Summary
Interstitial microdeletions in the proximal region of the long arm of chromosome 6 are rare. Herein we have reported 12 patients with developmental delays associated with interstitial microdeletions in 6q ranging from q12 to q22. The microdeletions were detected by chromosomal microarray testing. To confirm the clinical significance of these deletions, genotype-phenotype correlation analysis was performed using genetic and predicted loss-of-function data. SIM1 was recognized as the gene responsible for developmental delay, particularly in Prader-Willi syndrome-like phenotypes. Other genes possibly related to developmental delay were ZNF292, PHIP, KCNQ5, and NUS1. To further establish the correlation between the genotype and phenotype, more patient information is required.
Keywords: chromosomal microarray testing, 6q interstitial deletions, developmental delay
1. Introduction
Interstitial microdeletions in the proximal region of the long arm of chromosome 6 are rare. In 1997, Hopkins et al. reported three new cases and reviewed 57 previously reported cases of partial deletions on 6q and classified them into three phenotypic groups: proximal [del(6) (q11-q16)], intermediate [del(6)(q15-q25)], and distal [del(6)(q25-qter)] (1). Although there were some characteristic features unique to each phenotypic group, recognizable clinical entities were not established at that time. In 2007, Klein et al. reported three patients with 6q deletions (2). Two of them were obese and showed signs of hypotonia and developmental delay, which were described as Prader-Willi syndrome (PWS)-like phenotypes; SIM1 (MIM* 603128) located in the deleted region, was suspected to be a candidate gene for the PWS-like phenotype. In 2012, Rosenfeld et al. reported 12 new cases of interstitial 6q deletions and four of the cases had PWS-like phenotypes (3). These findings suggest that there is a correlation between the occurrence of the PWS-like phenotype and SIM1 deletion. This is further supported by other reports of patients with similar genotypic and phenotypic findings (4,5).
Similarly, deletions in certain regions were associated with characteristic findings. So far, in our ongoing research on genomic copy number analysis using chromosomal microarray testing, we have encountered 12 patients who showed interstitial microdeletions in 6q. Herein, the genotype-phenotype correlation was assessed.
2. Patients and Methods
This study was performed in accordance with the Declaration of Helsinki and approved by the ethics committee of this institution. Written informed consent was obtained from the families of the patients. Blood samples were collected from the patients; additionally, blood samples were also collected from the parents of the patients when it was necessary for further investigation. Genomic copy number variations were analyzed using the Agilent Microarray system (Agilent Technologies, Santa Clara, CA, USA), as previously described (6). Based on the results, patients with 6q interstitial microdeletions were included in this study. The clinical information of the patients was obtained from their attending doctors. The correlation between the genotype and phenotype was then investigated using a genome map in which the deleted regions were depicted (Figure 1). Gene information was evaluated using Online Mendelian Inheritance in Man® (OMIM; https://www. omim.org/). Predicted loss-of-function was also used for the evaluation. The genomic coordinate referred to was the GRCh37/hg19 genome build.
Figure 1.
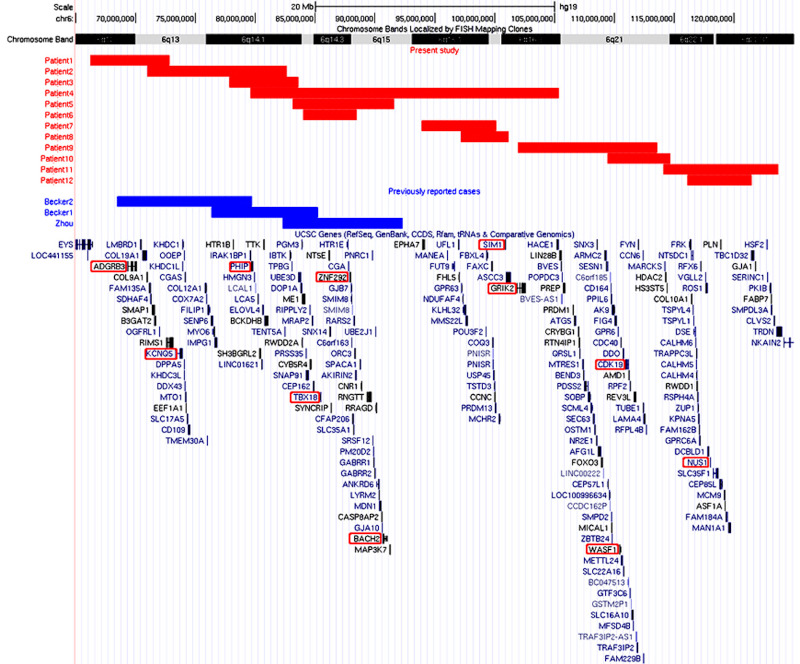
Genome map of 6q captured from the UCSC genome browser. Regions of the identified deletions are depicted by custom tracks with rectangles; the red and blue are for regions of the deletions identified in this study and previous studies, respectively. The genes discussed in the text are highlighted using red circles.
3. Results and Discussion
Interstitial deletions involving the long arm of chromosome 6 were identified in 12 patients: six males and six females, ranging in age from 1 to 21 years (Table 1). Although all the patients showed developmental delays, the extent of developmental delay varied among the patients. The deleted regions are shown on the genome map in Figure 1. For further evaluation, we summarized the genes from the deleted regions and the probability of loss-of-function intolerance (pLI), shown in Supplemental Table S1 (http://www.irdrjournal.com/ action/getSupplementalData.php?ID=112) (7). The candidate genes listed were those related to autosomal dominant traits and had a pLI score of 1. The genes listed are shown in Figure 1, in which genome map is captured from the UCSC genome browser (https://genome.ucsc. edu/).
Table 1. Clinical features of the patients in this study.
| Items | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | Patient 11 | Patient 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 8y9m | 1y2m | 1y5m | 21y | 1y6m | 3y2m | 2y3m | 16y4m | 5y7m | 3y10m | 4y10m | 13y9m |
| Gender | Female | Male | Female | Male | Male | Male | Female | Female | Male | Female | Male | Female |
| Chromosome band | 6q12q13 | 6q13q14.1 | 6q14.1 | 6q14.1q16.3 | 6q14.1q15 | 6q14.2q15 | 6q16.1 | 6q16.1q16.3 | 6q16.3q21 | 6q21q22.1 | 6q21q22.31 | 6q22.1q22.31 |
| Start* | 65,485,026 | 67,176,848 | 76,392,293 | 72,891,116 | 81,135,362 | 84,025,024 | 92,902,137 | 96,533,420 | 98,273,562 | 108,670,734 | 113,020,897 | 114,656,502 |
| End* | 73,838,151 | 85,971,380 | 84,606,179 | 110,736,632 | 93,665,050 | 88,452,718 | 101,255,262 | 101,724,035 | 117,068,093 | 115,143,797 | 125,341,725 | 123,074,922 |
| Delition Size (Mb) | 8.4 | 18.8 | 8.2 | 37.8 | 12.5 | 4.4 | 8.4 | 5.2 | 18.8 | 6.5 | 12.3 | 8.4 |
| Gestational age | NA | 39w1d | 39w3d | 38w0d | NA | 40w6d | 39w5d | 38w4d | 37w6d | 39w6d | 39w0d | 40w5d |
| Birth weight | NA | 2976 | 3135 | 1970 | NA | 3194 | 2860 | 3048 | 2156 | 3554 | 3165 | 2595 |
| Birth length | NA | 47.6 | 49.5 | NA | NA | 50.5 | 48 | NA | 46 | 52 | 49.1 | 46.2 |
| Birth occipitofrontal circumference | NA | 32.9 | 33 | NA | NA | 35 | 30.4 | NA | 32 | 34 | 32.5 | 31 |
| Developmental delay | + | + | + | + | + | + | + | ± | + | + | + | + |
| DQ/IQ | DQ 79 | DQ 38 | DQ below 35 | IQ 76 | DQ 35 | DQ60 | DQ below 35 | DQ54 | ||||
| Growth deficiency | - | - | + | NA | - | - | - | - | + | - | - | - |
| Microcephaly | - | - | + | - | - | - | -2.6SD | - | - | - | + | - |
| Epilepsy | + | - | - | + | - | - | - | - | - | - | + | - |
| MRI brain abnormalities | - | - | NA | NA | + | - | - | - | NA | + | NA | + |
| Gastrointestinal anomalies | - | - | - | + | + | - | - | - | + | - | - | - |
| Genital anomalies | - | - | - | + | + | + | - | - | - | - | - | - |
| Distinctive facia features | ||||||||||||
| Low-ser-ears | - | + | + | - | - | + | + | - | - | - | + | - |
| Epicanthus | - | - | + | - | - | + | - | - | - | + | + | - |
| Blepharophimosis | - | - | - | - | + | - | + | - | - | - | - | - |
| Micrognathia | - | + | + | - | + | + | + | - | + | - | - | - |
| Flat nasal bridge | - | - | - | - | - | + | - | - | - | - | + | - |
| Wide ala nasi | - | - | - | - | - | - | - | - | - | + | - | - |
| Hypertelorism | - | - | - | - | - | + | + | - | - | + | + | - |
| Other findings | Hypoplastic kidney, renal enuresis | Cryptorchidism, ptosis | Hypothyroidism, precocious puberty, obesity |
y, years; m, months; w, weeks; s, days; NA, not available, DQ, developmental quotient; IQ, inteligent quotient, *Genomic cordinate corresponds to GRCh37/hg19.
The first patient was an 8-year-9-month-old girl (patient 1) who was born by cesarean section owing to an unequal infant pelvis. The patient showed distinctive features. Her development was delayed; the patient started walking independently at 2 years of age and speaking at 4 years of age. At 6 years of age, she developed epilepsy, for which she had to take antiepileptic drugs. A radiological examination of the brain revealed no abnormalities.
The second patient, a 13-month-old boy (patient 2), could turn over, but not sit up; this indicated delayed motor function development. Furthermore, he had language delay. Physical examination revealed distinctive features, which included coarse scalp hair. Magnetic resonance imaging (MRI) of the brain showed no apparent abnormalities.
The third patient was a 17-month-old girl (patient 3) who could not walk unaided, which indicated a developmental delay. She also showed growth deficiency with -2 SD parameters and microcephaly with -3 SD parameters.
The prenatal history of a 21-year-old male patient (patient 4) revealed amniotic fluid overload in the 8th month of pregnancy. There were several congenital anomalies, such as congenital duodenal atresia, inguinal hernia, and cleft palate. In addition, there were also urogenital anomalies present that included bilateral hypoplastic kidneys, renal enuresis, and cryptorchidism. An external strabismus was observed later. His motor skills were limited to sitting, and he had not yet acquired substantial language skills; this indicated a severe psychomotor developmental delay. He developed epilepsy and neuropsychiatric features, including sleep disorder and self-injurious behavior, such as hitting his eyes with his hands. Episodes of hypothermia were also observed.
The fifth patient, an 18-month-old boy (patient 5), had no remarkable perinatal history. The patient showed psychomotor developmental delays from early infancy. There were several abnormal findings, such as blephaophimosis, small jaw, hypertrichosis on the back, tapered phalanges, long first toe, accessory ears, abnormal dentition, hypodontia, soft larynx, and left optic nerve hypoplasia. Gastroesophageal reflux disease was also observed. Brain MRI showed corpus callosum hypoplasia and delayed myelination. However, there were no abnormal results from the laboratory tests for inborn errors of metabolism, mitochondrial disease, Pompe's disease, Fabry disease, and mucopolysaccharidosis. Auditory testing revealed no abnormalities.
Motor development in a 3-year-old boy (patient 6) was delayed; he achieved head control at 8 months, independent sitting at 12 months, crawling at 12 months, and independent walking at 32 months. His language development was severely delayed, and he could not speak comprehensibly. He showed distinctive features that included flat occiput, hypertelorism, epicanthus, bilateral ptosis, strabismus, flat basal bridge, low-set ears, small mouth, short neck, and bilateral cryptorchidism.
The seventh patient, a 27-month-old girl (patient 7), had a small jaw as a distinctive feature. She could crawl, but could not grasp or speak significantly, indicating psychomotor developmental delay. Ophthalmological examination revealed interocular dissection. A neurological examination revealed generalized hypotonia. Brain MRI showed no abnormalities. Moreover, there were no abnormalities found upon routine laboratory examination. She is now 15 years of age. The patient had severe intellectual disability with microcephaly and was not obese.
The eighth patient was a 16-year-4-month-old girl (Patient 8) who was diagnosed with psychomotor developmental delay and hypothyroidism at 14 months of age. She developed precocious puberty and was treated with leuplin, but later she became amenorrheic. This patient was 151 cm tall (-1.3 SD) and weighed 79 kg (+2.6 SD), which indicated obesity.
The ninth patient was a 5-year-7-month-old boy (patient 9) who showed developmental delay. At 2 years of age, he started to walk independently and had no significant speech, indicating delayed development. The patient had a small jaw and was short in stature. Gastroesophageal reflux disease, sleep apnea syndrome, and scoliosis were also observed.
The tenth patient, a 3-year-10-month-old girl (patient 10), had occipital flatness, bilateral eye openings, lamina propria, auricular deformity, low nasal apex, wide nasal bridge, bilateral middle fingers, and curly hair. Brain MRI showed corpus callosum hypoplasia. At the time of examination, her height, weight, and occipitofrontal circumference were 107 cm (+2.3 SD), 18.1 kg (+1.7 SD), and 47.3 cm (-1.2 SD), respectively; this indicated a relatively large stature.
A 4-year-10-month-old boy (patient 11) had sufficient head and neck control; however, he was not ambulatory and has no significant speech, indicating a developmental delay. Physical examination revealed microcephaly, occipital flatness, bilateral eye openings, lamina propria, low auricular position, flat nasal dorsum, cleft palate, and a small mandible. He experienced epileptic seizures, with loss of consciousness for a few seconds, and was taking antiepileptic drugs.
The twelfth patient, a 13-year-9-month-old girl (patient 12), had developmental delay hand tremors. Brain MRI revealed an enlarged cerebellar fissure. EEG abnormalities were also observed. The patient did not experience any epileptic seizures.
According to the genome map (Figure 1), the deleted regions in two of the patients (patients 4 and 7) included SIM1, which was confirmed to contribute to obesity and PWS-like features when loss-of-function variants were present in the coding region (8). Indeed, patient 7 showed PWS-like phenotypes, including obesity; however, patient 4 showed a much more severe developmental delay than that of patient 7. The PWS-like phenotypes could not be distinguished owing to the large region of deletion in 6q, that was observed in this study.
Additionally, patient 4 exhibited urinary tract abnormalities. The overlapping regions of the deletions in patients 4, 5, and 6 included TBX18 (MIM* 604613), which is related to "congenital anomalies of kidney and urinary tract" (MIM# 143400). However, TBX18 variants identified in humans with congenital anomalies of kidney and urinary tract exert a dominant-negative effect rather than haploinsufficiency (9). Thus, we considered that the urinary tract abnormalities observed in patient 4 were not related to the deletion of TBX18, which explained why patient 5 did not show any urinary tract abnormalities.
The overlapping regions of deletion in patients 4, 5, and 6 included ZNF292 (MIM* 616213), which is related to "intellectual developmental disorder, autosomal dominant 64" (MIM# 619188). Therefore, the haploinsufficiency of ZNF292 may have contributed to the developmental delay observed in patients 4 and 5 (10,11). Furthermore, Zhou et al. (2017) reported a patient with developmental delay in association with an overlapping region of a deletion from 6q14.1 to 6q15; this suggested that the haploinsufficiency of ZNF292 is responsible for the delayed development (12).
Additionally, the overlapping region of the deletions in patients 4 and 5 included BACH2 (MIM* 605394), which is related to "immunodeficiency 60 and autoimmunity" (IMD60) (MIM# 618394). Previous reports determined that missense variations of BACH2 contributed to the dominant negative effects; therefore, BACH2 was excluded from this analysis (13).
The shortest region of overlapping deletions was observed in patients 2, 3, and 4. This region included PHIP (MIM* 612870), which is known to cause Chung- Jansen syndrome (CHUJANS; MIM# 617991), a clinical entity characterized by global developmental delay in infants, impaired intellectual development or learning difficulties, behavioral abnormalities, dysmorphic features, and obesity (14). Thus, it is plausible that PHIP haploinsufficiency contributed to the neurodevelopmental delay observed in patients 2, 3, and 4.
Becker e t a l . reported two patients with microdeletions in 6q (15). One of the patients (patient 2) showed a more proximal deletion than that observed in the patient reported by Zhou et al. (12). The deleted region included ADGRB3 (MIM* 602684). ADGRB3 is a member of the adhesion-G protein-coupled receptor family and is mostly expressed in the brain (16). Although the pLI score of ADGRB3 was "1", which indicated intolerance for haploinsufficiency, previously reported ADGRB3 variants were biallelic. Thus, it is unknown whether ADGRB3 is related to the developmental delay observed in patient 1.
The haploinsufficiency of the other genes located in the proximal regions of 6q may be related to the clinical features observed in this study. KCNQ5 (MIM * 607357), located on 6q13, is related to "intellectual developmental disorder, autosomal dominant 46" (MIM# 617601) (17). Loss-of-function variants in KCNQ5 were predicted to lower the seizure threshold by decreasing the repolarization reserve; therefore, it is possible that KCNQ5 haploinsufficiency contributed to the clinical features that were observed in patient 2.
Additionally, GRIK2 (MIM* 138244) was located in the deleted regions in patients 4 and 9. It is related to "neurodevelopmental disorder with impaired language and ataxia and with or without seizures" (MIN# 619580). However, the identified variants of GRIK2 were related to gain-of-function (18); thus, the haploinsufficiency of GRIK2 would not have contributed to the clinical features of these patients.
WASF1 (MIM* 605035) and CDK19 (MIM* 614720) were in the deleted regions that were observed in patients 9 and 10. WASF1 is responsible for "neurodevelopmental disorder with absent language and variable seizures" (MIM# 618707). Previously reported WASF1 variants were nonsense variants, and they were considered to have dominant negative effects, which were a consequence of evading nonsense mediated decay (19). CDK19 is associated with "developmental and epileptic encephalopathy 87" (MIM# 618916). All previously reported variants of CDK19 were dominant negative missense variants that resulted (20). Therefore, it is difficult to attribute the developmental delay observed in patients 8 and 9 to WASF1 or CDK19 haploinsufficiency.
The overlapping region of the deletions observed in patients 9 and 10 was proposed as the etiology for acro-cardio-facial syndrome (MIM 600460) (21,22); however, none of the patients showed any symptoms related to acro-cardio-facial syndrome.
Furthermore, NUS1 (MIM* 610463) was located in the deleted regions observed in patients 10 and 11. NUS1 is responsible for "intellectual developmental disorder, autosomal dominant 55, with seizures" (MIM# 617831). Because previously reported NUS1 variants were related to loss of function, haploinsufficiency was considered the pathogenic mechanism (23).
4. Conclusion
Among the genes in the long arm of chromosome 6 (chr6:65,485,026-125,341,725), only five genes (SIM1, ZNF292, PHIP, KCNQ5, and NUS1) were considered to be related to the developmental delay observed in the patients reported in this study.
Acknowledgements
We would like to express our gratitude to the patients and their families for their cooperation.
Funding:
his study was supported by KAKENHI (Grant Numbers 21K07873) from the Japan Society for the Promotion of Science, Initiative on Rare and Undiagnosed Diseases (grant number 20ek0109301) from the Japan Agency for Medical Research and Development (AMED), and a grant from the Ministry of Health, Labor, and Welfare Japan.
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- 1. Hopkin RJ, Schorry E, Bofinger M, Milatovich A, Stern HJ, Jayne C, Saal HM. New insights into the phenotypes of 6q deletions. Am J Med Genet. 1997; 70:377-386. [PubMed] [Google Scholar]
- 2. Klein OD, Cotter PD, Moore MW, Zanko A, Gilats M, Epstein CJ, Conte F, Rauen KA. Interstitial deletions of chromosome 6q: genotype-phenotype correlation utilizing array CGH. Clin Genet. 2007; 71:260-266. [DOI] [PubMed] [Google Scholar]
- 3. Rosenfeld JA, Amrom D, Andermann E, et al. Genotype-phenotype correlation in interstitial 6q deletions: a report of 12 new cases. Neurogenetics. 2012; 13:31-47. [DOI] [PubMed] [Google Scholar]
- 4. Izumi K, Housam R, Kapadia C, Stallings VA, Medne L, Shaikh TH, Kublaoui BM, Zackai EH, Grimberg A. Endocrine phenotype of 6q16.1-q21 deletion involving SIM1 and Prader-Willi syndrome-like features. Am J Med Genet A. 2013; 161a:3137-3143. [DOI] [PubMed] [Google Scholar]
- 5. Vignoli A, Scornavacca GF, Peron A, La Briola F, Canevini MP. Interstitial 6q microdeletion syndrome and epilepsy: a new patient and review of the literature. Am J Med Genet A. 2013; 161a:2009-2015. [DOI] [PubMed] [Google Scholar]
- 6. Yamamoto T, Wilsdon A, Joss S, Isidor B, Erlandsson A, Suri M, Sangu N, Shimada S, Shimojima K, Le Caignec C, Samuelsson L, Stefanova M. An emerging phenotype of Xq22 microdeletions in females with severe intellectual disability, hypotonia and behavioral abnormalities. J Hum Genet. 2014; 59:300-306. [DOI] [PubMed] [Google Scholar]
- 7. Fabre A, Mancini J. No preferential mode of inheritance for highly constrained genes. Intractable Rare Dis Res. 2022; 11:25-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bonnefond A, Raimondo A, Stutzmann F, et al. Loss-of-function mutations in SIM1 contribute to obesity and Prader-Willi-like features. J Clin Invest. 2013; 123:3037-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vivante A, Kleppa MJ, Schulz J, et al. Mutations in TBX18 cause dominant urinary tract malformations via transcriptional dysregulation of ureter development. Am J Hum Genet. 2015; 97:291-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Engwerda A, Frentz B, den Ouden AL, Flapper BCT, Swertz MA, Gerkes EH, Plantinga M, Dijkhuizen T, van Ravenswaaij-Arts CMA. The phenotypic spectrum of proximal 6q deletions based on a large cohort derived from social media and literature reports. Eur J Hum Genet. 2018; 26:1478-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mirzaa GM, Chong JX, Piton A, et al. De novo and inherited variants i n ZNF292 underlie a neurodevelopmental disorder with features of autism spectrum disorder. Genet Med. 2020; 22:538-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou Q, Wu XH, Yang YC, Zou CC. Clinical Features in Patients with Microdeletion at 6q14.1-q15. Indian J Pediatr. 2017; 84:883-886. [DOI] [PubMed] [Google Scholar]
- 13. Afzali B, Grönholm J, Vandrovcova J, et al. BACH2 immunodeficiency illustrates an association between super-enhancers and haploinsufficiency. Nat Immunol. 2017; 18:813-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Webster E, Cho MT, Alexander N, Desai S, Naidu S, Bekheirnia MR, Lewis A, Retterer K, Juusola J, Chung WK. De novo PHIP-predicted deleterious variants are associated with developmental delay, intellectual disability, obesity, and dysmorphic features. Cold Spring Harb Mol Case Stud. 2016; 2:a001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Becker K, Di Donato N, Holder-Espinasse M, et al. De novo microdeletions of chromosome 6q14.1-q14.3 and 6q12.1-q14.1 in two patients with intellectual disability - further delineation of the 6q14 microdeletion syndrome and review of the literature. Eur J Med Genet. 2012; 55:490-497. [DOI] [PubMed] [Google Scholar]
- 16. Scuderi C, Saccuzzo L, Vinci M, Castiglia L, Galesi O, Salemi M, Mattina T, Borgione E, Città S, Romano C, Fichera M. Biallelic intragenic duplication in ADGRB3 (BAI3) gene associated with intellectual disability, cerebellar atrophy, and behavioral disorder. Eur J Hum Genet. 2019; 27:594-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lehman A, Thouta S, Mancini GMS, et al. Loss-of-function and gain-of-function mutations in KCNQ5 cause intellectual disability or epileptic encephalopathy. Am J Hum Genet. 2017; 101:65-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guzmán YF, Ramsey K, Stolz JR, Craig DW, Huentelman MJ, Narayanan V, Swanson GT. A gain-of-function mutation in the GRIK2 gene causes neurodevelopmental deficits. Neurol Genet. 2017; 3:e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ito Y, Carss KJ, Duarte ST, et al. De novo truncating mutations in WASF1 cause intellectual disability with seizures. Am J Hum Genet. 2018; 103:144-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chung HL, Mao X, Wang H, et al. De novo variants in CDK19 are associated with a syndrome involving intellectual disability and epileptic encephalopathy. Am J Hum Genet. 2020; 106:717-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Milani D, Cagnoli GA, Baccarin M, Alfei E, Guerneri S, Esposito S. Insights into 6q21-q22: Refinement of the critical region for acro-cardio-facial syndrome. Congenit Anom (Kyoto). 2016; 56:187-189. [DOI] [PubMed] [Google Scholar]
- 22. Shukla A, Hebbar M, Harms FL, Kadavigere R, Girisha KM, Kutsche K. Phenotypic variability in patients with interstitial 6q21-q22 microdeletion and Acro-Cardio- Facial syndrome. Am J Med Genet A. 2016; 170:2998-3003. [DOI] [PubMed] [Google Scholar]
- 23. Hamdan FF, Myers CT, Cossette P, et al. High rate of recurrent de novo mutations in developmental and epileptic encephalopathies. Am J Hum Genet. 2017; 101:664-685. [DOI] [PMC free article] [PubMed] [Google Scholar]


