. 6B, strain 2 versus strain 1) and fliM strain LM4815 (strain 4 versus strain 3). Thus, the fliG, fliM, and fliN genes play essential roles in swimming motility. It should be noted that for IPTG-promoted induction of fli gene expression from plasmids pLM2294 (fliM+N+) and pLM2296 (fliN+), differences in motility for different mutant alleles were observed, e.g., insertions with TnphoA aligned with or opposed to transcription and fliM versus fliN mutants. We interpret these data in light of findings for E. coli (56, 57) to suggest that the ratio of FliM to FliN is critical for maximal motility.
Introduction of pLM2296 (fliM+) to strain LM4815 resulted in IPTG-controllable motility (Fig. 6B, strain 6); however, pLM2297 (containing fliMshort) failed to restore motility to LM4815 (strain 7). FliMshort appeared to interfere with flagellar assembly, for no flagellin was detected in immunoblots containing whole-cell preparations of IPTG-induced strain LM4815 with pLM2297 (data not shown). Moreover, IPTG induction of strain LM1017 with pLM2297, but not with the parental vector, pLM2296 (fliM+), or pLM2294 (fliM+N+), reduced movement in M agar (Fig. 6B, strain 9 compared to strains 8, 10, and 11). This suggests that in V. parahaemolyticus fliMshort is expressed, the product is stable, and FliMshort negatively interferes with the function of the wild-type switch complex.
Effects of switch and motor mutations on swarming and swarmer cell gene expression.
Although genetic evidence suggested that the flhA gene was required for polar but not lateral flagellar assembly, it seemed necessary to define the specificity of the roles of the upstream gene products, in particular, to determine whether the switch gene products were unique for polar motility or were shared by both flagellar systems. Gene disruption in the swarming-defective strain allowed evaluation of the role of the genes in swimming motility. To assess the contribution of the fli genes identified on cosmid pLM2047 to swarming motility, the transposons that were inserted into these genes were transferred to the chromosome of wild-type strain BB22.
Figure 7A shows polar and lateral flagellin profiles of wild-type and mutant strains harvested from plates. All of the strains produced lateral flagellin. The swarming motility over the surface of solidified swarm medium of strains with transposon-induced mutations in motA, motB, motX, motY, fliG, fliM, or fliN resembled wild-type swarming motility. Thus, lateral function is unaffected in the mutants, and these switch and motor genes are reserved for polar flagellar function. This panel also shows that mutant strains with mot defects produced polar flagellins, whereas strains with fliG, fliM, or fliN insertions failed to synthesize polar flagellins.
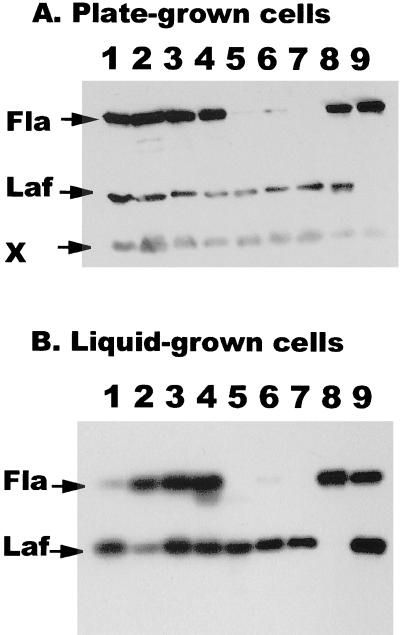
FIG. 7.
Immunoblot analysis of polar and lateral flagellin production by strains with polar switch and motor defects. All mutant strains were derived from the wild-type strain BB22. Blots were reacted with pooled antisera directed against polar (Fla) and lateral (Laf) flagellins. (A) Mutant strains were harvested from plates. Lanes: 1, LM4652 (motA); 2, LM4656 (motB); 3, LM4262 (motX); 4, LM4474 (motY); 5, LM4810 (fliG); 6, LM4830 (fliM); 7, LM4832 (fliN); 8, BB22 (from plates); 9, BB22 (from liquid). (B) Strains harvested from liquid cultures were loaded into lanes as in panel A, except that lane 8 contained BB22 from liquid and lane 9 contained BB22 from plates. The immunoblot in panel A was reacted with pooled antisera at a dilution of 1:5,000 during an overnight incubation. A minor, antiserum-reactive, nonflagellin band (X) served as a control for the amount of whole cells loaded in each lane. The immunoblot in panel B was reacted with a 1:1,000 dilution of antilateral serum and a 1:5,000 dilution of antipolar serum for a 2-h incubation.
Previous work has demonstrated that polar flagellar function is intimately related to induction of swarmer cell development (38). Conditions that slow the flagellar motor down, e.g., solid surfaces, viscosity, or use of the sodium channel-blocking drug phenamil, induce swarmer cell development (23, 38). Genetic disruption of polar flagellar function has also been shown to result in constitutive swarmer cell gene expression (38, 41). To further dissect the mechanism of surface sensing and signal transmission, we examined the effect of these mutations, which knock out all known components of the polar motor, on induction of lateral flagella (Fig. 7B). In contrast to the wild-type strain, which only produces lateral flagella when grown on solidified medium (lane 9) and not when grown in liquid medium (lane 8), all of the mutants produced lateral flagella when grown in liquid culture (lanes 1 to 7).
DISCUSSION
The rotary flagellar motor is a powerful molecular machine that couples the energy of membrane potential to rotation of the flagellum. This rotation is reversible, and the flagellum has been measured to turn at rates as fast as 100,000 rpm (33). The mechanism of conversion of chemical energy to work, i.e., transduction of the transmembrane potential of specific ions to generation of torque, is not fully understood. Two types of flagellar motors, driven by different coupling ions, are known. It is believed that there is highly efficient coupling of the passage of protons or sodium ions through the stationary part of the motor to the generation of torque (5), which occurs at the C ring found at the base of the flagellum. FliG, FliM, and FliN form the C ring of proton-type motors. The C ring is also known as the switch complex because it controls the direction of flagellar rotation. With respect to the proton-type motor, a structural model for the part of the rotor that interacts with the stator has been developed on the basis of extensive mutational analysis coupled with the crystal structure determination of the C-terminal domain of FliG (9, 18, 19, 21, 29, 31, 37, 50, 59). It is postulated that key charged residues on the face of a ridge of FliG interact with specific charged residues of MotA (31).
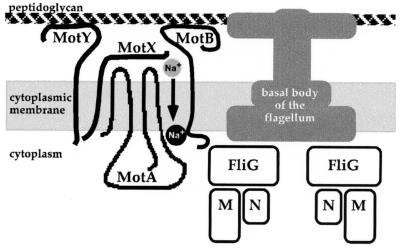
How does the sodium-type flagellar motor work? Seven components have been identified and are illustrated in Fig. 8. Four are membrane proteins and may comprise the stator. Two of these, MotB and MotY, possess domains likely to interact with peptidoglycan and may be the elements responsible for anchoring the force generator. These two proteins can be successfully fused to alkaline phosphatase with retention of function, which suggests that there is sufficient tolerance within the torque-generating complex to accommodate bulk near MotB and MotY. The proton-type motor has a single protein to perform the equivalent stabilizing function, i.e., MotB. In the proton-driven motor, transmembrane domains of MotA and MotB form the proton-conducting channel. For sodium-driven motors, phenamil resistance maps to sites in MotA and MotB, implicating these proteins in Na+ transfer (22, 26). The most unusual component of the motor is MotX, and its function is unknown, although it has been shown to recruit MotY to the membrane (40). When MotX is introduced into E. coli, its overexpression renders E. coli sensitive to killing by Na+. Potentially MotX might modify or specify MotA-MotB ion channel selectivity. It is postulated that on transfer of Na+ through the torque generator, force is transmitted from the stator to FliG, which is part of the rotor. Our discovery of the genes encoding components of the switching apparatus for the polar flagellum of V. parahaemolyticus provides insight into the workings of the sodium-type motor. The strong homology of the V. parahaemolyticus fliG, fliM, and fliN gene products with the proton-type switch proteins argues that the role of the switch complex in the sodium-type motor is similar to the role of the switch complex in the proton-type motor.
FIG. 8.
Model for the sodium-type flagellar motor. Seven genes that encode components of the motor have been identified. Allelic disruption using transposons demonstrates that all are essential for polar-type motility. Alkaline phosphatase fusions at the C termini of MotB or MotY interfere with, but do not abolish, polar motility. The function of MotX is unknown, although it is essential for torque generation and has been shown to interact with MotY. MotA and MotB resemble their homologs in the proton-driven motor; although they are not interchangeable with the motor parts of E. coli. Potential Na+ interaction sites on the cytoplasmic face of MotA and MotB (proximal to the Na+ that is indicated by the black circle) have been defined by mutations conferring phenamil resistance. On passage of sodium ions through the motor, torque is transmitted from the presumed stationary components (MotA, -B, -X, and -Y) to the flagellar switch components (FliG, -M, and -N) located at the base of the flagellar basal body. Switch components are reserved for polar, and not lateral, function in V. parahaemolyticus, although they can partially interact with the E. coli flagellar apparatus.
The deduced switch proteins of V. parahaemolyticus resemble their proton-type homologs. Each possesses conserved, charged residues previously shown to be required for motor function in E. coli and S. enterica serovar Typhimurium. The conservation of charged residues at the active-site ridge of proton-type FliG with residues in sodium-type FliG, coupled with the conservation of E. coli MotA residues shown to participate in key electrostatic interactions with FliG and amino acids in sodium-type MotA (22), suggests that the mechanisms of energy transfer at the rotor-stator interface in the two motors are similar, despite being powered by different ion flows.
In switch-defective E. coli mutant strains, low-level expression of V. parahaemolyticus fliM and fliN, but not fliG, induces trails of motility in M agar and the aberrant twitching motility observed in the microscope. High-level expression of Vibrio fliM or fliN impedes movement of the wild-type E. coli strain. Thus, when the sodium switch parts are expressed in E. coli, there is limited interaction between them and the proton-type motor-switch complex. V. parahaemolyticus FliG is unable to participate in the E. coli switching apparatus. The E. coli mutant with Vibrio fliG on a plasmid fails to produce flagella. It seems possible that the N-terminal domain of V. parahaemolyticus FliG precludes interaction with the E. coli switch-assembly complex. Studies with FliG of Thermotoga maritima have shown that although the full-length protein fails to interact properly with flagellar proteins of E. coli, replacement of the T. maritima FliG N terminus, which contains the domain required for flagellar assembly, with the equivalent domain from E. coli creates a functional hybrid protein (31). Sequence alignments of the C termini of T. maritima FliG and V. parahaemolyticus FliG exhibit the same degree of identity with E. coli FliG.
V. parahaemolyticus FliM and FliN possess charge domains not found in their proton-type counterparts. It will be of interest to determine whether these domains are functionally important and if so whether they are important with respect to motor, switching, or assembly function. To initiate such studies, we examined the C terminus of the FliM. C-terminal truncation of S. enterica serovar Typhimurium FliM does not impair protein function (60). On the basis of the nucleotide sequence, the V. parahaemolyticus FliM polypeptide was predicted to be more highly charged and longer than other FliM homologs. FliM production was examined in minicells, along with the product of truncated gene fliMshort, which coded for a protein prematurely terminating at residue 320. In contrast to what was found for a plasmid encoding full-length FliM, FliMshort failed to productively substitute when the truncated gene was introduced into V. parahaemolyticus fliM mutant strains, and mutant fliM/fliMshort merodiploid strains appear blocked in flagellar assembly for they failed to produce flagellin. FliMshort negatively interfered with motility when overexpressed from a plasmid in the wild-type strain. Taken together, these experiments support the idea that the charged C terminus of V. parahaemolyticus FliM is essential for motility.
V. parahaemolyticus achieves the assembly of two types of flagellar appendages simultaneously. Prior genetic analysis has suggested, but not proven, that polar and lateral flagellar structural and assembly components were distinct because mutants failing to swim could still swarm and vice versa (38, 42). However, when both flagellar systems are present, behavior is coordinated; therefore, at some level the two motility systems must be integrated. How is the flow of chemosensory information channeled? It is known that chemotaxis mutants with defects in a locus that encodes central, chemotaxis signal transduction proteins fail to productively swim or swarm (48). This work identifies a large locus of flagellar genes that encode components for the switching apparatus and assembly. Insertional inactivation of fliG, fliM, and fliN genes in this locus demonstrated that the genes encode products reserved for polar function. All of the mutants retained swarming motility. Thus, these switch genes are specialized for the polar system. It seems that integration of chemosensory signaling must occur prior to interaction with the switch. Perhaps phosphorylated CheY can interact with both polar and lateral switch complexes, or perhaps multiple CheY proteins exist. Recent studies have shown that a domain near the N terminus of S. enterica serovar Typhimurium FliM interacts with phospho-CheY (61), and this segment is quite well conserved in the V. parahaemolyticus polar FliM. The switch components for the Vibrio lateral motor remain to be identified.
This work also provides some insight into the hierarchy of polar flagellar gene control and assembly. Mutants with defects in the polar motor genes, motX, motY, motA, and motB, produce a polar flagellum, as determined by immunodetection of flagellins (shown in this work) and electron microscopy (not shown). Thus, the mot products are not required for flagellar assembly. Mutants with defects in the fliG, fliM, or fliN gene fail to produce polar flagellins. Immunoblot analysis of flagellin production was performed on whole cells; therefore, flagellin gene expression seems to also be affected. These results are consistent with the hierarchy of flagellar gene control and assembly that has been established for E. coli and S. enterica serovar Typhimurium (32). In addition, the phenotype of these mutants contributes to our understanding of the mechanism of surface sensing. The performance of the polar flagellum is coupled to the transcription of swarmer cell genes. When function is inhibited, for example, by physical constraint (38) or by using the sodium channel inhibitor phenamil (23), swarmer cell differentiation is induced. Polar flagellar rotation and swarmer cell induction are inversely correlated, and thus motor function is implicated in signal transduction. We have now determined that loss of function of any of the seven motor or switch genes creates mutant strains constitutive for expression of swarmer cell genes. Thus, none of these gene products are essential for triggering swarmer cell development.
ACKNOWLEDGMENTS
We thank David Blair for bacterial strains and counsel, May Macnab for antiserum to E. coli flagellin, and the DNA Core at the University of Iowa for excellent support.
This research was supported by Public Health Service grant GM43196 from the National Institutes of Health to L.L.M. and NSF and Howard Hughes Undergraduate Research Fellowships to B.R.B.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asai Y, Kojima S, Kato H, Nishioka N, Kawagishi I, Homma M. Putative channel components for the fast-rotating sodium flagellar motor of a marine bacterium. J Bacteriol. 1997;179:5104–5110. doi: 10.1128/jb.179.16.5104-5110.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atsumi T, McCarter L, Imae Y. Polar and lateral flagellar motors of marine Vibrio are driven by different ion membrane forces. Nature (London) 1992;355:182–184. doi: 10.1038/355182a0. [DOI] [PubMed] [Google Scholar]
- 4.Belas R, Simon M, Silverman M. Regulation of lateral flagella gene transcription in Vibrio parahaemolyticus. J Bacteriol. 1986;167:210–218. doi: 10.1128/jb.167.1.210-218.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg H C. Torque generation by the flagellar rotary motor. Biophys J. 1995;68:163s–167s. [PMC free article] [PubMed] [Google Scholar]
- 6.Blair D F. How bacteria sense and swim. Annu Rev Microbiol. 1995;49:489–522. doi: 10.1146/annurev.mi.49.100195.002421. [DOI] [PubMed] [Google Scholar]
- 7.Blair D F, Berg H C. The MotA protein of E. coli is a proton-conducting component of the flagellar motor. Cell. 1990;60:439–449. doi: 10.1016/0092-8674(90)90595-6. [DOI] [PubMed] [Google Scholar]
- 8.Blair D F, Berg H C. Mutations in the MotA protein of Escherichia coli reveal domains critical for proton conduction. J Mol Biol. 1991;221:1433–1442. doi: 10.1016/0022-2836(91)90943-z. [DOI] [PubMed] [Google Scholar]
- 9.Braun T F, Poulson S, Gully J B, Empey J C, Way S V, Putnam A, Blair D F. Function of proline residues of MotA in torque generation by the flagellar motor of Escherichia coli. J Bacteriol. 1999;181:3542–3551. doi: 10.1128/jb.181.11.3542-3551.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chun S Y, Parkinson J S. Bacterial motility: membrane topology of the Escherichia coli MotB protein. Science. 1988;230:276–277. doi: 10.1126/science.2447650. [DOI] [PubMed] [Google Scholar]
- 11.Dean G E, Macnab R M, Stader J, Matsumura P, Burks C. Gene sequence and predicted amino acid sequence of the motA protein, a membrane-associated protein required for flagellar rotation in Escherichia coli. J Bacteriol. 1984;159:991–999. doi: 10.1128/jb.159.3.991-999.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeMot R, Vanderleyden J. The C-terminal sequence conservation between OmpA-related outer membrane proteins and MotB suggests a common function in both Gram-positive and Gram-negative bacteria, possibly in the interaction of these domains with peptidoglycan. Mol Microbiol. 1994;12:333–334. doi: 10.1111/j.1365-2958.1994.tb01021.x. [DOI] [PubMed] [Google Scholar]
- 13.DeRosier D J. The turn of the screw: the bacterial flagellar motor. Cell. 1998;93:17–20. doi: 10.1016/s0092-8674(00)81141-1. [DOI] [PubMed] [Google Scholar]
- 14.Engebrecht J, Silverman M. Identification of genes and gene products necessary for bacterial bioluminescence. Proc Natl Acad Sci USA. 1984;81:4154–4158. doi: 10.1073/pnas.81.13.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francis N R, Sosinsky G E, Thomas D, DeRosier D J. Isolation, characterization and structure of bacterial flagellar motors containing the switch complex. J Mol Biol. 1994;235:1261–1270. doi: 10.1006/jmbi.1994.1079. [DOI] [PubMed] [Google Scholar]
- 16.Friedman A, Long S R, Brown S E, Buikema W J, Ausubel F. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982;18:289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- 17.Fuerste J P, Pansegrau W, Frank R, Blocker H, Scholz P, Bagdasarian M, Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48:119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- 18.Garza A G, Biran R, Wohlschlegel J A, Manson M D. Mutations in motB suppressible by changes in stator or rotor components of the bacterial flagellar motor. J Mol Biol. 1996;258:270–285. doi: 10.1006/jmbi.1996.0249. [DOI] [PubMed] [Google Scholar]
- 19.Garza A G, Harris-Haller L W, Stoebner R A, Manson M D. Motility protein interactions in the bacterial flagellar motor. Proc Natl Acad Sci USA. 1995;92:1970–1974. doi: 10.1073/pnas.92.6.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imae Y, Atsumi T. Na+ driven bacterial flagellar motors. J Bioenerg Biomembr. 1989;21:705–716. doi: 10.1007/BF00762688. [DOI] [PubMed] [Google Scholar]
- 21.Irikura V M, Kihara M, Yamaguchi S, Sockett H, Macnab R M. Salmonella typhimurium fliG and fliN mutations causing defects in assembly, rotation, and switching of the flagellar motor. J Bacteriol. 1993;175:802–810. doi: 10.1128/jb.175.3.802-810.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaques S, Kim Y-K, McCarter L L. Mutations conferring resistance to phenamil and amiloride, inhibitors of sodium-driven motility of Vibrio parahaemolyticus. Proc Natl Acad Sci USA. 1999;96:5740–5745. doi: 10.1073/pnas.96.10.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawagishi I, Imagawa M, Imae Y, McCarter L, Homma M. The sodium-driven polar flagellar motor of marine Vibrio as the mechanosensor that regulates lateral flagellar expression. Mol Microbiol. 1996;20:693–699. doi: 10.1111/j.1365-2958.1996.tb02509.x. [DOI] [PubMed] [Google Scholar]
- 24.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 25.Khan S, Dapice M, Reese T S. Effects of mot gene expression on the structure of the flagellar motor. J Mol Biol. 1988;202:575–584. doi: 10.1016/0022-2836(88)90287-2. [DOI] [PubMed] [Google Scholar]
- 26.Kojima S, Asai Y, Atsumi T, Kawagishi I, Homma M. Na+-driven flagellar motor resistant to phenamil, an amiloride analog, caused by mutations in putative channel components. J Mol Biol. 1999;285:1537–1547. doi: 10.1006/jmbi.1998.2377. [DOI] [PubMed] [Google Scholar]
- 27.Kovach M E, Phillips R W, Elzer P H, Roop II R M, Peterson K M. PBBR1MCS: a broad-host-range cloning vector. BioTechniques. 1994;16:800–802. [PubMed] [Google Scholar]
- 28.Larsen S H, Adler J, Gargus J J, Hogg R W. Chemomechanical coupling without ATP: the source of energy for motility and chemotaxis in bacteria. Proc Natl Acad Sci USA. 1974;71:1239–1243. doi: 10.1073/pnas.71.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lloyd S A, Blair D F. Charged residues of the rotor protein FliG essential for torque generation in the flagellar motor of Escherichia coli. J Mol Biol. 1997;266:733–744. doi: 10.1006/jmbi.1996.0836. [DOI] [PubMed] [Google Scholar]
- 30.Lloyd S A, Tang H, Wang X, Billings S, Blair D F. Torque generation in the flagellar motor of Escherichia coli: evidence of a direct role for FliG but not for FliM or FliN. J Bacteriol. 1996;178:223–231. doi: 10.1128/jb.178.1.223-231.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lloyd S A, Whitby F G, Blair D F, Hill C P. Structure of the C-terminal domain of FliG, a component of the rotor in the bacterial flagellar motor. Nature. 1999;400:472–475. doi: 10.1038/22794. [DOI] [PubMed] [Google Scholar]
- 32.Macnab R M. Flagella and motility. In: Neidhardt F C, Curtiss R III, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 123–146. [Google Scholar]
- 33.Magariyama Y, Sugiyama S, Muramoto K, Maekawa Y, Kawagishi I, Imae Y, Kudo S. Very fast flagellar rotation. Nature. 1994;371:752. doi: 10.1038/371752b0. [DOI] [PubMed] [Google Scholar]
- 34.Manoil C, Bailey J. A simple screen for permissive sites in proteins: analysis of Escherichia coli lac permease. J Mol Biol. 1997;267:250–263. doi: 10.1006/jmbi.1996.0881. [DOI] [PubMed] [Google Scholar]
- 35.Manoil C, Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci USA. 1985;83:8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manson M D, Tedesco P, Berg H C, Harold F M, van der Drift C. A protonmotive force drives bacterial flagella. Proc Natl Acad Sci USA. 1977;74:3060–3064. doi: 10.1073/pnas.74.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marykwas D L, Berg H C. A mutational analysis of the interaction between FliG and FliM, two components of the flagellar motor of Escherichia coli. J Bacteriol. 1996;178:1289–1294. doi: 10.1128/jb.178.5.1289-1294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarter L, Hilmen M, Silverman M. Flagellar dynamometer controls swarmer cell differentiation of V. parahaemolyticus. Cell. 1988;54:345–351. doi: 10.1016/0092-8674(88)90197-3. [DOI] [PubMed] [Google Scholar]
- 39.McCarter L L. MotY, a component of the sodium-type flagellar motor. J Bacteriol. 1994;176:4219–4225. doi: 10.1128/jb.176.14.4219-4225.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCarter L L. MotX, a channel component of the sodium-type flagellar motor. J Bacteriol. 1994;176:5988–5998. doi: 10.1128/jb.176.19.5988-5998.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCarter L L. Genetic and molecular characterization of the polar flagellum of Vibrio parahaemolyticus. J Bacteriol. 1995;177:1595–1609. doi: 10.1128/jb.177.6.1595-1609.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCarter L L. The multiple identities of Vibrio parahaemolyticus. J Mol Microbiol Biotechnol. 1999;1:51–57. [PubMed] [Google Scholar]
- 43.McCarter L L, Silverman M. Phosphate regulation of gene expression in Vibrio parahaemolyticus. J Bacteriol. 1987;169:3441–3449. doi: 10.1128/jb.169.8.3441-3449.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCarter L L, Wright M E. Identification of genes encoding components of the swarmer cell flagellar motor and propeller and a sigma factor controlling differentiation of Vibrio parahaemolyticus. J Bacteriol. 1993;175:3361–3371. doi: 10.1128/jb.175.11.3361-3371.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meister M, Lowe G, Berg H C. The proton flux through the bacterial flagellar motor. Cell. 1987;49:643–650. doi: 10.1016/0092-8674(87)90540-x. [DOI] [PubMed] [Google Scholar]
- 46.Oosawa K, Ueno T, Aizawa S I. Overproduction of the bacterial flagellar switch proteins and their interactions with the MS ring complex in vitro. J Bacteriol. 1994;176:3683–3691. doi: 10.1128/jb.176.12.3683-3691.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 48.Sar N, McCarter L, Simon M, Silverman M. Chemotactic control of the two flagellar systems of Vibrio parahaemolyticus. J Bacteriol. 1990;172:334–341. doi: 10.1128/jb.172.1.334-341.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schweizer H P. Small broad-host-range gentamicin resistance gene cassette for site-specific insertion and deletion mutagenesis. BioTechniques. 1993;15:831–833. [PubMed] [Google Scholar]
- 50.Sharp L L, Zhou J, Blair D F. Tryptophan-scanning mutagenesis of MotB, an integral membrane protein essential for flagellar rotation in Escherichia coli. Biochemistry. 1995;34:9166–9171. doi: 10.1021/bi00028a028. [DOI] [PubMed] [Google Scholar]
- 51.Sharp L L, Zhou J, Blair D F. Features of MotA proton channel structure revealed by tryptophan-scanning mutagenesis. Proc Natl Acad Sci USA. 1995;92:7946–7950. doi: 10.1073/pnas.92.17.7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silverman M, Showalter R, McCarter L. Genetic analysis in Vibrio. Methods Enzymol. 1991;204:515–536. doi: 10.1016/0076-6879(91)04026-k. [DOI] [PubMed] [Google Scholar]
- 53.Sockett H, Yamaguchi S, Kihara M, Irikura V M, Macnab R M. Molecular analysis of the flagellar switch protein FliM of Salmonella typhimurium. J Bacteriol. 1992;174:793–806. doi: 10.1128/jb.174.3.793-806.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stader J, Matsumura P, Vacante D, Dean G E, Macnab R M. Nucleotide sequence of the Escherichia coli motB gene and site-limited incorporation of its product into the cytoplasmic membrane. J Bacteriol. 1986;166:244–252. doi: 10.1128/jb.166.1.244-252.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stolz B, Berg H C. Evidence for interactions between MotA and MotB, torque-generating elements of the flagellar motor of Escherichia coli. J Bacteriol. 1991;173:7033–7037. doi: 10.1128/jb.173.21.7033-7037.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang H, Billings S, Wang X, Sharp L, Blair D F. Regulated underexpression and overexpression of the FliN protein of Escherichia coli and evidence for an interaction between FliN and FliM in the flagellar motor. J Bacteriol. 1995;177:3496–3503. doi: 10.1128/jb.177.12.3496-3503.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang H, Blair D F. Regulated underexpression of the FliM protein of Escherichia coli and evidence for location in the flagellar motor distinct from the MotA/MotB torque generators. J Bacteriol. 1995;177:3485–3495. doi: 10.1128/jb.177.12.3485-3495.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Togashi F, Yamaguchi S, Kihara M, Aizawa S-I, Macnab R M. An extreme clockwise switch bias mutation in fliG of Salmonella typhimurium and its suppression by slow-motile mutations in motA and motB. J Bacteriol. 1997;179:2994–3003. doi: 10.1128/jb.179.9.2994-3003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toker A S, Kihara M, Macnab R M. Deletion analysis of the fliM flagellar switch protein of Salmonella typhimurium. J Bacteriol. 1996;178:7069–7079. doi: 10.1128/jb.178.24.7069-7079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Toker A S, Macnab R M. Distinct regions of bacterial flagellar switch protein FliM interact with FliG, FliN, and CheY. J Mol Biol. 1997;273:623–634. doi: 10.1006/jmbi.1997.1335. [DOI] [PubMed] [Google Scholar]
- 62.Welch M, Oosawa K, Aizawa S-I, Eisenbach M. Phosphorylation-dependent binding of a signal molecule to the flagellar switch of bacteria. Proc Natl Acad Sci USA. 1993;90:8787–8791. doi: 10.1073/pnas.90.19.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woo T H S, Cheng A F, Ling J M. An application of a simple method for the preparation of bacterial DNA. BioTechniques. 1992;13:696–697. [PubMed] [Google Scholar]
- 64.Yamaguchi S, Fujita H, Ishihara A, Aizawa S-I, Macnab R M. Subdivision of flagellar genes of Salmonella typhimurium into regions responsible for assembly, rotation, and switching. J Bacteriol. 1986;166:187–193. doi: 10.1128/jb.166.1.187-193.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamaguchi S, Aizawa S-I, Kihara M, Isomura M, Jones C J, Macnab R M. Genetic evidence for a switching and energy-transducing complex in the flagellar motor of Salmonella typhimurium. J Bacteriol. 1986;168:1172–1179. doi: 10.1128/jb.168.3.1172-1179.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou J, Blair D F. Residues of the cytoplasmic domain of MotA essential for torque generation in the bacterial flagellar motor. J Mol Biol. 1997;273:428–439. doi: 10.1006/jmbi.1997.1316. [DOI] [PubMed] [Google Scholar]
- 67.Zhou J, Lloyd S A, Blair D F. Electrostatic interactions between rotor and stator in the bacterial flagellar motor. Proc Natl Acad Sci USA. 1998;95:6436–6441. doi: 10.1073/pnas.95.11.6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou J, Sharp L L, Tan H L, Lloyd S A, Billings S, Braun T F, Blair D F. Function of protonable residues in the flagellar motor of Escherichia coli: a critical role for Asp 32 of MotB. J Bacteriol. 1998;180:2729–2735. doi: 10.1128/jb.180.10.2729-2735.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]